Abstract
The mechanism underlying adenosinergic modulation of respiration was examined in vitro by applying the whole-cell patch-clamp technique to different types of respiration-related neurones located in the rostral ventrolateral medulla of neonatal rats (0-4 days old).
The adenosine A1-receptor agonist (R)-N6-(2-phenylisopropyl)-adenosine (R-PIA, 10 μM; n = 31) increased the burst distance of rhythmic C4 inspiratory discharges and decreased the duration of inspiratory discharges (control: 8·00 ± 2·49 s and 918 ± 273 ms; R-PIA: 12·10 ± 5·60 s and 726 ± 215 ms).
Expiratory neurones demonstrated a reversible decrease in input resistance (Rin), a depression of action potential discharges and a hyperpolarization of the membrane potential (Vm) during application of R-PIA (1-10 μM). Similar responses of Rin and Vm to R-PIA were evident after synaptic activity had been blocked by 0·5 μM tetrodotoxin (TTX).
Some of the biphasic expiratory (biphasic E) neurones, but none of the inspiratory neurones, demonstrated changes in Rin or Vm during R-PIA application. With TTX present, R-PIA did not alter Vm or Rin in biphasic expiratory or inspiratory neurones.
Furthermore, R-PIA decreased the spontaneous postsynaptic activities of all neurones examined. The effects of R-PIA on respiratory activity, Rin and Vm could be reversed by the A1-receptor antagonist 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX; 200 nM).
Our data suggest that the modulation of respiratory output induced by adenosinergic agents can be explained by (1) a general decrease in synaptic transmission between medullary respiration-related neurones mediated by presynaptic A1-receptors, and (2) an inactivation, via membrane hyperpolarization, of medullary expiratory neurones mediated by postsynaptic A1-receptors. Furthermore, our data demonstrate that inactivation of expiratory neurones does not abolish the respiratory rhythmic activity, but only modulates respiratory rhythm in vitro.
Neuronal networks in the medulla oblongata generate respiratory rhythm in mammals. The pre-Bötzinger complex (Smith et al. 1991) in the rostral ventrolateral medulla oblongata (RVL) (Onimaru et al. 1988) has been proposed to be the crucial site of this rhythmogenesis, but the detailed organization of this noed vitale is still unknown. The unique patterns of receptors and membrane channels expressed by specific types of neurones, and interaction between these neurones, maintains the central pattern generator for respiration. Thus it is important to increase our understanding of the receptors and membrane channels in each type of cell in this neuronal network.
One of the central modulators of respiration is adenosine, whose action is especially important around the time of birth (Runold et al. 1986; Irestedt et al. 1989; Herlenius et al. 1997). Adenosine has been proposed to be involved in the suppression of fetal and neonatal breathing (Lagercrantz et al. 1984; Bissonnette et al. 1991; Herlenius et al. 1997), particularly during hypoxia when extracellular levels of this nucleoside rapidly increase (Winn et al. 1981). Furthermore, blocking A1-receptors with xanthine derivatives abolishes or attenuates hypoxia-induced depression of breathing in newborn mammals (Runold et al. 1989; Neylon & Marshall 1991; Kawai et al. 1995). Thus blocking the action of endogenous adenosine is probably the mechanism by which theophylline exerts its clinical effects in the treatment of apnoea of prematurity.
Adenosine plays multiple roles throughout the nervous system (Brundege & Dunwiddie, 1997), e.g. inhibiting excitatory postsynaptic currents in hippocampal neurones (Katchman & Hershkowitz, 1993) and decreasing neurotransmitter release in prejunctional motoneurones (Mynlieff & Beam, 1994). As recently reported, stimulation of brainstem adenosine A1-receptors depresses the activity of inspiratory neurones in the neonatal RVL, as well as decreasing the respiratory frequency (Herlenius et al. 1997). However, the mechanism(s) underlying the central adenosinergic depression of respiration and the possible involvement of respiration-related neurones other than the inspiratory neurones in the neonate remain to be elucidated.
In the present study we have used adenosine analogues as tools for investigating brainstem respiratory rhythm. We have examined the possible involvement of activation of A1-receptors on different respiration-related neurones in the neonatal RVL by adenosine in modulation of the respiratory output. We have thus extended the characterization of these RVL neurones to include responsiveness to respiratory modulators.
In order to achieve this goal, a reduced preparation of brainstem-spinal cord from neonatal rats was used, allowing pharmacological manipulation while recording brainstem neurones simultaneously with respiratory activity at the spinal cord. This en bloc preparation generates respiration-related rhythmic activity for several hours when perfused with artificial cerebrospinal fluid (ACSF) (Suzue, 1984) and allows neurophysiological classification and subsequent morphological characterization of respiration-related neurones. Three major groups of respiration-related neurones have been described in this en bloc preparation, i.e. inspiratory, expiratory and biphasic expiratory (biphasic E) neurones (also classified as pre-inspiratory neurones; Onimaru et al. 1988). The respiratory rhythm generation in the neonatal in vitro models differs from that of adult in vivo models. In this neonatal model Cl−-mediated inhibition is not necessary for the respiratory rhythm, whereas it is in the adult (Onimaru et al. 1990; Feldman et al. 1991; Onimaru et al. 1997). However, the isolated respiratory network in the brainstem is sufficient to generate the essential features at the cellular and system levels that have previously been described for the intact respiratory system, i.e. both at the motor output level and within the respiratory network (Ramirez et al. 1998). Adenosine is released systemically during hypoxia and our study was designed to investigate the mechanism behind adenosine A1-receptor-induced modulation of respiration. This was conducted on a system level through recordings of C4 output, at a network level in recordings from individual neurones in a functional network, and finally at a cellular level through whole-cell recordings that were combined with tetrodotoxin (TTX) application, thus eliminating sodium-dependent synaptic transmission.
We have examined the effects of adenosine A1-receptors in the brainstem respiratory network and in individual respiratory neurones. The data document a general effect on synaptic transmission, respiratory rhythm and an inactivation of expiratory neurones. Part of this study has already been presented in a thesis by Eric Herlenius (Herlenius, 1998).
METHODS
Brainstem-spinal cord preparation
These experiments were performed on the brainstem and spinal cord of newborn (0- to 4-day-old) Wistar rats. Under deep ether anaesthesia, the brainstem-spinal cord was dissected and isolated as previously described (Suzue, 1984; Smith et al. 1990; Herlenius et al. 1997). The brainstem was then rostrally decerebrated between the VIth cranial nerve roots and the lower border of the trapezoid body. This preparation was subsequently transferred to a 2 ml chamber, in which it was continuously perfused at a rate of 3.0-3.5 ml min−1 with the following artificial cerebrospinal fluid (ACSF) (mM): 124 NaCl, 5.0 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3 and 30 glucose, equilibrated with 95 % O2 and 5 % CO2 at 26-27°C to give a pH of 7.4. Bath temperature was measured either directly in the bath or indirectly in a water-heating bath (Julabo UC, Julabo 5B), after calibration by direct measurements. The high extracellular [K+] compared with normal physiological concentrations is similar to that used by many researchers utilizing this in vitro preparation (Kawai et al. 1996; Onimaru et al. 1997), and is used to sustain a regular rhythmic activity as [Ca2+] is increased.
All animal experiments were approved by the Regional Animal Ethics Committee.
Recordings
Respiratory activity was recorded using suction electrodes applied to the proximal ends of cut C4 or C5 ventral roots containing respiratory motoneurone axons. The C4/C5 activity (C4) was either amplified and recorded directly or after 3 Hz high-pass filtering. Whole-cell recordings from respiratory neurones were obtained using the modified ‘blind’ patch-clamp technique (Blanton et al. 1989) (see Onimaru & Homma, 1992, for detailed description). Briefly, patch pipettes were pulled in one step from borosilicate glass (GC100TF-10, outer diameter 1.0 mm, with a filament; Clark Electromed, Reading, UK) using a vertical puller (PE-2; Narishige, Tokyo, Japan). The electrode tips had inner diameters of 1.2-2.0 μm and a DC resistance of 4-8 MΩ. The electrodes were filled with a solution consisting of (mM): 120 potassium gluconate, 10 EGTA, 10 Hepes, 1 CaCl2, 1 MgCl2 and 1 Na2-ATP. KOH was used to adjust the pH to 7.3.
Extracellular signals and intracellular membrane potentials were measured with a voltage-clamp amplifier (Nihon Koden, CEZ-311). Respiration-related neurones were sought in the RVL (Arata et al. 1990) in the region of the ventral respiratory group (Bianchi et al. 1995) and identified by monitoring extracellular action potential discharge. When such a neurone was detected, positive pressure (5-15 cmH2O) was released and a slight negative pressure was applied. The resulting formation of a gigaohm seal (> 1 GΩ) was monitored and confirmed by applying a hyperpolarizing current pulse (0.1 nA, duration 30 ms, 2 Hz). Rupture of the cell membrane was achieved by a single hyperpolarizing current pulse (0.5-0.8 nA, duration 30 ms). Membrane potentials were recorded after compensation of the series resistance (20-50 MΩ) and capacitance.
Only respiration-related neurones that had negative resting potentials of 40 mV or more, and an overshooting action potential after establishment of the whole-cell configuration, were included in this study. Neurones were identified and classified on the basis of their characteristic firing patterns and the temporal correlation of this activity to the respiratory cycle of C4/C5 activity. Inspiratory neurones (Insp) receive excitatory synaptic input and discharge action potentials during inspiratory phrenic (C4/C5) activity. Expiratory neurones (Exp) discharge action potentials between the inspiratory phases and are inhibited during the inspiratory phase (Arata et al. 1990; Smith et al. 1990; Shao & Feldman, 1997).
Biphasic expiratory neurones (biphasic E) are characterized by pre- and post-inspiratory excitation and inspiratory-related inhibition (Onimaru et al. 1990). Inspiratory neurones were further classified into three subtypes according to previous classifications performed with this preparation (Onimaru et al. 1996, 1997). Type I neurones (Insp I) receive excitatory postsynaptic potentials (EPSPs) prior to the onset, as well as after the termination of C4 activity, whereas type III neurones (Insp III) are hyperpolarized by synchronized inhibitory postsynaptic potentials (IPSPs) during the pre- and post-inspiratory phases. Insp neurones that only demonstrated EPSPs during the inspiratory phase and no hyperpolarization during the pre- or post-inspiratory phases were classified as type II neurones (Insp II). Membrane potentials (Vm) were not corrected for liquid junction potentials, determined to be ≤ -10 mV, in order to facilitate comparisons with earlier studies performed with this preparation (Smith et al. 1991; Onimaru & Homma, 1992; Ballanyi et al. 1994; Kawai et al. 1996; Onimaru et al. 1996, 1997). The current-voltage (I-V) relationship was determined by injection of an inward current (0.02-0.08 nA, duration 100 ms) during silent phases between bursts in the expiratory phase of the respiratory cycle, or during negative holding potentials (-50 mV) in the case of certain expiratory neurones. Input resistance (Rin) was calculated from the slope of a least-squares regression line fitted to the data. Quantification of spontaneous synaptic events (subthreshold EPSPs) was achieved by measurement of the Vm standard deviation during 5 s intervals in the expiratory phase between action potential discharges of Insp and biphasic E neurones. In eight neurones the effects of adenosinergic drugs on after-hyperpolarization (AHP) were examined by brief intracellular injection of a depolarizing current pulse (10-16 pA, duration 10 ms). A > 10 % change of AHP was used as the criterion for classifying a neurone as responsive (Bayliss et al. 1995). In addition, the extracellular unit activity and its frequency (action potentials per minute) were examined during drug application in five expiratory neurones.
During the experiments, signals were displayed on a chart recorder, monitored using an oscilloscope, digitized (Digidata 1200B, Axon Instruments) and stored on digital audio tape (RD-120TE, TEAC, Tokyo, Japan) or a hard disk for off-line analysis. A thermal head recorder (San-ei, Tokyo, Japan) was used for printing and preparing some of the figures included in this paper.
Drugs
The metabolically stable adenosine A1-receptor agonist (R)-N6-(2-phenylisopropyl)-adenosine (R-PIA; RBI, Natick, MA, USA) was dissolved in 5 % dimethyl sulphoxide (DMSO) and then further diluted in ACSF to obtain a concentration of 1.0 or 10.0 μM. A 1 mM stock solution of the adenosine A1-receptor antagonist 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX; Sigma) in ethanol was diluted in ACSF to obtain a final concentration of 200 nM. The final concentration of DMSO or ethanol in the ACSF was less than 0.01 %. Furthermore, control experiments performed with only DMSO (n = 5) or ethanol (n = 5) added to the ACSF did not change the respiratory activity. Tetrodotoxin (TTX, 0.5 μM; Sigma) was administered in some (n = 24) experiments to block presynaptic Na+-dependent action potentials. These drug solutions were freshly prepared and added to the ACSF superfusing the preparation, the pH being adjusted to 7.4 with 95 % O2 and 5 % CO2 prior to bath application. In each experiment, only a single neurone was examined for the effects of a drug because of difficulties encountered in removing applied drug. Drug effects were evaluated when steady-state drug response was achieved, after 15-20 min in the case of R-PIA and 10-12 min for DPCPX and TTX, as determined by 30-40 min drug exposure in pilot experiments.
Statistics
Off-line analysis was performed using a personal computer and the commercially available programs Axoscope (Axon Instruments), Origin (Microcal Software Inc., Northampton, MA, USA) and JMP (SAS Institute Inc., Cary, NC, USA). The results are presented as means ± standard deviations. After analysis of the variance by the F test, statistical analysis was performed using Student's two-tailed paired t test. When possible, Spearman rank non-parametric correlation was performed on measured variables with respect to postnatal age, in order to evaluate a possible dependency of the results on age. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Control conditions
The membrane properties of seventy-nine RVL neurones examined after establishing whole-cell patch-clamp configuration are documented in Table 1. Figure 1 depicts the major respiratory neurone subtypes: inspiratory, biphasic E and expiratory neurones. The neurones that exhibited a tonic expiratory phase activity that was inhibited during the inspiratory phase are referred to as expiratory. The discharge activity of sixteen out of twenty-six expiratory neurones was also inhibited prior to and after termination of C4 activity (Fig 1 and Fig 4B), similar to that recently reported by Arata et al. (1998). Two of these sixteen neurones were inhibited more than 2.5 s after C4 burst activity had ended, and only discharged action potentials in the later half of the expiratory phase. As the tonic and the peri-inspiratory-inhibited expiratory neurones had similar Vm and Rin values during control conditions, and responded in a similar manner to R-PIA, results from these expiratory neurones are combined and considered as a single group.
Table 1.
The resting membrane potential (Vm), amplitude of action potential (AP) and input resistance (Rin) of respiration-related and tonic RVL neurones measured during the expiratory phase of the respiratory cycle
| RVL neurone | n | Vm (mV) | AP (mV) | Rin (MΩ) |
|---|---|---|---|---|
| Inspiratory, I | 10 | −50.8 ± 5.1 | 51.2 ± 6.4 | 341 ± 119 |
| Inspiratory, II | 14 | −47.7 ± 5.3 | 53.0 ± 12.7 | 394 ± 189 |
| Inspiratory, III | 9 | −45.8 ± 3.1 | 46.4 ± 10.3 | 392 ± 147 |
| Biphasic E | 14 | −44.8 ± 4.7 | 53.3 ± 14 | 4 338 ± 154 |
| Expiratory | 21 | −44.2 ± 3.5 | 45.7 ± 8.5 | 489 ± 166 |
| Tonic | 11 | −45.1 ± 4.0 | 52.8 ± 12.6 | 554 ± 207 |
Figure 1. Respiration-related neurones in the neonatal rat brainstem.
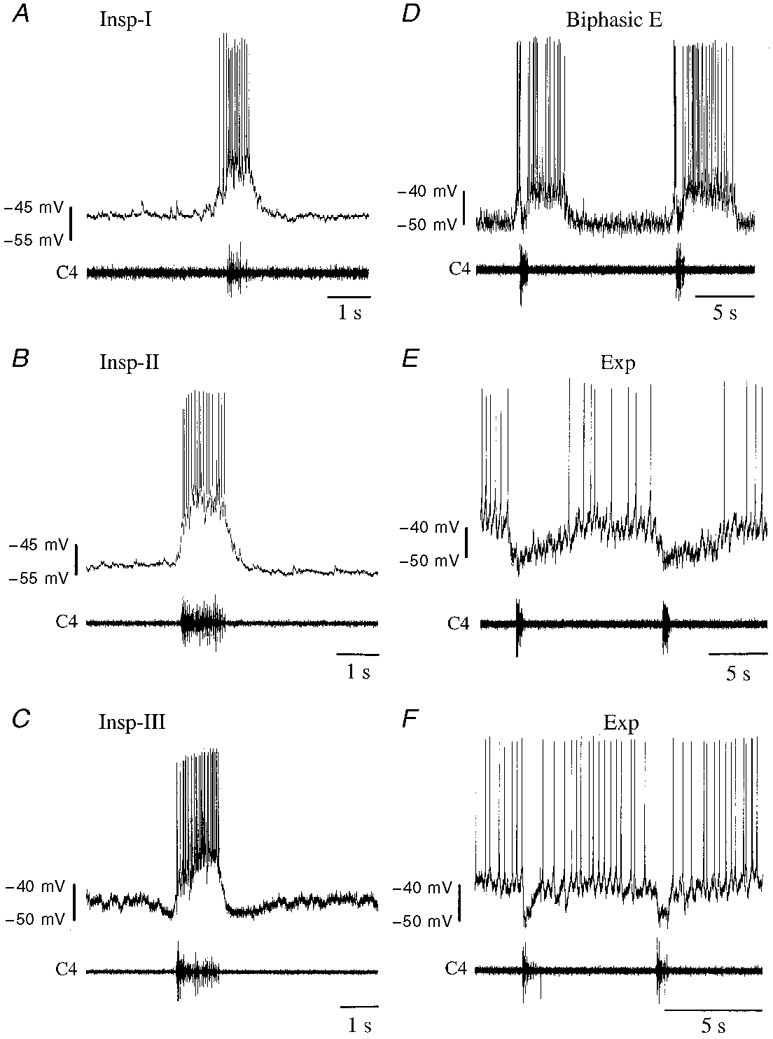
Membrane potential trajectories of different respiration-related neurones are shown. Upper traces: membrane potentials (mV); lower traces: accompanying C4 inspiratory activity. A-C, inspiratory neurones (Insp) receive excitatory synaptic input (EPSPs) and discharge action potentials during C4 ventral root inspiratory activity (C4). A, type I neurones (Insp-I) receive EPSPs prior to and after termination of C4 activity. B, type II neurones (Insp-II) exhibit abrupt transition from resting potential level to burst phase without EPSPs prior to and after C4 inspiratory activity, whereas type III neurones (Insp-III) (C) are inhibited during both these phases. D, biphasic expiratory neurones (Biphasic E) are characterized by pre- and post-inspiratory excitation and inspiratory-related inhibition. Expiratory (Exp) neurones are inhibited by hyperpolarizing IPSPs during the inspiratory phase and either (E) discharge tonically in the expiratory phase or (F) receive IPSPs also in the pre- and post-inspiratory phase, and have a decreased discharge rate during these phases. In the neurone shown, a prolonged inhibition after C4 burst activity makes this neurone discharge primarily in the late expiratory phase.
Figure 4. Inactivation of expiratory neurones by R-PIA-induced hyperpolarization.
A, R-PIA (10 μM) induces hyperpolarization of the membrane potential of an expiratory neurone and eliminates its slow but continuous expiratory phase discharge of action potentials. Administration of DPCPX (200 nM) reverses these changes. B, an expiratory neurone in which R-PIA (1 μM) induces a hyperpolarization. Subsequent DPCPX (200 nM) administration depolarizes the neurone before synaptic noise and C4 activity are increased. Upper traces, expiratory neurone; lower traces, C4 activity.
The rhythmic respiratory activity recorded from C4 had a burst interval of 8.2 ± 2.7 s and burst duration of 844 ± 263 ms (n = 111).
Adenosinergic effect on respiratory output
After exposure to the adenosine analogue R-PIA (10 μM; n = 31), the frequency of rhythmic C4 discharges decreased (C4 burst interval for controls was 8.00 ± 2.49 s and for R-PIA was 12.10 ± 5.63 s). The duration of inspiratory discharges also decreased (C4 burst duration for control: 918 ± 273 ms, and for R-PIA: 726 ± 215 ms). These differences were significant (P < 0.01, Student's paired t test). The steady state of drug effect was reached within 20 min (Fig 2 and Fig 7D). In some preparations with an irregular control respiratory rhythm, R-PIA could have a stabilizing effect (Fig. 2). DPCPX (n = 24) decreased the interval between respiratory bursts (control, 8.29 ± 2.66 s; DPCPX, 7.43 ± 1.86 s; P < 0.05), but burst duration was not affected (control, 942 ± 299 ms; DPCPX, 937 ± 167 ms). The changes in C4/C5 activity caused by R-PIA could be reversed by DPCPX (n = 15). During application of DPCPX, tonic and rhythmic non-respiratory C4 discharges occurred in some preparations (Fig 3C, Fig 4A and Fig 6). Postnatal age had a significant influence on the effect of R-PIA on burst interval (Spearman rank correlation of postnatal age vs. percentage change in burst distance; P = 0.012), but not on burst duration (P = 0.32). The R-PIA dose dependency of C4 effect differed with age (Fig. 2C). We decided to use 10 μM R-PIA in most experiments (n = 31), as a clear effect was observed with all postnatal ages, even though 1 μM was used in six preparations from young (< 48 h) rats. No significant correlation was observed between postnatal age and the effects of DPCPX (Spearman rank correlation of postnatal age vs. percentage change of burst distance, P = 0.12, and vs. percentage change of burst duration,P = 0.31).
Figure 2. Adenosinergic modulation of C4 respiratory output.
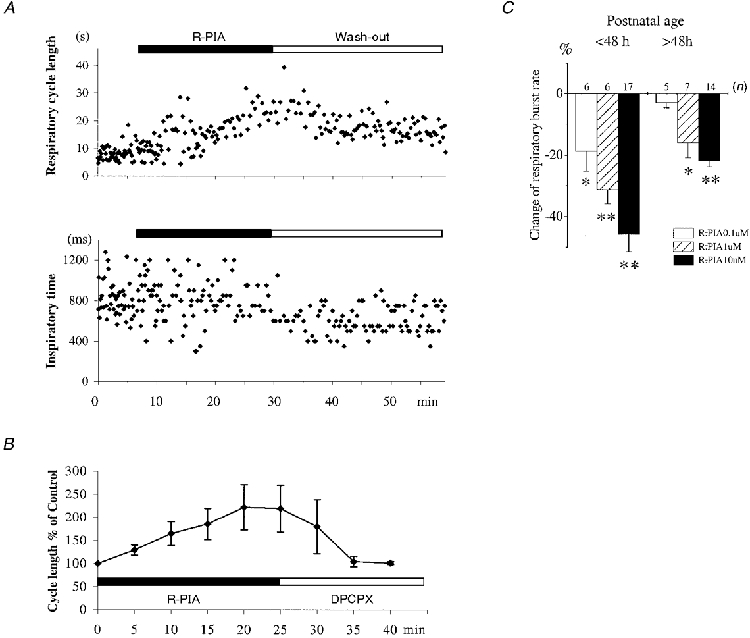
A, respiratory cycle length (top) and inspiratory time (bottom) during control, R-PIA (10 μM) and wash-out in a preparation from a 1-day-old rat (P1). B, R-PIA (10 μM) induces a DPCPX (200 nM) reversible decrease of C4 inspiratory activity. The steady-state effect was reached after 20 min. Data are means ±s.d. from ten preparations (P0-P3). C, dose dependency of R-PIA (0.1-10 μM)-induced change on frequency of C4 respiratory activity. The effect was apparently saturated at 10 μM in the older rats because higher concentrations of R-PIA did not increase the degree of respiratory inhibition further (* P < 0.05 and ** P < 0.01).
Figure 7. Adenosinergic modulation of inspiratory neurone and synaptic noise.
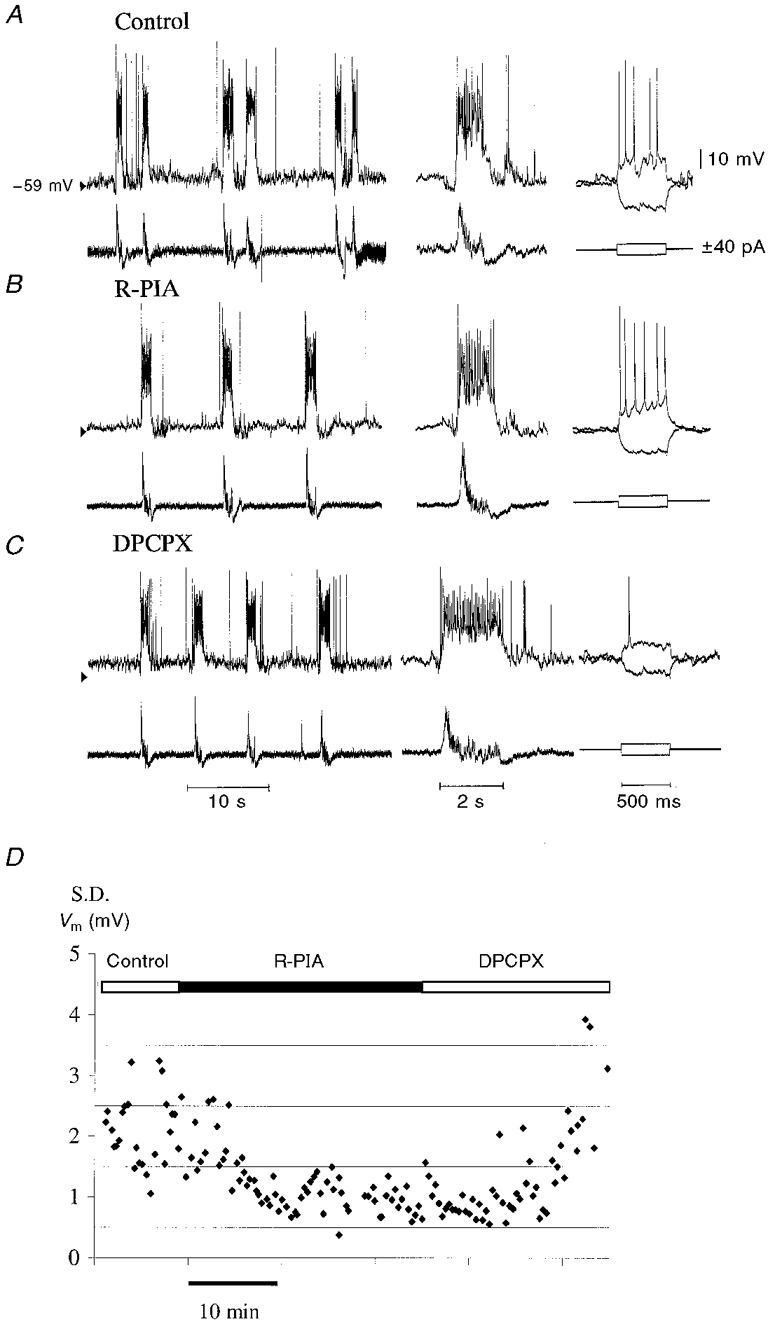
A, an inspiratory neurone (type III) from a 1-day-old rat (P1) perfused with normal ACSF demonstrates a magnified C4 burst of activity and response to an injection of current (±40 pA). B, the same neurone, after a 15 min perfusion with ACSF containing R-PIA (10 μM). Note the reduced synaptic noise between the inspiratory potentials. C, the same neurone after a 12 min perfusion with ACSF containing DPCPX (200 nM). Note a depolarization of the membrane potential, an increase in the synaptic noise and an increased membrane resistance. D, change of synaptic noise in a biphasic E neurone during application of R-PIA (10 μM) and subsequent DPCPX (200 nM). Quantification of subthreshold postsynaptic potentials was performed by measuring the standard deviation (s.d.) of resting membrane potential (Vm) in the expiratory phase.
Figure 3. Inactivation of an expiratory neurone by R-PIA.
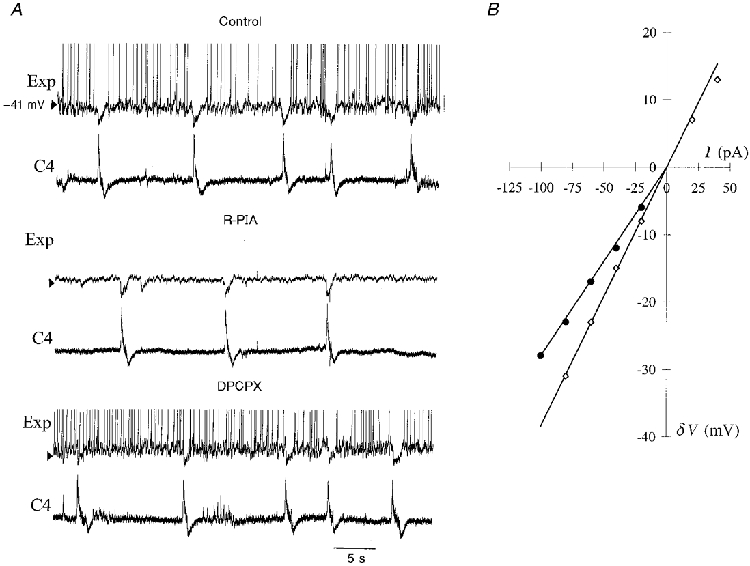
A, during control conditions (upper two traces), an expiratory neurone (Exp) demonstrates discharges of action potentials between the inspiratory phases of C4 discharge activity (C4) and hyperpolarization during the inspiratory phase; action potentials are truncated. When added to the ACSF, R-PIA (1 μM) eliminates the discharges of action potentials and reduces synaptic noise in the expiratory phase, despite depolarization of Vm (+2 mV). C4 activity remains, however, at a slower frequency (middle two traces). Addition of DPCPX (200 nM) to the ACSF reverses these changes caused by R-PIA (bottom two traces). B, current-voltage plots of the expiratory neurone illustrated in A; ⋄, control conditions; •, after administration of R-PIA; I, injected current (in pA); δV, resulting shift in membrane potential (in mV). R-PIA decreased input resistance, calculated from the slope of a least-squares regression line fitted to the data.
Figure 6. Uncoupling of biphasic E neuronal activity from C4 inspiratory-related activity.
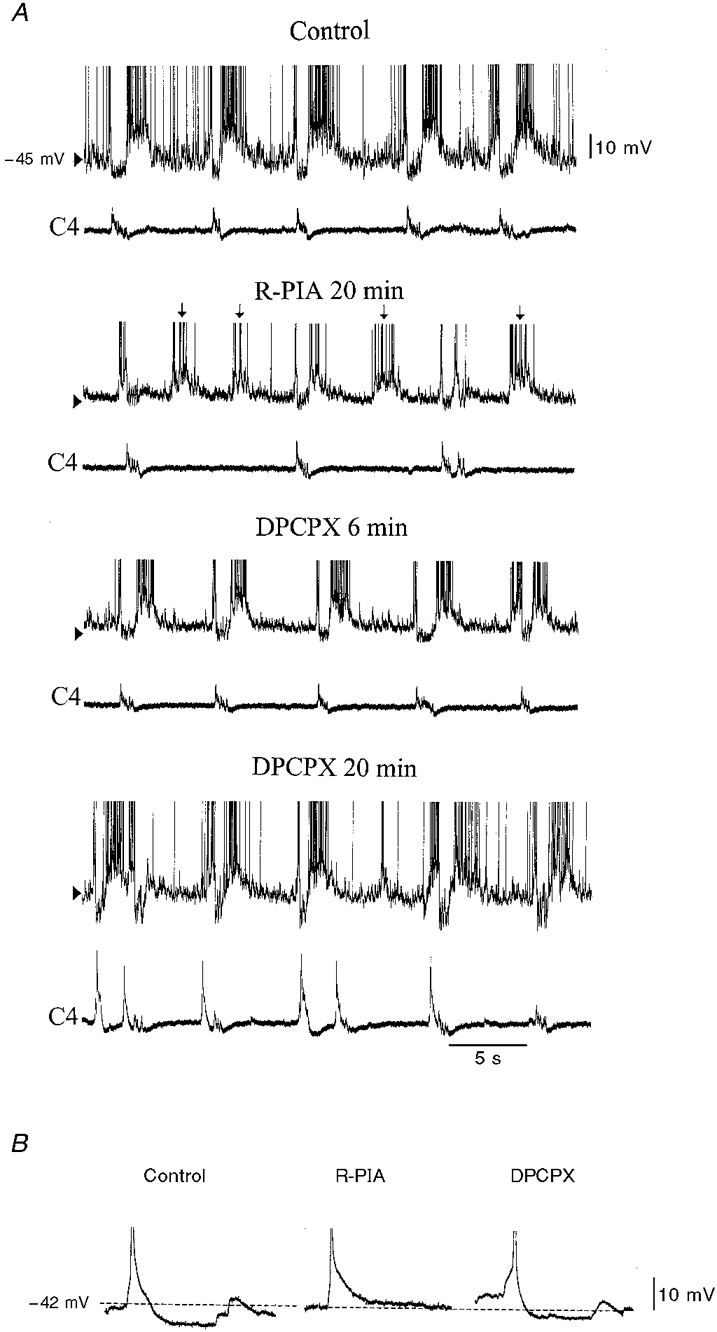
A, during R-PIA (10 μM) administration, the synaptic noise in the expiratory phase is reduced and the frequency of biphasic E membrane potential fluctuations increases. Note the uncoupling of C4 discharges, which decrease in frequency from every second biphasic E membrane depolarization. In the absence of C4 activity, the biphasic E neurone is not hyperpolarized during the depolarized phase (↓). DPCPX (200 nM) reverses the changes in synchronization and C4 respiratory rate induced by R-PIA. During continued perfusion, DPCPX reverses the R-PIA-induced synaptic noise reduction and elicits an increased C4 activity. Upper traces, biphasic E neurone; lower traces, C4 activity (C4). B, the after-hyperpolarization was DPCPX-reversibly reduced by R-PIA in biphasic E neurones that exhibited disturbed synchronization with C4 activity; a sample of action potentials during control, R-PIA and DPCPX application are truncated.
Adenosinergic effect on expiratory neurones
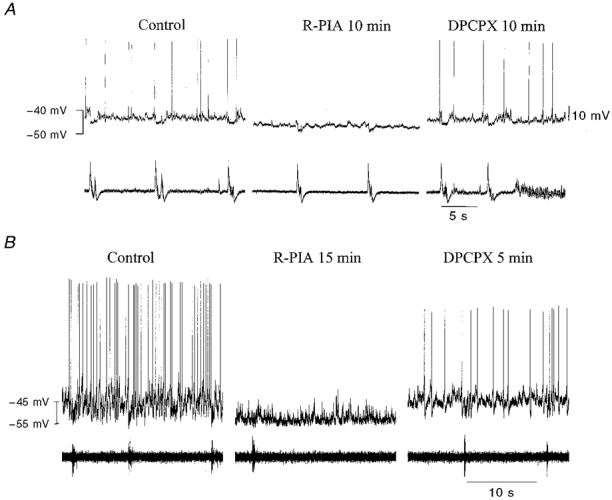
R-PIA caused a reversible decrease in the frequency (1/11) or, most often, complete discontinuation of action potential discharges (10/11) in Exp neurones (Fig 3 and Fig 4). This cease-fire was accompanied in most (5/6) neurones recorded in the whole-cell mode by Vm hyperpolarization and a reduction in Rin (Table 2). Furthermore, when synaptic transmission was blocked by TTX, R-PIA induced a hyperpolarization of Vm and a decrease in Rin in all neurones examined (Table 3). DPCPX could reverse the inactivation of action potential discharges induced by R-PIA (n = 7), and depolarized Vm, but did not alter Rin significantly compared with controls (n = 5, P = 0.04 and 0.37, respectively; Table 2). The after-hyperpolarization (AHP) was reduced by R-PIA in 3/4 of the expiratory neurones in which AHP was examined. DPCPX reversed this depression of AHP. To exclude time-dependent inactivation of expiratory neurones, control experiments were performed without drug application, Vm and Rin being stable during these conditions (n = 4, 20-50 min).
Table 2.
Effects of R-PIA and DPCPX on the resting membrane potential (Vm) and input resistance (Rin) of RVL respiration-related neurones
| Control | R-PIA | Control | DPCPX | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RVL neurone | n | Vm (mV) | Rin (MΩ) | Vm (mV) | Rin (MΩ) | n | Vm (mV) | Rin (MΩ) | Vm (mV) | Rin (MΩ) |
| Inspiratory | 8 | −47.9 ± 5.6 | 445 ± 154 | −47.5 ± 5.6 | 459 ± 176 | 6 | −46.8 ± 3.2 | 373 ± 62 | −44.5 ± 4.4 | 459 ± 219 |
| Biphasic E | 6 | −45.0 ± 5.3 | 369 ± 156 | −46.5 ± 8.1 | 320 ± 136 | 6 | −44.5 ± 5.4 | 375 ± 151 | −45.2 ± 6.3 | 318 ± 126 |
| Expiratory | 6 | −45.0 ± 3.0 | 505 ± 157 | −49.0 ± 6.0* | 381 ± 68* | 5 | −44.6 ± 3.2 | 475 ± 177 | −41.0 ± 2.0* | 364 ± 142 |
These values are means ± S.D. Since the number of samples was small, values for the subgroups of inspiratory neurones (classes I–III) were combined and considered as a single group compared for statistical analysis of the effects of R–PIA. Statistical significance was assessed by Student's paired t test
P < 0.05.
Table 3.
Effects of R-PIA on the resting membrane potential (Vm) and input resistance (Rin) of RVL respiration-related neurones after blockade of synaptic activity by TTX
| TTX | TTX + R-PIA | ||||
|---|---|---|---|---|---|
| RVL neurone | n | Vm (mV) | Rin (MΩ) | Vm(mV) | Rin (MΩ) |
| Inspiratory | 10 | −47.2 ± 6.2 | 314 ± 161 | −45.8 ± 5.0 | 295 ± 163 |
| Biphasic E | 4 | −46.3 ± 3.3 | 458 ± 384 | −44.3 ± 5.9 | 322 ± 157 |
| Expiratory | 6 | −45.0 ± 7.9 | 526 ± 323 | −49.3 ± 6.4* | 451 ± 241 |
These values are means ± S.D. Statistical significance was assessed by Student's paired t test, with
P < 0.05.
Adenosinergic effect on biphasic E neurones
Although administration of R-PIA did not change the mean Vm or Rin in biphasic E neurones (Table 2), the responses of individual neurones differed (see Fig 5 and Fig 6). In three of six biphasic E neurones, there was no change in Vm or Rin during superfusion with R-PIA. Under the same conditions, one biphasic E neurone depolarized (+2 mV), but there was no alteration in its Rin (Fig. 5). R-PIA induced reversible hyperpolarization in two of six biphasic E neurones (2-7 mV), but only one of these neurones had a decreased Rin. In two of six biphasic E neurones, the synchronization between neurone burst discharges and C4/C5 activity was reversibly disturbed by R-PIA (Fig. 6A). At the same time as the time interval between discharges of the biphasic E burst decreased, and consequently neuronal burst frequency increased, the C4/C5 respiratory activity decreased in frequency. Thus every second biphasic E burst was not followed by a C4/C5 discharge (Fig. 6A). Concurrently, the AHP was decreased (Fig. 6B). In ACSF containing TTX, R-PIA did not change Vm or Rin (Table 3). DPCPX did not alter Vm or Rin compared with control values (Table 2). However, DPCPX could reverse the changes in membrane potential and AHP depression and disturbances in coupling induced by R-PIA.
Figure 5. Effects of R-PIA on a biphasic E neurone.
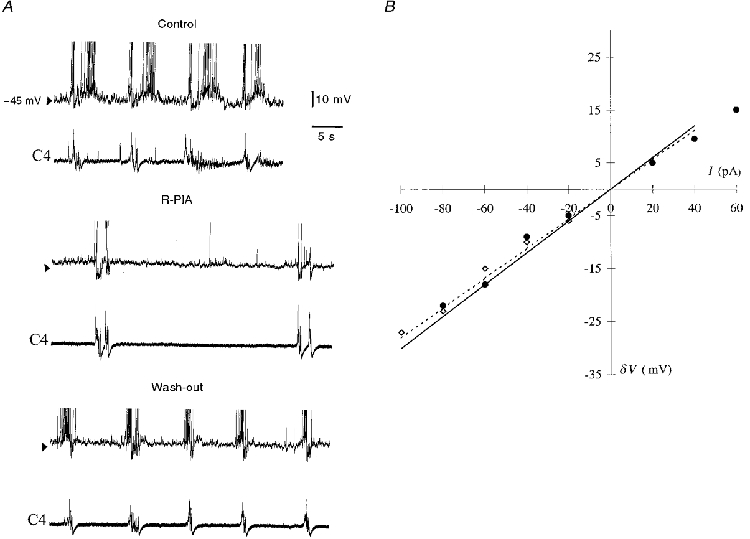
A, a biphasic E neurone is hyperpolarized during C4 inspiratory activity. Under control conditions, during administration of R-PIA (10 μM, 15 min) and after 7 min wash-out, action potentials are truncated. Note the decreased synaptic noise in the expiratory phase and the increased distance between membrane depolarization. B, current-voltage plots from this neurone, in which no changes in input resistance (Rin) were observed during R-PIA administration; I, injected current; δV, resulting shift in membrane potential. Rin was calculated from the slope of a least-squares regression line fitted to the data (⋄ and dotted line, control; •, R-PIA).
Adenosinergic effect on inspiratory neurones
Figure 7 depicts a typical recording of an inspiratory neurone before and during administration of R-PIA. Neither the Vm nor Rin was altered by R-PIA (Fig. 7 and Table 2). Furthermore, R-PIA did not induce changes in the Vm or Rin when synaptic transmission was blocked by the presence of TTX in the ACSF (Table 3). No differences in the responses to R-PIA of type I-III inspiratory neurones were observed (n = 6 for inspiratory type I, n = 7 for type II, and n = 5 for type III). Consequently, the values obtained during drug application to inspiratory neurones were combined in order to increase the validity of the statistical analysis. DPCPX induced a depolarization of Vm (+1-5 mV), probably due to increased synaptic input, since Rin did not change in most neurones (Table 2).
Adenosinergic modulation of spontaneous synaptic events
As illustrated in Fig. 3–Fig. 7, R-PIA consistently caused a reduction of spontaneous synaptic activities during the expiratory phase in all RVL neurones examined. In inspiratory neurones many overlapping EPSPs occurred during the inspiratory phase, and therefore no analysis of EPSPs during this phase was performed. In expiratory and biphasic E neurones the hyperpolarization (expressed in millivolts) induced by synchronized IPSPs did not change during exposure to R-PIA (expiratory values: control, -7.8 ± 2.6; + R-PIA, -6.7 ± 2.3 mV; biphasic E values: control, -6.8 ± 2.5; + R-PIA, -7.0 ± 2.6 mV). In all neurones examined the presence of DPCPX in the perfusate increased the EPSPs occurring during the expiratory phase.
DISCUSSION
We have demonstrated here that activation of presynaptic adenosine A1-receptors decreases synaptic transmission to all brainstem respiration-related neurones and thus decreases the overall activity of the brainstem respiratory circuit. Activation of postsynaptic A1-receptors of expiratory neurones also contributes to the inhibition of neuronal activity. Furthermore, our data also indicate that inactivation of expiratory neurones does not abolish C4 respiratory rhythmic activity in vitro.
C4 activity and adenosine
The increased C4 discharge frequency during application of the A1-receptor antagonist DPCPX suggests that endogenous adenosine has a small but significant influence on the respiratory rhythm during control conditions. This is consistent with previous studies (Dong & Feldman, 1995; Kawai et al. 1995; Herlenius et al. 1997) and indicates that, as in vivo, adenosine exerts a tonic inhibitory influence on the central respiratory activity. Furthermore, during antagonism of A1-receptors tonic and rhythmic non-respiratory C4 discharges occurred in some preparations (Fig 3C, Fig 4A and Fig 6). We did not pursue study of the mechanism(s) underlying these non-respiratory discharges. However, similar non-respiratory spinal nerve discharges can also be evoked by NMDA, dopamine D1 and cholinergic agonists (Smith et al. 1988). It is possible that increased excitation or disinhibition of neuronal circuits involved in locomotion may be involved in this non-respiratory activity. Increased activation of adenosine receptors by R-PIA decreased the respiratory output in an age-dependent manner in accordance with previous findings (Runold et al. 1986; Herlenius et al. 1997). DPCPX could reverse this depression, indicating that it is caused by A1-receptor activation.
Expiratory neurones
All expiratory neurones decreased their firing frequency and ten out of eleven neurones examined ceased firing completely in the presence of R-PIA. However, in none of these cases did the respiratory rhythmic output cease. The synaptic input in the expiratory phase of the respiratory cycle was reduced in all neurones examined, but in expiratory neurones Vm and Rin were also decreased. This hyperpolarization of expiratory neurones could reflect increased inhibitory synaptic inputs to these cells, decreased excitatory input or postsynaptic action of R-PIA on the membrane. The fact that R-PIA decreased Vm and Rin, even when synaptic transmission was blocked by TTX, indicates a postsynaptic mechanism of action. We suggest that this is due to an activation of K+ conductance via adenosine A1-receptors present on expiratory neurones. This would be in accordance with the well-characterized ability of adenosine to activate potassium conductance postsynaptically (Thompson et al. 1992). Furthermore, it has recently been reported that RVL expiratory neurones in the neonatal rat express three different potassium channels. One of these, which may be expressed specifically in the expiratory neurone, is voltage dependent and determines the neuronal level of depolarization (Jacquin et al. 1997).
Our present results agree with earlier findings concerning adenosinergic modulation of expiratory neurones in the adult cat by Schmidt et al. (1995), who demonstrated that expiratory neurones are hyperpolarized and cease to discharge action potentials by pre- and postsynaptic A1-receptor activation. Furthermore, our findings indicate that expiratory neurones are not an essential part of the respiratory rhythm generator in the neonatal rat brainstem in vitro.
In adult cats exposed to hypoxia, synaptic noise was reduced in the brainstem neuronal network for respiration and expiratory type 2 (E2) neurones ceased discharge of action potentials due to pre- and postsynaptic-induced changes of the membrane properties (Richter et al. 1993). Our findings are consistent with both of these findings, since a hypoxia-induced increase in extracellular adenosine levels could mediate both of these effects.
The respiratory network in the isolated neonatal rat medulla oblongata includes reciprocal inhibitory synaptic connections between expiratory and inspiratory neurones, as well as between biphasic E and inspiratory neurones (Onimaru et al. 1992; Arata et al. 1998). Thus at birth some of the synaptic connections among respiratory neurones that exist in the adult rat are already present. Extrapolation of these in vitro findings in neonatal rats to adult mammals in vivo has to be executed cautiously. In vivo the expiratory phase (E) of the respiratory cycle can be divided into two phases, E1 and E2 (Richter, 1982), and many expiratory neurones fire predominantly during stage 2 (E2) of expiration. However, the firing of several expiratory neurones in vivo also spans both phases (Ezure, 1990). In the neonatal in vitro preparation many expiratory neurones constantly fire during the expiratory phases, but about two-thirds of expiratory neurones are inhibited in the pre- and post-inspiratory phase (Arata et al. 1998). This subtype of expiratory neurones has been proposed to be equivalent to late expiratory (or E2) neurones in adult mammals.
It was recently reported that reciprocal (i.e. inspiratory-phase) inhibition of expiratory neurones is not necessary for the maintenance of respiratory rhythm generation (Shao & Feldman, 1997). Our results also suggest that action potential discharge of these expiratory neurones and input to other respiration-related neurones is not required for the generation of respiratory rhythm in vitro. This is consistent with the view that respiratory rhythm is generated primarily by inspiratory-related neurones (von Euler, 1980; Richter, 1982) and that medullary expiratory neurones are less important for the rhythmogenesis of respiration. This conclusion is also in accordance with the different pacemaker models for respiratory rhythm generation proposed for neonatal mammals (Onimaru et al. 1988; Feldman et al. 1990; Onimaru et al. 1997; Rekling & Feldman, 1998). It is contradictory to network models for the generation of respiratory rhythm in adult mammals, which have phasic and reciprocal inhibitory interactions between inspiratory and expiratory neurones as a key feature (Ezure, 1990; Bianchi et al. 1995; Rybak et al. 1997). However, respiratory generation matures during the first two postnatal weeks (Paton & Richter, 1995) and the neurones in the reduced neonatal in vitro preparation may respond differently from those in adult in vivo systems. Nevertheless, as demonstrated by Richter et al. (1993), most expiratory type 2 neurones in the adult cat are inactivated by membrane hyperpolarization and stop discharging action potentials during hypoxia. As adenosine levels increase during hypoxia (Winn et al. 1981), a possible explanation could be that expiratory neurones in vivo as well as in vitro are not essential for respiratory rhythm and can be inactivated by hypoxia-induced increased adenosine levels.
Biphasic E neurones
In biphasic E neurones the modulation induced by A1-receptors appeared to be primarily presynaptic, since neither R-PIA nor DPCPX altered the Vm or Rin in most of the neurones examined. A presynaptic mode of action is further supported by the absence of changes in Vm or Rin during administration of R-PIA after blocking synaptic activity with TTX. The relationship between biphasic E bursts and C4/C5 discharges was disturbed in two experiments, possibly due to an A1-receptor-mediated reduction of reciprocal synaptic transmission between excitatory biphasic E neurones. In both of the biphasic E neurones, which increased their burst frequencies, a reduction in after-hyperpolarization (AHP) was observed. Such a reduction in AHP could be due to direct inhibition of calcium-dependent potassium channels (KCa) or might represent an indirect effect, i.e. an inhibition of N-type Ca2+ channels and a consequent reduction in the entry of Ca2+ necessary to support the AHP. A reduction in AHP allows more rapid firing and thereby contributes to enhanced excitability. Different adenosinergic actions on the AHP of biphasic E neurones will decrease the synchronization between these neurones. A speculative explanation for this finding is that a disturbance of synchronization between biphasic E neurone bursts may lead to a failure to trigger inspiratory bursts (Onimaru et al. 1992), thereby causing a decrease in C4 respiratory burst rate. The different responses of biphasic E neurones to R-PIA indicates that biphasic E neuronal subtypes could have different adenosinergic receptor expression or coupling (Onimaru et al. 1997). Our sample of biphasic E neurones was too small to distinguish between these two possibilities.
Inspiratory neurones
All inspiratory neurones examined continued firing in the presence of R-PIA, which is in agreement with our previous findings (Herlenius et al. 1997). Since R-PIA did not change the Vm or Rin in inspiratory neurones, it would appear that these neurones do not express A1-receptors in or close to the soma. Although several in situ studies have demonstrated that the A1-receptor is expressed ubiquitously in the brain (Rivkees, 1995), the electrophysiological effects of adenosine are dependent on the cell type (Brundege & Dunwiddie, 1997). Thus different patterns of expression or coupling of A1-receptors, resulting in different neuronal electrophysiological effects of adenosinergic agents in neurones participating in the same functional respiratory network is plausible.
Functional implications
The modulation of spontaneous synaptic activities induced by R-PIA and DPCPX appears to be mediated by presynaptic A1-receptors, since this modulation was observed in all neurones examined with no correlation to changes in Vm or Rin. Our findings are in agreement with the known effects of presynaptic adenosine A1-receptors and with the established role of adenosine in regulating synaptic transmission under both normal and pathophysiological conditions (Katchman & Hershkowitz, 1993; Mynlieff & Beam, 1994; Brundege & Dunwiddie, 1997). In vivo interstitial levels of adenosine in adults are in the range of 0.03-0.3 μM and may rise 100-fold during hypoxia (Winn et al. 1981; Fredholm, 1995). Activation of A1-receptors induces a functional inactivation of expiratory neurones and may potentially serve an important protective function during hypoxia. A decrease or cessation of action potential discharges in the non-vital expiratory neurones together with decreased synaptic transmission and activity in the brainstem respiratory neuronal network would protect this vital network during hypoxia, by reducing the local energy and oxygen demands while these resources are scarce (Ballanyi et al. 1994). Hypoxia will, besides increasing adenosine levels, also decrease the intracellular levels of ATP and thus open more KATP channels in respiratory neurones (Pierrefiche et al. 1997; Mironov et al. 1998). This will further limit synaptic interactions and neuronal discharge activity (Richter et al. 1991).
It may be of functional significance that vital brainstem neurones and their resulting respiratory output remain active, while overall synaptic transmission in the network is decreased. This would attenuate local neuronal damage induced by hypoxia and at the same time increase the time for respiration to possibly restore oxygen saturation to normal levels.
We conclude that the decrease in frequency of respiratory motor output induced by adenosinergic agents is mediated via activation of pre- and postsynaptic A1-receptors on respiration-related neurones in the medulla oblongata. Furthermore, inactivation of expiratory neurones in vitro modulates but does not abolish the generation of the respiratory rhythm.
Acknowledgments
We gratefully acknowledge Professor Hiroshi Onimaru for methodological and technical support, as well as for his valuable advice, Dr Juri Shvarev for technical support and Dr R. A. Harris for linguistic advice. This work was supported by grants from the Swedish Medical Research Council (SMFR 14X-0907, 19X-5234 and B96-04R-11693) and the Fraenckel Foundation for Medical Research.
References
- Arata A, Onimaru H, Homma I. Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Research Bulletin. 1990;24:599–604. doi: 10.1016/0361-9230(90)90165-v. 10.1016/0361-9230(90)90165-V. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the rostral ventrolateral medulla of newborn rat brainstem-spinal cord preparation in vitro. NeuroReport. 1998;9:743–746. doi: 10.1097/00001756-199803090-00033. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Volker A, Richter DW. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. NeuroReport. 1994;6:165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Umemiya M, Berger AJ. Inhibition of N- and P-type calcium channels and the afterhyperpolarization in rat motoneurones. The Journal of Physiology. 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Hohimer AR, Knopp SJ. The effect of centrally administered adenosine on fetal breathing movements. Respiratory Physiology. 1991;84:273–285. doi: 10.1016/0034-5687(91)90123-z. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AJ. Whole-cell recordings from neurons in slices of reptilian and mammalian cerebral cortex. Journal of Neuroscience Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Advances in Pharmacology. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. Journal of Neuroscience. 1995;15:3458–3467. doi: 10.1523/JNEUROSCI.15-05-03458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between respiratory neurons and considerations on the genesis of respiratory rhythm. Progress in Neurobiology. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. 10.1016/0301-0082(90)90030-K. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC, Ellenberger HH, Connelly CA, Liu GS, Greer JJ, Lindsay AD, Otto MR. Neurogenesis of respiratory rhythm and pattern: emerging concepts. American Journal of Physiology. 1990;259:R879–886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC, Liu G. Respiratory pattern generation in mammals: in vitro en bloc analyses. Current Opinion in Neurobiology. 1991;1:590–594. doi: 10.1016/s0959-4388(05)80033-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors in the central nervous system. News in Physiological Sciences. 1995;10:122–128. [Google Scholar]
- Herlenius E. Vol. 152. Stockholm, Sweden: Department of Woman and Child Health, Karolinska Institutet; 1998. Respiratory activity in medulla oblongata and its modulation by adenosine and opioids. PhD Thesis http://diss.kib.ki.se/1998/91-628-3240-9. [Google Scholar]
- Herlenius E, Lagercrantz H, Yamamoto Y. Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatric Research. 1997;42:46–53. doi: 10.1203/00006450-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Irestedt L, Dahlin I, Hertzberg T, Sollevi A, Lagercrantz H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatric Research. 1989;26:106–108. doi: 10.1203/00006450-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Jacquin TD, Descoins C, Champagnat J, Denavit-Saubie M. K+ channels of expiratory neurones in the brainstem preparation of the newborn rat. NeuroReport. 1997;8:3673–3678. doi: 10.1097/00001756-199712010-00004. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Hershkowitz N. Adenosine antagonists prevent hypoxia-induced depression of excitatory but not inhibitory synaptic currents. Neuroscience Letters. 1993;159:123–126. doi: 10.1016/0304-3940(93)90814-2. 10.1016/0304-3940(93)90814-2. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Okada Y, Muckenhoff K, Scheid P. Theophylline and hypoxic ventilatory response in the rat isolated brainstem-spinal cord. Respiratory Physiology. 1995;100:25–32. doi: 10.1016/0034-5687(94)00124-i. 10.1016/0034-5687(94)00124-I. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, Euler C. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors. Pediatric Research. 1984;18:387–390. doi: 10.1203/00006450-198404000-00018. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. The Journal of Physiology. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynlieff M, Beam KG. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. Journal of Neuroscience. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. The Journal of Physiology. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Research. 1988;445:314–324. doi: 10.1016/0006-8993(88)91194-8. 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflügers Archiv. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Japanese The Journal of Physiology. 1997;47:385–403. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Richter D. Calcium-dependent responses in neurons of the isolated respiratory network of newborn rats. The Journal of Physiology. 1996;491:677–695. doi: 10.1113/jphysiol.1996.sp021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflügers Archiv. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I, Iwatsuki K. Excitation of inspiratory neurons by preinspiratory neurons in rat medulla in vitro. Brain Research Bulletin. 1992;29:879–882. doi: 10.1016/0361-9230(92)90159-u. [DOI] [PubMed] [Google Scholar]
- Paton JF, Richter DW. Maturational changes in the respiratory rhythm generator of the mouse. Pflügers Archiv. 1995;430:115–124. doi: 10.1007/BF00373846. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW, Spyer KM. Hypoxic response of hypoglossal motoneurones in the in vivo cat. The Journal of Physiology. 1997;505:785–795. doi: 10.1111/j.1469-7793.1997.785ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. The Journal of Physiology. 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons - hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. Journal of Experimental Biology. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. The Journal of Physiology. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Modulation of respiratory patterns during hypoxia. In: Speck DF, Dekin MS, Revelette WR, Frazier DT, editors. Respiratory Control: Central and Peripheral Mechanisms. Lexington, USA: The University Press of Kentucky; 1993. pp. 21–28. [Google Scholar]
- Rivkees SA. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Developmental Brain Research. 1995;89:202–213. doi: 10.1016/0165-3806(95)00120-3. [DOI] [PubMed] [Google Scholar]
- Runold M, Lagercrantz H, Fredholm BB. Ventilatory effect of an adenosine analogue in unanesthetized rabbits during development. Journal of Applied Physiology. 1986;61:255–259. doi: 10.1152/jappl.1986.61.1.255. [DOI] [PubMed] [Google Scholar]
- Runold M, Lagercrantz H, Prabhakar NR, Fredholm BB. Role of adenosine in hypoxic ventilatory depression. Journal of Applied Physiology. 1989;67:541–546. doi: 10.1152/jappl.1989.67.2.541. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JFR, Schwaber JS. Modelling neural mechanisms for genesis of respiratory rhythm and pattern. 2. Network models of the central respiratory pattern generator. Journal of Neurophysiology. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. The Journal of Physiology. 1995;483:769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex - differential roles of glycinergic and GABAergic neural transmission. Journal of Neurophysiology. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brainstem-spinal cord in vitro. FASEB Journal. 1988;2:2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu GS, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brainstem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. Journal of Neurophysiology. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Haas HL, Gahwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. The Journal of Physiology. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. American Journal of Physiology. 1981;241:235–242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- von Euler C. Central pattern generation during breathing. Trends in Neurosciences. 1980;3:275–277. [Google Scholar]


