Abstract
We studied the regulatory mechanism of Na+ transport by hyposmolality in renal epithelial A6 cells.
Hyposmolality increased (1) Na+ absorption, which was detected as an amiloride-sensitive short-circuit current (INa), (2) Na+-K+ pump activity, (3) basolateral Cl− conductance (Gb,Cl), and (4) phosphorylation of tyrosine, suggesting an increase in activity of protein tyrosine kinase (PTK).
A Cl− channel blocker, 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), which abolished Gb,Cl, blocked the INa by inhibiting the Na+-K+ pump without any direct effect on amiloride-sensitive Na+ channels. Diminution of Gb,Cl by Cl− replacement with a less permeable anion, gluconate, also decreased the hyposmolality-increased Na+-K+ pump activity.
The PTK inhibitors tyrphostin A23 and genistein induced diminution of the hyposmolality-stimulated Gb,Cl, which was associated with attenuation of the hyposmolality-increased Na+-K+ pump activity.
Taken together, these observations suggest that: (1) hyposmolality activates PTK; (2) the activated PTK increases Gb,Cl; and (3) the PTK-increased Gb,Cl stimulates the Na+-K+ pump.
This PTK-activated Gb,Cl-mediated signalling of hyposmolality is a novel pathway for stimulation of the Na+-K+ pump.
In a renal epithelial A6 cell line, hyposmolality increases amiloride-sensitive transepithelial Na+ transport mediated through apical amiloride-sensitive Na+ channels (Na+ entry step) and the basolateral Na+-K+ pump (Na+ extrusion step) by increasing the number of amiloride-sensitive Na+ channels (Wills et al. 1991) through stimulation of channel translocation from intracellular store sites to the apical membrane (Niisato & Marunaka, 1997c). It is, in general, thought that the entry step of Na+ across the apical membrane is the rate limiting step in transepithelial Na+ transport (see reviews by Eaton et al. 1992; Marunaka, 1997). Indeed, in A6 cells, the entry step of Na+ across the apical membrane is the rate limiting one in transepithelial Na+ transport under the basal condition (Marunaka, 1996, 1997), suggesting that an increase in Na+ entry is required to stimulate the transepithelial Na+ transport. However, under some conditions upregulation of the Na+-K+ pump activity is essential for stimulation of Na+ transport (Marunaka, 1996). However, no information is available on regulation of the Na+-K+ pump by osmolality. The present study investigated the effect of hyposmolality on the Na+-K+ pump activity, measuring short-circuit currents (Isc) of A6 cells. We report here that (1) hyposmolality activates protein tyrosine kinase (PTK), (2) Cl− conductances are increased by PTK, and (3) the increased Cl− conductance stimulates the Na+-K+ pump. Through this pathway, hyposmolality activates the Na+-K+ pump.
METHODS
Cell culture
A6 cells derived from Xenopus laevis were purchased from American Type Culture Collection (Rockville, MD, USA) at passage 68. Passages 76-84 were used for experiments. No differences were discernible between cells from different passages (Niisato et al. 1999). Cells were maintained in plastic tissue culture flasks at 27°C in a humidified incubator with 2 % CO2 in air in a culture medium which contained 75 % (v/v) NCTC-109 medium (Gibco, Grand Island, NY, USA), 15 % (v/v) distilled water and 10 % (v/v) fetal bovine serum (Gibco) (Niisato et al. 1999).
Solutions
An experimental hyposmotic solution contained (mM): 55 NaCl, 3·5 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes and 5 glucose (pH = 7·4). This solution was used for application of hypotonicity unless otherwise indicated. Cl− in the solution was replaced with gluconate or NO3− by substituting these at 55 mM for the 55 mM NaCl. The osmolalities of the hyposmotic and isotonic solutions were measured with a vapour pressure osmometer (model 5500, WESCOR Inc., Logan, UT, USA) and adjusted to 135 and 255 mosmol (kg H2O)−1, respectively, by adding appropriate amounts of sucrose. DMSO alone had no effects on currents and conductances.
Short-circuit current (Isc) measurement
Monolayers of cells subcultured on tissue culture-treated Transwell filter cups (Costar Corporation, Cambridge, MA, USA) for 10-14 days were transferred to a modified Ussing chamber (Jim's Instrument, Iowa City, IA, USA) designed to hold the filter cup. Transepithelial potential (PD) was continuously measured by a high-impedance millivoltmeter that could function as a voltage clamp with automatic fluid resistance compensation (VCC-600, Physiologic Instrument, San Diego, CA, USA) with a pair of calomel electrodes that were immersed in a saturated KCl solution and bridged to the modified Ussing chamber by a pair of polyethylene tubes filled with a solution of 2 % (w/v) agarose in 2 M KCl solution (Niisato & Marunaka, 1997a, b;Ito et al. 1997). Short-circuit current (Isc) was measured by the amplifier, VCC-600, with a pair of silver-silver chloride electrodes that were immersed in a 2 M NaCl solution and bridged to the modified Ussing chamber by a pair of polyethylene tubes filled with a solution of 2 % (w/v) agarose in a 2 M NaCl solution (Niisato & Marunaka, 1997a, b;Ito et al. 1997). When the Isc was measured, the PD was clamped to 0 mV for 1 s by the amplifier. Under a steady condition, the Isc was stable and did not change even if the transepithelial voltage was clamped to 0 mV for up to 1 min. In a non-steady state, the value of Isc measured at 1 s after clamping the PD to 0 mV is shown as Isc in the present study. A positive current represents a net flow of cations from the apical to the basolateral solution.
Measurement of ion conductance
Every 10 s we applied a pulse of ±1 μA constant current for 1 s to the monolayer under open-circuit conditions so that we could calculate the transepithelial conductance (Gt) from the change in the PD (ΔPD) caused by the constant current pulse (1 μA) using Ohm's law (Gt= 1 μA/ΔPD mV). Under a steady condition, the conductance was stable and did not change even if the transepithelial voltage was clamped to 0 mV for up to 1 min with application of a pulse of ±1 mV constant voltage for measurement of conductance under short-circuit conditions. In a non-steady state, the value of conductance measured at 1 s after applying a pulse of ±1 μA constant current for 1 s to the monolayer under open-circuit conditions or clamping the PD to 0 mV (Gt=Isc/PD) is shown as the conductance in the present study. As previously reported (Wills & Millinoff, 1990; Doi & Marunaka, 1995), the Isc measured directly by clamping the PD to 0 mV was identical to that calculated as Gt× PD (equivalent current); that is, the monolayer had a linear current-voltage relationship.
Measurement of the Na+-K+ pump current
To measure the current generated by the Na+-K+ pump (the pump current), 50 μM nystatin (an ionophore of monovalent ions) was applied to the apical side (Marunaka, 1996). This treatment increased the cytosolic Na+ concentration via elevation of the apical Na+ conductance, leading to a condition that the rate-limiting step of the transepithelial Na+ transport was the extrusion step across the basolateral membrane by the Na+-K+ pump (Lewis et al. 1977). The pump current was estimated by applying an inhibitor of the Na+-K+ pump, ouabain (1 mM), to the basolateral side. The ouabain-sensitive current is shown as the pump current in the present study.
Single channel current recording
Cells were subcultured on translucent porous Nunc filter inserts (Nunc tissue culture inserts, Nunc, Roskilde, Denmark) for 10-14 days before applying the single channel recording technique. Standard patch clamp techniques were used (Hamill et al. 1981; Marunaka et al. 1992, 1994, 1997, 1999a). Patch pipettes were made from LG 16 glass (Dagan Corporation, Minneapolis, MN, USA) and fire polished to produce tip diameters of about 0·5 μm. The patch pipette was applied from the apical side. Then, we made a gigaohm seal (> 100 GΩ) on the apical membrane of cells. Single channel currents were obtained with an Axopatch-1D patch clamp amplifier (Axon Instruments). Current signals were recorded on a digital video recorder (HF860D, Sony, Tokyo) with pulse-code modulation (1-DR-390, Neuro Data Instruments Corporation, New York, NY, USA). Current signals were digitized at a sampling rate of 5000 Hz, and analysed with a continuous-data acquisition program. A 100 Hz low-pass filter using a software Gaussian filter was used to present the actual traces. The open probability was measured at no applied potential in cell-attached patches (i.e. resting membrane potential) and at -100 mV in inside-out patches (Marunaka & Eaton, 1991; Marunaka & Tohda, 1993; Marunaka et al. 1998).
Western blotting
Cells were subcultured on translucent porous Nunc filter inserts (Nunc Tissue Culture Inserts, Nunc, Roskilde, Denmark) for 10-14 days before Western blot experiments. Cells with and without hypotonic treatment were lysed by lysis buffer (50 mM Hepes, 150 mM NaCl, 1·5 mM MgCl2, 1 mM EGTA, 10 % (v/v) glycerol, 1 % (v/v) Triton X-100, 100 mM NaF, 10 mM pyrophosphate, 200 μM sodium orthovanadate, 250 μg ml−1 leupeptin, 0·1 mM phenylmethylsulfonyl fluoride, 100 kallikrein inactivator units ml−1 aprotinin, pH 7·4) on ice. Cells were homogenized by sonication and centrifuged at 12 000 g for 10 min at 4°C to remove insoluble debris. The cell lysate containing 25 μg protein was boiled in SDS sample buffer (60 mM Tris-HCl, 2 % (w/v) SDS, 5 % (v/v) glycerol, pH 6·8) and then subjected to 10 % (w/v) SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred to nitrocellulose membranes. Non-specific binding was blocked by incubation in 5 % (w/v) bovine serum albumin for 60 min. Membranes were immunoblotted with a monoclonal anti-phosphotyrosine antibody, PY99 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). After overnight incubation at 4°C, the membrane was washed with Tris-buffered saline (TBS) and incubated for 60 min at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG. After washing, blots were developed with an enhanced chemiluminescence (ECL) detection kit from Amersham (Oakville, Ontario, Canada). The intensity of the bands was quantified with an Imaging Densitomer (GS-690, Bio-Rad Laboratories, Hercules, CA, USA).
Temperature
All experiments for electrophysiological and other measurements were performed at 24-25°C unless otherwise indicated.
Chemicals and materials
5-Nitro-2-(3-phenylpropylamino)-benzoate (NPPB) was obtained from Research Biochemicals International. Diphenylamine-2-carboxylate (DPC) was purchased from Fluka. Tyrphostin A23 and genistein were obtained from Calbiochem. Amiloride, ouabain, nystatin, Hepes and other chemical compounds were purchased from Sigma Chemical Company unless otherwise indicated. Amiloride, NPPB, DPC, tyrphostin A23, genistein, nystatin, and ouabain were dissolved in DMSO. The final concentration of DMSO did not exceed 0·1 %.
Data presentation
All data are presented as means ± standard error of the mean (s.e.m.). Where s.e.m. bars are not visible, they are smaller than the symbol. Student's t test, ANOVA and Duncan's multiple range comparison test were used for statistical analysis as appropriate and P < 0·05 was considered significant.
RESULTS
Effects of amiloride and NPPB on short-circuit current (Isc)
Isc increased with time after the cell was exposed to a hyposmotic solution (Fig. 1A). To study what kind of ion transport was stimulated in a solution with low osmolality, we applied amiloride (a blocker of epithelial Na+ channels; Kleyman & Cragoe, 1988, 1990) and NPPB (a Cl− channel blocker; Gögelein, 1988; Greger, 1990). Amiloride (10 μM) applied to the apical membrane almost completely abolished the increased Isc (Fig. 1Aa), suggesting that most (∼95 %) of the increased Isc is due to Na+ absorption. To determine whether the increased Isc contained Cl− transport, we added NPPB (100 μM) to both the apical and basolateral sides. NPPB also almost completely abolished the increased Isc (Fig. 1Ab) to a level similar to the amiloride-induced one (Fig. 1Aa); i.e. most (∼95 %) of the increased Isc was blocked by NPPB as well as amiloride. This observation suggests that NPPB blocks the amiloride-sensitive transepithelial Na+ transport in A6 cells. However, NPPB is thought to be a Cl− channel blocker, but not an amiloride-sensitive Na+ channel blocker. Therefore NPPB would not be expected to block an amiloride-sensitive Na+ channel.
Figure 1. Effects of amiloride and NPPB on hyposmolality-induced Isc and conductance.
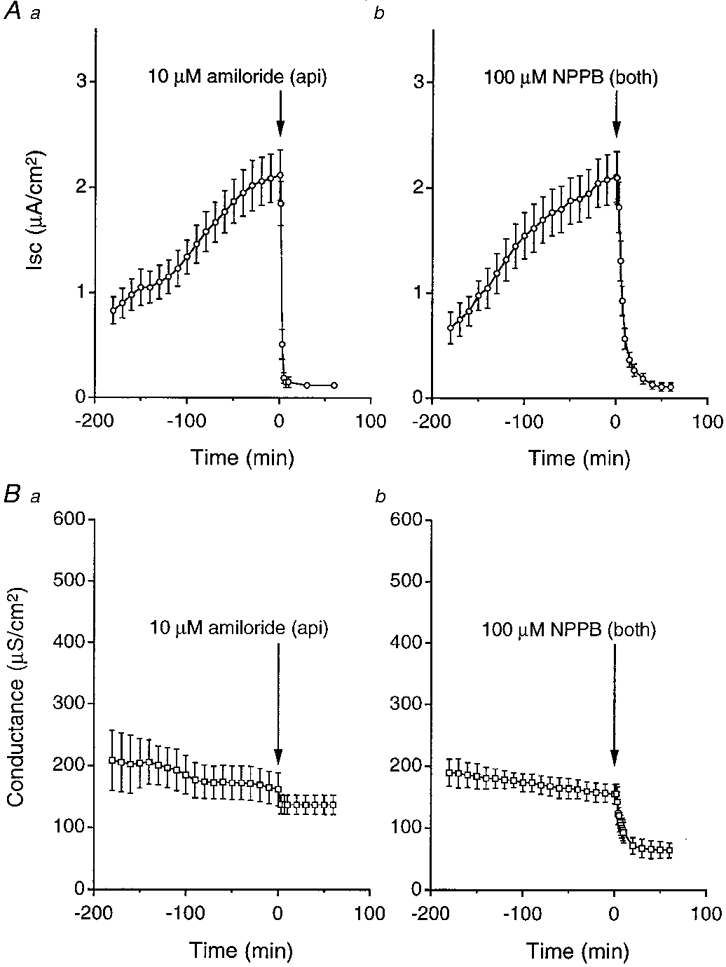
A, Isc increased gradually with time after exposure of cells to a hyposmotic solution. a, amiloride (10 μM) applied to the apical solution abolished the increased Isc. b, NPPB (100 μM) applied to the apical and basolateral solutions also abolished the increased Isc, reducing it to a level similar to that with amiloride. B, conductances did not significantly change during the period when cells were exposed to a hyposmotic solution. a, amiloride (10 μM) applied to the apical solution induced a small decrease in conductance. b, NPPB (100 μM) applied to the apical and basolateral solutions induced a much larger decrease in conductance than amiloride. Although the Isc increased with time after application of hypotonicity, the conductance did not significantly increase. The amiloride-sensitive conductance might increase, but the amiloride-sensitive conductance was much smaller than the total conductance (Fig. 1B a). Therefore, we could not see a significant increase in the total conductance even if the amiloride-sensitive conductance increases. Number of experiments (n) = 4.
We also measured transepithelial conductances. Hyposmolality did not significantly change the conductance (Fig. 1B). Amiloride and NPPB decreased the conductance. However, the amiloride-sensitive conductance (Fig. 1B a) was much smaller than the NPPB-sensitive conductance (Fig. 1B b).
The effect of NPPB on the amiloride-sensitive Na+ channel
Although NPPB is, in general, thought to be a Cl− channel blocker, but not an amiloride-sensitive Na+ channel blocker, we had no direct evidence that an amiloride-sensitive Na+ channel is not blocked by NPPB. To study whether NPPB blocks an amiloride-sensitive channel with a single channel conductance of 4 pS which contributes to transepithelial Na+ transport in A6 cells (Wills et al. 1991; Marunaka & Eaton, 1991; Marunaka et al. 1992), we applied the patch clamp (single channel recording) technique to cells 150 min after exposure to a hyposmotic solution. Figure 2A shows single channel currents through a 4 pS amiloride-sensitive Na+ channel (Marunaka & Eaton, 1991; Marunaka et al. 1992) in cell-attached patches without (Fig. 2Aa) and with (Fig. 2Ab) 200 μM NPPB in the pipette solution. Figure 2B shows the statistical result of NPPB action on the open probability of the channel. These single channel recordings clearly indicate that NPPB (200 μM) applied in the patch pipette (extracellular surface) had no significant effect on the open probability of the channel (Fig. 2). Further, we studied the effect of NPPB applied to the cytosolic surface on the open probability of the channel in inside-out patches. Figure 3A shows actual traces of single channel currents before (Fig. 3Aa) and 20 min after (Fig. 3Ab) application of 200 μM NPPB in an inside-out patch. Channel activity after NPPB application seems to be maintained at a level identical to that before its application. NPPB applied to the cytosolic surface had no significant effect on the open probability (Fig. 3B) similar to that in the case of NPPB applied to the extracellular surface (Fig. 2B). These observations indicate that NPPB does not block the 4 pS amiloride-sensitive Na+ channel that contributes to the Na+ transport in A6 cells (Wills et al. 1991; Marunaka & Eaton, 1991; Marunaka et al. 1992; Marunaka, 1997) but abolishes the amiloride-sensitive transepithelial Na+ transport. Accordingly, we should consider another inhibitory pathway of the amiloride-sensitive transepithelial Na+ transport by NPPB.
Figure 2. The effect of NPPB on single channel currents through 4 pS amiloride-sensitive Na+ channels in cell-attached patches.
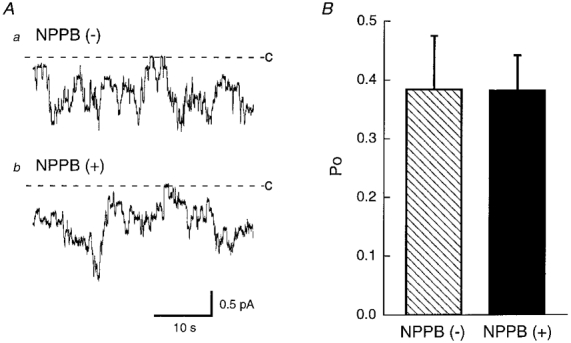
A, actual traces of single channel currents in the absence (a) and presence (b) of NPPB (200 μM) in the pipette solution obtained from cells 150 min after exposure to a hyposmotic solution. Even in the presence of NPPB in the pipette, the channel showed activity similar to that in the absence of NPPB. B, statistical results of the open probability (Po) of the channel in the absence ( ) and presence (▪) of NPPB (200 μM) in the pipette solution. NPPB had no significant effect on the open probability of the channel. n = 8. C and dashed line represent the closed level of the channel.
) and presence (▪) of NPPB (200 μM) in the pipette solution. NPPB had no significant effect on the open probability of the channel. n = 8. C and dashed line represent the closed level of the channel.
Figure 3. The effect of NPPB on single channel currents through 4 pS amiloride-sensitive Na+ channels in inside-out patches.
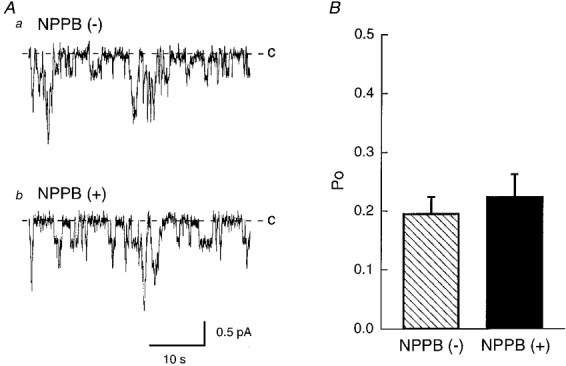
A, actual traces of single channel currents before (a) and 20 min after (b) 200 μM NPPB applied to the cytosolic surface in an inside-out patch obtained from a cell 150 min after exposure to a hyposmotic solution. Even after application of NPPB to the bathing solution (cytosolic surface), the channel showed activity similar to that before its application. B, statistical results of the open probability (Po) of the channel before ( ) and 20 min after (▪) application of 200 μM NPPB. NPPB had no significant effect on the open probability of the channel. n = 6.
) and 20 min after (▪) application of 200 μM NPPB. NPPB had no significant effect on the open probability of the channel. n = 6.
Effects of amiloride and NPPB on the Na+-K+ pump activity
Amiloride-sensitive transepithelial Na+ transport is mediated through two steps: a Na+ entry step through amiloride-sensitive Na+ channels at the apical membrane and a Na+ extrusion step by the Na+-K+ pump at the basolateral membrane. If either of these two steps is blocked, the amiloride-sensitive transepithelial Na+ transport is abolished. Since NPPB did not directly inhibit the 4 pS amiloride-sensitive Na+ channel, we considered whether NPPB blocks the Na+-K+ pump activity leading to abolishment of the amiloride-sensitive transepithelial Na+ transport.
To estimate the Na+-K+ pump activity, we treated cells with nystatin (50 μM) applied to the apical membrane, which would increase the cytosolic Na+ concentration, resulting in activation of the Na+-K+ pump (Lewis et al. 1977). Under this condition, the rate limiting step of the transepithelial Na+ transport was the extrusion step of Na+ by the Na+-K+ pump at the basolateral membrane. By measuring an ouabain-sensitive current, we estimated the Na+-K+ pump activity (Fig. 4A). The Isc reached a steady state by 30 min after application of 50 μM nystatin. Therefore, we applied ouabain (1 mM) to the monolayer 30 min after addition of nystatin. When amiloride was applied to the apical solution, the increased Isc was diminished due to blockade of the entry step of Na+ by amiloride (Fig. 4B). Application of nystatin in the presence of amiloride increased the Isc (Fig. 4B) to a level similar to that without amiloride (Fig. 4A), and ouabain abolished the nystatin-increased current (Fig. 4B) similar to control (Fig. 4A). NPPB also diminished the hyposmolality-induced Isc (Fig. 4C) similar to amiloride (Fig. 4B), but a much smaller effect of nystatin on the current in the presence of NPPB (Fig. 4C) was observed than for control (Fig. 4A) or with amiloride (Fig. 4B). NPPB significantly diminished the Na+-K+ pump activity (Fig. 5), suggesting that NPPB abolishes the amiloride-sensitive transepithelial Na+ transport by blocking the Na+-K+ pump activity.
Figure 4. Effects of amiloride and NPPB on ouabain-sensitive currents.
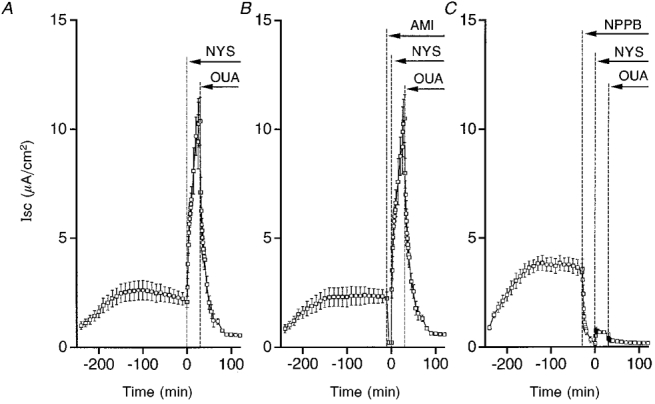
Time course of Isc. A, control. Nystatin (50 μM, NYS) applied to the apical solution quickly increased Isc. Isc reached a steady state by 30 min after application of nystatin. Ouabain (1 mM, OUA) applied to the basolateral solution abolished most of the Isc. n = 10. B, amiloride applied to the apical solution. Although amiloride (10 μM, AMI) abolished the Isc, nystatin (NYS) increased the Isc similar to control. Ouabain (1 mM, OUA) applied to the basolateral solution abolished most of the Isc. n= 4. C, NPPB applied to the apical and basolateral solutions. NPPB (100 μM) abolished the Isc similar to amiloride. However, unlike amiloride, nystatin induced a small increase in Isc, and a small effect of ouabain was observed. n = 5.
Figure 5. Statistical results of ouabain-sensitive currents.
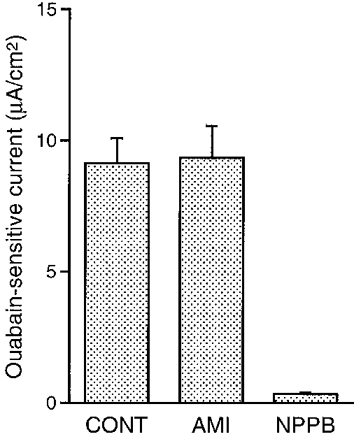
Amiloride (10 μM; apical application) had no significant effects on the ouabain-sensitive current, but NPPB (100 μM; bilateral application) almost completely abolished the ouabain-sensitive current. Control (CONT), n= 10; amiloride (AMI), n= 4; NPPB, n= 5.
Effects of DPC and replacement of Cl− on the Na+-K+ pump activity in a hypotonic solution
Since NPPB is a Cl− channel blocker, we studied whether the blocking action of NPPB is mediated through diminution of Cl− conductance. To decrease the Cl− conductance, we applied another Cl− channel blocker, DPC. Figure 6 shows the time course of Isc under various conditions and the results are summarized in Fig. 7. Application of DPC (2 mM) also diminished the ouabain-sensitive current similar to NPPB (Fig 6A and Fig 7). Further, to diminish Cl− conductances without using channel blockers, we replaced Cl− with a less membrane-permeable anion, gluconate. The nystatin-induced current in a gluconate solution (Fig. 6B) was smaller than control (Fig. 4A) (see also a summary shown in Fig. 7). The ouabain-sensitive current in a gluconate solution (Fig. 6B) was also smaller than control (Fig. 4A) (see also a summary shown in Fig. 7). However, replacement of Cl− with NO3−, which is a more membrane-permeable anion than gluconate, had no significant effect on the nystatin-induced ouabain-sensitive currents (Fig 6C and Fig 7). These observations suggest that Cl− conductances may play a crucial role in maintenance of the Na+-K+ pump activity.
Figure 6. Time course of Isc in a hyposmotic solution with DPC (A), gluconate (B) or NO3− (C).
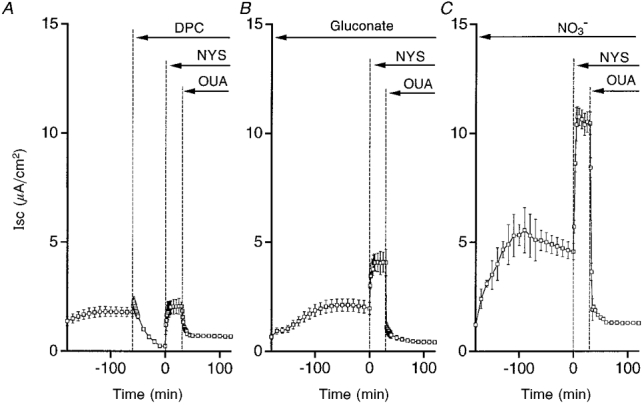
A, DPC (2 mM; bilateral application) decreased the Isc. In the presence of DPC, nystatin (50 μM, NYS; apical application) induced a small current. Ouabain (1 mM, OUA; basolateral application) diminished the nystatin-induced Isc. B, in a gluconate solution, nystatin induced an increase in Isc, which was larger than that in the presence of DPC (A) but much smaller than control (see Fig. 4A). Ouabain (1 mM, OUA; basolateral application) diminished the nystatin-induced Isc. C, in a NO3− solution, nystatin induced a large increase in Isc similar to control (see Fig. 4A). Ouabain (1 mM, OUA; basolateral application) diminished the nystatin-induced Isc. n = 4-8.
Figure 7. Effects of NPPB, DPC and Cl− removal on the ouabain-sensitive current in a hyposmotic solution.
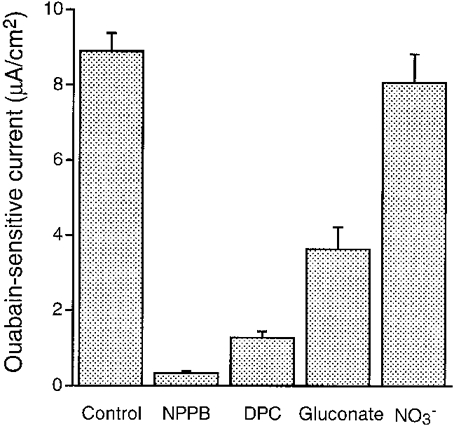
The ouabain-sensitive current was measured in cells treated with 50 μM nystatin (apical application). NPPB (100 μM) almost completely abolished the ouabain-sensitive current. DPC (2 mM), which was applied to the apical and basolateral solutions, also significantly diminished the ouabain-sensitive current. Replacement of Cl− with gluconate, which is a relatively impermeable anion, in the apical and basolateral solutions also significantly diminished the ouabain-sensitive current. Replacement of Cl− with NO3−, which generally has a permeability identical to Cl−, in the apical and basolateral solutions had no significant effects on the ouabain-sensitive current. n = 4-8.
Effects of DPC and replacement of Cl− on the NPPB-sensitive conductance
Although we suggest that Cl− conductances may play a key role in the Na+-K+ pump activity from results of experiments using Cl− channel blockers or removing Cl−, we did not measure Cl− conductances. After application of nystatin to the apical membrane, we estimated the Cl− conductance by measuring the NPPB-sensitive conductance. Figure 8A shows the time course of conductance change in control experiments. Nystatin (50 μM) applied to the apical membrane increased the conductance about 7-fold, which reached a steady state by 30 min after application of nystatin. We applied 100 μM NPPB to both the apical and basolateral membranes 30 min after application of nystatin. NPPB rapidly diminished the increased conductance and most of the conductance was sensitive to NPPB. We also studied the effect of DPC on the conductance (Fig. 8B). DPC (2 mM; bilateral application) decreased the basal conductance. The DPC treatment almost completely diminished the nystatin action on the conductance (Fig. 8B), and NPPB had a small effect on the conductance. To test the effect of Cl− replacement with gluconate on the conductance, we measured the conductance in a gluconate solution (Fig. 8C). In a gluconate solution, nystatin had a little effect on the conductance similar to DPC. The replacement of Cl− with gluconate decreased the basal conductance (compare the basal conductances in Fig. 8A and C), suggesting that the Cl− conductance significantly contributes to the basal conductance. However, in a NO3− solution, nystatin had an effect on the conductance identical to control (see Fig. 8A and D). In this case, NPPB drastically diminished the nystatin-induced conductance (Fig. 8D) similar to control (Fig. 8A). The NPPB-sensitive conductances of nystatin (apical application)-treated cells under the conditions shown in Fig. 8 are summarized in Fig. 9.
Figure 8. Time courses of conductance under various conditions.
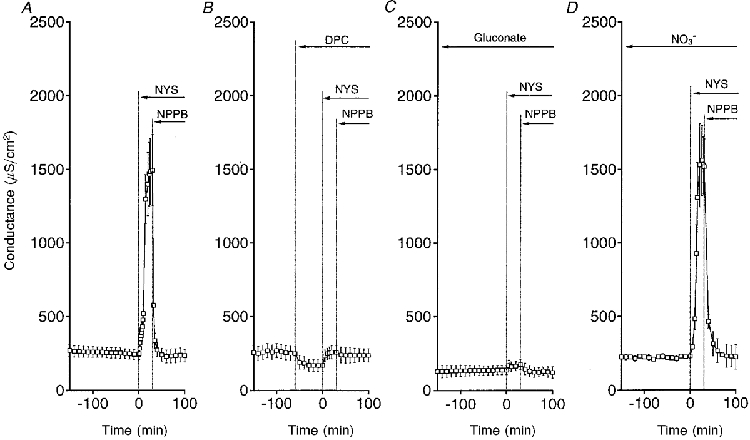
A, control. B, DPC (2 mM, bilateral application) decreased the basal conductance. Nystatin (50 μM, apical application) had a small effect on the conductance. NPPB decreased the conductance a little bit. C, in a Cl−-free gluconate solution, nystatin induced a small increase in conductance. NPPB decreased the conductance a little bit. D, in a Cl−-free NO3− solution, nystatin drastically increased the conductance, identical to control. NPPB also markedly inhibited the increased conductance similar to control. n = 4-6.
Figure 9. NPPB-sensitive conductances under various conditions.
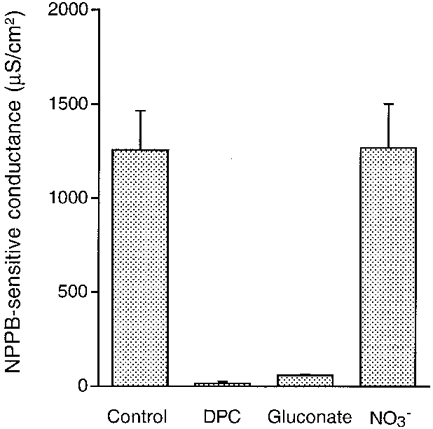
The NPPB-sensitive conductance was measured in cells treated with 50 μM nystatin (apical application). The NPPB-sensitive conductance observed in a hypotonic solution was abolished by DPC (2 mM; apical and basolateral application). The NPPB-sensitive conductance was also diminished in a Cl−-free solution with gluconate replacement, but replacement of Cl− with NO3− had no inhibitory effects on the NPPB-sensitive conductance, unlike gluconate. n = 4-6.
Correlation between NPPB-sensitive conductance and ouabain-sensitive current
Figure 10 shows a correlation between the NPPB-sensitive conductance and the ouabain-sensitive current. The NPPB-sensitive conductance and the ouabain-sensitive current did not have a linear correlation. Therefore, we tried to study whether the correlation between the NPPB-sensitive conductance and the ouabain-sensitive current is fitted by a Michaelis-Menten equation. As shown in Fig. 10, the correlation between the NPPB-sensitive conductance and the ouabain-sensitive current was well fitted by a Michaelis-Menten equation; Im (the maximum value of the ouabain-sensitive current) was estimated to be 9·8 μA cm−2 and Km (the value of the NPPB-sensitive conductance that is half the maximum value of the ouabain-sensitive current) was estimated to be 104·3 μS cm−2 (correlation coefficient = 0·86). This fitting result indicates that the NPPB-sensitive conductance and the ouabain-sensitive current have a correlation represented by a Michaelis-Menten equation.
Figure 10. Correlation between NPPB-sensitive conductance and ouabain-sensitive current.
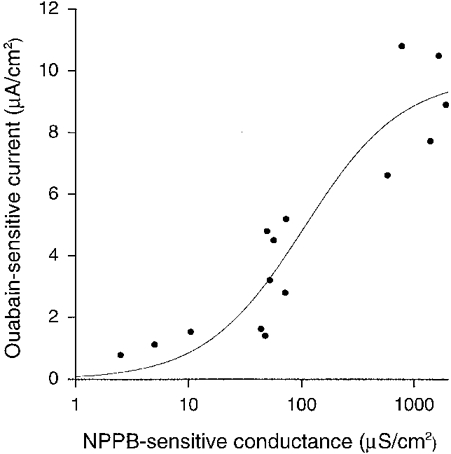
The ouabain-sensitive current did not increase linearly against the NPPB-sensitive conductance. The relationship between the NPPB-sensitive conductance and the ouabain-sensitive current was fitted by a Michaelis-Menten equation. The estimated value of the maximum ouabain-sensitive current (Im) was 9·8 μA cm−2; the conductance showing the half-maximum ouabain-sensitive current (Km) was estimated to be 104·3 μS cm−2. The continuous line is drawn by the equation with these values (Im of 9·8 μA cm−2, Km of 104·3 μS cm−2). Correlation coefficient = 0·86. The data fitting was done with a non-linear least-squares method.
The effect of hyposmolality on the Na+-K+ pump activity and the basolateral Cl− conductance
As indicated in previous reports using noise analysis and single channel current recording techniques (Wills et al. 1991; Niisato & Marunaka, 1997c), hyposmolality stimulates an amiloride-sensitive Isc by increasing the number of conducting amiloride-sensitive Na+ channels in the apical membrane. However, it is unknown whether hyposmolality has any effects on the Na+-K+ pump activity. Therefore, we studied the effect of hyposmolality on the Na+-K+ pump activity. The Na+-K+ pump activity was increased by hyposmolality (Fig. 11A). As demonstrated above, the NPPB-sensitive conductance is required to maintain the Na+-K+ pump activity. Therefore, hyposmolality may increase the Na+-K+ pump activity by increasing the NPPB-sensitive conductance. To study whether the hyposmolality-increased activity of the Na+-K+ pump is associated with an increase in the NPPB-sensitive conductance, we measured the NPPB-sensitive Cl− conductance. As shown in Fig. 11B, hyposmolality increased the NPPB-sensitive conductance. The statistical results are summarized in Fig. 12. These observations strongly suggest that hyposmolality increases the Na+-K+ pump activity by elevating the Cl− conductance. Further, this conductance was due to the basolateral Cl− conductance, since the apical membrane was permeabilized by nystatin (see details in Discussion).
Figure 11. Time courses of Isc and conductance after exposure of cells to a hyposmotic or isosmotic solution.
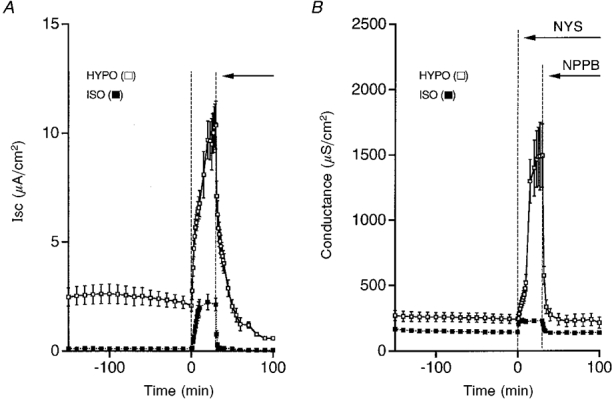
A, nystatin (NYS, 50 μM, apical application) increased Isc, but the magnitude of the increased Isc was much larger in a hyposmotic solution (□) than that in an isosmotic solution (▪). The ouabain-sensitive current was also much larger in a hyposmotic solution (□) than that in an isosmotic solution (▪). B, nystatin increased the conductance in both hyposmotic and isosmotic solutions, but the nystatin-induced increase in conductance was much larger in a hypotonic solution (□) compared with that in an isosmotic solution (▪). The NPPB-sensitive conductance was much larger in a hyposmotic solution (□) than that in an isosmotic solution (▪). n = 4-8.
Figure 12. The effect of hyposmolality on the ouabain-sensitive current and the NPPB-sensitive conductance.
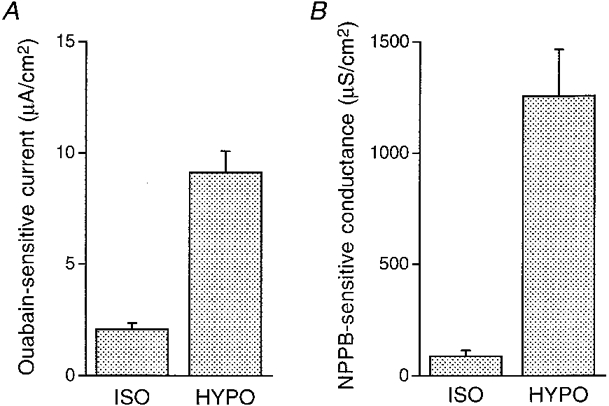
The ouabain-sensitive current and the NPPB-sensitive conductance were measured in cells treated with 50 μM nystatin (apical application). A, ouabain-sensitive currents. Hyposmolality increased the ouabain-sensitive current. B, NPPB-sensitive conductances. Hyposmolality increased the NPPB-sensitive Cl− conductance. n = 4-8.
Effects of inhibitors of protein tyrosine kinase (PTK) on the Isc, the Na+-K+ pump activity and the basolateral Cl− conductance
We suggest that the hyposmolality-induced increase in the Na+-K+ pump activity would be mediated through elevation of the basolateral Cl− conductance. However, the mechanism by which hyposmolality elevates the basolateral Cl− conductance is still unknown. Some studies have reported that hypotonic stress increases protein tyrosine phosphorylation in human intestine cells (Tilly et al. 1993) and in human neutrophils (Edashige et al. 1993), suggesting that hypotonic stress activates protein tyrosine kinase (PTK). Further, a study in cardiac myocytes (Sadoshima et al. 1996) has more recently provided evidence that hypotonic stress immediately activates PTK. We therefore considered the contribution of PTK to the hyposmolality-induced increases in the Na+-K+ pump activity and the basolateral Cl− conductance. To test for a role of PTK in the hyposmolality-increased Na+-K+ pump activity and basolateral Cl− conductance, we applied a PTK inhibitor, tyrphostin A23, to the cell. Tyrphostin A23 (100 μM; bilateral application) diminished the hyposmolality-induced Isc (Fig. 13A). In tyrphostin A23-treated cells, nystatin induced a much smaller increase in Isc than control (Fig. 13A) and the ouabain-sensitive current was also much smaller than control (Fig 13A and Fig 14). Tyrphostin A23 also abolished the NPPB-sensitive conductance (Fig 13B and Fig 14). Another PTK inhibitor, genistein (100 μM; bilateral application with the same time schedule as tyrphostin A23), also diminished the stimulatory effects of hyposmotic stress on the ouabain-sensitive current and the NPPB-sensitive conductance (Fig. 14).
Figure 13. Time courses of Isc and conductance in the presence and absence of a PTK inhibitor, tyrphostin A23 (TY23), after exposure of cells to a hyposmotic solution.
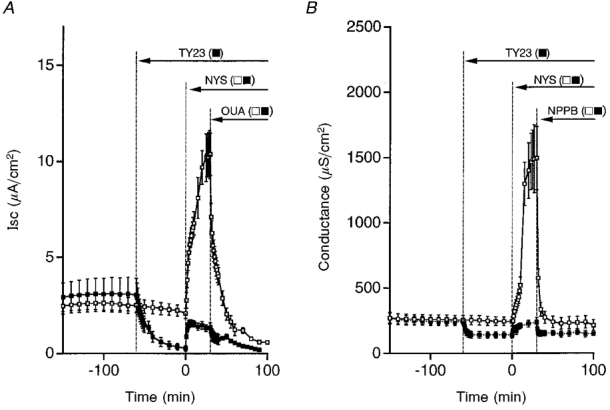
A, TY23 (100 μM, bilateral application) diminished the basal Isc (▪). The Isc increased by nystatin (NYS, 50 μM, apical application) was diminished by TY23 treatment. The ouabain-sensitive current was also much smaller in the presence (▪) than in the absence of TY23 (□). B, TY23 decreased the basal conductance. The magnitude of the nystatin-increased conductance was much smaller in the presence (▪) than in the absence of TY23 (□). The NPPB-sensitive current was also much smaller in the presence of TY23 (▪) than in the absence of TY23 (□). n = 5-8.
Figure 14. Effects of inhibitors of protein tyrosine kinase (PTK) on the ouabain-sensitive current and the NPPB-sensitive conductance.
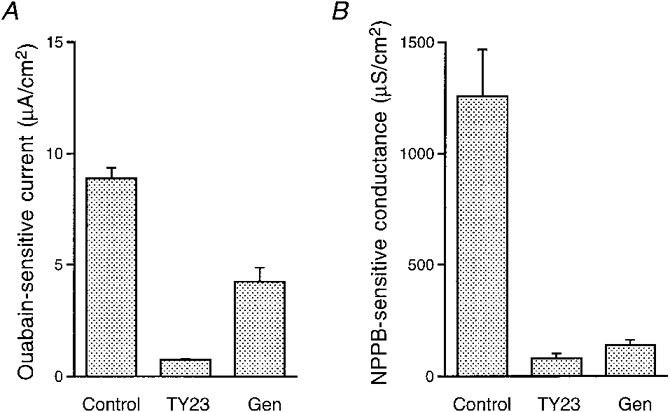
The ouabain-sensitive current and the NPPB-sensitive conductance were measured in cells treated with 50 μM nystatin (apical application) in a hypotonic solution. A, ouabain-sensitive currents. TY23 almost completely abolished the ouabain-sensitive current. Another PTK inhibitor, genistein (Gen; 100 μM; bilateral application), also significantly diminished the ouabain-sensitive current. Control, n= 8; TY23, n= 8; Gen, n= 4. B, TY23 and Gen significantly diminished the NPPB-sensitive conductance. Control, n= 5; TY23, n= 5; Gen, n= 3.
Effects of hyposmolality and PTK inhibitor on phosphorylation of tyrosine
PTK inhibitors diminished the hyposmolality-induced increases in the Na+-K+ pump activity and the basolateral Cl− conductance. Therefore, a possible signalling pathway of hyposmolality to activation of the Na+-K+ pump and the basolateral Cl− conductance would be mediated through activation of PTK. Activation of PTK would increase phosphorylation of tyrosine. Therefore, we studied whether hyposmolality stimulates phosphorylation of tyrosine. Incubation of cells in a hyposmotic solution increased phosphorylation of tyrosine in multiple proteins of various molecular weights (Fig. 15); especially, 60 kDa proteins showed a large increase in tyrosine phosphorylation (Fig 15 and Fig 16). The hyposmolality-induced increase in phosphotyrosine was blocked by treatment with tyrphostin A23 (Fig 15 and Fig 16), suggesting that hyposmolality may increase tyrosine phosphorylation by activating PTK.
Figure 15. Effects of hyposmotic shock and tyrphostin A23 (TY23) on phosphorylation of tyrosine.
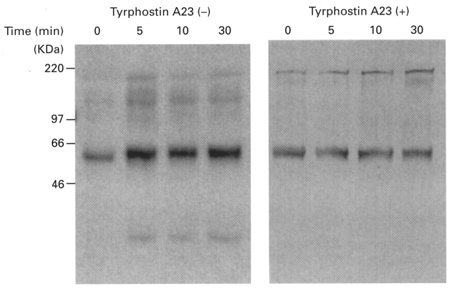
After exposure of cells to a hypotonic solution with or without pretreatment of tyrphostin A23 (100 μM, bilateral application, 1 h), cell lysates were prepared. The cell lysates containing 25 μg protein were subjected to 10 % SDS-PAGE and then immunoblotted with anti-phosphotyrosine antibody, PY99. Phosphorylation of tyrosine increased with time after cells had been exposed to a hyposmotic solution. Tyrosine of 60 kDa proteins was mainly phosphorylated by hyposmotic shock. Tyrphostin A23 (TY23, 100 μM) blocked the hyposmolality-induced phosphorylation of tyrosine.
Figure 16. Quantified results of tyrosine phosphorylation in proteins of around 60 kDa with an imaging densitometer.
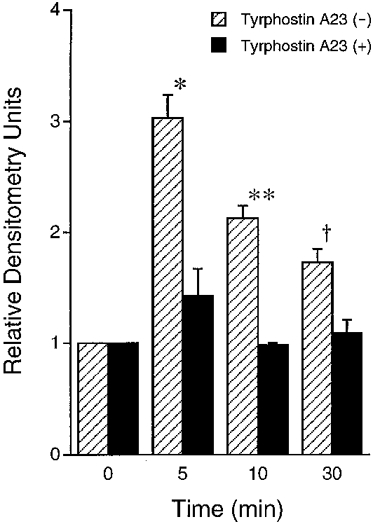
In the absence of tyrphostin A23 ( ), hyposmotic stress increased phosphotyrosine with a peak around 5 min after application of hyposmotic stress followed by a decline of phosphotyrosine. The amount of phosphotyrosine at 5, 10 and 30 min after application of hypotonic stress in the absence of tyrphostin A23 was larger than the basal amount at 0 min (* P < 0·01, **P < 0·01 and †P < 0·025 compared with 0 min). In the presence of TY23 (100 μM, bilateral application; ▪), hypotonic stress did not significantly increase the amount of phosphotyrosine; i.e. the amount of phosphotyrosine at 5, 10 or 30 min was not significantly different from that at 0 min. n = 3.
), hyposmotic stress increased phosphotyrosine with a peak around 5 min after application of hyposmotic stress followed by a decline of phosphotyrosine. The amount of phosphotyrosine at 5, 10 and 30 min after application of hypotonic stress in the absence of tyrphostin A23 was larger than the basal amount at 0 min (* P < 0·01, **P < 0·01 and †P < 0·025 compared with 0 min). In the presence of TY23 (100 μM, bilateral application; ▪), hypotonic stress did not significantly increase the amount of phosphotyrosine; i.e. the amount of phosphotyrosine at 5, 10 or 30 min was not significantly different from that at 0 min. n = 3.
DISCUSSION
The present study indicates that a Cl− conductance plays a crucial role in maintenance of the Na+-K+ pump activity and hyposmotic shock increases the Na+-K+ pump activity by increasing a Cl− conductance in a PTK-dependent pathway. The Na+-K+ pump current (Na+ extrusion step) in an isosmotic solution was not larger than the INa in a hypotonic solution. This observation suggests that if hyposmotic shock has no stimulatory effects on the Na+-K+ pump, the rate limiting step of transepithelial Na+ transport would be the extrusion step of Na+ across the basolateral membrane by the Na+-K+ pump and the hyposmotic shock could not increase the amiloride-sensitive Isc (INa) to a level shown in the present study. Therefore, the stimulatory action of hyposmotic shock on the Na+-K+ pump is essentially required to stimulate the transepithelial Na+ transport to a level shown in the present study.
A report (Féraille et al. 1997) has recently indicated that tyrosine phosphorylation regulates the Na+-K+ pump activity. Although this report (Féraille et al. 1997) indicates regulatory mechanisms of the Na+-K+ pump activity by PTK, it is still unclear how PTK activates the Na+-K+ pump. The present study suggests that the Na+-K+ pump activity would be regulated by a PTK-dependent Cl− conductance and hyposmolality would stimulate the Na+-K+ pump via activation of the basolateral Cl− conductance mediated through PTK, although we need to perform further experiments to determine which phosphoproteins are involved in stimulation of sodium transport by hyposmotic stress.
The Cl− conductance measured as the NPPB-sensitive conductance
As shown in Fig 8 and Fig 9, the NPPB-sensitive conductance was almost completely diminished by another Cl− channel blocker, DPC (Gögelein, 1988; Greger, 1990) or replacement of Cl− in the bathing solution with an anion less permeable through Cl− channels, gluconate (Marunaka & Eaton, 1990a, b;Eaton et al. 1992), but not by replacement of Cl− with NO3−, which has an identical permeability to Cl− (Eaton et al. 1992). Further, in the presence of NPPB, the conductance was not significantly changed by Cl− replacement with gluconate (compare the conductance after application of NPPB with nystatin shown in Fig. 8A and C; 233·7 ± 42·3 μS cm−2 in a Cl− solution (n= 5) vs. 121·3 ± 38·7 μS cm−2 in a gluconate solution (n= 6)), suggesting that NPPB completely abolishes the Cl− conductance. Further, our preliminary observation indicates that the NPPB-induced decrease in Gt was not affected by removal of Na+ or K+. These observations suggest that the NPPB-sensitive conductance represents the Cl− conductance.
The basolateral Cl− conductance
In the present study, we measured the Cl− (NPPB-sensitive) conductance applying 50 μM nystatin to the apical membrane. This apical application of nystatin increased the conductance (see Fig. 8A). This increase in conductance was due to the apical conductance. However, we did not know whether the permeabilization of the apical membrane by nystatin was complete enough to neglect the electrical parameter of the apical membrane in the present study. In other words, even with the apical application of nystatin, it was still unclear whether the measured Cl− conductance was the basolateral one. A study (Erlij et al. 1994) indicates that the apical application of nystatin (the same concentration in the present study) to A6 cells permeabilizes the apical membrane, but the electrical parameters of the apical membrane still significantly contribute to the electrical properties such as the membrane capacitance of the epithelium. Therefore, the Cl− conductance measured in A6 cells with the apical application of nystatin would not be concluded to be the basolateral Cl− conductance. To assess whether the NPPB-sensitive Cl− conductance measured in the present study was due to the basolateral one, we applied nystatin (100 μM) to the basolateral membrane after the apical application of nystatin (50 μM). Since the transepithelial conductance without nystatin treatment was negligibly small compared with that after bilateral application of nystatin (see details below), the paracellular conductance is negligibly small compared with the transcellular conductance. Therefore, the apical and basolateral conductances are represented as follows:
| (1) |
| (2) |
where Gt,n is the measured transepithelial (transcellular) conductance with the apical application of nystatin, Gt,nn is the measured transepithelial (transcellular) conductance with the apical and basolateral application of nystatin, Ga,n is the apical conductance with the apical application of nystatin, Gb is the basolateral conductance with the apical application of nystatin and Gb,n is the basolateral conductance with the apical and basolateral application of nystatin. From eqn (2), the following inequality is obtained:
| (3) |
From eqns (1) and (2), the following equation is obtained:
| (4) |
Then, the following inequality is obtained from eqn (4):
| (5) |
The following inequality (6) is obtained from inequalities (3) and (5), since all values of Gt,n, Gt,nn, Gb and (1/Gt,n - 1/Gt,nn) are positive (i.e. Gt,n > 0, Gt,nn > 0, Gb > 0 and Gt,n < Gt,nn):
| (6) |
Gt,n was 1330·1 ± 125·8 μS cm−2, and Gt,nn was 3387·7 ± 521·5 μS cm−2. Since Gt,nn was 2·7-fold (2·7 ± 0·4; P < 0·005; n = 8) larger than Gt,n, Ga,n is at least 1·7-fold larger than Gb, suggesting that 63 % of the measured conductance with apical application of nystatin shown in the present study is due to the basolateral one. Further, we applied NPPB (100 μM; bilateral application) after bilateral application of nystatin. This NPPB application did not significantly affect Gt,nn (3387·7 ± 521·5 μS cm−2 without NPPB vs. 3084·6 ± 585·5 μS cm−2 with NPPB; no significant difference; n = 8), suggesting that NPPB with apical nystatin application decreases the basolateral conductance. These observations and calculation suggest that the NPPB-sensitive Cl− conductance measured with the apical application of nystatin represents the basolateral Cl− conductance.
A role of basolateral Cl− channels (conductances)
Apical Cl− channels are well known to contribute to Cl− secretion (Eaton et al. 1992). However, it is unclear how basolateral Cl− channels (conductances) contribute to physiological events. The present study suggests that the basolateral Cl− conductance is essentially required to maintain the activity of the Na+-K+ pump, and that hyposmolality activates the Na+-K+ pump by increasing the basolateral Cl− conductance via a PTK-dependent pathway.
In the present study, we suggest a role of the basolateral Cl− conductance in the Na+-K+ pump activity. Some reports indicate a role of extracellular Cl− on the Na+-K+ pump activity. For example, a report (Sargeant et al. 1995) suggests that insulin activates the Na+-K+ pump by increasing the intracellular Na+ concentration due to an increase in Na+ influx via the bumetanide-sensitive Na+-K+-2Cl− cotransporter. Indeed, insulin stimulates the Na+-K+-2Cl− cotransporter (Longo, 1996; Marunaka et al. 1996; Marunaka et al. 1999b). Removal of extracellular Cl− diminishes the Na+ influx via the Na+-K+-2Cl− cotransporter, inhibiting the insulin action on the Na+-K+ pump (Sargeant et al. 1995). However, in the present study, unlike the report of Sargeant et al. (1995) the Na+-K+ pump activity was measured with apical application of nystatin, and therefore a Na+ influx via the Na+-K+-2Cl− cotransporter has no major contribution to regulation of the intracellular Na+ concentration. Therefore, the conclusion of our present study is that the Cl− conductance plays an essential role in maintenance of the Na+-K+ pump activity which is not mediated through the Na+-K+-2Cl− cotransporter or through modification of the intracellular Na+ concentration.
Action of NPPB on the Na+-K+ pump
We suggest that NPPB (100 μM) inhibits the hyposmolality-activated Na+-K+ pump. We would have to consider the inhibitory action of 100 μM NPPB on the Na+-K+ pump as being due to a non-specific effect. Although we cannot rule out a non-specific effect of NPPB at that concentration, the most important observation in the present study is that there is a correlation between the basolateral Cl− conductance and the Na+-K+ pump activity. NPPB at 100 μM might directly inhibit the Na+-K+ pump. However, the observation on the correlation between the basolateral Cl− conductance and the Na+-K+ pump activity is not changed, since we also showed a correlation between the Na+-K+ pump activity and the basolateral Cl− conductance by replacing Cl− with gluconate or NO3− in addition to application of NPPB. Further, we observed that NPPB dose dependently decreased the Isc; the NPPB-sensitive Isc was 1·1 ± 0·2 μA cm−2 at 25 μM NPPB, 1·6 ± 0·2 μA cm−2 at 50 μM NPPB, and 2·0 ± 0·2 μA cm−2 at 100 μM NPPB 60 min after application of NPPB (n= 10). However, the NPPB-sensitive Isc was 1·8 ± 0·2 μA cm−2 at 25 μM NPPB, 1·9 ± 0·2 μA cm−2 at 50 μM NPPB, and 2·0 ± 0·2 μA cm−2 at 100 μM NPPB (no significant differences among the effects of 25, 50 and 100 μM NPPB, n= 10) when the inhibitory effect of NPPB on the conductance was measured 210 min after application of NPPB. Further, it is suggested that NPPB blocks Cl− channels from the cytosolic side (Greger, 1990). These observations suggest that NPPB at a lower concentration (25 μM) is large enough to completely inhibit the Isc, and that NPPB at a lower concentration requires a longer time to accumulate into the intracellular space to reach an intracellular concentration completely blocking Cl− channels. Moreover, even 240 min after application of 100 μM NPPB the monolayer could still keep a high transepithelial resistance (Rt). Namely, the Rt was 4600 ± 694 Ω cm2 (n= 5) at 180 min after exposure of cells to a hypotonic solution, and apical application of 50 μM nystatin reduced the Rt to 709 ± 186 Ω cm2 (n= 5) at 30 min after the nystatin application in a hypotonic solution (210 min after exposure to a hypotonic solution). The reduced Rt increased to 4999 ± 974 Ω cm2 240 min after application of 100 μM NPPB (n= 5), suggesting that the monolayers can keep a high Rt with intact tight junctions at least from a view point of Rt. In general, application of toxic agents breaks the tight junction of the epithelial monolayer, resulting in a low Rt. Further, the inhibition of Isc by 100 μM NPPB was reversible when NPPB was washed out from the bathing solution. Taken together, we might suggest that the inhibitory effect of NPPB would not be non-specific.
Correlations between the basolateral Cl− conductance and the Na+-K+ pump activity
Since we estimated the Na+-K+ pump activity by measuring Isc, Cl− movements are not essentially required as a counter ion even at electrogenic exchange of Na+ and K+ by the Na+-K+ pump. Therefore, Cl− (anion) conductances would not act on the Na+-K+ pump as a counter ion at the movement of Na+ and K+. Although we need further studies to understand the regulatory mechanism of the Na+-K+ pump activity by the basolateral Cl− conductance, the present study suggests a role of the basolateral Cl− conductance in the regulation of the Na+-K+ pump activity as the first step in the study.
Time courses of phosphotyrosine and Isc induced by hyposmolality
A recent study (Liu et al. 1996) suggests that mechanical stretch of fetal lung cells increases activity of a PTK, pp60 c-Src. Further, more recently osmotic cell swelling has been reported to activate a Cl− channel via a PTK-dependent pathway (Lepple-Wienhues et al. 1998). These reports suggest that a change in membrane tension increases PTK activity. In A6 cells, hyposmolality induces an increase in cell volume followed by a regulatory volume decrease (RVD) (De Smet et al. 1995a, b). The change in membrane tension would transiently occur just after application of hyposmolality to the cell. On the other hand, the increase in Isc occurs gradually with time after application of hyposmolality. Time courses of cell volume (or membrane tension) and Isc after exposure of the cell to a hyposmotic solution were different. However, the change in cell volume (or membrane tension) would play a role as a trigger in producing a signal to activate PTK leading to an increase in Isc.
Effects of PTK inhibitors applied after exposure to hypotonicity on the Na+-K+ pump and the NPPB-sensitive conductance
Based upon the observation by Tilly and his colleagues (Tilly et al. 1993) it could be suggested that: (1) activation of PTK occurs in the initial phase of the hyposmotic stress, (2) the hyposmolality-activated PTK increases the Na+-K+ pump activity and the NPPB-sensitive conductance, and (3) since the effects of PTK have been transduced to the effectors, PTK inhibitors should not have any effects if PTK inhibitors are applied after hypotonic stress. In the present study, PTK inhibitors (tyrphostin A23 and genistein) which were applied after hyposmotic stress had increased the ouabain-sensitive current and the NPPB-sensitive conductance, showed inhibitory effects similar to NPPB, which directly blocked Cl− channels (conductances). This observation seems to be inconsistent with the signalling pathway described above based on the observation by Tilly et al. (1993). However, as shown in Fig 15 and Fig 16, an increase in phosphotyrosine caused by hyposmotic stress was not really transient, but rather sustained. This observation suggests that the increase in PTK activity would be not only transient but also maintained, although the sustained activity of PTK would be lower than that at the peak. This would result in the inhibitory action of PTK inhibitors which were applied even after hypotonic stress. Taken together, these observations suggest two roles of PTK: (1) the initial activation of PTK plays a role as a trigger in the hyposmolality-induced down-stream signalling, and (2) the sustained activation of PTK maintains continuous stimulation of effectors such as Na+ transport.
Effects of DMSO on the Na+-K+ pump activity and the NPPB-sensitive conductance
We used DMSO as a vehicle for application of the agents amiloride, NPPB, DPC, tyrphostin A23, genistein, nystatin and ouabain to the bathing solution in the present study. The maximum concentration of DMSO used in the present study did not exceed 0·1 %, which is 12 mM. When amiloride, NPPB, DPC, tyrphostin A23, genistein, nystatin or ouabain was applied to the bathing solution, DMSO might affect currents and/or conductances by increasing the solution osmolality. We tested the effects of 0·1 % DMSO on currents and conductances, reaching the conclusion that 0·1 % DMSO had no detectable effects. Therefore, even if application of DMSO would increase the osmolality of the bathing solutions, the effects of amiloride, NPPB, DPC, tyrphostin A23, genistein, nystatin or ouabain reported in the present study were not due to an increase in the osmolality of the bathing solution.
Conclusions
In summary, we conclude that: (1) hyposmolality activates PTK; (2) an increase in PTK activity stimulates the basolateral Cl− conductance; and (3) stimulation of the basolateral Cl− conductance activates the Na+-K+ pump (see Fig. 17), although it is still unclear if the Cl− conductance directly links the Na+-K+ pump activity. This signalling pathway of hyposmolality to activation of the Na+-K+ pump mediated through the basolateral Cl− conductance via a PTK-dependent pathway is a novel pathway for the regulation of Na+-K+ pump activity.
Figure 17. A hypothesized scheme of the hyposmolality-induced regulatory pathway of the Na+-K+ pump activity through a PTK-dependent Cl− conductance.
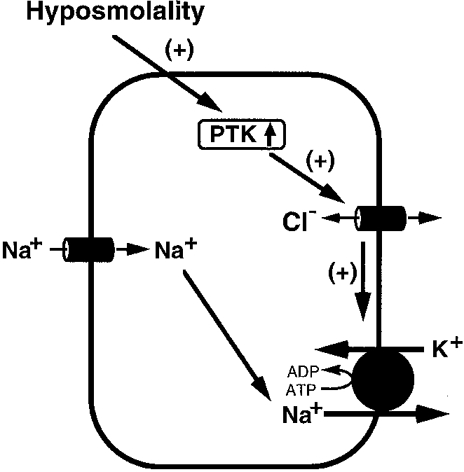
(1) Hyposmolality activates PTK. (2) PTK increases the basolateral Cl− conductance. (3) The basolateral Cl− conductance stimulates the Na+-K+ pump, although it is still unclear if the Cl− conductance directly links the Na+-K+ pump activity. (+) signifies a stimulatory effect.
Acknowledgments
This work was supported by grants from the Kidney Foundation of Canada, the Medical Research Council of Canada and the Ontario Thoracic Society (Block Term Grant) to Y. Marunaka, and by the International Scientific Research Program from the Ministry of Education, Science, Sports and Culture of Japan. N. N. is a Research Fellow and supported by a stipend from The Research Training Centre, The Hospital for Sick Children. Y. M. was a recipient of Scholar of the Medical Research Council of Canada.
References
- De Smet P, Simaels J, Van Driessche W. Regulatory volume decrease in a renal distal tubular cell line (A6). 1. Role of K+ and Cl−. Pflügers Archiv. 1995a;430:936–944. doi: 10.1007/BF01837407. [DOI] [PubMed] [Google Scholar]
- De Smet P, Simaels J, Van Driessche W. Regulatory volume decrease in a renal distal tubular cell line (A6). 2. Effect of Na+ transport rate. Pflügers Archiv. 1995b;430:945–953. doi: 10.1007/BF01837408. [DOI] [PubMed] [Google Scholar]
- Doi Y, Marunaka Y. Amiloride-sensitive and HCO3−-dependent ion transport activated by aldosterone and vasotocin in A6 cells. American Journal of Physiology. 1995;268:C762–770. doi: 10.1152/ajpcell.1995.268.3.C762. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Marunaka Y, Ling BN. Ion channels in epithelial tissue: Single channel properties. In: Schafer JA, Ussing HH, Kristensen P, Giebisch GH, editors. Membrane Transport in Biology. Berlin: Springer-Verlag; 1992. pp. 73–165. [Google Scholar]
- Edashige K, Watanabe Y, Sato EF, Takehara Y, Utsumi K. Reversible priming and protein tyrosine phosphorylation in human peripheral neutrophils under hypotonic conditions. Archives of Biochemistry and Biophysics. 1993;302:343–347. doi: 10.1006/abbi.1993.1221. [DOI] [PubMed] [Google Scholar]
- Erlij D, De Smet P, Van Driessche W. Effect of insulin on area and Na+ channel density of apical membrane of cultured toad kidney cells. The Journal of Physiology. 1994;481:533–542. doi: 10.1113/jphysiol.1994.sp020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Rousselot M, Favre H. Modulation of Na+, K+-ATPase activity by a tyrosine phosphorylation process in rat proximal convoluted tubule. The Journal of Physiology. 1997;498:99–108. doi: 10.1113/jphysiol.1997.sp021844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochimica et Biophysica Acta. 1988;947:521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Greger R. Chloride channel blockers. Methods in Enzymology. 1990;191:793–812. doi: 10.1016/0076-6879(90)91048-b. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ito Y, Niisato N, O'Brodovich H, Marunaka Y. The effect of brefeldin A on terbutaline-induced sodium absorption in fetal rat distal lung epithelium. Pflügers Archiv. 1997;434:492–494. doi: 10.1007/s004240050425. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. Journal of Membrane Biology. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Cation transport probes: The amiloride series. Methods in Enzymology. 1990;191:739–754. doi: 10.1016/0076-6879(90)91045-8. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. Journal of Cell Biology. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Eaton DC, Clausen C, Diamond JM. Nystatin as a probe for investigating the electrical properties of a tight epithelium. Journal of General Physiology. 1977;70:427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Qin Y, Liu J, Tanswell AK, Post M. Mechanical strain induces pp60src activation and translocation to cytoskeleton in fetal rat lung cells. Journal of Biological Chemistry. 1996;271:7066–7071. [PubMed] [Google Scholar]
- Longo N. Insulin stimulates the Na+, K+-ATPase and the Na+/K+/Cl− cotransporter of human fibroblasts. Biochimica et Biophysica Acta. 1996;1281:38–44. doi: 10.1016/0005-2736(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Marunaka Y. Physiological requirement of aldosterone action on Na+/K+ pump in ADH-stimulated Na+ absorption in renal epithelium. Japanese The Journal of Physiology. 1996;46:357–361. doi: 10.2170/jjphysiol.46.357. [DOI] [PubMed] [Google Scholar]
- Marunaka Y. Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Japanese The Journal of Physiology. 1997;47:499–511. doi: 10.2170/jjphysiol.47.499. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Effects of insulin and phosphatase on a Ca2+ dependent Cl− channel in a distal nephron cell line (A6) Journal of General Physiology. 1990a;95:773–789. doi: 10.1085/jgp.95.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Chloride channels in the apical membrane of a distal nephron A6 cell line. American Journal of Physiology. 1990b;258:C352–368. doi: 10.1152/ajpcell.1990.258.2.C352. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. American Journal of Physiology. 1991;260:C1071–1084. doi: 10.1152/ajpcell.1991.260.5.C1071. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Hagiwara N, Tohda H. Insulin activates single amiloride-blockable Na channels in a distal nephron cell line (A6) American Journal of Physiology. 1992;263:F392–400. doi: 10.1152/ajprenal.1992.263.3.F392. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, O'Brodovich H, Eaton DC. Regulation of amiloride-sensitive Na+-permeable channel by catecholamine via cytosolic Ca2+ and Cl− in fetal rat alveolar epithelium. The Journal of Physiology. 1999a;515:669–683. doi: 10.1111/j.1469-7793.1999.669ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, O'Brodovich H, Post M, Tanswell AK. Roles of Ca2+ and protein tyrosine kinase in insulin action on cell volume via Na+ and K+ channels and Na+/K+/2Cl− cotransporter in fetal rat alveolar type II pneumocyte. Journal of Membrane Biology. 1999b;168:91–101. doi: 10.1007/s002329900500. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, Shintani Y. Protein phosphatase 2B-dependent pathway of insulin action on single Cl− channel conductance in renal epithelial cells. Journal of Membrane Biology. 1998;161:235–245. doi: 10.1007/s002329900330. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Shintani Y, Downey GP, Niisato N. Activation of Na+-permeant cation channel by stretch and cAMP-dependent phosphorylation in renal epithelial A6 cells. Journal of General Physiology. 1997;110:327–336. doi: 10.1085/jgp.110.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y, Shintani Y, Sugimoto E, Niisato N. Roles of tyrosine kinase in insulin action on cell volume of fetal rat type II pneumocyte. Pflügers Archiv. 1996;432:571–573. doi: 10.1007/s004240050171. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Tohda H. Effects of vasopressin on single Cl− channels in the apical membrane of distal nephron epithelium (A6) Biochimica et Biophysica Acta. 1993;1153:105–110. doi: 10.1016/0005-2736(93)90281-4. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Tohda H, Hagiwara N, Nakahari T. Antidiuretic hormone-responding non-selective cation channel in distal nephron epithelium (A6) American Journal of Physiology. 1994;266:C1513–1522. doi: 10.1152/ajpcell.1994.266.6.C1513. [DOI] [PubMed] [Google Scholar]
- Niisato N, Ito Y, Marunaka Y. Activation of Cl− channel and Na+/K+/2Cl− cotransporter in renal epithelial A6 cells by flavonoids: genistein, daidzein, and apigenin. Biochemical and Biophysical Research Communications. 1999;254:368–371. doi: 10.1006/bbrc.1998.9952. [DOI] [PubMed] [Google Scholar]
- Niisato N, Marunaka Y. Regulation of Cl− transport by IBMX in renal A6 epithelium. Pflügers Archiv. 1997a;434:227–233. doi: 10.1007/s004240050389. [DOI] [PubMed] [Google Scholar]
- Niisato N, Marunaka Y. Cross talk of bumetanide-sensitive and HCO3−-dependent transporters activated by IBMX in renal epithelial A6 cells. Journal of Membrane Biology. 1997b;157:53–61. doi: 10.1007/s002329900215. [DOI] [PubMed] [Google Scholar]
- Niisato N, Marunaka Y. Hyposmolality-induced enhancement of ADH action on amiloride-sensitive Isc in renal epithelial A6 cells. Japanese The Journal of Physiology. 1997c;47:131–137. doi: 10.2170/jjphysiol.47.131. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO Journal. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- Sargeant RJ, Liu Z, Klip A. Action of insulin on Na+-K+-ATPase and the Na+-K+-2Cl− cotransporter in 3T3-L1 adipocytes. American Journal of Physiology. 1995;269:C217–225. doi: 10.1152/ajpcell.1995.269.1.C217. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Van Den Berghe N, Tertoolen LG, Edixhoven MJ, De Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. Journal of Biological Chemistry. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Wills NK, Millinoff LP. Amiloride-sensitive Na+ transport across cultured renal (A6) epithelium: Evidence for large currents and high Na:K selectivity. Pflügers Archiv. 1990;416:481–492. doi: 10.1007/BF00382680. [DOI] [PubMed] [Google Scholar]
- Wills NK, Millinoff LP, Crowe WE. Na+ channel activity in cultured renal (A6) epithelium: Regulation by solution osmolarity. Journal of Membrane Biology. 1991;121:79–90. doi: 10.1007/BF01870653. [DOI] [PubMed] [Google Scholar]


