Abstract
Electrical stimulation of digital nerves elicits short-latency excitatory and inhibitory spinal reflex responses in ongoing EMG in muscles acting on the fingers and thumb. Similar responses are elicited by stimulating a population of muscle spindles but not when a single muscle spindle is activated. The current study investigated whether short-latency EMG responses could be evoked from the discharge of a single cutaneous afferent.
Thirty-three tactile afferents were recorded via tungsten microelectrodes in the median nerve of awake humans. Spike-triggered averaging revealed EMG events time-locked to the afferent discharge. The afferents were activated by an external probe and the EMG was elicited by a weak voluntary contraction.
Eleven cutaneous afferents (33 %) showed a short-latency response in the ongoing EMG. Overt increases or decreases in EMG were observed for seven afferents (onset latency 20.0-41.1 11hms1h). For four slowly adapting (SA) type II afferents, EMG showed a periodicity that was correlated to the afferent interspike interval (r = 0.99).
The EMG associated with two rapidly adapting (FA) type I afferents (29 %) showed a short-latency excitation while five showed neither excitation nor inhibition. Seven SA II afferents (39 %) showed excitation and 11 no response; and none of the six SA I afferents showed any response.
We conclude that, unlike muscle spindle afferents, the input from a single cutaneous afferent is strong enough to drive, via interneurones, motoneurones supplying muscles acting on the digits. The potent short-latency response we found supports the important role of cutaneous mechanoreceptors in fine motor control of the human hand.
When we use our hands to manipulate objects peripheral feedback is of paramount importance in the fine control of grip and reaction forces (Johansson & Cole, 1992). It has long been known that electrical stimulation of the digital nerves can elicit a short-latency increase, followed by a short-latency decrease and a long-latency increase in ongoing electromyographic activity (EMG) of muscles acting on the fingers and thumb (Caccia et al. 1973; Buller et al. 1980; Datta & Stephens, 1981; Evans et al. 1989). The initial short-latency excitatory response in first dorsal interosseous muscle (FDI) occurs at (mean ±s.e.m.) 35 ± 1 ms, with the subsequent inhibition and excitation occurring at 49 ± 1 and 58 ± 1 ms, respectively (Evans et al. 1989). The spinally mediated nature of the short-latency excitation and short-latency inhibition was established by measuring conduction times to the spinal cord of both afferent and efferent signals with an allowance for interneuronal conductance (Jenner & Stephens, 1982), but there is some debate as to whether the long-latency excitation is mediated by a transcortical pathway or by a long-latency spinal reflex (Marsden et al. 1977a,b; Noth et al. 1983; Darton et al. 1985; Palmer & Ashby, 1992a,b; Macefield et al. 1996). Brisk mechanical stimulation of cutaneous receptors in the digits has also been shown to elicit short-latency (34 ± 1 ms) and long-latency (60 ± 1 ms) excitatory responses in FDI, with some evidence of an intervening inhibitory phase (Macefield et al. 1996), although the long-latency excitation has been the most consistently observed (Cole & Abbs, 1988; Johansson et al. 1992a). These reflexes, which are abolished by digital nerve block (Johansson et al. 1992a,b,c; Deuschl et al. 1996), are similar to those obtained by mechanical stimulation of muscle afferents in FDI, being 37 ± 2 and 59 ± 1 ms, respectively (Macefield et al. 1996).
Given that these short- and long-latency tactile reflexes are rather robust, one might expect that a reflex coupling could be demonstrated between a single cutaneous receptor in a digit and motoneurones supplying muscles acting on the digits. Interestingly, using spike-triggered averaging Gandevia et al. (1986) failed to find significant reflex coupling between single muscle spindle endings in human tibialis anterior muscle and motoneurones supplying this muscle, yet they and others (Buller et al. 1980; Garnett & Stephens, 1980; Gandevia et al. 1986) did find both short- and long-latency excitation when stimulating a population of muscle afferents. In the present study we have used spike-triggered averaging to assess whether the discharge of a single cutaneous receptor in a digit can modulate the ongoing EMG in human hand muscles. Some of this work has been presented in abstract form (McNulty et al. 1998).
METHODS
General procedures
Twenty-one experiments were performed on 17 healthy and apparently neurologically normal subjects (13 female, 4 male: age range 16-44 years); four subjects (including two of the authors) participated in two experiments. All subjects gave signed, informed consent and ethical approval was given by the Committee on Experimental Procedures Involving Human Subjects, University of New South Wales. Subjects reclined in a comfortable chair in a semi-recumbent position with the forearm supported in a supinated position on a stable platform. The elbow was flexed to ∼50 deg with the hand resting in a natural position, supported by modelling clay.
Neural recording
An insulated tungsten microelectrode (200 μm diameter, 5 μm length uninsulated tip, 1 μm tip diameter; type TM33B20, World Precision Instruments, USA) was inserted through the skin into the median nerve approximately 2 cm proximal to the wrist. A similar electrode with 1 mm of insulation removed from the tip was inserted subdermally 2 cm away and served as a reference electrode. Intraneural stimulation (0.1 ms pulse, 1 Hz, 0.01-1 mA) was delivered from an optically isolated, constant-current stimulator (ADInstruments, Australia). Once a cutaneous fascicle was penetrated (paraesthesiae generated below 0.025 mA) the microelectrode was connected to a preamplifier and advanced while the fascicular innervation territory was mechanically stimulated. Unitary action potentials, confirmed by uniform spike amplitude and shape, were recorded from cutaneous afferents that were classified according to the receptive field and adaptation characteristics of the four types of tactile mechanoreceptors found in glabrous skin (Johansson & Vallbo, 1978): rapidly adapting type I and II units (FA I, FA II), and slowly adapting type I and II units (SA I, SA II). Neural activity was filtered (bandwidth 0.3-5 kHz, gain 104), digitized (12.8 kHz) and stored on computer using the SC/ZOOM data acquisition and analysis system (Department of Physiology, University of Umeå, Sweden).
EMG recording
In the first series of experiments intramuscular EMG was recorded from the first dorsal interosseous muscle (FDI) using an insulated tungsten microelectrode from which 1 mm of insulation had been removed. Cutaneous afferents with receptive fields in the second digit were not studied in this series. In the second series, EMG was recorded with disposable 10 mm2 Ag-AgCl surface electrodes placed over the belly of the muscle acting on the digit where the receptive field of the mechanoreceptor had been identified. The muscles activated when recording from a receptor in the thumb were flexor pollicis longus (FPL) or the thenar muscles, FDI or flexor digitorum superficialis (FDS) in the index finger, and FDS in the middle and ring fingers. EMG activity was amplified (gain 2 × 103, bandwidth 10 Hz-5 kHz; ADInstruments), digitized at 3.2 kHz (surface EMG) or 6.4 kHz (intramuscular EMG) and stored on computer with the cutaneous afferent signal.
Experimental procedure
The receptive field of each afferent was stimulated throughout the experiment by a mechanical probe (contact surface diameter 1-2 mm). Stroking across the receptive field provided ongoing stimulation for the FA I units, whereas the SA I and SA II units were stimulated by a constant indentation of the receptive field. During the mechanical stimulation subjects maintained a weak voluntary contraction (< 20 % maximum voluntary contraction), either against a probe (SA I and SA II units) or against a fixed structure that did not contact the receptive field (FA I), as illustrated in Fig. 1.
Figure 1. Experimental set-up.
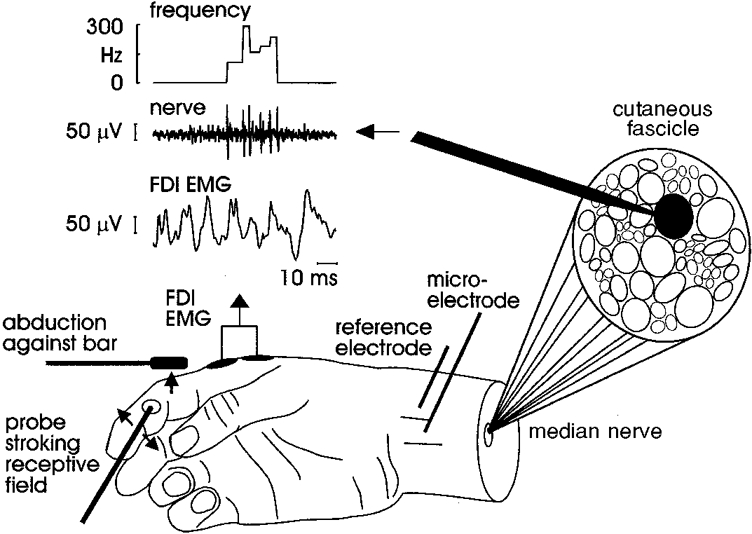
Data analysis
The unitary integrity of each recording was established using the spike recognition software incorporated in SC/ZOOM which allows the superimposition of individual spikes from the neural signal. The tight superimposition of these spikes, based on amplitude and morphology, is presented in each figure to illustrate the unitary nature of these recordings. Data were then analysed using spike-triggered averaging which samples the data with reference to each nerve spike so that the peak of the action potential becomes trigger-time zero (Buchthal & Schmalbruch, 1970; Milner-Brown et al. 1973). This temporally couples the EMG signals to the nerve-spike trigger, revealing any time-locked EMG event after averaging (number of spike triggers = 1235-9718). Averaged EMG was root-mean-square (RMS) processed, sampling over a sliding 6 ms window, to rectify and filter the signal while maintaining the integrity of the signal amplitude. Autocorrelograms (spike-to-spike) were constructed from the afferent spikes using 5 ms bins to illustrate the temporal profile of the neural signal in relation to the averaged EMG. Eleven units showed an overt response, that is, short-latency excitation or inhibition in the averaged EMG. The criterion for identifying an overt response is that there must be a visually apparent change evident in the ongoing EMG with a clearly defined peak projecting above the background activity, and that this change must occur at an appropriate latency for the known conduction velocities of these afferents (Johansson & Vallbo, 1983; Mackel, 1988). Latencies were measured using cursor placements at high temporal resolutions.
RESULTS
Afferent sample
Thirty-three afferents were recorded in the digits of the hand during 21 experiments (seven FA I afferents, six SA I afferents, 18 SA II afferents and two ectopically discharging afferent units that could not be type-identified). No FA II units were recorded. When recording intramuscular EMG from FDI, three afferents were located in the thumb, five in digit III, and eight in digit IV (including both ectopic units). When surface EMG was recorded from a muscle acting on the digit where the receptive field was located, 14 units were found in the thumb, with EMG in the thenar muscles, and three in digit II, with EMG in either FDI (1 unit) or FDS (2 units). As summarized in Table 1, two FA I units (29 %) showed short-latency excitation in the associated EMG, and five showed neither excitation nor inhibition. All the SA I units recorded showed no response. Conversely, seven SA II units (39 %) showed excitation in the ongoing EMG and 11 no response. One of the two ectopically discharging units showed a short-latency excitation and the other a short-latency inhibition. Short-latency EMG responses were evident from 19.0-73.8 ms after the afferent spike.
Table 1. Summary of afferents showing reflex responses.
| Unit no. | Unit type | Muscle | Digit | No. of spikes | Short-latency excitation(ms) | Short-latency inhibition(ms) | Long-latency excitation (ms) |
|---|---|---|---|---|---|---|---|
| 1 | FA I | FDS | II | 9718 | 20.8 | 74.5 | 113.9 |
| 2 | FA I | FDI | III | 1347 | 29.4 | 44.2 | 63.8 |
| 3 | SA II | Thenar | I | 2878 | 22.5 | — | — |
| 4 | SA II | Thenar | I | 7133 | 20.3 | — | — |
| 5 | SA II | FDI | I | 4121 | 24.8 | 54.3 | 58.9 |
| 6 | Ectopic | FDI | IV | 1702 | 41.1 | 60.0 | 93.1 |
| 7 | Ectopic | FDI | IV | 9027 | — | 46.3 | 78.8 |
| 8 | Cyclic SA II | Thenar | I nail | 1235 | 23.2 | — | — |
| 9 | Cyclic SA II | Thenar | I nail | 5542 | 26.5 | — | — |
| 10 | Cyclic SA II | FDI | II nail | 7405 | 15.2 | — | — |
| 11 | Cyclic SA II | FDS | II palm | 2097 | 73.8 | — | — |
Units arranged by afferent class. Number of spikes indicates the number of sweeps used in spike-triggered averaging.
Overt responses
Spike-triggered averaging revealed a time-locked increase or decrease in ongoing EMG for 11 afferents, i.e. the responses were evident without the need for further processing. Seven SA II afferents exhibited an overt short-latency EMG response, four of which will be considered separately (see section headed ‘Cyclic behaviour’). The range of onset latencies for the other three SA II units were 20.0-24.8 ms (peak latencies 27.9-33.4 ms). Figure 2 illustrates an SA II afferent recorded in the thenar eminence during a maintained indentation of the receptive field that elicited a regular discharge (mean ±s.e.m. 11.9 ± 0.08 Hz) while the subject sustained a weak voluntary abduction of the thenar muscles against the stimulating probe. The regularity of the afferent discharge can be seen in the autocorrelogram in Fig. 2B, in which the majority of spikes are clustered in one 5 ms bin. While EMG from four muscles was recorded, the excitatory response was only seen in the contracting thenar muscles. The onset latency for the initial excitation was 22.5 ms, the peak latency 30.6 ms. The apparent peak in the averaged prestimulus EMG may well reflect the input from the preceding spike, occurring as it does at an appropriate latency (see Discussion). As indicated in Table 1, onset latencies for the EMG associated with the other two SA II afferents showing overt responses were comparable.
Figure 2. Short-latency excitation in an SA II afferent.
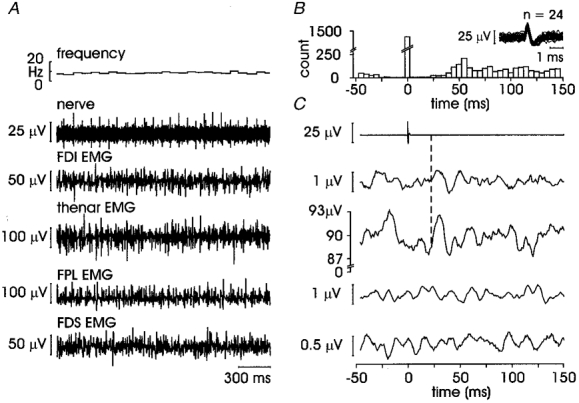
A, raw data for unit 3 in Table 1 with instantaneous frequency in top trace, neurogram in second trace and four EMG traces below; B, autocorrelogram of the afferent spikes; superimposed spikes in inset; C, averaged data from 2878 sweeps; dashed line to second trace indicates onset latency for response in thenar EMG (22.5 ms).
Figure 3 illustrates an FA I afferent located in the middle phalanx of the index finger, which was one of two FA I afferents showing an overt EMG response. It responded vigorously to stroking across the receptive field, discharging in brief (50-100 ms) high-frequency bursts (> 400 Hz), the burst duration reflecting the time taken for the probe to traverse the receptive field. EMG from four muscles was recorded in this experiment while the subject performed a weak flexion of the index finger against a rigid bar. However, as noted above, following spike-triggered averaging the response was only evident in the contracting muscle, despite intermittent spontaneously active motor units in the other muscles (not shown). The short-latency excitation clearly evident in the FDS EMG had an onset of 20.8 ms and a peak latency of 36.3 ms. Interestingly, this excitatory response was rather broad and it is apparent from the autocorrelogram (Fig. 3B) that this can be accounted for by the temporal spread of the spikes that comprise the high-frequency bursting pattern of this unit.
Figure 3. Short-latency excitation in an FA I afferent.
A, raw data for unit 1 in Table 1, with EMG from FDS (other EMG traces were flat); asterisks indicate the occurrence of spikes used to generate trace in D; B, afferent autocorrelogram and superimposed spikes; C, averaged data from 9718 sweeps; dashed line indicates onset latency 20.8 ms; D, repeated averaging using last spike from each burst (first 800 bursts of record); dotted line indicates onset latency of 18.1 ms.
To ensure that the EMG response was a real phenomenon and not a recording artefact, the averaging was repeated in this FA I unit using all spikes from the first half of the record only, and then again by triggering from the last spike only of each burst in the first part of the record (first from 4500 of 9718 spikes, and then from 800 spikes). Similar latencies for the same excitatory reflex were seen in each case and the second of these can be seen in Fig. 3D. Onset and peak latencies for the other FA I afferent with an overt EMG response were 29.4 and 37.4 ms, respectively.
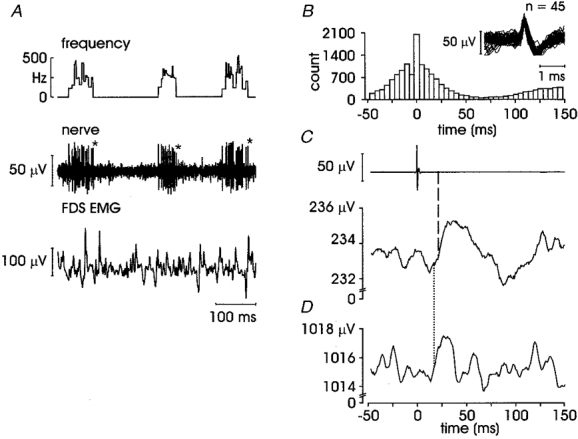
Two units were spontaneously active and showed the typical firing pattern of ectopically discharging myelinated axons (Macefield, 1998): short bursts of high-frequency firing. The unit illustrated in Fig. 4 discharged with 6.1 ± 0.1 spikes per burst, an average bursting frequency of 8.5 ± 0.1 Hz and a peak firing rate of 243.1 ± 1.8 Hz. The other generated 1.9 ± 0.1 spikes per burst, with an average bursting frequency of 9.3 ± 0.1 Hz and a peak firing rate of 230.0 ± 2.1 Hz. These units were not type-identified as their receptive fields cannot be located but both were in a cutaneous fascicle supplying digit IV; it is highly likely these units were tactile afferents, although we cannot exclude the possibility that one or both of these units originated in an interphalangeal joint. Overt EMG responses were seen in both ectopically discharging units. One of these (unit 6 in Table 1) showed a short-latency excitation, with onset and peak latencies of 41.1 and 54.2 ms, the other (unit 7) a short-latency inhibition (onset latency 46.3 ms, peak latency 68.4 ms). The unit illustrated in Fig. 4 (like that in Fig. 2) has a noticeable peak in the prestimulus EMG which we believe reflects the influence of the preceding burst of firing (see Discussion).
Figure 4. Ectopically discharging unit showing a short-latency inhibition, followed by a long-latency excitation.
Unit 7 in Table 1. A, afferent autocorrelogram and superimposed spikes; B, averaged data from 9027 sweeps with short-latency onset 46.3 ms and long-latency onset 78.8 ms.
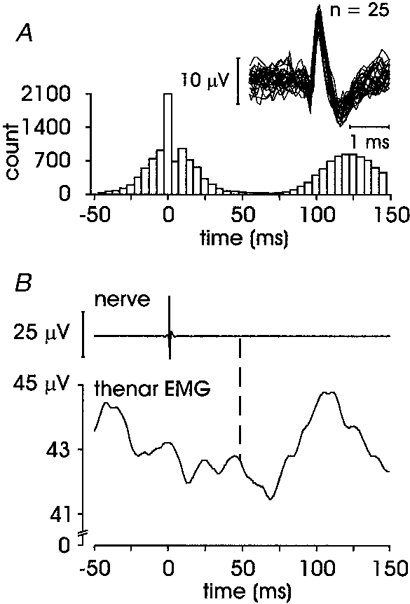
In addition to an overt short-latency excitation and/or inhibition, five units (two FA I, one SA II and two ectopic units) expressed a subsequent increase in the averaged EMG. An example of this long-latency response is illustrated in Fig. 5. This unit, an FA I afferent recorded in the pulp of the distal phalanx in digit III, shows a particularly large and well-defined triphasic pattern in FDI EMG: short-latency excitation at 29.4 ms, short-latency inhibition at 44.2 ms and long-latency excitation at 63.8 ms. This unit responded with a single spike to each tap over its receptive field, and it is clear from the autocorrelogram that the long-latency EMG response cannot be attributed to a later clustering of spikes from this unit. Data from four other units with a similar long-latency response are presented in Table 1. Note that unit 7 did not express a short-latency excitation but a short-latency inhibition was observed in the EMG.
Figure 5. FA I afferent showing short-latency excitation and inhibition, and long-latency excitation.
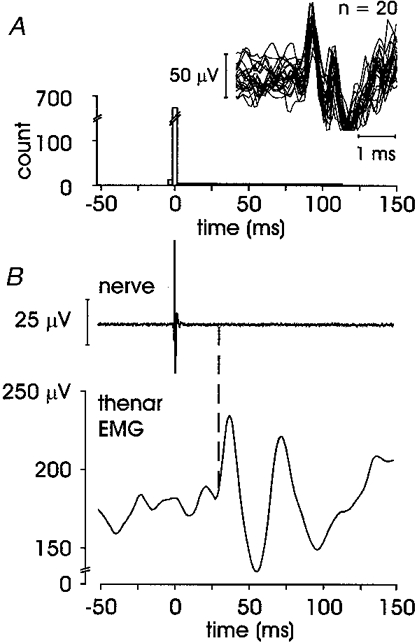
Unit 2 in Table 1. A, afferent autocorrelogram and superimposed spikes; B, averaged data from 1347 sweeps, short-latency excitation onset at 29.4 ms, short-latency inhibition at 44.2 ms and the long-latency excitation at 63.8 ms.
Cyclic behaviour
Interestingly, four SA II units showed an overt ‘cyclic’ response. For each unit EMG was recorded in a muscle acting on the digit on which the receptive field was located. These units (units 8-11 in Table 1) were located as follows: two in the nail bed of digit I (thenar muscles contracting), one in the nail bed of digit II (FDI contracting) and one just proximal to the medial side of the second metacarpophalangeal joint (FDS contracting). As illustrated in Fig. 6, while there was no apparent single peak in the ongoing EMG time-locked to the afferent spike, there was a rhythmic fluctuation in the averaged EMG activity. The average firing rates of these units were very regular and, in three units, higher than usual for SA II receptors (average firing rates (mean ±s.e.m.) 45.4 ± 0.17, 34.8 ± 0.28, 47.5 ± 0.02 and 15.8 ± 0.08 Hz). This regularity (variability 3.1 %), and its tight coupling to the EMG, is also apparent in the autocorrelogram in Fig. 6.
Figure 6. SA II unit displaying cyclic behaviour.
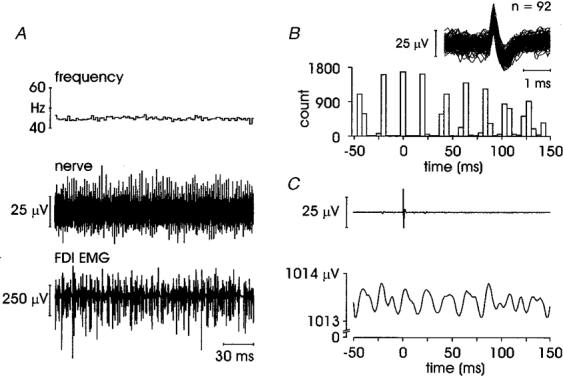
Unit 10 in Table 1, recorded in the nail bed of digit II. A, instantaneous firing rate (mean ±s.e.m.) 47.5 ± 0.02 Hz; B, afferent autocorrelogram and superimposed spikes; C, averaged data from 7405 sweeps.
As shown in Fig. 7, there was a very tight linear relationship between the interspike interval and the periodicity (peak to peak) of the cyclic EMG for the four units (r = 0.99). The number of afferent spike triggers ranged from 1235 to 5542. SA II units firing at ≤ 10 Hz are not discharging fast enough for cyclic behaviour to be detectable - their interspike intervals are greater than 100 ms - such that only one cycle will be observed within the sampling window. Once this cycling behaviour was taken into account, each of these units exhibited a short-latency excitation that was related to their firing rate: latencies from the spike to the subsequent trough of the averaged EMG were 23.2, 26.5, 15.2 and 73.8 ms, respectively. Note that the afferent generating the longest latency response also fired at a lower rate (15.8 Hz).
Figure 7. Linear regression of the periodicity of EMG output on the interspike interval in cyclic units (n = 4).
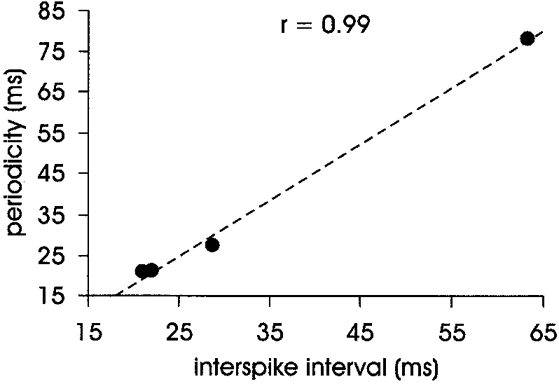
Periodicity is measured from cycle peak to peak in averaged EMG (see Fig. 6). Interspike interval is the reciprocal of the firing rate of the afferent.
DISCUSSION
In this study we have, for the first time, demonstrated a strong synaptic coupling between single cutaneous afferents and the motoneurone pool of muscles acting on the digits of the human hand. We have used spike-triggered averaging to reveal short-latency excitatory and inhibitory responses in ongoing EMG activity associated with both type-identified and ectopically discharging cutaneous afferent potentials. A triphasic pattern in the EMG of short-latency excitation followed by short-latency inhibition and long-latency excitation was seen in five units. A pattern of cyclic behaviour was also identified in four SA II units firing with a very regular discharge rate > 15 Hz.
Methodological considerations
The median nerve at the wrist was selected because it innervates a large proportion of the glabrous skin of the hand and the nerve is readily accessible to microneurography. At this level the nerve consists of purely cutaneous or motor fascicles, and only the former were selected. All unitary recordings were well isolated - we did not attempt to extract unitary action potentials from an oligounitary recording site. It has been shown that the presence of the microelectrode within the nerve fascicle does not cause appreciable axonal conduction block, indicating that afferent impulses are freely propagated to the central nervous system (Vallbo, 1976; Inglis et al. 1996,1998). However, we cannot exclude the possibility that our failure to demonstrate synaptic coupling between all tactile afferents and the motoneurone pool was in some instances due to the lack of propagation of the action potential beyond the recording site.
Stimulation of FA I afferents by stroking across the receptive field produces a pattern of short bursts of high frequency discharges. It is obvious from the known population densities of cutaneous afferents within the glabrous skin of the human hand (Johansson & Vallbo, 1979a) that, particularly for the FA I (and SA I) afferents, our stimuli could not have excited a single cutaneous mechanoreceptor, but rather a population. We did, however, record from a single tactile afferent. Every spike generated by the afferent was used to trigger averaging of the ongoing EMG, allowing us to observe EMG events time-locked to the unit's discharge. We have shown that the input from even a single tactile afferent can have a significant effect on the ongoing EMG of muscles acting on the digits. How much is due to an ensemble response is unclear, but within that ensemble response we can clearly identify the effect of the discharge from a single cutaneous mechanoreceptor onto the motoneurone pool. Moreover, such modulation of ongoing EMG could be observed even when the averaging was synchronized to the last spike of each burst, and could be seen with as few as 800 spike triggers.
SA I and SA II units were stimulated by sustained indentation of their receptive fields, i.e. by use of a static stimulus. SA II units respond with a very regular discharge pattern, while SA I units have a more irregular discharge. Given their extremely regular firing rate, we believe we are observing the synaptic input from a single SA II afferent, as it seems highly unlikely that multiple SA II units would fire with exact synchronicity in the same region. This is particularly so in units with a firing rate of ≤ 10 Hz, where the interspike interval is great enough for there to be a single neural spike per averaged sample. The sampling window we used for spike-triggered averaging was 250 ms, of which 50 ms was prestimulus. For SA II units firing around 10 Hz with an interspike interval of approximately 100 ms (i.e. 50 ms prestimulus and 50 ms post stimulus), there is no possibility of subsequent or preceding spikes interacting in the averaged result, at least with respect to the short-latency response.
Throughout each recording subjects used a single digit to exert a constant low-level force against the stimulating probe or a fixed structure. The low level of the contraction and the involvement of a single digit lessens the likelihood of afferents elsewhere in the hand being activated by factors associated with the contraction. These factors include skin stretch and additional cutaneous afferents activated by contact with the reaction surface. In FA I afferents the bursting pattern in response to stroking is quite different from that of SA I or SA II units that might be activated by contact with a structure to assist muscle contraction. Moreover, the receptive fields of FA I afferents are small and well defined. Therefore, there is no ambiguity between the firing of the FA I afferent we were recording and any associated activity due to contact with the reaction surface. For SA I and SA II units the stimulation of the receptive field and the reaction surface were one and the same.
Assessing synaptic coupling using spike-triggered averaging
Spike-triggered averaging has long been used to investigate synaptic coupling in the central nervous system, and in particular, reflex effects onto the motoneurone pool of peripheral muscles. Gandevia et al. (1986) used this technique to investigate the synaptic strength of single muscle spindle endings in tibialis anterior during ongoing contractions. Although they found no reflex activity from a single muscle afferent, it is known that activation of a population of spindles will elicit a short-latency monosynaptic excitation in the homonymous motoneurone pool of a contracting muscle (Buller & Stephens, 1980; Gandevia et al. 1986). We used this same approach to look for significant (oligo)synaptic coupling between single cutaneous afferents in the digits of the hand and muscles acting on these digits and, like Gandevia and co-workers, we stimulated a population of afferents but recorded from a single afferent. From within the response of a population of cutaneous mechanoreceptors we were able to demonstrate the synaptic connection between a single afferent and a muscle acting on the digits, regardless of the ensemble response. However, it is possible that the lack of reflex response in the study by Gandevia and colleagues reflects differences between leg and hand muscles, given that hand muscles are adapted for fine motor control. Important differences may include factors such as the strength and sensitivity of spindle input and the strength of the spinal connections.
Short-latency response to tactile afferent stimulation
Our short-latency reflex responses, using natural mechanical stimulation, fit well with the results of electrical stimulation of whole digital nerves, which elicits a short-latency excitation (E1) followed by a short-latency inhibition (I) and a long-latency excitation (E2) in related ongoing EMG (Caccia et al. 1973; Jenner & Stephens, 1982; Evans et al. 1989). Air-puff stimulation, a natural stimulus producing a purely cutaneous afferent input (but probably not involving SA II units), also produces a similar reflex response pattern (Deuschl et al. 1996), although the latencies are longer. However, it should be pointed out that the initial excitation observed in this study, occurring at a latency around 33-37 ms and termed cLLR I corresponds to E1 elicited by electrical stimulation; likewise, the longer latency excitation occurring between 56 and 60 ms (termed cLLR II) corresponds to E2. The longer latencies associated with air-puff stimulation reflect the difference between electrical stimulation of whole digital nerves and mechanical stimulation of the skin: these include the time taken for adequate stimulation of tactile mechanoreceptors, slow afferent conduction in the terminal axons and temporal dispersion of a non-synchronized afferent volley. Interestingly, short-latency excitation has not been observed during manipulation of objects in which incipient or overt slips elicit compensatory increases in grip force - only the long-latency responses are observed (Johansson & Westling, 1987, 1988; Johansson et al. 1992a,b,c). However, in similar experiments to those of Johansson's group (1992a,b,c), but using a single digit, Macefield et al. (1996) were able to evoke both short- and long-latency responses.
In the present study we observed increases in EMG of comparable latency to those reported above when synchronizing the averaging to the discharge of a single cutaneous receptor. Overt short-latency excitatory reflex responses in ongoing EMG were observed for five units, with onset latencies ranging from 20.0 to 29.4 ms for the type-identified cutaneous afferents, 41.1 and 46.3 for the ectopically discharging units, and 15.2 to 73.8 ms for the cyclic units. The wider range of onset latencies can largely be explained by the range of conduction velocities reported for single cutaneous afferents, 40-80 m s−1 (Johansson & Vallbo, 1983) and 20-60 m s−1 (Mackel, 1988). Furthermore, there are no significant differences in conduction velocity between the four types of cutaneous afferents - rather the afferent conduction velocity is highly correlated to axonal diameter, which is not receptor specific (Mackel, 1988). The largest onset latency, 73.8 ms, appears long for a spinal reflex, but given the range of potential conduction velocities above, an indeterminate number of interneuronal synapses, and that the EMG is time locked to the slower firing rate of this SA II cyclic unit, we believe it still constitutes a spinal reflex, albeit at the slowest end of the spectrum.
In several units a prestimulus peak was apparent in the averaged EMG that was broader and smaller than the short-latency reflex response reported (Figs 2 and 4). This was apparent in units that displayed a regular firing pattern, either in single spikes or regular bursts of spikes. When the interspike interval is taken into account, in addition to the variability of the firing rate of the unit, this peak reflects the influence of the preceding spike or burst of spikes. When there is a random pattern of bursts in the afferent discharge (Fig. 3) or there is a gap > 250 ms between presentations of the stimulus to the receptive field of the afferent (Fig. 5), there is no evidence of any prestimulus peaks. It is interesting to note that, when the unit in Fig. 3 was analysed again triggering only from the last spike of each burst, the response seen was very similar but slightly faster and narrower. Again this reflects the variability of the afferent's firing rate (which in this case represents presentation of the stimulus) and the altered influence of the afferent's pattern of discharge.
As pointed out above, electrical and mechanical stimulation of cutaneous receptors elicit long-latency EMG responses. Although we did not set out to study long-latency reflexes, we were able to see them in five of the seven overt responses. It is clear from Fig. 5 that this late response is temporally coupled to the trigger spike, and cannot be described as a short-latency response to a secondary clustering of spikes in the input signal.
Evidence for unitary input to the reflex response
As discussed earlier the reflex responses in the EMG associated with the discharge of SA II afferents emphasizes the strength of the synaptic coupling we have found, given that adjacent SA II receptors will not be firing synchronously and the sampling window for averaging will only allow the reflex response to the triggering spike to be observed. Subsequent or preceding spikes will not contribute to the short-latency averaged result when these afferents are discharging around 10 Hz.
More definitive evidence comes from the EMG averages synchronized to the spontaneous discharge of two ectopically active cutaneous axons. These units fire without cutaneous stimulation, with the hand completely relaxed and the palmar surface not in contact with any object; their discharge cannot be modified by mechanical stimulation of the skin or by voluntary effort (Macefield, 1998). The two ectopic units we recorded were spatially distant (digit IV) from the contracting muscle (FDI) and so unlikely to be activated by the voluntary effort. Given the scarcity of these ectopically active axons, it is highly unlikely that a similar discharge would be occurring within the same fascicle. Thus the high-frequency regular discharges generated by these axons reflect a highly synchronized unitary input to the central nervous system. Both units resulted in overt EMG responses, showing that the reflex response can be observed when only a single afferent is discharging.
Cyclic behaviour
The cyclic behaviour we saw in four units provides good evidence for the role of SA II afferents in motor control. The regular discharge of these receptors is tightly coupled to the output of the contracting muscle, there being a strong linear relationship between the interspike interval of the receptor's discharge and the periodicity of the averaged EMG response. Such a strong temporal relationship suggests that some of the muscle's activity could be determined by the input from SA II receptors in the skin. Presumably this linkage could be used advantageously in the control of objects being manipulated by the hand - a slip or some other perturbation in grip could then result in an increase in grip force long before the voluntary reaction time. Interestingly, these receptors have not been located in the glabrous skin of the cat footpad (Ferrington, 1985) or primate hand (Darian-Smith, 1984), which suggests a specialized adaptation in human glabrous skin.
Coupling to higher-order sensory neurones
Microstimulation of single cutaneous afferents can generate specific tactile sensations (Torebjörk et al. 1987; Macefield et al. 1990), indicating a strong synaptic security to the cortex. For instance, subjects can detect a single stimulus applied to the afferent of an FA I receptor (see also Johansson & Vallbo, 1979b) which is perceived as flutter or vibration as the frequency of stimulation increases. Likewise, microstimulation of a single SA I afferent can elicit a frequency-dependent perception of sustained indentation, but selective stimulation of SA II afferents does not generally elicit a perceptual response. This would suggest that the role of SA II afferents may be more related to motor control than to tactile sensation. However, it has been shown that SA II afferents located around the nail bed may be able to generate meaningful sensations of finger movement (Macefield et al. 1990). Interestingly, three of the four SA II afferents associated with cyclic oscillations in the EMG were located around the nail bed, leading one to speculate that these receptors possess a strong synaptic coupling to spinal motoneurones (via interneurones) as well as to higher-order sensory neurones. This strong synaptic security from single mechanoreceptors to higher-order sensory neurones has been shown more directly by Rowe and colleagues in the cat: Gynther et al. (1995) have established a 1:1 linkage between a single SA II receptor in the cat paw and its target neurone in the cuneate nucleus. Even at the low discharge rates typical of SA II receptors, reliable transmission is apparent without the need for temporal or spatial summation. Such high-fidelity transmission has also been demonstrated for SA I afferents (Vickery et al. 1994) and FA II afferents (Ferrington et al. 1986).
Given such a strong transmission security to higher-order sensory neurones it is perhaps not surprising that we have demonstrated a strong synaptic coupling between tactile afferents and spinal motoneurones, at least if we assume that the synaptic strength is comparable at spinal and supraspinal synapses. This would fit with the observation that microstimulation of single human muscle spindle afferents does not elicit a perceptual response (Macefield et al. 1990) - the synaptic strength at the spinal level is also weak, as revealed by spike-triggered averaging (Gandevia et al. 1986). It is worth emphasizing that the synaptic coupling between muscle spindles and spinal motoneurones is monosynaptic, but also includes di- and trisynaptic components (Watt et al. 1976), whereas there is no evidence that the coupling between tactile afferents and the motoneurone pool is monosynaptic. Despite this, spike-triggered averaging is capable of revealing connections composed of more than one synapse, provided that there is continuous activity in the interneurones (Stauffer et al. 1976; Watt et al. 1976; Kirkwood et al. 1987). The involvement of spinal interneurones in the cutaneous afferent-motoneurone pool pathway may allow not only a greater number of synaptic connections but also an increased gain in the strength of the signal. Spike-triggered averaging for one unit revealed a short-latency inhibition without a preceding excitation: whether this reflects weaker net excitatory synaptic coupling, stronger inhibitory coupling, or the interaction of more interneurones inhibiting the initial excitatory responses we cannot say.
Conclusion
In this study we used spike-triggered averaging to demonstrate the strength of synaptic coupling between single, type-identified cutaneous afferents and the motoneurone pool of muscles acting on the hand. Unlike muscle spindle afferents in the leg, the input from single cutaneous afferents in the hand is sufficiently strong to be able to drive not only the higher-order sensory neurones but also, via interneurones, spinal motoneurones. The contribution of these reflex responses will need to be considered when other inputs onto the motoneurone pool are being examined. This potent, short-latency synaptic coupling between tactile afferents in the hand and muscles acting on the digits supports the important role of cutaneous mechanoreceptors in motor control of the human hand.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Programme Grant 963206). We are grateful to Dr Jun Yang for assistance in the initial experiments.
References
- Buchthal F, Schmalbruch H. Contraction times and fibre types in intact human muscle. Acta Physiologica Scandinavica. 1970;79:435–452. doi: 10.1111/j.1748-1716.1970.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Buller NP, Garnett R, Stephens JA. The reflex responses of single motor units in human hand muscles following muscle afferent stimulation. The Journal of Physiology. 1980;303:337–349. doi: 10.1113/jphysiol.1980.sp013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia MR, McComas AJ, Upton MRM, Blogg T. Cutaneous reflexes in small muscles of the hand. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ, Abbs JH. Grip force adjustments evoked by load force perturbations of a grasped object. Journal of Neurophysiology. 1988;60:1513–1522. doi: 10.1152/jn.1988.60.4.1513. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I. The sense of touch: performance and peripheral neural processes. In: Darian-Smith I, editor. Handbook of Physiology, section 1, The Nervous System. Bethesda: American Physiological Society; 1984. pp. 739–788. [Google Scholar]
- Darton K, Lippold OC, Shahani M, Shahani U. Long-latency spinal reflexes in humans. Journal of Neurophysiology. 1985;53:1604–1618. doi: 10.1152/jn.1985.53.6.1604. [DOI] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. The effects of digital nerve stimulation on the firing of motor units in human first dorsal interosseous muscle. The Journal of Physiology. 1981;318:501–510. doi: 10.1113/jphysiol.1981.sp013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Feifel E, Guschlbauer B, Lücking CH. Hand muscle reflexes following air puff stimulation. Experimental Brain Research. 1996;105:138–146. doi: 10.1007/BF00242189. [DOI] [PubMed] [Google Scholar]
- Evans AL, Harrison LM, Stephens JA. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. The Journal of Physiology. 1989;418:1–12. doi: 10.1113/jphysiol.1989.sp017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG. Functional properties of slowly adapting mechanoreceptors in cat footpad skin. Somatosensory Research. 1985;2:249–261. doi: 10.3109/07367228509144567. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ, Tarvin RPC. High gain transmission of single impulses through dorsal column nuclei of the cat. Neuroscience Letters. 1986;65:277–282. doi: 10.1016/0304-3940(86)90274-0. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D, Mckeon B. Coupling between human muscle spindles endings and motor units assessed using spike-triggered averaging. Neuroscience Letters. 1986;71:181–186. doi: 10.1016/0304-3940(86)90555-0. [DOI] [PubMed] [Google Scholar]
- Garnett R, Stephens JA. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. The Journal of Physiology. 1980;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gynther BD, Vickery RM, Rowe MJ. Transmission characteristics for the 1:1 linkage between slowly adapting type II fibres and their cuneate target neurons in cat. Experimental Brain Research. 1995;105:67–75. doi: 10.1007/BF00242183. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Leeper JB, Burke D, Gandevia SC. Morphology of action potentials recorded from human nerves using microneurography. Experimental Brain Research. 1996;110:308–314. doi: 10.1007/BF00228561. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Leeper JB, Wilson LR, Gandevia SC, Burke D. The development of conduction block in single human axons following a focal nerve injury. The Journal of Physiology. 1998;513:127–133. doi: 10.1111/j.1469-7793.1998.127by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner JR, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. The Journal of Physiology. 1982;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Current Opinion in Neurobiology. 1992;2:815–823. doi: 10.1016/0959-4388(92)90139-c. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Häger C, Bäckström L. Somatosensory control of precision grip during unpredictable pulling loads. III. Impairments during digital anesthesia. Experimental Brain Research. 1992a;89:204–213. doi: 10.1007/BF00229017. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Häger C, Riso R. Somatosensory control of precision grip during unpredictable pulling loads. II. Changes in load force rate. Experimental Brain Research. 1992b;89:192–203. doi: 10.1007/BF00229016. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Riso R, Häger C, Bäckström L. Somatosensory control of precision grip during unpredictable pulling loads. I. Changes in load force amplitude. Experimental Brain Research. 1992c;89:181–191. doi: 10.1007/BF00229015. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo Å B. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. The Journal of Physiology. 1978;281:101–123. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo Å B. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. The Journal of Physiology. 1979a;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo Å B. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. The Journal of Physiology. 1979b;297:405–422. doi: 10.1113/jphysiol.1979.sp013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo Å B. Tactile sensory coding in the glabrous skin of the human hand. Trends in Neurosciences. 1983;6:27–32. [Google Scholar]
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Experimental Brain Research. 1987;66:141–154. doi: 10.1007/BF00236210. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Experimental Brain Research. 1988;71:72–86. doi: 10.1007/BF00247523. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Schomburg ED, Steffens H. Facilitatory interaction in spinal reflex pathways from nociceptive cutaneous afferents and identified secondary spindle afferents in the cat. Experimental Brain Research. 1987;68:657–660. doi: 10.1007/BF00249808. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. The Journal of Physiology. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG. Spontaneous and evoked ectopic discharges recorded from single human axons. Muscle and Nerve. 1998;21:461–468. doi: 10.1002/(sici)1097-4598(199804)21:4<461::aid-mus4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Rothwell JC, Day BL. The contribution of transcortical pathways to long-latency stretch and tactile reflexes in human hand muscles. Experimental Brain Research. 1996;108:147–154. doi: 10.1007/BF00242912. [DOI] [PubMed] [Google Scholar]
- Mackel R. Conduction of neural impulses in human mechanoreceptive cutaneous afferents. The Journal of Physiology. 1988;401:597–615. doi: 10.1113/jphysiol.1988.sp017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty PA, Macefield VG, Türker KS. Evidence for strong synaptic coupling between single tactile afferents and motoneurones supplying the human hand. Proceedings of the Australian Neuroscience Society. 1998;9:27. doi: 10.1111/j.1469-7793.1999.0883p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam JER. The effect of lesions of the sensorimotor cortex and capsular pathways on servo responses from the human long thumb flexor. Brain. 1977a;100:503–526. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam JER. The effect of posterior column lesions on servo responses from the human long thumb flexor. Brain. 1977b;100:185–200. doi: 10.1093/brain/100.1.185. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. The Journal of Physiology. 1973;228:285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth J, Friedemann HH, Podoll K, Lange HW. Absence of long-latency reflexes to imposed finger displacements in patients with Huntington's disease. Neuroscience Letters. 1983;35:97–100. doi: 10.1016/0304-3940(83)90533-5. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long-latency stretch reflex in humans is transcortical. The Journal of Physiology. 1992a;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. The transcortical nature of the later reflex responses in human small hand muscle to digital nerve stimulation. Experimental Brain Research. 1992b;91:320–326. doi: 10.1007/BF00231665. [DOI] [PubMed] [Google Scholar]
- Stauffer EK, Watt DGD, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. Journal of Neurophysiology. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Vallbo Å B, Ochoa JL. Intraneural microstimulation in man. Its relation to specificity of tactile sensations. Brain. 1987;110:1509–1529. doi: 10.1093/brain/110.6.1509. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B. Prediction of propagation block on the basis of impulse shape in single unit recordings from human nerves. Acta Physiologica Scandinavica. 1976;97:66–74. doi: 10.1111/j.1748-1716.1976.tb10236.x. [DOI] [PubMed] [Google Scholar]
- Vickery RM, Gynther BD, Rowe MJ. Synaptic transmission between single slowly adapting type I fibres and their cuneate target neurones in cat. The Journal of Physiology. 1994;474:379–392. doi: 10.1113/jphysiol.1994.sp020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt DGD, Stauffer EK, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. Journal of Neurophysiology. 1976;39:1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


