Abstract
The gastric corpus and antrum contain interstitial cells of Cajal (ICC) within the tunica muscularis. We tested the hypothesis that ICC are involved in the generation and regeneration of electrical slow waves.
Normal, postnatal development of slow wave activity was characterized in tissues freshly removed from animals between birth and day 50 (D50). Slow wave amplitude and frequency increased during this period. Networks of myenteric ICC (IC-MY) were present in gastric muscles at birth and did not change significantly in appearance during the period of study as imaged by confocal immunofluorescence microscopy.
IC-MY networks were maintained and electrical rhythmicity developed in organ culture in a manner similar to normal postnatal development. Electrical activity was maintained for at least 48 days in culture.
Addition of a neutralizing antibody (ACK2) for the receptor tyrosine kinase, Kit, to the culture media caused progressive loss of Kit-immunoreactive cells. Loss of Kit-immunoreactive cells was associated with loss of slow wave activity. Most muscles became electrically quiescent after 3-4 weeks of exposure to ACK2.
In some muscles small clusters of Kit-immunoreactive IC-MY remained after culturing with ACK2. These muscles displayed slow wave activity but only in the immediate regions in which Kit-positive IC-MY remained. These data suggest that regions without Kit-immunoreactive cells cannot generate or regenerate slow waves.
After loss of Kit-immunoreactive cells, the muscles could not be paced by direct electrical stimulation. Stimulation with acetylcholine also failed to elicit slow waves. The data suggest that the generation of slow waves is an exclusive property of IC-MY; smooth muscle cells may not express the ionic apparatus necessary for generation of these events.
We conclude that IC-MY are an essential element in the spontaneous rhythmic electrical and contractile activity of gastric muscles. This class of ICC appears to generate slow wave activity and may provide a means for regeneration of slow waves.
Interstitial cells of Cajal (ICC) are small spindle-shaped or stellate cells with numerous mitochondria and long processes that form networks between and within smooth muscle layers in the gastrointestinal (GI) tract (Thuneberg, 1982; Sanders, 1996). Populations of ICC are found in pacemaker regions of gastrointestinal muscles (Suzuki et al. 1986; Berezin et al. 1988). Isolated ICC are electrically rhythmic and express ionic conductances consistent with a role in pacemaking (Langton et al. 1989; Lee & Sanders, 1993). More recent studies have shown that ICC retain electrical rhythmicity in culture via generation of spontaneous transient inward currents (Tokutomi et al. 1995; Thomsen et al. 1998; Koh et al. 1998), display Ca2+ oscillations (Publicover et al. 1992), and maintain responsiveness to physiological agonists (Publicover et al. 1992). Loss of myenteric ICC (IC-MY) from the pacemaker region in the small bowel results in loss of spontaneous electrical rhythmicity (Ward et al. 1994, 1995; Huizinga et al. 1995; Torihashi et al. 1995), supporting the hypothesis that these cells are a critical element in intestinal pacemaking.
While the pacemaker function of ICC in intestinal muscles is strongly supported by experimental results, practically nothing is known about the role of gastric ICC in pacemaking. Two classes of ICC have been observed in the mammalian stomach (e.g. mouse: Burns et al. 1996; guinea-pig: Burns et al. 1997; cat, dog, ferret, opossum, rat, guinea-pig and rabbit: Christensen et al. 1992; human: Faussone-Pellegrini et al. 1989; Torihashi et al. 1999): IC-MY, found between the circular and longitudinal muscle layers from the orad corpus to the pyloric sphincter (Burns et al. 1996, 1997), and intramuscular ICC (IC-IM), found in close apposition to varicose nerve processes within the circular and longitudinal muscle layers (Burns et al. 1996). Previous studies have shown that IC-IM mediate enteric inhibitory neurotransmission, and loss of these cells did not affect electrical rhythmicity (Burns et al. 1996). IC-MY may generate electrical rhythmicity in the stomach; however, this hypothesis has not been tested directly.
The use of c-kit mutants (e.g. W/WV) has provided a means of understanding the function of specific types of ICC. For example, IC-MY are lost in the small intestine (Ward et al. 1994; Huizinga et al. 1995) and IC-IM are lost in the stomach (Burns et al. 1996), lower oesophageal sphincter and pyloric sphincter (Ward et al. 1998) in these mutants. However, several classes of ICC are resistant to the subtotal loss of function of the receptor tyrosine kinase, Kit, in W/WV mutants. Gastric IC-MY appear to develop normal morphology and function in W/WV mice (Burns et al. 1996; Ward et al. 1998).
Recent studies show that ICC and electrical rhythmicity develop in an apparently normal manner in tissues isolated at or before birth and placed in organ culture (Ward et al. 1997). Inclusion of ACK2, a neutralizing monoclonal antibody to Kit (Nishikawa et al. 1991) in cultures of murine small intestine prevented or reversed development of ICC, resulting in disruption and eventually complete disappearance of ICC networks and slow wave activity (Ward et al. 1997). This raises the possibility that neutralizing antibody treatment might be used to inhibit ICC development in regions of the GI tract where they survive in W/WV animals. In the present study we utilized this technique to investigate the role of gastric IC-MY in electrical pacemaking in the murine corpus and antrum.
METHODS
Animals and tissue preparation
BALB/c mice of either sex were obtained from breeder pairs purchased from Harlan Sprague-Dawley (Indianapolis, IN, USA). Between 0-50 days (D) of postnatal age, the animals were anaesthetized by chloroform inhalation and killed by decapitation (D0-10) or cervical dislocation followed by decapitation (> D10). The animals were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada.
Stomachs were excised and opened along the lesser curvature. The tissue was pinned, mucosal side up, onto the surface of a dish coated with Sylgard 184 silicone elastomer (Dow Corning Corp., Midland, MI, USA), and the gastric contents were washed away with Krebs-Ringer bicarbonate (KRB; for composition see below) solution. The mucosa was removed from the underlying tunica muscularis by peeling. The fundus was removed and the entire gastric corpus and antrum were used in subsequent steps.
Organotypic culture
Tissues from newborn (≤ 1 day old) animals were pinned with the mucosal side of the circular muscle up to the Sylgard-coated base of sterile 35 mm polypropylene dishes (Corning Glass Works, Corning, NY, USA). The muscles were washed with sterile culture medium at least seven times and incubated at 37°C in a humidified atmosphere (90 %) of 95 % O2-5 % CO2 in M199 medium (Sigma) supplemented with an antibiotic-antimycotic mixture (penicillin G sodium, 200 i.u. ml−1; streptomycin sulfate, 200 μg ml−1; amphotericin B, 0.5 μg ml−1; Gibco BRL) plus L-glutamine (2 mM; Gibco BRL). Experimental tissues were incubated in media containing rat anti-murine c-Kit neutralizing antibody (ACK2, 5 μg ml−1, Gibco BRL); control muscles were incubated in media only. In additional control experiments muscles from newborn animals (n = 3) were cultured in the presence of 5 μg ml−1 normal rat serum (Sigma) for 23-28 days. Slow wave frequency and amplitude and resting membrane potentials recorded from these tissues (n = 9 cells) were not statistically different from those recorded in control muscles incubated in serum-free media for 16-30 days (n = 25 cells in 5 tissues; see Results). Culture media were changed every second day.
Electrophysiology
Cultured or freshly dissected stomachs (entire corpus and antrum) were pinned to the Sylgard floor of a recording chamber with the mucosal side of the circular muscle facing upward. Pt electrodes were placed in parallel on either side of the tissue to provide electrical field stimulation (EFS). Circular muscle cells were impaled with glass microelectrodes filled with 3 M KCl with resistances of 40-100 MΩ. Transmembrane potential was measured with a standard microelectrode amplifier (Duo 773; WPI, Sarasota, FL, USA), displayed on an oscilloscope (HM 205-3, Hameg GmbH, Frankfurt am Main, Germany), and simultaneously recorded on video tape (VTR Model 420 M) and chart paper. EFS was performed with an S48 square-wave pulse generator (Grass Medical Instruments, Quincy, MA, USA). As both the amplitude of slow waves and the resting membrane potential (RMP) vary along the axis of the gastric corpus and antrum (Szurszewski, 1987) every attempt was made to record from at least four areas in each preparation. The number of cells from which recordings were made is denoted by n. The number of tissues from which the n value was obtained is also provided.
Immunohistochemistry
Following electrophysiological recording, tissues were fixed in acetone (4°C, 10 min) and then washed in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) for 4 × 15 min. Non-specific antibody binding was reduced by incubation in 1 % (w/v) bovine serum albumin (Sigma) in PBS for 1 h at room temperature (21-22°C). Tissues were then incubated with ACK2 (5 μg ml−1 in PBS containing 0.3 % (v/v) Triton X-100 (Sigma)) at 4°C for 48 h. Immunoreactivity was detected using a fluorescein-conjugated secondary antibody (anti-rat IgG (H+L), Vector Laboratories, Burlingame, CA, USA; 1 : 150 in PBS, 1 h, room temperature). In control tissues the primary antibody was omitted from the incubation solution. Tissues were examined with a Bio-Rad MRC 600 confocal microscope (Hercules, CA, USA) with an excitation wavelength of 488 nm. The confocal micrographs, constructed with CoMOS software (version 7.0a; Bio-Rad), are digital composites of Z- series scans of 10-18 optical sections through a depth of 10-22 μm.
Solutions and drugs
During experiments, tissues were maintained in KRB solution (37.5 ± 0.5°C; pH 7.3-7.4) containing (mM): Na+, 137.4; K+, 5.9; Ca2+, 2.5; Mg2+, 1.2; Cl−, 134; HCO3−, 15.5; H2PO4−, 1.2; and dextrose, 11.5; bubbled with 97 % O2-3 % CO2. Solutions of acetylcholine chloride (ACh, Sigma) and KCl were prepared in deionized water at 10 mM and 3 M, respectively, and further diluted in KRB to the final concentrations indicated in the text.
Statistical analyses
Slow wave parameters (RMP, slow wave frequency, amplitude and duration) from developing and cultured muscles were expressed as means ±s.e.m. and grouped as follows: days 0-5, 6-10, 11-15, 16-30 and 31-50. Between days 0-15, a bin size of 5 days was selected because this matched the dynamic changes occurring during this period. After 15 days of age or 15 days of culture, the size of the time bins was increased to 15 (days 16-30) and 20 days (days 31-50) because changes occurred more slowly during this period.
All statistical analyses were performed using the SigmaStat Statistical Software for Windows Version 2.0 (Jandel Corp., Sausalito, CA, USA). Non-parametric tests were used when the assumptions for the parametric test were violated. The following tests were used: unpaired Student's t test, Mann-Whitney rank sum test, one-way ANOVA followed by multiple comparisons (Bonferroni's t test or Student-Newman-Keuls test) and Kruskal- Wallis ANOVA on ranks followed by multiple comparisons (Dunn's test). A probability value of P < 0.05 was used as a cut-off for statistical significance.
RESULTS
Kit-positive cells in the gastric corpus and antrum
ICC in whole-mount preparations of tunica muscularis from murine gastric corpus and antrum were visualized with antibodies to c-Kit, a specific marker for these cells (ACK2; Nishikawa et al. 1991; Burns et al. 1996; Ward et al. 1997) (Fig. 1). Consistent with earlier reports (Burns et al. 1996; Ward et al. 1997), Kit-like immunoreactivity (Kit-LI) was located in specific cell populations. In the myenteric regions of the muscle, Kit-positive cells had fusiform cell bodies and multiple processes, and the density of these cells increased from corpus to antrum (myenteric plexus ICC; IC-MY). Within the circular and longitudinal muscle layers, Kit-LI was present in spindle-shaped cells running with the long axis of the muscle fibres (intramuscular ICC; IC-IM).
Figure 1. Effects of neutralizing Kit antibodies on ICC networks of the murine gastric corpus and antrum.
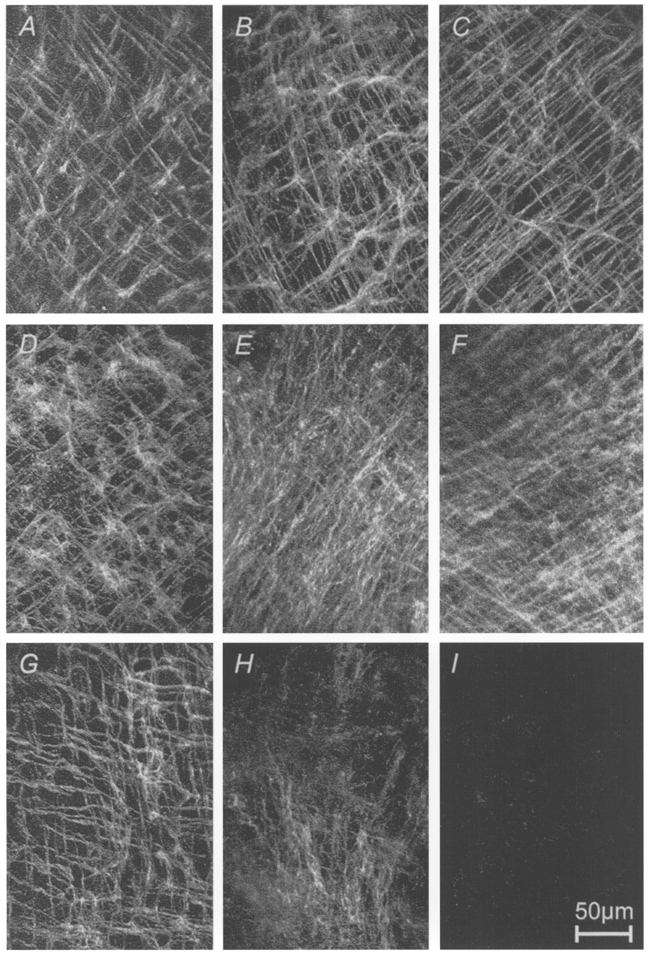
ICC networks in gastric muscle preparations were visualized by Kit immunohistochemistry and confocal microscopy. A-C, tissues freshly removed from normal mice on D1, D13 and D18. D-F, muscles removed from newborn mice (<= 1 day of postnatal age) and cultured for 0, 13 and 21 days under control conditions; G-I, muscles removed from newborn mice and cultured in the presence of neutralizing Kit antibodies for 0, 9 and 43 days. ICC networks were fully developed at birth in each animal (see A, D and G). Culturing did not appear to significantly alter the structure or distribution of ICC networks. Inclusion of neutralizing Kit antibody (ACK2) caused a time-dependent loss of ICC in the cultured muscles. Scale bar is 50 μm and applies to each panel.
Previous studies have suggested that the structure of IC-MY networks and the distribution of IC-IM at birth is similar to these structures in adult animals (Burns et al. 1996; Ward et al. 1997). In the present study we detected no change in the structure and distribution of IC-MY during postnatal development (D0-30, n = 12; Fig. 1A-C). When gastric corpus and antrum from newborn (≤ 1 day old) mice were placed in organ culture, ICC morphology and the structure of ICC networks were maintained for at least 48 days (n = 12; Fig. 1D-F). We often found that background immunofluorescence increased under organ culture conditions, and this tended to produce a less distinct appearance of the ICC networks in composite confocal images (compare Fig. 1B and C with E and F).
Inclusion of ACK2 (5 μg ml−1), a neutralizing monoclonal antibody to Kit, to the culture medium resulted in a profound reduction in Kit-LI (Fig. 1G-I). In seven tissues cultured for 14-48 days, Kit-LI completely disappeared (e.g. Fig. 1I). In seven other tissues (cultured for 9-41 days), Kit-LI could still be observed in small patches surrounded by areas of tissue with little or no staining (e.g. Fig. 1H). Areas that retained Kit-LI displayed relatively faint immunofluorescence. Upon careful examination of the individual optical sections making up the composite confocal images, the patches with Kit-positive cells contained all classes of ICC normally found within the gastric corpus and antrum (i.e. IC-MY and IC-IM in both the circular and longitudinal muscle layer; images not shown).
Role of Kit-positive cells in the electrical activity of gastric muscles
Intracellular electrical activity was recorded from 109 circular muscle cells of 25 tissues during normal postnatal development, from 99 cells of 25 muscles cultured under control conditions, and from 131 cells of 32 tissues cultured in the presence of ACK2. The data from developing and cultured muscles were grouped as follows: days 0-5, 6-10, 11-15, 16-30 and 31-50 (see Methods; and see Figs 2 and 3). Each group contained recordings from at least five tissues.
Figure 2. Effects of neutralizing Kit antibody on gastric slow waves.
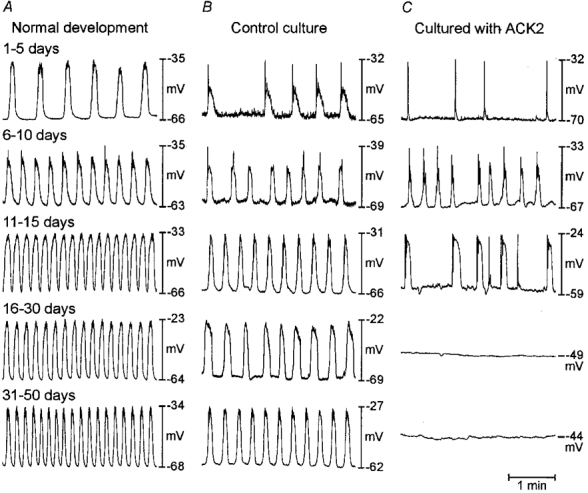
Representative electrical activity recorded from muscles freshly removed from animals on various days after birth (as noted) and from muscles cultured for various periods in the absence and presence of ACK2. (Electrical recordings were made from cultured muscles at the same time periods as the records from freshly dissected muscles in A.) At birth regular slow wave activity was recorded in gastric muscles. The frequency increased with age. A similar trend was observed in cultured muscles (B); however, the maximum frequency did not reach the same level as in normal development (see text and Fig. 3 for details). In the presence of ACK2, slow waves began to develop, but in many muscles slow waves disappeared and the muscles became electrically quiescent (C). Loss of slow waves corresponded to the loss of ICC as shown in Fig. 1.
Figure 3. Summary of the effects of ACK2 on electrical and slow wave parameters.
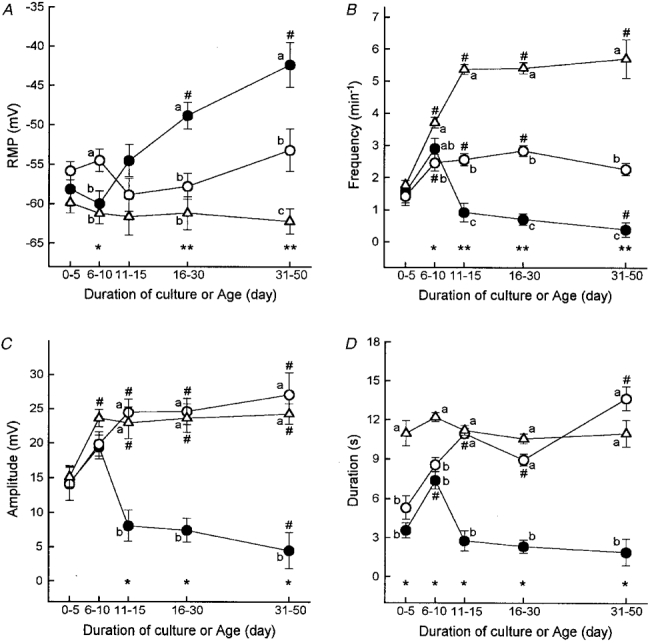
Resting membrane potential (RMP; A), and frequency (B), amplitude (C) and duration (D) of electrical slow waves recorded in freshly dissected gastric muscles during normal postnatal development (▵) and in preparations cultured from birth in the absence (○) or presence of Kit antibodies (•). Data were grouped into time bins, and data points in each panel are means ±s.e.m. from an average of 23 cells (range, 11-36) of at least 5 tissues from each time bin. # Significant difference from the first time bin (days 0-5) in each group of muscles (using one-way ANOVA followed by Bonferroni's t test or Kruskal-Wallis ANOVA on ranks followed by Dunn's test; ANOVA P values: A, ACK2-treated cultures, P < 0.001; B, normal development and ACK2-treated cultures, P < 0.001; control cultures, P < 0.003; C, all groups, P < 0.001; D, control and ACK2-treated cultures, P < 0.001; in multiple comparisons P < 0.05 was considered statistically significant). Asterisks indicate statistical significance between groups of muscles within the same time bins (one-way ANOVA followed by Student-Newman-Keuls test or Kruskal-Wallis ANOVA on ranks followed by Dunn's test). ANOVA P values were: A: *P < 0.005, **P < 0.001; in B: *P < 0.002, **P < 0.001; and in C and D: *P < 0.001. Data points within time bins designated by different lower case letters are significantly different (P < 0.05 by multiple comparisons; for example, a is different from b, and a and b are different from c).
Slow wave activity was monitored from muscles removed from animals between D0 and D50 (Fig. 2A). Several parameters of electrical activity i.e. RMP (most negative potential between slow waves) and the frequency, amplitude and duration of slow waves were tabulated during the postnatal period of development (Fig. 3A-D). At birth, RMP averaged -60 ± 1 mV (mean ±s.e.m.) and did not change significantly during postnatal development (Fig. 3A). With the exception of three cells of two tissues impaled on postnatal days 0 and 1 (9.4 % of cells impaled in D0-5 tissues), all cells displayed regular slow wave activity. Between D1-5 we also observed occasional irregular slow wave activity. The frequency of slow waves increased from 1.8 ± 0.1 min−1 from D0-5 to 5.4 ± 0.2 min−1 at D11-15 (Fig. 3B). The amplitude of slow waves also increased from 15 ± 1 mV to 24 ± 1 mV by D10 (Fig. 3C). The duration of slow waves remained constant between D0 and D50 (Fig. 3D).
Tissues obtained from newborn mice and placed into organ culture for 1-50 days had generally less negative resting potentials than tissues removed from animals of equivalent ages. The differences in resting potentials reached statistical significance only in groups of muscles cultured for 6-10 days or 31-50 days (Fig. 3A). After 5 days in culture all cells impaled in control cultured muscle strips displayed spontaneous, regular slow wave activity (n = 20 tissues). Before 5 days of culture, some cells were quiescent (6 cells of 3 tissues; 28.6 %) and arrhythmias in slow wave activity were also noted (see an example in Fig. 2B). Thus cultured muscles displayed similar irregularities in slow wave activity as muscles freshly removed from animals on D1-5 (see above).
Other parameters of slow wave activity also developed in organ culture in a manner similar to normal postnatal development. For example, slow wave amplitude increased in cultured muscles to the same extent as in normal development between D0-50 (Fig. 3C). The duration of slow waves also reached normal adult levels by days 11-15 in culture (Fig. 3D). In contrast, slow wave frequency in cultured muscles did not keep pace with the increase observed during normal postnatal development. The frequency of slow waves reached a maximum of 2.6 ± 0.1 min−1 in cultured muscles (in contrast to a frequency of 5.4 ± 0.2 min−1 in muscles freshly removed from mature animals; Fig. 3B).
In order to evaluate the role of ICC in generating slow wave activity, muscles were cultured with ACK2. The behaviour of tissues treated with ACK2 (5 μg ml−1) was similar to that of control cultures for up to 10 days in culture (Fig. 2C and summarized in Fig. 3A-D). Of the cells impaled in muscles treated with ACK2 for 1-5 days in culture, 35.7 % (10 cells in 4 tissues) were quiescent; however, 95.7 % of cells displayed spontaneous slow wave activity in muscles cultured for 6-10 days. The number of quiescent cells increased with time of exposure to ACK2 (e.g. from 1 cell of 12 (8.3 %) after 9-10 days of culture to 13 of 16 cells (81.2 %) after 31-50 days of culture; Fig. 2). The inhibition of slow waves was accompanied by significant reductions in RMP to an average of -42 ± 3 mV after 31-50 days of culture (Fig. 3A). Figure 3B-D shows a summary of the decrease in slow wave amplitude, duration and frequency as a function of ACK2 exposure time.
After incubations with ACK2, slow waves could still be recorded in a few isolated regions of some muscles. The remaining slow wave activity was often organized into clusters or occurred in a highly arrhythmic fashion. For example, after 9 days of exposure to ACK2, 51.2 % of the cells in which slow waves were still recorded displayed episodes of arrhythmic activity (see examples in Fig. 4). In contrast, only 15.3 % of cells in control cultures from 9- 50 days had measurable variations in the intervals between slow waves.
Figure 4. Arrhythmic slow wave activity in gastric muscles cultured with neutralizing Kit antibodies.
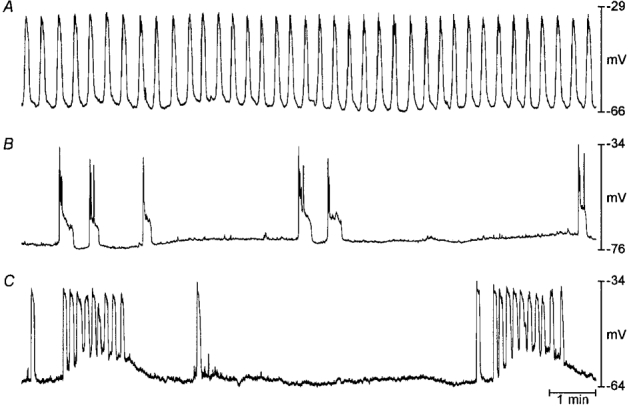
A shows typical slow wave activity recorded from a gastric muscle preparation cultured under control conditions for 15 days beginning at birth. B and C show patterns of arrhythmic slow wave activity recorded from muscles cultured with neutralizing Kit antibodies for 12 and 28 days, respectively.
Despite the arrhythmias observed in ACK2-treated muscles, when statistical analyses were performed on the electrical events of cells in which slow wave activity persisted (i.e. after omitting all quiescent cells), the average slow wave frequency and amplitude in control and ACK2-treated cultured muscles were not significantly different (not shown). Duration of residual slow waves in ACK2-treated muscles was, however, consistently shorter than in control muscles between D11-50 (P < 0.03; Student's t test or Mann-Whitney rank sum test).
The progressive depolarization during culturing in the presence of ACK2 occurred in parallel with loss of slow wave activity (Fig. 3A). In cells in which slow waves persisted after culturing with ACK2 for 9-50 days, RMP (-57 ± 2 mV; n = 39) was not statistically different from cells in control cultures (-57 ± 1 mV, n = 57). In contrast, the RMP of cells that became quiescent after culturing with ACK2 (i.e. -46 ± 1 mV, n = 53) had significantly less depolarized membrane potentials than cells in which slow wave activity persisted. (P < 0.001; one-way ANOVA followed by all-pairwise multiple comparisons, Student- Newman-Keuls method).
After electrophysiological recordings, 16 tissues that had been cultured with ACK2 were examined immunohistochemically. Kit-LI was not detected in any of the seven preparations that were electrically quiescent after culturing with ACK2 (Fig. 1I). In nine additional muscles slow waves were observed after culturing with ACK2. In seven of these muscles Kit-LI was apparent only in patches of cells. The networks of Kit-positive cells, however, were disrupted in most areas of the muscles (e.g. Fig. 1H). In three of these muscles with patchy Kit-LI, slow wave activity was recorded only in the regions where Kit-LI was observed (Fig. 5). For example, the muscle depicted in Fig. 5 was cultured 41 days with ACK2. In this muscle slow waves, occurred regularly or in ‘bursts’ (see above). These events were recorded from a small area in the mid-corpus along the greater curvature. Immunohistochemistry revealed Kit-LI in the same region (Fig. 5A and B). No specific immunoreactivity could be detected in the electrically quiescent regions of the tissue (an example of the immunoreactivity and the electrical activity in the orad antrum close to the lesser curvature is shown in Fig. 5C and D, respectively). In two of the muscles treated with ACK2, Kit-LI and slow wave activity were unaffected.
Figure 5. Correlation between Kit-like immunoreactivity and electrical slow wave activity.
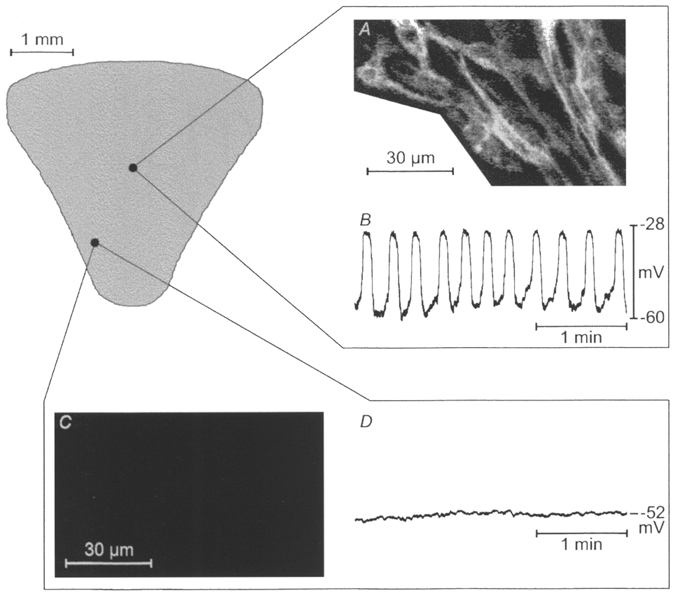
Kit-LI (A and C) and intracellular electrical activity (B and D) from a gastric muscle preparation cultured with neutralizing Kit antibodies for 41 days beginning at birth. Slow waves were recorded from a small area in the mid-corpus and the co-ordinates were recorded as a percentage of length and width of the tissue with a micrometer eyepiece. The muscles were then fixed and processed for immunohistochemistry. The region from which slow waves were recorded also displayed Kit-LI. Other areas, including that shown in the antrum in C and D, were electrically quiescent and lacked Kit-LI.
Role of Kit-positive cells in responses of gastric muscles to electrical and pharmacological stimulation
Membrane potential responses to electrical field stimulation (EFS), ACh and elevated extracellular K+ ([K+]o), were determined in control tissues and in muscles incubated with ACK2 (Figs 5 and 6). In freshly dissected gastric muscles, single square-wave electrical pulses elicited premature slow waves and caused slight depolarization of RMP (n = 10 cells in 6 tissues; Fig. 6A). The minimum pulse duration required for inducing slow waves varied from 20 to 200 ms (mean ±s.e.m.: 122 ± 19 ms). Addition of tetrodotoxin did not affect these responses, suggesting that the slow waves elicited by EFS resulted from direct stimulation of the slow wave pacemaker apparatus. EFS also induced slow waves and depolarized RMP in cultured gastric muscles (n = 28 cells in 15 control cultures; Fig. 6B) and in tissues in which slow waves persisted after culturing with ACK2 (n = 22 cells in 10 ACK2 cultures). Slow waves could not be induced with pulses of any duration tested (i.e. 0.05-500 ms) in tissues or in tissue regions that were electrically quiescent and devoid of Kit-positive cells after culturing with ACK2 (n = 38 cells in 13 tissues; e.g. Fig. 6C and D).
Figure 6. Effects of ACK2 on responses to electrical field stimulation.
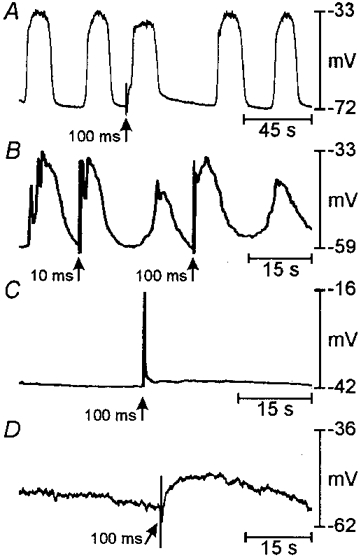
Examples of responses to single square-wave electrical pulses (150 V, 10-100 ms). In A, slow waves were evoked prematurely by 100 ms pulses in a freshly dissected muscle obtained from a D10 mouse. B shows a record from a muscle cultured under control conditions from birth for 21 days. Premature slow waves were evoked by 100 ms pulses. C and D show records from muscles cultured from birth for 12 and 18 days, respectively, with ACK2. These muscles were electrically quiescent and 100 ms pulses (and longer pulses up to 500 ms) failed to evoke slow waves.
ACh (10−6-10−5 M) caused a depolarization of RMP and increased slow wave frequency in freshly dissected gastric muscles (ΔRMP = 9 ± 2 mV; Δfrequency = 141 ± 10 % of control; n = 6 cells in 5 tissues; age: 7-50 days; means ±s.e.m.; Fig. 7A) and in control cultured muscles (ΔRMP = 13 ± 5 mV; Δfrequency = 181 ± 15 %; n = 5 cells in 4 tissues; duration of culture: 13-20 days; Fig. 7B). In muscles cultured with ACK2 in which spontaneous electrical activity was inhibited and ICC were absent, ACh failed to elicit slow waves. ACh continued to elicit depolarization of the RMP in quiescent muscles treated with ACK2 (ΔRMP = 12 ± 2 mV; n = 9 cells in 5 tissues; duration of culture: 12-43 days; Fig. 7C), which was statistically not different from the depolarization observed in freshly dissected gastric muscles or in control cultures (P > 0.05; one-way ANOVA). These findings suggest that the smooth muscle cells remaining after loss of Kit-positive cells were responsive to stimulation by ACh. During recording from one cell in this series of experiments ACh (10−5 M) elicited fast action potentials, which formed a continuous spike train during the rising and plateau phases of the RMP response and short bursts during repolarization (Fig. 7D). KCl, in concentrations up to 100 mM, also failed to induce slow waves in tissues in which spontaneous slow wave activity was inhibited by ACK2 treatment (ΔRMP = 20 ± 1 mV; n = 7 cells in 3 tissues; Fig. 7E).
Figure 7. Effects of ACK2 on responses of gastric muscle to pharmacological stimulation.
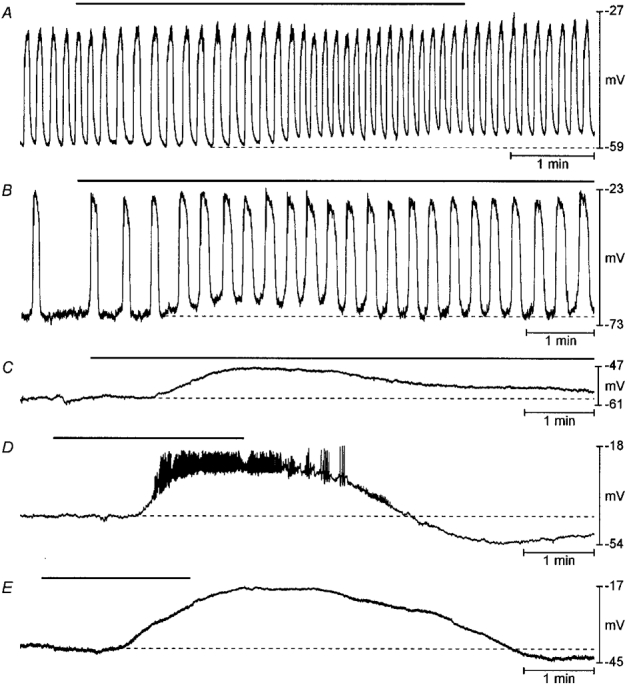
Examples of responses to 10−5 M acetylcholine (A-D) or to 100 mM KCl (E) in gastric muscle preparations. A shows the response of a freshly dissected muscle obtained from a 44-day-old mouse exposed to ACh (10−5 M; black bar). Resting membrane potential depolarized (note dotted line) and slow wave frequency was increased in response to ACh. B shows the response of a muscle cultured under control conditions beginning at birth for 13 days to ACh (10−5 M). Resting membrane potential was depolarized (dotted line) and the frequency of slow waves increased in response to ACh. C and D show responses of muscles cultured with ACK2 for 19 and 18 days, respectively. In these electrically quiescent muscles ACh (10−5 M) depolarized resting membrane potential but never elicited slow wave activity. In the example in D, brief, spike-like activity was superimposed upon the tonic depolarization. In E a muscle treated with ACK2 for 41 days was exposed to elevated external K+. This also caused tonic depolarization without stimulating oscillatory slow wave activity.
DISCUSSION
The stomach is normally paced by electrical slow waves generated in the orad corpus (Kelly & Code, 1971). Actually, all regions of the stomach distal to the pacemaker region (with the exception of the innermost band of muscle in the pyloric sphincter; see Sanders & Vogalis, 1989) are capable of generating spontaneous, ‘myogenic’ slow waves (see El-Sharkawy et al. 1978), but the orad corpus is the dominant pacemaker in the stomach because it generates slow waves at the most rapid frequency. Normal gastric peristalsis requires: (i) regulation of pacemaker frequency and maintenance of the proximal to distal frequency gradient; and (ii) orderly propagation of slow waves around the stomach and distally from the pacemaker in the corpus toward the pylorus. This study suggests that these critical aspects of gastric motility are functions provided by ICC because these cells generate pacemaker activity and provide the means of regeneration of this activity. Our experiments show that when Kit-immunopositive cells are lost, the stomach becomes non-rhythmic and non-phasic, and therefore would be non-peristaltic. Thus, functional ICC appear to be critical for the production and maintenance of normal gastric motility. Since loss of the intramuscular class of ICC (IC-IM) was previously shown not to affect electrical slow waves (Burns et al. 1996), rhythmicity is likely to be produced by IC-MY.
The basis for electrical rhythmicity in GI muscles has been investigated for a number of years, and several theories have been put forward to explain spontaneous activity in gastric tissues (Ohba et al. 1975; Connor et al. 1976; El-Sharkawy et al. 1978). These older studies did not consider the possibility that electrical slow waves might be generated and propagated by specialized cells in gastric muscles; however, one group suggested that slow waves recorded from the muscle consisted of two, mechanistically distinct components (see Tomita, 1981). A recent study has shown that gastric IC-MY and smooth muscle cells are both part of a common electrical syncytium, and these cells produce distinct electrical events (Dickens et al. 1999). These authors suggested that slow waves are generated by ICC and these events initiate responses in electrically coupled smooth muscle cells. Our data support and extend this concept by showing that ICC are essential for generation and regeneration of slow wave activity of gastric muscles, and smooth muscle lacks the mechanisms required for generation and propagation of slow waves. Smooth muscle cells, while electrically coupled to IC-MY, produce distinct electrical events and discrete functions in the process of generating peristaltic contractions in gastric muscles.
Recent work on small intestinal muscles has suggested that ICC provide pacemaker input by generating electrical slow waves. The present study generalizes this concept by showing that the electrical rhythmicity of gastric muscles also depends upon the presence and function of ICC. The number and distribution of cells with Kit immunoreactivity was greatly reduced by treatment with the neutralizing Kit antibody ACK2. Previous studies have shown that the loss of Kit immunoreactivity in the murine small bowel, colon and stomach after treatment with ACK2 or in Kit mutants (W/WV) is due to loss of ICC and not merely to a reduction in the expression of Kit (Ward et al. 1994; Torihashi et al. 1995; Burns et al. 1996). After treatment with ACK2 and loss of Kit-positive cells, gastric muscles were incapable of generating slow waves either spontaneously or in response to agonist or direct electrical stimulation of the muscle. After the antibody, slow waves were observed in a few muscles in which patches of IC-MY remained. In these tissues slow waves were only recorded within regions of muscle containing Kit-positive cells, and these events did not spread into regions devoid of Kit immunoreactivity. These findings suggest that ICC may also be required for regeneration or propagation of slow waves. After losing Kit-positive cells, normally phasic gastric muscles behaved like muscles of the fundus; the cells had less polarized resting potentials and were tonically depolarized by cholinergic stimulation (see Morgan et al. 1981). These findings suggest a new concept in GI motility: electrical slow waves are not only paced by ICC, but this aspect of gastric electrical activity is a unique property of ICC.
Pacemakers in GI muscles have been considered as initial inward current sources that depolarize adjacent (electrically coupled) smooth muscle cells. It has been thought that if threshold is reached in the smooth muscle cells, slow waves are regenerated and active propagation occurs (see Connor et al. 1976). Thus, the smooth muscle syncytium has generally been considered the major propagation pathway for slow waves. There have been hints in the literature, however, that the slow wave mechanism is mainly a feature of ICC. In intact GI muscles electrical slow waves actively propagate over many centimetres. From studies of canine tissues (1-3 mm in thickness) we reported that slow wave amplitude decays as a function of distance from the pacemaker region through the thickness of the circular muscle. This is suggestive of non-regenerative conduction of slow waves through the thickness of these muscles (see Smith et al. 1987a,b). Slow waves normally propagate without decrement around and in the longitudinal axis of the stomach, small bowel and colon. Removal of a thin strip of tissue from the innermost aspect of the tunica muscularis of the canine colon (i.e. the site of slow wave pacemaker ICC in that organ) blocked active propagation, and slow waves decayed in amplitude in all axes (see Sanders et al. 1990). The obvious conclusion from these studies was that the smooth muscle cells remaining after removal of the pacemaker regions were incapable of regenerating slow waves. Later studies showed that loss of IC-MY (pacemaker ICC) from the small bowels of W/WV and steel (Sl/Sld) mutants resulted in loss of electrical slow waves (Ward et al. 1995; Malysz et al. 1996; Mikkelsen et al. 1998), but smooth muscle tissues in these mutants remained excitable and generated Ca2+ action potentials. However, without IC-MY the smooth muscle never produced electrical events with the waveform or characteristics of slow waves. When these data are considered along with the findings of the present study, a novel concept emerges suggesting distinct roles for ICC and smooth muscle cells in GI motility. ICC generate slow waves, and these events conduct via gap junctions to smooth muscle cells and cause depolarization. The depolarization activates voltage-dependent channels in smooth muscle cells. Our findings suggest that spread of slow waves through the thickness of the smooth muscle layers occurs by conduction, not by active propagation (e.g. see Smith et al. 1987a,b; Dickens et al. 1999). Depending upon the species and availability of voltage-dependent ionic conductances in a particular smooth muscle (i.e. channels that result in inward or outward currents), the response to slow wave depolarizations could either be fast, Ca2+ action potentials (as in the small intestine), or a more sustained rise in the open probability of Ca2+ channels throughout the duration of the slow wave (as in many gastric muscles). Regardless of the form of the response, the depolarization caused by slow waves elicits Ca2+ entry and excitation-contraction coupling. Thus, the electrical activity of GI muscles results from mechanistically distinct electrical events occurring in two electrically coupled cell types, IC-MY and smooth muscle cells. The response of the smooth muscle cells to slow wave depolarization is regulated by neural and hormonal inputs, and our previous work has shown that at least a portion of this modulation is mediated by the second class of ICC in the stomach, IC-IM (see Burns et al. 1996).
White-spotting (W) mutants have been useful in testing the physiological role of certain classes of ICC. The W mutation is a complete deletion of the sequence that codes for the tyrosine kinase portion of Kit (Nocka et al. 1990). Animals homozygous for W die in utero due to severe anaemia (Nocka et al. 1990). Other W mutations are non-lethal. For example, WV is a point mutation in the tyrosine kinase portion that reduces kinase activity. In our initial studies relating Kit function to development of ICC, we used compound heterozygotes (W/WV) because this combination resulted in viable offspring that survived to adulthood. Only IC-MY of the small bowel and intramuscular ICC (IC-IM) in the stomach and sphincteric regions were lost in W/WV animals (see Burns et al. 1996; Malysz et al. 1996), leading some investigators to conclude that Kit was not important in the development of certain types of ICC (Malysz et al. 1996). Treatment of newborn animals with neutralizing antibodies to Kit, however, showed that several classes of ICC not affected in W/WV animals were lost when Kit was blocked. These data suggest that different ICC display variable resistance to loss of Kit function. Results of the present study are consistent with this idea. Gastric IC-MY survive in W/WV animals, but treatment with a neutralizing Kit antibody (ACK2) caused eventual disappearance of Kit-positive cells. Gastric IC-MY were considerably more resistant to ACK2 than IC-MY of the small intestine. Previous studies showed disruption of ICC networks and loss of function within 2-3 days of exposure of small intestinal muscles to ACK2 (Ward et al. 1997), but 2-4 weeks of exposure to ACK2 did not cause loss of all Kit-positive gastric IC-MY in each tissue. It is unlikely that experimental conditions or access of ACK2 to gastric vs. intestinal IC-MY can explain the differences in sensitivity. It is possible that the Kit signalling pathway may be reinforced by parallel pathways in gastric IC-MY; however, eventual loss of detectable IC-MY when Kit is blocked suggests that this pathway is obligatory for development and/or phenotypic survival of these cells.
A significant number of human patients suffer from delayed gastric emptying and other functional disorders of the proximal GI tract (e.g. Kendall & McCallum, 1993). Some clinical scientists have suggested that gastric electrical dysrhythmias could be responsible for certain types of functional disorders, and gastric pacing has been considered as a form of therapy (McCallum et al. 1998). Although experimental, some success has been reported in relieving arrhythmias (such as tachygastria) and other symptoms. Our data suggest that entrainment of electrical slow waves by gastric pacing occurs through stimulation of IC-MY. Very short pulse protocols, also reported to be beneficial, may directly affect nerves (Miedema et al. 1992), but the final transducers of neural inputs may still be either IC-MY or IC-IM (see Burns et al. 1996).
Some gastric emptying disorders could be due to physical or functional defects in the gastric pacemaker apparatus. Morphological examinations of gastric samples of patients with gastric emptying disorders and gastroparesis may reveal defects in ICC networks as have been detected in some forms of intestinal pseudo-obstruction (Isozaki et al. 1997). Patients with defective ICC may experience little benefit from electrical pacing either because it becomes impossible to elicit slow waves (total loss of ICC) or because slow wave propagation is altered (patchy loss or defects in ICC). It is also apparent that gastric pacemakers, particularly those of the terminal antrum, are uniquely susceptible to aberrant slow wave frequencies. In tachygastria, this can result in ectopic pacemaker activity (see McCallum et al. 1998). Why gastric IC-MY are prone to dysrhythmic behaviour, how various stimuli and agonists are capable of altering slow wave frequency, and whether IC-MY of patients with enhanced tendency toward arrhythmias have increased susceptibility to altered slow wave frequency are important questions that might be answered by direct studies of the pacemaker mechanisms present in these cells.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Yulia R. Bayguinov. This project was supported by a grant from the NIH, DK 40569.
References
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. Journal of Comparative Neurology. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AEJ, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proceedings of the National Academy of Sciences of the USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Rick GA, Lowe LS. Distributions of interstitial cells of Cajal in stomach and colon of cat, dog, ferret, opossum, rat, guinea pig and rabbit. Journal of the Autonomic Nervous System. 1992;37:47–56. doi: 10.1016/0165-1838(92)90144-6. 10.1016/0165-1838(92)90144-6. [DOI] [PubMed] [Google Scholar]
- Connor JA, Connor C, Kreulen DL, Prosser CL, Weems WA. Pacemaker activity and propagation in intestinal smooth muscle. In: Worcel M, Vassort G, editors. Smooth Muscle Pharmacology and Physiology. Paris: INSERM; 1976. pp. 285–300. [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. The Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy TY, Morgan KG, Szurszewski JH. Intracellular electrical activity of canine and human gastric smooth muscle. The Journal of Physiology. 1978;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. Journal of Submicroscopic Cytology and Pathology. 1989;21:439–460. [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit + cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. American Journal of Gastroenterology. 1997;92:332–334. [PubMed] [Google Scholar]
- Kelly KA, Code CF. Canine gastric pacemaker. American Journal of Physiology. 1971;220:112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- Kendall BJ, McCallum RW. Gastroparesis and the current use of prokinetic drugs. Gastroenterologist. 1993;1:107–114. [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. The Journal of Physiology. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Sanders KM. Comparison of ionic currents from interstitial cell and smooth muscle cells of canine colon. The Journal of Physiology. 1993;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. American Journal of Physiology. 1996;271:G387–399. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- Miedema BW, Sarr MG, Kelly KA. Pacing the human stomach. Surgery. 1992;111:143–150. [PubMed] [Google Scholar]
- Mikkelsen HB, Malysz J, Huizinga JD, Thuneberg L. Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterology and Motility. 1998;10:11–26. doi: 10.1046/j.1365-2982.1998.00082.x. 10.1046/j.1365-2982.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- Morgan KG, Muir TC, Szurszewski JH. The electrical basis for contraction and relaxation in canine fundal smooth muscle. Journal of Physiology. 1981;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S-I, Kunisada T, Era T, Sakakura T, Nishikawa S-I. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit dependency during melanocyte development. EMBO Journal. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus. W37, WV, W41 and W. EMBO Journal. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. The Journal of Physiology. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover NG, Horowitz NN, Sanders KM. Calcium oscillations in freshly dispersed and cultured interstitial cells from canine colon. American Journal of Physiology. 1992;262:C589–597. doi: 10.1152/ajpcell.1992.262.3.C589. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stevens R, Burke E, Ward SM. Slow waves actively propagate at submucosal surface of circular layer in canine colon. American Journal of Physiology. 1990;259:G258–263. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Vogalis F. Organization of electrical activity in the canine pyloric canal. The Journal of Physiology. 1989;416:49–66. doi: 10.1113/jphysiol.1989.sp017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. American Journal of Physiology. 1987a;252:C215–224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in the muscularis of the canine proximal colon. American Journal of Physiology. 1987b;252:C290–299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. American Journal of Physiology. 1986;250:G287–294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Medicine. 1998;4:848–851. doi: 10.1038/nm0798-848. 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemakers. Advances in Anatomy, Embryology and Cell Biology. 1982;71:1–130. [PubMed] [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit-positive cells. Pflügers Archiv. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscle. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: An Assessment of Current Knowledge. Austin, TX, USA: University of Texas Press; 1981. pp. 127–156. [Google Scholar]
- Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. Journal of the Autonomic Nervous System. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S-I, Nishi K, Kobayashi S, Sanders KM. c-kit- dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell and Tissue Research. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. American Journal of Physiology. 1995;269:C1577–1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. The Journal of Physiology. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang X-Y, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]


