Abstract
We have previously investigated P2X receptor-mediated synaptic currents in medial habenula neurones and shown that they can be calcium permeable. We now investigate the receptor properties of glutamate, the other, more abundant excitatory transmitter, to determine its receptor subtypes and their relative calcium permeability. This may have implications for the physiological role of the P2X receptors which mediate synaptic currents.
Using fast application of ATP, L-glutamate or kainate to nucleated patches, glutamate receptors were determined to be of the AMPA subtype but no functional P2X receptors were detected.
The deactivation and desensitization rates of the AMPA channel were determined to have time constants of 1·77 ± 0·21 ms (n= 10) and 4·01 ± 0·85 ms (n= 9) at -60 mV, respectively. AMPA receptors recovered from desensitization with two exponential components with time constants of 21·08 ± 2·95 and 233·60 ± 51·1 ms (n= 3). None of the deactivation or desensitization properties of the GluR channels depended on membrane potential.
The current-voltage relationship under different ionic conditions revealed that the GluR channel was equally permeable to Cs+ and Na+ but relatively impermeable to Ca2+ (PCa/PCs= 0·13, n= 6).
For both synaptic currents and somatic currents activated by fast application of L-glutamate to nucleated patches, decay time constants were similar at ±60 mV in the presence of Mg2+ ions. Thus GluR channels appear to be of the AMPA subtype and not the NMDA subtype.
Thus, under the conditions of this study, neurones of the medial habenula lack functional NMDA receptors and possess AMPA receptors that have low permeability to Ca2+. We conclude that the P2X receptor-mediated synaptic currents are the only calcium-permeable fast-transmitter gated currents in these neurones which may be important for their physiological function.
Although the role of P2X receptors in the peripheral nervous system is well established, it is only in the last few years that P2X receptors have been shown to be involved in synaptic transmission in the CNS, both mediating fast transmission (Edwards et al. 1992; Bardoni et al. 1997; Nieber et al. 1997) and as a modulator of central transmission (Gu & MacDermott, 1997). P2X receptors belong to a family of ligand-gated cation channels (for review see Buell et al. 1996a) which have a widespread distribution in the central nervous system (for example see Kidd et al. 1995; Séguéla et al. 1996; Collo et al. 1996; Buell et al. 1996b,Vulchanova et al. 1996; Soto et al. 1996; Lêet al. 1998) suggesting that these receptors may have an important role in central transmission. The major excitatory transmitter in the CNS is glutamate. The neurones in the medial habenula that receive ATP-mediated synaptic input also feature a dense glutamatergic innervation. However, we have previously shown that ATP and glutamate are not cotransmitters in the rat medial habenula (Robertson & Edwards, 1998). One of the major questions regarding purinergic transmission in the CNS is the physiological necessity of having two fast excitatory transmitters. We have shown that, like several of the cloned P2X receptors (Evans et al. 1996; Virginio et al. 1998), the P2X receptors that mediate purinergic transmission in the rat medial habenula are also calcium permeable (Edwards et al. 1997). Could this calcium permeability reflect the functional role of this purinergic synapse in the medial habenula?
Based on pharmacological criteria there are three classes of ionotropic receptors for glutamate (GluRs): AMPA, kainate and NMDA receptors (for review see Edmonds et al. 1995). These receptors can be separated into two families (Mayer & Westbrook, 1987a;Burnashev, 1993). In general the AMPA-kainate receptors mediate the fast components of the excitatory postsynaptic current (EPSC) while the NMDA receptors mediate slower currents. The NMDA receptors are highly calcium permeable in a voltage-dependent manner, allowing an influx of calcium at depolarized potentials. Recently several AMPA receptors have been identified that are also calcium permeable (for review see Jonas & Burnashev, 1995). Calcium permeability of these receptors depends on the relative abundance of the mRNA encoding the glutamate receptor subunit GluR-B (Burnashev et al. 1992; Bochet et al. 1994; Barnes-Davies & Forsythe, 1995; Geiger et al. 1995).
Very little is known about the glutamatergic component of transmission in the medial habenula. The main purpose of this work was to examine the functional properties of these glutamate receptors, in particular to study the calcium permeability of the AMPA receptors and determine whether these neurones also contain NMDA receptors. This information may help to suggest a physiological role for purinergic transmission in the medial habenula nucleus.
METHODS
Habenula slices
Standard methods were used to prepare parasagittal slices from 15- to 24-day-old male rats (Edwards et al. 1989; Stuart et al. 1993). Animals were killed by decapitation (under Home Office licence) and the brain was quickly removed and placed in cold Krebs solution. After hemisection along the mid-line, the front third of the brain was removed with a scalpel. The lateral portion of the hemisphere was then trimmed so that it could be glued to the tissue block of a Camden Vibra-slice (Loughborough, UK) with the mid-line surface uppermost. Parasagittal slices (200 μm thick) were cut under a dissecting microscope and the habenula region dissected free using the position of the hippocampus, and the stria medularis and fasciculus retroflexus of Meynert fibre tracts as guides. Two slices where taken from each hemisphere starting ∼100 μm from the mid-line to ensure that only the medial habenula was included. After cutting, slices were maintained in a standard Krebs solution bubbled with 95 % O2-5 % CO2 for around 45 min at 34°C before recordings began. All recordings were made at room temperature (21-24°C).
Recording and analysis of currents
Neurones were visualized with infrared differential interference contrast (IR-DIC) microscopy using a CCD camera (Hitachi KP-M1E/K) mounted on an upright microscope (Olympus BX50WI). Whole-cell recording and nucleated patch recordings were made with an Axopatch 1D patch clamp amplifier (Axon Instruments). Thick-walled borosilicate glass electrodes (World Precision Instruments 1B150F-3) were pulled to give a resistance of approximately 5 MΩ for whole-cell recordings and 10 MΩ for nucleated patch recordings.
Solutions
The composition of the solutions was as follows. Krebs solution (mM): NaCl, 125; KCl, 2·5; NaHCO3, 26; NaH2PO4, 1·25; glucose, 25; CaCl2, 2; MgCl2, 1; bubbled with 95 % O2-5 % CO2. Intracellular solution (mM): CsCl, 140; Hepes, 5; EGTA, 10; Mg-ATP, 2; pH 7·4 with CsOH (2·6 mM). Extracellular solution A (mM): NaCl, 140; KCl, 2·4; Hepes, 10; glucose, 25; MgCl2, 1; CaCl2, 2; pH 7·4 with NaOH (8 mM). Extracellular solution B (mM): CaCl2, 30; NMDG, 100; Hepes, 10; glucose, 25; pH 7·4 with Ca(OH)2.
Whole-cell recordings
Whole-cell recordings were performed on slices continuously superfused with standard Krebs solution containing bicuculline (10 μM), to block GABAA receptor-mediated events and in addition for P2X receptor-mediated synaptic responses, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 μM) to block AMPA receptors. In order to verify the stability and quality of the seal, a test pulse was monitored regularly and the recording terminated if the shape or size of the pulse was significantly altered.
Stimulated currents were evoked by applying bipolar rectangular stimulus voltage pulses of 100-200 μs duration (stimulus supplied by a Grass SD9 stimulator) to a stimulating electrode. Stimulating electrodes were pulled in the same way as recording electrodes and filled with normal Krebs solution. The stimulation voltage used was chosen by placing the stimulating electrode in the slice and slowly increasing the voltage from 1 V until a response was seen. For minimal stimulation the voltage chosen was the lowest voltage at which a response was clearly seen, in an attempt to stimulate only one or a few fibres. For multiple fibre stimulation the voltage of the stimulator was increased to approximately 30 V above the minimal value.
Currents were digitized at 44 kHz and recorded at a bandwidth of 10 kHz (4 pole Bessel) with a modified video cassette recorder (Vetter model 200, A.R. Vetter Co. Inc., Rebersburg, PA, USA). Currents were replayed through a 2 kHz filter (Frequency Devices, 8 pole Bessel) and sampled at 10 kHz with a CED1401 interface (CED, Cambridge, UK) using the programs WinWCP (evoked currents) or WinCDR (spontaneous currents) (kindly supplied by Dr J. Dempster, University of Strathclyde Electrophysiology Software: http://www.strath.ac.uk/Departments/PhysPharm/ses.htm).
Spontaneous currents were detected using the following selection criteria: to be classified as a spontaneous event the putative current had to cross an amplitude threshold, set as twice the root mean square (r.m.s.) noise of the recording, for a period of 1 ms. The currents were then viewed manually to avoid inclusion of artefacts. Spontaneous events were aligned, by the rising phase, and averaged for further analysis.
Nucleated patch recordings
After formation of a standard whole-cell recording, suction was applied to the pipette and the electrode withdrawn to form a nucleated patch (Sather et al. 1992). The nucleated patch was then placed near the mouth of the fast application pipette. Data were recorded using the standard intracellular solution and extracellular solution A. In experiments to determine the calcium permeability of the AMPA receptors a high Ca2+ extracellular solution (solution B) was used. Currents were recorded online, filtered at 2 kHz (4 pole Bessel) and sampled at 10 kHz (unless otherwise stated) with a CED1401 interface using the program WinWCP.
Fast applicator system
Fast application of agonists was carried out by the method of Colquhoun et al. (1992). Four-barrelled pipettes were fabricated from glass tubing (Vitro Dynamics Inc.; outside diameter 1·5 mm, inner walls 0·17 mm). Solutions were perfused through control and test barrels from four independent reservoirs by gravity feed. The nucleated patch was positioned near the interface of the two solutions. The application pipette was fixed to a Burleigh piezo fast application device (PZ150, Burleigh Instruments Inc., NY, USA). Pulses (1-100 ms duration) of agonists were applied to the patches by applying a voltage step to the piezo with the program WinWCP. ATP (100 μM or 1 mM), L-glutamate (1 mM) or kainate (1 mM) were applied to the patch every 5 s. Data from at least three sweeps were used for each data point. In some experiments to facilitate the activation of NMDA receptors, glycine (10 μM) was also added to both control and test barrels. While testing the calcium permeability of the AMPA receptors, the NMDA receptor antagonist 7-chlorokynurenate (10 μM) was added to both test and control barrels.
The properties of individual application pipettes were tested using an open pipette with Hepes-based extracellular solution A in the test barrel and a 50 % dilute Hepes solution in the test barrel. The 10-90 % rise time of solution exchange was typically around 0·5 ms.
Drugs
All drugs were from Sigma unless otherwise stated, but Na-ATP was from Boehringer Mannheim, and CNQX, suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), 7-chlorokynurenate, L-glutamate and kainate were from Research Biochemicals Inc., now Sigma. Unless otherwise stated, drugs were dissolved in distilled water (typically 1000 × final required concentration) and stored in aliquots at -18°C. Bicuculline was made up in distilled water and used the same day. CNQX was dissolved at 1000 × final concentration in DMSO and stored at -20°C. DMSO was included in control solutions for all comparisons with CNQX.
Data analyses
Data are represented as the means ± standard error (s.e.m.). Current traces were fitted with a single exponential curve, using a modified Levenberg-Marquardt least-square minimization algorithm with the software winWCP (Dempster, 1993). Data were analysed using the Prism statistics package (GraphPad Software Inc., San Diego, CA, USA). Results were considered significant if the probability of chance occurrence was less than 0·05.
Estimation of calcium permeability of AMPA receptor channels
Reversal potentials, corrected for the measured liquid junction potential of 3·4 mV, were used to estimate ionic permeability ratios by fitting the extended Goldman-Hodgkin-Katz equation (Mayer & Westbrook, 1987b;Otis et al. 1995; Edwards et al. 1997) to the reversal potential. The free calcium and monovalent ion concentrations were then converted to activities using activity coefficients of 0·55 for 30 mM Ca2+ and 0·72 for Cs+ interpolated from tabulated values (Robinson & Stokes, 1965). The monovalent ions were found to be equipermeable (see Results). The data were fitted with the following equation (Mayer & Westbrook, 1987b):
| (1) |
where R, T and F have their usual meaning and
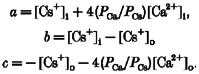 |
Thus there was one parameter to fit: the permeability ratio for Ca2+ to Cs+ (PCa/PCs). The permeability ratio was estimated by a non-linear least-square fit to the data.
Calcium entry during a synaptic current at -60 mV was calculated according to the methods described in Schneggenburger et al. (1993), Burnashev et al. (1995) and Edwards et al. (1997). Activity coefficients of 0·57 for 1·8 mM Ca2+, and 0·72 for monovalent ions and Cs+ were used.
RESULTS
Currents activated by fast application of ATP or L-glutamate to nucleated patches
Rapid application of ATP (100 ms pulse) to a nucleated patch resulted in no inward current at -60 mV (1 mM ATP, n= 9 or 100 μM, n= 4; Fig. 1). Subsequent fast application of L-glutamate (1 mM) activated currents with fast rise times < 1 ms and decay time constants of a few milliseconds (n= 13), confirming the viability of the patches (Fig. 1). ATP-evoked currents were not observed using two different commercial sources of ATP: Mg-ATP, (Sigma; n= 5) or Na-ATP (Boehringer Mannheim; n= 8). The lack of ATP-activated currents on nucleated patches was additionally confirmed in experiments in which ATP was omitted from the internal solution to avoid any possibility of current desensitization occurring while the electrode was approaching the cell (n= 4).
Figure 1. The AMPA (and not kainate) subtype of glutamate receptors but not P2X receptors are found in nucleated patches.
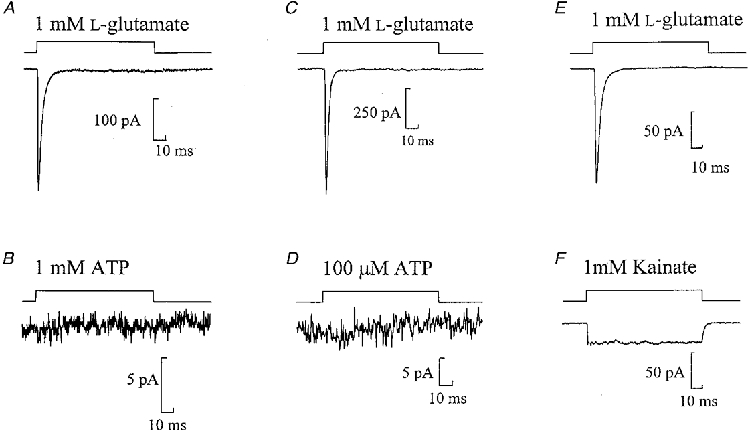
Currents evoked in three nucleated patches by 100 ms pulses of 1 mM L-glutamate (A, C and E), 1 mM ATP (B), 100 μM ATP (D) or 1 mM kainate (F). A and B, C and D, and E and F are from the same patches. The period of agonist application to the patch is represented by the top trace. Note the different current amplitude scales. Here and in subsequent figures each trace is the average of at least three sweeps.
The somatic glutamate receptors are of the AMPA receptor subtype
Fast application of kainate (1 mM) to nucleated patches held at -60 mV resulted in a sustained inward current with average amplitude of -29·2 ± 4·6 pA (n= 15) (Fig. 1F). This suggests that these currents are mediated by the AMPA subtype of glutamate receptor and not by the kainate subtype of glutamate receptor.
Current-voltage relationship of somatic GluR
The current-voltage relationship of the somatic GluR-mediated currents was determined in four patches with a Cs+-rich intracellular solution and a Na+-rich extracellular solution. Two millisecond pulses of 1 mM L-glutamate were applied at membrane potentials ranging from -50 to +50 mV (Fig. 2A and B). In some cells a wider voltage range was used. The order of voltage was varied during the experiment and at least three jumps were performed at each potential. The current amplitude ranged from -31 to -454 pA at -50 mV in different cells. The current-voltage relationship was linear (r2= 0·9984) with a reversal potential of 0 mV suggesting that the GluR channel was approximately equally permeable to Cs+ and Na+. No obvious rectification was observed with a rectification index (γ+ 50/γ - 50) of 0·96 ± 0·11 (n= 4). (Note the lack of NMDA component which will be discussed below in relation to more detailed experiments on this point.)
Figure 2. Current-voltage relationship and calcium permeability of somatic GluR channels.
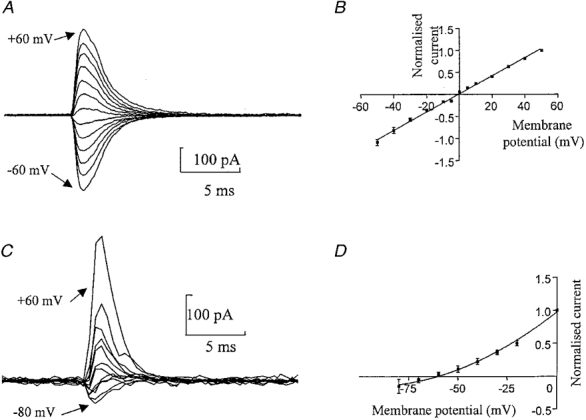
A, currents from a nucleated patch activated by 2 ms pulses of L-glutamate (1 mM) in normal Hepes-based solution. In this cell the responses were measured at different membrane potentials ranging from -60 to +60 mV in 10 mV steps. Each trace is an average of at least three responses. B, normalized current- voltage relationship of the peak glutamate-activated current for 4 patches. The data were fitted by a straight line giving an estimate for the reversal potential of -0·6 mV (corrected for liquid junction potential). C, currents from a nucleated patch activated by a 2 ms pulse of L-glutamate (1 mM) recorded in a Ca2+-rich extracellular solution, using a Cs+-rich intracellular solution. The membrane potential was held at 60, 40, 20, 0, -10, -20, -30, -40, -50, -60, -70 and -80 mV. Currents were digitized at 0·5 ms. D, normalized current-voltage relationship for all 6 patches. The data were fitted by a second order polynomial (r2= 0·9994), giving a reversal potential of -63·25 mV (n= 6) (corrected for liquid junction potential). Currents were normalized to +50 mV in B and 0 mV in D.
Calcium permeability of somatic GluRs
To determine the relative calcium permeability of the GluR channels, reversal potential measurements were carried out with an external solution in which calcium replaced all monovalent cations (Fig. 2C and D). The current-voltage relationship was best described by a second order polynomial (r2= 0·9989). The reversal potential of the GluR-activated currents calculated from this fit was shifted to negative values (-63·25 ± 5·98 mV, n= 6) compared with that in normal external solutions (Fig. 2). From this value the relative permeability of these channels to Ca2+ relative to monovalent ions was determined (eqn (1)), which gave a PCa/PCs of 0·13. Thus the GluR receptors from the soma of medial habenula neurones have low calcium permeability.
To test if the somatic receptors have similar relative calcium permeability to the synaptic glutamate receptors, we have performed experiments which show that bath application of the polyamine spermine (100 μM) reduces the average amplitude of evoked glutamatergic EPSCs in these neurones by 12·8 ± 9·4 % (n= 5). This is consistent with the reduction in amplitude observed in AMPA receptors with low calcium permeability (Mahanty & Sah, 1998).
Kinetic properties of somatic glutamate receptors
To determine the deactivation rate (defined here as the decay of the current after removal of glutamate) and the desensitization rate of the GluR channels (in the continuous presence of glutamate), experiments were performed in the standard Hepes solution with no magnesium and with 10 μM glycine added. Pulses of 1 and 2 ms duration of L-glutamate (1 mM) were used to determine the deactivation rate of the channels. Pulses of 10 and 100 ms duration were used to determine the desensitization rate of the channels. A short pulse of L-glutamate (either 1 or 2 ms) resulted in currents with fast rise times (-60 mV, 10-90 % values: 2 ms pulse, 0·77 ± 0·06 ms, n= 11; 1 ms pulse, 0·66 ± 0·07 ms, n= 6) and decay times that were fitted with a single exponential component (-60 mV; 2 ms pulse, τ= 1·77 ± 0·21 ms, n= 10; 1 ms pulse, τ= 2·05 ± 0·46 ms, n= 5). This reflects the channel deactivation rate. There was no statistically significant difference between either the rise time or decay kinetics of currents activated by a 1 or 2 ms pulse of L-glutamate. The pulse duration did not alter the amplitude of the current. The decay time course of the current was fitted to the data immediately after the pulse for a 1 or 2 ms application of agonist.
The longer pulses of L-glutamate (10 and 100 ms) resulted in currents with similar rise times (10-90 % values: 100 ms pulse, 0·74 ± 0·07, n= 10; 10 ms pulse, 0·69 ± 0·05, n= 10) to the currents evoked by the shorter pulses (see above). However, the currents activated by the longer pulses exhibited slower decay rates (P < 0·05) (100 ms pulse, 4·01 ± 0·85 ms, n= 9; 10 ms pulse, 3·27 ± 0·37 ms, n= 10). The decay of the currents evoked by the 10 and 100 ms pulses of L-glutamate reflect the desensitization rate of the channel. Note that the currents decayed back to baseline within the 100 ms pulse, suggesting that these channels have a negligible steady-state conductance (Fig. 1).
Recovery from desensitization of AMPA channels
To determine the rate of recovery from desensitization, two brief (2 ms) pulses of L-glutamate were applied at varying time intervals. Even a very brief exposure of the receptors to glutamate produced a considerable depression of the subsequent response, with the receptors requiring several hundred milliseconds to recover (Fig. 3). The rate of recovery from desensitization was fitted by two exponential components, which were similar at both ±60 mV (-60 mV, τ1= 21·08 ± 2·95 ms (55 %), τ2= 233·60 ± 51·1 ms (45 %), r2= 0·9996; +60 mV, τ1= 16·7 ± 3·2 ms (58 %), τ2= 240·38 ± 78·7 ms (42 %), r2= 0·9987). The maximum extent of desensitization was 36 ± 2·6 % (n= 3) of the current amplitude at -60 mV and 42 ± 4·9 % (n= 3) at +60 mV.
Figure 3. Recovery from desensitization of somatic GluR channels.
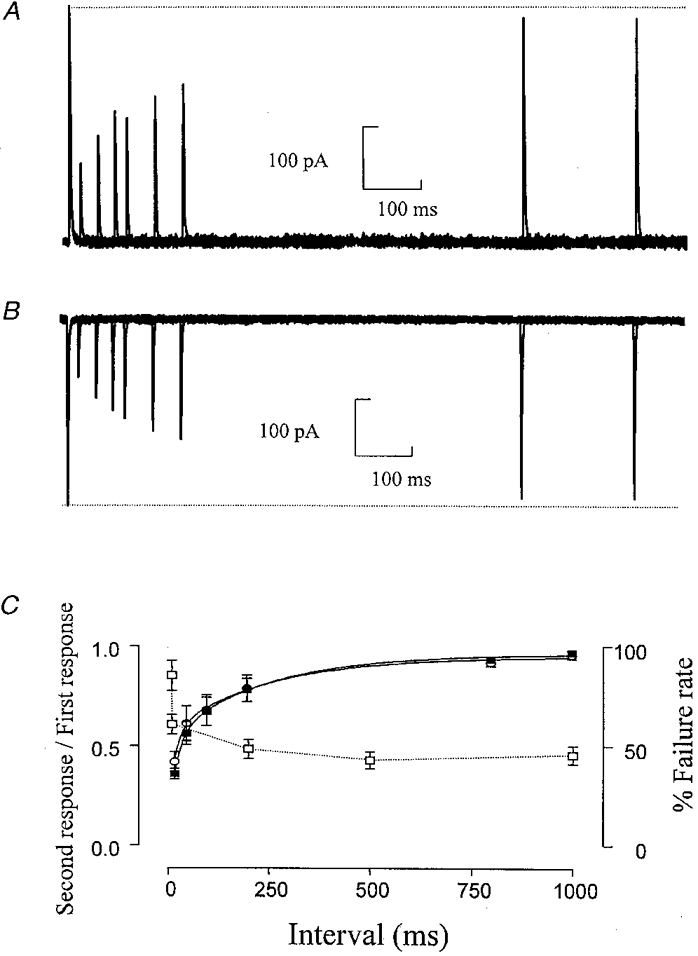
A and B, superimposed responses to the application of two 2 ms pulses of L-glutamate (1 mM) at +60 mV (A) and -60 mV (B). The pulses are separated by a variable time interval of 20-1000 ms. Responses in A and B are from the same patch. C, depression of the amplitude of the second response expressed as a fraction of the amplitude of the first response, plotted against the time interval between the pulses. ▪, -60 mV; ○, +60 mV (n= 3). □, % failure rate of glutamatergic synaptic responses at -70 mV; failure rate from Robertson & Edwards (1998).
NMDA receptor activation does not contribute to the GluR-mediated response
To determine the contribution of NMDA receptors to the current activated by 1 mM L-glutamate, currents were recorded from nucleated patches with the standard Hepes solution. Glycine (10 μM) was added to facilitate the activation of NMDA channels.
Positive potentials should relieve magnesium block of NMDA receptors if these channels are present, resulting in outward currents with much slower decay time constants. At +60 mV the currents activated by a 10 ms pulse of L-glutamate (1 mM) showed very similar kinetics to those activated at -60 mV (Fig. 4). The decay of the current was fitted by a single exponential with time constants of 3·60 ± 0·48 ms (n= 6) at -60 mV compared with that of 3·25 ± 0·63 ms (n= 4) at +60 mV. The similar decay time constants at both ±60 mV suggests that these patches do not contain NMDA receptors. The rise time of the L-glutamate-activated current was also similar. At -60 mV the 10-90 % rise time was 0·78 ± 0·07 ms (n= 6) compared with 0·95 ± 0·17 ms (n= 4) at +60 mV.
Figure 4. Nucleated patches contain AMPA receptors but not NMDA receptors.
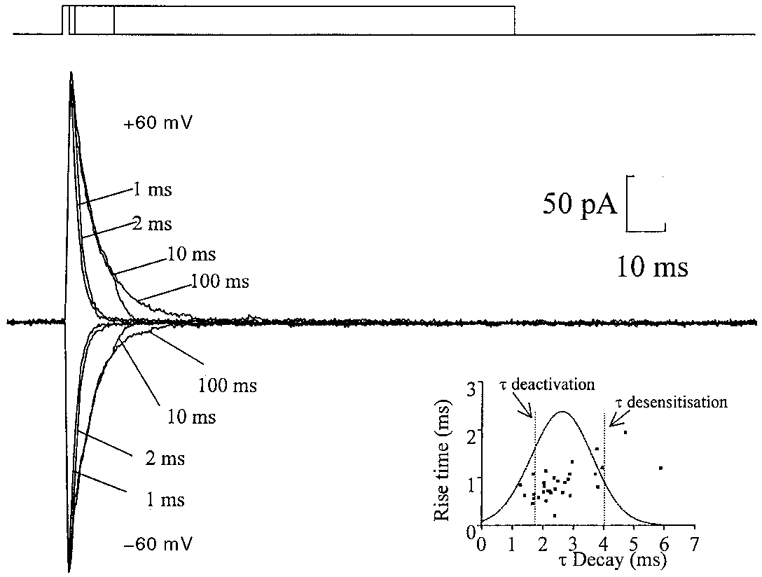
Currents evoked from a nucleated patch, recorded in the presence of extracellular magnesium (1 mM) and glycine (10 μM). L-Glutamate (1 mM) was applied in pulses of 1, 2, 10 and 100 ms at holding potentials of both +60 and -60 mV. The top trace represents the duration of the agonist application. Note the similarity in time course of the inward and outward current, indicating a lack of an NMDA-mediated component. Each trace is the average of three sweeps. Inset, plot of the average rise time of evoked glutamatergic synaptic currents against the average decay time constant in 31 cells; membrane potential, -70 mV, ▪. For comparison a Gaussian distribution of the mean evoked synaptic decay time constant (2·60 ± 1·01 ms, mean ±s.d.) and lines representing both the deactivation time constant (1·77 ± 0·21 ms, n= 10) and desensitization time constant (4·01 ± 0·85 ms, n= 9) are superimposed on the graph.
Several experiments were performed with the Hepes-based extracellular solution which contained no added magnesium to compare the kinetics of the currents with those in the presence of magnesium (Fig. 2A). A 100 ms pulse of L-glutamate (1 mM) activated currents with a 10-90 % rise time of 0·74 ± 0·06 ms (n= 9) at -60 mV and the current decay was fitted by a single exponential with τ= 4·02 ± 0·8 ms (n= 9). There was no statistical difference between the decay in the presence or absence of magnesium at -60 mV. This again suggests that these patches do not contain any NMDA channels.
Synaptic responses do not contain an NMDA component
The mean rise time (10-90 %) of evoked glutamatergic EPSCs, in the presence of extracellular Mg2+, at -60 mV was 0·88 ± 0·06 ms with a mean decay time of 2·60 ± 0·18 ms (n= 31). To determine if there was an NMDA component of the synaptic response in these neurones, whole-cell recordings were performed in normal Krebs solution containing bicuculline (10 μM). With minimal stimulation protocols (see Methods) synaptic currents were evoked at a range of potentials and the decay time constant analysed (Fig. 5A and B). In all cells tested (n= 9) the decay of the evoked currents was not significantly longer at positive potentials than at negative potentials. The decay of the evoked current was best described by a single exponential component with τ= 4·02 ± 0·21 ms at -60 mV and 5·70 ± 0·62 ms at +60 mV. In four of these cells, synaptic currents were also evoked in a magnesium-free extracellular solution. In this magnesium-free extracellular solution there was no difference in the current decay at ±60 mV (τ= 3·76 ± 0·38 ms, -60 mV; τ= 4·50 ± 0·36 ms, +60 mV). CNQX (20 μM) abolished the current at both ±60 mV (n= 8).
Figure 5. Synaptic responses do not have an NMDA receptor component.
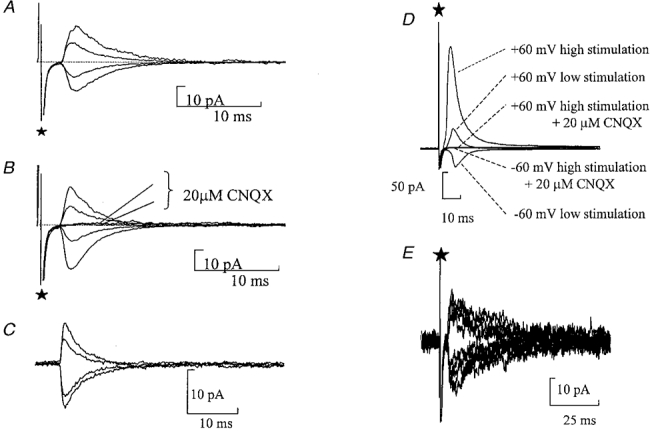
A and B, average evoked glutamatergic synaptic responses in the presence of bicuculline (10 μM) with minimal stimulation. ⋆marks the stimulus artefact. A, currents recorded when the membrane potential was held at +60, +30, -30 and -60 mV with 1 mM extracellular Mg2+. B, average evoked currents recorded when the membrane potential was held at +60, +30, -30 and -60 mV with no Mg2+ added to the extracellular solution. CNQX (20 μM) abolished the evoked glutamatergic synaptic currents. C, spontaneous glutamatergic currents recorded in the normal extracellular solution containing Mg2+. The membrane potential was held at 60, 40, -30 and -60 mV. D, evoked glutamatergic synaptic responses in the presence of bicuculline (10 μM) with multiple fibre stimulation. Low stimulation is 22 V, high stimulation is 50 V. Currents were recorded at a membrane potential of -60 and +60 mV. CNQX (20 μM) abolished the evoked glutamatergic synaptic currents. E, raw data showing evoked P2X receptor-mediated synaptic currents in the presence of CNQX (20 μM) and bicuculline (20 μM). Recording of superimposed traces at membrane potentials of ±60 mV. Note the similar decay time at both membrane potentials showing a lack of any NMDA receptor-mediated component.
In 10 cells where protocols were used to stimulate greater than one input, evoked synaptic currents were recorded at ±60 mV. CNQX (20 μM) abolished the current at both ±60 mV under these conditions (n= 10) (Fig. 5D). The absence of an NMDA component was also confirmed in four cells where the decay of spontaneous currents was analysed at various potentials (Fig. 5C). The spontaneous currents were aligned by their rising phase and averaged. Note no selection on the basis of rise time was performed. At all potentials tested the spontaneous currents exhibited a fast decay, e.g. τ= 4·40 ± 0·1 ms at -60 mV and τ= 5·15 ± 0·79 ms at +60 mV. These data support the findings from the nucleated patches suggesting that these neurones lack NMDA receptors.
Finally we tested whether there could be an NMDA component selectively localized with the P2X receptors. P2X receptor-mediated inputs were stimulated in the presence of 20 μM CNQX and 20 μM bicuculline. Initially currents were recorded at -60 mV with 1 mM extracellular magnesium. At +60 mV where the magnesium block of NMDA receptors would be relieved, there was no evidence of an additional slowly decaying synaptic component (Fig. 5E, n= 4). Thus synapses mediated by P2X receptors do not have a functional NMDA receptor-mediated component. (Note that we have found P2X-mediated currents in 66 % of neurones in which stimulation was attempted. This is, however, undoubtedly an underestimate of the occurrence of the synapse as the input is sparse and finding the correct place for stimulation is limited by loss of cells during attempts to stimulate, as well as orientation of the slice in the bath.)
DISCUSSION
The medial habenula nucleus is one of the few regions of the brain where ATP has been identified as a fast transmitter. The object of this study was to try and establish the physiological relevance of this excitatory purinergic synapse. We have previously shown that about half of the purinergic synapses are calcium permeable (Edwards et al. 1997) and that ATP is not a co-transmitter with glutamate in this nucleus (Robertson & Edwards, 1998).
This P2X receptor-mediated influx of calcium may be an important postsynaptic modulator of transmission in these neurones. The purinergic inputs are, however, sparse compared with glutamatergic inputs, hence any calcium influx through glutamate receptors would be expected to swamp the calcium influx through P2X receptors. In order to determine if influx of calcium through glutamate receptors contributes to calcium entry in these neurones, it was of interest to look at the glutamate receptor subtypes and their Ca2+ permeability. This may help determine the likely functional role of calcium influx through the purinergic synapse.
P2X receptors are the only route of transmitter-gated Ca2+ entry in medial habenula neurones
Our previous observation that iontophoretic application of αβ-methylene ATP resulted in only a small whole-cell current in these neurones (n= 10) (Edwards et al. 1992) suggested that the receptors were virtually absent on the soma of these cells. Moreover in this original study, outside-out patches revealed a channel in only one of the six patches tested. Subsequent attempts showed no channels (n > 10, F. C. Halliday & A. J. Gibb, personal communication). This finding was confirmed using nucleated patches pulled from the soma of these cells. These experiments showed the absence of any functional P2X receptors on the soma of these cells whereas functional GluRs were consistently observed. This confirmed that the absence of currents observed in the previous studies was due to an absence of channels rather than rapid desensitization of the response.
We exploited this relative abundance of somatic GluR channels to investigate the types of GluRs present and the Ca2+ permeability of these receptors using fast application of glutamate. The soma of these neurones were found to have only AMPA glutamate receptors with no evidence for functional NMDA receptors. The lack of NMDA receptors was also confirmed with both glutamatergic-evoked synaptic currents and spontaneous currents. Indeed the AMPA/kainate antagonist CNQX totally blocked the evoked synaptic currents, confirming that these fast decaying synaptic events were mediated by AMPA glutamate receptors. In addition, no currents characteristic of NMDA receptor activation were observed while recording at +60 mV from P2X receptor-mediated synapses. Although we cannot exclude that in a small population of neurones within the medial habenula or at a different developmental stage, there is an NMDA component to the synaptic currents, most neurones and indeed those which receive ATP inputs lack functional NMDA inputs in 21-day-old rats. Where present, NMDA receptors are highly calcium permeable and are thought to have a physiological role allowing Ca2+ entry at depolarized potentials (Ascher & Johnson, 1989). This source of Ca2+ signalling is clearly not available in medial habenula neurones. This is fairly unusual in the brain, with cerebellar Purkinje cells and interneurones in the amygdala being two of the few other central neurones where there are no functional NMDA receptors in mature neurones (Konnerth et al. 1990; Mahanty & Sah, 1998). Another possible source of calcium influx in these cells is through the AMPA channels themselves. Recent studies have shown that some AMPA channels are Ca2+ permeable (e.g. Müller et al. 1992; Jonas et al. 1994; Otis et al. 1995; for review see Jonas & Burnashev, 1995). However, this present study shows that the somatic AMPA receptors are of low Ca2+ permeability (PCa/PM= 0·13). If the somatic GluRs are similar to those that underlie the synaptic currents, then a single unitary GluR-mediated synaptic event has a calcium influx of 0·74 fC at -70 mV, compared with a calcium influx of 25 fC at -70 mV for a unitary P2X receptor-mediated synaptic current in these neurones, under similar recording conditions (Edwards et al. 1997). Bath application of the polyamine spermine reduces the average amplitude of evoked glutamate EPSCs in these neurones by 12·8 ± 9·4 % (n= 5), consistent with the 18·5 % decrease observed in AMPA receptors with low calcium permeability (Mahanty & Sah, 1998). Calcium-permeable AMPA receptors show a 63 ± 9 % decrease (Mahanty & Sah, 1998); thus it seems highly probable that the synaptic AMPA receptors have a similar low permeability to calcium as the somatic AMPA receptors in these medial habenula neurones.
Our previous paper failed to show any glycinergic or 5-HT3 responses or any nicotinic synapses (Edwards et al. 1992); thus it appears that P2X receptors are the only ionotropic receptors which mediate a synaptic calcium influx in these neurones. This suggests that, in contrast to dorsal root ganglion neurones (Gu & MacDermott, 1997) in which Ca2+ entry through ATP-gated channels presynaptically modulates spontaneous glutamate release, in habenula neurones it may contribute to elevation of Ca2+ in postsynaptic neurones. The fact that our previous findings (Edwards et al. 1997) showed that the calcium permeability was very varied between cells further suggests that this feature of the synapse might be important for its physiological role.
The molecular composition of the somatic GluR
The relatively rapid recovery from desensitization of the glutamate receptors and the sustained current when activated by high concentrations of the agonist kainate suggest that these receptors are of the AMPA rather than the kainate subtype (Burnashev, 1993; Lerma et al. 1993). The channel permeability and gating kinetics of AMPA receptor channels vary between AMPA receptor subtypes. The subunits GluR-A, -C and -D feature a high calcium permeability and a rectifying current-voltage relationship whereas the edited GluR-B subunit confers low calcium permeability and a linear current-voltage relationship (Burnashev et al. 1992; Geiger et al. 1995). The linear current-voltage relationship of these GluR channels in nucleated patches from neurones of the medial habenula is consistent with the finding that these receptors have low calcium permeability. In this recording mode we have previously observed that washout of polyamine is poor during the recording time (Rozov et al. 1998). Indeed the habenula has been shown to stain densely with antibodies to the C-terminal amino acid sequence of GluR-A, -B/C and -D (Petralia & Wenthold, 1992).
The GluR channels in the habenula are unusual in that they display both low calcium permeability and exhibit rapid deactivation and desensitization. The deactivation and desensitization kinetics are similar to those found in interneurones whereas the calcium permeability is similar to that found in principal neurones (Geiger et al. 1995; Götz et al. 1997). Recently AMPA receptors with both fast kinetics and low calcium permeability have been identified in cerebellar Purkinje cells (Hausser & Roth, 1997). Clearly, calcium permeability and gating kinetics can be determined independently. The calcium permeability and the fast receptor kinetics suggest that the receptor is composed of GluR-B/Glu-D subunits and the lack of any sustained current in the continued presence of agonist suggests that these subunits are likely to be of the flop splice variant (Burnashev, 1993).
The recovery from desensitization of the AMPA receptors on these neurones was of a similar order to that found in rat hippocampal dentate gyrus neurones but was significantly slower than AMPA receptors in CA3 and CA1 pyramidal neurones and cerebellar Purkinje cells (Colquhoun et al. 1992; Koh et al. 1995; Hausser & Roth, 1997). The recovery from desensitization should play an important part in synaptic integration, leading to a decrease in the amplitude of the postsynaptic response with higher firing rates of the incoming pathway. In a previous study looking at evoked glutamatergic EPSCs (Robertson & Edwards, 1998), we noted that increasing the stimulation frequency from 0·5 to 5 Hz caused very little change in postsynaptic responses whereas at 10 and 100 Hz the response appeared to fail almost constantly. We suggested that at lower stimulation frequencies, the failure rate observed was due to failure to release transmitter. However, after a brief period at 100 Hz the failure rate did not recover immediately and thus we suggested another factor was involved which determined the ‘failure rate’ at higher stimulation frequencies. One possibility is that the 100 Hz stimulation depletes the readily releasable pool of vesicles. However, it seems this is more likely to reflect release of transmitter onto desensitized receptors as the increase in failure rate with increased stimulation frequency (Robertson & Edwards, 1998) closely mirrors the time course of recovery from desensitization observed with the somatic AMPA receptors (Fig. 3C).
Comparison of evoked synaptic currents and currents evoked by brief glutamate pulses
Previous studies have shown that the decay of the EPSCs between mossy fibres and CA3 neurones are much closer to the deactivation time constant of the AMPA channels than to the desensitization rate constant, suggesting that the EPSC time course is largely determined by channel deactivation following rapid removal of glutamate from the cleft (Colquhoun et al. 1992). In this study the decay of the EPSC fell between the desensitization rate of the GluR channels and the deactivation rate. There was a fairly wide range of values for mean evoked EPSC decay time constants between different cells (1·25-5·88 ms), which presumably reflects variation in the position of recorded synapses in the extensive dendritic trees of these cells. Indeed there was a positive correlation between rise time and decay (n= 31, P < 0·0001, inset in Fig. 4) with the average decay of 2·6 ± 0·18 ms and the average rise time (10-90 %) of 0·88 ± 0·06 ms (n= 31). Considering the fastest synaptic currents are at least as fast as the deactivation time constant (1·77 ± 0·21, n= 10, 2 ms pulse) and the majority are considerably faster than the desensitization time constant (4·01 ± 0·85 ms, n= 9) it seems likely that, similar to the mossy fibre EPSC, rapid removal of glutamate from the cleft determines the EPSC decay kinetics.
Conclusion
Neurones of the medial habenula do not contain functional NMDA receptors. The AMPA receptors of these neurones have low calcium permeability. Thus the purinergic synapse is the only transmitter-gated Ca2+ entry pathway in these neurones which may reflect the functional significance of the purinergic synapse.
Acknowledgments
We would like to thank Richard Hodkinson for expert technical assistance and Dr Alasdair J. Gibb for critical reading of an earlier version of the manuscript. This work was funded by The Wellcome Trust.
References
- Ascher P, Johnson JW. The NMDA receptor, its channel, and its modulation by glycine. In: Watkins JC, Collingridge GL, editors. The NMDA Receptor. Oxford: Oxford University Press; 1989. pp. 109–122. [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, Macdermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. The Journal of Physiology. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Lino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: An emerging channel family. European Journal of Neuroscience. 1996a;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996b;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnashev N. Recombinant ionotropic glutamate receptors: functional distinctions imparted by different subunits. Cellular Physiology and Biochemistry. 1993;3:318–331. [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. The Journal of Physiology. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. The Journal of Physiology. 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster J. Biological Techniques: Computer Analyses of Electrophysiological Signal. London: Academic Press Ltd; 1993. [Google Scholar]
- Edmonds B, Gibb AJ, Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annual Review of Physiology. 1995;57:495–519. doi: 10.1146/annurev.ph.57.030195.002431. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ, Gibb AJ. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. The Journal of Physiology. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JRP, Melcher T, Koh D-S, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Götz T, Kraushaar U, Geiger J, Lübke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. Journal of Neuroscience. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, Macdermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Hausser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. The Journal of Physiology. 1997;501:77–95. doi: 10.1111/j.1469-7793.1997.077bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CBA, Simon J, Michel AD, Barnard EA, Humphrey PPA. Localization of P2X purinoreceptor transcripts in the rat nervous system. Molecular Pharmacology. 1995;48:569–573. [PubMed] [Google Scholar]
- Koh D-S, Geiger JRP, Jonas P, Sakmann B. Ca2+-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. The Journal of Physiology. 1995;485:383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê KT, Villeneuve P, Ramjaun AR, Mcpherson PS, Beaudet A, Séguéla P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. The Journal of Physiology. 1987a;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Progress in Neurobiology. 1987b;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Müller T, Möller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. British Journal of Pharmacology. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Raman IM, Trussell LO. AMPA receptors with high calcium permeability mediate synaptic transmission in the avian auditory pathway. The Journal of Physiology. 1995;482:309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. Journal of Comparative Neurology. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Edwards FA. ATP and glutamate are released from separate neurones in the rat medial habenula nucleus: frequency dependence and adenosine-mediated inhibition of release. The Journal of Physiology. 1998;508:691–701. doi: 10.1111/j.1469-7793.1998.691bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths; 1965. [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. The Journal of Physiology. 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Dieudonne S, Macdonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. The Journal of Physiology. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. Journal of Neuroscience. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP activated ionotropic receptor cloned from rat brain. Proceedings of the National Academy of Sciences of the USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurons. The Journal of Physiology. 1998;510:27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]


