Abstract
The study aimed to describe in cat forelimb and shoulder motoneurones the convergence and projection patterns from large muscle spindle afferents (Ia). In 11 chloralose-anaesthetized cats maximum Ia EPSPs evoked by electrical stimulation of ipsilateral forelimb nerves were obtained in 309 intracellularly recorded α-motoneurones.
Groups of motor nuclei displayed similar Ia patterns. As in the distal forelimb they were often interconnected by bidirectional pathways, which were used to combine Ia synergistic groups. Three such groups are described at the shoulder.
The first group was composed of the main flexors of the scapulo-humeral joint. Regular disto-proximal Ia excitation from elbow extensors (and median afferents) indicates a coupling of flexion in the scapulo-humeral joint to the angular position of the elbow.
The second group comprised the outward rotators of the humerus with differentiated Ia convergence onto the different group members. The patterns of Ia excitation received and sent by the group members demonstrate that the outward rotators are incorporated in versatile synergisms and may occupy a central position in steering forelimb movements.
The third group was formed by the spinatus muscle and the subscapularis. This arrangement is suggested by the common convergence onto them from the elbow extensors and flexors. The pattern may serve to guide and keep the humeral head in the joint capsule.
The Ia synergistic groups receive Ia convergence from muscles acting at distant joints and also project to distant muscles. This is discussed as part of an extended pattern of Ia connections along the forelimb. In this way the shoulder muscles would be incorporated in flexor and extensor oriented synergisms which are needed to co-ordinate the muscular activation along the multijoint forelimb during locomotion. When the shoulder Ia pathways are compared with those in the distal forelimb, organization of the Ia system apparently follows a few basic principles which have adapted to the mechanical situation at the particular joints and their mechanical interaction.
The shoulder joint, which consists of two components (Reighard & Jennings, 1966), is of special importance for the movements of the upper extremity. It is the mechanical base for any movement of the forearm against the trunk. The trunk-scapular joint is of purely muscular origin and allows the scapula to glide on the surface of the trunk. The humero-scapular joint has a very small bony articular surface and the humerus is kept inside the glenoid cavity by muscular and ligamenteous forces. This construction between forearm and trunk poses special demands on the nervous system to regulate the muscular forces. On one hand, the muscles hold the different components of the shoulder together. On the other hand, they allow mobility of the forearm against the trunk, as is needed in normal motor behaviour. There is a further, more functionally related, aspect which underlines the importance of the shoulder in the control of the forelimb during reaching and pointing movements. Several arguments indicate that it may act as the centre of a reference frame organizing the movements of the forelimb in space (e.g. Soechting & Flanders, 1991).
Not much is known about the segmental neuronal mechanisms assisting these functions. The shoulder region is equipped with a high density of muscle spindle receptors (e.g. Körner 1960), which project into the spinal cord and relay their message about the local conditions in the shoulder area to spinal and supraspinal systems. It is to be expected that the peripheral demands on the neural control of the shoulder joints are reflected in the specific connectivity patterns of these afferents. With regard to the importance of a co-ordinated distribution of contractile force between the muscles acting on the two shoulder joints, the connections from the large muscles spindle afferents (Ia) from the shoulder muscles are of particular interest. One of the main functions of this afferent system is to supply balanced and co-ordinated excitation in motor nuclei to agonists and close functional synergists (Eccles et al. 1957; for review see Baldissera et al. 1981). They could thus play an important role in organizing shoulder function.
We have performed a detailed analysis of the pattern of monosynaptic Ia connections between the cat shoulder muscles. Beyond this particular topic the study is of general interest for the organization and function of the afferent Ia system since, for the first time, it deals with joints which in their construction are largely dependent on muscular forces. Furthermore, the shoulder joint is the last of the large limb joints which has not yet been studied with respect to the Ia pathways (ankle: Eccles et al. 1957; hip, knee: Eccles & Lundberg, 1958; elbow, wrist: Fritz et al. 1989). It is to be expected that the comprehensive database now available on the Ia system of the cat fore- and hindlimb will lead to a deeper insight into the organization of the Ia afferent system, particular in its adaptability to specific biomechanical needs, in the local strategies of this reflex system, in its participation in motor behaviour and its contribution to motor control. Preliminary results have been published in abstract form (Hohn et al. 1993).
METHODS
The report is based on experiments on 11 cats operated upon under ether and subsequently anaesthetized with α-chloralose (50-80 mg kg−1i.v.) and supplementary small doses of Nembutal (5-10 mg i.p., adding to a total amount of 10-20 mg kg−1). After dissection the animals were immobilized with pancuronium bromide, a pneumothorax was performed and artificial ventilation adjusted to an end-tidal [CO2] close to 4 %. The arterial blood pressure was measured, the rectal temperature was kept within 36-38°C. The depth of the anaesthesia was monitored by the degree of pupillary constriction and the stability of blood pressure. It was kept constant by additional injections of chloralose and Nembutal. At the end of the experiments the animals were killed by an intravenous overdose of Nembutal. The project was approved by the ethical committee of Schleswig-Holstein and the experiments performed according to rules of the Tierschutzgesetz of Germany.
We performed a medial incision along the lateral side of the left forelimb to dissect the nerves to the first group of seven muscles listed in Table 1 and schematically illustrated in Fig. 1. The nerves were cut at their entrance into the respective muscles. The nerve stems of the median (Med), ulnar (Ul) and deep radial (DR) nerves were dissected through the same lateral approach. A medial approach through an incision between trunk and medial ridge of the scapula was used in the second group of muscles (8th to 13th) to dissect the nerves (Table 1, Fig. 1). Since the different branches of the Ser nerve have a distributed anatomical course we could dissect those to the caudal half of this muscle only. The limb was pronated and placed in a metal bath that was filled with liquid and temperature stabilized (37°C) using paraffin oil. For stimulation (square wave voltage pulses of 0.1 ms duration) the nerves indicated with an asterisk in Table 1 were mounted on buried electrodes, the remainder were placed on silver hook electrodes positioned in the paraffin pool. A dorsal laminectomy was performed from C3 to Th1. Spinal cord surface potentials were recorded from the cord dorsum near the dorsal root entry zone to monitor the stimulation strength in multiples of threshold. α-Motoneurones were intracellularly recorded with glass microelectrodes (2-4 MΩ resistance; 3 M potassium acetate). The amplified signals were displayed on an oscilloscope, A/D converted, averaged on-line (at least 12 sweeps) and stored sweep by sweep for off-line analysis.
Table 1.
List of dissected muscle nerves, nerve stems and abbreviations
| M. biceps brachii | Bi |
| M. brachialis | Br |
| M. triceps brachii | |
| caput longum | TLo |
| caput mediale | TM |
| caput laterale | TLa |
| anconeus | An |
| M. cleidobrachialis | ClB* |
| M. acromiodeltoideus1 | AcD* |
| M. teres minor1 | TMi |
| M. spinodeltoideus1,2 | SpD |
| M. infraspinatus2,3 | ISp* |
| M. supraspinatus3 | SSp* |
| M. teres major2,4 | TMa* |
| M. latissimus dorsi2,4 | LD* |
| M. subscapularis4 | SSc* |
| M. serratus anterior5 | Ser* |
| N. ulnaris | Ul |
| N. medianu | Med |
| N. radialis profundus | DR |
| M. extensor carpi radialis | ECR |
| M. supinator | Sup |
The numbers indicate the main actions of the muscles on the shoulder joint:
outward rotation
flexion
abduction
inward rotation
depression of scapula. The nerves indicated with an asterisk were mounted on buried electrodes, the remainder on hook electrodes.
Figure 1. Origin and insertion of the investigated shoulder and forearm muscles.
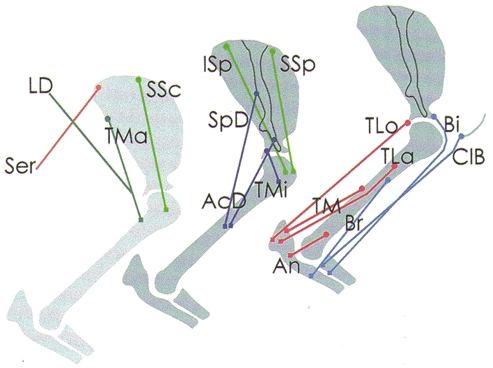
The left-hand drawing shows the scapula and the humerus from the medial, and the middle and right-hand drawings from the lateral side. • indicates the origin, and ▪ the insertion of the muscles. The course of the muscles is based on the descriptions by Reighard & Jennings (1966), Crouch (1969) and on our own observations in post-mortem preparations. The muscles are indicated with the abbreviations listed in Table 1. The colour coding denotes the muscles of different Ia synergistic groups. Light blue: Bi, Br, ClB (group of elbow flexors); red: TLo, TLa, TM, An (group of elbow extensors); dark blue: SpD, AcD, TMi (group of outward rotators of humerus, including ISp); light green: ISp, SSp, SSc (group stabilizing the humeral head in the glenoid fossa); dark green: TMa, LD (group of flexors in the humero-scapular joint, including SpD); brown: Ser.
In tracking for the different limb and shoulder motor nuclei we followed the available anatomical maps (Fritz et al. 1986a, b; Hörner & Kümmel, 1993). Motoneurones were identified by antidromic invasion from one of the dissected nerves (Brock et al. 1953; see e.g. Fig. 4B and H). In all cases the antidromic action potentials occurred before onset of the homonymous EPSPs. When a motoneurone had been identified, the peripheral muscle nerves were stimulated one after the other for the presence of EPSPs. The EPSPs were classified as being monosynaptic and of Ia origin when their intraspinal latencies were shorter than 1.2 ms and their thresholds between 1.0 and 1.4. The maximal size of the Ia EPSPs was established by grading the stimulation strength. In the case of homonymous connections this assessment of the maximal Ia EPSPs was complicated by antidromic invasion from the stimulated nerve. In about a third of the neurones the homonymous EPSP was maximal at a stimulus strength subthreshold for antidromic invasion; in other neurones it was possible to prevent antidromic invasion by hyperpolarizing the membrane potential. In these latter cells the amplitude of the maximal homonymous EPSP was calculated as described by Eccles et al. (1957). The presence of motoneuronal or interneuronal extracellular fields could be a complicating factor in assessing the size of the maximal EPSPs. After withdrawal of the microelectrode from the intracellular position the extracellular signals were recorded and, if necessary, subtracted from the intracellular potential.
Figure 4. Convergence patterns onto the motor nuclei of group 2 (SpD, AcD, TMi, ISp).
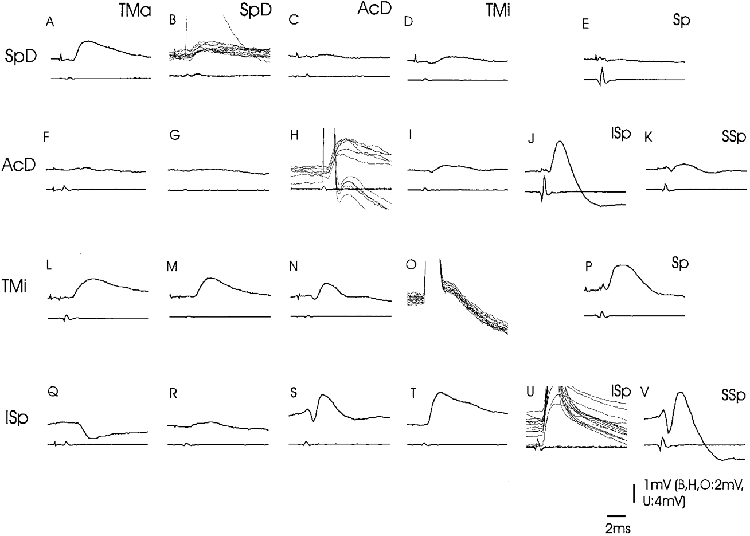
Intracellular records from four different motoneurones (SpD: A-E; AcD: F-K; TMi: L-P; ISp: Q-V). The stimulated nerves are indicated above the specimen records. The upper traces are intracellular records, the lower traces are records from the spinal cord surface at the level of the respective motoneurones. The traces give averaged responses (n = 12 records) except in B, H, O and U, where superimposed sweeps illustrate the antidromic action potentials and the homonymous EPSP. The voltage calibrations apply to the intracellular records. In E and P, the nerves to ISp and SSp were mounted together (indicated as ‘Sp’).
The data from the neurones of a common motor nucleus were pooled. The projection from a nerve onto a motoneurone was treated as positive when the EPSP size was equal to or exceeded 0.1 mV (doubtful cases were treated as not tested). The Ia EPSPs were analysed with regard to the connectivity pattern of the Ia afferents (qualitative analysis) and to the absolute EPSP sizes (quantitative analysis). For the quantitative analysis (Tables 3–6) motoneurones with an antidromic action potential larger than 50 mV were included. The EPSP amplitudes are presented as mean values with standard deviations (in a given nerve-nucleus combination the amplitudes of the EPSPs recorded in the different cells were added then divided by the number of cells tested in this combination, i.e. including the cells without an EPSP). For the qualitative description of the connectivity pattern (Table 2) motoneurones with an action potential larger than 40 mV were included. Compared with quantitative analysis the selection criterion for the acceptance of motoneurones was reduced, but this allowed the inclusion of a larger number of neurones from the small shoulder nuclei that are difficult to locate in the spinal cord (Hörner & Kümmel, 1993).
Table 3.
Mean amplitude of the Ia EPSPs evoked in the motoneurones of group 1
| Nerves | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | LD | TMa | SpD | TLo | TM | Med | ||||||
| LD | 2.7 ± 0.4 | (3) | 0.2 ± 0.2 | (17) | 0.3 ± 0.3 | (9) | 0.2 ± 0.3 | (17) | 0.1 ± 0.3 | (17) | — | (17) |
| TMa | 2.0 ± 1.6 | (8) | 3.4 | (1) | 0.3 ± 0.3 | (8) | 0.5 ± 0.4 | (11) | — | (11) | 0.3 ± 0.7 | (11) |
| SpD | n.t. | 0.7 ± 0.3 | (5) | 0.8 | (1) | 0.1 ± 0.2 | (5) | — | (2) | 0.1 ± 0.1 | (5) | |
| AcD | 0.1 ± 0.3 | (9) | 0.1 ± 0.2 | (16) | 0.1 ± 0.2 | (12) | — | (17) | < 0.1 | (10) | < 0.1 | (12) |
| TMi | 0.4 ± 0.1 | (5) | 0.6 ± 1.0 | (6) | 0.9 ± 0.9 | (4) | — | (6) | — | (6) | — | (5) |
| Br | 0.1 ± 0.2 | (9) | 0.1 ± 0.2 | (12) | 0.1 ± 0.2 | (6) | — | (12) | — | (10) | 0.4 ± 0.2 | (11) |
Material of the quantitative analysis. The motor nuclei are listed from top to bottom, the stimulated nerves from left to right. Values are mean EPSPs in mV ± S.D.; numbers in parentheses are the number of cells tested. —, Ia excitation not found in any cell. n.t., combination not tested.
Table 6.
Mean amplitude of the Ia EPSPs evoked in the motoneurones of group 6
| Nerves | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | Bi | Br | ClB | AcD | DR | Med | ||||||
| Bi | 5.7 ± 2.1 | (16) | 1.9 ± 1.0 | (28) | 0.5 ± 0.4 | (25) | — | (26) | 1.0 ± 0.6 | (28) | 0.6 ± 0.7 | (27) |
| Br | 0.7 ± 0.4 | (11) | 2.8 ± 0.8 | (4) | 0.5 ± 0.4 | (9) | — | (11) | 1.7 ± 0.9 | (10) | 0.4 ± 0.2 | (11) |
| ClB | < 0.1 | (8) | < 0.1 | (9) | 3.8 ± 2.4 | (4) | 0.2 ± 0.2 | (9) | — | (9) | 0.2 ± 0.2 | (9) |
| AcD | — | (16) | < 0.1 | (16) | 0.2 ± 0.3 | (15) | 2.8 ± 1.6 | (5) | < 0.1 | (16) | < 0.1 | (12) |
Material of the quantitative analysis. The motor nuclei are listed from top to bottom, the stimulated nerves from left to right. Values are mean EPSPs in mV ± S.D.; numbers in parentheses are the number of cells tested. —, Ia excitation not found in any cell.
Table 2.
Monosynaptic Ia excitation in identified motoneurones to shoulder and forearm muscles
| Nerves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | LD | TMa | SpD | AcD | TMi | ISp | Sp | SSp | SSc | Ser* |
| LD | 19 (19) | 14 (19) | 8 (11) | — (7) | — (7) | — (19) | n.t. | — (19) | — (19) | — (19) |
| TMa | 8 (8) | 11 (11) | 4 (8) | — (7) | — (7) | — (11) | n.t. | — (11) | — (11) | — (8) |
| SpD | n.t. | 5 (5) | 5 (5) | 2 (5) | 5 (5) | — (5) | n.t. | — (5) | — (5) | n.t. |
| AcD | 2 (10) | 5 (17) | 3 (13) | 18 (18) | 14 (17) | 6 (6) | 11 (12) | 2 (6) | — (13) | 2 (10) |
| TMi | 1 (6) | 3 (7) | 5 (5) | 4 (6) | 7 (7) | n.t. | 5 (7) | n.t. | — (7) | — (6) |
| ISp | — (12) | — (47) | 5 (34) | 44 (46) | 41 (43) | 49 (49) | n.t. | 30 (33) | — (42) | 3 (12) |
| SSp | — (16) | — (39) | — (34) | 7 (42) | — (40) | 16 (16) | n.t. | 44 (44) | 38 (41) | 16 (17) |
| SSc | — (8) | 2 (31) | — (28) | 1 (29) | — (30) | — (11) | 3 (20) | 3 (11) | 32 (32) | — (8) |
| Ser | — (6) | — (6) | — (5) | 1 (5) | — (5) | — (6) | n.t. | — (6) | — (6) | 6 (6) |
| TLo | — (1) | — (9) | — (8) | — (8) | — (8) | n.t. | 1 (8) | n.t. | 1 (9) | — (1) |
| TM | 1 (5) | 1 (5) | — (5) | n.t. | n.t. | — (5) | n.t. | — (5) | — (5) | — (5) |
| TLa | — (4) | 1 (5) | — (2) | — (4) | — (4) | 1 (1) | 4 (4) | 1 (1) | 4 (5) | 4 (4) |
| An | — (1) | 1 (3) | — (3) | — (2) | — (2) | — (1) | 2 (2) | 1 (1) | 2 (3) | — (1) |
| Bi | — (11) | — (31) | — (26) | — (30) | — (29) | — (27) | n.t. | — (27) | — (32) | — (11) |
| Br | 3 (10) | 6 (14) | 2 (6) | — (12) | 2 (12) | — (12) | n.t. | — (12) | — (14) | — (10) |
| ClB | — (4) | — (9) | — (8) | 8 (10) | — (8) | — (8) | n.t. | — (8) | 1 (9) | 1 (4) |
| Nerves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | TLo | TM | TLa | An | Bi | Br | ClB | DR | Med | Ul |
| LD | 9 (19) | 4 (19) | — (19) | — (18) | — (19) | — (18) | — (11) | — (18) | 1 (19) | — (19) |
| TMa | 8 (11) | — (11) | — (11) | — (11) | — (11) | 2 (11) | — (8) | — (11) | 3 (11) | — (10) |
| SpD | 2 (5) | — (2) | — (5) | — (5) | — (5) | — (5) | — (5) | — (5) | 1 (5) | — (5) |
| AcD | — (17) | 1 (10) | — (17) | — (12) | — (17) | 1 (16) | 9 (17) | 1 (17) | 1 (13) | — (17) |
| TMi | — (7) | — (7) | — (7) | — (7) | — (7) | — (7) | — (7) | — (5) | — (6) | — (5) |
| ISp | 9 (39) | 14 (30) | 12 (32) | 9 (32) | — (45) | — (45) | 2 (43) | — (47) | 3 (39) | — (45) |
| SSp | 4 (41) | 12 (29) | 17 (28) | 3 (25) | 10 (37) | — (41) | 10 (41) | 2 (39) | 5 (39) | 2 (40) |
| SSc | 2 (32) | 2 (25) | 2 (22) | — (21) | 12 (30) | 5 (30) | 3 (31) | — (30) | 8 (29) | 2 (30) |
| Ser | — (6) | — (6) | — (6) | — (6) | — (6) | — (6) | — (4) | — (5) | — (6) | 1 (6) |
| TLo | 12 (12) | 2 (7) | 2 (7) | 2 (8) | — (5) | — (7) | — (10) | — (11) | 1 (12) | 5 (12) |
| TM | 4 (5) | 5 (5) | 2 (5) | 1 (2) | — (1) | — (5) | n.t. | — (5) | — (5) | — (5) |
| TLa | 4 (5) | 5 (5) | 5 (5) | 3 (5) | — (4) | — (4) | — (4) | — (5) | 2 (5) | — (4) |
| An | 3 (3) | 3 (3) | 3 (3) | 3 (3) | — (3) | — (3) | — (3) | — (3) | 1 (3) | — (3) |
| Bi | — (30) | — (23) | — (21) | — (21) | 32 (32) | 32 (32) | 27 (31) | 30 (32) | 19 (32) | — (32) |
| Br | — (14) | — (11) | — (13) | — (12) | 13 (14) | 14 (14) | 9 (11) | 11 (11) | 4 (12) | — (10) |
| ClB | — (10) | — (10) | — (10) | — (10) | 2 (10) | 1 (10) | 10 (10) | — (10) | 4 (10) | — (10) |
Material of the qualitative analysis. The motor nuclei are listed from top to bottom, the stimulated nerves from left to right. Values given are the number of cells with a monosynaptic Ia EPSP from the tested nerve; numbers in parentheses are the number of cells tested. —, Ia excitation not found in any cell. n.t., combination not tested.
Not all branches to this muscle were dissected.
The Ia connections are described by the following terms, which have been used previously (Eccles et al. 1957; Eccles & Lundberg, 1958; Hongo et al. 1984; Fritz et al. 1989). The term Ia relation between muscles describes the heteronymous Ia connections from the muscle in question to the respective motor nuclei. Such relations may be unidirectional (Ia afferents of one muscle project onto motoneurones of another without a corresponding reciprocal projection), or bidirectional (mutual connections between Ia afferents and motoneurones of both muscles). Bidirectional relations are termed balanced when the frequencies and the mean amplitudes of the EPSPs are rather similar; they are termed skewed when one reciprocal projection substantially exceeds the other one.
RESULTS
Data are presented from 309 α-motoneurones projecting to different shoulder and forelimb muscles. Table 2 lists 272 α-motoneurones which act on the shoulder and elbow joints. It does not include 37 neurones to muscles which act on joints distal to the elbow. The small number of TMi and SpD neurones reflects the small size of the respective nuclei (Hörner & Kümmel, 1993); the small number of Ser cells is due to the fact that this nerve was not mounted in all experiments.
In 87 of the motoneurones the duration of the after-hyperpolarization (AHP) was established. The histogram of Fig. 2A shows a normal distribution with a mean value of 67 ± 21 ms. Due to the difficult intracellular recording situation in the brachial enlargement the sample may be biased in favour of fast motoneurones. Regarding the duration of the AHP there was no obvious difference between the investigated motor nuclei (Fig. 2B). One group of motor nuclei consisting of ISp, SSp and SSc had mean values between 70 and 80 ms, a second group consisting of AcD, Ser, ClB, LD and TMa had mean values below 60 ms. This may reflect that muscles of the former group include a higher percentage of slow motoneurones than the muscles of the second group, but histochemical data on the fibre type composition of the respective muscles are not available.
Figure 2. Duration of after-hyperpolarization in the motoneurones and motor nuclei.
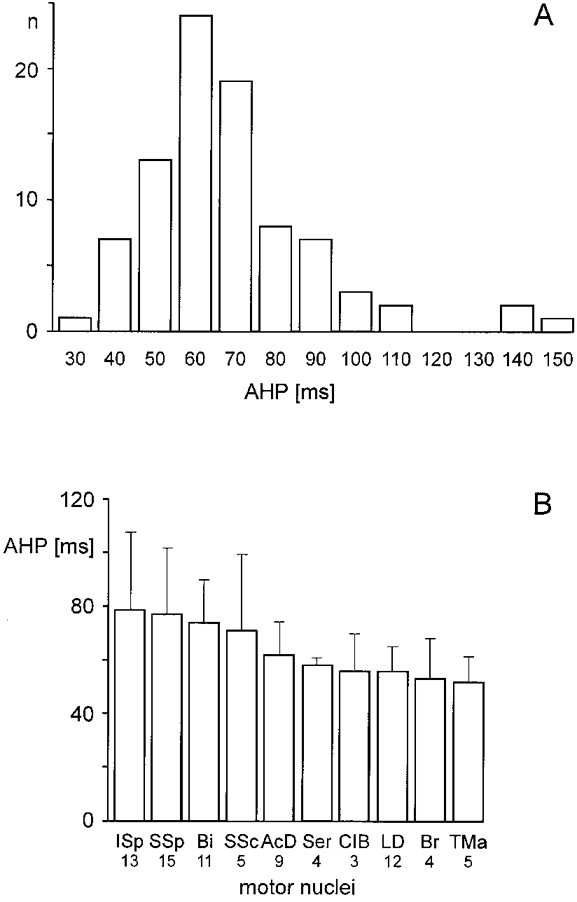
A, histogram from 87 cells (ordinate) with the duration of the after-hyperpolarization (AHP; abscissa). B, the motor nuclei are ranked according to the mean AHP duration (±s.d.). The number of cells included in the samples is given below the respective nuclei.
Amount of Ia excitation
Figure 3A shows that the total aggregates of the Ia EPSPs (sum of homonymous and heteronymous EPSPs) ranged from 9.7 to 2.8 mV (the actions from Ser Ia afferents were not included in these values, since branches to the caudal half of this muscle only were mounted on stimulating electrodes). These values are comparable with those reported for the motoneurones to the distal cat forelimb (Fritz et al. 1989) and in the same range as the values recorded in the cat hindlimb (summarized in Hongo et al. 1984). The motor nuclei to the shoulder muscles were evenly distributed within that range. The heteronymous-homonymous relations were above or near 1.0 in the elbow (Bi, Br, TLo), but well below 1.0 in the shoulder muscles (in the TMa, TMi and SpD motor nuclei the number of motoneurones was too small to establish this relation). Nevertheless, convincing differences between the shoulder and the distal limb motor nuclei seemed not to be present. This impression changed when the cells without Ia convergence from the nerves were excluded from the material (Fig. 3B). In this situation the total aggregates increased in the big shoulder muscles Bi (12.3 mV), ISp (11.3 mV), SSp (11.3 mV) and SSc (7.0 mV), whereas there was not much difference in the elbow muscles (Br, TLo, ClB). A corresponding increase was present with respect to the heteronymous-homonymous relations. This finding indicates the presence of subpopulations of motoneurones in these shoulder motor nuclei with different Ia receptivness. In the LD, SpD and TMi nuclei only minor differences were found.
Figure 3. Aggregate Ia EPSPs in the shoulder and forearm motor nuclei.
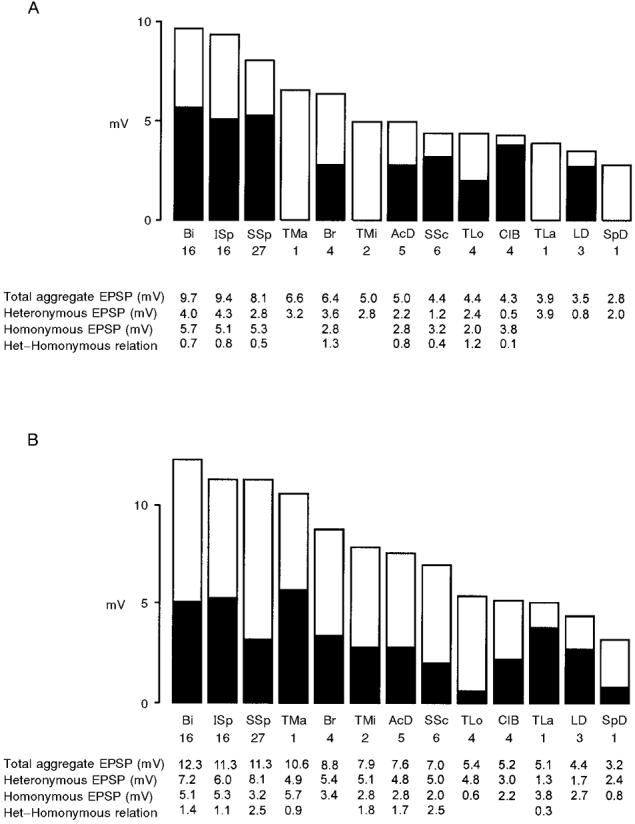
To obtain the total aggregate Ia EPSPs the mean values of the heteronymous EPSPs (ordinate, mV) were added (open area) to the maximal homonymous EPSPs (filled area). The numerical relations between heteronymous and homonymous EPSPs are given. The motor nuclei are ranked according to the size of the total aggregate EPSP. A, histogram giving the data of Tables 3–6 with all tested cells (i.e. with and without convergences). B, histogram comprising all cells with Ia EPSPs (i.e. excluding cells without EPSPs). Note that the ranking of the motor nuclei differs in each situation. The number of cells included in the samples is given below the respective nuclei.
Patterns of Ia connections
The number of neurones with monosynaptic EPSPs in the various nerve-nucleus combinations are listed in Table 2. Wide receptive fields of heteronymous Ia excitation were found in many motor nuclei. The distribution of the pathways is highly organized. It seems that groups of motor nuclei have similar, if not identical, Ia patterns. Nuclei with a similar Ia pattern are mostly interconnected by bidirectional, in many cases balanced, pathways. To simplify the description of the complex relations and to promote a functional interpretation the different motor nuclei will be described in the context of their Ia synergistic group (for definition see Fritz et al. 1989). The groups are illustrated in the summarizing Table 7.
Table 7.
Ia synergistic groups at the shoulder
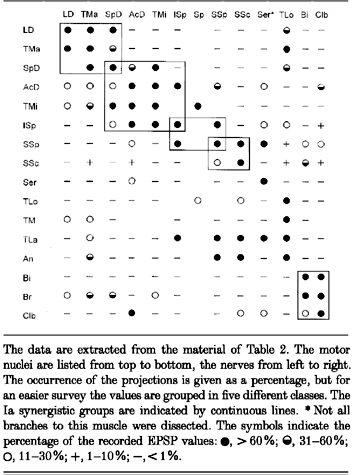
The data are extracted from the material of Table 2. The motor nuclei are listed from top to bottom, the nerves from left to right. The occurrence of the projections is given as a percentage, but for an easier survey the values are grouped in five different classes. The Ia synergistic groups are indicated by continuous lines. *Not all branches to this muscle were dissected. The symbols indicate the percentage of the recorded EPSP values: •, > 60 %;  , 31-60%; ○, 11-30%; +, 1-10%; −, < 1 %.
, 31-60%; ○, 11-30%; +, 1-10%; −, < 1 %.
Group 1 - LD, TMa and SpD
This group is composed of the main flexors of the scapulo-humeral joint (Table 2 and Table 3). The SpD motor nucleus, which is tightly connected in this group, is at the same time a member of group 2 as well.
The motor nuclei had bidirectional relations with each other with frequencies above 70 % (Table 2). The strength of the connections was skewed in all investigated combinations (Table 3). Thus the TMa receives stronger excitation from the LD than it emits to this nucleus (mean EPSP 2.0 versus 0.2 mV), and the SpD receives stronger excitation from TMa than in the reverse direction (mean EPSP 0.7 versus 0.3 mV). This skewedness seems to reflect a medio-lateral gradient from the trunk to the scapula (LD to TMa) and from the deep to the superficial muscular layers (TMa to SpD). Future recordings of the LD actions in SpD motoneurones will help to decide whether this gradient reflects a general principle or rather the specific co-ordination of the muscles involved in humeral flexion.
Skewed connections were present between this group and the triceps muscles (Table 2 and Table 3). Most frequently they originated from the TLo which supplied Ia excitation to all group members (in a few cases the TM excited LD). The TLo action was strong in TMa motoneurones (mean EPSP 0.5 mV), of moderate size in LD (mean EPSP 0.2 mV) and weak in SpD neurones (mean EPSP 0.1 mV). This disto-proximal organization of Ia excitation would couple flexion in the scapulo-humeral joint to the angular position of the elbow joint, as monitored and controlled by the triceps muscular complex. The reverse relations from the group to the other triceps muscles were weak and mainly originated from TMa (Table 2).
Ia afferents from the Med nerve supply a further source of distal Ia excitation to the flexors of the scapulo-humeral joint. The frequency of these projections was weak (Table 2), but the individual effects could be sizeable, especially in the TMa nucleus (mean EPSP 0.3 mV, Table 3). Since we have not dissected the Med nerve branches we can not ascribe the effects to particular distal muscles. It is also not known if this is a unidirectional or bidirectional relation. In the five Med motoneurones recorded (mostly located in C7/C8, for species see Fritz et al. 1986a) we have never recorded any Ia actions from the shoulder. However, we have not done a systematic survey of all Med motor nuclei.
There was a consistent projection from the members of group 1 onto the outward rotators of the humerus, except on ISp (Table 2 and Table 3). The relation was bidirectional in the case of the SpD, which correlates with the function of this muscle as an outward rotator. The relations to LD and TMa were unidirectional, TMi received stronger Ia excitation than did AcD (Table 3). Regarding the emitting connections from the shoulder flexors the distant LD supplied stronger excitation than did the nearer SpD. This connectivity indicates a close functional synergism between humeral flexion and outward rotation. The unidirectional organization suggests that outward rotation is controlled by the angular position of the scapulo-humeral joint. It should be noted, however, that the strongest outward rotator, ISp, is not included in this Ia control from shoulder flexors (Table 2).
All group members excited the Br motor nucleus, with a moderate frequency (Table 2) and a weak strength (Table 3). The relation seemed to be unidirectional, since in the reverse direction we recorded effects from Br afferents in only two out of 34 tested LD, TMa and SpD cells.
Group 2 - SpD, AcD, TMi and ISp
Outward rotation of the humerus is the common mechanical function of the muscles combined in this group. Three of them are members of other groups as well, suggesting that they have additional mechanical actions: the SpD is a member of group 1, the ISp is a member of group 3 and the AcD is a member of an additional group formed by ClB and AcD.
The relations within the group are illustrated in Fig. 4 with records from typical neurones, including the Ia actions from the shoulder flexor TMa. The quantitative data on the Ia convergence and projection are listed in Table 2 and Table 4. The relations between the motor nuclei were bidirectional (except for the unidirectional projection from SpD onto ISp). They displayed a differentiated pattern with regard to the strength of the connections (Table 4). A weak relation was present between AcD-SpD, a moderate one between AcD-TMi and a strong one between TMi-SpD and AcD- ISp. This organization suggests the presence of several local Ia synergies, which are adapted to specific mechanical situations. The local pattern seems to be overlaid by a more general distribution of Ia excitation. The AcD, for example, received stronger Ia excitation from the deeper and more distant muscles than from the immediate neighbour SpD (mean EPSP from SpD: 0.1 mV; from TMi: 0.4 mV; from ISp: 1.2 mV; Table 4, see also Fig. 4). A similar gradient was found in the SpD motoneurones (Table 4 and Fig. 4; the lack of Ia excitation from ISp in SpD is not contradictory to this hypothesis, but reflects the additional affiliation of ISp and SpD to two further functional groups). The excitation distributed from these muscles followed the same gradient. It was small in the immediate neighbours, but larger in the more distant muscles (e.g. mean EPSP from AcD in SpD: 0.1 mV; in TMi: 0.3 mV; in ISp 0.9 mV; Table 4 and Fig. 4).
Table 4.
Mean amplitude of the Ia EPSPs evoked in the motoneurones of group 2
| Nerves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | SpD | AcD | TMi | ISp | SSp | |||||
| SpD | 0.8 | (1) | 0.1 ± 0.2 | (5) | 1.1 ± 1.0 | (4) | — | (5) | — | (5) |
| AcD | 0.1 ± 0.2 | (12) | 2.8 ± 1.0 | (5) | 0.4 ± 0.4 | (16) | 1.2 ± 0.2 | (6) | 0.1 ± 0.2 | (6) |
| TMi | 0.9 ± 0.9 | (4) | 0.3 ± 0.3 | (5) | 2.2 ± 1.0 | (2) | 0.6 ± 0.6 | (6) | ||
| ISp | 0.1 ± 0.3 | (30) | 0.9 ± 0.7 | (39) | 1.2 ± 1.3 | (36) | 5.1 ± 2.5 | (16) | 1.4 ± 1.4 | (27) |
Material of the quantitative analysis. The motor nuclei are listed from top to bottom, the stimulated nerves from left to right. Values are mean EPSPs in mV ± S.D.; numbers in parentheses are the number of cells tested. —, Ia excitation not found in any cell. In the case of the TMi motoneurones the nerve branches to the ISp and SSp heads were not separated; the EPSP amplitude therefore refers to stimulation of the common spinatus nerve.
The Ia convergence onto this group was broad and not homogeneous (Table 2). It fell apart in different patterns reflecting that SpD, AcD and ISp are members of different Ia groups. The only Ia convergence that was specific for the group proper seemed to be the projection from SSp (in the TMi motor nucleus the effects from the separate Sp branches have not been investigated, but following the parallel projection from both branches onto AcD, TMi and ISp it is likely that SSp projects to TMi as well). Excitation from SSp was restricted to the outward rotators of the group, with the strength increasing from AcD to ISp (Table 4; Fig. 4). The SpD, which has a similar location to AcD but also acts as a flexor at the scapulo-humeral joint, was not reached by the SSp convergence. A strong unidirectional excitation onto the outward rotators AcD and TMi originated from the flexors of the humerus (group 1; Table 3). The ISp was not included in this connection scheme. The extensive Ia projection onto this motor nucleus from Ser, the different triceps heads and from median afferents (Table 2) reflects the affiliation of this muscle to group 3 and will therefore be treated in that context.
The AcD had bidirectional and balanced relations with the ClB (Table 2) which will be discussed below. In addition the nucleus had weak bidirectional connections with Ser. Strength and regularity of this relation are difficult to estimate since only part of the Ser afferents and motoneurones were investigated. On the other hand, this connection is interesting since it demonstrates an influence from the trunk-scapular onto the scapulo-humeral joint.
Group 3 - ISp, SSp and SSc
Although the distribution of the bidirectional relations (Table 2) indicates the presence of two groups of muscles, i.e. ISp/SSp and SSp/SSc, respectively, we combined these motor nuclei into a common group, since the convergence from other muscles onto them suggests a common functional synergism. Part of this convergence is illustrated in Fig. 4; Fig. 5 displays the convergence pattern received by a typical ISp motoneurone; the quantitative data of this group are listed in Table 4 and Table 5.
Figure 5. Convergence onto a single ISp motoneurone.
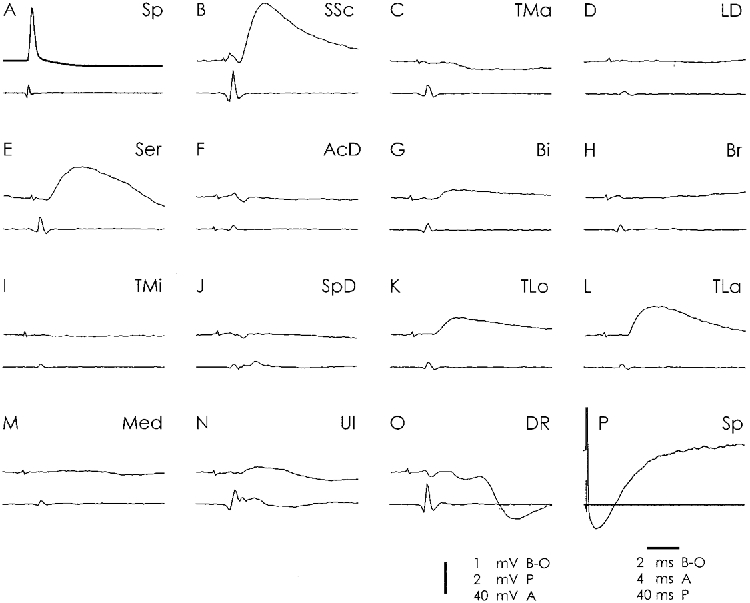
The structure of the figure is as in Fig. 4. The SP neurone was identified as projecting to the ISp by the Ia convergence from the Ser muscle (E, see Table 2).
Table 5.
Mean amplitude of the Ia EPSPs evoked in the motoneurones of group 3
| Nerves | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | LD | TMa | SpD | AcD | TMi | ISp | SSp | SSc | Ser |
| ISp | — | — | 0.1 ± 0.3 | 0.9 ± 0.7 | 1.2 ± 1.3 | 5.1 ± 2.5 | 1.4 ± 1.4 | — | 0.1 ± 0.2 |
| (9) | (42) | (30) | (39) | (36) | (16) | (27) | (37) | (9) | |
| SSp | — | — | — | < 0.1 | — | 1.4 ± 1.4 | 5.3 ± 2.6 | 0.7 ± 0.8 | 0.6 ± 0.4 |
| (14) | (35) | (29) | (36) | (34) | (14) | (12) | (31) | (14) | |
| SSc | — | 0.1 ± 0.6 | — | — | — | — | 0.4 ± 0.8 | 3.2 ± 3.6 | — |
| (6) | (28) | (26) | (25) | (27) | (10) | (10) | (6) | (6) | |
| Ser | — | — | — | < 0.1 | — | — | — | — | 1.7 ± 0.2 |
| (6) | (6) | (5) | (5) | (5) | (6) | (6) | (6) | (3) | |
| Nerves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Motoneurones | TLo | TM | TLa | An | Bi | Br | ClB | DR | Med | Ul |
| ISp | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.4 | — | — | — | — | < 0.1 | — |
| (34) | (26) | (28) | (26) | (40) | (40) | (36) | (42) | (34) | (40) | |
| SSp | < 0.1 | 0.1 ± 0.2 | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.4 | — | 0.1 ± 0.1 | < 0.1 | < 0.1 | < 0.1 |
| (34) | (24) | (24) | (24) | (30) | (35) | (34) | (33) | (33) | (34) | |
| SSc | < 0.1 | 0.1 ± 0.2 | < 0.1 | — | 0.2 ± 0.3 | 0.1 ± 0.2 | < 0.1 | — | 0.3 ± 0.1 | < 0.1 |
| (29) | (22) | (19) | (18) | (27) | (27) | (28) | (27) | (26) | (26) | |
| Ser | — | — | — | — | — | — | — | — | — | < 0.1 |
| (6) | (6) | (6) | (6) | (6) | (6) | (4) | (5) | (6) | (6) | |
Material of the quantitative analysis. The motor nuclei are listed from top to bottom, the stimulated nerves from left to right. Values are mean EPSPs in mV ± S.D.; numbers in parentheses are the number of cells tested. —, Ia excitation not found in any cell.
The bidirectional relations between ISp and SSp were balanced; those between SSp and SSc were strongly skewed in direction to SSp, both with respect to frequency and strength (from SSc to SSp: 93 %, mean EPSP 0.7 mV; from SSp to SSc: 27 %, mean EPSP 0.4 mV; Table 2 and Table 5). No Ia relations were present between ISp and SSc. All three muscles have different mechanical actions on the scapulo-humeral joint. Following Reighard & Jennings (1966), the humerus is rotated outwards by the ISp, protracted by the SSp (in addition the muscle may act as an abductor) and adducted and rotated inwards by the SSc. Accordingly, these muscles are antagonists in some functions, ISp and SSc in outward/inward rotation, SSp and SSc in abduction/ adduction. On the other hand, they have the common feature of acting on the capsule of the scapulo-humeral joint; the SSc and SSp by directly inserting on it, the ISp by passing over it. It is hypothesized that during shoulder movements the bidirectional Ia connections between these muscles and the Ia convergence onto them serve to guide the head of the humerus in the capsule and to keep it in the glenoid fossa.
The three motor nuclei received monosynaptic convergence from the triceps muscles (Table 2 and Table 5; Fig. 5), which was stronger in ISp and SSp than in SSc (Table 5). This projection indicates that the angular position of the elbow, as monitored by the different triceps muscles, may control the position of the humeral head in the glenoid fossa. Ia excitation will reach the two Sp heads and thus prevent gliding in a dorso-lateral direction. A second convergent system from the elbow originated from the flexors Bi, Br and ClB (Table 2). It was focused onto the SSc in the case of Bi and/or Br, and onto the SSp in the case of the ClB (Table 5). This convergence should accomplish the same stabilizing function. It would prevent a downward gliding of the humerus during the swing phase (following the downward orientation of the force vector) by acting on the SSc and SSp which both retract the bone into the fossa. In SSp neurones with Ia excitation from the elbow, Ia convergence from the flexors and extensors was regularly present in the same neurone. This is illustrated in the cell of Fig. 5 with Ia excitation from TLo and TLa in K and L, and from Bi in G. Of the 23 SSp neurones (out of 44) with Ia excitation from the elbow, eight cells were exclusively excited from one of the elbow extensors, two from one of the elbow flexors, but 13 displayed Ia excitation from both elbow systems. This double projection would allow a direct and continuous excitatory input from the elbow to stabilize the humeral head in the scapulo-humeral joint.
The unidirectional Ia convergence from Ser (Table 2 and Table 5) which was prominent and strong in the SSp motoneurones (94 %, mean EPSP 0.6 mV) is interpreted in the same way. Activation of these afferents during the stance phase, particularly during E2, could assist the SSp and ISp in preventing a dorso-lateral gliding of the humeral head in the joint capsule. The weak but consistent unidirectional Ia projection from unidentified median muscles (Table 2 and Table 5) would serve the same purpose.
The Ia relations between the members of this group and the triceps motor nuclei are complex. Whereas all motor nuclei were Ia excited from all triceps muscles, the reverse pattern was more differentiated (Table 2). The relations were unidirectional for TM and TLo, but bidirectional for TLa and An. The connection with TLa was strongly skewed in favour of TLa (mean EPSP from ISp: 0.3 mV; from SSp: 0.5 mV; from SSc: 0.4 mV; for the reverse relation see Table 5). The connections to TLa and An probably serve the control of elbow extension from the scapular muscles, since neither the double joint TLo is reached nor the multi-headed TM, but only the single joint and single-headed TLa and An.
Group 4 - Ser
No consistent bidirectional connections were found between Ser and the investigated limb or shoulder muscles (Table 2). Ser was rather characterized by unidirectional projections from its Ia afferents onto other motor nuclei. A strong unidirectional Ia projection was present to both Sp heads (Fig. 5; Table 5). It would assist Sp in stabilizing the dorso-lateral part of the capsule in conditions when Ser is passively stretched or actively contracted. In this context it is understandable that Ser Ia excitation does not reach the SSc. This latter muscle serves the same stabilizing task, but the SSc force vector will pull the humerus into dorsal direction. The unidirectional Ia excitation from Ser onto the TLa (Table 2; mean EPSP: 0.6 mV), together with the same projection from the two Sp heads, could serve the control of elbow extension during the E-epochs of swing and stance. Since we have investigated only a part of the Ser motor nucleus future research has to establish if the connectivity of the Ser motoneurones and of its Ia afferents as described here is representative for the complete nucleus.
Despite its weak strength the bidirectional relation between AcD and Ser (Table 2, mean EPSP below 0.1 mV) is interesting since it couples the trunk-scapular joint to the scapulo-humeral joint. The action of AcD is an outward rotation of the humerus, but the close relations with ClB (see below) might indicate that AcD is also involved in protraction of the humerus and elbow flexion. The bidirectional relations between AcD and Ser could be a mechanism to control and steer these humeral functions from the scapula.
Group 5 - TLo, TM, TLa and An
Fritz et al. (1989) (see also Eccles et al. 1957) have performed a systematic analysis of the Ia connections between the different triceps muscles. They found close bidirectional relations between all group members, which were skewed in the case of TLo and balanced between the others. Our material is mostly in agreement with those previous data. The few discrepancies (e.g. low number of TLo motoneurones activated from TM, TLa and An) are most probably due to the smaller sample of triceps neurones recorded in the present analysis, which was focused on the projection from the triceps Ia systems onto the shoulder nuclei and on the projection from the latter onto triceps motoneurones.
The triceps Ia system has two extensive projection fields onto the motor nuclei acting on the shoulder muscles, one reaching the flexors of the scapulo-humeral joint, the other reaching the ISp, SSp and SSc motor nuclei (Table 2). The projection to the flexors of the scapulo-humeral joint mainly originated from the double joint muscle TLo, which in itself has a flexor function on this joint (Fig. 1). The TLo projection was focused onto the TMa (73 %, mean EPSP: 0.5 mV; Table 3). It was unidirectional, and thus could reflect a mechanical synergism at the scapulo-humeral joint between TLo and the shoulder flexors. In addition it could be a functional mechanism to couple and control from the elbow the retraction in the shoulder joint during forward movement of the animal during the stance phase. The reverse and weak Ia projections emitted from the shoulder flexors onto the triceps nuclei mainly originated from TMa and reached the single joint muscles TM, TLa and An (Table 2). The material is not large enough to discuss these unidirectional relations in quantitative and functional details. In the second projection field from the elbow extensors to the ISp, SSp and SSc nuclei the excitation originated mainly from TM and TLa. It was focused on the ISp and SSp nuclei (Table 5). Activation of this pathway during the stance phase would prevent a dorso-lateral gliding of the humeral head by opposing the dorsal oriented force vector acting onto the scapulo-humeral joint. The Ia projections from the ISp, SSp and SSc muscles onto the triceps motor nuclei are specific for TLa and An (bidirectional but skewed) and have been presented in the frame of group 3.
Group 6 - Bi, Br and ClB
The elbow flexors Bi, Br and ClB had bidirectional Ia relations with each other (Fig. 5; Table 6). The relations between Bi and Br were skewed in direction to the Bi nucleus (Table 6; see also Fritz et al. 1989). The relations of the ClB motor nucleus to both these muscles were bidirectional, but strongly skewed (ClB Ia afferents evoked a mean EPSP of 0.5 mV in the Bi and Br motoneurones, ClB motoneurones received a mean Ia EPSP from Bi and Br of less than 0.1 mV). This skewedness is astonishing when considering that ClB is a mechanical agonist to Bi and Br in elbow flexion (Reighard & Jennings, 1966).
Differences exist in the Ia convergence from other muscles onto this group (Table 2). In the projection from the distal forelimb the effects in Bi and Br motoneurones from DR and Med are consistent with previous results. They should originate from the extensor carpi radialis muscle in the case of the DR, and from the pronator teres muscles in the case of the Med (Fritz et al. 1989). The ClB motor nucleus received no effect from the DR nerve. About half of the ClB motoneurones were Ia excited from the Med nerve, but it remains to be established whether this action originates from the pronator teres muscles, as it does in the case of the Bi and Br motoneurones.
The Ia excitation from the shoulder muscles was directed towards the Br and ClB motoneurones, and not to the Bi nucleus. Br received a wide convergence from both outward and inward rotators of the humerus (TMi/SpD, TMa/LD). The projections from TMi, SpD and LD were unidirectional, those of the TMa bidirectional (Table 3). The ClB motor nucleus received only a weak Ia convergence from the shoulder (SSc, Ser) except for excitation from the AcD (Table 2, see below). In the reverse direction two shoulder motor nuclei were Ia excited from the biceps group: the abductor SSp and the inward rotator SSc. These projections were unidirectional, and mostly maintained by Bi (see group 3).
Close bidirectional relations were present between ClB and AcD (Table 2). Because of the balanced nature of these relations both muscles were put together into one group (Table 6). However, the motor nuclei are clearly different in the extended Ia pattern received by the motoneurones and emitted by the respective Ia afferents (Table 2). This suggests that the bidirectional relations between AcD and ClB rather serve a local synergism in elbow flexion or outward rotation of the humerus.
Various DR motor nuclei
In this investigation 32 DR motoneurones were investigated in some detail. Based on the identification from the Ia convergence pattern (Fritz et al. 1989) six motoneurones innervated the extensor carpi radialis muscle, six the supinator muscle and three the extensor carpi ulnaris muscle. The remaining 17 DR motoneurones could not be identified further.
The three motoneurones to the extensor carpi ulnaris were devoid of any Ia excitation from the shoulder muscles. Out of the six ECR motoneurones, three received Ia excitation from the shoulder and two from the ClB. Figure 6A-H illustrates such a neurone (excitation from ClB in H) which was in addition excited from the Sp branches (D). Convergence from the shoulder was more frequent in the Sup motoneurones. Five of six motoneurones received Ia excitation. They displayed a broader convergence pattern than the one observed in the ECR motoneurones. The convergence differed between the different Sup motoneurones and many combinations were found. Two motoneurones were Ia excited from the SSc, three from Sp, two from TMi and one each from ClB and Ser. The number of recorded motoneurones is too small to detect any systematic rule, but it is interesting that no Ia excitation was present from either TMa or AcD in these six neurones. Figure 6I-P illustrates a neurone with monosynaptic excitation from TMi, SSc, An and Bi and with large disynaptic inhibition from Sp and SSc.
Figure 6. Convergence onto two different DR motoneurones.
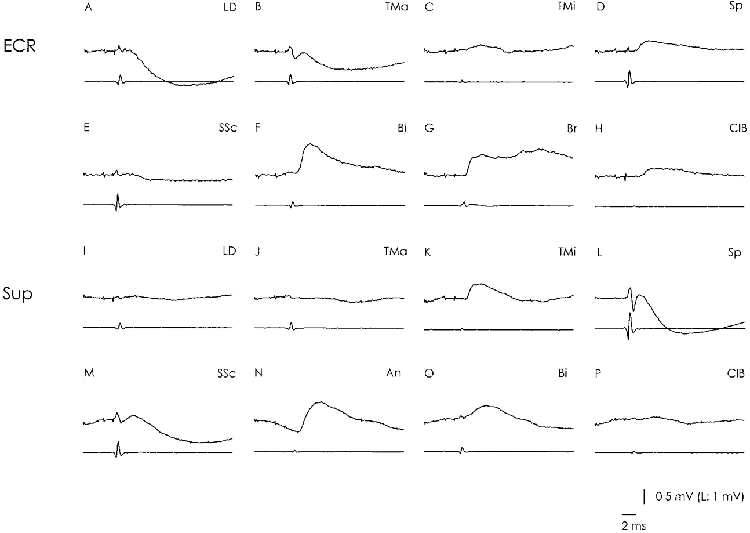
The structure of the figure is as in Fig. 4. Both motoneurones (A-H; I-P) were located in caudal C6. The cell in A-H was identified as an extensor carpi radialis motoneurone (ECR) because of the typical monosynaptic convergence from Bi and Br (F and G; Fritz et al. 1989). It received monosynaptic excitation from SP, TMi and ClB (C, D and H) and distinct disynaptic inhibition from LD, TMa and SSc (A, B and E). The cell in I-P was identified as a supinator motoneurone (Sup) because of the typical monosynaptic convergence from An and Bi (N and O; Fritz et al. 1989). It received monosynaptic excitation from TMi and SSc (K and M) and disynaptic inhibition from Sp and SSc (L and M).
DISCUSSION
A summary of the Ia connections described in this paper is presented in Table 7. It displays the main pathways from Table 2 in a reduced and schematic version.
Ia synergistic groups at the shoulder
The shoulder motor nuclei are integrated in a pattern of local Ia connections. Bidirectional relations combine motor nuclei into Ia synergistic groups, in a manner similar to that described in the distal forelimb (Fritz et al. 1989) and in the hindlimb (Eccles et al. 1957; Baldissera et al. 1981). At the shoulder three such Ia synergistic groups can be distinguished (Table 7).
In two groups (shoulder flexors; outward rotators) the bidirectional relations seem to express a mechanical synergism between the interconnected muscles. The relations are skewed, which results in graded Ia connections (e.g. from trunk to scapula or from deep to superficial muscles, Table 3). Such a connectivity is different from the pattern of balanced bidirectional relations as they are present between muscles working in the same direction onto a common joint (e.g. elbow, radio-ulnar joint; Fritz et al. 1989). The skewedness in the Ia relations within the shoulder flexor and outward rotator groups probably characterize adaptations of the Ia system to joints with changing synergistic-antagonistic relations. Comparable skewed relations are present between the extensors acting on the ball like wrist joint. There the connections are restricted to the anatomical neighbours (Fritz et al. 1989), which allows a precise coupling of muscles that are synergists in the intended movement, but an uncoupling of those which are antagonists. At the shoulder a similar detailed functional interpretation of the connections is not possible, particularly with regard to the medio-lateral and deep-superficial gradients present in these groups, since kinematic and electromygraphical investigations of the respective muscles during defined motor behaviour are missing.
The third group (ISp, SSp and SSc) shows balanced bidirectional relations. In movements of the humerus the muscles are synergists in some actions, but antagonists in others. Data correlating the kinematics of such humerus movements with activation of the muscles are not available, conclusions on the functional basis of the described Ia connections are therefore speculative. It seems, however, that any interpretation based on a mechanical synergism of the respective muscles would not meet the described Ia connectivity. Independent of their action onto the humerus all three muscles stabilize the position of the humeral head in the glenoid cavity and this very important task of securing the glenoid action might be the main common function subserved by the bidirectional Ia connections.
Monosynaptic Ia pathways within the forelimb
With the data reported in this paper the set of monosynaptic Ia connections in the forelimb is now well described. Although some pathways are not yet investigated (e.g. between scapula and trunk) and others not yet understood in sufficient detail (relations between shoulder and distal forelimb muscles), the picture is nevertheless reasonably complete. It seems that a few basic principles are used in the forelimb which have adapted to the mechanical properties of the particular joints and to the synergistic- antagonistic relationships of the muscles in motor behaviour.
Myotatic principle
This term, which was developed in the cat hindlimb describes muscles acting in the same direction onto a common joint to have bidirectional and balanced Ia relations with each other (Lloyd, 1946a, b; Eccles et al. 1957; Eccles & Lundberg, 1958). Usually the interconnected muscles are anatomical neighbours and act at hinge joints with just one degree of freedom. In the forelimb such a connectivity is present in the elbow flexors or extensor groups (Fritz et al. 1989). The connections between the muscles acting on the radio-ulnar joints are organized according to the myotatic principle as well, although they are not anatomically neighbouring muscles (supinator /abductor pollicis longus; pronator teres/pronator quadratus) and act on different joints, but with just one degree of freedom. At the shoulder, myotatic relations have been found between ISp and SSp. These muscles are anatomical neighbours and act as synergists onto a common joint. Further Ia connections following the myotatic principle have not been found, which reflects the complex situation at the shoulder joint with its ball-like construction.
Neighbouring principle
At the wrist, Fritz et al. (1989) described a set of graded Ia connections between the extensor muscles which they called the neighbouring principle. They regarded this as a modification of the myotatic principle reflecting the graded mechanical synergism between the muscles acting on a ball joint. This principle connects muscles with similar, yet slightly different mechanical actions. At the same time it disconnects muscles, which are synergists in some movements, but antagonists in others. The Ia relations between the outward rotators of the shoulder and the shoulder flexors seem to follow such a connectivity principle. In these groups the architecture is less clear than at the wrist, partly because the anatomical- mechanical relation is more complex in the shoulder than at the wrist and partly because the pattern is overlaid by additional gradients in the Ia connections.
Unidirectional Ia relations
The large number of such projections in the distal forelimb differs from their rarity in the hindlimb (Fritz et al. 1989). At the shoulder, numerous skewed or unidirectional pathways are present from elbow extensor and flexor muscles onto the SSp, ISp and SSc motor nuclei, particularly from the double joint TLo and Bi muscles. These connections most probably regulate the membrane potential in the respective shoulder motoneurones in dependency of the elbow position. A second group of Ia connections is directed proximal from the distal forelimb. It originates from unknown muscles innervated by the median and ulnar nerves and reaches both shoulder and trunk motor nuclei. Similarly to the distal-proximal elbow- shoulder relations these pathways may be viewed in the framework of controlling a proximal function from a distal mechanical event (discussion in Fritz et al. 1989). A third set of unidirectional Ia connections is directed from proximal muscles to distally located motoneurones. Such proximo- distal Ia relations are frequent in the distal forelimb, where they assist the adjustment of the position of a distal joint which is secondarily affected by contraction of a prime mover (readjustment hypothesis; Fritz et al. 1989). One of the key arguments for this hypothesis is the projection from the antagonistic flexor and extensor muscles of the elbow onto one and the same supinator motoneurone. This projection would secure the position of the radio-ulnar plane in space and make it independent from the elbow position (Caliebe et al. 1990). Similar pathways have now been found from antagonistic shoulder muscles onto the same supinator motoneurone.
Ia pathways of the shoulder and motor behaviour
It is self evident that the pattern of Ia connections is correlated with a functional meaningful synergism. For the cat hindlimb it has long been hypothesized that these connections may have evolved to assist stepping (Eccles & Lundberg, 1958). On the assumption of an α-γ-co-activation during voluntary movements this was inferred from the parallelism in certain phases of locomotion between the set of Ia pathways and the groups of co-activated muscles (Engberg & Lundberg, 1969; Lundberg, 1969). The data obtained in the hindlimb of freely locomoting cats with recordings from presumed Ia afferents demonstrate a weaker skeletomotor influence on the fusimotor signal than was hitherto assumed (e.g. Hulliger et al. 1989). Correlation of Ia firing with muscle length and tension disclosed in all cases that the afferent Ia discharge primarily signalled the time course of the changes in muscle length during the step cycle (Prochazka & Gorassini, 1998a). The contribution of linked fusimotor activity was significant in some muscles, but negligible in others (Prochazka & Gorassini, 1998b, compare triceps surae with hamstring), which seems to be correlated with the recruitment of the involved muscles in locomotion. These data show that, for an assessment of the functional contribution of Ia pathways to motor behaviour, detailed knowledge of the kinematic parameters and of the recruitment of the respective muscles during movement would be necessary. Such data are not available for the cat shoulder. Although X-ray investigations describe the kinematics of the scapula and of the various forelimb joints during locomotion and target reaching (Caliebe et al. 1990; Boczek-Funcke et al. 1996, 1998) they do not allow measurements of the time course of the length changes of the involved muscles. Qualitative EMG recordings show a strong recruitment of some shoulder muscles during walking and trot (English, 1978a, b) but do not allow a precise correlation with the angular changes between forelimb segments. In view of this incomplete database and of the general question as to what extent the above conclusions may be transferred from the hindlimb to the complex situation at the shoulder, it seems reasonable to us to first discuss the described Ia connections on the simplified assumption of an α-γ-co-activation during voluntary movements. This will have to be modified later when more differentiated EMG and kinematic data become available.
The different shoulder Ia pathways would easily fit into an extensor-flexor pattern of muscular activation found during locomotion. One set of Ia connections may support co-ordination between muscles that are active during the stance phase. The group of shoulder flexors (TMa, LD) shows bidirectional, skewed interconnections with the elbow extensors TLa, TM and An. Both muscle groups are active during the E2/E3 phases; the bidirectional connections could thus assist propulsion of the animal and extension against gravity (English, 1978b). The unilateral projection from TLo onto the shoulder flexors, particularly onto the LD, could work in a similar way. Biomechanical considerations and EMG data suggest that in early stance LD plays an important role for stabilization of the muscular girdle between body and forelimb, thus antagonizing the body weight and pulling the body through the shoulder girdle (English, 1978b). Since elbow flexion during E2 will stretch TLo and probably activate discharges in its Ia afferents, the unilateral Ia projection from TLo onto the shoulder flexors would increase the activity of the latter muscles and thus oppose the yield caused by the body weight. The connections from the elbow extensors to the SSp, ISp and SSc muscles would stabilize the humeral head in the glenoid cavity during stance. This interpretation is supported by the kinematic data that the force vector produced by the body load is opposite to the force vector of the spinatus muscles (Boczek-Funcke et al. 1996). The Ia relations from the SSp, ISp and SSc muscles to the elbow extensors would thus adjust the elbow joint during the stance phase in dependence of the angular situation at the humero-scapular complex.
The second major set of Ia connections at the shoulder may assist initiation of the extensor activity at the end of the swing phase and during the E1 phase to prepare the joints for loading with the body weight. During E1 the humerus is abducted (Boczek-Funcke et al. 1996). In an α-γ-co-activated condition the ensuing stretch of the adductor SSp will probably activate the Ia afferents of this muscle to the elbow extensors. This projection would support or initiate the elbow extension that begins around 40 ms after abduction and precedes ground contact (Boczek-Funcke et al. 1996). EMG data indicate that during E1 the elbow extensors are activated earlier than LD (English 1978a), which might control the timing of the LD activity. A comparable interpretation could explain the unilateral projection from TMa onto the other elbow extensors. During E1 TMa is activated earlier than TLa, TM, An, and thus could control the timing of the activation of the single joint elbow extensor muscles (English, 1978a, b).
The Ia projections from the shoulder to the elbow flexors are simpler. All shoulder flexors project to Br, reflecting the need to activate this muscle before the beginning of the flexion phase. The slowly increasing activity of the shoulder flexors in E3 (English, 1978a) will activate Br motoneurones during the late stance phase and thus initiate the flexion movement of the elbow during the swing phase.
The connections at the shoulder fit into a system of Ia pathways along the multijoint forelimb which assist organization of the flexor and extensor phases. Figure 7 gives a very schematic diagram which is based on a simplified version of the present data on the shoulder and those of Fritz et al. (1989) on the distal forelimb. The figure shows the elbow extensors in the centre of the locomotion related Ia connections. The connections sent and received by them occur with high frequency and considerable strength and include a large number of nuclei, from very distal to very proximal, some even located at the trunk. The signals originating from these muscles during the stance phases are distributed to the other extensor motor nuclei throughout the whole limb. A distal group of muscles acts at the wrist and digit joints. The palmar flexors are interconnected with each other, with a strong excitatory convergence onto the extensor carpi ulnaris muscle (ECU). Their signal is additionally fed to the triceps group. This group builds the connections to the Ia synergistic groups at the shoulder, which subserve the functions of shoulder flexion, propulsion, stance against gravity and stabilization of the humero-scapular joint.
Figure 7. Schematic overview of the Ia relations in the forelimb possibly related to locomotion.
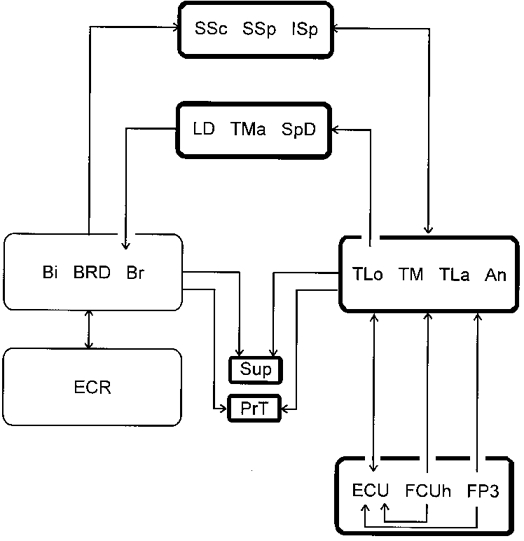
The connections are extracted from Tables 3–6 of the present paper and from Figs 6 and 7 of Fritz et al. (1989). The boxes comprise groups of motor nuclei or single motor nuclei. The lines and arrows indicate the connections; lines from or onto one box indicate a common projection or convergence, lines from or onto single motor nuclei indicate a specialized projection or convergence. The thin lines around ECR, Bi, Br and BRD nuclei indicate that the respective connections are weaker than those between the other motor nuclei. For a functional interpretation see Discussion in the present paper and in Fritz et al. (1989). Note that on the flexor side a projection comparable to that between elbow extensors and palmar extensors is not present.
The pattern on the flexor side is simpler, the connections weaker and the number of participating motor nuclei smaller. It is further remarkable that the few relationships present between elbow flexors and shoulder muscles are unidirectional. The set of connections from the shoulder flexors to the brachio-radialis muscle (BRD) may initiate the swing phase. A second set, mainly from Bi onto SSp and SSc, has been discussed to subserve stabilization of the humero-scapular joint. The apparent lack of connections from elbow flexors to shoulder muscles would indicate that the information about the angular position of the elbow for the control of the ongoing activation of the shoulder muscles is primarily taken from the elbow extensors.
The unidirectional projection from the elbow extensors and flexors onto the supinator and pronator motoneurones has been discussed to serve the stabilization of the radio-ulnar plane during locomotion (see Illert, 1996). The present study has now demonstrated a comparable projection from the shoulder muscles onto the supinator motoneurones. This would be reasonable since the changing force vector during the stance phase of locomotion might perturb or destabilize the radio-ulnar position of the limb. The details of this projection remain to be established, as well as their connections to the pronator muscles.
The Ia connections of the forelimb must also be considered in relation to target reaching (e.g. Gorska & Sybirska, 1980). The set of connections specialized for paw and digit movements during target reaching and object manipulation comprises primarily the DR innervated extensors of the paw and digits and the pronator and supinator muscles, together with the intrinsic paw muscles (Fritz et al. 1989). These muscles show specific EMG patterns before and during object taking (Hoffmann et al. 1986; Illert, 1996). Lacking a recurrent Renshaw system they have additional specific properties which separate them from the other forelimb muscles (Hörner et al. 1991; Illert et al. 1996). EMG data from proximal muscles during target reaching and object manipulation would be needed to extract what Ia relations at the shoulder may be specific for this motor behaviour. It may be doubtful if there are any, since the task of holding the limb in space is a more general one which applies to other kinds of motor behaviour as well. On the other hand, the kinematic studies on the shoulder joint, the movement of the scapula and the kinematics of the humerus during locomotion and target-reaching tasks show distinct qualitative differences between these behaviours (Boczek-Funcke et al. 1995, 1996, 1998), which must find their expression in the activation patterns of the relevant muscles and also in the Ia connectivity of their motor nuclei.
Acknowledgments
Ms Marion Wendisch provided excellent technical assistance. We thank Dr D. Wietelmann for participation in preliminary experiments and Drs Boczek-Funcke and Kuhtz-Buschbeck for allowing us to cite from unpublished material. The study was supported by the Deutsche Forschungsgemeinschaft.
References
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems, chapter 12. In: Brooks VB, editor. Handbook of Physiology, section 2, The Nervous System, Motor Control. Vol. 1. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. part. 1. [Google Scholar]
- Boczek-Funcke A. Röntgenkinematische Untersuchungen zur motorischen Steuerung von Ziel- und Greifbewegungen bei der Katze. Habilitationsschrift, Medizinische Fakultät der Christian-Albrechts-Universität zu Kiel. 1997:1–91. [Google Scholar]
- Boczek-Funcke A, Illert M, Kuhtz-Buschbeck J. Kinematic analysis of the shoulder girdle during treadmill locomotion: an X-ray study. European Journal of Neuroscience. 1996;8:261–272. doi: 10.1111/j.1460-9568.1996.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A, Kuhtz-Buschbeck J, Illert M. X-ray kinematic analysis of the shoulder girdle during target-reaching and food-taking in the cat. Pflügers Archiv. 1995;429(suppl. 6):R38. [Google Scholar]
- Boczek-Funcke A, Kuhtz-Buschbeck JP, Raethjen J, Paschmeyer B, Illert M. Shaping of the cat paw for food taking and object manipulation: an X-ray analysis. European Journal of Neuroscience. 1998;10:3885–3897. doi: 10.1046/j.1460-9568.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. Intracellular recording from antidromically activated motoneurones. The Journal of Physiology. 1953;122:429–462. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe F, Häußler J, Hoffmann P, Illert M, Schirrmacher J, Wiedemann E. Cat distal forelimb joints and locomotion: an X-ray study. European Journal of Neuroscience. 1990;3:18–31. doi: 10.1111/j.1460-9568.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Crouch JE. Text-Atlas of Cat Anatomy. Philadelphia, PA, USA: Lea & Febiger; 1969. [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents onto many different species of alpha motoneurones. The Journal of Physiology. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. The Journal of Physiology. 1958;144:271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiologica Scandinavica. 1969;75:614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- English AWM. An electromyographic analysis of forelimb muscles during overground stepping in the cat. Journal of Experimental Biology. 1978a;76:105–122. doi: 10.1242/jeb.76.1.105. [DOI] [PubMed] [Google Scholar]
- English AWM. Functional analysis of the shoulder girdle of cats during locomotion. Journal of Morphology. 1978b;156:279–292. doi: 10.1002/jmor.1051560209. [DOI] [PubMed] [Google Scholar]
- Fritz N, Illert M, de la Motte S, Reeh P, Saggau P. Pattern of monosynaptic Ia connections in the cat forelimb. The Journal of Physiology. 1989;419:321–351. doi: 10.1113/jphysiol.1989.sp017875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz N, Illert M, Reeh P. Location of motoneurones projecting to the cat distal forelimb. II. Median and ulnar motonuclei. Journal of Comparative Neurology. 1986a;244:302–312. doi: 10.1002/cne.902440304. [DOI] [PubMed] [Google Scholar]
- Fritz N, Illert M, Saggau P. Location of motoneurones projecting to the cat distal forelimb. I. Deep radial motonuclei. Journal of Comparative Neurology. 1986b;244:286–301. doi: 10.1002/cne.902440303. [DOI] [PubMed] [Google Scholar]
- Gorska T, Sybirska E. Effects of pyramidal lesions on forelimb movements in the cat. Acta Neurobiologica Experimentalis. 1980;40:843–859. [PubMed] [Google Scholar]
- Hoffman P, Illert M, Wiedemann E. EMG pattern of cat forelimb muscles during target reaching and food taking movements. Neuroscience Letters. 1986;26:S215. [Google Scholar]
- Hohn A, Illert M, Jänike R, Wietelmann D. Ia pathways interconnecting forelimb and shoulder muscles in the cat. Pflügers Archiv. 1993;422(suppl. 1):R33. [Google Scholar]
- Hongo T, Lundberg A, Phillips CG, Thompson RF. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proceedings of the Royal Society B. 1984;221:261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Hörner M, Illert M, Kümmel H. Absence of recurrent axon collaterals in motoneurones to the extrinsic digit extensor muscles of the cat forelimb. Neuroscience Letters. 1991;122:183–186. doi: 10.1016/0304-3940(91)90853-l. [DOI] [PubMed] [Google Scholar]
- Hörner M, Kümmel H. Topographical representation of shoulder motor nuclei in the cat spinal cord as revealed by retrograde fluorochrome tracers. Journal of Comparative Neurology. 1993;335:309–319. doi: 10.1002/cne.903350302. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Dürmüller N, Prochazka A, Trend P. Flexible fusimotor control of muscle spindle feedback during a variety of natural movements. In: Allum JH, Hulliger M, editors. Afferent Control of Posture and Locomotion, Progress in Brain Research. Vol. 80. Amsterdam: Elsevier; 1989. pp. 87–101. [DOI] [PubMed] [Google Scholar]
- Illert M. Monosynaptic Ia pathways and motor behaviour of the cat distal forelimb. Acta Neurobiologica Experimentalis. 1996;56:423–433. doi: 10.55782/ane-1996-1145. [DOI] [PubMed] [Google Scholar]
- Illert M, Kümmel H, Scott JJA. Beta innervation and recurrent inhibition: a hypothesis for manipulatory and postural control. Pflügers Archiv. 1996;432:R61–67. [PubMed] [Google Scholar]
- Körner G. Untersuchungen über Zahl, Anordnung und Länge der Muskelspindeln in einigen Schulter- und Oberarmmuskeln und im Muskulus sternalis des Menschen. Anatomischer Anzeiger. 1960;108:99–103. [Google Scholar]
- Lloyd DPC. Facilitation and inhibition of spinal motoneurons. Journal of Neurophysiology. 1946a;9:421–438. doi: 10.1152/jn.1946.9.6.421. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC. Integrative pattern of excitation and inhibition in two neuron reflex arcs. Journal of Neurophysiology. 1946b;9:439–444. doi: 10.1152/jn.1946.9.6.439. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Reflex Control of Stepping. Oslo: The Nansen Memorial Lecture V. Universitetsforlaget; 1969. [Google Scholar]
- Prochazka A, Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. The Journal of Physiology. 1998a;507:277–291. doi: 10.1111/j.1469-7793.1998.277bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. The Journal of Physiology. 1998b;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reighard J, Jennings HS. Anatomy of the Cat. New York: Holt, Rinehart & Winston; 1966. [Google Scholar]
- Soechting JF, Flanders M. Deducing central algorithms of arm movement control from kinematics. In: Humphrey DR, Freund H-J, editors. Motor Control: Concepts and Issues. Chichester, UK: John Wiley & Sons; 1991. pp. 293–306. [Google Scholar]


