Abstract
The cardiac effects of the NO donors sodium nitroprusside (SNP), S-nitroso-N-acetyl-penicillamine (SNAP) and 3-morpholino-sydnonimine (SIN-1) were studied in frog fibres to evaluate the contribution of cyclic GMP-dependent mechanisms.
SNP and SNAP (0·1-100 μM) reduced the force of contraction in a concentration-dependent manner in atrial and ventricular fibres. This effect was associated with a reduction in the time to peak (TTP) and the time for half-relaxation of contraction (T½).
SIN-1 (100 μM) also reduced the force of contraction in two-thirds of the atrial fibres. However, it exerted a positive inotropic effect in the remaining atrial fibres, as well as in most ventricular fibres.
The guanylyl cyclase inhibitor 1H-[1,2,4]oxidiazolo[4,3-a]quinoxaline-1-one (ODQ, 10 μM) antagonized the negative inotropic effects of SIN-1 (50 μM) and SNAP (25 μM) but had no effect on the positive inotropic response to SIN-1 (100 μM).
In the presence of SIN-1, superoxide dismutase (SOD, 50-200 U ml−1) either potentiated the negative inotropic effect or turned the positive inotropic effect of the drug into a negative effect. SOD had no effects when applied alone or in the presence of SNAP.
6-Anilino-5,8-quinolinedione (LY 83583, 3-30 μM), a superoxide anion generator also known as a cyclic GMP-lowering agent, exerted a positive inotropic effect, which was antagonized by SOD (200-370 U ml−1) but not by ODQ (10 μM).
We conclude that SNP, SNAP and SIN-1 exert cyclic GMP-dependent negative inotropic effects, which are attributed to the generation of NO. In addition, SIN-1 and LY 83583 exert cyclic GMP-independent positive inotropic effects, which require the generation of superoxide anion.
The endogenous production of nitric oxide (NO) within the heart plays an important role in the regulation of cardiac contractility, both under physiological and pathological conditions (Shah, 1996; Wolin et al. 1997). Several sources of NO have been identified. Constitutive NO synthase (NOS) activity is present in cardiac vascular and endocardial endothelial cells and cardiac intrinsic neurones as well as in cardiac myocytes (Kelly et al. 1996). A specific NOS isoform (iNOS or NOS2) is also induced in most cardiac cell types upon in vitro or in vivo challenge with extracellular messengers, such as pro-inflammatory cytokines (Kelly et al. 1996). The heterodimeric guanylyl cyclase appears to be one of the main targets for NO in the heart. Indeed, numerous studies have demonstrated the participation of cGMP in the contractile effects of NO (Kojda et al. 1996; Shah, 1996). The stimulation of cGMP production by NO leads in turn to the regulation of the activity of several proteins, including the cGMP-dependent protein kinase (cGMP-PK) and the cGMP-stimulated and cGMP-inhibited phosphodiesterases (PDE2 and PDE3), or to the regulation of the pacemaker If current (Iino et al. 1997; Méry et al. 1997; Musialek et al. 1997). In addition, NO may act in a cGMP-independent manner (Stamler, 1994; Crow & Beckman, 1995; Beckman & Koppenol, 1996). In the heart, thio-nitrosylation of the lateral chain of cysteine residues may play a role in the regulatory effects of NO on L-type Ca2+ channels (Campbell et al. 1996), creatine kinase (Gross et al. 1996) and ryanodine receptors (Xu et al. 1998). NO can also compete with oxygen for binding onto cytochrome oxidase (reviewed in Wolin et al. 1997).
Although NO-regulated targets have been identified in about every type of cardiac myocyte studied so far, the relative importance of each target may vary depending on the cardiac cell type and/or the animal species. For instance, NO was found to exert positive chronotropic effects in rat hearts (Kojda et al. 1997) and guinea-pig isolated nodal preparations (Musialek et al. 1997) and positive inotropic effects in rat isolated myocytes and multicellular cardiac preparations from rat and cat (Kojda et al. 1996, 1997; Mohan et al. 1996). However, NO was found to exert negative inotropic effects in papillary muscles or whole hearts and to abbreviate myocardial relaxation in various cardiac preparations (Meulemans et al. 1988; Brutsaert & Andries, 1992; Mohan et al. 1996; Shah, 1996). Moreover, a number of studies have demonstrated that authentic NO, NO donors and endogenous NO production had negligible effects on either single-cell shortening, papillary muscle contractility or whole-heart contractility and beating frequency (Kennedy et al. 1994; Weyrich et al. 1994; Nawrath et al. 1995; Wyeth et al. 1996; MacDonell & Diamond, 1995, 1997; Crystal & Gureviscius, 1996).
Part of this discrepancy may derive from the complexity of the biological chemistry of NO and the heterogeneous states of the cardiac preparations studied. Although NO radical is synthesized and released by NOS, the extent to which NO radical is indeed the final messenger is still an open question (Stamler, 1994). NO has been shown to undergo a large variety of bioconversions, both under in vitro and in vivo conditions, leading to the generation of a number of different compounds, including nitrosothiols, iron-nitrosyl complexes, peroxynitrite and nitrosotyrosine (Stamler, 1994; Butler et al. 1995; Crow & Beckman, 1995; Beckman & Koppenol, 1996). The occurrence of these NO derivatives, as well as their stability and metabolism, are greatly dependent on the experimental environment. For instance, the in vivo generation of peroxynitrite, from the combination of NO and superoxide anion, appears to occur primarily in the context of oxidative stress (Crow & Beckman, 1995; Beckman & Koppenol, 1996). In turn, peroxynitrite may aggravate the oxidative damage under these conditions. In contrast, in a reducing environment, peroxynitrite may itself become a source of NO and exert physiological effects unrelated to oxidation (Lizasoain et al. 1996; Mayer et al. 1998). Thus the heterogeneity of NO effects may be explained in part by the chemistry of the intermediates carrying the NO.
In an attempt to understand the functional diversity of NO donors in the heart, we have compared the effects in the same cardiac preparation of three NO donors possessing different chemical properties. The drugs used were sodium nitroprusside (SNP), S-nitroso-N-acetylpenicillamine (SNAP) and 3-morpholino-sydnonimine (SIN-1). SNAP is a naturally occurring nitrosothiol and as such shares chemical properties with a family of compounds found in living cells (Stamler, 1994; Butler et al. 1995; Crow & Beckman, 1995; Mayer et al. 1998). SNP and SNAP were shown to generate NO although the two drugs exhibit quite different mechanisms of NO release (Stamler, 1994; Butler et al. 1995). SIN-1 is unique in that it simultaneously generates superoxide anion and NO, the instantaneous combination of which gives rise to peroxynitrite (Feelisch et al. 1989; Crow & Beckman, 1995; Beckman & Koppenol, 1996; Mayer et al. 1998). However, in the presence of superoxide dismutase (SOD), which competes with NO binding on the superoxide anion, SIN-1 will become a NO donor (Crow & Beckman, 1995; Beckman & Koppenol, 1996). The effects of the NO donors were tested in frog heart since this preparation is devoid of a coronary system, thus allowing examination of the effects of the drugs in the absence of vascular changes (Page & Niedergerke, 1972). In frog atrial fibres, SNP was shown to exert a negative inotropic effect (Flitney et al. 1980) much larger than that of glycerol trinitrate, another NO donor (Nawrath et al. 1995). In contrast, SIN-1 had no effect on this preparation (Nawrath et al. 1995). This last finding is surprising since: (1) SIN-1 and SNP regulate the L-type Ca2+ current in frog ventricular myocytes in a cGMP-dependent manner (Méry et al. 1993, 1997) and (2) the contraction of frog heart is highly dependent upon calcium influx through the L-type Ca2+ channels (Morad & Cleemann, 1987). To examine whether tissue differences may explain this discrepancy, we compared the effects of the three NO donors in atrial and ventricular fibres. For comparison, we have also characterized the effect of LY 83583, a superoxide anion generator also known as a cGMP-lowering agent (see Abi Gerges et al. 1997 for a review).
A preliminary report of part of this work has appeared in abstract form (Chesnais et al. 1997).
METHODS
The investigation conformed with the European Community and French national guidelines in the care and use of animals.
Contraction recordings
Frogs (Rana esculenta) were killed by decapitation and double pithed. Thin atrial or ventricular fibres (150-300 μm in diameter, 2-4 mm length) were dissected out from the endocardial surface of the hearts. We selected fibres well individualized from the mass of the heart, in which no branching occurred except at the extremities. Only those fibres exhibiting a minimal attachment (< 30 %) to the rest of the tissue were studied. Since isolation of atrial fibres mostly consisted of dissecting their extremities, most of the endocardial surface of the fibres was intact (see Page & Niedergerke, 1972). In the ventricle, trabeculae of the desired size are difficult to find so that most of the preparations were obtained after longitudinal dissection of the papillary muscles. Following dissection, the fibres were pinned at one end to the bottom of a perfusion chamber and hooked at the other end with a fine silk thread to the force transducer (model AE 801, SensoNor, Horten, Norway). Before each experiment, the resting fibre length was set by releasing the muscle just enough so there was no resting tension and then increasing the length by 20 %. Because of their low stiffness, atrial fibres were stretched in a more progressive manner than ventricular fibres and the equilibration period was generally longer in atrial compared with ventricular fibres. Muscles were allowed to equilibrate to a steady-state isometric active contraction amplitude in the control Ringer solution for 30 to 60 min, during which minor modifications of the passive tension were observed. In the present study (99 atrial and 118 ventricular fibres), the amplitude of the passive tension (atria: 3·68 ± 0·25 mg; ventricle: 10·99 ± 0·77 mg) was well below the amplitude of the active tension (atria: 27·64 ± 2·12 mg; ventricle: 52·83 ± 2·78 mg). Fibres in which active tension was less than twice the passive tension were discarded. All experiments were performed at room temperature (19-26°C). In long-lasting experiments, room temperature could eventually drift (increase) with a slow time course, but never by more than 2°C, accounting for a slow decrease in the active tension.
Field stimulation was applied with platinum electrodes at a constant frequency of 0·2 Hz, the amplitude being adjusted to 10 % above threshold value. The volume of the perfusion chamber (150 μl) was kept constant throughout an experiment in order to avoid modifications of the tension due to fluid level variations. The solutions were perfused by gravity into the chamber. The perfusion rate was > 3 ml min−1, allowing a complete renewal of the bathing solution in less than 80 s.
Solutions
Control Ringer solution contained (mM): 88 NaCl; 0·513 NaH2PO4; 2·5 KCl; 1·8 MgCl2; 1·8 CaCl2; 2·4 NaHCO3; 5 glucose; 5 sodium pyruvate; 5 creatine; 10 Hepes, adjusted to pH 7·4 with NaOH. Working solutions were made fresh daily. 1H-[1,2,4]oxidiazolo[4,3-a]quinoxaline-1-one (ODQ, 100 mM) was prepared in dimethylsulphoxide (DMSO). DMSO (up to 0·1 μl ml−1) had no effect on the contraction of frog cardiac fibres (data not shown). All other agents tested were diluted in control Ringer solution < 10 min before application onto the fibre studied, i.e. only fresh solutions were tested. All solutions were protected from natural light.
Data analysis
During contraction experiments, the recordings were displayed simultaneously on an oscilloscope and on a chart recorder. Isometric active tension, passive tension, time to peak (TTP) and half-relaxation time (T½) were measured on-line (sampling frequency 0·5-2 kHz) and stored on a PC-compatible microcomputer programmed in Pascal language (Microsoft). Individual contraction traces could also be stored on line for further analysis.
The results are expressed as means ±s.e.m. Differences between means were tested for statistical significance by Student's paired t test. The effects of the compounds used, alone or in combination, are measured as percentage variations over the basal level. Calculations of the effects of agonists were corrected for the spontaneous course of the baseline level, when present. The resting tension was unaffected by the various treatments reported here.
Drugs
SIN-1 and SIN-1C were generous gifts from Dr J. Winicki (Hoechst Houdé Laboratories, La Défense, France). LY 83583 was a gift from E. Lilly Pharmaceuticals (Indianapolis, IN, USA), or Calbiochem. SNAP was from Calbiochem or Tocris Cookson. ODQ was from Tocris Cookson. Superoxide dismutase (SOD, from erythrocytes) and xanthine oxidase (from buttermilk) were from Sigma Chemical Co. All other drugs were from Sigma Chemical Co.
RESULTS
Contractile effects of SNP and SNAP in frog atrial fibres
Figure 1 shows typical experiments in which the effects of two NO donors, SNP (Fig. 1A) and SNAP (Fig. 1B), were tested on the contractility of frog atrial fibres. Both compounds induced a reduction in the amplitude of contraction, with an ∼80 % inhibition observed at 100 μM concentration. At this concentration, the two drugs also modified the kinetics of contraction, reducing both the time to peak (TTP) and the time for half-maximal relaxation of contraction (T½).
Figure 1. Contractile effects of SNP and SNAP in frog atrial fibres.
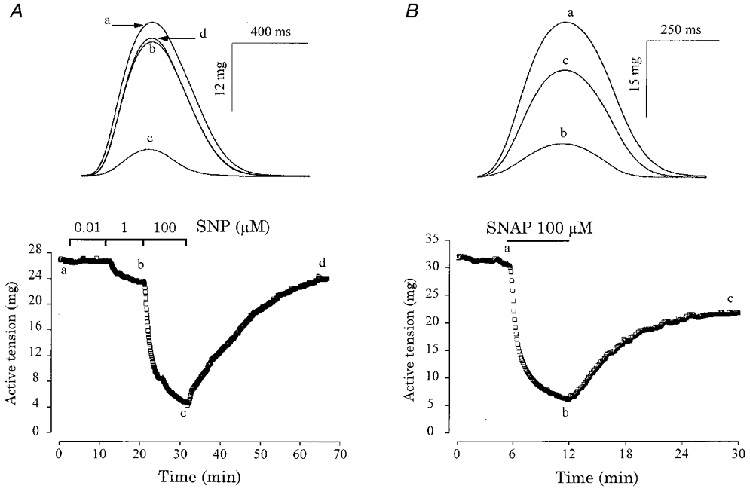
A and B, atrial fibres were initially superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. Superfusion of the fibres with SNP (0·01, 1 and 100 μM) in A or with SNAP (100 μM) in B is indicated by the lines. Top panels, individual contractions were recorded at the times indicated by the corresponding letters on the main graphs.
The results of several similar experiments are summarized in Fig. 2. SNP and SNAP produced concentration-dependent negative inotropic effects in frog atrial fibres, with a threshold concentration in the range of 0·1-1 μM (Fig. 2A). At the highest concentration tested (100 μM), the inhibitory effect of SNAP (-73·8 ± 2·9 % over control, mean ±s.e.m., n= 17, P < 0·005) was higher than that of SNP (-55·7 ± 7·2 %, n= 15, P < 0·005vs. control, P < 0·05vs. SNAP). The negative inotropic effects of SNP and SNAP were accompanied by significant accelerations in the relaxation phase, as seen by the concentration-dependent reduction in T½ (Fig. 2B). Furthermore, SNAP and SNP (at 100 μM concentration) also reduced TTP by, respectively, 8·9 ± 2·8 % (n= 17, P < 0·005) and 8·4 ± 2·0 % (n= 15, P < 0·0001).
Figure 2. Summary of the effects of SNP and SNAP in frog fibres.
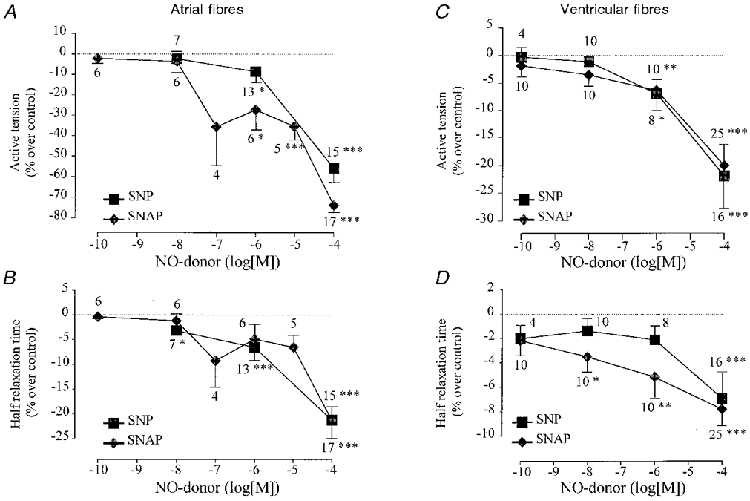
A-D summarize the effects of SNP (▪) and SNAP (♦) on the active tension (A and C) and on the half-relaxation time (T½) (B and D) of atrial (A, B) and ventricular fibres (C, D), in experiments similar to those of Fig. 1. Active tension and half-relaxation time in the presence of SNP and SNAP were normalized to their respective amplitudes in the absence of the drug. The symbols and the lines are the means ±s.e.m. of the number of experiments indicated near the lines. Statistical differences from the control level are indicated as: *P < 0·05; **P < 0·01; ***P < 0·005.
To investigate whether the inotropic effects of SNAP and SNP were mediated by NO rather than by some by-product(s) co-released with NO, we examined the effect of ferrocyanide ion, which is released by SNP, and N-acetyl-penicillamine, which is released by SNAP. In three experiments, 1 μM and 100 μM ferrocyanide ion had negligible effects on the force of contraction (+1·0 ± 4·6 % and -6·7 ± 3·4 % variation, respectively), TTP (-3·5 ± 1·1 % and -3·0 ± 1·1 %, respectively) and T½ (-3·2 ± 1·2 % and -6·9 ± 2·2 %, respectively). Similarly, in four other experiments, 100 μM N-acetyl-penicillamine barely affected peak tension (-10·3 ± 5·2 %), TTP (-2·0 ± 0·9 %) and T½ (-1·9 ± 2·1 %). Thus, the inotropic effects of SNAP and SNP in frog atrial fibres most probably involved the liberation of NO.
Contractile effects of SNP and SNAP in frog ventricular fibres
To investigate whether NO donors exert different inotropic effects in different cardiac tissues, we examined the effects of SNP and SNAP in ventricular tissue. Figure 2C shows that both drugs produced concentration-dependent inhibitory effects, which were essentially identical for the two drugs both in terms of efficacy and potency. These effects were accompanied by a progressive acceleration in the relaxation phase (Fig. 2D) and a 4-8 % reduction in TTP (not shown). Thus, the inotropic effects of SNP and SNAP are qualitatively similar in ventricular and atrial fibres. However, at any given SNP or SNAP concentration, ventricular fibres responded ∼2-fold less than atrial fibres to the NO donors, both in terms of peak tension and kinetics of contraction. As in atrial fibres, we checked for the effects of ferrocyanide ion (1 and 100 μM, 3 experiments) and N-acetyl-penicillamine (100 μM, 5 experiments) in frog ventricular fibres but these compounds had no significant effect on either peak tension, TTP or T½ (not shown).
Contractile effects of SIN-1 in frog atrial fibres
We next tested the effects of SIN-1, another classically used NO donor, on the force and kinetics of contraction in frog atrial fibres. As shown in Fig. 3A and B, SIN-1 produced two types of effects. In the experiment shown in Fig. 3A, a low concentration of SIN-1 (10 nM) slightly increased peak tension while a larger concentration (100 μM) produced a net inhibition. However, this inhibitory effect was ∼2-fold smaller than that of SNP (or SNAP, not shown) used at the same concentration and tested after washout of SIN-1. In the experiment of Fig. 3B, SIN-1 produced only positive inotropic effects, both at 1 and 100 μM, which were fully reversible upon washout of the drug. Several similar experiments were performed and the results, in terms of variations in peak tension and T½, are summarized in Fig. 3C and D, respectively. When all the results were pooled together (symbols in Fig. 3C), the mean inotropic effects of SIN-1 appeared not to be statistically significant. However, the bimodal response of SIN-1 illustrated in Fig. 3A and B allowed us to separate the experiments into two groups, those with positive inotropic effects (upper bars) and those with negative inotropic effects (lower bars). Thus, at 100 μM concentration, SIN-1 exerted a 28·4 ± 3·5 % inhibition in peak tension in 28 out of 40 atrial fibres, i.e. in about two-thirds of experiments. This effect was ∼50 % smaller than that of the same concentration of SNP or SNAP (Fig. 2A) and was also accompanied by a net acceleration in the relaxation phase of contraction (filled bar, Fig. 3D) and a small (< 4 %) reduction in TTP (not shown). However, in about a third of the fibres (12 out of 40), 100 μM SIN-1 exerted a robust positive inotropic effect (+52·7 ± 11·7 % increase in peak tension), which was not accompanied by any significant alteration in the kinetics of contraction (open bar in Fig. 3D, for T½).
Figure 3. Contractile effects of SIN-1 in frog atrial fibres.
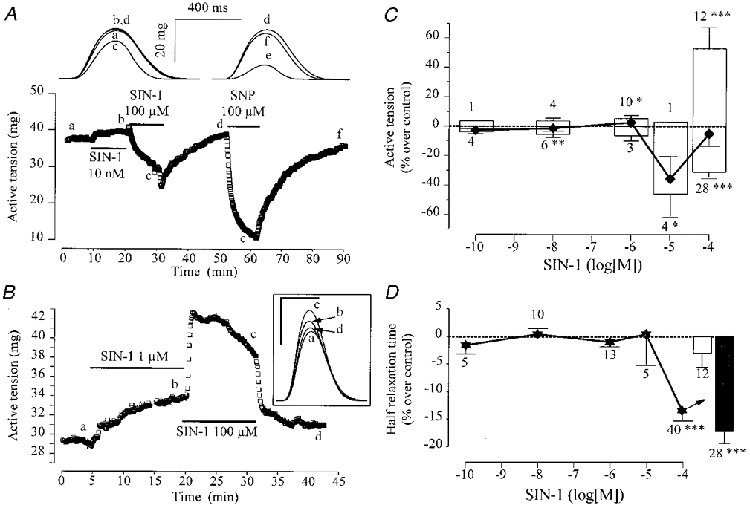
In A and B, atrial fibres were first superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. The application of SIN-1 or SNP is indicated by the lines. The individual traces shown in A (upper part) and B (inset) were recorded at the times shown by the corresponding letters on the main graphs. In the inset to B, the scale bars are: horizontal, 400 ms; vertical, 20 mg. C summarizes the effects of SIN-1 (♦) on the active tension of atrial fibres in independent experiments. The active tension in the presence of SIN-1 was normalized to the amplitude of the active tension in the absence of the drug. Positive and negative (open bars, up or down) inotropic effects were dissociated. D summarizes the effects of SIN-1 on the half-relaxation time in atrial fibres (same experiments as in C). Half-relaxation times in the presence of SIN-1 were normalized to their respective amplitudes in the absence of the drug in fibres where SIN-1 exerted positive (open bar) or negative inotropic effects (filled bar). In C and D, symbols and bars are the mean values and the lines are the s.e.m. of the number of experiments indicated near the lines. Statistical differences from the control level are indicated as: *P < 0·05; **P < 0·01; ***P < 0·005.
Upon release of NO, SIN-1 is transformed into SIN-1C, which might exert effects of its own. Thus, we tested the effect of SIN-1C on the contractility of frog atrial fibres. However, in five individual experiments, SIN-1C (100 μM) had no effect on peak tension (+2·9 ± 5·1 % variation), TTP (-1·1 ± 0·9 %) or T½ (-2·2 ± 1·3 %).
Contractile effects of SIN-1 in frog ventricular fibres
The quantitative differences in the inotropic responses to SNP and SNAP between atrial and ventricular tissues prompted us to compare the effects of SIN-1 in both tissues. Figure 4A shows a typical experiment performed in a frog ventricular fibre. While SNP (100 μM) produced a net and reversible negative inotropic effect as shown above, the same concentration of SIN-1 produced a strong and reversible increase in peak tension. However, as shown in Fig. 4B, lower concentrations produced negligible effects. At 100 μM concentration, SIN-1 increased the force of contraction by, on average, ∼20 % in 82 out of 85 experiments (Fig. 4B). This effect was accompanied by a small but significant prolongation of the relaxation phase (upper open bar in Fig. 4C). In the three remaining fibres, SIN-1 induced a negative inotropic response (Fig. 4B) and an acceleration in the relaxation phase (lower open bar in Fig. 4C), which were both ∼2-fold smaller than in atrial fibres (compare Figs 3 and 4). As in atrial fibres, SIN-1C (100 μM, 3 experiments) had no effect on either peak tension, TTP or T½ (not shown). Thus, the responses to SIN-1 were qualitatively similar in frog atrial and ventricular fibres in that both tissues responded to only high concentrations (100 μM), which induced either positive or negative inotropic effects. However, both effects were ∼2-fold smaller in ventricular compared with atrial fibres. Besides, 96 % of the fibres in the ventricle, compared with 30 % in the atria, elicited a positive inotropic response to SIN-1.
Figure 4. Contractile effects of SIN-1 in frog ventricular fibres.
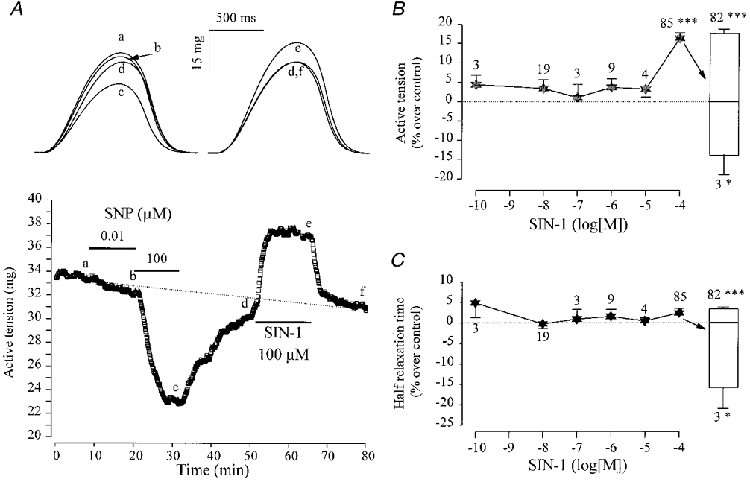
A, a ventricular fibre was superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. Applications of SNP (0·01 μM and 100 μM) and SIN-1 (100 μM) were performed as indicated by the lines. The individual traces shown in the upper part of A were recorded at the times indicated by the corresponding letters on the main graph. B summarizes the effects of SIN-1 on the active tension of ventricular fibres in separate experiments. The active tension in the presence of SIN-1 was normalized to the amplitude of the active tension in the absence of the drug. C summarizes the effects of SIN-1 on the half-relaxation time in ventricular fibres (same experiments as in B). Half-relaxation time in the presence of SIN-1 was normalized to its amplitude in the absence of the drug. In B and C, the symbols and lines are the means ±s.e.m. of the number of experiments indicated near the lines. Positive (upper bars) and negative (lower bars) inotropic effects were dissociated. Statistical differences from the control level are indicated as: *P < 0·05; ***P < 0·005.
Role of guanylyl cyclase in the inotropic effects of NO donors
From the results above, it appears that different NO donors may exert different inotropic effects in frog cardiac fibres. In particular, the effects of SIN-1 clearly differed from those of SNAP and SNP in that SIN-1 was able to produce two opposite responses, positive and negative, while the effects of SNAP and SNP were always negative. In the following, our aim was to get some insights into the mechanisms involved in these responses. We first examined the role of guanylyl cyclase in the effects of NO donors using ODQ, a direct guanylyl cyclase inhibitor. ODQ was recently shown to compete with the NO-dependent activation of the enzyme (Schrammel et al. 1996) and to antagonize the effect of NO donors on the cardiac L-type Ca2+ current (Abi Gerges et al. 1997).
In the experiment of Fig. 5A, a frog atrial fibre was first exposed to SNAP (25 μM), which induced a large negative inotropic effect. Further application of 10 μM ODQ in the continuing presence of SNAP totally abolished the effect of the NO donor. Similarly, ODQ also abolished the negative inotropic response to SIN-1 (Fig. 5B). In this experiment, application of SIN-1 (50 μM) to an atrial fibre produced a transient stimulatory effect followed by a sustained inhibition of peak tension. Upon application of ODQ (10 μM), the contraction amplitude recovered fully and even increased above the control level. Several similar experiments were performed and are summarized in Fig. 5D. While ODQ (10 μM) alone had no effect on contractility, either in atrium (n= 5) or ventricle (n= 4), the drug fully antagonized the inhibitory responses to either SNAP (25 μM) or SIN-1 (50 or 100 μM) in both tissues. In contrast, ODQ was found to have no effect on the positive inotropic response to SIN-1. Indeed, Fig. 5C shows a typical experiment in which SIN-1 (100 μM) produced a stimulatory effect on the contractility of a frog ventricular fibre which was barely affected by the addition of ODQ (10 μM) to the external solution. Similar results were obtained in a total of eight experiments and are summarized in Fig. 5D. Altogether, these results demonstrate that the negative inotropic effect of NO donors is due to activation of guanylyl cyclase. However, the positive inotropic effect of SIN-1 appeared to involve a cGMP-independent mechanism.
Figure 5. Effect of ODQ on the contractile response of frog fibres to NO donors.
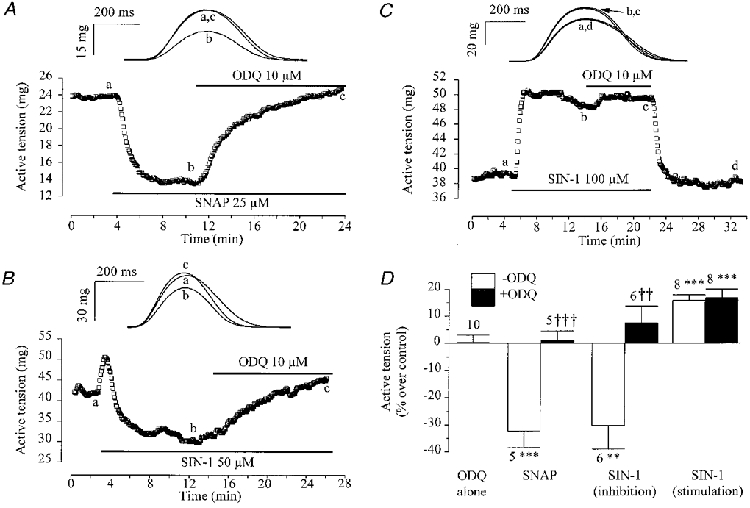
A-C, typical experiments performed with atrial fibres first superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. ODQ (10 μM) was applied in the presence of 25 μM SNAP (A), or SIN-1 (50 μM in B, 100 μM in C), as indicated by the lines. The individual traces (upper panels) were recorded at the times indicated by the corresponding letters on the main graphs. D summarizes the effects of ODQ (10 μM, filled bars) on the active tension in frog fibres (pooled data) when applied alone, or in the presence of SNAP (25 μM) or SIN-1 (50 or 100 μM). The effects of NO donors alone are shown (open bars). Positive (6 ventricular plus 2 atrial fibres) and negative (6 atrial fibres) inotropic effects of SIN-1 are represented separately. The active tension in the presence of the drugs was normalized to the respective amplitude in the absence of the drugs. Bars and lines are the means ±s.e.m. of the number of experiments indicated near the lines. Statistical differences from the control level are indicated as: **P < 0·01; ***P < 0·005. Differences between groups in the absence and presence of ODQ are indicated as: ††P < 0·01; †††P < 0·005.
Role of superoxide anion in the inotropic effects of NO donors
Unlike SNP or SNAP, SIN-1 releases superoxide anion in addition to NO (Feelisch et al. 1989; Crow & Beckman, 1995; Beckman & Koppenol, 1996; Mayer et al. 1998). Thus, an attractive hypothesis is that generation of superoxide anion participates in the positive inotropic effect of SIN-1. To test this hypothesis, we examined the effect of SIN-1 in the presence of superoxide dismutase (SOD), the superoxide anion scavenger (Beckman & Koppenol, 1996).
When applied alone, SOD (50-200 U ml−1, 17 ventricular and 16 atrial fibres) did not significantly modify the active tension (-2·4 ± 1·0 % and 2·4 ± 2·1 %, respectively), the time to peak (-0·5 ± 0·5 % and -0·5 ± 0·6 %) or the half-relaxation time (-3·7 ± 1·7 % and -0·4 ± 1·1 %). However, in both tissues, application of SOD strongly modified the contractile effects of SIN-1. Figure 6A shows a typical experiment performed in a frog atrial fibre, in which SIN-1 alone produced a positive inotropic effect at 100 μM concentration (see above). The positive inotropic effect of 100 μM SIN-1 was transformed into a large negative effect by the addition of 50 U ml−1 SOD. Figure 6B summarizes the results of similar experiments performed in atrial fibres. The diagram shows that while only six out of nine fibres responded negatively to 100 μM SIN-1 under control conditions, all seven fibres tested responded by a strong decrease in peak tension when SIN-1 was applied in the presence of SOD. Under this condition, the negative inotropic effect was comparable to that produced by 100 μM SNAP alone or in the presence of SOD. Similarly, SOD also potentiated by ∼2-fold the accelerating effect of 100 μM SIN-1 on the contraction kinetics, reducing T½ (Fig. 6C) and TTP (data not shown) to values which were comparable to those obtained by the same concentration of SNAP. Moreover, most atrial fibres responded to 1 μM SIN-1 in the presence of SOD, while a 10- to 100-fold higher concentration was necessary to elicit a response when SIN-1 was used alone (Fig. 6B). Thus, in frog atrial fibres, SOD either potentiates the negative inotropic effect of SIN-1 or changes the positive inotropic effect into a negative one. The presence of SOD also induced an increase in the sensitivity of the contractile response to SIN-1. In contrast, SOD does not modify the inhibitory effects of SNAP (Fig. 6B and C) and SNP (0·1 nM to 100 μM, data not shown).
Figure 6. Effect of SOD on the contractile response of frog atrial fibres to NO donors.
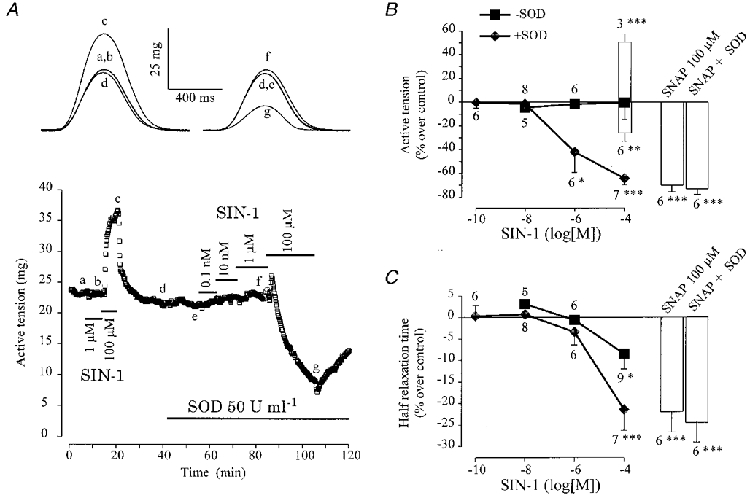
A, an atrial fibre was initially superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. SIN-1 was applied alone or in the presence of SOD (50 U ml−1), as indicated by the lines. The individual traces were recorded at the times indicated by the corresponding letters on the main graph. B and C summarize the effects of SIN-1 (symbols and open bars) on the active tension (B) and the half-relaxation time (C), in the absence (▪) or presence of 50 U ml−1 SOD (♦). Positive and negative inotropic effects of 100 μM SIN-1 are presented separately (bars, mean values; lines, s.e.m.). The effects of SNAP (100 μM) in the absence or presence of SOD (50-200 U ml−1) are also summarized (bars, mean effects; lines, s.e.m.). Active tension and half-relaxation time in the presence of the drugs was normalized to the respective amplitude in the absence of the drug. Statistical differences from the control level are indicated as: *P < 0·05; **P < 0·01; ***P < 0·005.
Next, we examined the effect of SOD in ventricular fibres where SIN-1 induces a mainly positive inotropic response. In the experiment shown in Fig. 7A, SIN-1 (100 μM) induced a large positive inotropic effect under control conditions. However, addition of 50 U ml−1 SOD transformed this positive effect into a negative one, which was further increased when the concentration of SOD reached 200 U ml−1. Figure 7B summarizes the results of several similar experiments in which the effect of 100 μM SIN-1 was tested in the absence or presence of increasing concentrations of SOD. In each of the ventricular fibres tested, SIN-1 alone produced a positive inotropic effect (Fig. 7B), associated with negligible modifications in the kinetics of contraction (not shown). In the presence of SOD, the average contractile response (▪) of the fibres to SIN-1 was progressively changed into a negative inotropic effect. The histograms summarize the mean amplitude and the number of positive and negative responses at each SOD concentration. They indicate that the relative proportion of negative responses increased with the concentration of SOD, although the amplitude of the response was essentially independent of the concentration. Unlike the effects of SIN-1 alone, the inhibitory effect of 100 μM SIN-1 in the presence of SOD (50-200 U ml−1) was accompanied by a decrease in both TTP (not shown) and T½ (Fig. 7C). These changes in kinetics were similar to those obtained with similar concentrations of SNAP (Fig. 7C) or SNP (not shown), both in the absence and presence of SOD.
Figure 7. Effect of SOD on the contractile response of frog ventricular fibres to NO donors.
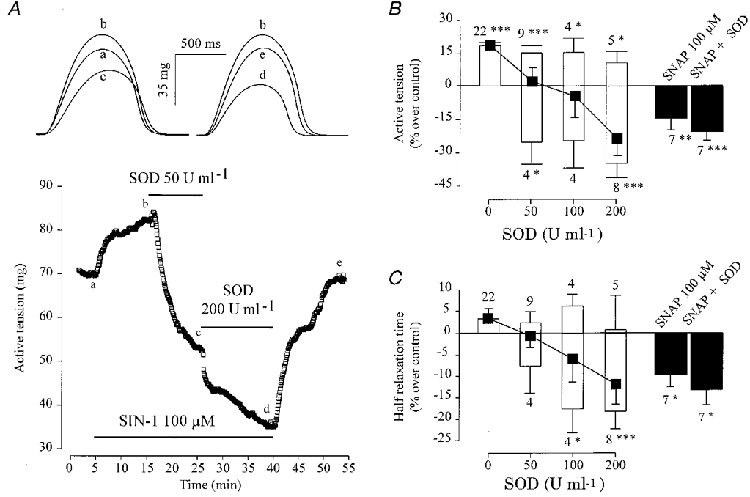
A, a ventricular fibre was first superfused with control solution. Each symbol is the measurement of the maximal active tension, every 5 s. SIN-1 (100 μM) was applied in the absence or presence of SOD (50 or 200 U ml−1), as indicated by the lines. The individual traces shown in the upper part of A were recorded at the times indicated by the corresponding letters on the main graph. B and C summarize the effects of SIN-1 on the active tension (B) and the half-relaxation time (C) in the absence or presence of 50, 100 or 200 U ml−1 SOD (▪). Positive and negative inotropic effects of 100 μM SIN-1 are presented separately (bars, mean values; lines, s.e.m.). Symbols indicate the means, and lines the s.e.m. of the overall effects of SIN-1. The effects of SNAP (100 μM) in the absence or presence of SOD (50-200 U ml−1) are also summarized (filled bars, mean effects; lines, s.e.m.). Active tension and half-relaxation time in the presence of the drugs were normalized to their respective values in the absence of the drugs. Statistical differences from the control level are indicated as: *P < 0·05; **P < 0·01; ***P < 0·005.
Both in atrial and ventricular fibres, the effect of SOD appeared to be specific to SIN-1 since SOD did not modify the contractile response to SNAP (Figs 6B and 7B) or SNP (not shown) at any concentration tested. This specificity of action suggests that endogenous superoxide anion does not mediate the effect of SOD. Rather, it indicates that SIN-1, unlike the two other NO donors, generates superoxide anion which contributes to the overall inotropic effect of SIN-1 in frog cardiac muscle.
Contractile effects of LY 83583, a superoxide anion generator
To examine this hypothesis further, we tested the effects of LY 83583, a superoxide anion generator (reviewed in Abi Gerges et al. 1997). Application of LY 83583 alone produced a positive inotropic effect both in atrial and ventricular fibres. A typical experiment is shown in Fig. 8A. Application of 30 μM LY 83583 to this ventricular fibre produced a net increase in peak tension, about twice as large as the stimulatory effect of SIN-1 (100 μM). On average (Fig. 8B), 10-30 μM LY 83583 produced a similar 20-30 % positive inotropic effect in atrial and ventricular fibres. This effect was accompanied by a slight prolongation of the contraction, as evidenced by an increase in TTP and T½ (data not shown). Thus, LY 83583 mimicked the stimulatory effects of SIN-1. However, LY 83583 is not only a superoxide anion generator but has also been described as a guanylyl cyclase inhibitor (reviewed in Abi Gerges et al. 1997). Therefore, additional experiments were needed to examine the respective contribution of these two mechanisms to the overall effects of the drug.
Figure 8. Contractile effects of LY 83583, the superoxide anion generator.
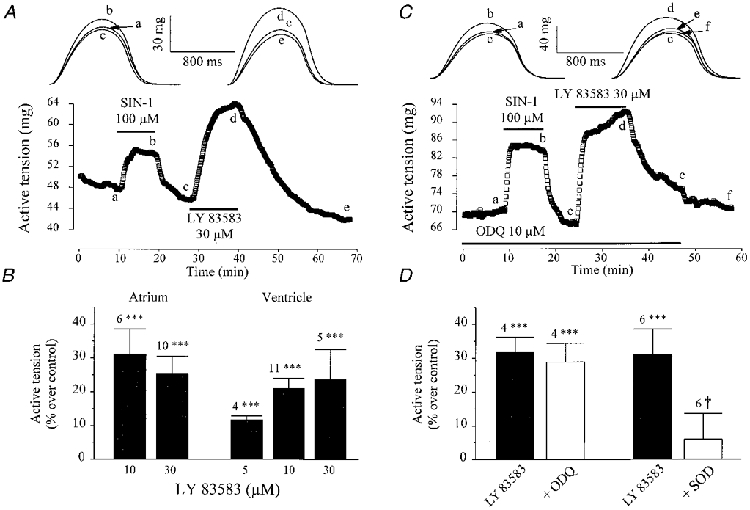
A, a ventricular fibre was first superfused with the control solution. Each symbol is the measurement of the maximal active tension, every 5 s. SIN-1 (100 μM) and LY 83583 (30 μM) were applied as indicated by the lines. C, same as in A, except that SIN-1 and LY 83583 were applied in the presence of ODQ (10 μM). The individual traces shown in the upper part of A and C were recorded at the times indicated by the corresponding letters on the main graphs. B summarizes the effects of different concentrations of LY 83583 in atrial and ventricular fibres. D summarizes the effects of LY 83583 (30 μM) in the absence (filled bars) or presence (open bars) of ODQ (10 μM), or SOD (200-370 U ml−1) in atrial and ventricular fibres (pooled data). Active tension in the presence of the drugs was normalized to its value in the absence of the drug(s). Bars and lines are the means ±s.e.m. of the number of experiments indicated near the lines. Statistical differences from the control level are indicated as ***P < 0·005. Difference between groups in the absence and presence of SOD is indicated as †P < 0·05.
As shown above, the effects of LY 83583 were not mimicked by ODQ, another guanylyl cyclase inhibitor, since ODQ alone had no effect on contractility. Moreover, in the ventricular fibre of Fig. 8C, both LY 83583 (30 μM) and SIN-1 (100 μM) induced strong stimulatory effects in the presence of ODQ (10 μM). This result is typical of four experiments (Fig. 8D). On the contrary, as summarized in Fig. 8D, SOD (200-370 U ml−1) counteracted the positive inotropic effects of LY 83583 (30 μM). Furthermore, the stimulatory effect of LY 83583 was mimicked by exposure of frog cardiac fibres to another superoxide anion generating system (100 μM xanthine plus 10 mU ml−1 xanthine oxidase, data not shown). Altogether, these experiments demonstrate that both LY 83583 and SIN-1 induce positive inotropic effects in a superoxide anion-dependent and cGMP-independent manner in frog cardiac fibres.
DISCUSSION
We have examined the contractile effects of three NO donors, SNP, SNAP and SIN-1, in frog cardiac fibres. We found that SNP and SNAP exert only negative inotropic effects, while SIN-1 can give rise to either positive or negative effects on cardiac contractility. The occurrence of these opposite effects was not related to any obvious morphological (diameter, length) or functional (active tension amplitude, resting tension amplitude, kinetics of contraction) property of the fibres. The negative inotropic effect of all NO donors required the activation of a NO-sensitive guanylyl cyclase. In contrast, the positive inotropic effect of SIN-1 appeared unrelated to cGMP production but involved the superoxide anion. Thus, the chemical properties of the NO donor determine the overall effect on cardiac contractility. In addition, atrial tissue appeared more sensitive than ventricular tissue to the negative inotropic effect of the drugs, while positive inotropic effects of SIN-1 were more consistently found in ventricular than atrial tissues, indicating that intrinsic factors also govern the macroscopic response to NO derivatives.
Negative inotropic effect of NO donors
In frog cardiac fibres, SIN-1, SNP and SNAP were able to exert a negative inotropic effect, which was accompanied by a reduction in the time to peak and the half-time of relaxation. A similar effect of NO donors was reported in mammalian cardiac muscle (Brutsaert & Andries, 1992; Shah, 1996; Flesch et al. 1997). This negative inotropic effect was not mimicked by the by-products of the NO donors (SIN-1C, ferrocyanide ion and N-acetyl-penicillamine). Since the only common property of the three drugs used in this study is to release NO, it is reasonable to assume that NO mediates the negative inotropic effect. At the largest concentrations used (100 μM), the negative inotropic effect of SIN-1 was ∼2-fold smaller than that of SNAP or SNP. However, application of SOD enhanced the inhibitory effect of SIN-1 to a level which became comparable to that observed with SNAP or SNP. SOD is well recognized as catalysing the dismutation of superoxide anion into hydrogen peroxide (see Beckman & Koppenol, 1996). An accumulation of hydrogen peroxide might participate in the effect of SOD (Mao et al. 1993; Yim et al. 1993). However, this oxygen species exerted a positive inotropic effect in frog fibres (data not shown), opposite to the effect of SOD in the presence of SIN-1. Accordingly, catalase did not modify the effects of SOD (authors’ unpublished observations). A confounding effect of the Cu+ ion, located in the active site of SOD, was also ruled out because Cu+ ions (10 μM CuSO4) did not mimic the effects of SOD (data not shown). Taken together, these findings suggested that the negative inotropic effect of SIN-1 was attenuated by a simultaneous release of superoxide anion (see Feelisch et al. 1989; Beckman & Koppenol, 1996).
The negative inotropic effects of NO donors were antagonized by ODQ, an inhibitor of the NO-sensitive guanylyl cyclase (Schrammel et al. 1996). Indeed, NO donors increase guanylyl cyclase activity and cGMP levels in frog heart (see Flitney et al. 1980; Méry et al. 1993; Abi Gerges et al. 1997). Furthermore, SIN-1, SNP and SNAP inhibited the L-type Ca2+ current in a cGMP-dependent and ODQ-sensitive manner, in isolated frog ventricular myocytes (Méry et al. 1993; Abi Gerges et al. 1997 and references therein). Recently, ODQ, like Methylene Blue, appeared to ‘display properties other than inhibition of soluble guanylyl cyclase’ in rat aorta (Muller et al. 1998 and references therein). However, ODQ did not mimic the cGMP-independent effects of Methylene Blue in isolated frog myocytes (Abi Gerges et al. 1997). Furthermore, ODQ had no effects on the basal contraction in frog fibres (this study), while Methylene Blue increased the active and the passive tensions in this preparation (data not shown). Altogether, our findings support the view that ODQ was acting as a guanylyl cyclase inhibitor in the frog heart.
Positive inotropic effect of SIN-1 in cardiac fibres
SIN-1 produced a positive inotropic effect in one-third of atrial fibres and in most of the ventricular fibres with little or no changes in the kinetics of contraction. The positive inotropic effect of SIN-1 was not antagonized by ODQ, suggesting that this effect did not require cGMP production (see Schrammel et al. 1996). For several reasons, we believe that the positive inotropic effect of SIN-1 involved superoxide anion. First, SIN-1, unlike SNP and SNAP, can generate superoxide anion when dissolved in physiological saline solutions (Feelisch et al. 1989; Crow & Beckman, 1995; Beckman & Koppenol, 1996). The positive inotropic effect of SIN-1 was indeed reduced and/or abolished in the presence of SOD in frog fibres. Second, the positive inotropic effect of SIN-1 was mimicked by LY 83583, a superoxide anion generator (Abi Gerges et al. 1997). Accordingly, the effect of LY 83583 was blocked by SOD. Superoxide anion generation via the xanthine-xanthine oxidase system also induced positive inotropic effects in frog fibres (data not shown). Nevertheless, LY 83583 is known as a cGMP-lowering agent, since it was reported to lower guanylyl cyclase and/or NOS activities (see Luo et al. 1995; Abi Gerges et al. 1997 and references therein). However, the positive inotropic effect of LY 83583 was never mimicked by ODQ (this study), nor by NOS inhibitors in frog fibres (see Méry et al. 1997). Thus, a modulation of cGMP levels might hardly account for the effects of LY 83583 reported here.
While the positive inotropic effects of LY 83583 suggest that superoxide anion directly modulates cardiac contractility, we cannot exclude the possibility that some superoxide anion by-product(s) contribute(s) to the positive inotropic effect of SIN-1. Superoxide anion is a precursor for hydrogen peroxide, which can in turn give rise to hydroxyl radical. Besides, SIN-1 releases NO in addition to superoxide anion and those two radicals react instantaneously (at a rate of about 7 × 109 M s−1, i.e. close to the diffusion rate) to form peroxynitrite (Crow & Beckman, 1995; Beckman & Koppenol, 1996). Therefore further experiments, e.g. using free radical scavengers, catalase and/or authentic peroxynitrite, are needed to examine the respective role of these various chemical species in the superoxide anion-mediated positive inotropic effect of SIN-1.
The relevance of our results to the physiology of the mammalian heart may be questioned. Indeed, intracellular calcium mobilization plays a minor role in the excitation- contraction coupling of the frog heart, as compared with that of higher vertebrates (Morad & Cleemann, 1987). However, the intracellular calcium channel-ryanodine receptor is modulated by NO donors and this modulation can involve cGMP-dependent as well as cGMP-independent mechanisms in vitro (Takasago et al. 1991; Iino et al. 1997; Zahradnikova et al. 1997; Xu et al. 1998). Furthermore, positive inotropic effects of NO donors were documented earlier in mammalian cardiac preparations (Mohan et al. 1996; Kojda et al. 1996, 1997). Some of these effects were antagonized by ODQ (Kojda et al. 1997) or Methylene Blue (Mohan et al. 1996) suggesting the involvement of a cGMP-dependent NO pathway. NO donors also increased the L-type Ca2+ current in frog (Méry et al. 1993) and human (Kirstein et al. 1995) cardiac myocytes through a cGMP-dependent inhibition of PDE3. However, other positive inotropic (Musialek et al. 1997; see also Kennedy et al. 1994) and chronotropic (Kojda et al. 1997) effects of NO donors were found to be insensitive to ODQ, suggesting the involvement of cGMP-independent mechanisms. In the light of our findings, we speculate that superoxide anion may participate in these ODQ-resistant stimulatory effects of NO donors.
Differences between atrial and ventricular tissues
This study illustrates differences between atrial and ventricular tissues from frog heart in the response to NO donors. First, the inhibitory effects of SNP, SNAP and SIN-1 + SOD were ∼2-fold higher in the atria than in the ventricle. Second, SIN-1 more consistently elicited positive inotropic effects in the ventricle than in the atria. An earlier study also showed differences between the effects of NO donors in ventricles and atria from rabbit heart (Ishibashi et al. 1993). Ventricular tissue, which has a larger myoglobin content than atrial tissue (Lin et al. 1990; Ishibashi et al. 1993), may scavenge NO at a faster rate and this would lead to a reduced negative inotropic effect due to NO. Hence, when SIN-1 is used, a reduced NO-mediated inhibitory response could favour the occurrence of a superoxide anion-mediated positive response.
An interaction between NO donors and the cardiac innervation might also be considered. Indeed, NOS activity as well as NO donors modulate cardiac neurotransmission in the mammalian heart (Schwarz et al. 1995 and references therein; Jumrussirikul et al. 1998). In addition, the effects of NO donors are dependent upon the presence of β-adrenergic agonists (or β-adrenergic mimetics) in isolated frog myocytes (see Méry et al. 1997). Since the innervation of the heart is heterogeneous (Hartzell, 1988), a modulation of neurosecretion by NO donors might contribute to the differential effects of these compounds in the atria and the ventricle. Ongoing experiments in our laboratory are aimed at illustrating this hypothesis.
Acknowledgments
We are very grateful to Dr J. Winicki (Hoechst Houdé Laboratories) for the generous gift of SIN-1 and SIN-1C. We thank Patrick Lechêne for excellent computer programming and Jacqueline Hoerter, Renée Ventura-Clapier, Vladimir Veksler and Philippe Matéo for helpful discussions. This work was supported by a grant from Hoechst Pharma.
References
- Abi Gerges N, Hove-Madsen L, Fischmeister R, Méry P-F. A comparative study of the effects of three guanylyl cyclase inhibitors on the L-type Ca2+ and muscarinic K+ currents in frog cardiac myocytes. British Journal of Pharmacology. 1997;121:1369–1377. doi: 10.1038/sj.bjp.0701249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good, the bad, and the ugly. American Journal of Physiology. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, Andries LJ. The endocardial endothelium. American Journal of Physiology. 1992;263:H985–1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- Butler AR, Flitney FW, Williams DLH. NO, nitrosonium ions, nitroxide ions, nitrothiols and iron-nitrosyls in biology: a chemist's perspective. Trends in Pharmacological Science. 1995;16:18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Journal of General Physiology. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnais J-M, Fischmeister R, Méry P-F. Contractile effects of nitric oxide (NO)-donors in frog cardiac muscle. Journal of Molecular and Cellular Cardiology. 1997;29:A114. [Google Scholar]
- Crow JP, Beckman JS. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. In: Ignarro L, Murad F, editors. Nitric Oxide Biochemistry, Molecular Biology, and Therapeutic Implications, Advances in Pharmacology. Vol. 34. San Diego: Academic Press; 1995. pp. 17–43. [DOI] [PubMed] [Google Scholar]
- Crystal GJ, Gurevicius J. Nitric oxide does not modulate myocardial contractility acutely in in situ canine hearts. American Journal of Physiology. 1996;270:H1568–1576. doi: 10.1152/ajpheart.1996.270.5.H1568. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Ostrowski J, Noack E. On the mechanism of NO release from sydnonimines. Journal of Cardiovascular Pharmacology. 1989;14:S13–22. [PubMed] [Google Scholar]
- Flesch M, Kilter H, Cremers B, Lenz O, Südkamp M, Kuhn-Regnier F, Böhm M. Acute effects of nitric oxide and cyclic GMP on human myocardial contractility. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1340–1349. [PubMed] [Google Scholar]
- Flitney FW, Moshiri M, Singh J. Effects of sodium nitroprusside on isolated frog ventricle. The Journal of Physiology. 1980;305:25–26P. [Google Scholar]
- Gross WL, Bak MI, Ingwall JS, Arstall WA, Smith TW, Balligand J-L, Kelly RA. Nitric oxide inhibits creatine kinase and regulates heart contractile reserve. Proceedings of the National Academy of Sciences of the USA. 1996;93:5604–5609. doi: 10.1073/pnas.93.11.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine, and second messenger systems. Progress in Biophysics and Molecular Biology. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Iino S, Cui Y, Galione A, Terrar DA. Actions of cADP-ribose and its antagonists on contraction in guinea pig isolated ventricular myocytes. Influence of temperature. Circulation Research. 1997;81:879–884. doi: 10.1161/01.res.81.5.879. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Hamaguchi M, Kato K, Kawada T, Ohta H, Sasage H, Imai S. Relationship between myoglobin contents and increase in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn-Schmiedeberg's Archives of Pharmacology. 1993;347:533–561. doi: 10.1007/BF00166750. [DOI] [PubMed] [Google Scholar]
- Jumrussirikul P, Dinerman J, Dawson TD, Dawson VL, Ekelund U, Georgakopoulos D, Schramm PL, Calkins H, Snyder SH, Hare JM, Berger RD. Interaction between neuronal nitric oxide synthase and inhibitory G protein activity in heart rate regulation in conscious mice. Journal of Clinical Investigation. 1998;102:2179–1285. doi: 10.1172/JCI2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circulation Research. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- Kennedy RH, Hicks KK, Brian JE, Jr, Seifen E. Nitric oxide has no chronotropic effect in right atria isolated from rat heart. European Journal of Pharmacology. 1994;255:149–156. doi: 10.1016/0014-2999(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Rivet-Bastide M, Hatem S, Bénardeau A, Mercadier JJ, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. Journal of Clinical Investigation. 1995;95:794–802. doi: 10.1172/JCI117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Nix P, Schlüter KD, Piper H-M, Noak E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circulation Research. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Noak E. Inhibition of nitric oxide synthase and soluble guanylate cyclase induces cardiodepressive effects in normal rat hearts. European Journal of Pharmacology. 1997;334:181–190. doi: 10.1016/s0014-2999(97)01168-0. [DOI] [PubMed] [Google Scholar]
- Lin L, Sylven C, Sotonyi P, Somogyi E, Kaijser L, Jansson E. Myoglobin content and citrate synthase activity in different parts of the normal human heart. Journal of Applied Physiology. 1990;69:899–901. doi: 10.1152/jappl.1990.69.3.899. [DOI] [PubMed] [Google Scholar]
- Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione and glucose. Biochemical Journal. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Das S, Vincent SR. Effects of methylene blue and LY 83583 on neuronal nitric oxide synthase and NADPH-diaphorase. European Journal of Pharmacology. 1995;290:247–251. doi: 10.1016/0922-4106(95)00084-4. [DOI] [PubMed] [Google Scholar]
- MacDonell K, Diamond J. Cyclic GMP-dependent protein kinase activation in the absence of negative inotropic effects in the rat ventricle. British Journal of Pharmacology. 1997;122:1425–1435. doi: 10.1038/sj.bjp.0701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell K, Tibbits GF, Diamond J. cGMP elevation does not mediate muscarinic agonist-induced negative inotropy in rat ventricular cardiomyocytes. American Journal of Physiology. 1995;269:H1905–1912. doi: 10.1152/ajpheart.1995.269.6.H1905. [DOI] [PubMed] [Google Scholar]
- Mao GD, Thomas PD, Lopaschuk GD, Poznanski MJ. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the fenton reaction in SOD toxicity. Journal of Biological Chemistry. 1993;268:416–420. [PubMed] [Google Scholar]
- Mayer B, Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Brunner F. A new pathway of nitric oxide/cyclic GMP signalling involving S-nitrosoglutathione. Journal of Biological Chemistry. 1998;273:3264–3270. doi: 10.1074/jbc.273.6.3264. [DOI] [PubMed] [Google Scholar]
- Méry P-F, Abi Gerges N, Vandecasteele G, Jureviscius J, Fischmeister R. Muscarinic regulation of the L-type calcium current in isolated cardiac myocytes. Life Science. 1997;60:1113–1120. doi: 10.1016/s0024-3205(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Méry PF, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current - Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. Journal of Biological Chemistry. 1993;268:26286–26295. [PubMed] [Google Scholar]
- Meulemans AL, Sipido KR, Sys SU, Brutsaert DL. Atriopeptin III induces early relaxation of isolated mammalian papillary muscle. Circulation Research. 1988;62:1171–1174. doi: 10.1161/01.res.62.6.1171. [DOI] [PubMed] [Google Scholar]
- Mohan P, Brutsaert DL, Paulus WJ, Sys SU. Myocardial contractile response to nitric oxide and cGMP. Circulation. 1996;93:1223–1229. doi: 10.1161/01.cir.93.6.1223. [DOI] [PubMed] [Google Scholar]
- Morad M, Cleemann L. Role of Ca2+ channel in development of tension in heart muscle. Journal of Molecular and Cellular Cardiology. 1987;19:527–553. doi: 10.1016/s0022-2828(87)80360-7. [DOI] [PubMed] [Google Scholar]
- Muller B, Kleschyov AL, Malblanc S, Stoclet J-C. Nitric oxide-related cyclic GMP-independent relaxing effect of N-acetylcysteine in lipopolysaccharide-treated rat aorta. British Journal of Pharmacology. 1998;123:1221–1229. doi: 10.1038/sj.bjp.0701737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musialek P, Lei M, Brown HF, Paterson DJ, Casadei B. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f) Circulation Research. 1997;81:60–68. doi: 10.1161/01.res.81.1.60. [DOI] [PubMed] [Google Scholar]
- Nawrath H, Bäumer D, Rupp J, Oelert H. The ineffectiveness of the NO-cyclic GMP signalling pathway in the atrial myocardium. British Journal of Pharmacology. 1995;116:3061–3067. doi: 10.1111/j.1476-5381.1995.tb15964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SG, Niedergerke R. Structures of physiological interest in the frog heart ventricle. Journal of Cell Science. 1972;11:179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterisation of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Molecular Pharmacology. 1996;50:1–5. [PubMed] [Google Scholar]
- Schwarz P, Diem R, Dun NJ, Forstermann U. Endogenous and exogenous nitric oxide inhibits norepinephrine release from rat heart sympathetic nerves. Circulation Research. 1995;77:841–848. doi: 10.1161/01.res.77.4.841. [DOI] [PubMed] [Google Scholar]
- Shah AM. Paracrine modulation of heart cell function by endothelial cells. Cardiovascular Research. 1996;31:847–867. [PubMed] [Google Scholar]
- Stamler JS. Redox signalling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K-I, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. Journal of Biochemistry. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Ma X-L, Buerke M, Murohara T, Armstead VE, Lefer AM, Nicolas JM, Thomas AP, Lefer DJ, Vinten-Johansen J. Physiological concentrations of nitric oxide do not elicit an acute negative inotropic effect in unstimulated cardiac muscle. Circulation Research. 1994;75:692–700. doi: 10.1161/01.res.75.4.692. [DOI] [PubMed] [Google Scholar]
- Wolin MS, Hintze TH, Shen W, Mohazzab-H KM, Xie Y-W. Involvement of reactive oxygen and nitrogen species in signalling mechanisms that control tissue respiration in muscle. Biochemical Society Transactions. 1997;25:934–939. doi: 10.1042/bst0250934. [DOI] [PubMed] [Google Scholar]
- Wyeth RP, Temma K, Seifen E, Kennedy RH. Negative inotropic actions of nitric oxide require high doses in rat cardiac muscle. Pflügers Archiv. 1996;432:678–684. doi: 10.1007/s004240050185. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Yim MB, Chock PB, Stadtman ER. Enzyme function of copper, zinc superoxide dismutase as free radical generator. Journal of Biological Chemistry. 1993;268:4099–4105. [PubMed] [Google Scholar]
- Zahradnikova A, Minarivic I, Venema RC, Meszaros LG. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22:447–453. doi: 10.1016/s0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]


