Abstract
The lipid bilayer technique is used to characterize the biophysical and pharmacological properties of a novel, fast, cation-selective channel formed by incorporating platypus (Ornithorhynchus anatinus) venom (OaV) into lipid membranes.
A synthetic C-type natriuretic peptide OaCNP-39, which is identical to that present in platypus venom, mimics the conductance, kinetics, selectivity and pharmacological properties of the OaV-formed fast cation-selective channel. The N-terminal fragment containing residues 1-17, i.e. OaCNP-39(1-17), induces the channel activity.
The current amplitude of the TEACl-insensitive fast cation-selective channel is dependent on cytoplasmic K+, [K+]cis. The increase in the current amplitude, as a function of increasing [K+]cis, is non-linear and can be described by the Michaelis-Menten equation. At +140 mV, the values of γmax and KS are 63·1 pS and 169 mM, respectively, whereas at 0 mV the values of γmax and KS are 21·1 pS and 307 mM, respectively. γmax and KS are maximal single channel conductance and concentration for half-maximal γ, respectively. The calculated permeability ratios, PK:PRb:PNa: PCs:PLi, were 1:0·76:0·21:0·09:0·03, respectively.
The probability of the fast channel being open, Po, increases from 0·15 at 0 mV to 0·75 at +140 mV. In contrast, the channel frequency, Fo, decreases from 400 to 180 events per second for voltages between 0 mV and +140. The mean open time, To, increases as the bilayer is made more positive, between 0 and +140 mV. The mean values of the voltage-dependent kinetic parameters, Po, Fo, To and mean closed time (Tc), are independent of [KCl]cis between 50 and 750 mM (P > 0·05).
It is proposed that some of the symptoms of envenomation by platypus venom may be caused partly by changes in cellular functions mediated via the OaCNP-39-formed fast cation-selective channel, which affects signal transduction.
Envenomation by platypus (Ornithorhynchus anatinus) venom (OaV) causes severe local effects, including intense pain, hyperalgesia and plasma extravasation (oedema) (de Plater et al. 1995), as well as hypotension and peripheral vasodilation in systemically administered experimental animals (Fenner et al. 1992). The molecular mechanisms underlying these OaV-induced effects are not known. Using the lipid bilayer technique it has been shown that platypus venom (OaV), which contains 19 peptide-protein components (de Plater et al. 1995; de Plater, 1998), formed inward anion and outward slow and fast cation-selective channels (J. I. Kourie unpublished data). The OaV-formed anion channel has a maximum conductance of 857 ± 23 pS in 250/50 mM KCl cis/trans. The current-voltage relationship of this channel shows strong inward rectification. The channel activity undergoes time-dependent inactivation that can be removed by depolarizing voltage steps more positive than +40 mV, the equilibrium potential for chloride (ECl). The conductance values for the slow and fast channels are 22·5 ± 2·6 and 38·8 ± 4·6 pS in 250/50 mM KCl cis/trans and 41·38 ± 4·2 and 60·7 ± 7·1 pS in 750/50 mM KCl cis/trans, respectively. The kinetics of the slow ion channels are voltage dependent. The channel open probability (Po) is between 0·1 and 0·8 at potentials between 0 and +140 mV. The channel frequency (Fo) increases with depolarizing potentials between 0 and +140 mV, whereas the mean open time (To) and mean closed time (Tc) decrease. The channel has conductance values of 21·47 ± 2·3 and 0·53 ± 0·1 pS in 250 mM KCl and choline chloride, respectively. The amplitude of the single channel current is dependent on cytoplasmic [K+] ([K+]cis) and the reversal potential (Erev) value responds to increases in [K+]cis by shifting to more negative voltages. The increase in current amplitude as a function of increasing [K+]cis is described by the Michaelis-Menten equation. At +140 mV, γmax and KS (maximal single channel conductance and concentration for half-maximal γ, respectively), have values of 38·6 pS and 380 mM and decline at 0 mV to 15·76 pS and 250 mM, respectively. The permeability values for PK:PNa:PCs:Pcholine are 1:1:0·63:0·089, respectively.
The fast cation channel has not yet been studied in detail. The aims of this study are: (a) to characterize the biophysical properties of the OaV-formed fast cation-selective channel; (b) to compare these properties with those of a fast cation-selective channel formed by a synthetic C-type natriuretic peptide (OaCNP-39), which has been identified as a major component of Ornithorhynchus anatinus venom (de Plater, 1998); and (c) to determine the role of the 17 residue N-terminal fragment, i.e. OaCNP-39(1-17) of OaCNP-39 in the channel activity.
METHODS
Solutions
Unless otherwise stated, the initial experimental solution for incorporating OaV, OaCNP-39 and OaCNP-39(1-17) into the bilayers contained KCl (250 mM cis and 50 mM trans) plus 1 mM CaCl2 and 10 mM Hepes buffer (pH 7·4, adjusted with 4·8 mM KOH).
Synthesis of OaCNP-39 and OaCNP-39(1-17) and amino acid analysis
Small synthetic peptides are powerful tools for the study of structure-function relationships of ion channel-forming proteins. The procedures for synthesis of OaCNP-39 and OaCNP-39(1-17) and amino acid analysis have been detailed previously (de Plater et al. 1998a, b).
The total sequence of OaCNP-39 is shown below:
 OaCNP-39 and its fragments are routinely synthesized at the Centre for Molecular Structure and Function at the Australian National University, using an Applied Biosystems 470A solid phase peptide synthesizer (de Plater et al. 1998a). Disulphide bridges are formed using a 10-fold molar excess of EKATHIOXTM resin (Ekagen, Palo Alto, CA), stirred at room temperature for 4 h. The peptide is purified by preparative RP-HPLC (BioRad Gradient Module 850 M), using a Dynamax C8 column (300A, 21·4 mm × 25 cm, Ranin Associates) and analysed by amino acid analysis (see below) and TOF-MALDI (VG Analytical, Fisons) mass spectrometry to confirm authenticity. Peptide concentrations are determined by quantitative amino acid analysis (de Plater, 1998). A lyophilized sample is subjected to gas-phase hydrolysis under N2 at 110°C for 20 h, using 6 N HCl with 0·1 % phenol. Analyses are performed using an H-P AminoQuant Series II (Hewlett-Packard) amino acid analyser. Amino acids are subjected to pre-column derivitization with ortho-pthalaldehyde (OPA with 3-mercaptopFropionic acid in 400 mM sodium borate, pH 10·4) and 9-fluorenylmethyl chloroformate, and derivitized amino acids are detected using an HP1046 (Hewlett-Packard) fluorescence detector.
OaCNP-39 and its fragments are routinely synthesized at the Centre for Molecular Structure and Function at the Australian National University, using an Applied Biosystems 470A solid phase peptide synthesizer (de Plater et al. 1998a). Disulphide bridges are formed using a 10-fold molar excess of EKATHIOXTM resin (Ekagen, Palo Alto, CA), stirred at room temperature for 4 h. The peptide is purified by preparative RP-HPLC (BioRad Gradient Module 850 M), using a Dynamax C8 column (300A, 21·4 mm × 25 cm, Ranin Associates) and analysed by amino acid analysis (see below) and TOF-MALDI (VG Analytical, Fisons) mass spectrometry to confirm authenticity. Peptide concentrations are determined by quantitative amino acid analysis (de Plater, 1998). A lyophilized sample is subjected to gas-phase hydrolysis under N2 at 110°C for 20 h, using 6 N HCl with 0·1 % phenol. Analyses are performed using an H-P AminoQuant Series II (Hewlett-Packard) amino acid analyser. Amino acids are subjected to pre-column derivitization with ortho-pthalaldehyde (OPA with 3-mercaptopFropionic acid in 400 mM sodium borate, pH 10·4) and 9-fluorenylmethyl chloroformate, and derivitized amino acids are detected using an HP1046 (Hewlett-Packard) fluorescence detector.
Lipid bilayer membranes and incorporation of OaV, OaCNP-39 and OaCNP-39(1-17)
Bilayers were formed across a 150 μm hole in the wall of a 1 ml delrinTM cup, using a mixture of palmitoyl-oleoyl-phosphatidylethanolamine, palmitoyl-oleoyl-phosphatidylserine and palmitoyl-oleoyl-phosphatidylcholine (5:3:2, by volume) (Miller & Racker, 1976; Kourie et al. 1996), obtained from Avanti Polar Lipids (Alabama). The lipid mixture was dried under a stream of N2 and redissolved in n-decane at a final concentration of 50 mg ml−1. OaV, OaCNP-39 and OaCNP-39(1-17) were incorporated into the lipid bilayer by addition to the cis chamber up to a final peptide concentration of 0·1-1 μg ml−1. The side of the bilayer to which the OaV, OaCNP-39 and OaCNP-39(1-17) were added is defined as cis, and the other side as trans. The experiments were conducted at room temperature (between 20 and 25°C).
Recording single channel activity
The pCLAMP6 program (Axon Instruments Inc.) was used for voltage command and acquisition of Cl− current families with an Axopatch 200 amplifier (Axon Instruments Inc.). The current was monitored on an oscilloscope and stored on a compact disc recorder (CD-R). The cis and trans chambers were connected to the amplifier head stage by Ag-AgCl electrodes in agar salt-bridges containing the solutions present in each chamber. Voltages and currents were expressed relative to the trans chamber. Data were filtered at 1 kHz (4-pole Bessel, -3 dB) and digitized via a TL-1 DMA interface (Axon Instruments Inc.) at 2 kHz. The optimal bilayers that were formed had specific capacitance values larger than 0·42 μF cm−2 but lower than the ∼1·0 μF cm−2 value for biological membranes, because the bilayer area includes some of the thick film of the annulus, which has much lower capacitance than is found in biological membranes (Kourie, 1996).
Data analysis
CHANNEL 2 (developed by P. W. Gage & M. Smith, at The John Curtin School of Medical Research, Australian National University), an in-house analysis program (Kourie et al. 1996a), was used to measure the parameters of single channel activity (Colquhoun & Hawkes, 1983). These include: (a) mean open time, To (the total time that the channel was not closed and including openings to all conductance levels, divided by the number of events); (b) mean closed time, Tc (the total time that the channel was closed divided by the number of events); (c) frequency of the channel opening, Fo; (d) the open probability, Po (the sum of all open times as a fraction of the total time). The value of the current amplitude was obtained by measuring the distance (in pA) between two lines. The first line was set on the maximum baseline noise of the closed level, where the current amplitude was considered 0 pA, and the second was set on the noise of the majority of distinct open events longer than 0·5 ms. The threshold level for the detection of single channel events was set at 50 % of the current amplitude (Patlak, 1993). The reversal potential was corrected for the liquid junction potential by using the JPCalc software (Barry, 1994). Assuming that the only permeant ions in the system were K+ and the substituting cation, X+, this shift (ΔErev=EX - EK) can be used to estimate the selectivity from the following equation (Hodgkin and Katz, 1949):
Statistics
Unless otherwise stated, each ion channel was used as its own control and the comparison was made between biophysical parameters of the channel before and after changing the cis or trans solutions. Data are reported as means ±s.e.m. (see Table 1).
Table 1.
Parameters of cation-selective channels recorded in 250/50 mM KCl cis/transand clamped at +80 mV
| Channel-forming peptide | Conductance(pS) | Open probability | Selectivity sequence PK:PRb:PNa:PCs:PLi |
|---|---|---|---|
| OaV | 38.8 ± 4.6 (n= 11) | 0.52 ± 0.11 (n= 10) | — |
| OaCNP-39 | 37.6 ± 4.3 (n= 5) | 0.47 ± 0.09 (n= 5) | 1:0.64:0.28:0.13:0.06 (n= 1) |
| OaCNP-39(1-17) | 41.1 ± 4.7 (n= 8) | 0.45 ± 0.13 (n= 7) | 1:0.76:0.21:0.09:0.03 (n= 2) |
RESULTS
One hundred and seventy-four fast channels were recorded after the incorporation of OaV (65 channels), OaCNP-39 (62 channels) and OaCNP-39(1-17) (47 channels) into bilayers of specific capacitance > 0·42 pF cm−2. These channels were recorded at different voltages ranging between -140 and +160 mV and in solutions of different composition and concentrations. The lifespan of the channel was, on average, 30 min and varied between less than 1 min to 2 h. The activity of these channels was lost, mainly because of bilayer breakage, after: (a) application of large voltages, particularly positive voltages; (b) electronic mixing of the cis and/or trans solutions after the addition of a treatment; (c) perfusion of the cis or trans solutions with new solutions. Also, to a lesser extent, loss of activity was due to the bilayer thickening that results from the increase in the volume of the solvent separating the monolayers of the painted artificial bilayer. The OaV-formed channels were stable and irreversibly associated with those lipid bilayers that maintained their specific capacitance of > 0·42 pF cm−2. This indicates that the formed channels are not likely to be due to peptide-induced structural disturbances of the bilayer. Furthermore, OaV and OaCNP-39, when inactivated by being boiled in 250 mM KCl, failed to form channels in the lipid bilayer (n= 3 and 5, respectively), when used at concentrations and for periods similar to those in which the active peptides produced channels. This indicates that certain natural features of the bioactive peptides in the venom are needed for channel formation. In order to ascertain that this was not due to a low probability of peptide incorporation into the lipid bilayer, liposomes (Arispe et al. 1993) were used to detect the incorporation of the peptide into the bilayer by monitoring the specific capacitance of the lipid bilayer. Although the liposomes were incorporated as indicated by the increase in the specific capacitance, no channel activities were observed for OaCNP-39 and OaV (n= 7).
Cationic nature of the OaV-formed fast ion channel
To determine whether the outward current was the result of an efflux of cations or an influx of anions, the cis cytoplasmic side of the bilayer was successively exposed to 250 mM KCl, 250 mM choline chloride and 250 mM potassium gluconate, while the trans side of the bilayer remained exposed to 50 mM KCl. A voltage protocol was then used to obtain families of currents under these different ionic compositions. From an initial holding potential of 0 mV, the membrane potential (Vm) was stepped to voltages ranging from -160 to +140 mV, in steps of +20 mV, for periods lasting 6·25 s. Figure 1A shows typical current traces recorded for the OaV-formed fast channels at voltages between -160 and +140 mV. The activity of this channel is characterized by ‘bursts’ in the outward currents at voltages between 0 and +140 mV. These bursts are separated by long periods of channel inactivation or closure. The time course of the single channel activity is voltage independent. It is apparent that when the 250 mM KCl in the cis chamber was totally replaced by 250 mM choline chloride the outward currents were eliminated (Fig. 1B). Furthermore, replacement of choline chloride with potassium gluconate led to the recovery of the outward currents at all voltages between -20 and +140 mV (Fig. 1C). The current-voltage relationships constructed from these current families show the changes in the current amplitude and Erev (Fig. 1D). In contrast to choline chloride, substituting potassium gluconate for KCl had relatively little effect on either channel kinetics (Fig. 1B and C) or the current-voltage (i-V) relationships (Fig. 1D), suggesting that the cation, rather than the anion, carries the current.
Figure 1. The cationic nature of the OaV-formed fast ion channels.
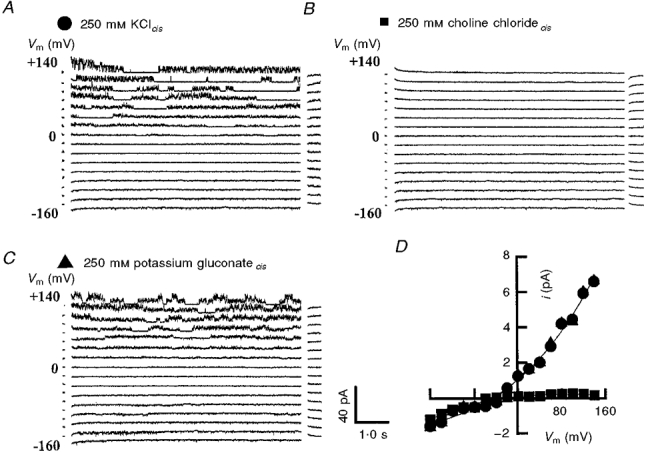
Representative families of current traces illustrating the activity of fast channels recorded from a voltage-clamped optimal bilayer, i.e. specific bilayer capacitance > 42 μF cm−2. The cis solutions were 250 mM KCl (A), 250 mM choline chloride (B) and 250 mM potassium gluconate (C.) The trans solution was 50 mM KCl in A-C. Membrane voltages between -160 and +140 in +20 mV steps are shown on the left of the current traces in A-C. Following convention, the upward deflections denote activation of outward potassium current, i.e. potassium ions moving from the cis chamber to the trans chamber. For a better display, the data are filtered at 1 kHz, digitized at 2 kHz and reduced by a factor of five. The current traces are separated by a 10 pA offset. D, current-voltage relationships for the treatments in A-C. For these treatments the values for Erev were -44·2 mV (KCl), -5·6 mV (choline chloride) and -43·8 mV (potassium gluconate); mean of 1-3 experiments. KCl, •; choline chloride, ▪and potassium gluconate, ▴.
Potassium-dependence of the OaV-formed fast ion channel
The dependence of the fast outward current on [K+]cis was examined at several concentrations between 50 and 750 mM. The cis solution was successively perfused with 50, 150, 250, 350, 450, 550, 650 and 750 mM K+cis and families of current traces were obtained at voltages between -160 and +140 mV (e.g. Fig. 2). The time course of the single channel activity was independent of [K+]cis where the burst characteristics of the channel activity remained unaffected. However, it is apparent that the amplitude of the outward current increased as a function of increasing [K+]cis.
Figure 2. Dependence of the OaV-formed fast cation-selective channels on [KCl]cis between 50 and 750 mM.
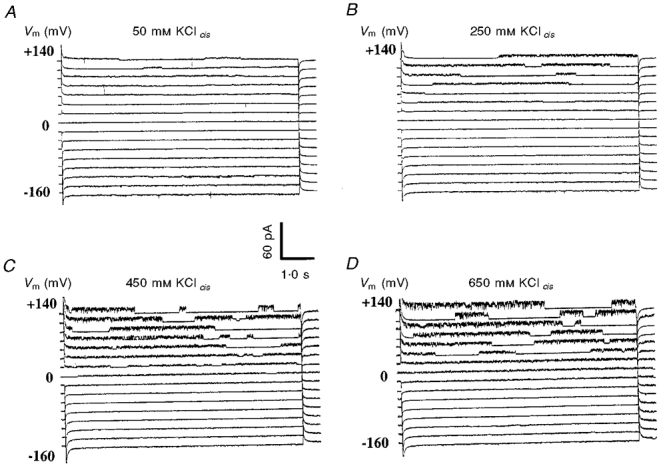
For clarity, only four families of current traces are shown. A, 50/50 mM KCl; B, 250/50 mM KCl; C, 450/50 mM KCl; D, 650/50 mM KCl. The current traces are separated by a 15 pA offset. The rest of the conditions for data display are as in Fig. 1.
The current-voltage relationships constructed from these current families show outward rectification and confirm the increase in current amplitude at depolarizing voltages (Fig. 3A). The current reversal potential (Erev) responded to changes in [K+]cis (Fig. 3B) in a manner similar to EK, suggesting that this fast outward current is carried by K+. For example, the reversal potentials of the OaV-formed fast cation-selective channels in 50/50, 250/50 and 750/50 mM KCl cis/trans are 0, -44·2, and -75·7 mV, close to EK values of 0, -41·2 and -69·4 mV, respectively. The increase in current amplitude as a function of increasing [K+]cis was non-linear (Fig. 3C), indicating K+ binding to sites in the channel pore. The outward current is described by the Michaelis-Menten equation:
where KS is the concentration for half-maximal γ. At +140 mV, the values of γmax and KS are 63·1 pS and 169 mM whereas at 0 mV the values of γmax and KS are 21·1 pS and 307 mM, respectively. It is thought that saturation is due to a binding and an unbinding step of K+ to the channel protein which makes the permeation rate limiting at high ionic concentration (Hille, 1992).
Figure 3. Voltage dependence of the current amplitude of the OaV-formed fast cation-selective channel.
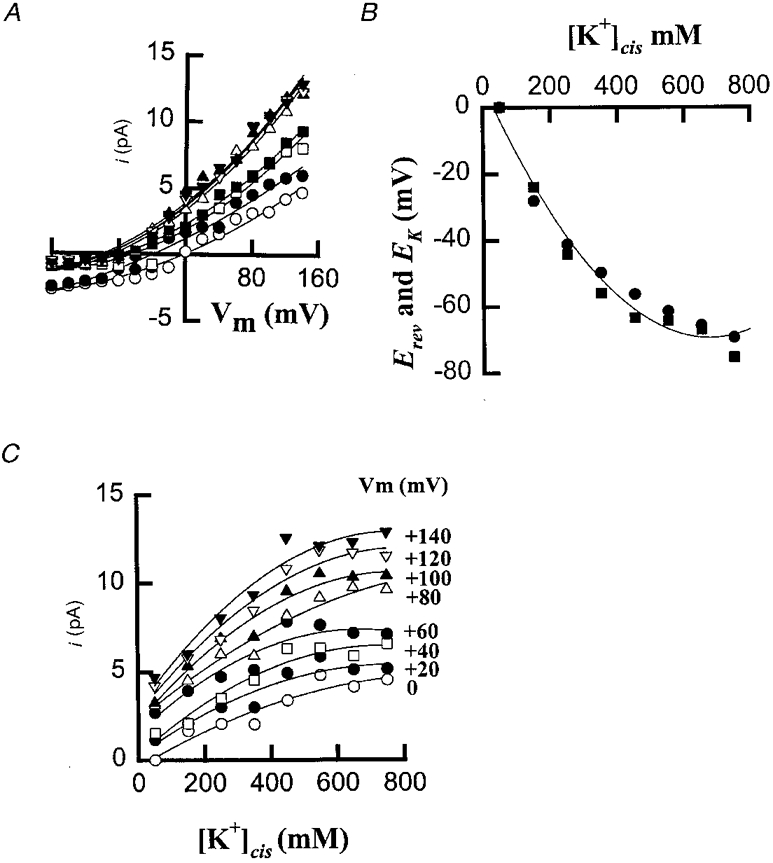
A, current-voltage relationships in: 50/50 mM KCl (○); 150/50 mM KCl (•); 250/50 mM KCl (□); 350/50 mM KCl (▪); 450/50 mM KCl (▵); 550/50 mM KCl (▴); 650/50 mM KCl (▿) and 750/50 mM cis/trans KCl (▾). The values for Erev were 0 mV (50); -23·9 mV (150); -44·2 mV (250); -56·0 mV (350); -63·4 mV (450); -64·0 mV (550); -67·0 mV (650) and -75·5 mV (750); mean of 1-5 experiments. B, EK (•) and Erev (▪) plotted against [KCl]cis. C, Michaelis-Menten curves for the single-channel current amplitude as a function of [KCl]cis at different voltages between 0 (bottom) and +140 mV (top) in +20 mV steps. The continuous lines are drawn to a second-order polynomial fit.
Kinetics of the OaV-formed single fast ion channels
The kinetic parameters of the OaV-formed fast channels were obtained by analysing channel activity within bursts at positive voltages and [KCl]cis ranging between 50 and 750 mM (Fig. 4). The probability of the channel being open, Po, is voltage dependent, with mean values between 0·15 and 0·75 at voltages between 0 and +140 mV, respectively (Fig. 4A). This increase is mainly due to increases in the mean values of the average open time, To (Fig. 4C), rather than to increases in the values of Fo (Fig. 4B) or changes in the mean values of the mean closed time, Tc (Fig. 4D). In contrast, the channel frequency, Fo, decreased with depolarization of the bilayer. The mean values of Fo were between 357 and 155 events s−1 for voltages between 0 and +140 mV, respectively. For the same voltage range, the mean values of the mean open time, To, were between 0·75 and 4·5 ms, while no clear pattern emerged for the values of the mean closed time, Tc, at voltages between 0 and +140 mV. The voltage dependency of Po was not affected by [KCl]cis (Fig. 4A). This implies that the increase in the probability of opening of the outward cation-selective channels at depolarizing voltages will allow the efflux of K+ at a wide range of [KCl]cis. Like Po, the values of Fo, To and Tc were independent of [KCl]cis ranging between 50 and 750 mM (P > 0·05). The kinetic parameters of the OaCNP-39-formed channels were also obtained at the steady state by analysing channel activity at different depolarizing voltages and for different [K+]cis (data not shown; cf. Figure 5). The kinetic parameters, voltage- and potassium-dependence of the OaCNP-39-formed channels (n= 5) were similar to those of the OaV-formed channels (see also Table 1).
Figure 4. Voltage- and [K+]cis-dependence of the kinetic parameters of OaV formed fast cation-selective channel.
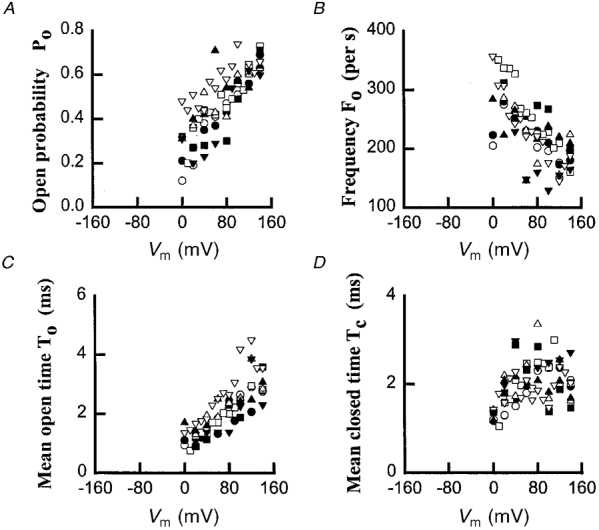
A, open probability (Po); B, frequency (Fo); C, mean open time (To); D, mean closed time (Tc). 50/50 mM KCl, (○); 150/50 mM KCl, (•); 250/50 mM KCl, (□); 350/50 mM KCl, (▪); 450/50 mM KCl, (▵); 550/50 mM KCl, (▴); 650/50 mM KCl (▿) and 750/50 mM KCl cis/trans (▾). The threshold for channel detection was set at 50 % of the current amplitude
Figure 5. Effects of TEACl on synthetic OaCNP-39-formed channel.
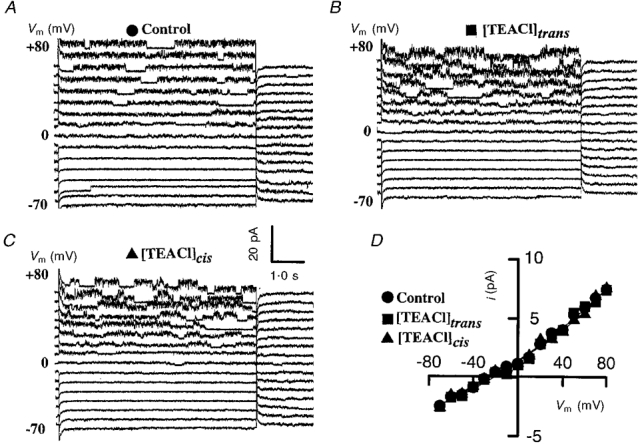
Effects of TEACl on synthetic OaCNP-39 (a major peptide found in the platypus venom OaV)-formed fast cation channel: control (A), 50 mM [TEACl]trans (B) and 50 mM [TEACl]cis (C). D, current-voltage relationships control (•), 100 mM [TEACl]trans (▪) and 50 mM [TEACl]cis (▴). The current traces are separated by a 5 pA offset. Similarly, TEACl had no effect on the OaV-formed fast cation channel.
Voltage-dependence of the OaCNP-39 formed ion channel
OaCNP-39 is a major component of platypus venom and thus its ability to form ion channels was examined to see whether it is involved in the formation of channels similar to those of the OaV-formed channels. It was found that, like OaV, the incorporation of synthetic OaCNP-39 into lipid bilayer membranes in 250/50 mM KCl cis/trans produced bursts of channel activity. The time course of channel activity showed no inactivation even after periods of up to 15 min (data not shown). A voltage protocol was used to examine the voltage dependence of single-channel currents in 250/50 mM KCl cis/trans. From an initial holding potential of 0 mV, the membrane potential (Vm) was stepped to voltages ranging from -70 mV to +80 mV, in steps of +10 mV, for periods lasting 6·25 s.
There was no delay in the channel activity and the channel opened immediately after the application of the depolarizing voltage steps (Fig. 5A). Current-voltage relationships were constructed to examine the voltage dependence of the conductance of the OaCNP-39-formed ion channel conductance (Fig. 5D). The current-voltage relationship exhibited weak outward rectification that is characteristically fitted with two exponentials. The reversal potential (Erev) of -44·2 mV is close to the EK value of -41·2, calculated from the Nernst equation (Hodgkin & Katz, 1949), which indicates that under these experimental conditions the outward current is primarily due to the movement of K+. Previously, it was shown that the activity of the OaV-formed fast cation-selective channel was not affected by 100 mM [TEACl]trans (a blocker of outwardly rectifying K+ channels) (J. I. Kourie unpublished data), nor by 50 mM [TEACl]cis (data not shown). Similarly, neither the conductance of the OaCNP-39-formed fast cation-selective channel nor the Erev were affected by 50 mM [TEACl]trans or/and 50 mM [TEACl]cis (Fig. 5B -D). Several other agents also failed to affect the channel conductance (see below).
The role of the N-terminus of OaCNP-39 in the formation of the fast cation-selective channel
To ascertain the role of the OaCNP-39 components which formed the fast cation channel, the 17 residues of the N-terminal fragment, i.e. OaCNP-39(1-17) and the fragment of amino acids from residue 18 to 39, i.e. OaCNP-39(18-39), of the OaCNP-39 were synthesized and incorporated into lipid bilayer membranes. It was found that the OaCNP-39(18-39) forms a large cation channel (Kourie, 1999) which is different in its conductance, selectivity and kinetics from the fast cation-selective channel formed by either the parent peptide, OaCNP-39, or OaV. On the other hand, the N-terminal OaCNP-39(1-17) formed fast cation-selective channels (Fig. 6A) that have properties similar to those of the channel formed by both the parent peptide, OaCNP-39, and OaV (see Table 1). The cationic nature of the channel was also determined by successively exposing the cis (cytoplasmic) side of the bilayer to 250 mM KCl, 250 mM choline chloride and 250 mM potassium gluconate. In contrast to choline chloride, substituting potassium gluconate for KCl had no effect on either channel kinetics or the i-V relation (data not shown). These findings suggest that the cation rather than the anion carries the current through the OaCNP-39(1-17)-formed fast channel.
Figure 6. Monovalent cation selectivity of the OaCNP-39(1-17) (a fragment of the parent peptide OaCNP-39 found in OaV)-formed fast cation-selective channel at voltages between -160 and +140 mV.
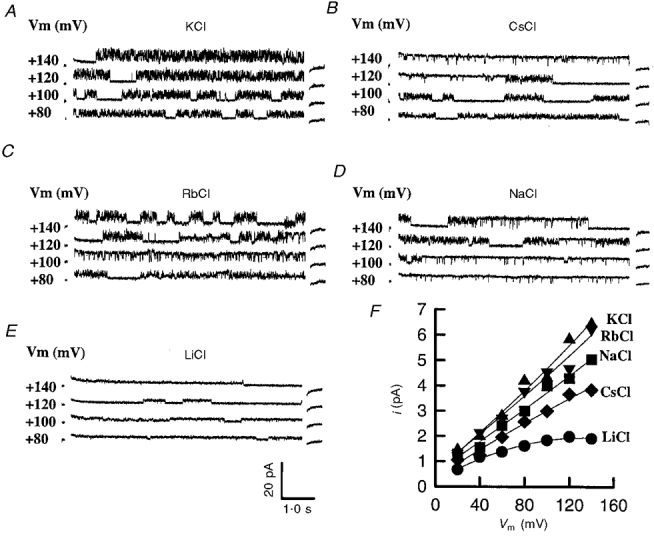
For clarity, only examples of the single channel currents activated at +80, +100, +120 and +140 mV are shown in: A, 250 mM [KCl]cis; B, 250 mM [CsCl]cis; C, 250 mM [RbCl]cis; D, 250 mM [NaCl]cis and E, 250 mM [LiCl]cis. The current traces are separated by a 10 pA offset. F, current-voltage relationships for the monovalent cations Li+ (•), Na+ (▪), K+ (▴), Rb+ (▾), and Cs+ (♦).
Ion selectivity of the OaCNP-39(1-17)-formed fast cation-selective channel
The ion selectivity of the channel was determined in ion substitution experiments that involved the use of the OaCNP-39(1-17), because of its availability and easier synthesis. The 250 mM KCl in the cis chamber was totally replaced by 250 mM of each of NaCl, CsCl, RbCl and LiCl, and families of current traces were obtained at voltages between -160 and +140 mV. The current-voltage relationships constructed from these current families show changes in the current amplitude and Erev (Fig. 6B). The conductance of the channel decreases with increasing dehydration energy of the permeant monovalent cations in the order K+ > Rb+ > Na+ > Cs+ > Li+. The shift in the reversal potential for the single unitary currents to more negative values when Rb+, Na+, Li+or Cs+ is substituted for K+ indicates that these ions are less permeable than K+ (Fig. 6B). The calculated relative permeability values for PK:PRb:PNa:PCs:PLi were 1:0·76:0·21:0·09:0·03, respectively (see also Table 1).
Negative data
The conductance of the OaCNP-39(1-17)-formed a fast cation-selective channel and the Erev were affected by 25-50 mM [TEACl]trans or/and [TEACl]cis (each n= 5). Furthermore, the addition to the cis solution of 2·5 mM neflumic acid; 0·36 mM cAMP; 20 μM IP3 or IP4; 40 μg ml−1 prostigmin; 20 μl anti-cGMP; 20 μl putrescine; 20 μM ATP; 10 mM AlCl3 or 50 mM NH4Cl, failed to affect the conductance of the OaCNP-39(1-17)-formed channel (n= 2-8).
DISCUSSION
Characteristics of OaV, OaCNP-39(1-17) and OaCNP-39-formed fast channels
The results presented in this study show that platypus venom forms a fast cation-selective channel. The activity of this channel is characterized by bursts in the outward currents at voltages between -20 and +140 mV in 250/50 mM KCl cis/trans. The bursts are separated by long periods of channel inactivation or closure. The conductance values of the fast cation-selective channel in lipid bilayer membranes were 38·8 ± 4·6 and 60·7 ± 7·1 pS in 250/50 and 750/50 mM cis/trans, respectively. The current reversal potential of -44 mV in 250/50 mM KCl cis/trans is closer to the EK value than that of ECl, indicating that this channel is more selective for cations than for anions. The Erev responded to changes in [K+]cis in a manner similar to EK, suggesting that this fast outward current is carried by K+. The increase in current amplitude as a function of increasing [K+]cis was non-linear and can be described by the Michaelis-Menten equation. At +140 mV, the values of γmax and KS are 63·1 pS and 169 mM, whereas at 0 mV, the values of γmax and KS are 21·1 pS and 307 mM, respectively. On the other hand, the conductance values for the slow channels are 22·5 ± 2·6 pS and 41·38 ± 4·2 pS in 250/50 and 750/50 mM KCl cis/trans, respectively. At +140 mV, γmax and KS values were 38·6 pS and 380 mM and decreased to 15·76 pS and 250 mM at 0 mV, respectively. These conductance values are much smaller than the previously reported value of 546 ± 23 pS for the synthetic human CNP-22 and the OaCNP-39(18-39) venom. The cationic selectivities of the OaV-, OaCNP-39(18-39)- and OaCNP-39(1-17)-formed channels were PK:PRb:PNa:PCs:PLi, 1:0·76:0·21:0·09:0·03, respectively. By comparison, the values for the OaV-formed slow cation channel were PK:PNa:PCs:Pcholine, 1:1:0·63:0·089, respectively and for the CNP-22 and OaCNP-39(18-39)-formed cation channel were PK:PNa:PCs:Pcholine, 1:0·88:0·76:0·13, respectively. These findings suggest that the selectivity filter of this fast cation-selective channel discriminates on the basis of charge, size and energy required for dehydration of monovalent cations. However, the amino acids in the oxygen rings-based filter (Eisenman & Dani, 1987) of the OaCNP-39(1-17) have yet to be determined.
The time courses of the current transitions of the OaV-, OaCNP-39-, and OaCNP-39(1-17)-formed channels, unlike those of the CNP-22 and OaCNP-39(18-39)-formed channels, reveal no inactivation at voltages between -160 and +140 mV. The kinetic parameters of the OaV-, OaCNP-39(18-39)-, and OaCNP-39(1-17)-formed channels reported here are different from those of the CNP-22- and OaCNP-39(18-39)-formed channels. At positive voltages between 0 and +80 mV, the probability of the CNP-22- or OaCNP-39(18-39)-formed channels being open, Po, was virtually constant and had a value of 1. At negative voltages between -70 and -10 mV, the Po of the CNP-22 and OaCNP-39(18-39)-formed channels had a bell-shaped curve with a peak at -60 mV. The channel frequency, Fo, decreased from 14·96 events s−1 at -70 mV to 0·8 events s−1 at +80 mV. The mean open time, To, increased from 65 ms at -70 mV to 201 ms at -50 mV and to 335 ms at -30 mV. In contrast, the mean closed time, Tc, decreased exponentially from 16·4 ms at -70 mV to 0·5 ms at -20 mV. The potassium independence of the kinetic parameters of the fast channel indicates that voltage rather than K+ underlies the conformational changes in the amino acids of the channel proteins controlling the gating mechanism.
The unitary conductance of the fast channel was not affected by 50 mM [TEACl]cis or 100 mM [TEACl]trans. The OaV-, OaCNP-39(18-39)-, and OaCNP-39(1-17)-formed fast channels reported here, like the CNP-22-formed channel (Kourie, 1999) but unlike the OaV-formed slow cation channel, are TEACl insensitive. These biophysical properties of the fast channel point to a novel channel that is different from the previously reported peptide- or toxin-formed, voltage-dependent cation channels. These include the staphylococcal δ-toxin-induced 70-100 and 450 pS channels (Mellor et al. 1988), the tetanus toxin-induced 89 pS channel (500 mM KCl) (Gambale & Montal, 1988) and the Clostridium botulinum C2-II toxin-induced 55 pS channel (100 mM KCl) (Schmid et al. 1994).
Mechanisms involved in OaCNP-39-channel formation
The possibility that natriuretic peptides actually form ion channels, as opposed to merely regulating them, has not previously been examined. The structures of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) incorporate a 17-residue intra-molecular disulphide loop (between residues 6 and 22). The amino acid sequence of the loop is identical in ANP and BNP, whilst CNP is the same, except for two residues (Kojima et al. 1990; Sudoh et al. 1990; Komatsu et al. 1991; Mukoyama et al. 1991; Suga et al. 1992; 1993). In contrast to ANP and BNP, which possess C- and N-terminal sequence extensions beyond this loop, CNP extends only N-terminally (Suga et al. 1993; de Plater et al. 1995; 1998a). The results reported here suggest that the components of the C-type natriuretic peptides can form ion channels via different mechanisms. Previously, it was reported that acidic conditions play an important role in toxin-formed ion channel formation. It is thought that acidic conditions allow some domains to dock onto and insert into the membrane, as suggested for colicin E1 (Merrill et al. 1997) and Bcl-2-formed channels (Minn et al. 1997). The fact that OaCNP-39 forms cation-selective channels in negatively charged lipid bilayer membranes at pH 7·4 and voltages between -80 and +80 mV suggests that acidic conditions are not required for conformational changes.
Under conditions of ion channel formation, the regions most likely to bind to the negatively charged bilayer are those containing lysine and arginine. Similarly, the charged polar residues will line the channel pore in contact with aqueous solvent. The unitary conductance of this channel is likely to be determined by rings of negatively charged amino acids, i.e. aspartate and histidine residues. The large number of positive charges, i.e. arginine and lysine, may have a role in gating the channel by sensing the electrical field across the bilayer in a manner similar to that suggested for the S4 segment of voltage-dependent channels (for review see Catterall, 1992). The most likely regions that will be in the interior of the channel protein or hidden in the membrane lipid away from the aqueous solvent are those containing proline, leucine and alanine. These peptides contain the cysteines needed to form disulphide bridges, which are used for joining separate peptide chains or linking two cysteines in the same chain. The significance of the disulphides in channel activity is not yet known. We are currently using NMR and ion channel-recording techniques to examine the physicochemical and structural properties of OaCNP-39(1-17) such as polarity, basicity, bulk, ability to hydrogen bond, structural propensities and conformational flexibility, and to relate these properties to the electrophysiological properties of the channel (P. Pallaghy & J. I. Kourie, unpublished data).
Pathophysiological significance of OaV-formed ion channels
Here we report that the C-type natriuretic peptide, which has been identified as OaCNP-39, a component of platypus venom, can function directly by forming novel voltage-gated cation-selective channels in artificial lipid bilayer membranes. The N-terminal fragment OaCNP-39(1-17) of this peptide is involved in forming this fast cation channel. Although we have characterized the biophysical properties of the fast cation-selective channel, its in vivo function remains to be determined. It is known that C-type natriuretic peptides are distributed widely in the mammalian central nervous system, the brain, endothelial cells, the lower part of the gastro-intestinal tract and the kidney (Sudoh et al. 1990; Komatsu et al. 1991; Suga et al. 1992; 1993). They form, together with ANP and BNP, a family of peptides, which exhibit potent natriuretic, diuretic, hypotensive and vaso-relaxant properties (Kojima et al. 1990; Sudoh et al. 1990; Komatsu et al. 1991; Suga et al. 1992; 1993; Hama et al. 1994; de Plater et al. 1995). There is evidence to suggest that C-type natriuretic peptides may act as potentially potent toxins. They are found in the venom of the South American pit viper (Bothrops jararaca) (Murayama et al. 1997). It has also been shown that the OaCNP-39 from platypus venom is associated with sustained tonic relaxation of the rat uterus in vitro (de Plater et al. 1998a, b). There is also evidence to suggest that C-type natriuretic peptides have a pathological role in cytokine-associated disorders, septic shock and renal failure (Suga et al. 1992; Murayama et al. 1997).
The molecular mechanisms underlying the action of these peptides are not well known. It is thought that they act via the ANPB receptor, particulate guanylate cyclase-B (Koller et al. 1991; Suga et al. 1992), leading to an increase in the level of cGMP (Stingo et al. 1992), which regulates ion transport pathways (Solomon et al. 1992; White et al. 1993; Wei et al. 1994; Kelley, Cotton & Drumm, 1997; 1998). In muscle cells, the cGMP regulation of ion transport pathways results in the inhibition of muscle contraction, vasodilatation and modification of fluid and electrolyte homeostasis (Sudoh et al. 1990; Morita et al. 1992; de Plater et al. 1995). The findings reported here indicate that, in addition to their known cGMP-mediated interaction with intrinsic ion transport mechanisms (see Kourie & Rive 1999), C-type natriuretic peptides may also exert their effects on signal transduction by directly forming ion transport pathways. This mechanism of action may explain the potent actions of these peptides. Ion channels are the most efficient ion transport pathways, capable of catalysing the permeation of 106 to 108 ions per second (Hille, 1992). The OaCNP-39-formed channels could mediate their effects via changes in Vm and second messenger systems (e.g. Ca2+ homeostasis). It is reasonable to propose that the findings reported here indicate that the symptoms of envenomation by platypus venom may be partly due to channel formation affecting signal transduction. The findings support previous suggestions that both the OaV-formed slow outward cation channel and the large conductance channel formed by CNP-22 may exert their pathological effects by rapidly depleting the ionic and osmotic gradients across the membrane (Kourie & Rive, 1999; Kourie, 1999).
In conclusion, data obtained using the lipid bilayer technique show that OaV and OaCNP-39, which is a major component peptide in platypus venom, form (in 250/50 mM KCl cis/trans) a TEACl-insensitive fast cation-selective channel. The N-terminal fragment (1-17 amino acids) of this 39 amino acid, C-type natriuretic peptide forms this channel. These findings are important to the development of strategies for therapies of clinical cases of both platypus venom-induced symptoms (e.g. pain and oedema) and CNP-associated pathologies.
Acknowledgments
I would like to thank Drs P. J. Milburn and G. M. de Plater for their encouragement and generous gift of platypus venom, OaCNP-39 and OaCNP-39(1-17). I also thank Mr R. McCart and Mr H. Wood for numerous discussions, suggestions and critical reading of the manuscript. The laboratory assistance of Mss A. Culverson, C. Horan and E. Sturgiss of the CSIRO Research Scheme is greatly appreciated. This research work is supported by a National Health and Medical Research Council project grant (no. 970122) and Australian Research Council Small Research Grant (F 99123).
References
- Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by alzheimer disease amyloid β-protein. Proceedings of the National Academy of Sciences of the USA. 1993;90:10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Catterall W. Cellular and molecular biology of voltage-gated sodium channels. Physiological Reviews. 1992;72:S15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. Fitting and statistical analysis of single-channel recording. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1983. pp. 135–175. [Google Scholar]
- De Plater GM. 1998. Fractionation, primary structural characterisation and biological activities of polypeptides from the venom of the platypus (Ornithorhynchus anatinus) PhD thesis. Australian National University, Canberra, Australia. [Google Scholar]
- De Plater GM, Martin RL, Milburn PJ. A pharmacological and biochemical investigation of the venom from the platypus (Ornithorhynchus anatinus) Toxicon. 1995;33:157–169. doi: 10.1016/0041-0101(94)00150-7. [DOI] [PubMed] [Google Scholar]
- De Plater GM, Martin RL, Milburn PJ. The natriuretic peptide (ovCNP) from platypus (Ornithorhynchus anatinus) venom relaxes the isolated rat uterus and promotes oedema and mast cell histamine release. Toxicon. 1998a;36:847–857. doi: 10.1016/s0041-0101(97)00176-1. [DOI] [PubMed] [Google Scholar]
- De Plater GM, Martin RL, Milburn PJ. A C-type natriuretic peptide from the venom of the platypus (Ornithorhynchus anatinus): structure and pharmacology. Comparative Biochemistry and Physiology. 1998b;C 120:99–110. doi: 10.1016/s0742-8413(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Eisenman G, Dani JA. An introduction to molecular architecture and permeability of ion channels. Annual Review of Biophysics and Biophysical Chemistry. 1987;16:205–226. doi: 10.1146/annurev.bb.16.060187.001225. [DOI] [PubMed] [Google Scholar]
- Fenner PJ, Williamson JA, Myers D. Platypus envenomation - a painful learning experience. Medical Journal of Australia. 1992;157:829–832. doi: 10.5694/j.1326-5377.1992.tb141302.x. [DOI] [PubMed] [Google Scholar]
- Gambale F, Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophysical Journal. 1988;53:771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama N, Itoh H, Shirakami G, Suga S, Komatsu Y, Yoshimasa T, Tanaka I, Mori K, Nakao K. Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochemical and Biophysical Research Communications. 1994;198:1177–1182. doi: 10.1006/bbrc.1994.1166. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. USA: Sinauer Associates; 1992. [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. The Journal of Physiology. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TJ, Cotton CU, Drumm ML. In vivo activation of CFTR-dependent chloride transport in murine airway epithelium by CNP. American Journal of Physiology. 1997;273:L1065–1072. doi: 10.1152/ajplung.1997.273.5.L1065. [DOI] [PubMed] [Google Scholar]
- Kelley TJ, Cotton CU, Drumm ML. Regulation of amiloride-sensitive sodium absorption in murine airway epithelium by C-type natriuretic peptide. American Journal of Physiology. 1998;273:L990–996. doi: 10.1152/ajplung.1998.274.6.L990. [DOI] [PubMed] [Google Scholar]
- Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analysis of a cDNA encoding precursor for rat C-type natriuretic peptide (CNP) FEBS Letters. 1990;276:209–213. doi: 10.1016/0014-5793(90)80544-s. [DOI] [PubMed] [Google Scholar]
- Koller KJ, Lowe DG, Bennett GL, Minamino W, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Nakao K, Suga S, Ogawa Y, Mukoyama M, Arai H, Shirakami G, Hosoda K, Nakagawa O, Hama N, Kishimoto I, Imura H. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology. 1991;129:1104–1106. doi: 10.1210/endo-129-2-1104. [DOI] [PubMed] [Google Scholar]
- Kourie JI. Vagaries of artificial bilayers and gating modes of the SCl channel from the sarcoplasmic reticulum of skeletal muscle. Journal of Membrane Science. 1996;116:221–227. [Google Scholar]
- Kourie JI. Synthetic mammalian C-type natriuretic peptide forms large cation channels. FEBS Letters. 1999;445:57–62. doi: 10.1016/s0014-5793(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Laver DR, Junankar PR, Gage PW, Dulhunty AF. Characteristic of two types of chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Biophysical Journal. 1996;70:202–221. doi: 10.1016/S0006-3495(96)79564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI, Rive MJ. Role of natriuretic peptides in ion transport mechanisms. Medicinal Research Reviews. 1999;19:75–94. doi: 10.1002/(sici)1098-1128(199901)19:1<75::aid-med4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mellor IR, Thomas DH, Sansom MS. Properties of ion channels formed by Staphylococcus aureus delta-toxin. Biochimca et Biophysica Acta. 1988;942:280–294. doi: 10.1016/0005-2736(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Merrill AR, Steer BA, Prentice GA, Weller MJ, Szabo AG. Identification of a chameleon-like pH-sensitive segment within the colicin E1 channel domain that may serve as the pH-activated trigger for membrane bilayer association. Biochemistry. 1997;36:6874–6884. doi: 10.1021/bi970222i. [DOI] [PubMed] [Google Scholar]
- Miller C, Racker E. Ca2+-induced fusion of fragmented sarcoplasmic reticulum with artificial planar bilayers. Journal of Membrane Biology. 1976;30:283–300. doi: 10.1007/BF01869673. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Morita H, Hagike M, Horiba T, Miyake K, Ohyama H, Yamaouchi H, Hosomi H, Kangawa K, Minamino N, Matsuo H. Effects of brain natriuretic peptide and C-type natriuretic peptide infusion on urine flow and jejunal absorption in anesthetized dogs. Japanese Journal of Physiology. 1992;42:349–353. doi: 10.2170/jjphysiol.42.349. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, Kambayashi K, Inouye K, Imura H. Brain natriuretic peptide as a novel cardiac hormone in humans: evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. Journal of Clinical Investigations. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama N, Hayashi M, Ohi H, Ferreira LAF, Hermann V, Saito H, Fujita Y, Higuchi S, Fernandes BL, Yamane T, de Camargo ACM. Cloning and sequence analysis of a Bothrops jararaca cDNA encoding a precursor of seven bradykinin - potentiating peptides and a C-type natriuretic peptide. Proceedings of the National Academy of Sciences of the USA. 1997;94:1189–1193. doi: 10.1073/pnas.94.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB. Measuring kinetics of complex single ion channel data using mean-variance histograms. Biophysical Journal. 1993;65:29–42. doi: 10.1016/S0006-3495(93)81041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Benz R, Just I, Aktories K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. Formation of cation-selective channels and inhibition of channel function by chloroquine. Journal of Biological Chemistry. 1994;269:16706–16711. [PubMed] [Google Scholar]
- Solomon R, Protter A, McEnroe G, Porter JG, Silva P. C-type natriuretic peptides stimulate chloride secretion in the rectal gland of Squalus acanthias. American Journal of Physiology. 1992;262:R707–771. doi: 10.1152/ajpregu.1992.262.4.R707. [DOI] [PubMed] [Google Scholar]
- Stingo AJ, Clavell AL, Arthus L, Burnett JC., Jr Cardiovascular and renal actions of C-type natriuretic peptide. American Journal of Physiology. 1992;262:H308–312. doi: 10.1152/ajpheart.1992.262.1.H308. [DOI] [PubMed] [Google Scholar]
- Sudoh T, Minamino N, Kenji K, Matsuo H. C-type natriuretic peptide (CNP): A new member of natriuretic peptide family identified in porcine brain. Biochemical and Biophysical Research Communications. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, Nakao K. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells: evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology. 1993;133:3038–3041. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, Imura H. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- Wei CM, Hu S, Miller VM, Burnett JCJ. Vascular actions of C-type natriuretic peptide in isolated porcine coronary arteries and coronary vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1994;205:765–771. doi: 10.1006/bbrc.1994.2731. [DOI] [PubMed] [Google Scholar]
- White RE, Lee AB, Shcherbatko AD, Lincoln TM, Schonbrunn A, Armstrong DL. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993;361:263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]


