Abstract
We have recently shown that neurones in the rostral region of the medial vestibular nucleus (MVN) develop a sustained increase in their intrinsic excitability within 4 h of a lesion of the vestibular receptors of the ipsilateral inner ear. This increased excitability may be important in the rapid recovery of resting activity in these neurones during ‘vestibular compensation’, the behavioural recovery that follows unilateral vestibular deafferentation. In this study we investigated the role of the acute stress that normally accompanies the symptoms of unilateral labyrinthectomy (UL), and in particular the role of glucocorticoid receptors (GRs), in the development of the increase in excitability in the rostral MVN cells after UL in the rat.
The compensatory increase in intrinsic excitability (CIE) of MVN neurones failed to occur in animals that were labyrinthectomized under urethane anaesthesia and kept at a stable level of anaesthesia for either 4 or 6 h after UL, so that they did not experience the stress normally associated with the vestibular deafferentation syndrome. In these animals, ‘mimicking’ the stress response by administration of the synthetic GR agonist dexamethasone at the time of UL, restored and somewhat potentiated CIE in the MVN cells. Administration of dexamethasone in itself had no effect on the intrinsic excitability of MVN cells in sham-operated animals.
In animals that awoke after labyrinthectomy, and which therefore experienced the full range of oculomotor and postural symptoms of UL, there was a high level of Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus over 1·5-3 h post-UL, indicating a strong activation of the stress axis.
The GR antagonist RU38486 administered at the time of UL abolished CIE in the rostral MVN cells, and significantly delayed behavioural recovery as indicated by the persistence of circular walking. The mineralocorticoid receptor (MR) antagonist spironolactone administered at the time of UL had no effect.
Vestibular compensation thus involves a novel form of ‘metaplasticity’ in the adult brain, in which the increase in intrinsic excitability of rostral MVN cells and the initial behavioural recovery are dependent both on the vestibular deafferentation and on the activation of glucocorticoid receptors, during the acute behavioural stress response that follows UL. These findings help elucidate the beneficial effects of neuroactive steroids on vestibular plasticity in various species including man, while the lack of such an effect in the guinea-pig may be due to the significant differences in the physiology of the stress axis in that species.
Ablation of the vestibular receptors of one inner ear (unilateral labyrinthectomy, UL) or unilateral vestibular neurectomy disfacilitates the second-order neurones in the medial vestibular nucleus (MVN) ipsilateral to the lesion. The normally high resting activity of these cells is largely abolished immediately after UL as a result of this disfacilitation but presumably also because of enhanced commissural inhibition from contralateral MVN neurones, which become hyperactive due to the loss of inhibitory drive from the lesioned side (Smith & Curthoys, 1988a,b; Newlands & Perachio, 1990a,b; Ris et al. 1995, 1997; for reviews, see Smith & Curthoys, 1989; Dieringer, 1995; Curthoys & Halmagyi, 1995). The consequent profound imbalance in the resting activity of MVN cells on the lesioned and intact sides is believed to cause the severe oculomotor and postural symptoms that immediately follow UL (spontaneous ocular nystagmus, barrel rolling, circular walking, and yaw- and roll-head tilt; Smith & Curthoys, 1989; Curthoys & Halmagyi, 1995). Remarkably, these severe symptoms abate rapidly so that, in the rat, barrel rolling and circular walking abate within 6-8 h of the lesion, and spontaneous nystagmus disappears within 48 h, as ‘vestibular compensation’ (VC) occurs. This behavioural recovery is accompanied by the re-appearance of resting activity in the ipsilateral MVN cells, which rises to near-normal levels (rat: Hamann & Lannou, 1988; guinea-pig: Smith & Curthoys, 1988a,b; Ris et al. 1995, 1997). The initial, rapid stage of VC is followed by a much slower process in which the dynamic reflex responses to vestibular stimulation recover, though never completely (Curthoys & Halmagyi, 1995). Since there is no regeneration of the lesioned labyrinth or nerve, the behavioural recovery after UL is attributed to lesion-induced plasticity in the central vestibular pathways.
While many studies have sought evidence for synaptic or neurochemical changes in the vestibular nuclei and related central structures during VC, the specific cellular mechanisms that bring about the initial rapid recovery of resting activity in the ipsilateral MVN cells after UL are largely unknown (Smith & Curthoys, 1989; Curthoys & Halmagyi, 1995). We have recently shown, using slices of the ipsilateral MVN prepared from animals that had been labyrinthectomized at various times beforehand, that there is a significant increase in the intrinsic excitability of the deafferented MVN neurones between 2 and 4 h after UL (Cameron & Dutia, 1997). This is observed as an increase in the mean spontaneous firing rates of the ipsilateral cells when the MVN of the two sides are isolated in vitro, removed from the influence of the commissural inhibitory system that normally links them in vivo. This ‘compensatory increase in excitability’ (CIE) is restricted to the MVN cells located in the rostral part of the ipsilateral nucleus, while the in vitro firing rates of the cells in the ipsilateral caudal MVN and the contralateral MVN are no different after UL. We have proposed that the development of CIE in the rostral MVN neurones may be a cellular mechanism for the recovery of resting activity in these cells after UL in vivo, as it would serve to counteract their initial disfacilitation and overcome the enhanced commissural inhibition that follows UL (Cameron & Dutia, 1997).
In this study we investigated the effect of the acute behavioural stress that normally accompanies UL on the development of CIE in the ipsilateral MVN cells. Several lines of evidence suggest that stress-related neuroactive steroids influence the rate of vestibular compensation. Yamanaka et al. (1995) have shown that administration of the synthetic glucocorticoid receptor (GR) agonist dexamethasone promotes behavioural recovery after UL in the rabbit, while the GR antagonist RU38486 delays it. In the guinea-pig, Jerram et al. (1995) reported that systemic administration of methylprednisolone reduced the frequency of spontaneous nystagmus after UL, and we have shown that the opioid antagonist naloxone has a similar effect (Dutia et al. 1996). In man, short-term treatment with methylprednisolone has been reported to reduce vertigo in conditions such as peripheral vestibular neuritis (Ariyasu et al. 1990). The immunocytochemical demonstration of glucocorticoid receptors in the MVN and the results from recent studies using iontophoretic application of neurosteroids in vivo indicate that steroids may modulate the membrane properties and synaptic function in vestibular neurones (Ahima & Harlan, 1990; Yamamoto et al. 1998). However, Alice et al. (1998) reported that dexamethasone had no effect on the firing rate of the majority of guinea-pig MVN neurones in vitro and did not influence the rate of behavioural compensation after UL. In the present experiments, we investigated whether the development of CIE in the rostral MVN cells in the rat was dependent on the animal's experience of stress after UL, by comparing the in vitro firing rates of MVN cells in slices from animals that had regained wakefulness and experienced stress following labyrinthectomy with those in slices from animals that were maintained at a stable level of anaesthesia after UL. The results show that activation of GRs, either through the endogenous release of glucocorticoids or by exogenous administration of dexamethasone, is required both for the development of CIE in the rostral MVN cells and for the behavioural recovery after UL. Vestibular compensation is therefore a novel instance of metaplasticity in the adult brain, where the plastic changes in the properties of rostral MVN neurones that cause the compensatory increase in their excitability depend not only on the initial vestibular imbalance after UL but also on the activation of GRs in the course of the acute stress response that accompanies UL.
METHODS
Slice preparation and recording
Extracellular recordings of the spontaneous tonic activity of MVN neurones were made in horizontal slices of the rat dorsal brainstem in vitro. Slices were prepared as described in detail in previous reports (Dutia et al. 1992; Cameron & Dutia, 1997). Briefly, the animals were anaesthetized in a mixture of 3-4 % halothane in oxygen and decapitated. The whole brain was removed into ice-cold artificial cerebrospinal fluid (aCSF; composition (mM): NaCl, 124; KCl, 5; KH2PO4, 1.2; MgSO4, 1.3; CaCl2, 2.4; NaHCO3, 26.0; D-glucose, 10.0). The brainstem extending from the inferior colliculi to the obex was isolated, and the cerebellum removed to expose the MVN on the floor of the fourth ventricle. The brainstem was cemented with the fourth ventricle uppermost to the stage of a Vibroslice (Camden Instruments, London, UK), and slices of the dorsal brainstem (350-400 μm thickness) containing the MVN were prepared. Each slice was trimmed and cut along the midline, to give two isolated medial vestibular nuclei. Care was taken to unambiguously identify the left (ipsilateral) and right (contralateral) vestibular nuclei. The ipsilateral MVN was transferred to an interface-type incubation chamber that was continuously perfused with aCSF bubbled with 95 % O2-5 % CO2 (pH 7.4; flow rate 1.5 ml min−1) and maintained at 33 ± 0.2°C. All slices were incubated for at least 1 h before recording. Single unit extracellular recordings were made from tonically active vestibular neurones in the rostral region of the MVN using conventional glass micropipettes filled with 2 M sodium gluconate (impedance 10-30 MΩ) connected to the headstage of an Axoclamp 2A amplifier (Axon Instruments). The resting discharge of tonically active MVN neurones was displayed and analysed on-line using a CED 1401 plus laboratory interface (Cambridge Electronic Design, Cambridge, UK) linked to a microcomputer.
In these experiments MVN slices were prepared from animals that had previously undergone a left unilateral labyrinthectomy either under avertin anaesthesia (300 mg kg−1 tribromoethanol i.p., Dyer et al. 1981; ‘avertin-UL’ group), or under urethane anaesthesia (ethyl carbamate, 1.25 g kg−1; ‘urethane-UL’ group). The labyrinthectomy was carried out by opening the horizontal semicircular canal duct in the temporal bone, drilling through the horizontal and anterior semicircular canal ampullae and aspirating the vestibule, which was then rinsed with 100 % ethanol as described in detail previously (Cameron & Dutia, 1997). The avertin-UL animals began to recover from anaesthesia about 50-60 min following induction, typically 20-30 min after the labyrinthectomy was completed. They showed the characteristic severe symptoms of unilateral vestibular loss including spontaneous nystagmus, barrel rolling, circular walking, loss of ipsilateral extensor tone, and head yaw- and roll-tilt. These animals were allowed to recover for 4 h in their home cages after UL, at which point they were re-anaesthetized with halothane and decapitated for the preparation of MVN slices as described above. The urethane-UL animals underwent an identical surgical labyrinthectomy, and then remained anaesthetized in a warm environment for either 4 or 6 h after UL, when they were decapitated and the brainstem removed for the preparation of MVN slices. Although it was not possible to confirm the success of the labyrinthectomy in the urethane-UL animals by observing the characteristic symptoms of vestibular deafferentation as they did not recover from anaesthesia, in each case the completeness of the labyrinthectomy was confirmed visually under the operating microscope.
Control experiments were done in MVN slices prepared from normal (labyrinth intact) animals, and from animals that underwent a sham operation either under avertin anaesthesia (‘avertin-sham’ group), or urethane anaesthesia (‘urethane-sham’ group). In sham-operated animals the horizontal canal duct was exposed using the same surgical approach as for the labyrinthectomy, but the duct was not opened and the inner ear remained intact (Cameron & Dutia, 1997). As with the labyrinthectomized animals, avertin-sham animals were allowed to recover for 4 h post-operation, after which MVN slices were prepared for electrophysiological recording. None of the avertin-sham animals showed symptoms of inner ear damage during the recovery period. The urethane-sham group of animals were kept in a warm environment for 4 h post-operation, after which MVN slices were prepared as above.
Some of the urethane-UL animals were treated with either the synthetic glucocorticoid agonist dexamethasone, or the mineralocorticoid antagonist spironolactone (Sigma, 5 mg kg−1i.p., 30 min before UL and 2 h post-UL). Some of the avertin-anaesthetized animals were treated with the glucocorticoid receptor antagonist RU38486 (5 mg kg−1i.p., 30 min before UL).
Fos staining
The expression of Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus (PVN) was quantified as an index of the degree of activation of the hypothalamo-pituitary-adrenal stress axis after UL. Avertin-sham animals or avertin-UL animals were anaesthetized with halothane and decapitated at either 1.5, 3 or 6 h post-operation. The brains were rapidly removed and cooled by immersion in 2-methylbutane at -30°C. Coronal sections (15 μm) at the level of the PVN were cut and thaw-mounted on gelatin-coated slides, and stored at -70°C until required. The sections were fixed in 4 % paraformaldehyde and 0.1 % glutaraldehyde for 30 min, and washed in 0.2 % Triton X-100 in 0.1 M phosphate buffer, pH 7.4 (PBT, 4 × 15 min). Endogenous peroxidase activity was reduced by immersion in methanol for 20 min, followed by washing in PBT (3 × 15 min). The sections were incubated with a primary antibody to Fos (Oncogene Science, NY, USA, 1:1000 in normal goat serum) for 24 h at 4°C, washed with PBT (2 × 10 min), and incubated with avidin-conjugated anti-goat secondary antibody for 30 min (Vector Laboratories, Peterborough, UK). Fos-like immunoreactivity was visualized using the Vectastain ABC kit followed by incubation in peroxidase substrate solution (VIP, Vector UK). The number of Fos-positive cells per square millimetre in six alternate sections of the PVN from each animal were counted under bright-field illumination using an image analysis system (NIH Image), and the mean number of Fos-positive PVN cells per square millimetre was calculated for each group of animals.
The experiments in this study were carried out in accordance with the provisions of the UK Animals (Scientific Procedures) Act, 1986.
RESULTS
Effects of anaesthesia on the expression of CIE in MVN neurones after UL
The spontaneous in vitro discharge rates of MVN cells were systematically sampled in the rostral region of the ipsilateral nucleus, in slices prepared from animals that had regained wakefulness after either a sham operation or a unilateral labyrinthectomy (UL) under avertin anaesthesia 4 h previously (‘avertin-sham’ group, n = 9 animals, and ‘avertin-UL’ group, n = 9 animals). The mean firing rates of rostral MVN cells in slices from avertin-sham animals were no different from those in slices from normal animals (15.22 ± 1.4 spikes s−1, n = 49 cells in avertin-sham slices vs. 15.15 ± 1.1 spikes s−1, n = 62 cells in normal (labyrinth-intact) controls; data from Cameron & Dutia, 1997; Fig. 1A). In slices prepared from avertin-UL animals, the mean discharge rate of the rostral MVN cells was significantly higher than that in avertin-sham controls (21.61 ± 1.8 spikes s−1, n = 61 cells, P < 0.05, Mann-Whitney ranked sum test). This result confirms our earlier report of a significant increase in intrinsic excitability of ipsilateral rostral MVN cells within 4 h after unilateral labyrinthectomy in the rat (Cameron & Dutia, 1997).
Figure 1. Compensatory increase in intrinsic excitability of rostral MVN cells after UL under various experimental conditions.
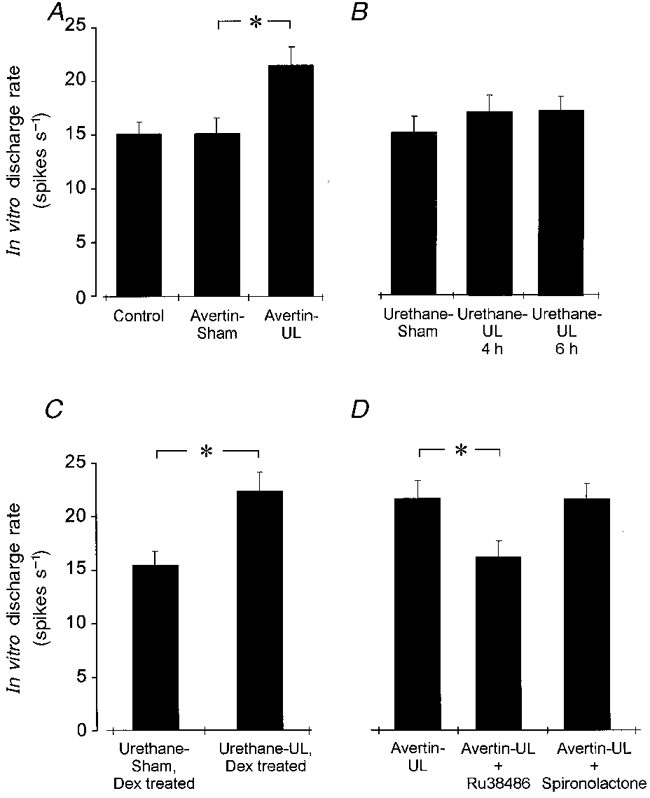
Mean (±s.e.m.) spontaneous firing rates of rostral MVN neurones in slices of the ipsilateral medial vestibular nucleus in vitro, under various experimental conditions. A, mean spontaneous firing rates of rostral MVN neurones in slices from control animals and animals that were sham operated (Avertin-sham) or labyrinthectomized (Avertin-UL) under avertin anaesthesia. Note the significant increase in the mean spontaneous firing rate of MVN cells in the avertin-UL group compared with the avertin-sham controls (* P < 0.05, Student's t test). B, mean spontaneous firing rates of rostral MVN cells in slices from urethane-anaesthetized animals that underwent a sham operation (urethane-Sham), or a left labyrinthectomy either 4 h earlier (‘urethane-UL, 4 h’ group) or 6 h earlier (‘urethane-UL, 6 h’ group). There was no significant change in the spontaneous discharge rate of the rostral MVN cells after UL in these animals. C, mean spontaneous firing rates of rostral MVN cells in slices from urethane-anaesthetized animals that were treated with the glucocorticoid receptor agonist dexamethasone, and subjected either to a sham operation (‘urethane-Sham, Dex-treated’ group) or to a left labyrinthectomy (‘urethane-UL, Dex-treated’ group) 4 h earlier. The significant increase in the spontaneous firing rate of rostral MVN cells after UL is restored in the urethane-anaesthetized animals by dexamethasone. D, mean spontaneous firing rates of rostral MVN cells in slices from animals labyrinthectomized under avertin anaesthesia 4 h earlier, which were treated with either the GR antagonist RU38486 (‘avertin-UL, RU-treated’ group) or the mineralocorticoid receptor antagonist spironolactone (‘avertin-UL, Sp-treated’ group). The increase in spontaneous firing rate of the rostral MVN cells after UL is abolished by the GR antagonist, while the MR antagonist has no effect.
By contrast, in slices prepared from animals that were unilaterally labyrinthectomized under urethane anaesthesia and which then remained anaesthetized for 4 h after UL (‘urethane-UL, 4 h’ group, n = 6 animals), this increase in intrinsic excitability of the rostral MVN cells did not occur (Fig. 1B). The mean in vitro firing rates of the rostral MVN cells in these slices were not significantly different from urethane-sham controls (16.79 ± 1.49 spikes s−1, n = 58 cells in ‘urethane-UL, 4 h’ slices vs. 15.35 ± 1.31 spikes s−1, n = 76 cells in urethane-sham controls, Fig. 1B). To test the possibility that the development of CIE in the rostral cells in the urethane-UL animals was simply delayed compared with that in avertin-UL animals, a further six animals were labyrinthectomized under urethane and kept anaesthetized for 6 h post-UL (‘urethane-UL, 6 h’, Fig. 1B). In slices prepared from these animals, the mean in vitro firing rate of the rostral MVN cells was no different from that in the ‘urethane-UL, 4 h’ animals (16.98 ± 1.2 spikes s−1, n = 48 cells, Fig. 1B).
Effects of GR activation by exogenous dexamethasone in urethane-anaesthetized animals after UL
In a further group of five animals that were labyrinthectomized under urethane anaesthesia, we ‘mimicked’ the acute activation of the stress response after UL by administering the synthetic GR agonist dexamethasone (5 mg kg−1, 30 min before UL and 2 h post-UL (Yamanaka et al. 1995;‘urethane-UL, Dex-treated’ group). As shown in Fig. 1C, administration of dexamethasone restored CIE in the rostral MVN cells in these animals. In slices prepared 4 h after UL from these animals, the mean firing rate of the rostral MVN cells was close to that seen in avertin-UL animals (22.31 ± 1.8 spikes s−1, n = 42 cells; Fig. 1C). This was again significantly higher than the mean spontaneous firing rate in urethane-UL controls (Fig. 1B,P < 0.05), and also significantly higher than that in ‘urethane-sham, Dex-treated’ controls (Fig. 1C). Dexamethasone administration to urethane-sham animals (‘urethane-sham, Dex-treated’ group) had no effect (Fig. 1C; mean in vitro firing rate of rostral MVN cells 15.8 ± 1.59 spikes s−1, n = 34 cells, n = 5 animals).
Effects of the GR antagonist RU38486 on the expression of CIE in rostral MVN cells in avertin-UL animals
In the converse experiment, we investigated whether the endogenous secretion of glucocorticoids after UL in the awake animal was important for the development of the increase in excitability of the rostral MVN cells, by administering the GR antagonist RU38486 (5 mg kg−1i.m., 30 min before UL), to animals labyrinthectomized under avertin anaesthesia (‘avertin-UL, RU-treated’ group, n = 4 animals). In slices prepared 4 h after UL from these animals, the mean in vitro firing rates of the rostral MVN cells were no different from those in avertin-sham controls (Fig. 1D; 16.1 ± 1.5 spikes s−1, n = 42 cells). By contrast the administration of the mineralocorticoid receptor (MR) antagonist spironolactone to avertin-UL animals (5 mg kg−1i.p., 30 min before UL; ‘avertin-UL, Sp-treated’ group, n = 4 animals) did not prevent the development of CIE in the rostral MVN cells after UL (Fig. 1D; mean 20.5 ± 1.4 spikes s−1, n = 31 cells). Thus the activation of GR, but not MR, in the awake animals after UL is necessary for the increase in intrinsic excitability of the rostral MVN cells in the early stage of vestibular compensation.
Effects of the GR antagonist RU 38486 on behavioural recovery after UL
The effects of the GR antagonist RU38486 on the behavioural recovery after UL were further studied in two groups of 12 animals, using the decline in the incidence of circular walking as an index of behavioural compensation after UL. We have previously shown that circular walking in the rat declines with a time course similar to that for ocular nystagmus rather than the longer-lasting postural symptoms (Cameron et al. 1997). One group of 12 animals was labyrinthectomized under avertin anaesthesia and treated with RU38486 (5 mg kg−1i.m., 30 min before UL), while a control group of 12 animals was similarly labyrinthectomized and injected with saline. In the RU38486-treated group, there was a significantly lower incidence of circular walking 2 h after UL compared with that in the control group (Fig. 2). This presumably is due to a slower recovery from the effects of anaesthesia in these animals. However over the following 3 h, the incidence of circular walking in the RU38486-treated animals was not significantly different from that in the control animals. Subsequently, the incidence of circular walking in the control animals decreased significantly between 5 and 6 h post-UL (Fig. 2; Cameron et al. 1997), but this did not occur in the RU38486-treated animals which continued to show undiminished levels of circular walking until 7 h after UL (Fig. 2). On the following day (∼22 h post-UL), circular walking was not observed in either the RU38486-treated or the control groups.
Figure 2. Effects the glucocorticoid receptor antagonist RU38486 on the incidence of circular walking in avertin-UL animals.
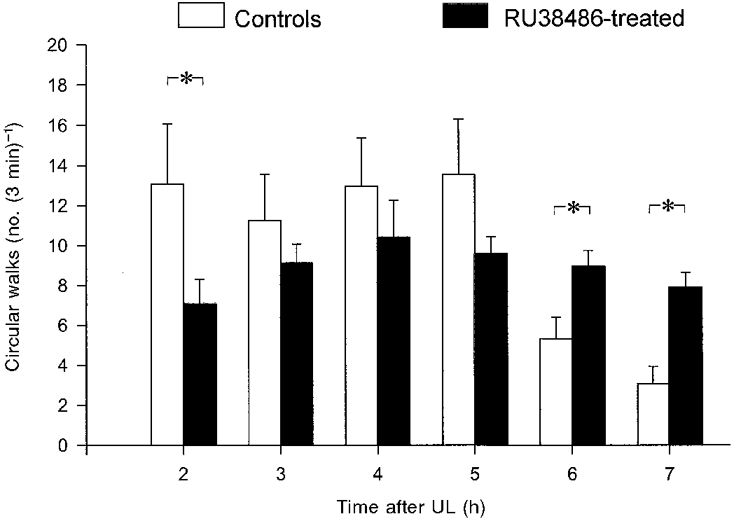
□, control group, n = 12 animals; ▪, RU38486 treated group, n = 12 animals. The number of circular movements carried out by the labyrinthectomized animals over a 3 min period when placed in an open enclosure, were measured at hourly intervals after recovery from avertin anaesthesia. The bars show the mean (±s.e.m.) number of circular walks for the two groups at each time point. Asterisks indicate a significant difference between the RU38486-treated and control groups (P < 0.05, Mann-Whitney ranked sum test).
Evidence for the activation of the stress axis after UL: Fos expression in the paraventricular nucleus of the hypothalamus after UL
As illustrated in Fig. 3, Fos-immunoreactive neurones were observed in both parvocellular and magnocellular regions of the PVN in brains from avertin-sham and avertin-UL animals (Fig. 3A). The numbers of Fos-positive cells in the PVN from avertin-UL animals at 1.5 h and at 3 h post-UL were significantly higher than those in the avertin-sham controls (Fig. 3B). This significant difference was not present at 6 h post-UL, when the number of Fos-positive cells in avertin-UL animals declined to the same level as in the sham-operated controls.
Figure 3. Evidence for the activation of the stress axis after unilateral labyrinthectomy.
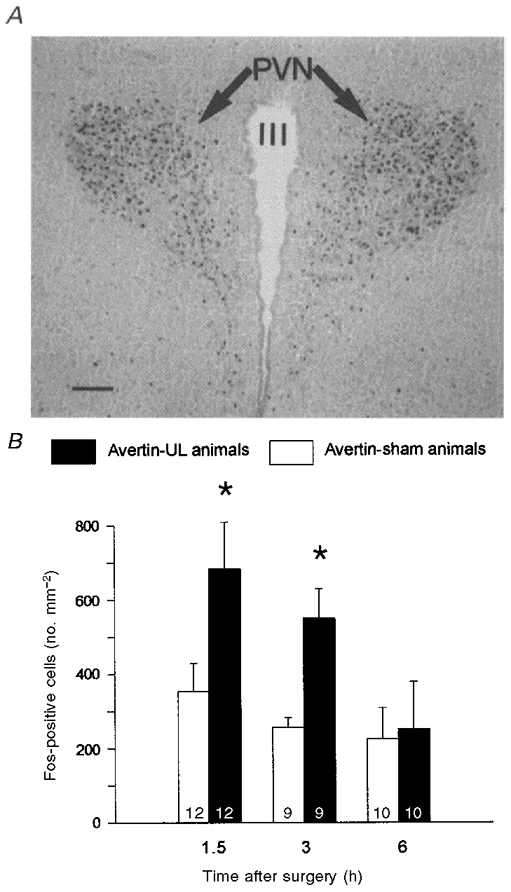
A, micrograph of the paraventricular nuclei of the hypothalamus showing immunoreactivity for Fos 1.5 h after an avertin-UL. Calibration bar, 100 μm. B, mean (±s.e.m.) numbers of Fos-immunoreactive cell bodies in the paraventricular nucleus of avertin-sham animals (□) and avertin-UL animals (▪), at various times post operation. The numbers of animals in each group are indicated at the foot of each column. Asterisks indicate significant differences between the avertin-UL and avertin-sham groups (P < 0.01, Mann-Whitney ranked sum test).
DISCUSSION
These results show that the increase in intrinsic excitability of rostral MVN neurones after unilateral vestibular deafferentation in the rat and the behavioural recovery that takes place in the initial rapid stage of vestibular compensation are dependent on the activation of glucocorticoid receptors (GRs). Thus the compensatory increase in intrinsic excitability (CIE) in the rostral MVN cells fails to occur in animals that remain anaesthetized after UL so that they do not experience the vestibular deafferentation symptoms and the accompanying stress, but ‘mimicking’ the activation of the acute stress response in these animals by administration of the GR agonist dexamethasone restores CIE in these neurones. Conversely, in animals that recover wakefulness after UL under avertin anaesthesia and so experience the severe oculomotor and postural symptoms of UL, the GR antagonist RU38486 abolishes CIE in the rostral MVN cells. Using circular walking as an index of behavioural recovery after UL, which declines with a time course similar to that of ocular nystagmus rather than the postural symptoms (Cameron et al. 1997), RU38486 also delays behavioural recovery as evidenced by the persistence of circular walking. Taken together, these findings indicate that the activation of GRs has an important role in the initial rapid stage of vestibular compensation in the rat, and that the acute stress response that accompanies unilateral vestibular deafferentation is necessary for the induction of the neuronal plasticity that underlies the behavioural recovery after UL. The high levels of Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus in avertin-UL animals over the first 3 h after UL confirm a strong activation of the stress axis over this time (Fig. 3; Cirelli et al. 1996).
It is interesting that the increase in excitability of the MVN cells is observed at 4 h post-UL, and the decrease in circular walks in control (non-RU treated) animals occurs between 5 and 6 h after UL (Fig. 2). Assuming that the decrease in circular walking is related to a ‘re-balancing’ of the in vivo firing rates of the MVN cells on the lesioned and intact sides, and given that this has to take place from an initial situation where there is a profound disparity in the firing rates on the two sides (near silence on the lesioned side, hyperactivity on the intact side; Curthoys & Halmagyi, 1995), the time lag of an hour or so is not unreasonable. Although we have used circular walking as a convenient index of behavioural recovery, it is possible that other initial symptoms of UL may recover more or less rapidly than this; at present the precise relationship between neuronal activity in the MVN and behaviour is unknown. Nevertheless, the effects of GR blockade on behaviour and the cellular responses are consistent, so that circular walking does not diminish, and the increase in intrinsic excitability does not occur in RU-treated animals.
Our findings in the rat are in agreement with those of Yamanaka et al. (1995), who demonstrated that administration of dexamethasone accelerates behavioural compensation after UL in the rabbit, while the GR antagonist RU38486 retards it. Our findings also suggest that the beneficial effects of neurosteroids on vestibular vertigo in man (Ariyasu et al. 1990) may be mediated at least in part through their actions on the cellular mechanisms that regulate the excitability of MVN neurones. In contrast, Alice et al. (1998) observed that dexamethasone had no effect on the firing rates of the majority of guinea-pig MVN cells in vitro, and had no influence on the rate of behavioural recovery after UL in the guinea-pig. In slices of the rat MVN, we have also found that brief (60 s) pulses of dexamethasone do not affect the spontaneous in vitro firing rates of MVN cells (M. R. Sulaiman, T. Yamanaka & M. B. Dutia, unpublished observations). This indicates that dexamethasone does not have rapid membrane effects on MVN cells, and that instead its facilitation of CIE in the rostral MVN cells after UL probably involves a genomic response (Joels, 1997). The time course of the increase in intrinsic excitability over 2-4 h after UL in vivo (Cameron & Dutia, 1997) and its sensitivity to the antagonist RU38486 as demonstrated in the present experiments also indicate a genomic mechanism of action.
In the guinea-pig, the lack of effect of dexamethasone on vestibular compensation is likely to be due to the significant differences in various components of the pituitary-adrenal stress axis in this species (for reviews, see Funder, 1994; Keightley & Fuller, 1996). The guinea-pig has very high circulating levels of cortisol, and low-affinity, low-capacity transcortin so that free cortisol levels are relatively much higher than in other species. The guinea-pig also has very low-affinity glucocorticoid receptors, with approximately one-twentieth the affinity for dexamethasone as those in the mouse and rat (Kraft et al. 1979; Funder, 1994). Dexamethasone administration to the guinea-pig may therefore have little effect on the rate of vestibular compensation, as it is likely that almost all GRs are normally already occupied in this species. While the guinea-pig has been extensively used in cellular and behavioural studies of vestibular compensation (e.g. Smith & Curthoys, 1989; Ris et al. 1995, 1997; Alice et al. 1998), the present results suggest that there may exist, at the cellular level, some fundamental differences in the mechanisms underlying the recovery of neuronal function after UL in this species. It is possible that another component of the stress response may substitute for the role normally played by glucocorticoids. Indeed the finding that adrenocorticotrophic hormone (ACTH) accelerates vestibular compensation in the guinea-pig (Gilchrist et al. 1994, 1996) suggests that ACTH may be a candidate for this role. Thus, as with its adaptations to other endocrine systems that are affected (Keightley & Fuller, 1996), the guinea-pig may have evolved an alternative, equivalent but GR-independent mechanism to achieve normal behaviour, in this case compensation. The precise differences between the species will become clear only with further characterization of the initial cellular processes that take place after UL, and when the role of GRs in vestibular compensation is understood.
As we have proposed earlier, the increase in intrinsic excitability in the rostral MVN cells may be due to a downregulation of GABA receptor efficacy in these cells (Cameron & Dutia, 1997). In recent experiments we have confirmed that the functional efficacy of both GABAA and GABAB receptor-mediated inhibition in the rostral MVN cells is significantly downregulated in rostral MVN cells within 4 h after UL (Dutia, 1999). In addition, there are significant changes also in the intrinsic membrane properties of Type B, but not Type A, MVN cells within 4 h after UL (Johnston et al. 1994; Dutia, 1999). The present results suggest that the activation of GRs is necessary in order to initiate these adaptive changes in the properties of the rostral MVN cells. The initial rapid stage of vestibular compensation is therefore a novel instance of ‘metaplasticity’ in the adult brain (Abraham & Bear, 1996; Abraham & Tate, 1997; Fischer et al. 1997), where the neuronal plasticity that occurs after UL is dependent not only on the imbalance in afferent input and commissural inhibition between the vestibular nuclei of the lesioned and intact sides, but also on the concomitant activation of GRs. While the concept of metaplasticity has been elaborated largely in relation to glutamatergic NMDA receptor function and synaptic efficacy, in the present case GR activation appears to be a necessary process which in itself does not modify the cellular properties of the rostral MVN cells in sham-operated animals, but enables the subsequent plastic changes in these cells to take place after vestibular deafferentation. The acute stress response that follows UL in vivo therefore appears to play a functionally important role in enabling the cellular processes that mediate the restoration of resting activity in the deafferented MVN cells, and the behavioural recovery that follows.
Acknowledgments
We are grateful to Professor Jonathan Seckl for his advice and encouragement, and for his gift of RU38486. This work was supported by The Wellcome Trust, project grant 043132.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends in the Neurosciences. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. 10.1016/S0166-2236(96)80018-X. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Progress in Neurobiology. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. 10.1016/S0301-0082(97)00018-X. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of Type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. 10.1016/0306-4522(90)90244-X. [DOI] [PubMed] [Google Scholar]
- Alice C, Paul AE, Sansom AJ, Maclennan K, Darlington CL, Smith PF. The effects of steroids on vestibular compensation and vestibular nucleus neuronal activity in the guinea pig. Journal of Vestibular Research. 1998;8:201–207. 10.1016/S0957-4271(97)00032-3. [PubMed] [Google Scholar]
- Ariyasu L, Byl FM, Sprague MS, Adour KK. The beneficial effects of methylprednisolone in acute vestibular vertigo. Archives of Otolaryngology Head and Neck Surgery. 1990;116:700–704. doi: 10.1001/archotol.1990.01870060058010. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Cellular basis of vestibular compensation: changes in intrinsic excitability of MVN neurones. NeuroReport. 1997;8:2595–2599. doi: 10.1097/00001756-199707280-00035. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Turner JA, Maltwood DMR, Dutia MB. Circular walking as an index of behavioural recovery after unilateral labyrinthectomy in the rat. The Journal of Physiology. 1997;504.P:217P. [Google Scholar]
- Cirelli C, Pompieano M, D'Ascanio P, Arrighi P, Pompieano O. c-fos expression in the rat brain after unilateral labyrinthectomy and its relation to the uncompensated and compensated stages. Neuroscience. 1996;70:515–546. doi: 10.1016/0306-4522(95)00369-x. [DOI] [PubMed] [Google Scholar]
- Cuthroys IS, Halmagyi GM. Vestibular compensation: A review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. Journal of Vestibular Research. 1995;5:67–107. [PubMed] [Google Scholar]
- Dieringer N. ‘Vestibular Compensation’ - neural plasticity and its relation to functional recovery after labyrinthine lesions in frogs and other vertebrates. Progress in Neurobiology. 1995;46:97–129. 10.1016/0301-0082(94)00063-N. [PubMed] [Google Scholar]
- Dutia MB. Cellular mechanisms of vestibular compensation. Archives Italiennes de Biologie. 1999;137(suppl.):8–9. [Google Scholar]
- Dutia MB, Gilchrist DPD, Sansom AJ, Smith PF, Darlington CL. The opioid receptor antagonist, naloxone, enhances ocular motor compensation in guinea-pig following peripheral vestibular deafferentation. Experimental Neurology. 1996;141:141–144. doi: 10.1006/exnr.1996.0147. 10.1006/exnr.1996.0147. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Johnston AR, McQueen DS. Tonic activation of rat medial vestibular nucleus neurones in vitro and its inhibition by GABA. Experimental Brain Research. 1992;88:466–472. doi: 10.1007/BF00228176. [DOI] [PubMed] [Google Scholar]
- Dyer RG, Weick WF, Mansfield S, Corbet H. Secretion of lutenizing hormone in ovarectomized adult rats treated neonatally with monosodium glutamate. Journal of Endocrinology. 1981;91:341–346. doi: 10.1677/joe.0.0910341. [DOI] [PubMed] [Google Scholar]
- Fischer TM, Blazis DE, Priver NA, Carew TJ. Metaplasticity at identified inhibitory synapses in Aplysia. Nature. 1997;389:860–865. doi: 10.1038/39892. [DOI] [PubMed] [Google Scholar]
- Funder JW. Corticosteroid hormones and signal specificity. Annals of the New York Academy of Sciences. 1994;746:1–6. doi: 10.1111/j.1749-6632.1994.tb39202.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist DP, Darlington CL, Smith PF. A dose-response analysis of the beneficial effects of the ACTH-(4-9) analogue, Org 2766, on behavioural recovery following unilateral labyrinthectomy in guinea-pig. British Journal of Pharmacology. 1994;111:358–363. doi: 10.1111/j.1476-5381.1994.tb14068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist DP, Darlington CL, Smith PF. Evidence that short ACTH fragments enhance vestibular compensation via direct action on the ipsilateral vestibular nucleus. NeuroReport. 1996;7:1489–1492. doi: 10.1097/00001756-199606170-00009. [DOI] [PubMed] [Google Scholar]
- Hamann KF, Lannou J. Dynamic characteristics of vestibular nuclear neurones responses to vestibular and optokinetic stimulation during vestibular compensation in the rat. Acta Otolaryngology Supplement. 1988;455:1–19. doi: 10.3109/00016488809099006. [DOI] [PubMed] [Google Scholar]
- Jerram AH, Darlington CL, Smith PF. Methylprednisolone reduces spontaneous nystagmus following unilateral labyrinthectomy in guinea pig. European Journal of Pharmacology. 1995;275:291–293. doi: 10.1016/0014-2999(95)00039-n. 10.1016/0014-2999(95)00039-N. [DOI] [PubMed] [Google Scholar]
- Joëls M. Steroid hormones and excitability in the mammalian brain. Frontiers in Neuroendocrinology. 1997;18:2–48. doi: 10.1006/frne.1996.0144. 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Macleod NK, Dutia MB. Ionic conductances contributing to spike repolarisation and after-potentials in rat medial vestibular nucleus neurones. The Journal of Physiology. 1994;481:61–77. doi: 10.1113/jphysiol.1994.sp020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley MC, Fuller PJ. Anomalies in the endocrine axes of the guinea pig: relevance to human physiology and disease. Endocrine Reviews. 1996;17:30–44. doi: 10.1210/edrv-17-1-30. 10.1210/er.17.1.30. [DOI] [PubMed] [Google Scholar]
- Kraft N, Hodgson AJ, Funder JW. Glucocorticoid receptor and effector mechanisms: a comparison of the corticosensitive mouse with the corticoresistant guinea-pig. Endocrinology. 1979;104:344–349. doi: 10.1210/endo-104-2-344. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil: I. Type I neurones. Experimental Brain Research. 1990a;82:359–372. doi: 10.1007/BF00231255. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil: II. Type II neurones. Experimental Brain Research. 1990b;82:373–383. doi: 10.1007/BF00231256. [DOI] [PubMed] [Google Scholar]
- Ris L, Capron B, DeWaele C, Vidal PP, Godaux E. Dissociations between behavioural recovery and restoration of vestibular activity in the unilabyrinthectomized guinea-pig. The Journal of Physiology. 1997;500:509–522. doi: 10.1113/jphysiol.1997.sp022037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, DeWaele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsiateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. Journal of Neurophysiology. 1995;74:2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Neuronal activity in the contralateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Research. 1988a;444:295–307. doi: 10.1016/0006-8993(88)90938-9. 10.1016/0006-8993(88)90938-9. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Research. 1988b;444:308–319. doi: 10.1016/0006-8993(88)90939-0. 10.1016/0006-8993(88)90939-0. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Research Reviews. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yamanaka T, Matsunaga T. Effects of the neurosteroid dehydropepiandrosterone sulphate on medial vestibular nucleus neurones. Acta Otolaryngologica. 1998;118:185–191. doi: 10.1080/00016489850154883. 10.1080/00016489850154883. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Sasa M, Amano T, Miyahara H, Matsunaga T. Role of glucocorticoids in vestibular compensation in relation to activation of vestibular nucleus neurones. Acta Otolaryngologica. 1995;519(supplement):168–172. doi: 10.3109/00016489509121895. [DOI] [PubMed] [Google Scholar]


