Abstract
The N-methyl-D-aspartate (NMDA) glutamate receptor, widely distributed in the mammalian nervous system, has recently been identified in bone. In this study, we have investigated whether NMDA receptors expressed by osteoclasts have an electrophysiological activity.
Using the patch clamp technique two agonists of the NMDA receptor, L-glutamate (Glu) and NMDA, were shown to activate whole-cell currents recorded in isolated rabbit osteoclasts.
The current-voltage (I-V) relationships of the currents induced by Glu (IGlu) and NMDA (INMDA) were studied using Mg2+-free solutions. The agonist-induced currents had a linear I-V relationship with a reversal potential near 0 mV, as expected for a voltage independent and non-selective cationic current.
IGlu and INMDA were sensitive to specific blockers of NMDA subtype glutamate receptors, such as magnesium ions, (5R, 10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a, d]cyclohepten -5,10-imine (MK-801) and 1-(1,2-diphenylethyl) piperidine (DEP). The block of IGlu and INMDA by these specific antagonists was voltage dependent, strong for negative potentials (inward current) and absent for positive potentials (outward current).
These results demonstrate that NMDA receptors are functional in rabbit osteoclasts, and that their electrophysiological and pharmacological properties in these cells are similar to those documented for neuronal cells. Active NMDA receptors expressed by osteoclasts may represent a new target for regulating bone resorption.
Different factors and mechanisms such as the endocrine system, mechanical stimuli, local growth factors and cytokines have been shown to participate in the control of bone remodelling. More recently, several studies have demonstrated that neurotransmitters, including neuropeptides and excitatory amino acids such as glutamate (Bjurholm et al. 1989; Lerner 1996; Chenu et al. 1998; Patton et al. 1998), may also actively contribute to the local regulation of bone metabolism. Bone is innervated (Calvo & Forteza-Vila, 1969), and nerve fibres containing neuropeptides or glutamate (Hohmann et al. 1986; Hill & Elde 1991; Serre et al. 1998) have been found in this tissue, in the vicinity of bone cells which express receptors for these neurotransmitters (Hohmann & Tashjian 1984; Bjurholm et al. 1992; Konttinen et al. 1996; Chenu et al. 1998; Patton et al. 1998). Although neuromediators might be involved in a local paracrine signalling between bone cells, these findings strengthen the hypothesis of a neuronal control of bone metabolism, previously suggested by a number of clinical observations and in vitro studies (Konttinen et al. 1996; Lerner 1996).
l-Glutamate (Glu) is a widely used neuromediator in the central and peripheral nervous system that acts through two types of membrane receptors; ionotropic receptors (NMDA, α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), kainate) which have a ionic channel structure, and metabotropic receptors which are members of the seven transmembrane family receptors coupled to protein G. Different subtypes of glutamate receptors (GluR) have been identified in bone, expressed by both bone-forming osteoblasts and bone-resorbing osteoclasts (Chenu et al. 1998; Patton et al. 1998). In particular, we have previously demonstrated that osteoclasts express the NMDA subtype of GluR and that specific antagonists of these receptors block in vitro bone resorption (Chenu et al. 1998), suggesting that activation of NMDA receptors is required for the resorptive function of these cells.
In this report, we first demonstrate, using electrophysiological studies, that NMDA receptors expressed by normal mammalian osteoclasts are functional and regulated by specific modulators, and that they may be involved in osteoclast signalling activated by Glu during bone remodelling.
Initial results from this work have been reported in abstract form (Espinosa et al. 1998).
METHODS
Materials
Medium 199 (M199) and α minimal essential medium (αMEM), fetal calf serum (FCS), penicillin, streptomycin and Hepes buffer were purchased from Life Technologies (Grand Island, NY, USA). Chemicals were obtained from ICN Biomedicals Inc. (Aurora, OH, USA). Agonists and antagonists of glutamate receptors (GluR) were purchased from Tocris Cookson (Bristol, UK).
Osteoclast isolation and culture
Osteoclasts were isolated from long bones of 1-day-old New Zealand rabbits that were killed by decapitation. The bones were removed, cleaned of soft tissues and bone marrow, then split and scraped into M199 containing 10 % heat-inactivated fetal calf serum (FCS), 20 mM Hepes and 50 U ml−1 penicillin-streptomycin. Cells were centrifuged at low speed, resuspended in the same medium and seeded onto glass coverslips for 1 h at 37°C, 5 % CO2. After this adhesion period, non-adherent cells were removed and coverslips were placed for 24 h in αMEM containing 2 % heat-inactivated FCS, at pH 7.2.
Cells were always studied within 30 h of isolation. Electrophysiological recordings were performed on spread osteoclasts, identified as cells having multiple nuclei (> 3).
All experiments were carried out according to the guidelines defined by the animal welfare committee of the National Institute of Health and Medical Research (INSERM).
Electrophysiology
The conventional whole-cell patch-clamp configuration has been used. Macroscopic currents were recorded with RK-400 amplifier (Bio-Logic, Grenoble, France), and data were registered and analysed using pCLAMP6 software (Axon Instruments). Current signals were filtered at 3 kHz and digitized at 10 kHz using an analog/digital converter (DigiData 1200, Axon instruments). Borosilicate glass pipettes had a resistance of 2-3 MΩ and seal resistances were always superior to 1 GΩ. Current-voltage (I-V) relationships were obtained using a voltage ramp protocol defined by a 15 ms pulse from -30 mV holding potential (Vh) to -130 mV, followed by a ramp from -130 mV to +60 mV over 150 ms. This protocol was compared with a conventional step protocol, composed of four pulses of 85 ms from Vh = -30 mV to -100, -50, 0 and +50 mV, with a short return to Vh during 15 ms between each pulse. Each protocol was applied every 3 s. Experiments were performed at room temperature (21-25°C).
Cells were continuously superfused at 3 ml min−1 using a bath solution flowing by gravity from a set of five capillaries. Agonists and antagonists of GluR were applied using this system. The time to switch between different solutions was ≤ 3 s. Na+ external control solution (Tyrode solution) contained (mM): NaCl 145, CsCl 5, CaCl2 2, glucose 5, Hepes 10, pH at 7.4 adjusted with NaOH. The pipette-filling solution contained (mM): CsCl 15, NaCl 5, aspartic acid 100, EDTA 5, Hepes 20, pH at 7.2 adjusted with CsOH. Glutamate and NMDA were added to Tyrode solution at 100 μM with 20 μM glycine.
RESULTS
Glutamate and NMDA-induced currents in osteoclasts
Osteoclasts were voltage clamped at a holding potential (Vh) of -30 mV that allowed experiments over a long time period (> 30 min) to be performed. However, because the Glu (IGlu)- or NMDA (INMDA)-elicited current at -30 mV was small, the induced current modifications were followed using a voltage ramp protocol applied every 3 s in Cs+ solution. The mean holding current for all tested cells, measured at -30 mV, was -272 ± 54 pA. In order to minimize possible stretch activated currents, cells were continuously superfused with control solution and we have checked that application of the vehicle was not affecting the current. Glutamate (100 μM) and glycine (20 μM) activated a current, measured at different membrane potentials (-130 to +60 mV), which was inward at negative potentials and outward at positive potentials (Fig. 1). Three to ten seconds after agonist addition, the current increased and reached a peak in 30 to 60 s representing 15 -20 % of the control current (Glu: 15 ± 6 %, n = 12; NMDA: 18 ± 17 %, n = 10). No significant differences were observed between currents elicited by Glu and NMDA, the specific agonist of the NMDA subtype GluR. Responses to GluR agonists were observed in 12/39 cells (30.7 %) for Glu and 10/34 (29.4 %) for NMDA. Glu- and NMDA-induced currents had variable activation time courses and different reversal phases which could be attributed to the various times needed to reach homogeneous concentrations of agonists due to their high concentrations (100 μM) and/or to different times required for their complete wash out.
Figure 1. Extracellular L-glutamate (Glu) and NMDA activate a transient current in rabbit osteoclasts.
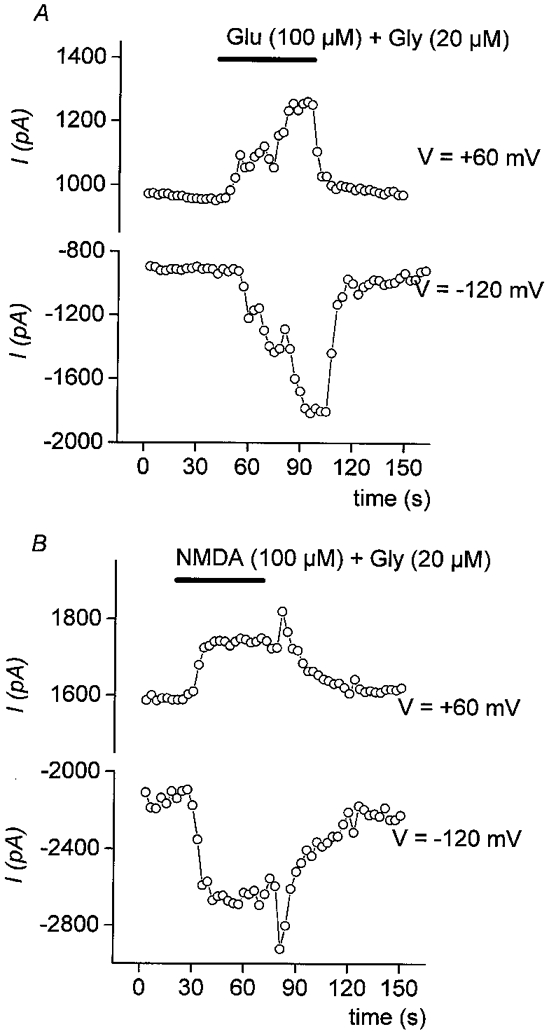
Each point plotted in this figure represents the whole-cell current (expressed in pA) measured every 3 s at -120 mV or +60 mV during a voltage ramp protocol (-130 to +60 mV in 150 ms, from holding potential of -30 mV). A, Glu (100 μM + Gly 20 μM) and B, NMDA (100 μM + Gly 20 μM) were applied during the time indicated by the bar. They both induced an inward current at -120 mV and an outward current at +60 mV. The current was reaching a peak in ≈60 s and was reversible after complete washout of the agonists.
No dynamic currents were observed. The time courses of the whole-cell currents had not shown dynamic components in control conditions and after Glu application (Fig. 2A and B). The agonist-induced currents (IGlu and INMDA) were calculated by subtracting the control current elicited by a ramp before agonist application (trace 1) from the agonist-induced current at its peak response (trace 2). The mean currents and standard deviations, calculated for 20 mV intervals, are reported in Fig. 2C and D. Similar amplitudes and I-V relationships were obtained for Glu- and NMDA-induced currents. Using internal Cs+ solution, I-V relationships of IGlu and INMDA were linear with a reversal potential of 0 mV, indicative of a cationic non-selective current; in K+ solution, the reversal potential was similarly close to 0 mV (not shown). As described for neuronal cells (Nowak et al. 1984), no rectification was observed for IGlu and INMDA in conditions where intracellular magnesium was buffered by 5 mM EDTA added in the pipette solution (Johnson & Ascher, 1990) (Fig. 2C and D). In Cs+ solution, chloride concentrations were adjusted to shift the chloride equilibrium potential to negative potentials (EClca -50 mV), hence suppressing a possible contribution of a chloride current.
Figure 2. Glutamate and NMDA elicit a voltage-independent non-selective cation current.
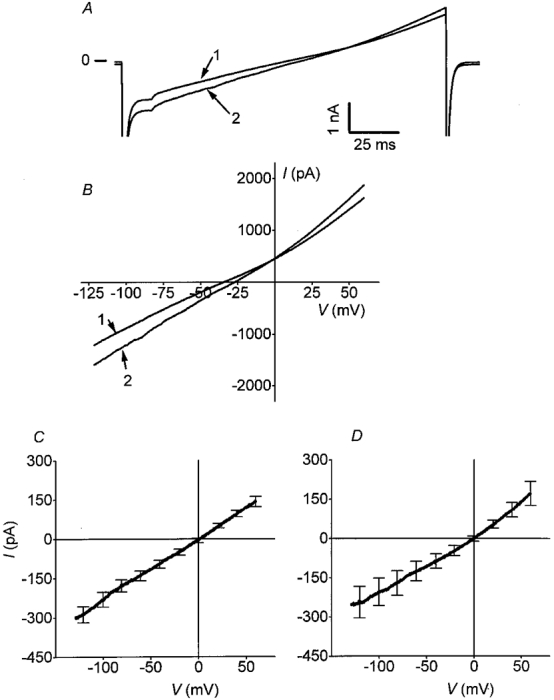
A, time courses of the whole-cell currents measured during the voltage ramp stimulation for control (1) and Glu application (2). B, I-V relationships of the whole-cell currents determined by a voltage ramp protocol, in control condition (1) and at the peak response to Glu (2). Mean currents (means ±s.e.m.) induced by Glu (C) (100 μM + Gly 20 μM, n = 12) and NMDA (D) (100 μM + Gly 20 μM, n = 10) were calculated by subtracting the control current from the agonist-induced current at the peak of response. They were both linear with a reversal potential close to 0 mV (minimum and maximum values being -25 and +21 mV, respectively).
Pharmacology
To confirm the involvement of the NMDA subtype GluR, we have tested the sensitivity of Glu- and NMDA-elicited currents to three well known NMDA specific channel blockers: divalent cations Mg2+ which have been well characterized as NMDA channel blockers (Ascher & Nowak 1988); (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo [a, d]cyclohepten-5,10-imine (MK-801), the most largely used non-competitive NMDA receptor antagonist in neuronal studies (MacDonald et al. 1991); and 1-(1,2-diphenylethyl) piperidine (DEP), a high affinity antagonist at the ion channel on NMDA receptor which is a conformationally flexive homeomorph of MK-801 (Rogawski 1993). When applied to the external face, these antagonists interact with the opened channel pore and block it in a voltage-dependent manner, with a stronger blockade at hyperpolarized potentials (Nowak et al. 1984; Honey et al. 1985).
NMDA receptor antagonists were tested during a continuous application of Glu or NMDA, and were added during the activation phase of the current in order to differentiate their effects clearly from the current desensitization observed during long-time exposure to agonists. Figure 3 illustrates the effects of these compounds on agonists-induced currents. External magnesium (2 mM) induced a strong voltage-dependent block of the NMDA-induced current, demonstrated by voltage step (Fig. 3A and B) or voltage ramp (Fig. 3C) protocols. The current was highly blocked at strong negative potentials while unaffected or slightly reduced at potentials superior to -50 mV (Fig. 3A). I-V relationships of INMDA measured with both protocols showed a negative slope between -100 and -50 mV (Fig. 3B and C). The outward current at strong negative potentials and the mild block of INMDA at potentials superior to +50 mV observed in the presence of magnesium might be the consequence of an inhibition of the outwardly rectifying chloride current by these ions, previously shown to be activated in osteoclasts (Sakai et al. 1999).
Figure 3. Mg2+, DEP and MK-801 induce a voltage-dependent block of NMDA receptor activation in osteoclasts.
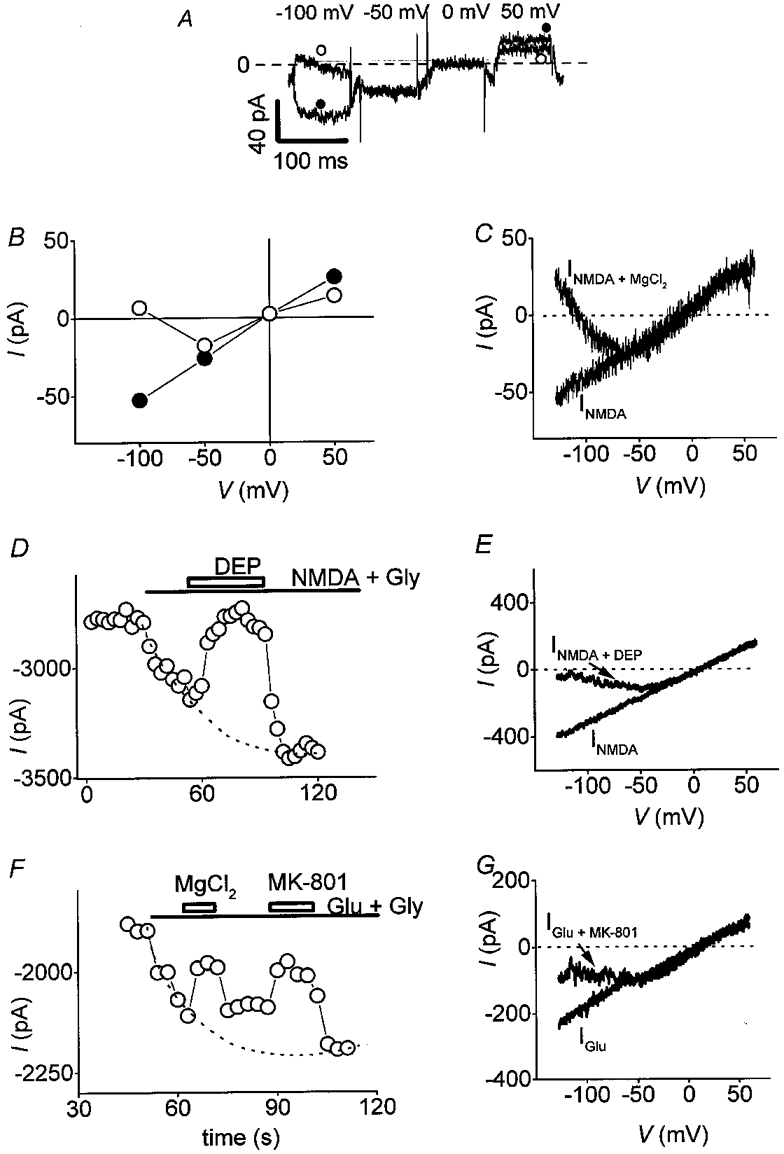
A, osteoclast was held at -30 mV and submitted to various potentials. Traces represent NMDA-induced current (INMDA) in the presence (○) or absence (•) of 2 mM extracellular Mg2+. INMDA was calculated by subtracting the control current from INMDA at the peak of response. B and C, I-V relationships of INMDA recorded in the presence (○) or absence (•) of 2 mM extracellular Mg2 (Vh = -30 mV), using a voltage step (B) or a voltage ramp (C) protocol. Both I-V curves showed a strong voltage-dependent block of INMDA by external magnesium, illustrated by the negative slope of the current between -120 mV and -50 mV. D and F, NMDA (100 μM + Gly 20 μM) or Glu (100 μM + Gly 20 μM) were applied continuously (as indicated by the bar) and currents were measured at -120 mV (Vh = -30 mV). Channel blockers, 50 μM DEP (D) and 50 μM MK-801 (F), were added during the time designated by rectangles. These compounds blocked INMDA and IGlu respectively and this inhibition was reversible after removal of the antagonists. Dotted lines in D and F were fitted by eye. E and G,I-V relationships of INMDA (E) obtained with a voltage ramp protocol, in absence (trace marked INMDA) or in presence of DEP (trace marked INMDA+ DEP); I-V relationships of IGlu (G) obtained with a voltage ramp protocol, in absence (trace marked IGlu) or in presence of MK-801 (trace marked IGlu+ MK-801).
DEP (50 μM; Fig. 3D) and MK-801 (50 μM; Fig. 3F) also reversibly blocked currents elicited by NMDA and Glu. DEP was a stronger blocker of the current than MK-801. The effects of these NMDA channel blockers on INMDA and IGlu obtained with a voltage ramp protocol are illustrated on Fig. 3E and G. The inward part of the current was blocked while its outward part was unaffected. As for magnesium, I-V relationships obtained with MK-801 and DEP had a negative slope for potentials inferior to -50 mV (J-shaped curve). These data clearly demonstrate a voltage-dependent block of INMDA by non-competitive antagonists, a major characteristic of the electrophysiological behaviour of NMDA Glu receptors.
DISCUSSION
Our results demonstrate for the first time the presence of active NMDA receptors in rabbit osteoclasts. We have shown that in Mg2+-free medium, extracellular application of NMDA receptor agonists activated a cationic non-selective and non-voltage-dependent conductance in these cells. In the presence of extracellular magnesium, the NMDA-induced current showed a strong outward rectification. This voltage-dependent block of current by external magnesium ions is also a main characteristic of NMDA receptors, the accepted mechanism being that Mg2+ ions block the open channel when the driving force is negative (inward current). Other non-competitive specific NMDA channel antagonists have been described in neuronal cells, which block these receptors in a voltage-dependent way (MacDonald et al. 1991). Our pharmacological studies have demonstrated a strong voltage-dependent inhibition of Glu- and NMDA-induced currents by MK-801 and DEP, two well known antagonists of NMDA receptors, confirming the involvement of this subtype of GluRs in the electrophysiological responses of osteoclasts to Glu and NMDA. The similarity between IGlu and INMDA, as well as the abilities of magnesium, MK-801 and DEP to block Glu-induced currents, are in favour of a prevalent activation of NMDA receptors in these cells. PreviouslyAMPA receptors have also been shown to be expressed by bone cells (Chenu et al. 1998). Although we have not yet any electrophysiological evidence for activation of AMPA GluR in osteoclasts, we cannot totally exclude a possible contribution of non-NMDA GluRs to Glu-induced currents in these cells.
Activation kinetics of NMDA receptors studied in neuronal cells using fast agonist application systems have shown two components: a rapid transient peak (100 ms duration) and a stable steady state (Sather et al. 1992). As we used a slow switch system to apply the agonists, it has not been possible to compare Glu-induced peaks of response. It is also possible that this slow application of agonists could have induced a pre-desensitization of receptors, reducing the maximum current amplitude (Sather et al. 1992). However, we have shown that the steady states of NMDA- and Glu-elicited currents were similar in osteoclasts compared with neuronal cells.
While the role of functional NMDA receptors in neuronal cells has been well described, the physiological significance of these receptors on osteoclasts is presently unknown. Although GluRs are the major neurotransmitter receptors in the vertebrate central nervous system, they have been recently discovered in non-neuronal tissues such as pancreas (Gonoi et al. 1994; Inagaki et al. 1995; Weaver et al. 1996), lung (Said et al. 1995) and in plants (Lam et al. 1998). Moreover, they could play a physiological role in these tissues since GluRs expressed by pancreatic cells are involved in the control of insulin and glucagon secretion by Glu (Bertrand et al. 1993). There is not yet any evidence for a mechanism involved in a local secretion of excitatory amino acids in bone. Glutamate may originate from bone cells and act as a paracrine signal between them, but it may also be released under specific stimuli from nerve fibres located in the vicinity of bone cells.
In neuronal cells, the activity of NMDA receptors depends on a pre-depolarization of the post-synaptic cell to abolish the voltage-dependent block by magnesium, induced by stimulation of non-NMDA glutamate receptors. One important characteristic of osteoclasts is that they can alternate between two stable membrane potentials of approximately -75 and -15 mV (Sims & Dixon, 1989). At -75 mV NMDA receptors are blocked by magnesium, while at -15 mV they allow a calcium influx into the cell. The switch from -75 mV to -15 mV could be triggered by a depolarizing current, due to activation of other types of receptors such as non-NMDA receptors if they are expressed, or P2X ATP receptors which have been recently described in osteoclasts (Weidema et al. 1997). The discovery of functional NMDA receptors in osteoclasts opens an interesting putative pathway for regulating bone resorption. Previous results by our group have shown that some antagonists of NMDA Glu receptors block in vitro bone resorption (Chenu et al. 1998). NMDA receptor activation induces changes in internal calcium concentration and modifications of membrane potential which may modulate the driving force for H+ and facilitate its electrogenic pumping out of the cell, as it may also change the driving force for calcium ions. Calcitonin which inhibits bone resorption has been shown to modify both membrane potential and internal calcium concentration (Okabe et al. 1998).
Active NMDA receptors are heterodimers of NR1 and NR2A, B, C or D subunits and the properties of these channel receptors have been shown to be modulated by their subunit composition (Williams 1997). NR1 is the major subunit that has been identified in bone cells but the composition of functional NMDA receptors on these cells is still unknown, although NR2D has been identified in bone marrow cells by RT-PCR (Chenu et al. 1998; Patton et al. 1998).
In this study, applications of NMDA receptor agonists have elicited a current in 30 % of tested osteoclasts. This heterogeneity of responses is in line with a variability of expression of NR1 previously demonstrated in these cells using immunocytochemistry. The relationship between the expression of functional NMDA receptors by osteoclasts and their bone resorbing activity will be very important to determine in the future. The demonstration of active NMDA glutamate receptors in osteoclasts represents a novel possibility for regulating bone resorption in physiological and pathological conditions such as osteoporosis.
Acknowledgments
We thank Dr L. Malaval and Dr P. Salin for helpful comments on the manuscript. This work was supported by the Institut de la Santé et de la Recherche Médicale and by a grant from Les Fonds de Recherche Hoechst Marion Roussel.
References
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartic acid responses of mouse central neurones in culture. The Journal of Physiology. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. European Journal of Pharmacology. 1993;237:45–50. doi: 10.1016/0014-2999(93)90091-u. [DOI] [PubMed] [Google Scholar]
- Bjurholm A, Kreicbergs A, Schultzberg M. Fixation and demineralization of bone tissue for immunohistochemical staining of neuropeptides. Calcified Tissue International. 1989;45:227–231. doi: 10.1007/BF02556042. [DOI] [PubMed] [Google Scholar]
- Bjurholm A, Kreicbergs A, Schultzberg M, Lerner UH. Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR-106-01, ROS 17/2.8, MC3T3-E1, and Saos2) and bone primary cells. Journal of Bone and Mineral Research. 1992;7:1011–1019. doi: 10.1002/jbmr.5650070903. [DOI] [PubMed] [Google Scholar]
- Calvo W, Forteza-Vila J. On the development of bone marrow innervation in new-born rats as studied with silver impregnation and electron microscopy. American Journal of Anatomy. 1969;126:355–371. doi: 10.1002/aja.1001260308. [DOI] [PubMed] [Google Scholar]
- Chenu C, Serre CM, Raynal C, Burt-Bichat B, Delmas PD. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–299. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Itzstein C, Delmas PD, Chenu C. Rabbit osteoclasts express active glutamate receptor channels. Bone. 1998;23:SA100. [Google Scholar]
- Gonoi T, Mizuno N, Inagaki N, Kuromi H, Seino Y, Miyazaki J, Seino S. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. Journal of Biological Chemistry. 1994;269:16989–16992. [PubMed] [Google Scholar]
- Hill EL, Elde R. Distribution of CGRP-, VIP-, DbH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell and Tissue Research. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- Hohmann EL, Tashjian AH., Jr Functional receptors for vasoactive intestinal peptide on human osteosarcoma cells. Endocrinology. 1984;114:1321–1327. doi: 10.1210/endo-114-4-1321. [DOI] [PubMed] [Google Scholar]
- Honey CR, Miljkovic Z, MacDonald JF. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neuroscience Letters. 1985;61:135–139. doi: 10.1016/0304-3940(85)90414-8. 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, Kaneko T, Iwanaga T, Seino S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB Journal. 1995;9:686–691. [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophysical Journal. 1990;57:1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Imai S, Suda A. Neuropeptides and the puzzle of bone remodeling. Acta Orthopaedica Scandinavia. 1996;67:632–639. doi: 10.3109/17453679608997772. [DOI] [PubMed] [Google Scholar]
- Lam H, Chiu J, Hsieh M, Meisel L, Oliveira IC, Shin M, Coruzzi G. Glutamate-receptor genes in plants. Nature. 1998;396:125–126. doi: 10.1038/24066. 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- Lerner UH. Kinins and neuropeptides. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. San Diego, CA, USA: Academic Press; 1996. pp. 199–205. [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. The Journal of Physiology. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Okabe K, Takada K, Okamoto F, Kajiya H, Soeda H. Calcitonin regulates channel activities in rat osteoclasts via protein kinase A. Bone. 1998;23:W076. abstract. [Google Scholar]
- Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM. Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone. 1998;22:645–649. doi: 10.1016/s8756-3282(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends in Pharmacological Sciences. 1993;14:325–331. doi: 10.1016/0165-6147(93)90005-5. 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Said SI, Berisha HI, Pakbaz H. N-methyl-D-aspartate receptors outside the central nervous system: activation causes acute lung injury that is mediated by nitric oxide synthesis and prevented by vasoactive intestinal peptide. Neuroscience. 1995;65:943–946. doi: 10.1016/0306-4522(95)00021-a. 10.1016/0306-4522(95)00021-A. [DOI] [PubMed] [Google Scholar]
- Sakai H, Nakamura F, Kuno M. Synergetic activation of outwardly rectifying Cl− currents by hypotonic stress and external Ca2+ in murine osteoclasts. The Journal of Physiology. 1999;515:157–168. doi: 10.1111/j.1469-7793.1999.157ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. The Journal of Physiology. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre CM, Farlay D, Delmas PD, Chenu C. Distribution and characterization of nerve fibers in long bones of growing rats. Bone. 1998;23:SA044. abstract. [Google Scholar]
- Sims SM, Dixon SJ. Inwardly rectifying K+ current in osteoclasts. American Journal of Physiology. 1989;256:C1277–1282. doi: 10.1152/ajpcell.1989.256.6.C1277. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic islets. Journal of Biological Chemistry. 1996;271:12977–12984. doi: 10.1074/jbc.271.22.12977. [DOI] [PubMed] [Google Scholar]
- Weidema AF, Barbera J, Dixon SJ, Sims SM. Extracellular nucleotides activate non-selective cation and Ca2+- dependent K+ channels in rat osteoclasts. The Journal of Physiology. 1997;503:303–315. doi: 10.1111/j.1469-7793.1997.303bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]


