Abstract
Interleukin (IL)-1 is a potent endogenous pyrogen which causes fever when injected into a number of brain sites. However, the brain sites at which endogenous IL-1 acts to influence body temperature remain equivocal. The aim of this study was to determine the effect of local administration of the interleukin-1 receptor antagonist (IL-1ra) into specific sites in the hypothalamus, and other brain regions known to contain receptors for IL-1, on the febrile response of rats to peripheral injection of lipopolysaccharide (LPS) into a subcutaneous air pouch (intrapouch, i.p.o.) that does not lead to LPS appearance in the circulation.
Injection of LPS (100 μg kg−1, i.p.o.) induced a rise in body temperature which commenced 1·5 h after injection and was maximal at 3 h (38·9 ± 0·2 °C, compared with 37·0 ± 0·1 °C at 0 h, n= 6, P < 0·001). Intracerebroventricular (i.c.v.) IL-1ra (500 μg in 5 μl) significantly attenuated LPS fever (IL-1ra, 37·7 ± 0·2 °C; saline, 38·9 ± 0·2 °C; n= 6, P < 0·001). Unilateral microinjection of IL-1ra (50 μg in 0·5 μl at 0 + 1 h) into the anterior hypothalamus (AH), paraventricular hypothalamic nucleus (PVH), peri-subfornical organ, subfornical organ (SFO) or hippocampus (dentate gyrus and CA3 region) also significantly reduced the fever induced by LPS.
The same dose of IL-1ra had no effect on fever when administered into the ventromedial hypothalamus (VMH), organum vasculosum lamina terminalis (OVLT), CA1 field of the hippocampus, striatum or cortex.
These data indicate that the action of endogenous IL-1 in the brain during fever is site specific, acting at the AH, PVH, SFO and hippocampus, but not the VMH, OVLT and striatum or cortex.
Fever, an adaptive host defence response to viral and bacterial infections, and other inflammatory stimuli, relies on co-ordinated neuroimmune interactions between the periphery and the brain (Kluger, 1991). The pro-inflammatory cytokines (interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)-α), which are synthesized and released from activated peripheral immune cells after exposure to pathogens, play a pivotal role in this co-ordination (Kluger, 1991; Hopkins & Rothwell, 1995). Although several pro-inflammatory cytokines have been implicated in fever, IL-1 is believed to play an important role in both the afferent and central signalling mechanisms (see Kluger, 1991).
There is now considerable evidence that IL-1 acts directly in the brain to cause fever: (i) IL-1 is synthesized in the brain (mainly in the hypothalamus) in response to peripheral injection of bacterial lipopolysaccharide (LPS) (Bandtlow et al. 1990; Ban et al. 1992; Nguyen et al. 1998), (ii) injection of low (picomolar) quantities of recombinant IL-1 into the brain elicits marked fever in rodents (Anforth et al. 1998), (iii) IL-1 administration in vivo and in vitro alters the activity of hypothalamic thermosensitive neurons, the characteristics of which are consistent with the development of fever (Hori et al. 1988; Nakashima et al. 1989), and (iv) blocking the action of brain IL-1 by central administration of neutralizing anti-IL-1β antiserum (Klir et al. 1994; Gourine et al. 1998) or the naturally occurring interleukin-1 receptor antagonist (IL-1ra) (Luheshi et al. 1996; Miller et al. 1997) attenuates fever.
The anterior hypothalamus is the major site of thermoregulation in the brain (see Kluger, 1991), and is believed to be a primary target of IL-1 (Klir et al. 1994; Gourine et al. 1998). Several pieces of evidence suggest, however, that it is not the only brain region involved in thermoregulation, but is a component of a more complex central thermoregulatory process (Satinoff, 1978). For example, animals without a hypothalamus can respond to thermal stresses (see Satinoff, 1978), and animals with the connections between forebrain and hindbrain structures severed are capable of developing fever (Liu & Shyy, 1980). Indeed, peripheral LPS injection induces mRNA, bioactive and immunoreactive IL-1 expression in a number of extrahypothalamic regions, such as striatum, cortex and hippocampus (Ban et al. 1992; Quan et al. 1994; Nguyen et al. 1998) and receptors for IL-1 have been identified in the cortex and hippocampus (Farrar et al. 1987; Yabuuchi et al. 1994; Gayle et al. 1997). Moreover, injection of IL-1 into the striatum (Grundy et al. 1998) leads to a marked and reproducible fever in rats.
Earlier studies, which set out to localize brain IL-1 action, have utilized injection of the recombinant cytokine (Morimoto et al. 1989; Walter et al. 1989; Murakami et al. 1990; Lesnikov et al. 1991; Sellami & de Beaurepaire, 1995). However, the action of recombinant cytokines may reflect pharmacological effects, which do not necessarily equate with sites of action of endogenous IL-1. Similarly, expression of IL-1 in the brain has been studied in response to intraperitoneal or intravenous injection of LPS, usually at high doses (1 mg kg−1), which is likely to have direct actions on the brain or influence blood-brain barrier permeability to circulating cytokines.
The purpose of this study was to identify the sites of action in the brain of endogenous IL-1 in fever in rats, using a stimulus which does not influence the brain directly. We achieved this by administering LPS into a sterile, subcutaneous air pouch. This procedure elicits marked fever in rats, of comparable magnitude to that seen in response to intraperitoneal injection of the same dose of LPS (see Rothwell, 1997), in the absence of detectable LPS in the circulation (Cartmell et al. 1998). The sites of action of endogenous IL-1 were tested by pre-treatment of specific brain sites with human recombinant IL-1ra (hrIL-1ra).
METHODS
Male Sprague-Dawley rats (Charles River) (250-350 g) were used in all experiments. Animals were housed at a constant ambient temperature of 21 ± 2°C on a 12 h:12 h light-dark cycle (light on from 08.00 to 20.00 h). Food (pelleted rat chow, Beekay International, UK) and water were provided ad libitum. All animal procedures conformed with the requirements of the British Home Office Animal Licensing Inspectorate (UK).
Surgery
Following induction of anaesthesia (3 % halothane in oxygen; Fluothane, Zeneca) and complete areflexia, each animal was placed in a stereotaxic frame (Stoelting, IL, USA) with the incisor bar height set at 3·3 mm below the interaural line (according to Paxinos & Watson, 1986). A heating pad was placed beneath the animal to maintain core body temperature. A 21-gauge thin-walled stainless steel guide tube was implanted stereotaxically such that the tip of the guide was positioned 1 mm above the microinjection site. The following stereotaxic co-ordinates (relative to bregma; Paxinos & Watson, 1986) were used for microinjection: organum vasculosum lamina terminalis (OVLT; A+0·8, L0·0, V8·2 and A0·0, L0·0, V8·6 mm), peri-subfornical organ (septohippocampal nucleus, A+0·8, L0·0, V4·5 mm), cortex (A+0·7, L4·8, V3·0 mm), striatum (P-0·3, L3·6, V5·5 mm), lateral cerebral ventricle (i.c.v.; P-0·8, L1·5, V3·5 mm), subfornical organ (SFO; P-0·9, L0·0, V4·9 mm), anterior hypothalamus (AH; P-1·4 to -1·8, L0·6, V8·5 mm), paraventricular hypothalamic nucleus (PVH; P-1·7, L0·6, V7·8 mm), ventromedial hypothalamus (VMH; P-2·3 to -2·8, L0·5, V9·5 mm) and hippocampus (dentate gyrus, P-2·8, L1·0, V4·0 mm and P-3·3, L0·5, V3·6 mm; CA1 region, P-3·3, L2·2, V3·0 mm; CA3 region, P-3·3, L2·2, V3·7 mm; Fig. 1). Each animal was given an intramuscular injection of terramycin (5 mg (0·1 ml)−1, Pfizer) immediately after surgery and allowed a minimum of 7 days for recovery.
Figure 1. Localization of sites of injection of IL-1ra.
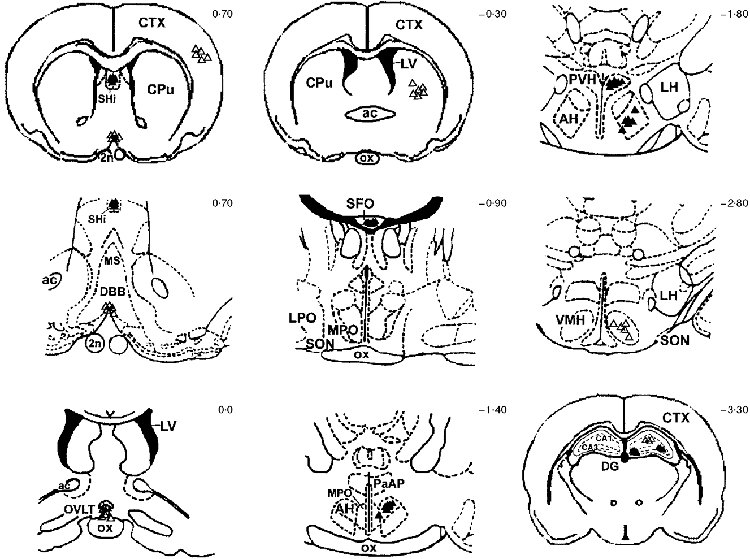
Frontal sections of the rat brain showing the distribution of individual sites microinjected with 0·5 μl saline or 50 μg hrIL-1ra in 0·5 μl, at time 0 h and 1 h, illustrated according to the magnitude of the suppression in body temperature in response to LPS i.p.o. and hrIL-1ra into a particular brain site. ▵, < 0·5 °C; ▴, > 0·5 °C. CPu, caudate putamen (striatum); CTX, cortex; SHi, septohippocampal nucleus (peri-subfornical organ); ac, anterior commissure; MS, medial septal nucleus; DBB, diagonal band of Broca; 2n, optic nucleus; OVLT, organum vasculosum lamina terminalis; LV, lateral ventricle; ox, optic chiasm; SFO, subfornical organ; LPO, lateral preoptic area; MPO, medial preoptic area; SON, supraoptic nucleus; AH, anterior hypothalamus; PaAP, paraventricular hypothalamus, anterior part; PVH, paraventricular hypothalamus; LH, lateral hypothalamus, VMH, ventromedial hypothalamus; DG, dentate gyrus, CA1 & CA3, fields of the hippocampus. The numbers on the right of the individual frontal sections indicate the distances in millimetres from bregma (based on the anterior-posterior orientation of Paxinos & Watson, 1986).
Six days before the start of the experiment a subcutaneous air pouch, for peripheral injection of LPS, was formed according to the method described by Edwards et al. (1981). Briefly, animals were anaesthetized (3 % halothane in oxygen) until complete areflexia and 20 ml of sterile air (0·2 μM Acrodisc, Gelman Sciences, Ann Arbor, MI, USA) was injected into the subcutaneous tissue of the dorsal midline, caudal to the scapulae. Three days after the initial pouch formation, animals were briefly re-anaesthetized (approximately 1 min, with halothane as above) and the air pouch was reinflated with 10 ml of sterile air, to maintain an open cavity. On day 6, LPS or saline was injected directly into the air pouch of conscious animals.
Measurement of body temperature
Core body temperature of the rats was measured by remote biotelemetry (Data Quest IV system, Data Sciences, St Paul, MN, USA), using pre-calibrated radiotransmitters (TA10TA-F40, Data Sciences) implanted intraperitoneally at the same time as the stereotaxic implantation of the guide cannulae (see above). Animals were housed individually 24 h before the experiments. Transmitter output frequency (in hertz) was monitored, at 10 min intervals, by an antenna mounted in a receiver board situated beneath the cage of each animal, and data were channelled into a peripheral processor (BCM 100, Data Sciences) connected to an IBM-compatible personal computer.
Experimental protocol
For all experiments, animals were injected intrapouch (i.p.o.) with either LPS (100 μg kg−1 in a final concentration of 100 μg ml−1; Escherichia coli serotype 0128:B12; Sigma) or sterile, non-pyrogenic saline (1 ml kg−1), followed by central injection of either saline or hrIL-1ra. Different groups of animals were used for each brain site under investigation. In the dose-response phase of the experiments, hrIL-1ra (a gift from Amgen, Thousand Oaks, CA, USA) was injected into the cerebral ventricle of conscious, unrestrained rats at a final dose of 1, 10 or 50 μg in 0·5 μl saline, 100 μg in 1 μl or 500 μg in 5 μl. Saline injections were given in an identical manner. All subsequent experiments, which involved microinjection into discrete brain tissue sites, used a final injection volume of 0·5 μl. The dose of hrIL-1ra chosen for these experiments (50 μg in 0·5 μl, microinjections given at the time of LPS administration (0 h, 10.00 h) and again 1 h later (+1 h); total dose of hrIL-1ra injected, 100 μg) was the minimum dose of intracerebroventricular (i.c.v.) hrIL-1ra which significantly attenuated the 6 h thermal response to LPS injection i.p.o. (Fig. 2B). The two injections of hrIL-1ra were given 1 h apart as this time course was deemed successful in a previous study (Luheshi et al. 1996). For microinjection, a 31-gauge needle, connected by polyethylene tubing (0·38 mm i.d., 1·09 mm o.d.) to a 5 μl Hamilton gas-tight microlitre syringe (Hamilton, GB) was lowered into the guide cannula so that it protruded 1·0 mm beyond its tip into the tissue of the region under investigation. Each microinjection was delivered unilaterally over a period of 30 s in a volume of 0·5 μl of either the saline vehicle or hrIL-1ra (50 μg in 0·5 μl). The needle was left in the guide tube for an additional 1 min to ensure dispersion of the injected substance within the tissue. Data were excluded from analysis if: (i) the core body temperature of the animal at the beginning of the experiment was below 36·8°C or above 37·3°C, (ii) the guide cannula was obstructed prior to central injection or (iii) an Evans Blue microinjection at the end of the experiment (see below) showed that the injection was not into the designated site. Less than 5 % of the original animals were excluded from data analysis for these reasons.
Figure 2. Effect of intracerebroventricular injection of hrIL-1ra (various doses) on peripheral LPS-induced fever in rats.
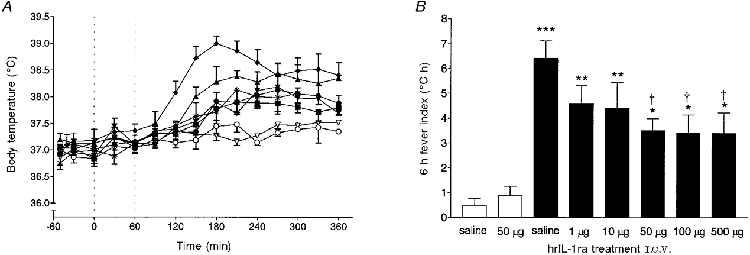
Injection of lipopolysaccharide (LPS, 100 μg kg−1) (filled symbols) or saline (open symbols) into a subcutaneous air pouch (i.p.o.) at time 0 h, followed immediately by unilateral microinjection into the cerebral ventricle (i.c.v.) of saline or various doses of human recombinant interleukin-1 receptor antagonist (hrIL-1ra), at time 0 h and 1 h. Baseline temperatures were not significantly different from one another at time 0 h (P > 0·05, ANOVA). The results are presented as follows. A, time curves (means ±s.e.m.) of core body temperature (°C) for: LPS i.p.o., saline i.c.v. (♦, n= 10); LPS i.p.o., 1 μg hrIL-1ra in 0·5 μl i.c.v. (▴, n= 4); LPS i.p.o., 10 μg hrIL-1ra in 0·5 μl i.c.v. (▾, n= 4); LPS i.p.o., 50 μg hrIL-1ra in 0·5 μl i.c.v. (•, n= 5); LPS i.p.o., 100 μg hrIL-1ra in 1 μl i.c.v. (▪, n= 5); LPS i.p.o., 500 μg hrIL-1ra in 5 μl i.c.v. (▪, n= 5); saline i.p.o., saline i.c.v. (○, n= 6); saline i.p.o., 50 μg hrIL-1ra in 0·5 μl i.c.v. (▿, n= 5). Dotted lines indicate injection times into the particular brain site. B, 6 h fever indices (°C h) for: LPS i.p.o., various doses of hrIL-1ra or saline i.c.v. (▪); saline i.p.o., hrIL-1ra or saline i.c.v. (□). The order of injections was randomized. For clarity, columns indicating the s.e.m. are shown only at 30 min intervals. ***P < 0·001; **P < 0·01; *P < 0·05 compared with saline i.p.o. and saline or hrIL-1ra i.c.v., ANOVA. †P < 0·05 compared with LPS i.p.o. and saline i.c.v., ANOVA.
Histology
At the end of the experiments, each rat was killed by terminal anaesthesia (halothane, as before) and 0·5 μl Evans Blue was injected into the respective brain site. Brains were rapidly removed, snap-frozen in isopentane (approximately -40°C) on dry ice, and stored at -70°C. Each brain was sectioned on a cryostat through the plane of the microinjection sites. Coronal sections (rostro-caudal; 20 μm every 100 μm throughout the area of injection) were mounted onto 3-aminopropyltriethoxysilane-coated slides and stained with Cresyl Fast Violet, following standard histological procedures. The position of each cannula track and locus of microinjection were identified under light microscopy and ‘mapped’ in the coronal plane on anatomical reconstructions (Fig. 1).
Statistical and data analysis
All data are presented as means ±s.e.m. for the number of animals given. Body temperature responses to microinjection of IL-1ra were compared with body temperature responses to i.p.o. injection of LPS and saline alone at that site as well as i.p.o. saline and saline or hrIL-1ra alone at that site. Responses were analysed for statistical significance according to: (a) maximum body temperature attained after each treatment and (b) the integrated hyperthermic response, calculated as the deviation from baseline over the 6 h period following peripheral (i.p.o.) LPS injection (the 6 h fever index, in°C h). Differences between the groups were determined by analysis of variance (ANOVA) followed by a Tukey-Kramer multiple comparisons post hoc test. A two-tailed probability of P < 0·05 was considered statistically significant.
RESULTS
Temperature responses
In all experiments, mean baseline temperature ranged between 36·9 and 37·2°C and was similar for all groups (data not shown). Intrapouch injection of saline alone and saline or hrIL-1ra i.c.v. caused no significant change in core temperature (Fig. 2A and B). Injection of LPS i.p.o. (0 h) and saline i.c.v. (0 + 1 h) induced a rise in core body temperature which commenced 1·5 h after injection and was maximal at 3 h (38·9 ± 0·2°C, n= 6, P < 0·001 compared with pre-injection body temperature). These data were not significantly different from those after i.p.o. injection of LPS alone (P > 0·05, data not shown). The duration of the febrile response, from the peak body temperature to the completion of defervescence, exceeded 3 h (data not shown).
Effect of intracerebroventricular injection of hrIL-1ra on body temperature
Injection of hrIL-1ra i.c.v. (at the time of LPS i.p.o. and again 1 h later) induced a dose-dependent suppression of the febrile response to LPS i.p.o. (Fig. 2). The highest dose of hrIL-1ra (500 μg in 5 μl) suppressed mean body temperature to 37·7 ± 0·2°C (n= 5, P < 0·001, ANOVA) at 3 h after i.p.o. injection of LPS, compared with animals injected with LPS i.p.o. and saline i.c.v. (38·9 ± 0·2°C, n= 6). This was not significantly different from animals injected with saline i.p.o. and saline (at 3 h: 37·4 ± 0·1°C, n= 6, P > 0·05, ANOVA) or hrIL-1ra i.c.v (50 μg, 0 + 1 h; at 3 h: 37·3 ± 0·1°C, n= 5, P > 0·05, ANOVA). The body temperature responses to 10, 50 or 100 μg of hrIL-1ra i.c.v. were significantly lower (at 3 h: 37·9 ± 0·2°C, n= 4, P < 0·001; 37·9 ± 0·1°C, n= 5, P < 0·001; and 37·8 ± 0·2°C, n= 5, P < 0·001, ANOVA, respectively) than that observed in rats injected with LPS i.p.o. plus saline i.c.v. (Fig. 2A), but were not significantly different from each other or from the group of animals injected with the highest dose of hrIL-1ra (500 μg i.c.v.). The mean maximum body temperature of rats injected with LPS i.p.o. and 1 μg of hrIL-1ra was 38·3 ± 0·2°C (Fig. 2A). There was no significant difference in body temperature between animals injected with LPS i.p.o. and the lowest dose of hrIL-1ra (1 μg) or saline, i.c.v. (P > 0·05, ANOVA). Compared with animals injected with saline i.p.o. and saline or hrIL-1ra i.c.v., however, body temperature in this group of animals (LPS i.p.o. and 1 μg hrIL-1ra i.c.v.) was significantly higher (P < 0·01, ANOVA).
The febrile response over the time course investigated (6 h fever index, in°C h) of rats injected with LPS i.p.o. and hrIL-1ra (50-500 μg i.c.v.) was significantly attenuated (P < 0·05, ANOVA) compared with those injected with LPS i.p.o. and saline i.c.v. (Fig. 2B). Doses of 10 or 1 μg hrIL-1ra i.c.v. had no effect on the febrile response when compared with animals injected with LPS i.p.o. and saline i.c.v. (P > 0·05, ANOVA), but were significantly enhanced in comparison with animals injected with saline i.p.o. and saline (P < 0·01, ANOVA) or hrIL-1ra (P < 0·01, ANOVA) i.c.v. (Fig. 2B). The minimum dose of hrIL-1ra i.c.v. (50 μg in 0·5 μl, 0 + 1 h) that significantly attenuated the 6 h thermal response to LPS i.p.o. (Fig. 2B) was chosen for all subsequent experiments which involved microinjection into discrete brain tissue sites.
Effect of hrIL-1ra injection into different brain sites on LPS-induced fever
The maximum core temperature induced by LPS (i.p.o.) was 38·9 ± 0·2°C, and this febrile response was not affected by injection of saline into any brain site (Fig. 3A-E). There were no significant differences between the latency to maximum level of fever (data not shown), nor the mean maximum rise in body temperature, for groups injected i.p.o. with LPS and saline into any brain site. Injection of saline or hrIL-1ra alone (in response to saline i.p.o.) also had no effect on body temperature (Fig. 3A-E). Unilateral microinjection of hrIL-1ra (50 μg in 0·5 μl, 0 + 1 h) into the AH, including the preoptic region (IL-1ra, 37·7 ± 0·2°C; saline, 38·8 ± 0·1°C; n= 6, P < 0·001; Fig. 3A), PVH (IL-1ra, 37·7 ± 0·1°C; saline, 38·9 ± 0·2°C; n= 5, P < 0·001; Fig. 3B), peri-SFO (IL-1ra, 37·6 ± 0·1°C; saline, 38·8 ± 0·1°C; n= 5, P < 0·001; Fig. 3C), SFO (IL-1ra, 37·7 ± 0·1°C; saline, 38·7 ± 0·1°C; n= 5, P < 0·01; Fig. 3D), dentate gyrus (IL-1ra, 38·0 ± 0·1°C; saline, 38·9 ± 0·2°C; n= 5, P < 0·01, Fig. 3E), or CA3 field of the hippocampus (IL-1ra, 37·7 ± 0·3°C; saline, 38·6 ± 0·2°C; n= 5, P < 0·01) significantly decreased body temperature to i.p.o. LPS to a similar extent. The elevation in body temperature at 4-6 h was similar in animals injected with LPS i.p.o. and either saline or hrIL-1ra into the hippocampus (Fig. 3E), but was significantly lower in animals injected with hrIL-1ra into one of the other four ‘effective’ sites, compared with saline injected at that same site (Fig. 3A-D). No single site resulted in complete abolition of fever after hrIL-1ra injection.
Figure 3. Changes in body temperature of rats following microinjection of hrIL-1ra into various brain sites.
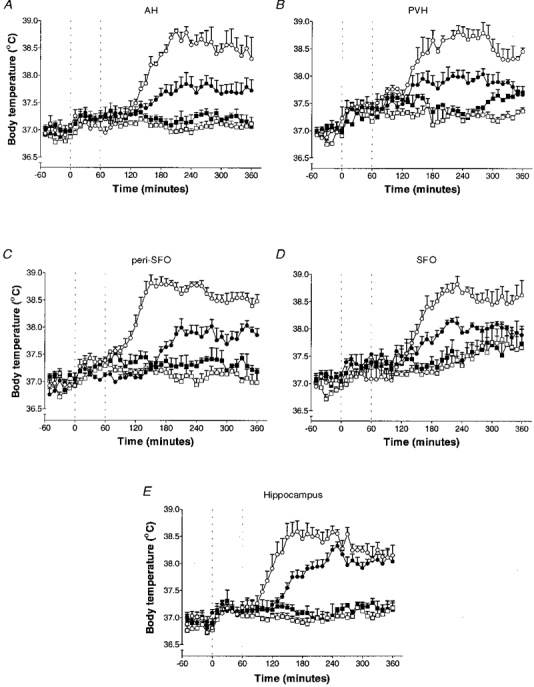
Changes in body temperature (means ±s.e.m.) of rats following microinjection of saline (0·5 μl, ○) or hrIL-1ra (50 μg in 0·5 μl, •) into the anterior hypothalamus (AH, n= 6) (A), paraventricular hypothalamus (PVH, n= 5) (B), peri-subfornical organ (peri-SFO, n= 5) (C), subfornical organ (SFO, n= 5) (D) or hippocampus (dentate gyrus, n= 5) (E) immediately (0 h) and at time 1 h after LPS injection i.p.o. Dotted lines indicate injection times into the particular brain site. Injection of saline (□) or hrIL-1ra (▪) alone into each particular brain site, after saline (1 ml kg−1) i.p.o., had no effect on body temperature.
In contrast, unilateral microinjection of hrIL-1ra into the VMH (IL-1ra, 38·7 ± 0·2°C; saline, 38·7 ± 0·3°C; n= 5), OVLT (IL-1ra, 38·6 ± 0·2°C; saline, 38·9 ± 0·2°C; n= 5), CA1 field of the hippocampus (IL-1ra, 38·8 ± 0·2°C; saline, 38·6 ± 0·3°C; n= 5), striatum (IL-1ra, 38·4 ± 0·3°C; saline, 38·5 ± 0·1°C; n= 6) or cortex (IL-1ra, 38·7 ± 0·1°C; saline, 38·7 ± 0·1°C; n= 6) had no effect on LPS-induced fever. Injection of saline or hrIL-1ra (in response to saline i.p.o.) had no effect on body temperature when injected into the VMH, OVLT, striatum or cortex (data not shown).
The integrated thermal response over the experimental period (6 h fever index in°C h) was similar for all groups injected with LPS i.p.o. and saline into each brain site (Fig. 4). Injection of hrIL-1ra into the AH, PVH, SFO, dentate gyrus (Fig. 4A) or CA3 field of the hippocampus significantly attenuated (by 42, 41, 37, 39 and 37 %, respectively) the 6 h thermal response to LPS i.p.o. but had no effect when microinjected into the VMH, OVLT, striatum or cortex (Fig. 4B).
Figure 4. 6 h fever indices of rats.
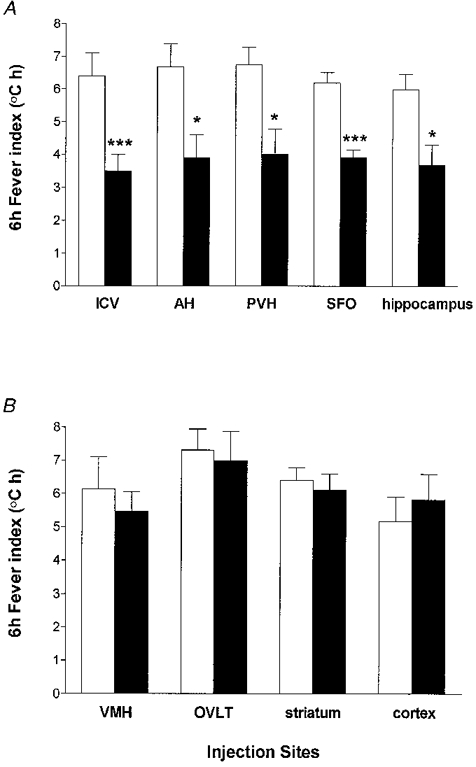
Six hour fever indices of rats injected peripherally (i.p.o.) with LPS (0 h), followed immediately by unilateral microinjection of 0·5 μl saline (□) or 50 μg hrIL-1ra in 0·5 μl (▪) into a discrete brain region at time 0 h and 1 h. Injection of saline or hrIL-1ra alone (after i.p.o. saline) has no lasting effect on body temperature (data not shown). i.c.v., lateral cerebral ventricle; AH, anterior hypothalamus; PVH, paraventricular hypothalamic nucleus; SFO, subfornical organ; VMH, ventromedial hypothalamus; OVLT, organum vasculosum lamina terminalis. Data are presented as means ±s.e.m.***P < 0·001; *P < 0·05 compared with LPS i.p.o. and saline into the particular brain region, ANOVA.
DISCUSSION
We have reported previously that central (i.c.v.) administration of hrIL-1ra significantly attenuates the fever induced by peripheral LPS in rodents (Luheshi et al. 1996; Miller et al. 1997), thereby supporting the involvement of endogenous brain IL-1 in the development of fever. The results from the present study confirm these data and show that IL-1ra injected i.c.v. attenuated (by 47 %), but did not prevent, fever induced by injection of LPS into a subcutaneous air pouch.
Injection of hrIL-1ra into the AH, PVH, SFO and hippocampus also significantly attenuated the febrile response to peripheral injection of LPS, by a similar extent to i.c.v. injection, though injection of IL-1ra into the hippocampus appeared to delay the febrile response. IL-1ra had no effect when injected into the VMH, OVLT or extrahypothalamic regions, namely the striatum or cortex. Injection of hrIL-1ra alone into any of the brain sites investigated did not alter core body temperature. It is unlikely that the attenuation of the fever response, induced by injection of IL-1ra into the brain, is due to diffusion into the periphery, since the same dose was ineffective when given systemically (data not shown).
Despite the attenuation of the febrile response, LPS-induced fevers were not abolished by our manipulations. It is unlikely that the depression of the febrile response was incomplete simply because the dose of IL-1ra finally used was insufficient, since this dose of IL-1ra completely suppresses IL-1-induced fever when IL-1 is administered centrally (G. N. Luheshi, personal communication). Moreover, i.c.v. administration of a 10-fold higher dose of hrIL-1ra suppressed fever to a similar extent as the final dose (100 μg) used in this study (Fig. 2). The absence of a linear dose-response relation between IL-1ra and suppression of the fever suggests a maximal level at which IL-1ra can suppress peripheral LPS-induced fever. Lipopolysaccharide induces an array of cytokines, in particular TNF-α, IL-1 and IL-6, which are elevated in a temporal and causal manner (Fong et al. 1989; Givalois et al. 1994; Luheshi et al. 1996). The data presented here suggest that while endogenous brain IL-1 contributes significantly to LPS-induced fever, other pyrogenic mediators (such as IL-6) are probably also involved.
The results of the current study provide further evidence that endogenous IL-1 acts within the AH to mediate LPS-induced fever. Our data further indicate that the PVH is a potential site of endogenous IL-1 action during fever. Indeed it has been shown recently that cells projecting from the PVH are activated by peripheral (intravenous) LPS (Elmquist & Saper, 1996) and lesions of the PVH reduce the febrile response to peripheral (intraperitoneal) LPS (Horn et al. 1994). However, endogenous IL-1 does not appear to act at all hypothalamic sites. Despite the close proximity of the PVH and VMH, injection of IL-1ra into the VMH had no effect on the febrile response induced by peripheral LPS. These data, coupled with microinjection of dye into the various brain sites, indicate that attenuation of the peripheral LPS-induced febrile response, by IL-1ra, is not due to diffusion to other brain sites. The VMH is traditionally associated with hunger and satiety, and is a site that is particularly reactive to several thermally active substances (Myers, 1974), including prostaglandin E2 (Morimoto et al. 1988a;Simpson et al. 1994). However, it is thermally insensitive to direct injection of exogenous cytokines (Miñano & Myers, 1991).
Injection of IL-1ra into the SFO, but not the OVLT, significantly attenuated fever induced by peripheral LPS. Both brain sites are highly vascularized circumventricular organs (CVOs) (Dellmann, 1985; Gross et al. 1986), and are potential routes whereby peripherally produced, circulating pyrogens could access the brain to initiate fever (Blatteis, 1992; Zeisberger & Merker, 1992; Maness et al. 1998). The present data suggest that endogenous IL-1, therefore, acts at sites in the SFO, but not the OVLT, to elicit fever in response to peripheral administration of LPS. This observation indicates the involvement of neural afferent rather than humoral signals (which have been proved to act at the OVLT) in the development of febrile responses to systemic stimuli (see Elmquist et al. 1997). While the OVLT does not appear to be a site of action for endogenous IL-1 in response to local inflammation, our data do not preclude the importance of the OVLT as a potential access point for other pyrogens and/or neuroactive substances (Morimoto et al. 1988b; Stitt, 1991; Vallières & Rivest, 1997; Scammell et al. 1998) which influence body temperature. Several studies are consistent with the hypothesis that this region translates blood-borne signals conveyed by the endogenous pyrogen into brain signals (see Blatteis, 1992; Elmquist et al. 1997). However, data from the present study are supported by findings of reduced systemic (intravenous) LPS-induced fevers in rats with lesions of the SFO but not the OVLT (Takahashi et al. 1997), induction of immunoreactive IL-1β in the SFO during endotoxin fever (Nakamori et al. 1994) and changes in protein synthesis in the SFO within 1 h after subcutaneous injection of IL-1β (Williams et al. 1994).
Although the hypothalamus is considered to be the primary site of thermoregulatory control in the brain, actions at other brain sites cannot be excluded. Receptors for IL-1 are distributed widely throughout the rat brain (Farrar et al. 1987; Yabuuchi et al. 1994; Gayle et al. 1997) and the extensive distribution of mRNA (Ban et al. 1992; Buttini & Boddeke, 1995), immunoreactive (van Dam et al. 1992; Nguyen et al. 1998) and bioactive (Quan et al. 1994) IL-1 expression, in response to systemic LPS, suggests multiple potential sites of endogenous IL-1 action in the rat brain. Although there is no direct evidence from this study, other studies have shown CVOs, meninges and choroid plexus to be sites of IL-1 expression after systemic LPS (Nakamori et al. 1994, 1995). The cell types expressing IL-1 in these regions include macrophages, microglia and perivascular cells. Marked induction of IL-1 in microglia throughout the entire brain parenchyma has also been observed (van Dam et al. 1992; Wong et al. 1997), but in these studies the doses of LPS injected were > 25-fold the dose used to induce fever in the present study. The observed IL-1 expression, therefore, may be due to the direct action of LPS and/or endogenous pyrogens on the brain.
Injection of hrIL-1ra into the hippocampus (dentate gyrus or CA3 region), but not the striatum or cortex, significantly attenuated the LPS-induced fever. It is unlikely that IL-1ra injected into the hippocampus diffused to the AH and thereby attenuated the hyperthermia, since the equivalent volume of injected dye was subsequently localized in the hippocampus. Stimulation of the hippocampus may directly influence neurones in the AH (Hori et al. 1982) and this region is particularly sensitive to IL-1 action in response to peripheral LPS injection (Weidenfeld et al. 1995). The failure of IL-1ra, when injected into the striatum, to attenuate LPS-induced fever further suggests that the sites of action of endogenous IL-1 in fever may be dissociated from the sites of action of exogenous IL-1. We have found, as with i.c.v. injection, that direct administration of exogenous IL-1 into the striatum induces a pronounced increase in body temperature (Grundy et al. 1998), which can be inhibited by pretreatment with flurbiprofen, a cyclooxygenase inhibitor (R. I. Grundy, personal communication). These findings dissociate the effects of IL-1ra in neurodegeneration, which appear to be localized in the striatum (Lawrence et al. 1988; Stroemer & Rothwell, 1997), from its actions in fever. The various actions of endogenous IL-1 in the brain, therefore, appear to be both stimulus dependent and site specific.
In conclusion, the present data indicate that the febrile response to subcutaneous injection of LPS results from the action of endogenous IL-1 at several specific brain sites. Our data do not negate the importance of the action of additional pyrogens and/or neuroactive substances, which may participate in the febrile response.
Acknowledgments
This research was supported by a Wellcome Trust International Travelling Postdoctoral Fellowship (T. C.). We are grateful to Mrs Anthea Hughes for technical support.
References
- Anforth HR, Bluthe R-M, Bristow A, Hopkins S, Lenczowski MJP, Luheshi GN, Lundkvist J, Michaud B, Mistry Y, Van Dam A-M, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJH, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1α and interleukin-1β. European Cytokine Network. 1998;9:279–288. [PubMed] [Google Scholar]
- Ban E, Haour F, Lenstra R. Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4:48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Meyer M, Lindholm D, Spranger M, Heumann R, Thoenen H. Regional and cellular codistribution of interleukin-1β and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. Journal of Cell Biology. 1990;111:1701–1711. doi: 10.1083/jcb.111.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis CM. Role of the OVLT in the febrile response to circulating pyrogens. Progressive Brain Research. 1992;91:409–412. doi: 10.1016/s0079-6123(08)62360-2. [DOI] [PubMed] [Google Scholar]
- Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1β messenger RNA in rat brain microglial cells. Neuroscience. 1995;65:523–530. doi: 10.1016/0306-4522(94)00525-a. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Miller AJ, Mistry Y, Rothwell NJ, Luheshi GN. Circulating IL-6 is derived from the site of inflammation in response to a localized injection of lipopolysaccharide in the rat. The Journal of Physiology. 1998;513.P:146P. [Google Scholar]
- Dellmann H-D. Fine structural organization of the subfornical organ. A concise review. Brain Research Bulletin. 1985;15:71–78. doi: 10.1016/0361-9230(85)90063-2. [DOI] [PubMed] [Google Scholar]
- Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. Journal of Pathology. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Saper CB. Activation of neurons projecting to the paraventricular hypothalamic nucleus by intravenous lipopolysaccharide. Journal of Comparative Neurology. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends in Neurosciences. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualisation and characterisation of interleukin 1 receptors in brain. Journal of Immunology. 1987;139:459–463. [PubMed] [Google Scholar]
- Fong Y, Tracey KJ, Moldawer LL, Hesse DG, Manogue KB, Kenney JS, Lee AT, Kuo GC, Allison AC, Lowry SF, Cerami A. Antibodies to cachectin/tumor necrosis factor reduce interleukin-1 beta and interleukin-6 appearance during lethal bacteremia. Journal of Experimental Medicine. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Plata-Salamán CR. Interleukin-1 receptor type I mRNA levels in brain regions from male and female rats. Brain Research Bulletin. 1997;42:463–467. doi: 10.1016/s0361-9230(96)00373-5. [DOI] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud I, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. American Journal of Physiology. 1994;267:R164–170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Rudolph K, Tesfaigzi J, Kluger MJ. Role of hypothalamic interleukin-1 beta in fever induced by cecal ligation and puncture in rats. American Journal of Physiology. 1998;275:R754–761. doi: 10.1152/ajpregu.1998.275.3.R754. [DOI] [PubMed] [Google Scholar]
- Gross PM, Sposito NM, Pettersen SE, Fenstermacher JD. Differences in function and structure of the capillary endothelium in gray matter, white matter and a circumventricular organ of rat brain. Blood Vessels. 1986;23:261–270. doi: 10.1159/000158652. [DOI] [PubMed] [Google Scholar]
- Grundy RI, Rothwell NJ, Allan SM. Interleukin-1 modifies excitotoxic cell death independently of effects on body temperature. British Journal of Pharmacology. 1998;123:85P. [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: expression and recognition. Trends in Neurosciences. 1995;18:83–88. [PubMed] [Google Scholar]
- Hori T, Osaka T, Kiyohara T, Shibata M, Nakashima T. Hippocampal input to preoptic thermosensitive neurons in the rat. Neuroscience Letters. 1982;32:155–158. doi: 10.1016/0304-3940(82)90266-x. [DOI] [PubMed] [Google Scholar]
- Hori T, Shibata M, Nakashima T, Yamasaki M, Asami A, Asami T, Koga H. Effects of interleukin-1 and arachidonate on the preoptic and anterior hypothalamic neurons. Brain Research Bulletin. 1988;20:75–82. doi: 10.1016/0361-9230(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Horn T, Wilkinson MF, Landgraf R, Pittman QJ. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. American Journal of Physiology. 1994;267:R323–328. doi: 10.1152/ajpregu.1994.267.1.R323. [DOI] [PubMed] [Google Scholar]
- Klir JJ, McClellan JL, Kluger MJ. Interleukin 1β causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. American Journal of Physiology. 1994;266:R1845–1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiological Reviews. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1 beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. European Journal of Neuroscience. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Lesnikov VA, Efremov OM, Korneva EA, Van Damme J, Billiau A. Fever produced by intrahypothalamic injection of interleukin-1 and interleukin-6. Cytokine. 1991;3:195–198. doi: 10.1016/1043-4666(91)90016-7. [DOI] [PubMed] [Google Scholar]
- Liu JC, Shyy TT. Pyrogenic responses in the decerebrate monkey (Macaca cyclopis) Experimental Neurology. 1980;67:481–491. doi: 10.1016/0014-4886(80)90120-x. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Miller AN, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. American Journal of Physiology. 1996;270:E91–95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- Maness LL, Kastin AJ, Banks WA. Relative contributions of a CVO and the microvascular bed to delivery of blood-borne IL-1α to the brain. American Journal of Physiology. 1998;275:E207–212. doi: 10.1152/ajpendo.1998.275.2.E207. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. British Journal of Pharmacology. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miñano FJ, Myers RD. Anorexia and adipsia: dissociation from fever after MIP-1 injection in ventromedial hypothalamus and preoptic area of rats. Brain Research Bulletin. 1991;27:273–278. doi: 10.1016/0361-9230(91)90081-t. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Sakata Y, Watanabe T. Brain regions involved in the development of acute phase responses accompanying fever in rabbits. The Journal of Physiology. 1989;416:645–657. doi: 10.1113/jphysiol.1989.sp017782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Watanabe T. Ventromedial hypothalamus is highly sensitive to prostaglandin E2 for producing fever in rabbits. The Journal of Physiology. 1988a;397:259–268. doi: 10.1113/jphysiol.1988.sp016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Watanabe T. Multiple control of fever production in the central nervous system of rabbits. The Journal of Physiology. 1988b;397:269–280. doi: 10.1113/jphysiol.1988.sp017000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Sakata Y, Watanabe T. Central action sites of interleukin-1β for inducing fever in rabbits. The Journal of Physiology. 1990;428:299–312. doi: 10.1113/jphysiol.1990.sp018213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD. Handbook of Drug and Chemical Stimulation of the Brain. New York: Van Nostrand Reinhold; 1974. [Google Scholar]
- Nakamori T, Morimoto A, Yamaguchi K, Watanabe T, Murakami N. Interleukin-1 beta production in the circumventricular organs during fever in rabbits. In: Pleschka K, Gertsberger R, editors. Integrative and Cellular Aspects of Autonomic Functions: Temperature and Osmoregulation. Paris: John Libbey Eurotext; 1994. pp. 153–162. [Google Scholar]
- Nakamori T, Sakata Y, Watanabe T, Morimoto A, Nakamura S, Murakami N. Suppression of interleukin-1 beta production in the circumventricular organs in endotoxin-tolerant rabbits. Brain Research. 1995;675:103–109. doi: 10.1016/0006-8993(95)00045-r. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hori T, Mori T, Kuriyama K, Mizuno K. Recombinant human interleukin-1β alters the activity of preoptic thermosensitive neurons in vitro. Brain Research Bulletin. 1989;23:209–213. doi: 10.1016/0361-9230(89)90149-4. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. Journal of Neuroscience. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain areas after peripheral and central injections of lippolysaccharide. Journal of Neuroimmunology. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Neuroimmune interactions: the role of cytokines. British Journal of Pharmacology. 1997;121:841–847. doi: 10.1038/sj.bjp.0701248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. American Journal of Physiology. 1998;274:R783–789. doi: 10.1152/ajpregu.1998.274.3.R783. [DOI] [PubMed] [Google Scholar]
- Sellami S, de Beaurepaire R. Hypothalamic and thalamic sites of action of interleukin-1β on food intake, body temperature and pain sensitivity in the rat. Brain Research. 1995;694:69–77. doi: 10.1016/0006-8993(95)00763-g. [DOI] [PubMed] [Google Scholar]
- Simpson CW, Ruwe WD, Myers RD. Prostaglandins and hypothalamic neurotransmitter receptors involved in hyperthermia: a critical evaluation. Neuroscience and Biobehavioral Reviews. 1994;18:1–20. doi: 10.1016/0149-7634(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Stitt JT. Differential sensitivity in the sites of fever production by prostaglandin E1 within the hypothalamus of the rat. The Journal of Physiology. 1991;432:99–110. doi: 10.1113/jphysiol.1991.sp018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer RP, Rothwell NJ. Cortical protection by localised striatal injection of IL-1ra following cerebral ischaemia in the rat. Journal of Cerebral Blood Flow and Metabolism. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Smith P, Ferguson A, Pittman QJ. Circumventricular organs and fever. American Journal of Physiology. 1997;273:R1690–1695. doi: 10.1152/ajpregu.1997.273.5.R1690. [DOI] [PubMed] [Google Scholar]
- Vallières L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1β. Journal of Neurochemistry. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- van Dam A-M, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness. Brain Research. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- Walter JS, Meyers P, Kreuger JM. Microinjection of interleukin-1 into brain: separation of sleep and fever responses. Physiology and Behavior. 1989;45:169–176. doi: 10.1016/0031-9384(89)90181-9. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Crumeyrolle-Arias M, Haour F. Effect of bacterial endotoxin and interleukin-1 on prostaglandin biosynthesis by the hippocampus of mouse brain: role of interleukin-1 receptors and glucocorticoids. Neuroendocrinology. 1995;62:39–46. doi: 10.1159/000126986. [DOI] [PubMed] [Google Scholar]
- Williams LM, Ballmer PE, Hannah LT, Grant I, Garlick PJ. Changes in regional protein synthesis in rat brain and pituitary after systemic interleukin-1β administration. American Journal of Physiology. 1994;267:E915–920. doi: 10.1152/ajpendo.1994.267.6.E915. [DOI] [PubMed] [Google Scholar]
- Wong M-L, Bongiorno PB, Rettori V, McCann SM, Lucinio J. Interleukin (IL)-1β, IL-1 receptor antagonist, IL-10 and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proceedings of the National Academy of Sciences of the USA. 1997;94:227–232. doi: 10.1073/pnas.94.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi K, Minami M, Katsumata S, Satoh M. Localisation of type I interleukin-1 receptor mRNA in the rat brain. Molecular Brain Research. 1994;27:27–36. doi: 10.1016/0169-328x(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Zeisberger E, Merker G. The role of OVLT in fever and antipyresis. Progressive Brain Research. 1992;91:403–408. doi: 10.1016/s0079-6123(08)62359-6. [DOI] [PubMed] [Google Scholar]


