Abstract
The influence of 30 mM inorganic phosphate (Pi) and pH (6.2-7.4) on the rate of ATP utilization was determined in mechanically skinned bundles of myofibrils from the iliofibularis muscle of Xenopus laevis at approximately 5 °C.
BDM (2,3-butanedione monoxime; 10 mM) depressed isometric force production and actomyosin (AM) ATPase activity equally. Therefore sarcoplasmic reticular (SR) ATPase activity could be determined by extrapolation of the total ATPase activity to zero force.
The SR ATPase activity without added Pi at pH 7.1 was 42 ± 2 % of the total ATPase activity. Addition of 30 mM Pi reduced SR ATPase activity slightly, by 9 ± 5 %, and depressed force by 62 ± 2 % and AM ATPase activity by 21 ± 6 %.
At pH 6.2, force, SR ATPase activity and AM ATPase activity were reduced by 21 ± 5, 61 ± 5 and 10 ± 4 % of their respective values at pH 7.1.
The SR ATPase activity at 30 mM Pi and pH 6.2 was reduced markedly to 20 ± 6 % of the value under control conditions, suggesting that the maximum rate of Ca2+ uptake during muscle fatigue was strongly depressed. This reduction was larger than expected on the basis of the effects of Pi and pH alone.
Prolonged activation of skeletal muscle tissue which leads to muscle fatigue is directly associated with a decline in force production and a slowing of relaxation. For a recent review describing the cellular mechanism of muscle fatigue, see Allen et al. (1995). The reduction in maximum force has been ascribed to an increase in intracellular inorganic phosphate (Pi) concentration and a fall in internal pH. Other factors influencing force development as well as the rate of relaxation are alterations in Ca2+ handling, i.e. a reduction of the intracellular calcium transient and an increase in the free calcium ion concentration at rest (Allen et al. 1989). Furthermore, it has been shown that the Ca2+ sensitivity of the contractile apparatus is depressed by both Pi and pH (e.g. Godt & Nosek, 1989).
Fabiato & Fabiato (1978) studied the effect of pH on force production and sarcoplasmic reticulum (SR) function in skinned cardiac and skeletal muscle cells. They found that the pH optimum for Ca2+ loading into the SR was decreased when the free Ca2+ concentration during loading was increased. This clearly indicates that Ca2+ handling by the SR is influenced by pH.
Further evidence that pH affects SR function has been provided by studies on isolated SR ATPase (MacLennan, 1970), SR fragments (Shigekawa et al. 1978; Rousseau & Pinkos, 1990; Wolosker & De Meis, 1994), skinned fibres (Lamb et al. 1992) and intact fibres (Curtin, 1986; Edman & Lou, 1990).
Less is known about the effect of Pi on Ca2+ handling. For a recent review of the mechanism of Ca2+ transport by the SR, see MacLennan et al. (1997). Fruen et al. (1994) showed that Pi stimulated the in vitro activity of the ryanodine receptor (RyR) Ca2+ release channel of the SR. Smith & Steele (1992) found that Pi decreases the Ca2+ content of cardiac SR. They could not distinguish whether this decrease was due to a reduction in Ca2+ uptake or to an increase in Ca2+ leakage caused by Pi. Zhu & Nosek (1991) and Stienen et al. (1993) showed, in cardiac and skeletal muscle, respectively, that Pi caused a reduction of Ca2+ uptake by the SR during short exposures of the skinned fibres to Ca2+ lasting 30-60 s. The time- and concentration-dependent formation of Ca2+-Pi complexes inside the SR (Fryer et al. 1995) complicate this type of measurement. In general, it can be concluded that there is considerable information available concerning the influence of pH and Pi on Ca2+ release by the SR. However, the magnitude of the effects on Ca2+ uptake under physiological conditions is unknown. These effects of Pi and pH on the activity of the SR Ca2+ pump are of interest for understanding Ca2+ handling during fatigue and ischaemia. Furthermore, alterations in active transport of Ca2+ into the lumen of the SR are important for muscle function during intensive exercise because it represents a considerable fraction of the total energy utilization during muscle contraction, and therefore has important metabolic consequences.
Recently we have presented the results of a technique to study SR ATPase activity in skinned muscle fibres (Stienen et al. 1995). Since the composition of the intracellular milieu can be accurately controlled in skinned fibres, we used this technique to study the effects of Pi and pH on SR ATPase. The changes in intracellular Ca2+ transients and in pH during fatigue are well documented in fast-twitch Xenopus fibres (Westerblad & Lännergren, 1988; Allen et al. 1989). Therefore, we decided to investigate the effects of Pi and pH on SR ATPase activity in mechanically skinned iliofibularis muscle of Xenopus.
METHODS
The methods employed were as described previously (Stienen et al. 1995). Briefly, adult female Xenopus laevis (African clawed toad) were kept in tap water at room temperature and fed with SDS Amphibia diet 3 (Special Diet Services, Witham, UK) every second day. The animals were killed by rapid decapitation, followed by pithing, according to the guidelines of the local Ethics Committee. Thin fibre bundles of about 1 mm in diameter were dissected from the outer dorsal zone of the iliofibularis muscle. These bundles were stored in Ringer solution as described by Buschman et al. (1996) for up to 30 h at 4°C. From the fibre bundles, thick fast-twitch fibres were selected. These fibres are of type 1 or type 2, as described by Lännergren & Smith (1966). Fibre segments of about 6 mm in length were isolated in cold dissecting solution and skinned mechanically by splitting them into bundles of myofibrils of about 150 μm in diameter. The composition of the dissecting solution was (mM): Na2ATP, 7.3; MgCl2, 10.6; EGTA, 20; creatine phosphate, 10; and Bes, 100; pH 7.0 (adjusted with KOH), ionic strength 200 mM (adjusted with KCl). Preparation diameters were measured in two perpendicular directions by means of a dissection microscope at a magnification of × 50. Cross-sectional area was calculated assuming an elliptical cross-section. The length of the preparations was measured at a magnification of × 20. On average, four experiments on different preparations from one muscle could be performed.
The preparations were mounted in the experimental set-up (Stienen et al. 1990) by means of aluminium T-clips (Goldman & Simmons, 1984). The holes in these clips were passed over hooks, one of which connected to a carbon fibre extending a force transducer element (AE 801, SensoNor, Horten, Norway) and the other to a glass rod connected to a manipulator. Sarcomere length was measured in relaxing solution by means of He-Ne laser diffraction and was adjusted to 2.3 μm.
The apparatus used to measure the ATPase activity consisted of several temperature-controlled troughs (5°C) in which the fibre could be immersed. During the actual measurement of ATPase activity, the preparation was kept in a small trough with quartz windows, with a volume of 30 μl. Hydrolysis of ATP inside the fibre was linked to the oxidation of NADH, measured photometrically via the absorption at 340 nm of near UV light from a 75 W xenon arc lamp that passed beneath the fibre. The solution in the chamber was continuously stirred. The absorbance signal obtained was linearly related to the NADH concentration inside the measuring chamber and its slope was a measure of the ATPase activity of the preparation. Calibration of the absorption signal was carried out after each recording by adding a known amount of ADP to the solution via a stepper motor-controlled pipette. The rate of ATP hydrolysis was derived from the slope of the absorbance signal relative to the baseline found after exposure of the preparation to the solution. The composition of the solutions used during the experiments is shown in Table 1.
Table 1.
Composition of the solutions
| Solution name | MgCl2(mM) | Na2ATP(mM) | EGTA(mM) | HDTA(mM) | CaEGTA(mM) | KProp(mM) |
|---|---|---|---|---|---|---|
| P1 experiments (pH 7.1) | ||||||
| Control (0 P1) | ||||||
| Relaxing | 6.80 | 6.09 | 5 | 15 | — | 27.9 |
| Pre-activating | 6.75 | 6.14 | 0.5 | 19.5 | — | 28.0 |
| Activating (pCa 4.8) | 6.71 | 6.15 | — | 15 | 5 | 27.8 |
| 30 mM P1 | ||||||
| Relaxing | 7.77 | 6.09 | 5 | — | — | 13.3 |
| Pre-activating | 7.72 | 6.09 | 0.5 | 4.5 | — | 13.4 |
| Activating (pCa 4.8) | 7.68 | 6.15 | — | — | 5 | 13.3 |
| Fatigue (30 mM P1, pH 6.2) | ||||||
| Relaxing | 6.80 | 8.14 | 5 | 7 | — | 31.1 |
| Pre-activating | 6.79 | 8.14 | 0.5 | 11.5 | — | 31.1 |
| Activating (pCa 3.9) | 6.79 | 8.57 | — | 7 | 5 | 29.9 |
| pH series | ||||||
| Control (pH 7.1) | ||||||
| Relaxing | 7.32 | 6.14 | 5 | — | — | 91.0 |
| Pre-activating | 7.32 | 6.14 | 0.5 | 4.5 | — | 91.0 |
| Activating (pCa 4.8) | 7.29 | 6.20 | — | — | 5 | 90.8 |
| pH 6.2 | ||||||
| Relaxing | 6.79 | 8.15 | 5 | — | — | 116.4 |
| Pre-activating | 6.78 | 8.15 | 0.5 | 4.5 | — | 116.4 |
| Activating (pCa 3.9) | 6.78 | 8.60 | — | — | 5 | 115.0 |
| pH 7.4 | ||||||
| Relaxing | 7.59 | 5.97 | 5 | — | — | 74.4 |
| Pre-activating | 7.48 | 5.97 | 0.5 | 4.5 | — | 74.6 |
| Activating (pCa 5.0) | 7.40 | 6.00 | — | — | 5 | 74.6 |
All solutions contained in addition 4 mg ml−1 pyruvate kinase (500 U ml−1, Sigma) and 0.24 mg ml−1 LDH (870 U ml−1, Sigma), with 10 mM phosphoenol pyruvate, 5 mM sodium azide, 10 μM oligomycin B, 0.8 mM NADH, 0.2 mM p1,p5-di(adenosin-5’)pentaphosphate, 5 mM caffeine and 100 mM Bes. The pH was adjusted with KOH. Potassium propionate (KProp) and HDTA were added to adjust ionic strength. CaEGTA was made by dissolving equimolar amounts of CaCO3 and EGTA. The pH 6.2 and fatigue solutions contained elevated enzyme concentrations (see Methods). The solutions used at pH 6.8 and pH 6.5 were obtained by appropriate mixing of the control (pH 7.1) and the pH 6.2 solutions.
Three different bathing solutions were used: a relaxing solution, a pre-activating solution with a low EGTA concentration, and an activating solution. The composition of the solutions was calculated with a computer program similar to that of Fabiato & Fabiato (1979), using the equilibrium constants described previously (Stienen et al. 1996). The calculated free Mg2+ and MgATP concentrations were 1 and 5 mM, respectively. The pH was adjusted with KOH while the solutions were kept at the temperature at which the measurements were carried out. Formal ionic strength (Γ/2 =½ΣCiZi2, where Ci and Zi are the concentration and charge, respectively, of the i th ionic species) was adjusted by adding potassium propionate. This has the disadvantage that when the effects of inorganic phosphate (Pi) are studied, the ionic equivalent (Ie=½ΣCi| Zi|) varies between control and test solution. In these experiments, therefore, ionic strength was balanced with a mixture of hexamethylene diamine-tetraacetate (HDTA) and potassium propionate, such that Γ/2 and Ie were 200 and 154 mM, respectively. In the experiments in which pH was varied ionic strength was adjusted with potassium propionate only, because this did not cause variations in Ie. These measurements were performed at Γ/2 = 217 mM and Ie= 187 mM. The temperature in the measuring chamber was measured routinely. During the experiments, the preparation was incubated in relaxing solution for 4 min, in pre-activating solution for 4 min, and in activating solution until a steady force level was attained, and from there it was transferred back again into relaxing solution. All measurements were carried out in the presence of 5 mM caffeine to ensure that Ca2+ uptake into the SR was maximal (Stienen et al. 1995).
The experiments started with two activations in the standard solution (Table 1). After the first activation, the length of the preparation was readjusted, if necessary. After that the sarcomere length usually remained stable throughout the experiment. The second activation served as the first control measurement. Thereafter two test activation-relaxation cycles were performed at different [Pi], [BDM] (2,3-butanedione monoxime) or pH, which were followed by a control measurement in the standard solution. The results of the test contractions were normalized to the mean of the bracketing control values. All activations were performed at saturating Ca2+ concentrations. The free Ca2+ concentrations given in Table 1 are calculated values. It was verified that the Ca2+ concentration in the activating solutions was saturating by adding extra amounts of Ca2+ from a concentrated CaCl2 stock under the various experimental conditions used. The differences between successive control values for force and ATPase activity were about 5 % or less. Usually a series of measurements lasted several hours. The experiments were terminated when isometric force during the control measurements was < 80 % of the force of the first activation. Force and ATPase activity were recorded with a pen recorder and after analog-to-digital conversion by a personal computer at a sampling rate of 10 Hz.
The activity of the enzymes used in the ATP-linked assay was pH dependent. Pyruvate kinase (PK) activity was optimal in the pH range 7-7.5, while lactate dehydrogenase (LDH) from rabbit had an optimal activity around pH 6 (Biochemica Information, Boehringer Mannheim, Germany). The standard enzyme concentrations used in this study were as used previously, i.e. 4 mg ml−1 PK and 0.24 mg ml−1 LDH. We determined the activity of PK at pH 6.2 relative to its activity at pH 7.1 in the activation solutions by measuring the rate of NADH breakdown at various PK concentrations when 1.5 nmol ADP was injected into the measuring chamber. During these measurements LDH was present in excess (1.2 mg ml−1). It was found that the relative activity of PK at pH 6.2 was reduced about ninefold. Similar measurements in which the LDH concentration was varied and PK was present in excess showed, in agreement with the literature, that LDH activity was not reduced at pH 6.2. In view of the large drop in PK activity, we decided to perform the measurements at pH 6.2 in both the presence and absence of 30 mM added phosphate with 8 mg ml−1 PK and 1.2 mg ml−1 LDH (i.e. 2 and 5 times the standard concentration used at pH 7.1, respectively). This PK concentration is near the maximum value attainable in aqueous solution. In paired experiments at pH 6.2, the effect of a fourfold reduction of the PK concentration (to 1 mg ml−1) was studied in one fibre, while in three fibres the effect of a twofold increase in PK concentration to 8 mg ml−1 was determined. Mean force and total ATPase values in these latter three fibres differed by less than 3 % indicating that the elevated enzyme concentrations were more than sufficient for adequate measurements. The fourfold reduction in PK concentration was also not associated with a significant change in ATPase activity or force.
Data values are given as means ±s.e.m. of n experiments. Regression lines between force and ATPase activity obtained at different BDM concentrations were calculated with force as an independent variable. Differences between mean values were statistically tested by means of Student's two-tailed t test for paired observations at a 0.05 level of significance (P < 0.05).
RESULTS
Effects of Pi
The method used to determine the SR and AM ATPase activities in the presence and absence of 30 mM Pi is illustrated in Fig. 1. In this figure total ATPase activity and force development are shown for a mechanically skinned bundle of myofibrils. Previously, it has been shown that BDM causes a reduction in isometric force and in total ATPase activity and that the intercept of the ATPase-force relationship at zero force reflects the SR ATPase rate (Stienen et al. 1995). Therefore the experiments were carried out in the absence and presence of BDM (10 mM). It can be seen that 10 mM BDM caused a considerable reduction in force both at 0 and at 30 mM added Pi. The isometric force in the presence of 30 mM Pi and 10 mM BDM was markedly reduced but the depression of the ATPase activity was less pronounced.
Figure 1. Force and ATPase activity in a mechanically skinned bundle of myofibrils in the absence and presence of 30 mM Pi.
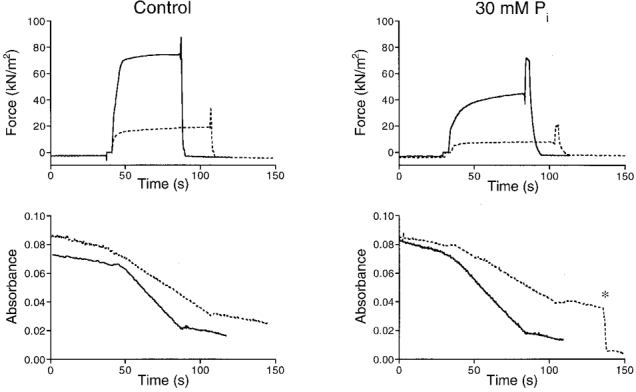
The dashed lines represent results obtained in the presence of 10 mM BDM. Upper recordings, force development; lower recordings, NADH absorbance. The preparation was activated by transferring it from the pre-activating solution (pCa 9) into the activating solution (pCa 4.8), and relaxed at the end of the measurement by transferring it back into relaxing solution (pCa 9). The final slope of the absorbance signal, relative to absorbance baseline (measured when the preparation was not in the measuring chamber), was used as a measure of the rate of ATP consumption. At the asterisk (lower right panel) a calibration of the absorbance signal is shown which corresponded to 0.5 nmol of ATP hydrolysed. The zero level in the absorbance signal was arbitrarily chosen. Preparation diameters (measured in two perpendicular directions), 160/140 μm; preparation length, 1.85 mm.
The data analysis is illustrated in Fig. 2A. In this figure isometric force and total ATPase activity, measured under the various circumstances, are plotted relative to the force and ATPase activity measured under control conditions, i.e. without Pi and BDM added. The small difference in the intercept at zero force with and without added Pi suggests that 30 mM Pi caused a minor decrease in the SR ATPase activity. A small decrease in SR ATPase activity was observed in 9 out of a total of 10 preparations. The mean decrease (±s.e.m.) in SR ATPase activity observed amounted to 9 ± 5 %, which was not quite significant (P = 0.06). The mean decrease in AM ATPase activity by 30 mM added Pi was 21 ± 6 %. An overview of these results is given in Table 2.
Figure 2. Effect of 10 mM BDM on force and ATPase activity in the absence (continuous line) and presence (dashed line) of 30 mM Pi.
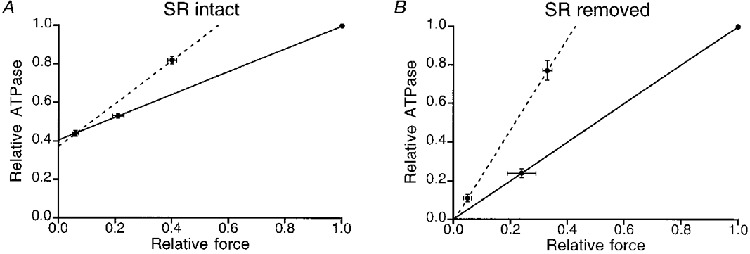
A, mean results (±s.e.m.) obtained from 10 mechanically skinned bundles of myofibrils. B, results obtained from 4 Triton X-100-treated skinned fibres. The intercepts at zero force in A reflect the SR ATPase activity as a fraction of the total ATPase activity without added Pi. The intercepts at zero force in B were not significantly different from zero, indicating that SR ATPase activity was completely abolished by the Triton X-100 treatment.
Table 2.
Overview of the effects of P1 and pH on ATPase activity and force development
| 30 mM P1 | Control (0 P1)(%) | 30 mM P1(%) | Relative change(%) |
|---|---|---|---|
| Total ATPase activity | 100 | 82 ± 3 | −18 ± 3* |
| AM ATPase activity | 58 ± 2 | 46 ± 3 | −21 ± 6* |
| SR ATPase activity | 42 ± 2 | 38 ± 4 | −9 ± 5 (P = 0.06) |
| Isometric force | 100 | 38 ± 2 | −62 ± 2* |
| pH 6.2 | Control (pH 7.1) (%) | pH 6.2(%) | Relative change(%) |
|---|---|---|---|
| Total ATPase activity | 100 | 72 ± 2 | −28 ± 2* |
| AM ATPase activity | 65 ± 2 | 59 ± 4 | −10 ± 4 (P = 0.07) |
| SR ATPase activity | 35 ± 2 | 14 ± 2 | −61 ± 5* |
| Isometric force | 100 | 79 ± 5 | −21 ± 5* |
| 30 mM P1, pH 6.2 | Relative change | |||
|---|---|---|---|---|
| Control(0 P1, pH 7.1) (%) | 30 mM P1 pH 6.2(%) | Experimental(%) | Calculated(%) | |
| Total ATPase activity | 100 | 47 ± 2 | −53 ± 2* | −41 ± 3† |
| AM ATPase activity | 59 ± 2 | 40 ± 3 | −32 ± 7* | −29 ± 3 |
| SR ATPase activity | 41 ± 2 | 8 ± 2 | −80 ± 6* | −64 ± 5† |
| Isometric force | 100 | 37 ± 3 | −63 ± 3* | −70 ± 3† |
The mean values for the total ATPase activity per volume, and force per cross-sectional area were, respectively, 0.26 ± 0.01 mM s−1 and 59 ± 3 kN m−1 (n = 44). The AM and SR ATPase activities are expressed relative to the total ATPase activity without BDM and added P1. The relative changes were calculated from individual values relative to the bracketing control values. Values are means ± S.E.M. from 10 (30 mM P1), 6 (pH 6.2) and 8 (30 mM P1, pH 6.2) different preparations. The calculated relative change with 30 mM P1 at pH6.2 was obtained from the product of the effects of 30 mM P1 and pH 6.2 alone.
P < 0.05 compared with control
no overlap between experimental and calculated values ± s.e.m.
Since BDM and Pi both affected isometric force development we investigated whether there existed some interference between the two agents which might compromise the separation between the SR and AM ATPase activities. For this purpose we also determined the influence of BDM and Pi in four Triton X-100-treated fibres. Triton X-100 treatment (1 % v/v; 30 min) disrupts all membranes including the SR, and the ATPase activity mainly reflects the AM ATPase activity (Stienen et al. 1995). The results obtained after Triton X-100 treatment are shown in Fig. 2B. These data clearly indicate that force and ATPase activity were reduced when 30 mM Pi was added. However, in this case the relative effects of 10 mM BDM in the absence and presence of 30 mM Pi were practically identical: in both cases BDM reduced force and ATPase activity in proportion. This confirmed our previous findings that the ATPase activity that remains after Triton X-100 treatment reflects the AM ATPase activity. The reduction in AM ATPase activity by 30 mM Pi derived from these experiments (21 ± 3 %) was not statistically different from the reduction in the AM ATPase activity observed in the mechanically skinned preparations not treated with Triton X-100. Hence it can be concluded that there was no interference between the effects of BDM and Pi on the SR and contractile apparatus.
Effects of pH
The effects of pH on force, and SR and AM ATPase activities were investigated in a similar way to the effects of Pi. In each fibre, total ATPase activity and maximum isometric force were measured in the presence and absence of 10 mM BDM under control conditions (pH 7.1) and at one ‘test’ pH. The number of fibres studied was seven at pH 6.2, 6 at pH 6.5, 6 at pH 6.8 and 7 at pH 7.4. An example of the recordings obtained on one preparation at pH 6.2 and pH 7.1 is shown in Fig. 3. It can be seen that force as well as total ATPase activity were reduced at pH 6.2 in comparison with the control measurement at pH 7.1. By extrapolation of the total ATPase activity at each pH to zero force, the relationship between pH and the SR and AM ATPase activities was obtained. The mean results obtained from a total of 26 preparations are shown in Fig. 4. In this figure the pH dependence of maximum isometric force is also plotted.
Figure 3. Effect of pH on force and ATPase activity in a mechanically skinned bundle of myofibrils.
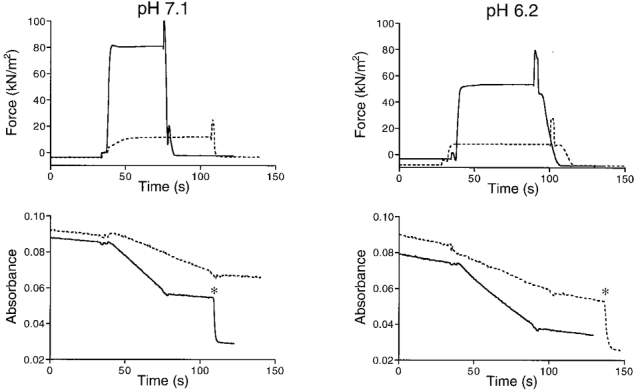
The results at pH 7.1 (left) are shown in the absence (continuous lines) and presence (dashed lines) of 10 mM BDM. In the right panels, the corresponding results obtained at pH 6.2 are shown. Upper recordings, force development; lower recordings, NADH absorbance. * Calibration of the absorbance signal. Preparation diameters, 180/140 μm; preparation length, 2.50 mm.
Figure 4. Dependence of force and ATPase activity on pH.
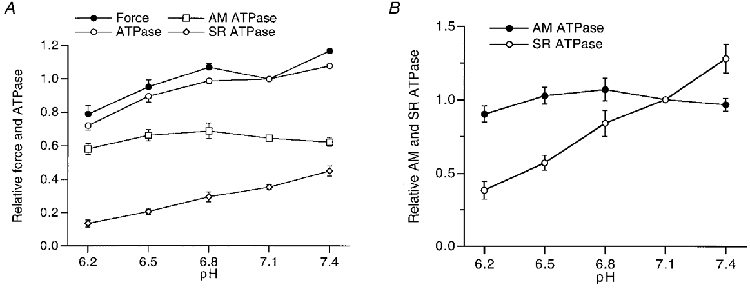
A, averaged results (±s.e.m.) of isometric force (•), and total (AM plus SR; ^), AM (□), and SR (⋄) ATPase activities as a function of pH. B, the effect of pH on AM (•) and SR (^) ATPase activities (±s.e.m.), normalized to the values obtained at pH 7.1. Number of observations at each pH, 6-7.
It can be seen that isometric force in the acidic range reached an optimum at pH 6.8 which was 7 % larger than that at pH 7.1. At pH 6.2 isometric force was reduced slightly to 79 ± 5 % of the value at pH 7.1. The total AM plus SR ATPase activity decreased gradually to 72 ± 2 % at pH 6.2. This reduction was mainly due to a decline in SR ATPase activity: at pH 6.2 the SR ATPase activity was reduced to 39 ± 5 % of its value at pH 7.1, whereas the AM ATPase activity was 90 ± 5 % of the activity found at pH 7.1. At pH 7.4, isometric force and total ATPase activity were slightly larger than at pH 7.1. It can be seen in Fig. 4B that the increase in total ATPase activity was due to an increase in the SR ATPase activity, which occurred in a linear fashion in the pH range studied.
To investigate possible interference between the effects of BDM and pH, we also studied the ATPase activity in Triton X-100-treated fibres at pH 6.2. It was found that the reduction in AM ATPase activity at pH 6.2 after Triton X-100 treatment was very similar to the reduction found without Triton treatment. Hence, it can be concluded that in the case of pH variations too, the method to discriminate between the SR and AM ATPase activities is valid.
As indicated in Methods, the activity of pyruvate kinase (PK) was markedly reduced at pH 6.2. Therefore, the experiments at pH 6.2 were performed with a PK concentration of twice the usual value. In these experiments the LDH concentration was increased fivefold. Control experiments (see Methods) indicated that these elevated concentrations were more than sufficient for adequate operation of the ATPase assay.
Combined effects of Pi and pH
During muscle fatigue of Xenopus fibres, the Pi concentration and pH change concomitantly. To mimic this condition, we studied the effects of 30 mM added Pi at pH 6.2 on force and ATPase activity in five preparations. An example of the recordings obtained is shown in Fig. 5. Isometric force under these conditions was markedly reduced to 37 ± 3 % of the value measured under control conditions at pH 7.1 without added Pi. The total (AM plus SR) ATPase activity was reduced to 47 ± 2 % of the control value. Most of this reduction was due to a decrease in the AM ATPase activity, which was reduced to 68 ± 7 % of the control value at pH 7.1 without added Pi. The relative decrease in SR ATPase activity to 20 ± 6 %, however, was even more pronounced than the reduction in total or AM ATPase activity. An overview of the combined effects of Pi and pH is shown in Table 2. In this table the results expected if Pi and pH were assumed to act independently are also shown. These expected values were obtained by multiplying the respective responses at 30 mM Pi with those at pH 6.2. It can be seen that the combined effects of 30 mM added Pi and pH 6.2 on total ATPase and SR ATPase activities are greater than the products of the independent effects. Small differences in the ionic composition of the solutions used for the pH series and the experiments in which Pi was present (see Methods) preclude a detailed comparison between the measured and calculated values. However, we do not think that this affects our general conclusion because the differences in the mean control ATPase activity values obtained in the two sets of solutions were negligible (results not shown).
Figure 5. Combined effects of 30 mM Pi and pH 6.2 on force and ATPase activity in a mechanically skinned bundle of myofibrils.
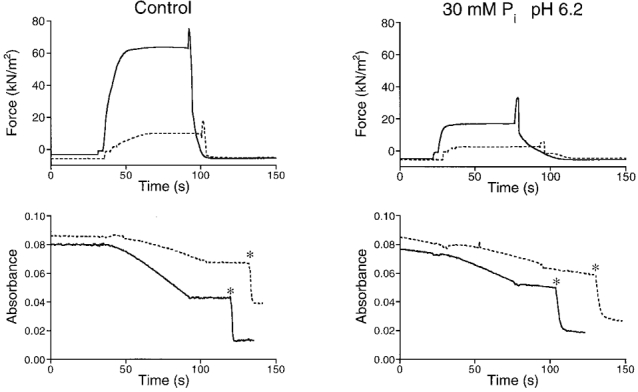
The results at pH 7.1 (left) are shown in the absence and presence (dashed lines) of 10 mM BDM. In the right panels, the corresponding results are shown obtained at 30 mM Pi and pH 6.2. Upper recordings, force development; lower recordings, NADH absorbance. The slower responses upon calibration injections (∗) in the right recordings, as compared with the left recordings, reflect the reduction in PK activity at pH 6.2. Preparation diameters, 140/130 μm; preparation length, 3.50 mm.
It is of interest to note that the control experiments performed before and after the incubations in 30 mM added Pi at pH 6.2 were not significantly different. This suggests that the long-lasting depression of the SR Ca2+ uptake rate observed in SR vesicles isolated after muscle recovery from high-intensity exercise (Byrd et al. 1989) was not caused by alterations in Pi and pH associated with fatigue.
Averaged values for the control experiments
Results for the different interventions (added Pi, altered pH, and pH 6.2 with 30 mM Pi) were obtained from 10, 26 and 8 different preparations, respectively. The mean values obtained for the control experiments (pH 7.1, no added Pi) of isometric force per cross-sectional area amounted to 59 ± 3 kN m−2 and total ATPase activity expressed per preparation volume corresponded to 0.26 ± 0.01 mM s−1. The relative SR ATPase activity expressed as a fraction of the total (AM plus SR) ATPase activity at pH 7.1 without added Pi amounted to 38 ± 1 % (n = 44). None of the control ATPase activity values in the various experimental groups differed significantly from the mean values from the complete series of experiments.
The mean basal ATPase activity (±s.e.m.) determined in relaxing solution (pCa 9) amounted to 1 ± 1 % of the maximal Ca2+-activated activity.
DISCUSSION
Comparison with previous results
The averaged values for the isometric force, the AM ATPase activity, and the SR ATPase activity are in good agreement with previous measurements for the maintenance heat production in intact fibres (e.g. Elzinga et al. 1987; Buschman et al. 1996) carried out at room temperature, when the measured temperature sensitivities (Stienen et al. 1995) are taken into account. This indicates that the results obtained from skinned fibres presented here can be compared quantitatively with those obtained from intact fibres.
Effects of Pi
Addition of 30 mM Pi caused a marked reduction in isometric force to 38 ± 2 %, which was similar to or even greater than the reduction found previously under similar conditions in frog (e.g. Stienen et al. 1990), mammalian (Potma et al. 1995) or cardiac muscle tissue (Kentish, 1986; Ebus et al. 1994). This reduction in force was accompanied by only a small decrease in the total (AM plus SR) ATPase activity to 79 ± 3 % of the control value found in the absence of added Pi. The SR ATPase activity in the absence of added Pi was 42 ± 2 % of the total ATPase activity. It decreased on average by 9 ± 5 % whereas the AM ATPase activity decreased on average by 21 ± 6 % when 30 mM Pi was added. These values are compatible with the heat measurements of Barclay et al. (1993). Our results indicate that the depression of the total ATP consumption by Pi is mainly due to the reduction in the AM ATPase activity. This moderate reduction in AM ATPase activity at 30 mM Pi is comparable to previous results in rabbit muscle fibres (Kawai et al. 1987; Potma et al. 1995) and in cardiac trabeculae from rat (Ebus et al. 1994).
The experiments suggest that addition of 30 mM Pi causes, if anything, only a slight reduction in the maximum SR ATPase activity, which may cause a minimal decrease in the Ca2+ content of the SR lumen.
Effects of pH
It was found that isometric force in fast-twitch Xenopus fibres was rather insensitive to changes in pH in the range studied. This is different from the results obtained in skinned mammalian muscle fibres at low temperature (∼15°C). In fast psoas fibres a linear relationship was found between force and pH. The increase in force with increasing pH in fast-twitch fibres is larger than in slow-twitch fibres (Chase & Kushmerick, 1988; Potma et al. 1994) but the pH dependence of force is less marked at higher temperatures (30°C; Pate et al. 1995). The slight increase in force at moderate degrees of acidification (from pH 7.1 to pH 6.8; Fig. 4) and this temperature effect may explain why caffeine and high K+ contractures in fatigued Xenopus fibres showed almost no reduction in maximum force (e.g. Allen et al. 1989). The decline in force expected on the basis of a rise in Pi concentration might at least in part be compensated by the pH-dependent increase in force between pH 6.8 and pH 7.1.
The observation that the AM ATPase activity was also rather insensitive to changes in pH provides further evidence that the contractile apparatus in Xenopus fibres is not very sensitive to changes in pH. This is in contrast to the SR ATPase activity, which increased by about a factor of 3 in the range from pH 6.2 to pH 7.4. Lamb et al. (1992) estimated the reduction of the SR Ca2+ uptake rate from the reduction in Ca2+ content of the SR after loading at pCa 6.0 and found, in skeletal muscle fibres from cane toad, a fourfold reduction when pH was reduced from 7.1 to 6.1, which is similar to our value.
Combined effects of Pi and pH
The combined effects of 30 mM Pi at pH 6.2 on force development and ATPase activity were studied to mimic the conditions during severe muscle fatigue. In agreement with previous studies in skeletal and cardiac muscle tissue (e.g. Godt & Nosek, 1989; Ebus et al. 1994; Potma et al. 1995), it was found that maximum isometric force was markedly depressed to 37 ± 3 % of the control force. AM ATPase activity was reduced less (to 68 ± 7 %) while the SR ATPase activity was reduced considerably (to 20 ± 6 %) compared with the control values. The reduction in the overall ATPase activity as well as of the AM ATPase activity is compatible with previous findings (e.g. Dawson et al. 1980; Nagesser et al. 1993; Ebus et al. 1994; Potma et al. 1995). With regard to the reduction in SR ATPase activity it should be noted that comparable measurements in intact muscle fibres under our experimental conditions (temperature, in particular) are not available. Westerblad et al. (1997) obtained a measure of the rate of Ca2+ uptake by the SR during relaxation following tetanic stimulation in unfatigued and fatigued fibres from Xenopus at room temperature (22°C). They provided evidence that during their fatigue protocol at the end of phase 2, where pH would have decreased and Pi would have increased considerably, the rate of SR Ca2+ uptake was depressed by a factor of 238/52.4, i.e. 4.5. This estimate is very similar to what we have found. Recently, it was observed in SR vesicles from fast skeletal muscle of rabbits that Pi attenuated the inhibition of Ca2+ uptake during acidosis (Wolosker et al. 1997). However, this might be due to a decrease in the stimulating effect of Pi on SR Ca2+ loading capacity.
It is interesting to see that the sensitivities of the contractile apparatus and the SR Ca2+ pump to Pi and pH differ. Our results show that force development and AM ATPase activity are more sensitive to an increase in Pi from 0 to 30 mM than to a decrease in pH from 7.1 to 6.2, while the reverse is true for SR Ca2+ uptake, and that the combined effects of Pi and pH on SR Ca2+ uptake are more pronounced than expected from an independent action of Pi and pH.
Acknowledgments
This study was supported by the Netherlands Organization for Scientific Research (NWO) and the Hungarian Research Fund (OTKA).
References
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. The Journal of Physiology. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ, Curtin NA, Woledge RC. Changes in crossbridge and non-crossbridge energetics during moderate fatigue of frog muscle fibres. The Journal of Physiology. 1993;468:543–555. doi: 10.1113/jphysiol.1993.sp019787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman HPJ, van der Laarse WJ, Stienen GJM, Elzinga G. Force-dependent and force-independent heat production in single slow- and fast-twitch muscle fibres from Xenopus laevis. The Journal of Physiology. 1996;496:503–519. doi: 10.1113/jphysiol.1996.sp021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd SK, McCutcheon LJ, Hodgson DR, Gollnick PD. Altered sarcoplasmic function after high-intensity exercise. Journal of Applied Physiology. 1989;67:2072–2077. doi: 10.1152/jappl.1989.67.5.2072. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophysical Journal. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NA. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. Journal of Muscle Research and Cell Motility. 1986;7:269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. The Journal of Physiology. 1980;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebus JP, Stienen GJM, Elzinga G. Influence of phosphate and pH on myofibrillar ATPase activity and force in skinned cardiac trabeculae from rat. The Journal of Physiology. 1994;476:501–516. doi: 10.1113/jphysiol.1994.sp020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Lou F. Changes in force and stiffness induced by fatigue and intracellular acidification in frog muscle fibres. The Journal of Physiology. 1990;424:133–149. doi: 10.1113/jphysiol.1990.sp018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G, Lännergren J, Stienen GJM. Stable maintenance heat rate and contractile properties of different single muscle fibres from Xenopus laevis at 20°C. The Journal of Physiology. 1987;393:399–412. doi: 10.1113/jphysiol.1987.sp016829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscle. The Journal of Physiology. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle fibres. Journal de Physiologie Paris. 1979;75:463–505. [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Shomer NH, Roghair TJ, Louis CF. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. Journal of Biological Chemistry. 1994;269:192–198. [PubMed] [Google Scholar]
- Fryer MJ, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. The Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. The Journal of Physiology. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman YE, Simmons RM. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. The Journal of Physiology. 1984;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Güth K, Winnikes K, Haist C, Rüegg JC. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflügers Archiv. 1987;408:1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Kentish JC. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. The Journal of Physiology. 1986;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Recupero E, Stephenson DG. Effect of myoplasmic pH on excitation-contraction coupling in skeletal muscle fibres of the toad. The Journal of Physiology. 1992;448:211–224. doi: 10.1113/jphysiol.1992.sp019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Smith RS. Types of muscle fibres in toad muscle. Acta Physiologica Scandinavica. 1966;68:263–274. [Google Scholar]
- MacLennan DH. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. Journal of Biological Chemistry. 1970;245:4508–4518. [PubMed] [Google Scholar]
- MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmatic reticulum Ca2+-ATPases. Journal of Biological Chemistry. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing intermittent stimulation of different types of single muscle fibres from Xenopus laevis. Journal of Muscle Research and Cell Motility. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned psoas muscle mechanics at high temperatures: implications for fatigue. The Journal of Physiology. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potma EJ, van Graas IA, Stienen GJM. Effects of pH on myofibrillar ATPase activity in fast and slow skeletal muscle fibers of the rabbit. Biophysical Journal. 1994;67:2404–2410. doi: 10.1016/S0006-3495(94)80727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potma EJ, van Graas IA, Stienen GJM. Influence of inorganic phosphate and pH on ATP ulitization in fast and slow skeletal muscle fibers. Biophysical Journal. 1995;69:2580–2589. doi: 10.1016/S0006-3495(95)80129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflügers Archiv. 1990;415:645–647. doi: 10.1007/BF02583520. [DOI] [PubMed] [Google Scholar]
- Shigekawa M, Dougherty JP, Katz AM. Reaction mechanism of Ca2+-dependent ATP hydrolysis by skeletal muscle sarcoplasmic reticulum in the absence of added alkali metal salts. Journal of Biological Chemistry. 1978;253:1442–1450. [PubMed] [Google Scholar]
- Smith GL, Steele DS. Inorganic phosphate decreases the Ca2+ content of the sarcoplasmic reticulum in saponin-treated rat cardiac trabeculae. The Journal of Physiology. 1992;458:457–473. doi: 10.1113/jphysiol.1992.sp019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. The Journal of Physiology. 1996;493:299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Roosemalen MCM, Wilson MGA, Elzinga G. Depression of force by phosphate in skinned skeletal muscle fibres of the frog. American Journal of Physiology. 1990;259:C349–357. doi: 10.1152/ajpcell.1990.259.2.C349. [DOI] [PubMed] [Google Scholar]
- Stienen GJM, van Graas IA, Elzinga G. Uptake and caffeine-induced release of calcium in fast muscle fibers of Xenopus laevis: effects of MgATP and Pi. American Journal of Physiology. 1993;265:C650–657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Stienen GJM, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. The Journal of Physiology. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiologica Scandinavica. 1988;133:83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J, Allen DG. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse. Contribution of [Ca2+] and cross-bridges. Journal of General Physiology. 1997;109:385–399. doi: 10.1085/jgp.109.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, De Meis L. pH-dependent inhibitory effects of Ca2+, Mg2+ and K+ on Ca2+ efflux mediated by sarcoplasmic reticulum ATPase. American Journal of Physiology. 1994;35:C1376–1381. doi: 10.1152/ajpcell.1994.266.5.C1376. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Rocha JBT, Engelender S, Panizutti R, De Miranda J, De Meis L. Sarco/endoplasmatic reticulum Ca2+-ATPase isoforms: diverse responses to acidosis. Biochemical Journal. 1997;321:545–550. doi: 10.1042/bj3210545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nosek TM. Intracellular milieu changes associated with hypoxia impair sarcoplasmic reticulum Ca2+ transport in cardiac muscle. American Journal of Physiology. 1991;261:H620–626. doi: 10.1152/ajpheart.1991.261.3.H620. [DOI] [PubMed] [Google Scholar]


