Abstract
Confocal laser scanning microscopy was used to visualize Ca2+ transients in the vascular smooth muscle cells (VSMC) of intact, pressurized rat mesenteric resistance arteries loaded with fluorescent calcium indicators. Vasoconstriction was assessed by measuring inner arterial diameter. All arteries were studied at 70 mmHg intralumenal pressure and 37 °C.
In the control condition of myogenic tone the arteries were constricted to 62 % (n = 10) of their passive diameter (p.d.). The [Ca2+]i in most VSMC of these arteries was constant over time. In a small percentage (< 10 %) of cells in each artery, [Ca2+]i oscillated regularly. Local calcium transients (Ca2+ sparks) were observed in five arteries studied with confocal linescan imaging.
Activation of α-adrenoceptors by phenylephrine (PE, 1.0 μM) induced further vasoconstriction of pressurized arteries (to 27 % of p.d.). In this condition, [Ca2+]i oscillations were prominent in a large percentage (83 %) of the VSMC. The Ca2+ oscillations ranged in frequency from 4 to 22 min−1, and were usually asynchronous between cells.
High [KCl]o (65 mM) induced nearly comparable vasoconstriction to PE (37 % of p.d.) but [Ca2+]i oscillated in only about 13 % of cells in each artery.
Block of L-type Ca2+ channels (with nifedipine) in arteries activated by PE caused nearly full vasodilatation, but did not abolish the Ca2+ oscillations. Subsequent block of the sarcoplasmic reticulum Ca2+ pump (with cyclopiazonic acid) abolished Ca2+ oscillations in all cells.
We conclude that Ca2+ entering VSMC via L-type Ca2+ channels has an obligatory role in force development, both in myogenic tone and during α1-adrenoceptor activation. The oscillatory pattern of [Ca2+]i that persists in the absence of Ca2+ entry via L-type Ca2+ channels is ineffective in activating contraction.
In vivo, the contractile state of individual vascular smooth muscle cells (VSMC) is regulated by neural, endothelial and mechanical influences. The ‘myogenic’ mechanism (Bayliss, 1902), by which arteries constrict to regulate flow in response to increases in intravascular pressure, involves an increase in ‘arterial wall’[Ca2+]i (Meininger et al. 1991; Zou et al. 1995; Knot & Nelson, 1998). Within individual VSMC, however, the molecular effectors of vasoconstriction (ions, receptors, channels, second messengers) are often localized spatially in microdomains, allowing for possible local control of function. In the present study we have utilized confocal imaging of Ca2+ indicator dyes to study individual, intact VSMC within the walls of resistance arteries having myogenic tone.
Previous confocal imaging studies have utilized either single isolated smooth muscle cells (Mironneau et al. 1996; Gordienko et al. 1998) or intact arteries stretched over glass cannulae (Kasai et al. 1997; Jaggar et al. 1998). Low resolution confocal imaging of arteries on cannulae (Kasai et al. 1997) revealed [Ca2+]i oscillations (but not Ca2+ sparks) during neuronal stimulation, and the modulation of VSMC activity by endothelial cells. The importance of local control of function is illustrated by the intriguing hypothesis that local Ca2+ transients (Ca2+ sparks) mediate vasodilatory effects during myogenic tone (Nelson et al. 1995; Knot et al. 1998). Indeed, a recent confocal imaging study of rat cerebral arteries showed that Ca2+ sparks are activated by Ca2+ influx via L-type Ca2+ channels, and that the sparks result from the opening of ryanodine receptor-coupled channels (Jaggar et al. 1998). These preparations, however, did not permit the actual administration of intralumenal pressure and the arteries could not develop ‘myogenic tone’ (because of the restriction of the glass cannula), so they were depolarized with 30 mM KCl ‘to simulate the effects of pressure’.
Arterial wall [Ca2+] has also been measured in pressurized arteries (Meininger et al. 1991; Zou et al. 1995; Knot & Nelson, 1998) and during α-adrenoceptor-mediated contractions (Meininger et al. 1991), but the [Ca2+]i within individual VSMC of intact arteries was not visualized and the possibility exists that patterns of changes of [Ca2+]i within individual cells are different from the ‘arterial wall Ca2+‘. The goal of our study, therefore, was to image intracellular Ca2+ and Ca2+ sparks (if possible) in single VSMC in intact resistance arteries under physiological conditions of intralumenal pressure and temperature.
We used distal arcading arteries of the rat mesentary (immediately proximal to the transmural arterioles) because these vessels exhibit myogenic tone in vitro (Sun et al. 1992) and contribute to vascular resistance in vivo (Fenger-Gron et al. 1995). The arteries were pressurized to 70 mmHg to induce myogenic tone (the ‘control condition’). Vasoconstriction resulting from the myogenic mechanism was then enhanced by exposure to an α-adrenoceptor agonist (phenylephrine, PE), or elevation of extracellular KCl. To determine the importance of intracellular and extracellular Ca2+, L-type Ca2+ channels were blocked with nifedipine. Ca2+ sequestration within the artery was inhibited with cyclopiazonic acid (CPA). The results strongly support an obligatory role of L-type Ca2+ channels in both myogenic tone and vasoconstriction after α-adrenoceptor activation (mimicking neuronal stimulation). The evidence for this is that even when Ca2+ oscillated within individual VSMC, vasoconstriction did not occur unless L-type Ca2+ channels were also functional. Ca2+ sparks were also recorded for the first time in pressurized arteries, providing direct evidence that sparks play a role in the regulation of myogenic tone.
METHODS
Animals
All experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Male Sprague-Dawley rats, weighing 180-300 g, were anaesthetized with intramuscular ketamine (50-100 mg kg−1), and killed by cervical dislocation. The mesenteric arcade was dissected from the abdominal cavity, cleaned free of blood and placed in a temperature-controlled dissection chamber containing a dissection solution (5°C) of the following composition (in mmol l−1): 3 Mops, 145 NaCl, 5 KCl, 2.5 CaCl2, 1 MgSO4, 1 KH2PO4, 0.02 EDTA, 2 pyruvate and 5 glucose, with 1.0 % albumin (pH 7.4).
Loading of resistance arteries with calcium indicators
Isolated arteries were dissected by methods similar to those described previously (Duling et al. 1981). Dissected segments of the distal arcading arteries (1-2 mm long) were transferred to a recording chamber where they were loaded with calcium indicator in dissection solution to which had been added 25 μM fluo-4 AM or 15 μM Calcium Green-1 AM, 0.5 % DMSO (v/v) and 0.03 % cremaphor EL (v/v). Loading was allowed to proceed for 3 h at room temperature. The arteries were then checked for the presence of intracellular dye by brief confocal imaging as described below. If the artery appeared to contain dye, the ends were then mounted on glass pipettes (tip diameter, 60-80 μm) and secured by 10/0 sutures. One pipette was attached to a servo-controlled pressure-regulating device (Living Systems, Burlington, VT, USA), while the other was attached to a closed stopcock in order to study the pressure-dependent effects in the absence of intralumenal flow. The arteries were equilibrated over ∼1 h to initial experimental conditions (37°C, 70 mmHg); those with significant leaks or branches were discarded. During this time, the arteries were continuously superfused with gassed Krebs solution (contents in mmol l−1): 112 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KHPO4, 11.5 glucose and 10 Hepes (pH 7.4 at 37°C), at 37°C (chamber PO2 = 90-100 mmHg, measured with an oxygen electrode; Microelectrodes Inc., Londonderry, NH, USA). Only vessels that exhibited stable myogenic tone (constriction to less than 75 % of maximal diameter) were studied further. Arterial diameter was measured using video calipers (AM Instrument, College Station, TX, USA) and a × 20 objective lens with transillumination. Figure 1A shows images of such an artery before (a) and after (b) the development of myogenic tone; the video calipers are seen as the vertical white bars. The arteries showed no detrimental effects of the dye loading; they produced the same amount of myogenic tone as unloaded arteries and were similarly responsive to acute changes in intralumenal pressure, PE and KCl.
Figure 1. Optical sectioning of the vascular wall of pressurized mesenteric arteries and determination of optical performance in situ.
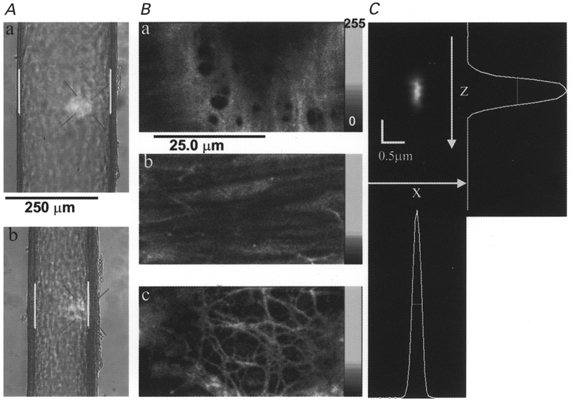
A, transmitted light images of a pressurized (70 mmHg) rat mesenteric artery mounted in a recording chamber on the microscope when fully relaxed (diameter, 224 μm) in Ca2+-free-2 mM EGTA Krebs solution (a) and after developing myogenic tone (136 μm) in normal Krebs solution (b). White bars are the video calipers used to measure lumen diameter. B, high-resolution optical sections of an artery loaded with Calcium Green-1 showing that discrete regions of the arterial wall can be visualized. The lumenal endothelium (a), media with smooth muscle cells (b), and perivascular neurons (c) are readily distinguished. C, point-spread function (PSF) of the microscope obtained by imaging a single fluorescent bead (0.1 μm diameter) that had been made to lodge on the inner surface of a typical arteriole not itself loaded with fluorescent dye. The x-z plane image was obtained by slicing vertically a 3-dimensional reconstruction of the bead obtained with pixels of 0.1 μm in the x-, y- and z-axes. The shifts of successive x-y planes apparent in the bead image were due to slight movement of the artery. These shifts, of approximately 0.1 μm, were not observed with beads adhering to the coverslips.
Drugs and solutions
PE, phentolamine, CPA and nifedipine were prepared as stock solutions and diluted in the superfusate reservoir. For KCl contractions, normal Krebs solution was replaced with a similar solution that contained 65 mM KCl in place of equimolar NaCl, and phentolamine (2 μM) was added. Maximal (passive) arterial diameter (p.d.) was determined after 10 min in Ca2+-free Krebs solution containing 2 mM EGTA. PE, phentolamine, nifedipine, CPA and cremaphor EL were obtained from Sigma; fluo-4 AM and Calcium Green-1 AM were purchased from Molecular Probes.
Calcium imaging and experimental protocol
An inverted, custom-built confocal laser scanning microscope, similar to that described previously (Parker et al. 1997; Mauban & Wier, 1998), was used. For Ca2+ imaging and optical sectioning, the artery was viewed through a × 60 water-immersion lens (n.a., 1.2). The dyes were excited with light at 488 nm from a 25 mW argon ion laser; fluorescent emission was collected at wavelengths > 510 nm (Fig. 1B). The point-spread function (PSF), a measure of spatial resolution, was determined by imaging single fluorescent beads (0.1 μm in diameter) adhering to the lumen of a mounted artery (Fig. 1C). It was important to characterize the optical performance of the microscope because of the unusual optical situation, in which the specimen is viewed through a thick aqueous layer and is itself relatively thick. The use of a water-immersion lens obviates the mismatch of refractive index that would occur using an oil-immersion lens in this situation. The water-immersion lens enabled correction for the different (from water) refractive index of the cover glass which forms the bottom of the recording chamber. The PSF had a full width at half-maximum (FWHM) in the x- and y-axesof 0.26 μm and an axial (z) FWHM of 0.70 μm. Several (usually 3-4) neighbouring VSMC were visualized by repeated scans (0.85 s frame−1) of a 51.2 μm × 25.4 μm area of the arterial wall. Images were 512 pixels by 256 pixels (or lines). Optical sectioning was adequate to exclude fluorescence from endothelial cells (Fig. 1Ba) and from the perivascular neurons (Fig. 1Bc). Individual VSMC could be observed clearly when focusing mid-way through the arterial wall (Fig. 1Bb). Several different areas of each artery were observed during each treatment. To improve temporal resolution when recording Ca2+ sparks, VSMC were also imaged in the linescan mode in which a line 50 μm in length was scanned every 3 ms.
Fluo-3 and Calcium Green-1 are non-ratiometric, Ca2+-sensitive dyes with solution Kd values of about 390 and 190 nm, respectively, although their reported in situ Kd values are considerably higher (Harkins et al. 1993). Fluo-4 has not been characterized intracellularly; it is chemically similar to fluo-3, but its better quantum yield makes it a more desirable calcium indicator. With these dyes, however, absolute Ca2+ concentration levels cannot be measured without knowing (or assuming) the [Ca2+] corresponding to a reference level of fluorescence, usually termed F0. This information is not available for the VSMC studied here, and thus the results will be reported simply as fluorescence changes. In the absence of dye loading, fluorescence levels were at least an order of magnitude lower than in the dye-loaded arteries we studied, indicating that the observed fluorescence did arise from the calcium indicator. Thus, changes in fluorescence can be ascribed unequivocally to changes in [Ca2+]. Because of sequestration of dye in organelles, however, changes in fluorescence cannot be ascribed entirely to changes in cytoplasmic free Ca2+ concentration.
[Ca2+] oscillations were characterized using a custom-made program written in the programming language IDL (Research Systems, Inc., Boulder, CO, USA). Consecutive frames (0.85 s each) were used to generate a continuous recording of [Ca2+] signals by measuring the mean pixel intensity in an area of interest (AOI) within individual cells of the arterial wall. Only continuous tracings of at least 30 s without significant arterial movement were analysed. The Ca2+ concentration within cells was considered ‘oscillating’ if repetitive [Ca2+] transients occurred during the recording period. [Ca2+] oscillations detected this way were confirmed by inspection using a movie playback program feature in IDL in order to exclude the possibility of a movement artifact.
RESULTS
Ca2+ in VSMC during myogenic tone (control conditions)
Ten arteries were studied in the control condition, in which arteries were subjected only to pressurization, and the vasoconstriction was by the myogenic mechanism. The mean (±s.e.m.) vasoconstriction in these arteries was to 61.6 ± 2.3 % (121 ± 6 μm, n = 10) of their passive diameter (p.d., 197 ± 7 μm). When images from a single optical section of the arterial media were obtained repetitively (0.85 s frame−1) for periods of 1 min or more in these arteries the fluo-4 fluorescence (or Calcium Green-1 fluorescence) appeared essentially constant in most VSMC (Fig. 2A). The small areas of interest (AOI, numbered 1-3) shown in Fig. 2A illustrate the typical pattern of [Ca2+] during myogenic tone in which the [Ca2+] appeared constant in all the visible VSMC throughout the recording period. Record 4 is derived from the entire image, and is expected to represent the fluorescence measurement that would be obtained without spatial resolution. In a few cells of each artery under the control conditions, however, fluorescence fluctuated at low amplitude or oscillated with more regular frequency. The low percentage of cells with Ca2+ oscillations in each artery (∼7 %, see summary of all data and statistics in Fig. 6) suggests that [Ca2+] oscillations are not frequent during myogenic tone. Their existence does indicate, however, that measurements of arterial wall [Ca2+]i are not an entirely accurate representation of events occurring in individual VSMC. More interestingly, the difference in behaviour of neighbouring VSMC implies that the Ca2+ transients in these cells are not necessarily coupled, although the cells are likely to be electrically coupled (Christ et al. 1996).
Figure 2. Ca2+-dependent fluorescence (fluo-4) in individual VSMC of an artery with myogenic tone and after exposure to KCl.
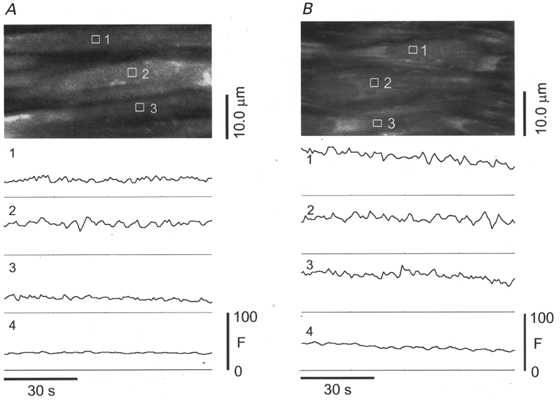
A, [Ca2+] in most VSMC of pressurized arteries with myogenic tone was effectively constant. This artery was constricted to 70 % of p.d. at the time of measurement. Traces below the image show the mean Ca2+-dependent fluorescence intensity within the areas of interest (AOI) outlined in white and numbered in the image. Trace number 4 is the mean fluorescence intensity from the entire image. AOI are 16 pixels or 1.6 μm on a side, for an area of 2.56 μm2. The small fluctuations in fluorescence intensity shown here may result from small movements of the artery and heterogeneous dye distribution. These were judged not to be true [Ca2+] oscillations. The heterogeneity of fluorescence intensity in cells arises from several factors, including compartmentalization and sequestration of dye in organelles. B, the artery was constricted with 65 mM KCl to 35 % of p.d. Under these conditions the [Ca2+] is effectively constant in all cells observed in this field of view. Traces below the image represent the mean Ca2+-dependent fluorescence intensity within the AOI outlined in white and numbered in the image. Trace number 4 is the mean fluorescence intensity from the entire image.
Figure 6. Summary of data on [Ca2+] oscillations and arterial diameter.
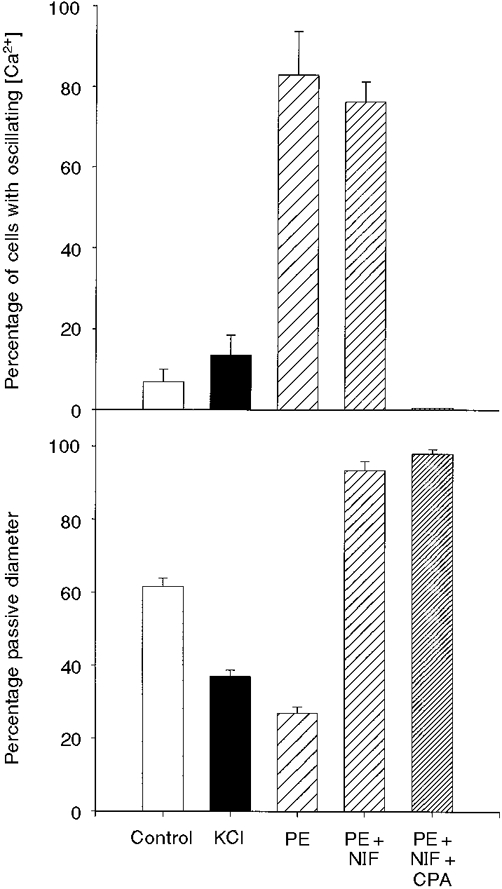
In the upper graph, the means and s.e.m. of the percentage of cells with oscillating [Ca2+] from each group are shown. The lower graph illustrates the means and s.e.m. of the diameters (percentage p.d.) of these arteries under the same conditions. The number of cells sampled under each condition was: Control, 95 cells from 10 arteries; KCl, 68 cells from 3 arteries; PE, 55 cells from 6 arteries; PE + NIF (nifedipine), 52 cells from 3 arteries; PE + NIF + CPA, 41 cells from 3 arteries.
Ca2+ in VSMC activated by KCl
KCl (65 mM) was highly effective in further constricting vessels (71 ± 13 μm or 31 ± 3 % of maximum diameter, n = 3). Exposure to 65 mM KCl increased slightly the number of cells showing Ca2+ oscillations (mean, 13 ± 3 %; 3 arteries, 68 cells). Figure 2B shows representative recordings. The spatial patterns of Ca2+ appear to be similar to that of myogenic tone. The spatially averaged [Ca2+] is reasonably representative of the patterns of [Ca2+] within the individual VSMC. Although the fluorescence levels are higher in the presence of KCl than during myogenic tone in the example shown, the absence of a comparable control measurement and the use of a non-ratiometric dye precludes knowing by how much [Ca2+] may have risen in these arteries after exposure to elevated [KCl].
Ca2+ in VSMC activated by PE
PE (1.0 μM) constricted the arteries to a mean diameter of 52 ± 5 μm, or 27 ± 2 % (n = 6) of maximum diameter. In contrast to the patterns of [Ca2+] observed during myogenic tone and KCl depolarization, PE induced large oscillatory fluorescence transients in the majority of VSMC. Images of such [Ca2+] oscillations are shown in Fig. 3, and the changes in fluorescence intensity in several AOI over a longer period of time are shown in Fig. 4. The oscillation frequency varied between 4 and 22 min−1, but the cells did not always oscillate synchronously. Therefore, when data from many cells were spatially averaged, the oscillation amplitudes were markedly damped (Fig. 4, record 4). When PE was washed out and 2.0 μM phentolamine was added to block further α-adrenoceptor activation, the amplitude and frequency of the Ca2+ oscillations declined. These oscillations also became less regular and, in most cells, eventually stopped (not shown).
Figure 3. Ca2+ oscillations in VSMC of pressurized arteries activated by PE.
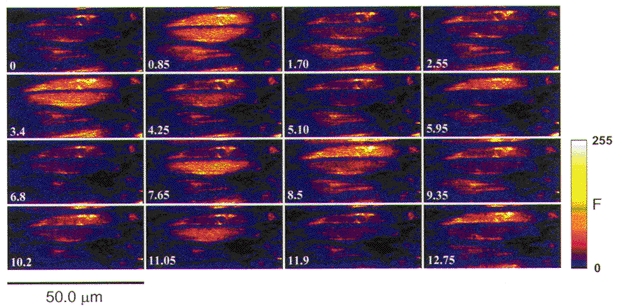
Frame-by-frame images (0.85 s frame−1) of fluo-4 fluorescence in a pressurized artery in the presence of 1.0 μM PE. This artery was constricted to 31 % of p.d. at the time of measurement. [Ca2+] oscillations are evident in all four cells in this field of view. The oscillations are distinguished from noise or movement by the fact that fluorescence changes in the entire cell, while other fluorescent structures in the image remain fixed in position. The [Ca2+] oscillations are not synchronous in all the cells, although oscillations do sometimes occur together.
Figure 4. Ca2+ oscillations within individual VSMC during activation by PE.
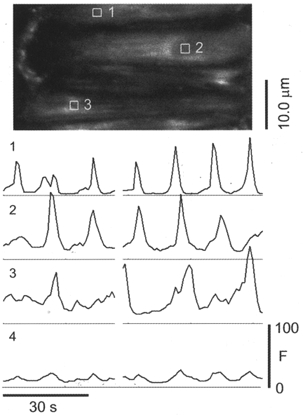
A fluo-4-loaded artery constricted to 29 % of p.d. following exposure to 1 μM PE. Traces below the image show the mean Ca2+-dependent fluorescence intensity within the AOI from individual cells outlined in white and numbered in the image. Trace number 4 is the mean fluorescence intensity from the entire image. The figure shows that Ca2+ oscillations are variable in character from cell to cell and are not always synchronous.
Mechanism of vasoconstriction in pressure and α-adrenoceptor activation
Physiologically, arteries such as those we used are subjected to intraluminal pressure (∼70 mmHg) and to neuronal activity (α-adrenoceptor activation). Vasoconstriction in response to pressure and to α-adrenoceptor activation involves elevation of [Ca2+]i via L-type Ca2+ channels (Nilsson et al. 1994; Knot & Nelson, 1998), synchronous and asynchronous Ca2+ oscillations (Figs 3 and 4) (Kasai et al. 1997), and oscillations of membrane potential (Vm) (Wesselman et al. 1997). Although block of L-type Ca2+ channels reduces arterial wall [Ca2+] and isometric force in arteries exposed to noradrenaline (Nilsson et al. 1998), such block had no effect on the amplitude or frequency of [Ca2+] oscillations induced by PE in freshly isolated pulmonary artery smooth muscle cells (Hamada et al. 1997). When we added nifedipine to vessels preconstricted with PE, the vessels relaxed nearly fully (185 ± 4 μm, or 93 ± 3 % of p.d., n = 3). Surprisingly, however, the Ca2+ oscillations persisted for periods of at least 15 min (the longest time observed), as shown clearly in the data of Fig. 5. When an inhibitor of the sarcoplasmic reticulum (SR) Ca2+ pump was added (CPA) these Ca2+ oscillations were rapidly abolished in all cells. All the data on cellular [Ca2+] transients and vasoconstriction are summarized in the bar graphs of Fig. 6.
Figure 5. PE-induced Ca2+ oscillations in the presence of nifedipine.
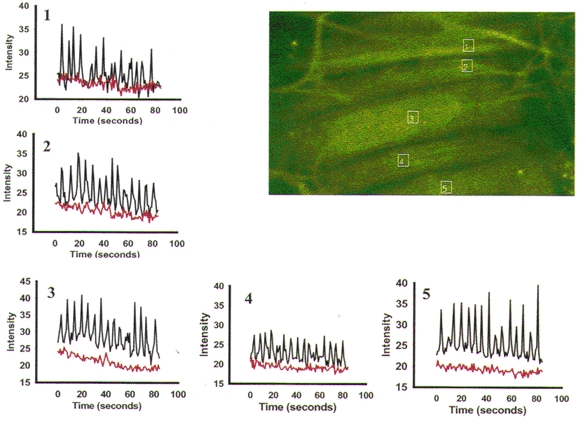
This representative Calcium Green-1-loaded artery developed myogenic tone (49 % of p.d. at 70 mmHg) and constricted in response to 1 μM PE (21 % of p.d.). After application of 1 μM nifedipine in the presence of PE the artery dilated to 98 % p.d. Confocal imaging began after the artery had dilated to a new steady-state diameter. The boxes are the AOI (1-5) measured during exposure to PE and nifedipine. Because the artery was near-maximally dilated by nifedipine and there was no vasomotion, it was possible to record from the same cell and AOI after the application of 20 μM of the SR Ca2+ pump inhibitor CPA. PE-induced [Ca2+] oscillations persisted (black tracing) despite the presence of nifedipine and near-maximal dilatation. CPA immediately abolished the [Ca2+] oscillations (red tracing) in all cells observed.
Ca2+ sparks
As mentioned above, it has been hypothesized that local Ca2+ transients (Ca2+ sparks) mediate vasodilatory effects during myogenic tone (Nelson et al. 1995; Knot et al. 1998). Nevertheless, for methodological reasons, Ca2+ sparks had not actually been recorded in arteries with myogenic tone. When we obtained images in the linescan mode, Ca2+ sparks were, in fact, evident in VSMC of arteries with myogenic tone (125 ± 10 μm, or 64 ± 3 % of p.d., n = 5). Representative single sparks from four different arteries are shown in Fig. 7A. The two sparks shown in the surface plot in Fig. 7B are from the same ‘frequent discharge site’. During 85 s of recording, this site produced 13 sparks. The Ca2+ sparks often occurred repetitively at the same site (Fig. 7A and B), as they do in isolated ileal myocytes (Gordienko et al. 1998). The mean peak fluorescence ratio (F/F0) of 210 sparks from five arteries was 3.9 ± 0.9 (Fig. 7C). This is a somewhat higher value than reported for isolated arterial myocytes (Nelson et al. 1995; Bonev et al. 1997), and substantially higher than in non-pressurized arteries (Jaggar et al. 1998). FWHM was 2.29 ± 0.7 μm (Fig. 7D). The distribution of amplitudes is not Gaussian, and has the shape expected from the random position of the scan line with respect to the origin of the spark (Izu et al. 1998). The mean half-time of decay was 37.4 ± 11.3 ms.
Figure 7. Ca2+ sparks in VSMC of arteries with myogenic tone.
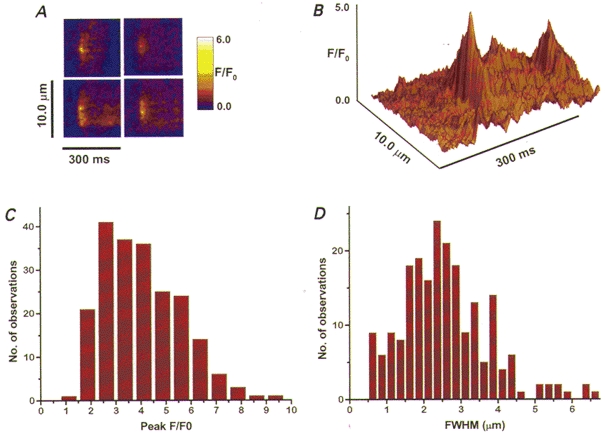
A, spatial and temporal characteristics of representative sparks from four different fluo-4-loaded arteries with myogenic tone (pressurized to 70 mmHg at 37 °C). B, surface plot of two sparks from another artery that originated from the same spatially localized region (frequent discharge site) in the cell. C and D, the frequency histograms of 210 sparks from five arteries showing the distribution of peak fluorescence (mean F/Fo = 3.9) and the spatial spread (mean FWHM = 2.29 μm).
DISCUSSION
The experiments described here demonstrate that optical sectioning by high-resolution confocal imaging with Ca2+-sensitive dyes is possible in intact, isolated, pressurized resistance arteries under conditions of myogenic and pharmacological stimulation. This preparation provides an opportunity to study local [Ca2+] in cells, as well as the relationships between changes in [Ca2+]i in neighbouring cells, in intact blood vessels at physiological temperature. Nevertheless, the calcium indicators used here have an important drawback in that they are non-ratiometric, so calibration of [Ca2+]i is difficult. In particular, it is impossible to calculate [Ca2+]i accurately in images obtained before and after significant changes in arterial diameter, when the microscope has to be refocused. Therefore, we have interpreted fluorescence changes in terms of changes in [Ca2+]i, rather than in absolute levels of [Ca2+]i. Also, dynamic changes in [Ca2+]i, such as those that occurred during the development of tone or immediately after the addition of PE, could not be followed because of vessel movement.
Sequestration of dye into organelles was evident, particularly with fluo-4. The relatively long loading period and high concentration of fluo-4 were required to ensure adequate loading of VSMC during the entire experiment. Significant loss of fluo-4 occurred during the equilibration period (∼1 h at 37°C), most probably as a result of active extrusion of dye from the cells at physiological temperature (arteries were always significantly more fluorescent at 21°C). The fact that fluo-4 fluorescence from some organelles was higher than in the cytoplasm of the cell (see Fig. 2) means that the changes in fluorescence we observed probably underestimate the true changes in cytoplasmic free [Ca2+]. Loading of organelles did enable us to observe occasional small frame-to-frame movements of cells. This may have been the result of slight vasomotion because of contraction and relaxation activity in neighbouring cells. This movement could not be easily quantified, and sometimes made it difficult to distinguish small [Ca2+]i (i.e. fluorescence) fluctuations from the background ‘noise’. The larger fluorescence changes, however, could be readily distinguished from such movement artifacts. The data described here were all obtained when the artery reached a maintained diameter and most cellular motion ceased.
Local Ca2+ transients: Ca2+ sparks
Transient, local release of Ca2+ from intracellular stores (Ca2+ sparks) has been described in several smooth muscle preparations (all at 20-28°C) including isolated rat arterial myocytes (Nelson et al. 1995; Bonev et al. 1997), rat venous myocytes (Mironneau et al. 1996) and guinea-pig ileal myocytes (Gordienko et al. 1998). Recently, Ca2+ sparks were observed in rat small cerebral arteries stretched over glass cannulae (Jaggar et al. 1998). In the latter study, spark frequency was increased 5.5-fold by raising external [KCl] from 6 to 30 mM to simulate pressure-dependent depolarization. While membrane depolarization is a component of pressure-dependent myogenic tone (Harder et al. 1987; Knot & Nelson, 1998), it is also clear that second-messenger systems other than calcium entry are activated by intralumenal pressure (reviewed by D'Angelo & Meininger, 1994). Thus the frequency of calcium sparks may reflect the balance of both positive and negative regulation by second messengers (Porter et al. 1998). For example, in cerebral artery smooth muscle cells calcium spark frequency is increased by L-type calcium channel activation, and decreased by protein kinase C activation. Both mechanisms have been implicated in the myogenic response; however, in the studies which used KCl to simulate myogenic tone only L-type activation would occur. The present study is the first to describe Ca2+ sparks in pressurized arteries with myogenic tone, thus providing direct support for the hypothesized role of Ca2+ sparks in regulating the myogenic response (Nelson et al. 1995; Knot et al. 1998). We observed somewhat higher values of F/F0 compared with those reported previously in non-pressurized rat cerebral arteries at 22°C (Jaggar et al. 1998), in isolated cells from rat portal veins (Mironneau et al. 1996) and in guinea-pig ileal myocytes (Gordienko et al. 1998). Direct comparison, however, of the Ca2+ sparks we recorded with those recorded previously is not possible because of the different experimental conditions used, including the use of different imaging techniques and different Ca2+ indicator dyes. The optical sections we obtained were slightly thinner (0.7 μm) than those studied previously (1.5 μm, Mironneau et al. 1996; 0.8-1.0 μm, Gordienko et al. 1998) and, in theory, the apparent amplitude of Ca2+ sparks will be reduced as the optical section becomes thicker (Izu et al. 1998). Although fluo-4 is reported to have the same affinity (Kd) for Ca2+ as fluo-3 in solution, no data are yet available on the Kd of fluo-4 in cytoplasm. If the Kd of fluo-4 in cytoplasm is actually higher than that of fluo-3 in cytoplasm, then larger fluorescence ratios may be expected for the same changes in [Ca2+]. In fact, such an effect has been reported (see data from Molecular Probes, Inc., in Bio Probes, vol. 30, Table 12, 1998). Finally, the parameter F/F0 is quite sensitive to small changes in background or ‘basal’[Ca2+] (i.e. the [Ca2+] at the time F0 is measured). This [Ca2+] could be different in the different preparations, because of the different conditions used.
Cellular Ca2+ transients
Our spatially resolved measurements indicate that arterial wall [Ca2+] cannot usually be characterized by a single value, since levels fluctuate in at least some cells during myogenic activation. Because the dyes we used are not ratiometric, we cannot estimate the degree of this error in temporally and spatially averaged measurements of the arterial wall. In the case of myogenic tone in our mesenteric arteries, this error is likely to be small, however, since [Ca2+]i oscillations occurred in relatively few cells. Nevertheless, it is possible that other arteries exhibit a substantially higher percentage of oscillating cells during myogenic tone, and thus contribute to the variation in the reported values of 50-200 nM in small arteries pressurized at ∼70 mmHg (Meininger et al. 1991; Zou et al. 1995; Knot & Nelson, 1998).
The responses to the two vasoconstrictors we tested were agent specific. KCl (65 mM) induced vasoconstriction in association with [Ca2+]i levels (i.e. fluo-4 fluorescence) that exhibited only minimal fluctuation (Fig. 2B). This is not to say that [Ca2+]i does not increase, but the pattern of [Ca2+]i is homogeneous. In marked contrast to KCl-induced vasoconstriction, however, PE-induced vasoconstriction was associated with marked spatial and temporal inhomogeneity of [Ca2+]i (Figs 3 and 4) while, following initial constriction, artery diameter remained relatively constant. Oscillations of [Ca2+]i have also been described in rat tail artery in response to electric field stimulation and noradrenaline (Kasai et al. 1997). Particularly noteworthy is our observation that the oscillations (measured as changes in fluorescence) sometimes varied in frequency as well as amplitude in adjacent cells, and thus were not always synchronous. This suggests that the [Ca2+]i transients in these cells are not strongly coupled together by diffusion of Ca2+ through gap junctions (Christ et al. 1996). The factors governing propagation through gap junctions are evidently not understood fully, since Ca2+ transients usually fail to propagate through electrically coupled cardiac muscle (Lamont et al. 1998), but it has been reported that Ca2+ waves do propagate through whole hearts (Hama et al. 1998). In the present study, rhythmic vasomotion was sometimes observed in the arteries, particularly in the presence of PE, and this might reasonably be ascribed to synchronous cellular [Ca2+] transients. We could observe the VSMC only when vasomotion was minimal, however. Thus, if synchronized [Ca2+] oscillations are associated with vasomotion, as seems likely, we would have been unable to observe them.
Because both myogenic and agonist-mediated contractions were sensitive to inhibition by nifedipine, our data support an obligatory role of L-type calcium channel activation in regulating arterial tone. The oscillatory pattern of [Ca2+], which continued in the presence of nifedipine, most probably involves SR calcium uptake and release, as it was blocked by CPA, similar to the block by ryanodine of PE-induced [Ca2+] oscillations in isolated pulmonary artery smooth muscle cells (Hamada et al. 1997). CPA could be acting indirectly on the VSMC, however, by activating endothelial cells, which can abolish neuronally stimulated [Ca2+] oscillations in VSMC (Kasai et al. 1997). In support of this interpretation is the fact that thapsigargin abolishes noradrenaline-induced force in intact, but not endothelium-denuded arteries (Nilsson et al. 1998). Because the calcium indicator dyes we used are not ratiometric, we do not know the amplitude of the [Ca2+] oscillations in fully dilated arteries in the presence of nifedipine. The possibility exists that vasoconstriction is absent because the [Ca2+] reached during oscillations falls below that required for contractile activation. In this case, the ‘obligatory’ role of Ca2+ entering via L-type Ca2+ channels could be that it provides a [Ca2+]‘pedestal’ on which oscillations derived from the SR are superimposed and/or amplified.
Finally, an important issue raised by our results is that spatially and/or temporally averaged arterial wall [Ca2+] measurements during agonist activation (Meininger et al. 1991) may markedly underestimate the actual [Ca2+]i levels achieved during oscillatory responses in individual cells. This could have important implications in studying [Ca2+]i- vasoconstriction relationships, particularly when the effects of receptor agonists, which produce oscillatory [Ca2+]i, are compared with the tonic increases produced by KCl contractions. While regulation of the calcium sensitivity of contraction by G-protein-coupled receptors is well accepted, it is likely that the spatio-temporal inhomogeneities of [Ca2+]i may also contribute to the apparently higher calcium sensitivity observed with receptor agonists in studies which rely on spatial and/or temporal averaging of arterial wall [Ca2+]. Similar concerns regarding spatial inhomogeneities have been raised by other investigators (Rembold et al. 1995; Van Riper et al. 1996).
In conclusion, application of high-resolution imaging methods to thick specimens in a state near to ‘physiological’ (temperature, pressure, intact cellular connections) demonstrates the spatio-temporal dynamics of intercellular, cellular and subcellular Ca2+. Since VSMC are the primary effectors of vascular tone, this ability to study Ca2+ in individual VSMC, as well as intercellular interactions, will be useful for elucidating mechanisms involved in regulating local blood flow and peripheral resistance.
Acknowledgments
This work was supported by NIH research grants HL-45215 to M. P. B. and HL-55280 to W. G. W., and by training grant AR-07592 to the University of Maryland Training Program in Muscle Biology.
References
- Bayliss WM. On the local reaction of the arterial wall to changes in internal pressure. The Journal of Physiology. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. American Journal of Physiology. 1997;273:C2090–2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, El-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circulation Research. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Meinenger GA. Transduction mechanisms involved in the regulation of myogenic activity. Hypertension. 1994;23:1096–1105. doi: 10.1161/01.hyp.23.6.1096. [DOI] [PubMed] [Google Scholar]
- Duling BR, Gore RW, Dacey RG, Damon DN. Methods for isolation, cannulation and in vitro study of single microvessels. American Journal of Physiology. 1981;241:H108–116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- Fenger-Gron J, Mulvaney MJ, Christensen KL. Mesenteric blood pressure profile of conscious, freely moving rats. The Journal of Physiology. 1995;488:753–760. doi: 10.1113/jphysiol.1995.sp021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB, Cannell MB. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle Cells. The Journal of Physiology. 1998;507:707–720. doi: 10.1111/j.1469-7793.1998.707bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama TA, Takahashi AA, Ichihara A, Takamatsu T. Real time in situ confocal imaging of calcium wave in the perfused whole heart of the rat. Cellular Signalling. 1998;10:331–337. doi: 10.1016/s0898-6568(97)00136-8. [DOI] [PubMed] [Google Scholar]
- Hamada H, Damron DS, Hong SJ, Van Wagoner DR, Murray PA. Phenylephrine-induced Ca2+ oscillations in canine pulmonary artery smooth muscle cells. Circulation Research. 1997;81:812–823. doi: 10.1161/01.res.81.5.812. [DOI] [PubMed] [Google Scholar]
- Harder DR, Gilbert R, Lombard JH. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. American Journal of Physiology. 1987;253:F778–781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with Fluo-3. Biophysical Journal. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu LT, Wier WG, Balke CW. Theoretical analysis of the Ca2+ spark amplitude distribution. Biophysical Journal. 1998;75:1144–1162. doi: 10.1016/s0006-3495(98)74034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. American Journal of Physiology. 1998;274:C755–761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- Kasai Y, Yamazawa T, Sakurai T, Taketani Y, Iino M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. The Journal of Physiology. 1997;504:349–357. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. The Journal of Physiology. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. The Journal of Physiology. 1998;508:211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Luther PW, Balke CWB, Wier WG. Intercellular Ca2+ waves in rat heart muscle. The Journal of Physiology. 1998;512:669–676. doi: 10.1111/j.1469-7793.1998.669bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauban JRH, Wier WG. A custom multi-photon and confocal laser scanning microscope optimized for physiological studies. Proceedings of Scanning 98. 1998;20:145. [Google Scholar]
- Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. American Journal of Physiology. 1991;261:H950–959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- Mironneau J, Arnaudeau S, Macrez-Leptretre N, Boittin FX. Ca2+ sparks and Ca2+ waves activate different Ca2+-dependent ion channels in single myocytes from rat portal vein. Cell Calcium. 1996;20:153–190. doi: 10.1016/s0143-4160(96)90104-9. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Jensen PE, Mulvany MJ. Minor role for direct adrenoceptor-mediated calcium entry in rat mesenteric small arteries. Journal of Vascular Research. 1994;31:314–321. doi: 10.1159/000159059. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Videbaek LM, Toma C, Mulvany MJ. Role of intracellular calcium for noradrenaline-induced depolarization in rat mesenteric small arteries. Journal of Vascular Research. 1998;35:36–44. doi: 10.1159/000025563. [DOI] [PubMed] [Google Scholar]
- Parker I, Callamaras N, Wier WG. A high-resolution, confocal laser-scanning microscope and flash photolysis system for physiological studies. Cell Calcium. 1997;21:441–452. doi: 10.1016/s0143-4160(97)90055-5. [DOI] [PubMed] [Google Scholar]
- Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. American Journal of Physiology. 1998;274:C1346–1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Van Riper DA, Chen XL. Focal [Ca2+]i increases detected by aequorin but not by fura-2 in histamine- and caffeine-stimulated swine carotid artery. The Journal of Physiology. 1995;488:549–564. doi: 10.1113/jphysiol.1995.sp020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. American Journal of Physiology. 1992;263:H1486–1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- Van Riper DA, Chen XL, Gould EM, Rembold CM. Focal increases in [Ca2+]i may account for apparent low Ca2+ sensitivity in swine carotid artery. Cell Calcium. 1996;19:501–508. doi: 10.1016/s0143-4160(96)90059-7. [DOI] [PubMed] [Google Scholar]
- Wesselman JPM, Schubert R, VanBavel E, Nilsson H, Mulvany MJ. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. American Journal of Physiology. 1997;272:H2241–2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolar tone. American Journal of Physiology. 1995;269:H1590–1596. doi: 10.1152/ajpheart.1995.269.5.H1590. [DOI] [PubMed] [Google Scholar]


