Abstract
We measured the rates of uptake of selected amino acids and betaine by primary cultures of chondrocytes from porcine articular cartilage after the cells had been incubated in ‘isotonic’ (0·3 osmol l−1) or hypertonic (0·5 osmol l−1) media.
Na+-dependent uptake of methylaminoisobutyric acid increased rapidly when the cells were exposed to hypertonic conditions, reached a peak after 6-9 h, and then gradually decreased so that after 24 h it was only slightly above the control value. Conversely, (Na++ Cl−)-dependent influx of γ-aminobutyric acid (GABA) remained low for the first 9 h of hypertonic incubation, but then increased markedly to reach a plateau value after 24-30 h. Betaine influx also increased in cells incubated in hypertonic medium, being mainly Na+ dependent after 6 h, but (Na++ Cl−)-dependent after 24 h.
This pattern indicates that exposure of the chondrocytes to hypertonicity induces first amino acid transport system A and then, as this decreases again, betaine-GABA transport activity.
Induction of betaine-GABA transport activity did not require continuous exposure of chondrocytes to hypertonicity; but the magnitude of the increase measured at the end of a 24 h incubation period was proportional to the length of time the cells had been exposed to hypertonicity during the 24 h.
Isolation and culture of chondrocytes in 0·4 osmol l−1 medium, instead of 0·3 osmol l−1, significantly increased their betaine-GABA transport activity, but not their system A activity.
Induction of betaine-GABA transport activity was prevented by addition of either actinomycin D or cycloheximide to the medium, but no mRNA for the betaine-GABA transporter known as BGT-1 was detected by Northern blot analysis of extracts of chondrocytes.
Some cartilage tissue is specialized to withstand not only very high hydrostatic pressures but also very rapid changes in pressure. In humans, for example, the pressure exerted on articular cartilage in the hip ‘may rise to 100-200 atm within milliseconds on standing and may cycle between resting values and 40-50 atm when walking’ (Afoke et al. 1987). When cartilage is continuously compressed by a static load the chondrocytes within the matrix are exposed to an hypertonic environment, because fluid is extruded but cations are preferentially retained by the charged proteoglycans, causing the osmotic pressure within the matrix to increase (Urban & Hall, 1992). Hence the question arises: how do chondrocytes cope with these conditions? Normally, of course, the initial effect of the imposition of hypertonic stress on animal cells is that they shrink, but most cells examined respond quickly with some regulatory volume control mechanism that involves ion transport, such as Na+-K+-Cl− co-transport (Hoffman & Simonsen, 1989). Changes in such ion transport activities were recently demonstrated in isolated chondrocytes exposed to either hypertonic or hypotonic conditions (Starks & Hall, 1995). Usually, however, cells cannot tolerate the presence of abnormally high intracellular concentrations of inorganic ions for long and so other mechanisms are required to cope with prolonged exposure to osmotic stress.
In several different kinds of cells, exposure to hypertonic conditions produces an increased activity of amino acid transport system A, presumably so that increased accumulation of amino acids can help to restore cell volume (Tramacere et al. 1984; Silvotti et al. 1991; Petronini et al. 1994). This is a slower response that depends on protein synthesis and, in the various cultured cells examined, is usually detectable after exposure to hypertonic conditions for about 2 h, shows maximum activity after about 6 h, and has largely disappeared by 24 h (Petronini et al. 1994). Recently reported variations in the rate of proline uptake by primary cultures of porcine chondrocytes after they had been incubated in hypertonic (0.48 osmol l−1) medium suggests that these chondrocytes also show induction of transport system A activity (Borghetti et al. 1995) following a time course similar to that noted previously in other cells (Petronini et al. 1994).
Comparison of chondrocytes with kidney cells is probably most appropriate, because cells of the inner medulla are exposed to marked variation in osmolarity during normal kidney function. Significant concentrations of ‘compatible osmolytes’, such as betaine and inositol, have been detected in renal medulla cells and the generally accepted picture is that these cells cope with hypertonic conditions by specifically accumulating compatible osmolytes, through increases in either their synthesis or uptake (Garcia-Perez & Burg, 1991; Burg, 1995; Burg et al. 1997). This theory received convincing support by the demonstration of the induction of a betaine-GABA transporter (BGT-1) in Madin-Darby canine kidney (MDCK) cells in response to hypertonic conditions (Yamauchi et al. 1992). Maximum transport activity of BGT-1 occurs only about 24 h after these cells have been subject to hypertonic conditions, although maximum induction of its mRNA is detectable after about 16 h (Yamauchi et al. 1992; Petronini et al. 1994). MDCK cells also display the more common and earlier induction of amino acid transport system A (Petronini et al. 1994; Chen et al. 1996; Kempson, 1998), but the BGT-1 system appears to be a more specific response to osmotic stress. Apart from these kidney-derived cells, analogous osmotic induction of betaine-GABA transport activity has since been demonstrated in the murine macrophage cell line RAW 264.7 (Warskulat et al. 1995) and some endothelial cells (Kempson et al. 1997; Weik et al. 1998). We have examined chondrocytes, and the results described below show that they behave similarly to MDCK cells, exhibiting induction of betaine-GABA transport activity, as well as amino acid transport system A, in response to imposed hypertonic conditions.
METHODS
Materials
[α-32P]dCTP, [methyl-3H]choline chloride and γ-amino[2,3-3H]butyric acid were obtained from Amersham International, Amersham. α-[1-14C]-methylaminoisobutyric acid (MeAIB) was obtained from New England Nuclear DuPont. Choline oxidase, betaine, γ-aminobutyric acid, methylaminoisobutyric acid, protease and collagenase type IA were bought from Sigma Chemical Co. [3H]Betaine was prepared from [3H]choline as described in detail previously for [14C]betaine (Petronini et al. 1993). A plasmid containing full length BGT-1 cDNA (Yamauchi et al. 1992) was kindly provided by Dr H. Moo Kwon, Division of Nephrology, The John Hopkins University School of Medicine, Baltimore, MD, USA, and a probe for β-actin by Dr Jeannette A. M. Maier, University of Milan, Italy. Media, fetal calf serum and antibiotics for culturing the cells were purchased from Gibco. Reagents for electrophoresis and blotting analysis were obtained from Bio-Rad Laboratories.
Cell cultures
Articular cartilage was obtained from elbow joints of 10-month-old pigs slaughtered at the local abbatoir. Cartilage was shaved from the articular surface under sterile conditions, finely diced and washed several times in phosphate-buffered saline. The tissue was then incubated at 37°C, first for 1 h in Dulbecco's modified Eagle's medium (D-MEM) containing protease (0.25 %), penicillin (100 u ml−1) and streptomycin (100 mg ml−1), and subsequently for 2 h in D-MEM containing 0.2 % collagenase. The digested material was filtered through nylon filters, first 100 μm and then 20 μm pore size, and the resulting cell suspension centrifuged at 350 g for 10 min. The supernatant fluid was discarded and the pellet washed several times by re-suspension and centrifugation in D-MEM containing fetal calf serum (10 %). The chondrocytes thus obtained were then seeded at high density (200 000 cells cm−2) in slightly modified (10 mmol l−1 NaHCO3, 25 mmol l−1 Hepes, pH 7.3) D-MEM plus 10 % fetal calf serum in plastic multiwell trays (Costar). They were cultured at 37°C in a water-saturated atmosphere of 5 % CO2 in air. The medium was changed every 3 days and the cells were used for experiments after 6 days.
Culture medium of altered osmolarity
Hypertonic medium was prepared by addition of extra NaCl or sucrose and the final osmolarity checked with a vapour-pressure osmometer (Wescor).
Cell phenotype and viability
Previous work established the conditions for culturing these chondrocytes and showed that one of the most important requirements for the maintenance of the phenotype is initial seeding at high density (Borghetti et al. 1995). Routine microscopic examination of the cultures readily reveals any de-differentiation of the cell into fibroblasts because the typical rounded shape of the chondrocytes is lost. Any cultures that showed evidence of such changes were discarded. The viability of the cultured cells was checked separately by measurement of their ability to exclude Trypan Blue. Ten samples were seeded at about 7 × 105 cells per dish and incubated for 24 h, half in 0.3 osmol l−1 medium and half in 0.5 osmol l−1 medium. The proportions of dead cells in the samples (mean ±s.e.m.) were 8.2 ± 1.3 % in 0.3 osmol l−1 medium and 9.8 ± 1.2 % in 0.5 osmol l−1 medium. Thus approximately 90 % of the cells were viable after 24 h incubation under both conditions.
Transport measurements
The rates of uptake of amino acids or betaine by the chondrocytes were measured after the latter had been incubated in control (0.3 osmol l−1) or test (0.5 osmol l−1) medium for the desired time. The cell monolayers were quickly washed in slightly modified (10 mmol l−1 NaHCO3 and 25 mmol l−1 Hepes, pH 7.3) Earle's basic salt solution and then incubated in this solution for 10 min at 37°C to diminish the cellular pool of amino acids. When required, choline or lithium was then used in place of Na+ in the medium, and either acetate or gluconate in place of Cl−. The cells were washed again and immediately incubated at 37°C for the desired time in the presence of labelled betaine, GABA or MeAIB. The incubations were stopped by removal of the medium and the cells were rapidly washed three times with fresh cold medium. Trichloroacetic acid (10 % w/v) was added to denature the cells and the radioactivity in samples of the acid extracts was measured by scintillation counting. Cell protein, precipitated by the trichloroacetic acid, was dissolved in 0.2 M NaOH and its concentration was determined by a dye-fixation method (Bio-Rad) with bovine serum albumin as standard (Bradford, 1976).
Northern blotting
Poly(A)+RNA was isolated from cultured cells with the use of a Quickprep Micro mRNA Purification Kit from Pharmacia Biotech. Samples (each 20 μg) of the poly(A)+RNA were fractionated by electrophoresis through 1 % agarose gels and the separated bands transferred to nylon filters. Full length BGT-1 cDNA (Yamauchi et al. 1992) and the β-actin probe were nick-translated (Amersham kit N.5000) with [α-32P]-dCTP (3000 Ci mmol−1). Hybridization, washing and autoradiography were then carried out exactly as described previously (Petronini et al. 1993).
RESULTS
Induction of amino acid transport activities
Under the standard conditions described in the experimental section, with an amino acid concentration of 0.1 mmol l−1, the uptake of both MeAIB and GABA by chondrocytes that had been incubated in either isotonic (0.3 osmol l−1) or hypertonic (0.5 osmol l−1) medium increased linearly with time for at least 20 min (results not presented). Thus the routine use of incubation periods of 5-10 min for influx measurements provided a good approximation to initial rates, although no kinetic analysis has been attempted here.
These preliminary experiments also showed that MeAIB influx was markedly increased in cells that had been incubated in hypertonic medium for 6 h, whilst GABA influx was similarly increased in cells exposed to hypertonic conditions for 20 h. The changes in amino acid transport activity were monitored more closely with samples of cells taken at various times during a 30 h incubation period in either the isotonic or hypertonic medium. The results (Fig. 1) revealed that uptake of MeAIB acid increased rapidly when the cells were exposed to hypertonic conditions, reaching a peak after 6-9 h and then gradually decreasing to a little above the control value at the 30 h time point. In contrast, GABA influx remained low for the first 9 h of hypertonic incubation, but then increased markedly to reach a plateau value after 24-30 h.
Figure 1. Sequential induction of amino acid transport activities.

Primary cultures of chondrocytes were incubated in either control (0.3 osmol l−1) or test (0.5 osmol l−1) medium for 3-30 h. At the indicated times samples of cells were then treated as described in the Methods section and their uptake of MeAIB and GABA was measured under standard conditions. The external concentration of both amino acids was 0.1 mmol l−1. The results are mean values (±s.e.m.) of 3 replicate measurements. Open symbols, control conditions; filled symbols, test conditions. Circles, MeAIB; squares, GABA.
Although there was no significant change in the influx of GABA into chondrocytes that had been incubated under isotonic conditions, Fig. 1 does reveal a slight but steady decrease in the rate of uptake of MeAIB by cells incubated in isotonic medium for long periods. This decrease was traced to the already known sensitivity of amino acid transport system A in cultured cells to the presence of fetal calf serum. Addition of fresh serum usually stimulates transport via system A, and this effect gradually declines during prolonged incubations. To circumvent this problem in some further studies of the effects of hypertonicity, the experimental protocols were modified in the following manner. When the effects of incubations for 6 and 24 h were to be compared, for example, fresh medium was added to cells at ‘zero time’ and again after 18 h, regardles of whether or not the composition of the medium was also changed. Then all transport measurements were made at 24 h after ‘zero time’. Typically, controls were incubated in 0.3 osmol l−1 medium for the first 18 h and then changed to fresh 0.3 osmol l−1 medium for the remaining 6 h. Test cells were either in 0.3 osmol l−1 medium for 18 h and then 0.5 osmol l−1 medium for the last 6 h, or in two lots of 0.5 osmol l−1 medium, for 18 h followed by 6 h. In this way a single control value was obtained for incubations under isotonic conditions, and all the cells not only spent the same time in culture but also were identically exposed to fresh serum.
To check that an osmotic effect, rather than a specific ionic effect, produces the increases in transport described above, media made hypertonic with sucrose were compared with those containing extra NaCl. After 6 h incubation, values (mean ±s.e.m. of 6 replicates) for MeAIB influx (nmol (mg protein)−1 (10 min)−1) were: 5.0 ± 0.2 (0.3 osmol l−1 medium), 13.2 ± 0.6 (0.5 osmol l−1 medium with NaCl) and 15.8 ± 0.7 (0.5 osmol l−1 medium with sucrose). After 24 h incubation, the corresponding values for GABA uptake were 2.0 ± 0.3, 10.0 ± 0.2 and 10.9 ± 0.2, respectively. Thus it seems clear that these stimulations of transport are caused by the increased osmotic pressure, not increased ionic strength or a specific effect of Na+ ions.
The ion dependencies of these induced transport activities were examined by replacement of either Na+ or Cl− ions during influx measurements, giving the data summarized in Table 1. About 70 % of MeAIB influx was dependent on Na+ ions under all conditions, with no dependency on the nature of the anion, in keeping with transport via amino acid system A. In contrast, the uptake of GABA was always partly dependent on Cl− as well as Na+, while the increased uptake induced by 24 h exposure of the cells to hypertonic medium was completely dependent on both Na+ and Cl− ions.
Table 1. Induction of two distinct transport activities.
| Uptake (nmol (mg protein)−1 (10 min)−1) | |||||
|---|---|---|---|---|---|
| Amino acid | Osmolarity(osmol l−1) | Time(h) | Total | Na+ dependent | Cl− dependent |
| MeAIB | 0.3 | — | 7.6 ± 0.2 | 5.3 ± 0.3 | 0.4 ± 0.6 |
| MeAIB | 0.5 | 6 | 20.0 ± 1.5 | 13.6 ± 1.5 | 2.9 ± 1.6 |
| MeAIB | 0.5 | 24 | 12.0 ± 0.5 | 8.2 ± 0.6 | 0 |
| GABA | 0.3 | — | 2.0 ± 0.1 | 1.3 ± 0.1 | 0.6 ± 0.1 |
| GABA | 0.5 | 6 | 2.2 ± 0.1 | 1.5 ± 0.1 | 0.8 ± 0.1 |
| GABA | 0.5 | 24 | 6.2 ± 0.4 | 5.4 ± 0.4 | 4.9 ± 0.4 |
Primary cultures of chondrocytes were incubated in either 0.3 osmol l−1 medium (18 h + 6 h), or 0.3 osmol l−1 medium (18 h) followed by 0.5 osmol l−1 medium for 6 h, or 0.5 osmol l−1 medium (18 h +6 h). The cells were then treated as described in the Methods section and their uptake of MeAIB and GABA was measured in the presence of either NaCl, choline chloride or sodium acetate as the main ions. Na+- dependent uptake is the difference between values in NaCl and choline chloride. Cl−-dependent uptake is the difference between values in NaCl and sodium acetate. The external concentration of both amino acids was 0.1 mmol l−1. The results are mean values (± S.E.M.) of 6 replicate measurements.
Induction of betaine influx
The results in Table 2 show that betaine influx into the chondrocytes was stimulated by their exposure to hypertonic conditions for either 6 or 24 h. Moreover, the addition of MeAIB strongly inhibited (ca. 90 %) the increase in influx occurring at 6 h, but caused only about a 40 % reduction of that measured at 24 h. Conversely, the addition of GABA inhibited betaine influx at 6 h by only 30 %, but by 90 % at 24 h. In keeping with these patterns of inhibition, betaine influx was partly dependent on Na+ and partly on Cl−, with the increase caused by exposure of the cells to hypertonic conditions for 24 h being completely dependent on both Na+ and Cl− (Table 3). The time courses of betaine uptake (not presented) again indicate that good approximations of initial rates were obtained under the standard conditions used. Thus, at least qualitatively, betaine mimics both MeAIB and GABA in its reactivity with both transport processes induced during exposure of the chondrocytes to hypertonicity
Table 2. Uptake of betaine by both induced transport systems.
| Uptake of betaine | |||||
|---|---|---|---|---|---|
| (nmol (mg protein)−1 (10 min)−1) | (%) | ||||
| Incubation time (h) | Addition to uptake medium | Control | Test | Increase | Induced |
| 6 | — | 0.4 ± 0.03 | 2.2 ± 0.11 | 1.8 ± 0.11 | 100 |
| 6 | MeAIB | 0.2 ± 0.02 | 0.4 ± 0.03 | 0.2 ± 0.04 | 11 |
| 6 | GABA | 0.3 ± 0.05 | 1.5 ± 0.02 | 1.2 ± 0.05 | 67 |
| 24 | — | 0.3 ± 0.03 | 2.6 ± 0.20 | 2.2 ± 0.20 | 100 |
| 24 | MeAIB | 0.4 ± 0.03 | 1.8 ± 0.38 | 1.4 ± 0.38 | 61 |
| 24 | GABA | 0.3 ± 0.02 | 0.5 ± 0.08 | 0.2 ± 0.08 | 9 |
Primary cultures of chondrocytes were incubated for 6 or 24 h in either control (0.3 osmol l−1) or test (0.5 osmol l−1) medium and then betaine uptake was measured under standard conditions in the absence and presence of unlabelled MeAIB or GABA, as indicated. The external concentration of betaine was 0.1 mmol l−1 and the added amino acids were present at 10 mmol l−1. Mean values (± S.E.M.) from 3 replicate measurements are given.
Table 3. Dependence of induced betaine uptake on Na+ and Cl−.
| Uptake (nmol (mg protein)−1 (10 min)−1) | ||||
|---|---|---|---|---|
| Osmolarity(osmol l−1) | Time(h) | Total | Na+ dependent | Cl− dependent |
| 0.3 | — | 0.63 ± 0.09 | 0.49 ± 0.09 | 0.36 ± 0.10 |
| 0.5 | 6 | 1.79 ± 0.07 | 1.38 ± 0.15 | 1.04 ± 0.07 |
| 0.5 | 24 | 2.67 ± 0.02 | 2.12 ± 0.08 | 2.13 ± 0.05 |
The experiment was similar to that described in the legend to Table 1 but betaine uptake was measured and LiCl was used to replace NaCl for estimation of Na+ dependence. The values given are means (± S.E.M.) from 3 replicate measurements.
The effects of several potential inhibitors on the uptake of betaine by chondrocytes were tested to obtain some idea of the specificity of the transport process induced during exposure of the cells to hypertonic medium for 24 h. Under the conditions used (0.1 mmol l−1 betaine and 10 mmol l−1 inhibitor) glycine, sarcosine and taurine each caused only 25-30 % inhibition of the induced transport, compared with the 95 % caused by GABA (Table 4). Proline, however, was also a potent inhibitor, decreasing betaine influx by about 75 % under the same conditions.
Table 4. Inhibition of betaine uptake.
| Betaine uptake | ||||
|---|---|---|---|---|
| (nmol (mg protein)− (10 min)−1) | (%) | |||
| Addition during flux measurement | Control | Test | Increase | Induced |
| None | 0.5 ± 0.1 | 2.7 ± 0.0 | 2.2 ± 0.1 | 100 |
| Glycine | 0.4 ± 0.1 | 2.0 ± 0.1 | 1.6 ± 0.2 | 73 |
| Taurine | 0.4 ± 0.01 | 2.0 ± 0.04 | 1.6 ± 0.04 | 73 |
| Sarcosine | 0.4 ± 0.01 | 1.9 ± 0.04 | 1.5 ± 0.04 | 68 |
| L-Proline | 0.2 ± 0.02 | 0.7 ± 0.03 | 0.5 ± 0.04 | 23 |
| GABA | 0.3 ± 0.02 | 0.5 ± 0.2 | 0.1 ± 0.2 | 5 |
Primary cultures of chondrocytes were incubated for 24 h in control (0.3 osmol l−1) or test (0.5 osmol l−1) medium before they were used for measurement of betaine uptake, from a 0.1 mmol l−1 concentration. The potentially inhibitory compounds added during the latter incubations were present at a final concentration of 10 mmol l−1. Mean values (± S.E.M.) from 3 replicate measurements are given.
Failure to detect BGT-1 mRNA
Northern blotting with full length BGT-1 cDNA readily revealed the presence of BGT-1 mRNA in MDCK cells that had been exposed to hypertonic (0.5 osmol l−1) conditions for 16-24 h, but not in control cells, incubated in isotonic (0.3 osmol l−1) conditions (Fig. 2). In contrast, repeated blotting tests performed with the total RNA fraction or with the total polyadenylate RNA fraction failed to detect any BGT-1 mRNA in similarly treated chondrocytes (Fig. 2).
Figure 2. Failure to detect mRNA for BGT-1 in chondrocytes.
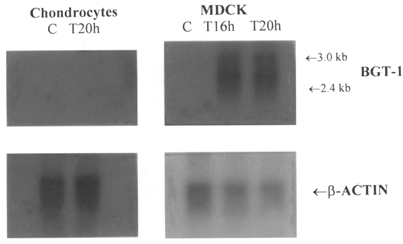
MDCK cells and primary cultures of chondrocytes were incubated, separately, in control medium (C, 0.3 osmol l−1) or test medium (T, 0.5 osmol l−1) for the indicated periods of time. Poly(A)+RNA was then extracted from the cells and analysed by Northern blotting for the presence of BGT-1 mRNA and β-actin mRNA, as described in the Methods section. The blot of β-actin mRNA was used to monitor the uniformity of loading the gel with the different samples of poly(A)+RNA: clearly, there were no significant differences between control and test samples in chondrocytes.
To check that the induced betaine-GABA transport activity is dependent on transcription and translation, the effects of actinomycin D and cycloheximide were tested. Cells were incubated for 16 h in either control (0.3 osmol l−1) or test (0.5 osmol l−1) conditions, in the presence and absence of either actinomycin D (0.1 μg ml−1) or cycloheximide (10 μg ml−1), before the uptake of GABA was measured. (A 16 h incubation was chosen instead of the usual 24 h to reduce possible general deliterious effects of the inhibitors.) In the first experiment, GABA influx (nmol (mg protein)−1 (10 min)−1) was: control, 2.1 ± 0.1 (4); test, 4.6 ± 0.2 (4); control plus actinomycin D, 1.8 ± 0.1 (4); test plus actinomycin D, 2.1 ± 0.1 (4). In the second experiment, the values were: control, 1.6 ± 0.1 (6); test, 7.6 ± 0.2 (6); control plus cycloheximide, 2.0 ± 0.4 (6); test plus cycloheximide, 2.1 ± 0.2 (6) (means ±s.e.m. (number of replicates)). Thus it seems clear that the induction of this transport activity is dependent on transcription and protein synthesis.
Magnitude and timing of induced transport activity in relation to the osmotic signal
Since the osmotically induced betaine-GABA transport activity is fully expressed only some 24 h after the chondrocytes have been exposed to hypertonic conditions, we checked to see if continuous exposure throughout the 24 h is necessary. Cells were incubated for a total of 24 h, first at 0.5 osmol l−1 for periods of 3-18 h, and then transferred back to 0.3 osmol l−1 for the remaining time. The results (Fig. 3) show clearly that continuous exposure of the cells to hypertonic conditions is not necessary. For example, although no significant change in GABA influx was detected after 6 h incubation of the cells at 0.5 osmol l−1, a clear increase was observed 18 h later, even though the cells had been at 0.3 osmol l−1 for those last 18 h. Similarly, the increase in GABA transport seen after 12 h incubation at 0.5 osmol l−1 was only half that recorded when the cells were incubated for another 12 h at 0.3 osmol l−1. On the other hand, Fig. 3 also shows that the magnitude of the induced transport activity is proportional to the duration of the exposure of the cells to hypertonic conditions, even when there is an ‘isotonic gap’ between exposure to the osmotic signal and measurement of transport activity.
Figure 3. Responses to interrupted osmotic signal.
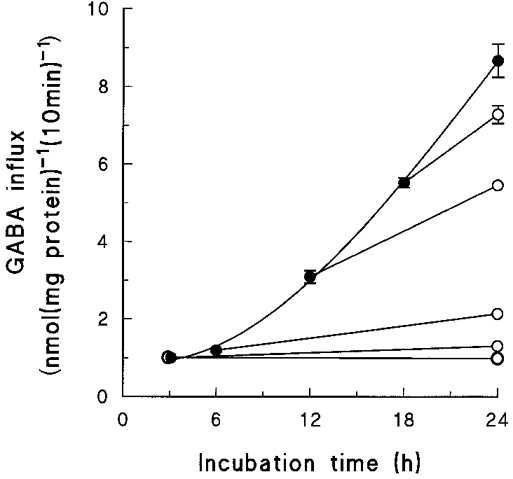
Primary cultures of chondrocytes were incubated for a total of 24 h, in 0.5 osmol l−1 medium for the first 0, 3, 6, 12, 18 or 24 h, and then in 0.3 osmol l−1 medium for the remaining period. At the indicated times samples of cells were treated as described in the Methods section and their uptake of GABA was measured under standard conditions. The external concentration of GABA was 0.1 mmol l−1. The results are mean values (±s.e.m.) of 6 replicate measurements. The error bars are contained within the symbols except where shown. •, 0.5 osmol l−1; ○, 0.3 osmol l−1
Cells cultured at different osmolarities
Although it is useful for comparisons with other cells and is common practice, culturing chondrocytes in media at 0.3 osmol l−1 as a control condition is probably not justified, since it is generally believed that the normal physiologically environment of chondrocytes in cartilage is nearer 0.4 osmol l−1 (Urban & Hall, 1992). Hence we also compared the properties of chondrocytes that had been isolated and cultured in a 0.4 osmol l−1 with those from a 0.3 osmol l−1 medium (Table 5). The results for MeAIB influx support the view that exposure of cells to an increase in osmolarity induces sytem A activity after a few hours, but that this activity declines again during prolonged exposure to the higher osmolarity. Also, the magnitude of the induced increase in transport activity appears to be directly proportional to the magnitude of the increase in osmolarity. Thus cells cultured for several days under either 0.4 or 0.3 osmol l−1 conditions had the same activity, but cells transferred from 0.3 to 0.4 osmol l−1, or from 0.4 to 0.5 osmol l−1, conditions for 6 h showed increases of 95 and 85 %, respectively, in transport activity. Similarly, an increase in osmolarity from 0.3 to 0.5 osmol l−1 produced an increase of almost 200 % in transport rate. In contrast, the rate of GABA transport in chondrocytes appears to depend more on the actual osmolarity maintained for long periods. For example, the GABA uptake into cells cultured for either 24 h or several days in 0.4 osmol l−1 medium was about double that into cells cultured in 0.3 osmol l−1. Similarly, incubation under 0.5 osmol l−1 conditions for 24 h gave about the same high rate of GABA transport regardless of whether the cells had been cultured initially under 0.3 or 0.4 osmol l−1 conditions.
Table 5. Effect of culturing cells at different osmolarities.
| Culture conditions (osmol l−1) | Initial rate of uptake (nmol (mg protein)−1 (10 min)−1) | ||
|---|---|---|---|
| Initial | Final | MeAIB(after 6 h at final osmolarity) | GABA (after 24 h at final osmolarity) |
| 0.3 | 0.3 | 5.7 ± 0.5 | 1.9 ± 0.2 |
| 0.3 | 0.4 | 11.1 ± 0.6 | 4.3 ± 0.2 |
| 0.3 | 0.5 | 17.0 ± 1.0 | 15.4 ± 0.5 |
| 0.4 | 0.4 | 5.5 ± 0.3 | 4.2 ± 0.1 |
| 0.4 | 0.5 | 10.2 ± 0.6 | 14.2 ± 0.2 |
Chondrocytes were initially cultured for several days in media at either 0.3 or 0.4 osmol l−1. Then, for the final 6 or 24 h, they were incubated at either 0.3, 0.4 or 0.5 osmol l−1, as indicated, before the influxes of MeAIB and GABA into them were measured under the standard conditions described in the Methods section. The external concentration of both amino acids was 0.1 mmol l−1. Mean values (±S.E.M.) of 6 replicate measurements are given.
DISCUSSION
It is generally accepted that betaine is accumulated in kidney medulla cells in response to hypertonicity via BGT-1, the amount of which is increased as a consequence of increased gene expression (Burg et al. 1997). Much of the evidence comes from experiments with MDCK cells and, in terms of both amino acid and betaine transport, the responses to hyperosmotic stress of primary cultures of chondrocytes parallel closely those of MDCK cells. Chondrocytes similarly show the induction of activity first of the Na+-dependent amino acid transport system A and later, as this activity again decreases, of a (Na++ Cl−)-dependent betaine-GABA transporter. Each of the solutes tested obviously permeates the cell membrane via more than one route, at least by both Na+-dependent and Na+-independent pathways. The Na+-dependent uptake of MeAIB, however, is not sensitive to the anion present under any condition tested, and the usual assumption that this component of flux goes exclusively via system A seems to hold for chondrocytes. In some other cells, at least, betaine also can be transported via system A, though with a very low affinity (Petronini et al. 1994). The results described above for chondrocytes are consistent with betaine being taken up via BGT-1, or a very similar (Na++ Cl−)-dependent transporter, as well as via system A. Under some conditions, the uptake of GABA, like that of betaine, is divided among pathways dependent on either Na+and Cl− ions, Na+ ions alone, or neither (Table 1). Hence it seems likely that GABA also can be transported to a limited extent via system A. The important finding, however, is that the increased influxes of both GABA and betaine induced by prolonged exposure of the chondrocytes to hypertonic conditions are dependent on Na+ plus Cl− ions (Table 1 and Table 3), which is characteristic of transporters such as BGT-1
The relevance of the induction of amino acid transport system A is unclear. From the results in Table 5 it appears that this induction depends only on an increase in osmolarity of the culture medium, not the actual osmolarity, and that the activity reverts to the basal value after prolonged incubation at any osmolarity. System A is similarly activated by several different stimuli in a wide variety of cells (Petronini et al. 1994; Chen et al. 1995) and hence appears to be a common and non-specific cell response to a variety of signals.
In spite of the remarkably similar responses to hypertonic stress by cultured chondrocytes and MDCK cells, the physiological interpretation is not so straightforward. At present there is no evidence that chondrocytes actually accumulate betaine, or any other compatible osmolyte, in vivo. Also, neither the magnitude nor the duration of static pressure on cartilage is likely to cause changes in vivo that mimic incubations of chondrocytes at 0.5 osmol l−1 for 6-24 h. On the other hand, the additional results obtained with chondrocytes cultured at 0.4 osmol l−1 do support the notion that the BGT-1-like transporter could be important in vivo. Under these conditions, which appear to mimic the in vivo situation quite closely (Urban & Hall, 1992), transport activity corresponding to the presence of BGT-1, or a similar transport protein, is always present in chondrocytes (Table 5). Moreover, when chondrocytes are isolated and incubated in the presence of betaine in a 0.38 osmol l−1 medium, subsequent transfer of the cells to a lower osmolarity stimulates the loss of accumulated betaine (Hall et al. 1998). Thus the available evidence does support the notion that betaine could act as a compatible osmolyte in chondrocytes, and the obviously important task is to find out if they do accumulate betaine, or any other compatible osmolyte, in vivo.
There are several possible explanations for the failure to detect any BGT-1 mRNA in chondrocytes. It is not caused by species dependency of the probe because we used the latter to demonstrate the presence of BGT-1 in a porcine endothelial cell culture (P. G. Petronini, R. R. Alfieri, M. N. Losio, A. E. Caccamo, A. Cavazzoni, M. A. Bonelli, A. F. Borghetti & K. P. Wheeler, unpublished results). However, the transporter in chondrocytes could be another isoform of BGT-1. Since BGT-1 was first cloned from MDCK cells (Yamauchi et al. 1992), eight isoforms of BGT-1 mRNA have been identified and classified into three groups, based on their usage of different promoter sites (Takenaka et al. 1995). Moreover, a liver-specific isoform has been identified in the rat (Burnham et al. 1996), so that a chondrocyte-specific betaine-GABA transporter would not be so remarkable. Alternatively, since the transport activity seems to be relatively stable in chondrocytes, the amount of BGT-1 mRNA could simply be too small for detection.
Acknowledgments
This work was supported by grants from MURS, the MURST/British Council agreement, CNR, Rome and the Associone Chiara Tassoni, Parma, Italy.
References
- Afoke NYP, Byers PD, Hutton WC. Contact pressures in the human hip joint. Journal of Bone Joint Surgery. 1987;69B:536–542. doi: 10.1302/0301-620X.69B4.3611154. quoted by Urban & Hall (1992) [DOI] [PubMed] [Google Scholar]
- Borghetti P, Della Salda L, De Angelis E, Maltarello MC, Petronini PG, Cabassi E, Marcato PS, Maraldi NM, Borghetti AF. Adaptive cellular response to osmotic stress in pig articular chondrocytes. Tissue and Cell. 1995;27:173–183. doi: 10.1016/s0040-8166(95)80020-4. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burg MB. Molecular basis of osmotic regulation. American The Journal of Physiology. 1995;268:F983–996. doi: 10.1152/ajprenal.1995.268.6.F983. [DOI] [PubMed] [Google Scholar]
- Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annual Review of Physiology. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- Burnham CE, Buerk B, Schmidt C, Bucuvalas JC. A liver-specific isoform of the betaine/GABA transporter in the rat: cDNA sequence and organ distribution. Biochimica et Biophysica Acta. 1996;1284:4–8. doi: 10.1016/0005-2736(96)00118-6. [DOI] [PubMed] [Google Scholar]
- Chen JG, Coe M, McAteer JA, Kempson SA. Hypertonic activation and recovery of system A amino acid transport in renal MDCK cells. American Journal of Physiology. 1996;270:F419–424. doi: 10.1152/ajprenal.1996.270.3.F419. [DOI] [PubMed] [Google Scholar]
- Chen JG, Strawbridge AB, Kempson SA. Microtubule disruption stimulates system A transport in cultured vascular smooth-muscle cells. American Journal of Physiology. 1995;268:C1512–1519. doi: 10.1152/ajpcell.1995.268.6.C1512. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiological Reviews. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- Hall AC, Wilson K, Wheeler KP. Stimulation of betaine efflux from articular chondrocytes following cell swelling. Transactions of the Orthopedic Research Society. 1998;23:921. [Google Scholar]
- Hoffman EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiological Reviews. 1989;69:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Kempson SA. Differential activation of system A and betaine/GABA transport in MDCK cell membranes by hypertonic stress. Biochimica et Biophysica Acta. 1998;1372:117–123. doi: 10.1016/s0005-2736(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Kempson SA, Hoshaw MJ, Hinesley RS, McAteer JA. Hypertonic stress up-regulates amino acid transport in vascular endothelial cells. Kidney International. 1997;52:1332–1339. doi: 10.1038/ki.1997.458. [DOI] [PubMed] [Google Scholar]
- Petronini PG, De Angelis EM, Borghetti AF, Wheeler KP. Effect of betaine on HSP70 expression and cell survival during adaptation to osmotic stress. Biochemical Journal. 1993;293:553–558. doi: 10.1042/bj2930553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronini PG, De Angelis E, Borghetti AF, Wheeler KP. Osmotically inducible uptake of betaine via amino acid transport system A in SV-3T3 cells. Biochemical Journal. 1994;300:45–50. doi: 10.1042/bj3000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvotti L, Petronini PG, Mazzini A, Piedimonte G, Borghetti AF. Differential adaptive response to hyperosmolarity of 3T3 and transformed SV3T3 cells. Experimental Cell Research. 1991;193:253–261. doi: 10.1016/0014-4827(91)90094-b. [DOI] [PubMed] [Google Scholar]
- Starks I, Hall AC. Activation of K+ transport pathways of isolated bovine articular chondrocytes following osmotic perturbation. The Journal of Physiology. 1995;482.P:18–19P. [Google Scholar]
- Takenaka M, Bagnasco SM, Preston AS, Uchida S, Yamauchi A, Kwon HM, Handler JS. The canine betaine γ-amino-n-butyric acid transporter gene - diverse messenger RNA isoforms are regulated by hypertonicity and are expressed in a tissue-specific manner. Proceedings of the National Academy of Sciences of the USA. 1995;92:1072–1076. doi: 10.1073/pnas.92.4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere M, Petronini PG, Severini A, Borghetti AF. Osmoregulation of amino acid transport activity in cultured fibroblasts. Experimental Cell Research. 1984;151:70–79. doi: 10.1016/0014-4827(84)90356-2. [DOI] [PubMed] [Google Scholar]
- Urban JPG, Hall AC. In: Articular Cartilage and Osteoarthritis. Kuettner K, Scheyerbach R, Peyron JC, Hascall VC, editors. New York: Raven Press Ltd; 1992. pp. 393–406. [Google Scholar]
- Warskulat U, Wettstein M, Häussinger D. Betaine is an osmolyte in RAW 264.7 mouse macrophages. FEBS Letters. 1995;377:47–50. doi: 10.1016/0014-5793(95)01317-2. 10.1016/0014-5793(95)01317-2. [DOI] [PubMed] [Google Scholar]
- Weik C, Warskulat U, Bode J, PetersRegehr T, Häussinger D. Compatible organic osmolytes in rat liver sinusoidal endothelial cells. Hepatology. 1998;27:569–575. doi: 10.1002/hep.510270235. 10.1002/hep.510270235. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Uchida S, Kwon HM, Preston AS, Robey BR, Garcia-Perez A, Burg MB, Handler JS. Cloning of a Na+-dependent and Cl−-dependent betaine transporter that is regulated by hypertonicity. Journal of Biological Chemistry. 1992;267:649–652. [PubMed] [Google Scholar]


