Abstract
The distribution of Na+ channels and development of excitability were investigated in vitro in purified spinal motoneurones obtained from rat embryos at E14, using electrophysiological, immunocytochemical and autoradiographical methods.
One hour after plating the motoneurones (DIV0), only somas were present. They expressed a robust delayed rectifier K+ current (IDR) and a fast-inactivating A-type K+ current (IA). The rapid neuritic outgrowth was paralleled by the emergence of a fast-activating TTX-sensitive sodium current (INa), and by an increase in both K+ currents.
The change in the three currents was measured daily, up to DIV8. The large increase in INa observed after DIV2 was accompanied by the onset of excitability. Spontaneous activity was observed as from DIV6.
The occurrence of axonal differentiation was confirmed by the fact that (i) only one neurite per motoneurone generated antidromic action potentials; and (ii) 125I-α-scorpion toxin binding, a specific marker of Na+ channels, labelled only one neurite and the greatest density was observed in the initial segment. Na+ channels therefore selectively targeted the axon and were absent from the dendrites and somas.
The specific distribution of Na+ channels was detectable as soon as the neurites began to grow. When the neuritic outgrowth was blocked by nocodazole, no INa developed.
It was concluded that, in spinal embryonic motoneurone in cell culture, Na+ channels, the expression of which starts with neuritic differentiation, are selectively addressed to the axonal process, whereas K+ channels are present in the soma prior to the neuritic outgrowth.
The nature of ionic channels and their distribution among the various cell compartments are crucial for defining the encoding properties of a neurone. In mammalian motoneurones, the mechanisms underlying the firing properties have been investigated using laminectomy (Barrett & Crill, 1980), and in hemisected in vitro spinal cord preparations (Walton & Fulton, 1986; Ziskind-Conhaim, 1988), spinal cord slices (Yarom et al. 1985; Takahashi, 1990; Safronov & Vogel, 1995, 1996) and cell cultures (McLarnon, 1995; Scamps et al. 1998). The authors of these studies have described the expression of Na+, Ca2+ and K+ conductances and established three major points: (i) Na+ channels are rarely present at the somatic level, (ii) several types of Ca2+ channels are expressed in variable proportions during development and (iii) various K+ channels are present in the somatic membranes, including voltage-gated, Ca2+- and Na+-activated and inward rectifier K+ currents.
Little is known, however, about the channel distribution between the various cell compartments during the first steps in the development of a mammalian motoneurone: are ionic channels initially distributed uniformly before being allocated to specialized compartments, or are they immediately routed to specific compartments?
In the case of the Na+ channels, this is a crucial question, since density of the Na+ channels must be particularly high at the site of spike initiation and the firing capacity of a motoneurone depends on this channel density (Traub & Llinas, 1977; Safronov & Vogel, 1995). In addition, the initial distribution of Na+ channels in embryonic motoneurones may subsequently influence the clustering of axonal Na+ channels in the node of Ranvier induced by Schwann cell contacts (Vabnick et al. 1996).
Based on the information now available about the conditions required for rat spinal motoneurones to survive and develop in vitro (for a rewiew, see Henderson, 1996; de Lapeyrière & Henderson, 1997), it has become possible to investigate the developmental pattern of ion channel expression in vitro. Motoneurone cell cultures have been found to develop axonal and somatodendritic compartments (Metzger et al. 1998). It has not yet been determined, however, whether these in vitro motoneurones are excitable, i.e. whether they express spike-generating Na+ and K+ currents.
It was therefore proposed to study the distribution of Na+ and K+ channels and their changes during the development of somato-dendritic and axonal polarity in this in vitro model. Using patch-clamp and autoradiographic procedures, it was observed that just after being plated, motoneurones restricted to their soma mainly expressed the delayed rectifier current IDR and, to a lesser extent, a fast-inactivating A-current. During the first hours in culture, the rapid outgrowth of the axon and dendrites was immediately followed by the expression of a TTX-sensitive Na+ current INa. Na+ channels were found to specifically target the axon, showing a greater density in the initial segment, and to be virtually absent from the soma and the dendrites.
METHODS
Motoneurone cell culture
Spinal motoneurones were purified from ventral cords of embryonic day 14 (E14) embryos as described by Henderson et al. (1995). Briefly, rats were anaesthetized by halathone inhalation and killed by inhalation of an excess of CO2. This procedure was in agreement with the French Ministry of Agriculture and the European Community Council Directive No. 86/609/EEC. Ventral spinal cells from embryo were dissociated after trypsin treatment and centrifuged over a 6.5% metrizamide cushion. The large cells remaining above the cushion were further selected using immune interaction between motoneurones and the 192 antibody coated on dishes, which recognizes the low affinity NGF receptor (Chandler et al. 1984) expressed only by ventral motoneurones at this age (Yan & Johnson, 1988).
Purified motoneurones were plated in 35 mm dishes (Nalge Nunc, USA) with polyornithine-laminin (Sigma). The culture medium was: Neurobasal (Gibco-BRL) supplemented with B27 (2% v/v; Gibco-BRL), horse serum (2% v/v; Sigma), L-glutamine (0.5 mM) and 2-mercaptoethanol (25 μM). Two growth-promoting factors were added: glial cell line-derived neurotrophic factor (100 pg ml−1 GDNF; Calbiochem) and ciliary neurotrophic factor (1 ng ml−1 CNTF; Calbiochem). The culture medium was changed every 5 days and L-glutamate (25 μM) was added to the culture medium during the first 5 days of growth. Motoneurones were maintained in vitro for 2 weeks.
Whole-cell patch-clamp recording
Motoneurones were studied using the conventional whole-cell patch-clamp technique. Patch electrodes with a 4 MΩ tip resistance were filled with the following intracellular pipette solution: 120 mM KCl, 10 mM NaCl, 2 mM MgCl2, 10 mM EGTA and 10 mM Hepes, pH 7.3 adjusted with KOH. In some experiments, CsCl was substituted for KCl in order to reduce the voltage-gated K+ currents. The bath solution contained: 140 mM NaCl, 2 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 0.4 mM Na2HPO4 and 10 mM Hepes, pH 7.3 adjusted with NaOH. Tetrodotoxin (TTX), tetraethylammonium (TEA), 4-aminopyridine (4-AP) and toxins were applied under pressure with a multi-barrelled pipette placed close to the soma of the motoneurone recorded from. Dendrotoxin (DTX) was from Latoxan (France), kaliotoxin (KTX) was a gift from J. Van Rietschoten (CNRS UMR 6560, Marseille, France), noxiustoxin (NTX) and BgK, a toxin extracted from sea anemones which acts on Kv1 channels (Cotton et al. 1997), were kindly provided by A. Ménez (CEA, Saclay, France). Nocodazole was from Sigma.
Experiments were carried out at room temperature (20-24°C) with a List EPC-7 patch-clamp amplifier. The software programs used for stimulation, data acquisition and analysis were drawn up by H. Chagneux (CNRS UPR 9024, Marseille, France). Current traces were low-pass filtered at 2 kHz through a 6-pole Bessel filter and digitized on a computer disk at 2-10 kHz. Capacity transient compensation was routinely performed in the cell-attached mode before patch membrane rupture. In the whole-cell voltage-clamp configuration, capacitive transients and leakage currents were subtracted using a factorized hyperpolarizing pulse, without additional transient compensation. The junction potential arising from the low ionic strength of the pipette saline was calculated as described by Barry & Lynch (1991) and found to be +5 mV. This value was subtracted from the observed and applied voltages. The values given are means ±s.e.m.
Electrical activity and antidromic stimulation
Spontaneous action potentials and the action potentials generated by applying current stimulation to the soma were recorded either in the cell-attached mode to avoid wash-out of the cytoplasmic content by the patch pipette solution or in the whole-cell configuration.
Antidromic activation was performed with bipolar extracellular electrodes. A patch electrode filled with bathing solution was placed on each side of a neurite and brief stimulations (0.1-0.5 ms) were delivered from an isolated stimulation unit. Stimulation artefacts and antidromically propagated action potentials were recorded at the soma with a cell-attached pipette.
Autoradiography
α-Scorpion toxin (α-ScTX), toxin II from Androctonus australis Hector, was a generous gift from Professor H. Rochat (CNRS UMR 6560, Marseille, France) and was radiolabelled by peroxidase iodination and purified by immunoprecipitation (Jover et al. 1988). Prior to autoradiography, cells were cultured in Permanox Lab-Tek chamber slides (Nalge Nunc). The cell cultures were incubated with 1 nM 125I-α-ScTX for 30 min at 37°C in the culture medium in the presence of 0.1 μM TTX. At the end of the incubation period, the cells were rinsed three times with 2 ml precooled buffer (140 mM choline chloride, 5 mM KCl, 0.8 mM MgSO4, 1.8 mM CaCl2, 0.1% BSA, 20 mM Hepes adjusted to pH 7.4 with Tris base), rinsed three times with 2 ml of precooled 0.1 M phosphate buffer (pH 7.4) and processed for autoradiography: fixation in 2.5% glutaraldehyde solution in phosphate buffer for 30 min at room temperature, post-fixation in 2% OsO4 solution in the same buffer and ethanol dehydration. Autoradiograms were prepared by dipping the slides into K5 Ilford emulsion, exposing them for 10-20 days at 4°C and developping them in Kodak D19. The slides were then mounted in Entellan (Merck) and the autoradiograms were viewed using interference contrast with a confocal microscope (Leica TCS). The pictures were prepared with Adobe Photoshop.
Immunocytochemistry
Cells grown in Permanox Lab-Tek chamber slides were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 15 min at 37°C, permeabilized in a 0.2% Triton X-100 solution containing 5% BSA, incubated with a monoclonal antibody against GAP 43 (Sigma) at a dilution of 1:2000 in 1% BSA in phosphate buffer solution. Rhodamine conjugated goat antimouse IgG (1:3000; Jackson) was used as the secondary antibody. Slides were mounted in Mowiol (Calbiochem) and the immunofluorescence staining was viewed with a confocal microscope (Leica TCS) using the 568 nm excitation line and bandpass filters.
RESULTS
Just after being plated, the motoneurones were devoid of any neurites. One hour after the plating, a few processes had begun to grow and 18 h after the plating, almost all the cells were multipolar. By day 5-6 in vitro (DIV5-6), the neurites were well developed and immunochemical labelling with antibodies against axonal GAP 43 and dendritic MAP2 showed the presence of one axonal process and several dendritic processes per cell (data not shown), as previously described by Metzger et al. (1998).
After 18 h in vitro, a fast transient inward current followed by a delayed outward current (IDR) was recorded from a holding potential (Vh) of -60 mV. The inward current was activated sharply between -50 and -40 mV, whereas the outward current was activated between -40 and -30 mV and increased with the voltage. At DIV5, i.e. after extensive neuritic development, the kinetics of the two currents showed no change, whereas their respective amplitudes increased (Fig. 1A).
Figure 1. Na+ and K+ currents in motoneuronal cell cultures purified from rat embryos at E14.
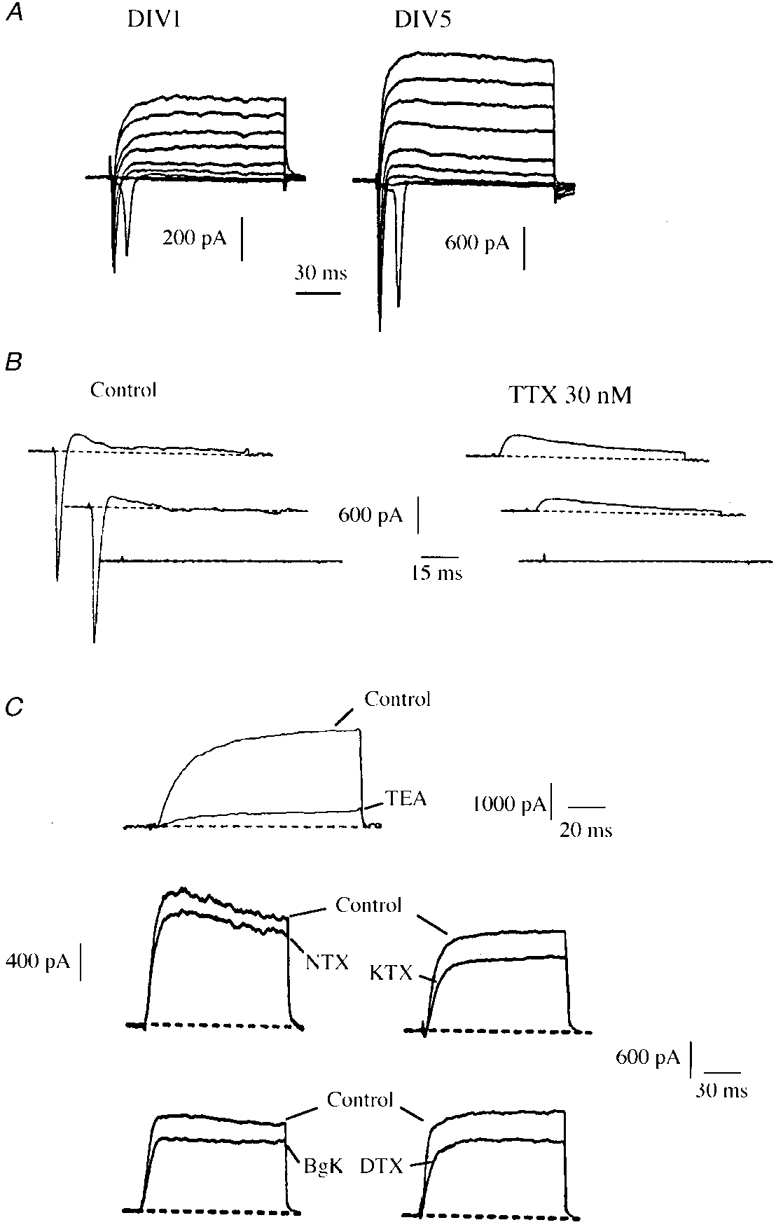
A, representative whole-cell currents recorded at DIV1 and DIV5. Currents were elicited by applying depolarizing pulses from -50 to 30 mV in 10 mV incremental steps from a holding potential Vh = -60 mV. B, fast transient sodium (INa) and outward potassium currents elicited at DIV4 in response to voltage pulses at -50, -40 and -30 mV, from Vh = -60 mV, under control conditions and in the presence of 30 nM TTX. C, delayed rectifier K+ currents (IDR) at 20 mV recorded from Vh = -60 mV. The majority (76%) of this component was blocked by 10 mM TEA (mean: 66 ± 5%, n = 5). The K+ current is poorly sensitive to toxins specific of Kv1 α-subunits: NTX, KTX, DTX and BgK were all applied at 200 nM (n = 46). Currents were elicited at 20 mV from a holding potential Vh = -60 mV (TEA, KTX, DTX) or Vh = -80 mV (NTX, BgK).
The inward current was blocked by 30 nM TTX (Fig. 1B). No evidence was found for the presence of a TTX-resistant Na+ current. The peak INa showed a 2-fold increase between DIV1 and DIV2 and a further 2-fold increase between DIV2 and DIV8. From DIV3 onwards, INa was larger than the outward currents (Fig. 2B). It was difficult, however, to exactly quantify INa due to the remote location of the Na+ channels (see below). The INa peak amplitude was therefore used as an index to the current activated during a spike, rather than the total Na+ current activated in response to a command voltage.
Figure 2. Evolution of INa, IA and IDR during the first 8 days of cell culture.
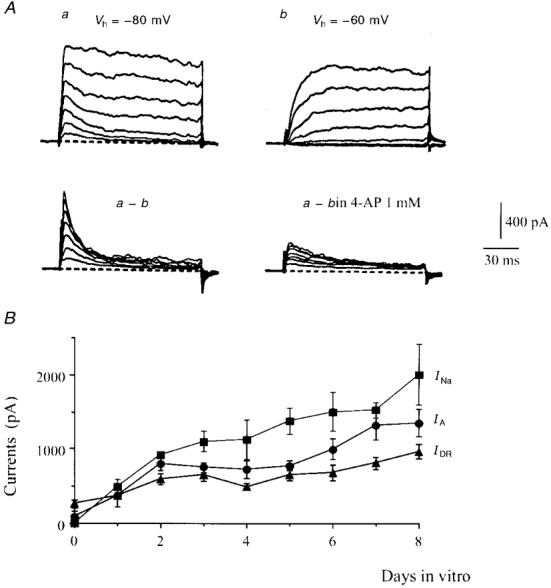
A, recordings performed in the presence of 30 nM TTX. Outward currents elicited at DIV2 by voltage pulses from -40 to 20 mV applied from two holding potentials, Vh = -80 mV (a) and Vh = -60 mV (b). The fast transient A-current was isolated by subtracting series b from series a. This current was partially blocked (64%) by 1 mM 4-AP. B, amplitude (mean ±s.e.m., n = 4 to 10 experiments) of INa, IDR and IA peaks at various days of growth. IA and IDR were recorded at +20 mV, and INa at -30 mV.
Attempts to characterize the delayed rectifier K+ component (IDR) were made using blockers with a wide (TEA) or more specific (DTX, NTX, KTX and BgK) range of activity. The latter four toxins are all blockers of voltage-gated K+ channels containing α-Kv1 subunits. The most sensitive subunits are: Kv1.1 and Kv1.2 to DTX, Kv1.2 and Kv1.3 to NTX, Kv1.1 and Kv1.3 to KTX, and Kv1.1, Kv1.2 and Kv1.3 to BgK (Grissmer et al. 1994; Cotton et al. 1997). TEA (10 mM) blocked 66 ± 5% (n = 5) of the total IDR and none of the toxins tested induced a block larger than 30% (n = 46, Fig. 1C).
A second voltage-gated K+ component was isolated by subtracting the outward currents obtained from a Vh of -60 mV from the records obtained from a Vh of -80 mV (Fig. 2A). This was a fast inactivating current which showed similar properties to those of the A-current (IA) described in neonatal rat motoneurones (Safronov & Vogel, 1995). It is blocked by 1 mM 4-AP (70 ± 2%, n = 5, Fig. 2A).
A few hours after the plating, the motoneurones were devoid of any neurite. The IDR component was consistently present, whereas the A-current was observed in only three out of the nine cells tested. The K+ currents were measured daily up to DIV8. IDR was increased 2-fold between DIV0 and DIV4 and again at DIV8 (Fig. 2B). IA was present in three out of the five motoneurones tested at DIV1 and in all the cells (n = 10) recorded at DIV2. At this stage, the peak A-current was larger than that of IDR, and reached a plateau between DIV3 and DIV5. Its amplitude increased again 2-fold between DIV5 and DIV8 (Fig. 2B).
In hemisected embryonic spinal cord, the motoneurones become excitable at E14 (Ziskind-Conhaim, 1988). Under our cell culture conditions, motoneurones isolated from E14 rat spinal cords were not excitable during the first 2 days in vitro. This lack of excitability may have resulted from the methods used to purify the motoneurones. From DIV3 onwards, somatic stimulation triggered an action potential (Fig. 3A). Starting from DIV5, a sustained stimulation (> 1 s) generated a train of action potentials with a maximum firing rate of 14 Hz. At this stage, spontaneous activity was observed in 13 cells out of the 17 cells recorded (Fig. 3B). This activity was sustained by irregular fluctuations of the membrane potential, the origin of which still remains to be determined (Fig. 3C).
Figure 3. Excitability and spontaneous activity of motoneurones in vitro.
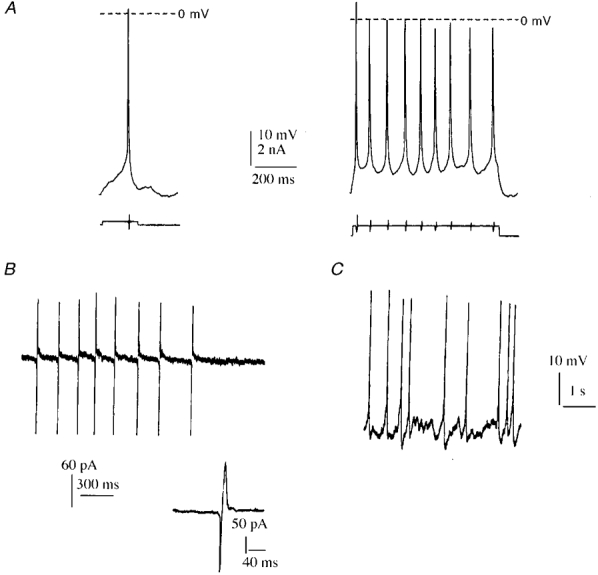
A, action potentials in the whole-cell configuration resulting at DIV5 from a suprathreshold depolarizing current pulse of 3 nA applied for 200 ms. A longer stimulation pulse of 3 nA applied for 1.25 s (right-hand trace) induced a tonic discharge at 14 Hz. B, spontaneous action potentials recorded at DIV6 in the cell-attached configuration. Inset: detail of one action potential. C, spontaneous action potentials recorded at DIV7 in the whole-cell configuration, just after seal rupture.
In all the recordings obtained between DIV1 and DIV14, INa was activated sharply. As shown in Fig. 4A, increasing the voltage pulse by only 1 mV (from -49 to -48 mV) triggered a full-sized INa, which contrasts with the gradual current increase which occurred in properly voltage-clamped Na+ channels. This suggested that the Na+ channels were located far from the clamped somatic space, presumably in the elongated processes. To test this assumption, Na+ currents were recorded using a patch pipette containing CsCl in order to partly block K+ currents (left panel in Fig. 4B). The longest and thinnest neurite (which we assumed to be the axon) was crushed 50-80 μm from the soma with the tip of a glass electrode (n = 4). More than 90% of the Na+ current was lost (right panel in Fig. 4B), whereas the somato-dendritic K+ currents which were not blocked by CsCl remained unchanged. Since performing the same procedure on the secondary processes of various motoneurones had no effect on the Na+ current, these processes were presumably not excitable.
Figure 4. Sharply activating sodium current.
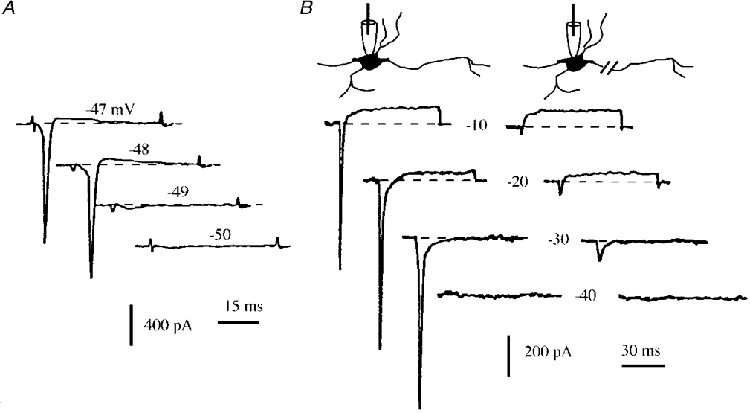
A, INa elicited at DIV8 by applying voltage pulses from -50 to -47 mV in 1 mV incremental steps, from Vh = -80 mV. The Na+ current was almost completely activated by changing the voltage pulse level from -49 to -48 mV. B, left panel: Na+ and K+ currents elicited at DIV2 by applying voltage pulses from -40 to 0 mV (10 mV increment) from a holding potential Vh = -60 mV. The intracellular pipette contained CsCl, which reduced the K+ currents. Right panel: same procedure performed after crushing the longest and thinnest neurite (presumed to be the axon).
The results of antidromic stimulation experiments supported this hypothesis. Stimulation was applied by placing extracellular electrodes filled with the bath solution on both sides of every neurite of a motoneurone. Brief (< 0.5 ms) stimuli were delivered at increasing voltages (1-15 V). Stimulation artefacts and antidromic potentials were recorded using a cell-attached electrode sealed to the soma. In 10 experiments, only one neurite generated an action potential in response to 0.1 ms, 7-8 V stimulations. This is illustrated in Fig. 5, which shows the effects of stimuli applied to all the neurites emerging from the soma. Insets 1 and 2 show the antidromic response of the excitable neurite. The other neurites were not excitable in spite of the increase in the duration and voltage of the stimulation (0.3 ms, 15 V). This experiment also illustrates the finding that antidromic potentials with an increasing latency (inset 2 in Fig. 5) were still recorded when the stimulating electrodes were moved along the excitable neurite, which shows that the action potentials were transmitted all along the whole excitable neurite.
Figure 5. Antidromic stimulation of the various neurites of a motoneurone.
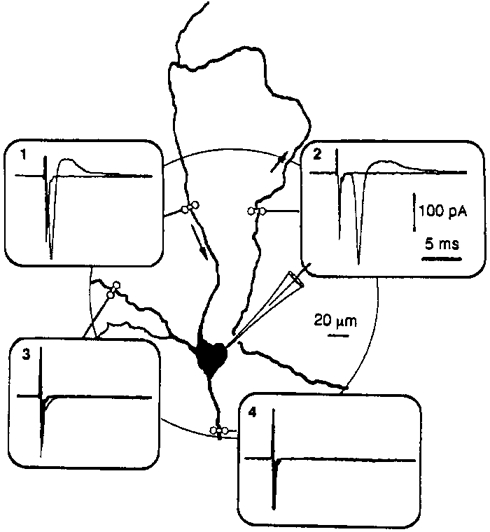
All the neurites of this motoneurone (DIV7) were stimulated with extracellular bipolar electrodes in order to generate an antidromic action potential. Somatic recordings were carried out in the cell-attached mode. A stimulation pulse of 0.1 ms, 8 V generated an antidromic potential in only one neurite (inset 1). When the stimulation was applied to the same neurite at a point approximately 600 μm from the soma, the antidromic potential reached the soma with a latency of 2.3 ms (inset 2), which corresponds to a propagation velocity of ≈0.3 m s−1. In insets 3 and 4, stimulation pulses of 0.3 ms, 15 V applied to the other processes were ineffective.
In order to detect the voltage-sensitive Na+ channels at the surface of the motoneurones, we used a radiolabelled α-scorpion toxin as a specific marker (Jover et al. 1988; Dargent et al. 1994). This toxin is known to selectively interact with pharmacological site 3 of Na+ channels in a voltage-dependent manner: the more depolarized the membrane, the lower the affinity becomes. After 6 days (Fig. 6A) or 2 days (Fig. 6B) in culture, cells were incubated with 1 nM 125I-α-scorpion toxin in the presence of 100 nM TTX which binds to site 1 and inhibits sodium influx through the pore, preventing any membrane depolarization from being induced by α-ScTX. The cells were subsequently processed for autoradiography. As shown in Fig. 6A and B, a single process was intensely labelled with silver grains, whereas almost no labelling was detected on the cell body or the other processes. When the incubation was carried out in the presence of an excess of unlabelled α-ScTX, none of the processes were labelled and the silver grains were very rare and randomly distributed on the surface of the slide (Fig. 6C). The density of the silver grains was highest at the initial segment of the axon and decreased distally from this region (Fig. 6A). The distance between the strongly labelled axonal domain and the cell body varied from cell to cell: in some cases the α-ScTX-labelled domain was directly adjacent to the soma whereas in other cases it was separated by an unlabelled segment. Since these data were obtained in vitro, it is difficult to interpret them in terms of axon hillocks and initial segments. The fact that the boundary between the two domains was always sharp indicates, however, that the localization of the Na+ channels is very precisely controlled in this area. Even after 2 weeks in vitro, we were unable to detect any Na+ channel aggregates of the kind described in retinal ganglion neurones grown in co-culture with oligodendrocytes (Kaplan et al. 1997), which indicates that, as in retinal ganglion neurones, the aggregation of Na+ channels probably requires extracellular signals.
Figure 6. Distribution of 125I-α-scorpion toxin binding sites at the surface of motoneurones in vitro.
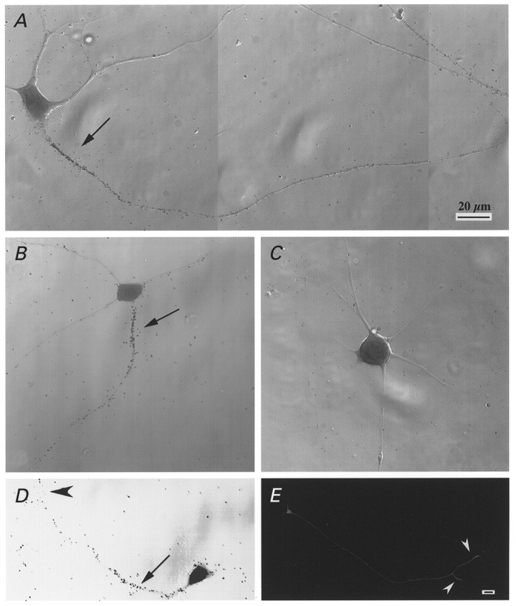
A-D, motoneurones cultured for 18 h (D), 2 days (B) and 6 days (A and C) were incubated with 1 nM 125I-α-scorpion toxin and 100 nM TTX, in the absence (A, B and D) or presence (C) of 100 nM native α-scorpion toxin. A high density of silver grains was observed at the initial segment of the axons (arrows), whereas the other neurites and the somata were not labelled. Growth cones (arrowheads) were also labelled. E, motoneurones cultured for 18 h were immunolabelled with a monoclonal anti-GAP 43 antibody. Growth cones (arrowheads) were heavily labelled. Scale bar in A also applies to B-D; scale bar in E, 20 μm.
The acquisition of this specific Na+ channel distribution occurred very early during in vitro neuronal development. As shown in Fig. 6D, the distribution of silver grains on a motoneurone after 18 h in culture was similar to that observed later on. It is noteworthy that the growth cone was also labelled. Figure 6E illustrates the distribution of GAP 43, a protein which has a high level of expression in growth cones and plays a key role in axonal growth and in modulating the formation of new connections (see for a review, Benowitz & Routtenberg, 1997). After 18 h in vitro, the density of the immunolabelled GAP 43 was highest at the distal segment of the growing axon, contrary to what occurred in the case of the Na+ channels.
These findings raise questions as to the presence of Na+ channels just after the plating, when the motoneurones were almost devoid of any processes. We therefore checked the presence of ionic currents at DIV0, 1 h after the plating (Fig. 7A). No INa was detected (n = 6) at that stage, whereas the IDR was present. Under these conditions, no specific labelling was detected upon performing autoradiography with 125I-α-ScTX (data not shown). Four hours after the plating, when the neurites had started to sprout, the motoneurones expressed a small but detectable Na+ current (n = 4, not illustrated). It can be seen from Fig. 7B that, after 18 h in vitro, motoneurones with neurites expressed an INa which was activated abruptly between -50 and -40 mV. These results suggest that the presence of Na+ currents was dependent on axon growth, whereas IDR expression did not require any sprouting.
Figure 7. INa is dependent on neuritic outgrowth.
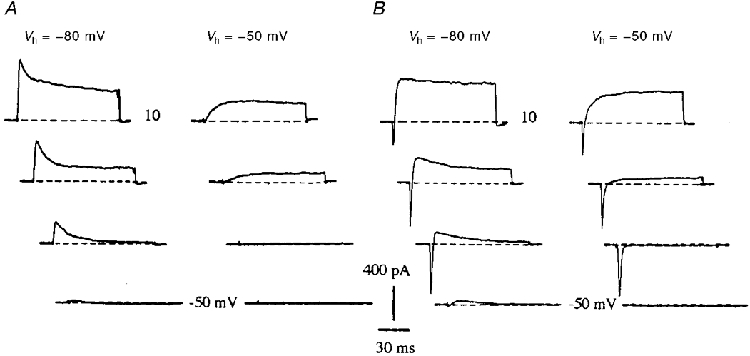
A, ionic currents recorded 1 h after the plating, when the motoneurones were devoid of neurites. INa was absent, whereas IA and IDR were already expressed. Voltage pulses: -50 to 10 mV in 20 mV incremental steps. B, same conditions as in A. Experiment performed 18 h after the plating when neurites had developed. Note the expression of INa.
We then examined the effects of nocodazole, which acts mainly on microtubule depolymerization and thus completely inhibits axonal growth in neuronal cell cultures (Lafont et al. 1993) probably by blocking the axonal transport of integral membrane proteins (Arregui et al. 1995). Nocodazole (30 μM) added to the culture medium immediately after the plating almost completely stopped the neuritic elongation. Some neurones were completely devoid of processes (Fig. 8B), whereas others had very small and less refringent neurites (Fig. 8C). Autoradiographic experiments with radiolabelled α-ScTX were carried out after 18 h in vitro in the absence (Fig. 8A) or presence of nocodazole (Fig. 8B and C). In the presence of the drug, no specific labelling was detected. Ionic currents were recorded under the same conditions (Fig. 8D). In the presence of nocodazole, no INa was detected (n = 4) whereas 74% (280 ± 57 pA) of the IDR level normally expressed at this stage was present. These results indicate that nocodazole completely abolished the transport of Na+ channels to the plasma membrane: only the K+ channels present in the plasma membrane before nocodazole addition were detected, which confirms the idea that the soma received no Na+ channels.
Figure 8. Effect of nocodazole on Na+ channel expression.
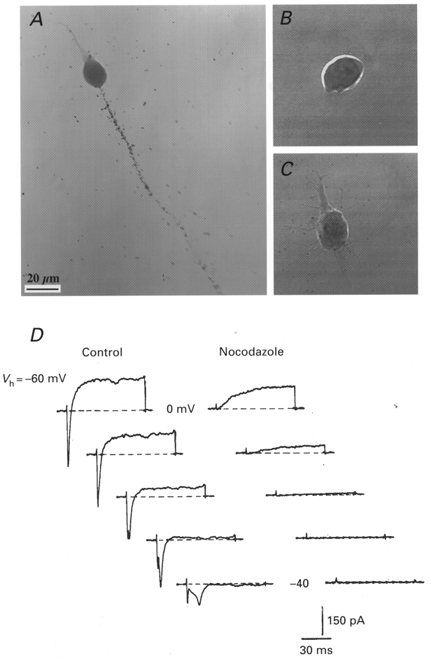
A-C, motoneurones grown for 18 h in the absence (A) or presence (B and C) of 30 μM nocodazole, labelled with 1 nM 125I-α-scorpion toxin in the presence of 100 nM TTX and processed for autoradiography. D, experiments performed at DIV1. Ionic currents elicited by voltage pulses at -40 to 0 mV in 10 mV incremental steps, under control conditions (left panel) and after treatment with nocodazole (30 μM, right panel). Nocodazole was added to the culture medium immediately after the plating. The nocodazole treatment abolished the Na+ current expression and partly reduced the delayed K+ current. Vh = -60 mV. Scale bar in A also applies to B and C.
DISCUSSION
In the present study we investigated the emergence and development of electrical excitability in cultured fetal rat spinal motoneurones. This cell model has been developed during the last few years (Pennica et al. 1996) and has been mainly used to study the mechanisms and factors involved in the survival and growth of mammalian motoneurones (Oppenheim, 1996). However, except for the recent analysis of somatic Ca2+ currents (Scamps et al. 1998), it has not yet been established whether these motoneurones are excitable and how and when the excitability may emerge.
The results of this study have shown that: (i) these cultured motoneurones are actually excitable; (ii) the excitability results from an increasing level of INa expression, which becomes large enough to generate action potentials as from DIV3; and (iii) the Na+ channels target only growing axons and are virtually absent from the soma and dendrites. This finding that Na+ channels are located in the axon alone is supported by (i) the absence of any detectable Na+ current prior to the neuritic outgrowth, when the neurite outgrowth was blocked by nocodazole, and after axotomy; (ii) the absence of any detectable α-ScTX labelling of somatic or dendritic membranes; and (iii) the absence of any somatic response to focal stimulation of non-axonal neurites.
In mice, the density of the Na+ channels in the soma of neuronal cell cultures obtained from embryonic spinal cords has been estimated to be less than two channels per square micrometre, and as we observed, the Na+ current elicited after 18 h in culture is activated abruptly (McDermott & Westbrook, 1986). These facts have been taken to indicate that the spike-generating Na+ channels may have a remote axonal localization. As far as the spinal motoneurones are concerned, Na+ channels have been detected in the soma of neonatal motoneurones (Safronov & Vogel, 1995). In this preparation, however, the whole-cell Na+ current is activated sharply (Takahashi, 1990), which suggests that the somatic Na+ channel density is far too low to generate an action potential. A similar distribution of Na+ channels was observed in rat spinal neurones of the dorsal horn, in which axotomy almost completely abolished the Na+ currents (Safronov et al. 1997). This steep somato-axonal Na+ channel gradient in spinal neurones seems to be specific to mammals, since in Xenopus motoneurones, the Na+ current was gradually activated, which suggests that Na+ channels were distributed in both somatic and axonal membranes (O'Dowd et al. 1988).
Unlike the Na+ current, the delayed rectifier current was present in the soma immediately after the plating when the motoneurones were completely devoid of processes. During the first days of growth, the rise in IDR presumably prevented the neurones from becoming excitable until INa overwhelmed IDR, which occurred after DIV3. In Xenopus embryonic spinal cord neurones, IDR was present at the earliest stages examined and IA was recorded only at DIV2 (O'Dowd et al. 1988; Ribera & Spitzer, 1990). Likewise, in our culture, the transient A-current was hardly detectable just after the plating. It then displayed a period of fast increase and was present in all the motoneurones tested at DIV2. In neonatal rat motoneurones, A-channels are present in the somatic membrane where they constitute the most frequently detected K+ channel in single recordings (Safronov & Vogel, 1995). This does not rule out the possible presence of A-channels in axons and dendrites, as described in the spinal neurones of the dorsal horn (Wolff et al. 1998) and hippocampal neurones (Sheng et al. 1992; Cooper et al. 1998). Further experiments involving for example cell-attached recordings of ionic currents from various part of the neurones are now required to determine the exact pattern of distribution of A-channels in our motoneuronal cell cultures.
In motoneurones dissociated from the spinal cord of chick embryos at various stages of development, IA and IDR also increased at different rates, with a later and larger increase in the case of IA, which suggests that the developmental pattern we observed in vitro mimics what occurs in vivo (McCobb et al. 1990). In other respects, in adult rats, a marked somato-dendritic immunoreactivity to the α-subunits Kv1.1 and Kv1.2 encoding DR channels has been described in ventral horn slices (Veh et al. 1995). In our cell cultures, we observed a low sensitivity to Kv1.1 and Kv1.2 channel blockers (NTX, KTX, DTX and BgK), which suggests that the proportion of the subunits encoding K+ channels may change at a later age than E14.
In addition to the above electrophysiological evidence, the existence of a higher Na+ channel density in the axon of mammalian neurones has been previously suggested by the results of experiments in which Na+ channels were directly labelled using either specific toxins (Catterall, 1981; Boudier et al. 1985) or antibodies (Wollner & Catterall, 1986). Our results clearly indicate that the difference in Na+ channel density between the axon and the soma does not result from the progressive redistribution of channel proteins during maturation, but is due to the targeting of axonal membranes by Na+ channels as soon as the axon begins to grow. In view of the high density of the Na+ channels present in the initial segment of the axon, it is also clear that the gradient is established at a very early stage. Another important result to emerge here is that this very precise distribution of Na+ channels does not result from interactions with glial cells or with other neurones, since at this stage of growth, motoneurones were completely isolated. No astrocytes or oligodendrocytes were present and no neural connections were yet established. This is in good agreement with recent data by Zhou et al. (1998) indicating that the clustering of Na+ channels at the initial segment is selectively controlled by ankyrin G, a cytoskeleton-associated protein. However, the factors that control this early targeting process are probably different from thoses that control Na+ channels clustering at the beginning of the formation of the nodes of Ranvier. This clustering was found to be induced by contact with Schwann cells in the dorsal root ganglion neurones (Joe & Angelides, 1992) and by oligodendrocytic factors in retinal ganglion cells (Kaplan et al. 1997).
In conclusion, in spinal motoneurones in vitro, K+ channels are present in the somatic membrane before and after the neuritic growth, whereas Na+ channels are expressed at a later stage and are distributed only to growing axons, showing a higher density in the proximal segment. These Na+ channels obviously give motoneurones their specific excitability and can be taken to constitute an early sign of axonal differentiation. In the absence of data on the distribution of ionic channels in motoneurones in vivo, this cell system should therefore be most useful for analysing the various subunits of Na+ and K+ channels in embryonic spinal motoneurones and for studying the factors that may serve to modulate their respective distribution.
Acknowledgments
We thank Dr C. Henderson and Dr M. Seagar for helpful comments and discussions, V. Arce and C. Henderson for help with the motoneurone cultures, Dr J. A. Boudier for help with the confocal microscopy, Dr R. Ben Khalifa for help with the electrophysiology, Ms F. Jullien and Mr G. Jacquet for technical assistance and J. Blanc for improving in the text. This research was supported by the Association Française contre les Myopathies (AFM, Grant No. 5975).
References
- Arregui AC, Parton RG, Simons K, Dotti CG. Nocodazole-dependent transport, and brefeldin A-sensitive processing and sorting, of newly synthesized membrane proteins in cultured neurons. Journal of Neuroscience. 1995;15:4259–4269. doi: 10.1523/JNEUROSCI.15-06-04259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JN, Crill WE. Voltage clamp of cat motoneurone somata: properties of the fast inward current. The Journal of Physiology. 1980;304:231–249. doi: 10.1113/jphysiol.1980.sp013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in Neurosciences. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Boudier JA, Berwald-Netter Y, Dellmann HD, Boudier JL, Couraud F, Koulakoff A, Cau P. Ultrastructural visualization of Na+ channel associated 125I-α-scorpion toxin binding sites on fetal mouse nerve cells in culture. Developmental Brain Research. 1985;20:137–142. doi: 10.1016/0165-3806(85)90097-5. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Localization of sodium channels in cultured neural cells. Journal of Neuroscience. 1981;1:777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. Journal of Biological Chemistry. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. Journal of Neuroscience. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton J, Crest M, Bouet F, Alessandri N, Gola M, Forest E, Karlsson E, Castañeda O, Harvey AL, Vita C, Menez A. A potassium-channel toxin from the sea anemone Bunodosoma granulifera, an inhibitor for Kv1 channels. European Journal of Biochemistry. 1997;244:192–202. doi: 10.1111/j.1432-1033.1997.00192.x. [DOI] [PubMed] [Google Scholar]
- Dargent B, Paillart C, Carlier E, Alcaraz G, Martin-Eauclaire MF, Couraud F. Sodium channel internalization in developing neurons. Neuron. 1994;13:683–690. doi: 10.1016/0896-6273(94)90035-3. 10.1016/0896-6273(94)90035-3. [DOI] [PubMed] [Google Scholar]
- De Lapeyrière O, Henderson CE. Motoneuron differentiation, survival and synaptogenesis. Current Opinion in Genetics of Development. 1997;7:642–650. doi: 10.1016/s0959-437x(97)80012-3. 10.1016/S0959-437X(97)80012-3. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz J, Auperin DD, Chandy G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5 and 3.1, stably expressed in mammalian cell lines. Molecular Pharmacology. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Henderson CE. Role of neurotrophic factors in neuronal development. Current Opinion in Neurobiology. 1996;6:64–70. doi: 10.1016/s0959-4388(96)80010-9. 10.1016/S0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W. Purified embryonic motoneurones. In: Cohen J, Wilkin G, editors. Nerve Cell Culture: A Pratical Approach. Oxford, London: University Press; 1995. pp. 69–81. [Google Scholar]
- Joe EH, Angelides K. Clustering of voltage-dependent sodium channels on axons depends on Schwann cell contact. Nature. 1992;356:333–335. doi: 10.1038/356333a0. 10.1038/356333a0. [DOI] [PubMed] [Google Scholar]
- Jover E, Massacrier A, Cau P, Martin MF, Couraud F. The correlation between Na+ channel subunits and scorpion toxin binding sites. Journal of Biological Chemistry. 1988;263:1542–1548. [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Lafont F, Rouget M, Rousselet A, Valenza C, Prochiantz A. Specific responses of axons and dendrites to cytoskeleton perturbations: an in vitro study. Journal of Cell Science. 1993;104:433–443. doi: 10.1242/jcs.104.2.433. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. The differentiation of excitability in embryonic chick limb motoneurons. Journal of Neuroscience. 1990;10:2974–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AB, Westbrook GL. Early development of voltage-dependent sodium currents in cultured mouse spinal cord neurons. Developmental Biology. 1986;113:317–326. doi: 10.1016/0012-1606(86)90167-3. [DOI] [PubMed] [Google Scholar]
- McLarnon JG. Potassium currents in motoneurones. Progress in Neurobiology. 1995;47:513–531. doi: 10.1016/0301-0082(95)00032-1. 10.1016/0301-0082(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Metzger F, Wiese S, Sendtner M. Effect of glutamate on dendritic growth in embryonic rat motoneurones. Journal of Neuroscience. 1998;18:1735–1742. doi: 10.1523/JNEUROSCI.18-05-01735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium and potassium currents in Xenopus spinal neurons. Journal of Neuroscience. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. 10.1016/S0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Pennica D, Arce V, Swanson TA, Vejsada R, Pollock RA, Armanini M, Dudley K, Phillips HS, Rosenthal A, Kato AC, Henderson CE. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurones. Neuron. 1996;17:63–74. doi: 10.1016/s0896-6273(00)80281-0. 10.1016/S0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Ribera AB, Spitzer NC. Differentiation of IKA in amphibian spinal neurons. Journal of Neuroscience. 1990;10:1886–1891. doi: 10.1523/JNEUROSCI.10-06-01886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. The Journal of Physiology. 1995;487:91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. The Journal of Physiology. 1996;497:727–734. doi: 10.1113/jphysiol.1996.sp021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Functional distribution of three types of Na+ channel on soma and processes of dorsal horn neurones of rat spinal cord. The Journal of Physiology. 1997;503:371–385. doi: 10.1111/j.1469-7793.1997.371bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F, Valentin S, Dayanithi G, Valmier J. Calcium channel subtypes responsable for voltage-gated intracellular calcium elevations in embryonic rat motoneurones. Neuroscience. 1998;87:719–730. doi: 10.1016/s0306-4522(98)00165-1. 10.1016/S0306-4522(98)00165-1. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur M-L, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. 10.1016/0896-6273(92)90166-B. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. The Journal of Physiology. 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Llinas R. The spatial distribution of ionic conductances in normal and axotomized motoneurons. Neuroscience. 1977;2:829–849. 10.1016/0306-4522(77)90110-5. [Google Scholar]
- Vabnick I, Novakovic D, Levinson R, Schachner M, Shrager P. The clustering of axonal sodium channels during development of the peripheral nervous system. Journal of Neuroscience. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: Contrasting subcellular locations and neuron-specific co-localizations in rat brain. European Journal of Neuroscience. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Walton K, Fulton BP. Ionic mechanisms underlying the firing properties of rat neonatal motoneurones studied in vitro. Neuroscience. 1986;19:669–683. doi: 10.1016/0306-4522(86)90291-5. 10.1016/0306-4522(86)90291-5. [DOI] [PubMed] [Google Scholar]
- Wolff M, Vogel W, Safronov BV. Uneven distribution of K+ channels in soma, axon and dendrites of rat spinal neurones: functional role of the soma in generation of action potentials. The Journal of Physiology. 1998;509:767–776. doi: 10.1111/j.1469-7793.1998.767bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proceedings of the National Academy of Sciences of the USA. 1986;83:8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. Journal of Neuroscience. 1988;9:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom Y, Sugimori M, Llinas R. Ionic currents and firing patterns of mammalian vagal motoneurones in vitro. Neuroscience. 1985;16:719–737. doi: 10.1016/0306-4522(85)90090-9. 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. Ankyrine G is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. Journal of Cellular Biology. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Electrical properties of motoneurones in the spinal cord of rat embryos. Developmental Biology. 1988;128:21–29. doi: 10.1016/0012-1606(88)90262-x. [DOI] [PubMed] [Google Scholar]


