Abstract
To help clarify the use of measurements of ‘excitability’, a simple model motoneurone receiving noisy tonic background excitation was tested with brief stimuli. Its response was determined from its PSTH (post-stimulus time histogram). The tonic background was varied from well below to well above the threshold for tonic firing. The conclusions should apply to many other neurones.
The response of the model to a stimulus depended upon a number of factors, including stimulus strength, synaptic membrane noise and especially whether or not the background drive elicited tonic firing. With the onset of firing, the shape of the stimulus-response curve changed drastically and the model then responded to the smallest stimulus without a threshold. When the drive was subthreshold, increasing the background excitation always increased the response to a given stimulus. However, what happened when the tonic drive exceeded the threshold for tonic firing depended upon the stimulus strength. With weak stimuli, the response increased with the drive to reach a plateau level where it was independent of the background firing rate; this occurred for stimuli comparable in size to the synaptic noise. With stronger stimuli, the response rose to a maximum for very low firing rates, but then decreased by up to 50 % to a plateau for high firing rates. Increasing the membrane noise reduced or abolished the maximum.
The model was also used to simulate a monosynaptic conditioning-testing paradigm. The effect of a given conditioning stimulus was then found to change with the onset of firing, including when the strength of the testing stimulus was adjusted to make the size of the test response the same in the presence and absence of firing.
The behaviour of real motoneurones can be expected to be at least as complex with the transition from silence to firing, so H reflex and other tests of ‘excitability’ must then be treated with caution. In particular, as has been observed experimentally, the response of a unit may decrease with increasing background excitation, as well as with inhibition.
Transferring the findings to corticospinal neurones makes it unlikely that the magnitude of the descending volley elicited by a given cortical stimulus (‘excitability’) will always increase with the initial level of cortical activity. In addition, the appreciable threshold for transcranial magnetic stimulation during voluntary contraction suggests that it first excites axons rather than the neural pacemakers.
The testing of the ‘excitability’ of human motoneurones by recording the surface EMG of a tendon jerk or H reflex remains invaluable. However, as most recently emphasized by Capaday (1997), the quantitative interpretation of the data is beset with pitfalls and the simple notion that the percentage increase in the response to a given stimulus provides a comprehensive linear measure of ‘excitability’ is long dead. The situation can be improved, with increased experimental effort, by studying single motor units; this removes the complications due to measuring a population response dependent upon the scatter in threshold etc. of its individual members. An excitatory effect then shows itself as an increased probability of firing in the post-stimulus time histogram (PSTH) of the studied unit, but the findings still need to be interpreted with care. The classical monosynaptic testing of excitability changes was developed for quiescent motoneurones (MNs) in anaesthetized animals, without initial recognition of the complications that arise on applying it to MNs that are physiologically active and already firing. The latter has certain advantages for routine testing (Burke et al. 1989), and occurs inevitably on studying overt motor action.
The introduction of transcranial stimulation of the motor cortex in man has provided a new impetus to the challenge of reliably interpreting such EMG responses, elicited by a brief excitatory input. First, the cortical response is normally viewed via its action on the motoneurones and their contribution has to be assessed before understanding can be achieved. Second, the background thinking underlying the analysis of ‘cortical excitability’ can gain much from studies on the motoneurone, which provides a useful example of a neurone whose behaviour can be analysed under a wide range of conditions. As with motoneurones, ‘cortical excitability’ tends to be conceptualized in terms of the overall magnitude of the output volley evoked by a fixed test stimulus. It then often seems to be assumed that an increase in ‘excitability’ should occur whenever the level of cortical activity increases with increased synaptic activation, as in the explicit statement that ‘the pyramidal neurones are more active and therefore more excitable’ (Datta et al. 1989).
The present paper aims to stimulate debate on how best to measure excitability, especially among experimentalists, by illustrating the response of a model motoneurone to a constant stimulus as the background synaptic activation is increased, starting well below threshold for firing. The model follows a long tradition of motoneurone modelling by restricting itself to certain key features of the MN, rather than attempting to incorporate everything that is currently known about its precise structure and numerous conductances (see references in Binder et al. 1996; Matthews, 1996). In this sense, it is a fairly generalized neuronal model and so potentially applicable also to corticospinal neurones. However, it remains primarily a motoneurone with an appropriate firing rate, recovery period and interval variability by virtue of choosing its various numerical values in relation to those known for spinal MNs (Binder et al. 1996; Matthews, 1996). It has an exponentially decaying after-hyperpolarization (AHP) conductance, so that the slope of the effective part of its membrane voltage ‘trajectory’ increases with the firing rate, and it mirrors the human situation by containing appreciable synaptic noise (Matthews, 1996). Its deliberate simplicity both helps conceptual clarity and allows a variety of conditions to be examined systematically without excessive computation. Using a more realistic compartmental model, requiring prolonged computation, Jones & Bawa (1997) found a reduction in response with increased firing rate for the few conditions studied.
In man, the balance of evidence argues that voluntarily increasing the background synaptic input and firing rate of the MN tends to decrease the response to a constant testing I a volley, and no-one has described an increase (Ashby & Zilm, 1982; Kudina, 1988; Miles et al. 1989; Piotrkiewicz et al. 1992; Nordstrom et al. 1992; Jones & Bawa, 1995). However, the matter remains controversial as the magnitude of the effect seems to be highly variable with some holding that it is normally absent or insignificant. This uncertainty over the effect of firing rate on ‘excitability’ made further modelling desirable to test whether the differences might depend simply upon minor variants in the experimental conditions. Piotrkiewicz et al. (1992), for example, considered that the reduction in ‘excitability’ only occurs when the initial firing is low. The main issue is how far response magnitude can be taken to provide a unique one-to-one measure of ‘activation’. Failure to do so questions the basis of the simplest concepts of ‘excitability’, including corticospinal neurones stimulated transcranially.
METHODS
A non-compartmental threshold-crossing model was employed for the simulations corresponding to a motoneurone lacking dendrites and with its pacemaker located on the soma. It had a single exponentially decaying conductance responsible for the post-spike AHP controlling its firing. Its various other conductances were independent of both voltage and time, and the threshold voltage for spike initiation was the same throughout the interspike interval. It had about 0.5 mV of synaptic noise (s.d.), and was tested with stimuli scaled in arbitrary units with a unit stimulus producing an EPSP of 0.3 mV. The model was run with a time step of 1 ms as the aim was simply to determine the overall size of the response, rather than to relate its time course to that of the underlying EPSP. Its firing behaviour approximated to that of a particular human MN studied previously (the biceps brachii MN of Figs 7–12, Matthews, 1996).
Figure 7. Modelling the uncertainties in the measurement of a ‘conditioning’ excitatory input by its facilitation of a testing ‘monosynaptic’ response.
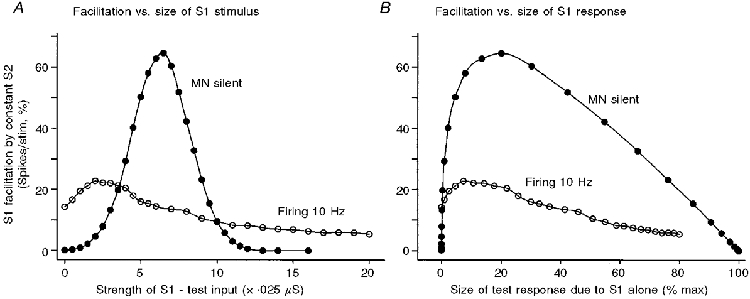
The S1 input, whose size was varied, simulated a test I a volley that produced a ‘monosynaptic’ response which was used to probe the facilitatory ‘excitation’ produced by a fixed ‘conditioning’ S2 input. The measure of facilitation used was the absolute increase in the combined ‘monosynaptic’ response to S1 + S2 above its value with S1 alone (see Methods). In A, the abscissa gives the size of the testing S1 stimulus, while in B it gives the size of the resultant testing ‘monosynaptic’ response. The transition from silence to firing can be seen to alter the amount of facilitation produced by S2 irrespective of whether S1 was kept constant or was adjusted to maintain a fixed unconditioned ‘monosynaptic test reflex’. The inputs were actually MN conductance changes. The outputs were the increase in the ‘firing index’ of the MN produced by the stimuli (i.e. the increase in the probability of a spike being discharged in response to the particular stimulus, above the value without the stimulus, expressed as a %). For both plots, the ordinate gives the amount of facilitation of the S1 response produced by a constant conditioning S2 stimulus (strength = 3 units or 0.075 μs), eliciting an ‘EPSP’ of 0.9 mV. •, MN initially silent as background drive insufficient for even large noise peaks to reach the firing threshold; ^, MN firing at 10 Hz after increasing the background excitatory drive (derived from data of Fig. 3).
Details of model
The resting potential was set by the leak conductance (0.5 μS) of the membrane and for convenience was given the value of 0 mV. The capacitance of the membrane (4 nF) gave it a resting time constant of 8 ms, which was then approximately halved by tonic synaptic activity (see below). The test excitation, corresponding to a I a testing volley, was provided by a 1 ms pulse of conductance with an equilibrium potential of +70 mV (relative to resting), producing a brief ‘depolarization’ (EPSP) of up to 10 mV with the largest stimuli. A spike was triggered whenever the EPSP exceeded +15 mV and was followed by an AHP due to a single ‘potassium’ conductance (initial value, 0.4 μS; equilibrium potential, -15 mV) which then decayed exponentially (time constant, 30 ms); the residual conductance from the preceding spike was wiped clean to eliminate any serial correlations in the interspike interval distribution (no material difference was found when it was preserved and summed with its successor). Immediately after a spike the membrane potential was reset to the arbitrary value of -10 mV. The precise value affected the shape of the earliest part of the AHP, but this is immaterial since, with the stimuli used, spikes were only generated well into the recovery process when the membrane potential depended simply upon the conductances. Algebra shows that the terminal part of the AHP in the region of threshold will have been closely exponential, but the membrane capacitance ensured that the voltage at any time lagged slightly behind the equilibrium value for the actual conductances. It will still have remained exponential at higher firing rates, but as the trajectory equilibrium then lies beyond threshold the segment involved in excitation is then relatively short, making its curvature less obvious (Fig. 11 of Matthews, 1996). The family of trajectories will have resembled those illustrated by Baldissera & Gustafsson (1974, their Fig. 2) who used a related conductance model; these departed from an exponential when well away from threshold in much the same way as do those of real MNs.
Figure 2. Mode of analysis of input-output relation to simulate monosynaptic testing of excitability with simultaneous stimuli.
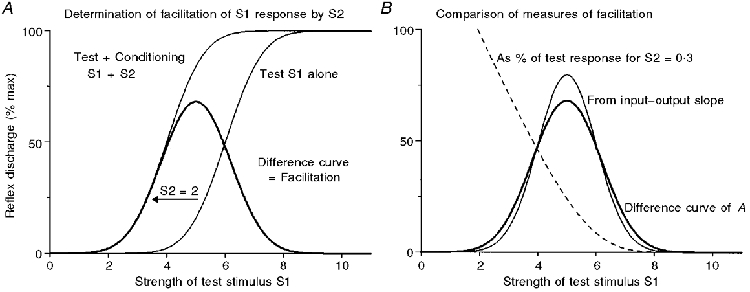
A, the facilitation produced by a fixed conditioning stimulus (S2 = 2, thick line) was obtained by shifting the input-output curve (thin) for the test stimulus S1 along the X-axis by 2 units and subtracting the old curve from the new (see text). The apparent ‘excitability’, as given by the amount of S2-induced facilitation, varies drastically with the strength of S1. B compares this excitability plot (thick line) with that obtained by scaling up the small-signal value given by the slope of the input-output curve (thin line); this shows that the exact shape of the relation between the amount of facilitation and the strength of the test stimulus S1 depends upon the strength of S2. The dashed line in B shows the quite different curve obtained on expressing the facilitation produced by S2 as a fraction of the S1 response; a much smaller S2 was used (S2 = 0.3) to make the scaling similar, but this does not change its shape (this and the slope plot were calculated from the input-output curve centred on S1 = 5). The S1 curve in A is a Gaussian integral and approximates to that for the silent MN in Fig. 3, except for the X-scaling. (The present abscissa is scaled in units of the underlying s.d., whereas that in Fig. 3 corresponds to arbitrary stimulus units, when the s.d. happened to be approximately 1.5.)
The model MN was bombarded by a mixture of tonic synaptic excitation and inhibition producing separate conductance changes (equilibrium values, +70 and -15 mV). Each conductance had a mean value with superimposed Gaussian noise (inhibition, always 0.2 ± 0.02 μS (mean ±s.d.); excitation varied either side of 0.25 ± 0.025 μS which elicited firing at 12 Hz). It is known that the sum of a large number of small independent synaptic inputs can be represented in this way (Kirkwood & Sears, 1991). Different firing rates and subthreshold excitation were produced by varying the excitatory conductance, with the excitatory noise normally varied in proportion to the square root of the excitatory conductance (Fig. 5 shows the very similar results obtained with a constant noise level). This gave a voltage noise, measured at threshold, with an s.d. of around 0.55 mV (no AHP conductance, membrane potential held at threshold by inactivating the spiking and injecting a steady current to counteract the net synaptic current). The stimulus strength in the various plots is scaled in units of 0.025 μS; a unit stimulus elicited an ‘EPSP’ of 0.305 mV. This value, which is the one cited in the main text, was measured at threshold in the absence of any residual AHP or noise for an excitatory conductance of 0.25 μS; under other conditions its exact value varied very slightly, depending upon the sum of the various conductances involved.
Figure 5. Increasing the synaptic noise acts somewhat like making the stimulus smaller.
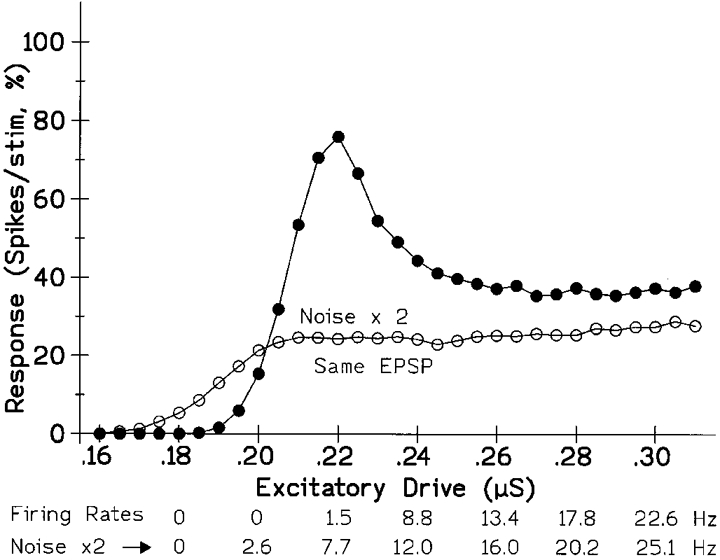
As in Fig. 4, the plots show the variation in the size of the response elicited by a fixed stimulus as the background excitation was altered. However, the absolute size of the stimulus was now the same for both curves (stimulation = 7 units; EPSP = 2 mV), while the noise conductances were doubled for the lower one (voltage noise s.d. approx. 0.55 and 1.1 mV). Thus the shape of the curves, and notably the occurrence of a maximum, depends upon the size of the stimulus relative to the noise rather than upon its absolute magnitude. Increasing the noise also increased the firing rate elicited by a given tonic excitation, as indicated below. (Excitatory and inhibitory noise conductances increased from 0.025 and 0.02 μS to 0.05 and 0.04 μS. As a simplification, for this figure, the conductance noise was kept the same for different levels of drive, rather than increasing as its square root; the voltage noise now decreased slightly with increasing conductance drive - from 0.573 to 0.525 mV for the peaked curve.)
The tonic behaviour of a model MN with noise is quite different from that of simpler models in which the trajectory simply rises smoothly until it reaches threshold; in contrast, when a noisy model is firing at low frequency most if not all of the spikes are elicited by noise transients before the mean voltage trajectory reaches threshold as also appears to be so for human MNs (Matthews, 1996). The inclusion of noise thus stops a model's discharge from being entirely regular, thereby matching noisy synaptic activation as in life; like a simpler model (Matthews, 1996), the present model's discharge variability decreased with firing rate in the same way as that of real MNs. The various parameters of the present model led it to behave like a carefully studied human biceps brachii MN, the estimated duration of whose AHP was close to that used in the model (Matthews, 1996, time constant 29 ms vs. the present 30 ms); for any given mean firing rate the real and the simulated interval histograms had a similar shape, with comparable coefficients of variation. Introducing noise also affects the shape of the standard f-I plot (firing rate vs. injected current) and smooths the otherwise abrupt upwards step to the minimum firing rate thereby permitting firing, albeit highly irregular, at lower frequencies than in its absence, as in a related model (Stein, 1967). This is illustrated in Fig. 1, which also helps validate the model by confirming that in the absence of noise the model's firing range and f-I slope of 4.4 I s−1 nA−1 falls within the range seen for noise-free cat MNs (Binder et al. 1996). As for real MNs the noise always increases the firing rate for a given level of drive (Poliakov et al. 1996).
Figure 1. Effect of noise on the form of the model's f-I plot of firing rate vs. injected stimulating current.
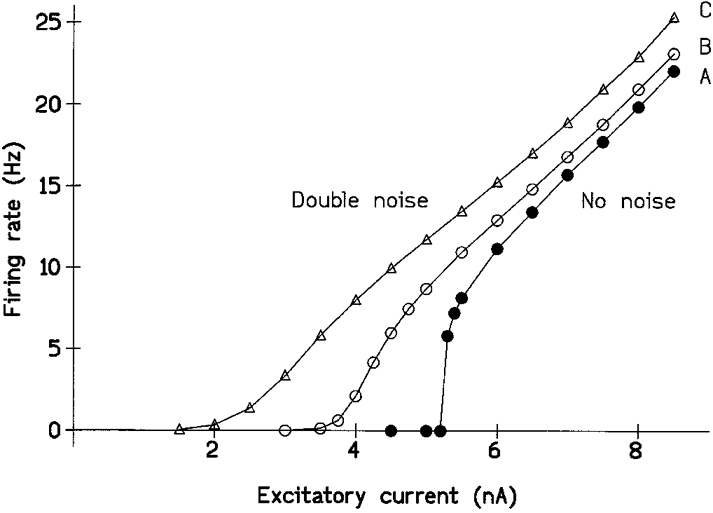
The conductance drive to the model was reduced to well below threshold and maintained firing was then induced by the ‘intracellular’ injection of a constant current. A, in the absence of noise; B, with the standard noise used in the modelling; C, with the noise doubled. Abscissa, the stimulating current. Ordinate, the resulting mean maintained firing rate. Noise increased the firing rate elicited by any given amount of current, dramatically so for currents that were otherwise subthreshold, and the model MN no longer showed the abrupt commencement of firing at the ‘minimum firing rate’ of 8 Hz seen in the absence of noise. The firing at low frequencies was notably irregular; at a firing rate of 2 Hz the s.d. of the interspike interval distribution was ±346 ms for the standard noise. Above the minimum firing rate, the noise had no appreciable effect on the slope of the f-I plot which was 4.4 I s−1 nA−1. (Noise in B and C as in Fig. 5. For B, background excitatory conductance drive was 0.15 ± 0.025 μS, inhibitory conductance 0.2 ± 0.02 μS; for C, mean conductances as in B, but both noise conductances doubled; membrane voltage noise s.d.s were 0.55 and 1.1 mV. A had the same mean conductances but over a thousand times less noise.)
Both the amplitude and the temporal distribution of the noise determine the firing rate with a given mean drive. In the present model, the amplitude distribution of the voltage noise was Gaussian (but with its amplitude varying slightly during the course of the AHP). Its temporal structure was a simple exponential decay and was determined principally by the capacitance smoothing inherent in converting the conductance transients into voltage changes (4 ms time constant (TC)). However, further smoothing occurred as a by-product of using the finite step of 1 ms to perform the calculations, since this cuts off high frequencies. In a related model, this had much the same effect as increasing the smoothing membrane time constant from 4 to 5 ms (Matthews, 1996). If an appreciably smaller computation interval were to be used then analogous filtering would have to be deliberately introduced to allow for the finite duration of the synaptic conductances which again limits the high-frequency noise. The membrane potential was updated for each successive 1 ms bin by integrating the rate of change of voltage with time, as described by MacGregor (1987).
Determination of response
The stimulus interval normally lay between 300 and 400 ms, the precise value varying randomly for each successive stimulus; thus the stimulus occurred largely at random in relation to the time of the immediately preceding spike. However, for strong stimuli given at high initial firing rates (when inter-spike variability is low) it emerged that the residual lack of randomness was producing effects just above the level of statistical significance; these were due to residual peaks in the model's autocorrelogram interacting with the patterning of the stimulus (cf. Matthews, 1997). The computation was then repeated using stimulus intervals of 600-800 ms. Each condition was run for just under 30 min of simulated time, entailing the delivery of some 5000 stimuli (2500 for the longer stimulus interval). The response of the model to the stimuli was estimated from standard post-stimulus time histograms (PSTHs) and accompanying cusums. The baseline firing rate was sometimes determined by counting the spikes in the 30 ms preceding the stimulus, and sometimes from 1 min of simulated firing without stimulation; the results agreed. The response was defined as the number of additional spikes elicited by the stimulus, over and above the baseline level. This was done for the 1 ms bin containing the stimulus, which is where any evoked excitation normally occurred followed by a period of absolute or relative silence; this approximates to the standard practice of summing the area of a PSTH based on a smaller bin width (Jones & Bawa, 1997). During firing the maximum possible response was thus very slightly less than 100 %, even though a spike occurred immediately after every stimulus, because the background was subtracted. This had an insignificant effect on the form of the curves illustrated (maximum reduction = 2.5 %, for a firing rate of 25 Hz). The error bars in Fig. 3 were obtained by computing the results in 10 separate sub-sets and taking their mean. They seemed too small to be worth including in the other figures.
Figure 3. The shape of the stimulus-response curve changes when the model MN starts firing.
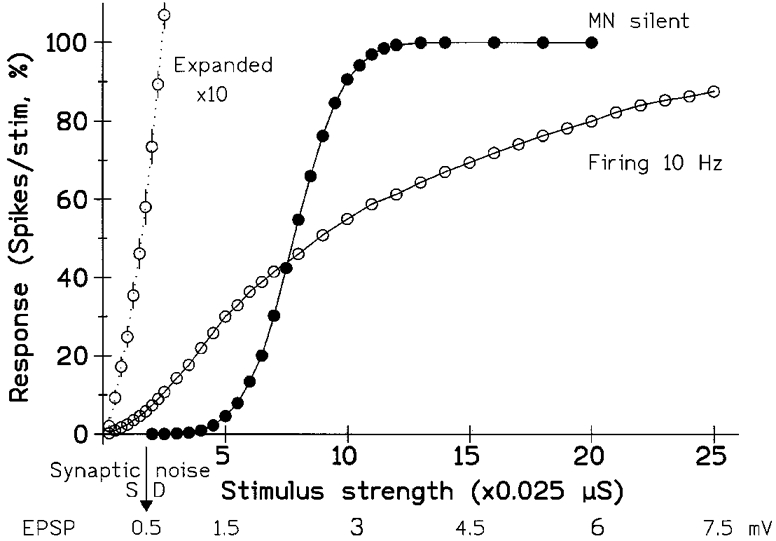
The stimulus consisted of a pulse of increased excitatory conductance, mimicking a synaptic input. The abscissa shows both the conductance change and the peak size of the resulting depolarization (approx. linearly related over range studied). The ordinate gives the response of the unit, determined from the PSTH; it shows the number of extra spikes, above the resting level, produced by the stimulus and is expressed as the percentage probability of the unit responding to the stimulus (i.e. the increase in its ‘firing index’). •, tonic synaptic input insufficient to produce background firing (maximum response then 100). ^, background firing at 10.0 Hz (the super-added response would now saturate at 99, since the unit had a 1 % probability of firing spontaneously in the 1 ms bin tested). The initial part of the 10 Hz curve is also plotted with a 10-fold expansion of the vertical scaling (20 % on scale = 2 %); the standard error bars are now visible (otherwise smaller than points for both plots). The arrow (bottom) indicates the s.d., in millivolts, of the synaptic noise. With the MN firing and using the present number of stimuli (approx. 5000) responses down to 0.5 % were significant; this minimal response was elicited by an EPSP of 0.1 mV or 20 % of the noise. In contrast, the quiescent MN required an EPSP of over 1 mV to give a similar minimal response of 0.5 %. However, with large EPSPs/stimuli the transition from silence to firing reduced the response of the MN. (Background excitatory conductances, 0.205 and 0.245 μS giving noise s.d.s of 0.52 and 0.54 mV with fixed inhibition of 0.2 ± 0.02 μS; noise and EPSP voltages measured with mean membrane potential held at threshold by injecting steady current and inactivating spiking.)
The analysis ignored a minor, quantitatively unimportant, complication so as to simplify understanding of the various comparisons. Because of the membrane noise, the response to the weakest stimuli might be spread over more than a single 1 ms bin; this occurred when a subsequent noise pulse triggered a spike with the help of the decayed EPSP when it would not otherwise have done so. Measuring the response at the peak of the cusum slightly increased its value for the weakest stimuli, but this made no difference to the general pattern of results and otherwise complicated matters. The timing of the cusum peak for small stimuli is subject to statistical variation, and the errors increase and become hard to estimate if the number of bins used varies from trial to trial. If the measurements are routinely made over several bins for all stimuli then most responses are artefactually reduced; the background firing in the extra bins must now be subtracted, including when they make no contribution to the response.
Interaction of conditioning and testing monosynaptic responses
Figure 2 illustrates graphically the way in which the input-output curves of Fig. 3 were used to produce the plots of Fig. 7 describing how the effects of two simultaneous stimuli interact in a monosynaptic conditioning-testing paradigm simply as a result of the non-linearities in the input-output relation, ignoring all potential biophysical complexities. Similar arguments are readily applicable when the test stimulus follows the conditioning stimulus. The inputs are conductance changes which then sum linearly and the output is expressed in spikes (firing index), not EPSP amplitude (membrane depolarization). The matter requiring examination is the way in which changing the size of the test stimulus S1 affects the amount of facilitation produced by a constant conditioning stimulus S2. For a given S1 the facilitation produced by a subthreshold S2 is obtained by subtracting the response to S1 alone from that for S1 + S2. The sigmoid plot on the right of Fig. 2A shows the input-output relation for S1. The sigmoid on the left is the same curve shifted by 2 units, and corresponds to the relation for the two stimuli combined. The thick curve gives their difference, measured in the Y direction, and relates the non-linear facilitation of the S1 response to the strength of the S1 stimulus. When the conditioning stimuli (S2) are weak, simple algebra shows that the facilitation is directly proportional to the slope (differential) of the input-output curve for S1 at each value of S1 (cf. Capaday, 1997, his Fig. 8). Figure 2B compares this small-signal estimate (thin, more highly peaked) with that actually found with the large stimulus (thick); the larger the S2, the greater the difference. Thus, for geometrical reasons, the exact shape of the curve depends on the strength of S2. Finally, the dashed line in Fig. 2B shows the classical neurophysiological way of plotting the data to illustrate why this has not been currently employed, although its use continues; such plotting of the additional response evoked by S2 as a percentage of that evoked by S1 alone introduces yet further complications (Crone et al. 1990; Capaday, 1997).
Ancillary modelling
The behaviour of the model was not dependent upon the particular choice of its parameters. Similar results were obtained on changing the values of the various conductances, including those responsible for excitation and inhibition, or the time constant or size of the AHP. It was then noteworthy that the net voltage noise across the membrane proved to be the important factor, rather than the particular values of the inhibitory and excitatory noise conductances per se.
Two simpler standard, basically similar, models were also tested more briefly to extend the generality of the findings and exclude any possibility that the present conclusions arose from the particularities of the present model. In the first (Matthews, 1996), all input variables were voltages with the time structure of the constant amplitude noise (± 1 mV s.d.) obtained by temporally smoothing a Gaussian series (4 ms TC); the membrane had neither conductance or capacitance. In the second, the membrane was given the present leak and AHP conductances, but was excited by injecting various steady currents to which was added a constant amount of noise (6-9.5 nA, ± 2 nA s.d., giving 1 mV membrane noise); the requisite temporal smoothing was now introduced by giving the membrane capacitance (again 4 nF). In both cases, the test stimulus was again a 1 ms pulse, of voltage or current (1-10 mV or 1-20 nA, respectively), and again similarly complex curves were obtained, as in Fig. 4 for the fuller model. Such behaviour can thus be attributed to two key features: that the membrane had very appreciable noise, so that firing began well before the mean value of the trajectory reached threshold, and that the effective portion of the post-spike voltage trajectory was approximately exponential and so approached threshold with a slope that increased with the firing rate.
Figure 4. The effect on the response of increasing the level of tonic excitation depends upon the size of the stimulus.
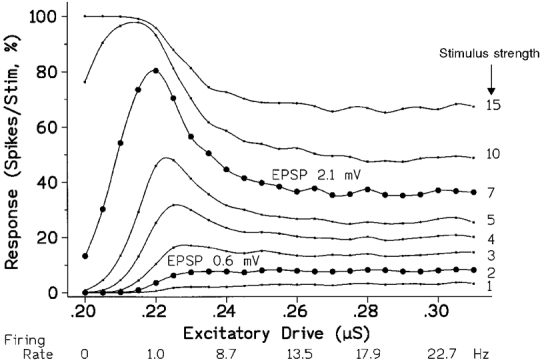
Each curve is for a different sized stimulus, increasing from below upwards (labelled on the right). Two curves have been picked out with larger symbols to emphasize their difference in shape; the EPSPs for these curves are noted for comparison with the background synaptic noise of around 0.5 mV (s.d.). The abscissa plots the background excitatory conductance; the consequential tonic firing rate is also indicated. As in Fig. 3, the ordinate gives the response to the brief stimulus, expressed as an increase in the ‘Firing index’ of the MN. For the weakest stimuli the response started by increasing progressively as the background drive was increased and then became approximately constant once steady firing was established. For stronger stimuli the response rose to a maximum as the MN began to be excited by the combination of noise and the increasing background drive, but then fell back to a lower plateau as tonic firing became established. (Unit stimulus = 0.025 μS, giving an EPSP of 0.3 mV.)
RESULTS
The contrasting effects of increasing stimulus strength with and without firing
The model motoneurone was stimulated by superimposing a brief increase of excitatory conductance on top of tonic excitatory and inhibitory inputs with their accompanying synaptic noise, all of which were expressed as conductances to mimic synaptic inputs. A number of identical stimuli were given and the probability of a single stimulus discharging a spike determined to give the response as a ‘firing index’ (Hunt, 1955), after subtracting any background firing. A probabilistic measure is needed because in the presence of synaptic noise the successive stimuli will vary as to whether or not they bring the membrane potential up to threshold. Thus, on increasing the size of the stimulus, the resulting stimulus-response plot is a curve rather than a step-function (Hunt, 1955). Figure 3 illustrates two such curves for the model MN which, for simplicity, will hereafter usually be referred to simply as the MN. The shape of the input-output relation can be seen to depend crucially upon whether or not the background synaptic drive, coupled with its noise, made the MN fire.
When the mean initial depolarization is well below spike threshold and the MN is silent (•), then the curve approximates to a Gaussian integral. Weak stimuli fail to excite (i.e. act with an excessively low probability) showing it has a threshold, while sufficiently large stimuli invariably do excite. Both the modelling and simple theory show that increasing the background excitation shifts the sigmoid to the left (not illustrated) without otherwise altering it, so that a given stimulus elicits a larger response. Increasing the amplitude of the noise increases the lateral spread of the curve without shifting its centre (i.e. expands it along the X-axis); thus the effect of a given stimulus will then become larger or smaller depending upon its size. The curve is inherently non-linear, but a linear approximation gives a reasonable fit over its central two thirds. A linear extrapolation of either this psuedo-linear central segment or any lower curved portion of the sigmoid will indicate a definite threshold for excitation. From this point of view increasing the drive decreases the threshold, and vice versa, while increasing the noise reduces the slope (gain).
When the model is already firing tonically, the curve ceases to be a simple sigmoid and spans a much wider range of stimulus strengths. Its clear inflections and changes of slope contradict any suggestion that it can be considered to be truly linear; but no attempt has been made to fit an equation to it, partly because its shape changes on varying the firing rate. However, for many practical purposes, a linear approximation would be acceptable with the fitted line passing through the origin (i.e. limited data would be readily fitted by a straight line constrained to pass through the origin). As emphasized by the expanded plot, the model MN no longer has a threshold and excitation occurs with the weakest stimulus. However, there will be a statistical threshold, as the response blends into the underlying fluctuating background of the PSTH, since any PSTH is based on a limited number of trials.
Following from all this, the crucial point is that when the stimulus is small the model MN gives a much larger response when it is already firing than when it is initially silent. This is because small stimuli, well below the threshold of the silent MN, will have a reasonable probability of triggering a spike when they arrive late in the firing recovery cycle of the MN and the membrane potential is close to threshold. In contrast, when the stimulus is strong the firing MN responds less vigorously than the silent MN. This is due to the inability of even a strong stimulus to excite the firing MN when it arrives at the beginning of its recovery cycle. Thus, when the model MN starts firing its ‘excitability’, as judged by its response to a given testing volley, might be variously taken to become larger or smaller depending upon the chosen stimulus and the background synaptic input to the silent MN.
Effect of changing the background synaptic drive and the firing rate of the model
Figure 4 extends the observation that stimulus size is crucial in determining the effect of firing on the model's ‘excitability’, as can be rapidly appreciated by comparing the marked pair of curves (•). Each curve shows the effect of increasing the background synaptic input on the magnitude of the response elicited by a stimulus of a particular size. The abscissa gives the synaptic drive rather than the resulting firing rate, since this allows subthreshold actions to be included in the same plot; the firing rate increases monotonically with the drive, as labelled. When the stimulus is small the response rises progressively to a low plateau as the drive increases, but when the stimulus is large the response rises to a maximum, as the model begins to fire, before falling back to a plateau. The prominence of this initial peak increases with stimulus size until it is limited by saturation at 100 %; the curve for the strongest stimulus lacks a peak simply because the abscissa does not start at zero.
Thus there is no general relation between ‘excitability’ as assessed by the response of the model MN to a fixed stimulus, and its initial level of activity. If very small stimuli are used, the relation is reasonably monotonic so that the observation of an increase in the response does betoken an increase in the background input to the MN. However, the plateau develops so rapidly with the inception of firing that the finding that some particular manoeuvre leaves the MN's ‘excitability’ unchanged provides no indication as to whether or not it has influenced the MN. With medium to large stimuli the situation is worse since, depending upon the range studied, increasing the tonic excitatory input to the MN may variously cause an increase, a decrease or no change in its ‘excitability’. The only safe situation is when it can be guaranteed that the MN remains silent under all conditions studied; the excitability then increases with the background drive, whatever the stimulus strength. But once the MN is firing, the literal interpretation of changes in ‘excitability’ may be quite misleading, since it may decrease with more background excitation as well as with inhibition.
Figure 6 (continuous curve) replots the responses of the upper marked curve (stimulus, 7 units) against the firing rate rather than the drive. This emphasizes that the maxima occur at firing rates of around only 1 Hz, rather than in the physiological firing range over which the response falls to an approximate equilibrium. It bears emphasis that, with the present realistic duration of AHP (30 ms TC, see Methods and Fig. 2), discharges below 8 Hz only occur by virtue of the noise, are highly irregular and the lowest firing rates are unlikely to be physiologically realisable in man (first, the slope of the drive-response, or f-I, relation may become steeper requiring overall input of the MN to be held more constant to elicit a constant firing rate, as readily achievable in the model; second, the discharge progressively approximates to random firing making it impossible for a subject to control the mean firing rate by feedback). However, the low-frequency peak will contribute to the gross EMG since statistically some contributory units will be receiving the appropriate level of drive.
Figure 6. The effects of changing the AHP can be normalized when plotting the response against the firing rate.
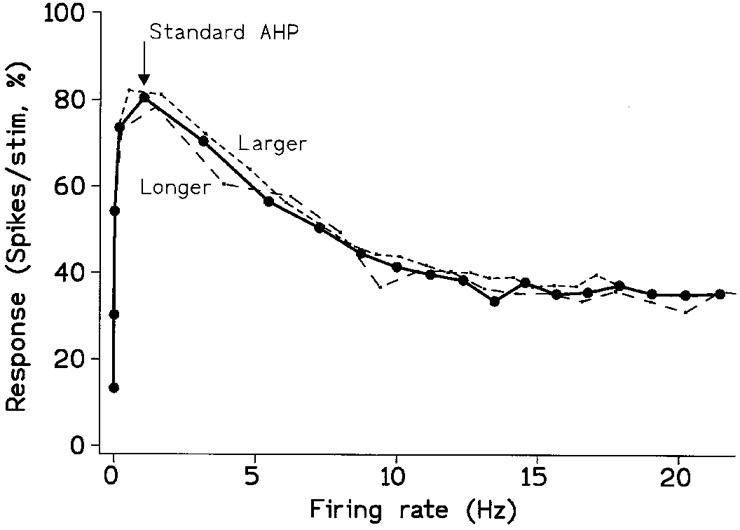
The continuous curve with symbols replots data from Fig. 4 to emphasize that the maximum response occurs at a very low firing rate (stimulus strength = 7). The dashed lines show the effect of increasing either the duration or the size of the AHP by 50 %, with suitable normalization. Both fall within the error range. On increasing the duration of the AHP the firing rate was scaled up by 50 % before plotting (long dash), showing that if all else is equal the behaviour of fast and slow MNs can be equalized simply by temporal scaling. The effect of increasing the size of the AHP by 50 % was compensated for by increasing both the noise and the stimulus by 50 % (short dash), without altering the scaling for plotting.
Effect of changing the synaptic noise
The behaviour of the present model MN depends crucially upon its containing synaptic noise. As with real MNs, increasing the noise amplitude increased the firing rate for a given drive as indicated at the bottom of Fig. 5 (also Fig. 1 and Poliakov et al. 1996). In addition, the extent to which a given stimulus gave a maximum on varying the background drive, as in Fig. 4, depended upon its size relative to the prevailing noise rather than upon its absolute magnitude. In the example of Fig. 5 the peak disappeared on doubling the noise, as with small stimuli in Fig. 4, although now both the stimulus and its consequential EPSP were unchanged. However, the responsiveness of the MN over a range of drives does not depend simply upon the stimulus-noise ratio. Decreasing the ratio by decreasing the stimulus always reduces the response, whatever the drive; but for low drives, below 0.20 in Fig. 5, reducing the stimulus-noise ratio by increasing the noise enhances the response rather than depressing it. However, things are simplified when the responses for the two noise levels are compared at the same firing rate; increasing the noise then always decreased the response (not plotted, see values at bottom of Fig. 4); the largest reduction occurred at low firing rates, since only the low-noise curve peaked. In the model of Jones & Bawa (1997) the introduction of noise to an initially noise-free MN likewise decreased the response at a given firing rate, and real MNs behave similarly (Poliakov et al. 1996). Thus the noise level affects the ‘excitability’ of the MNs irrespective of whether its mean input remains the same or is adjusted to standardize its firing rate. This effect is potentially physiologically significant; the noise level of γ motoneurones in the cat, for example, is thought to increase on spinalization (Ellaway, 1972).
The effect of abolishing the noise was also tested to confirm the expectation that the peak was due to the AHP and consequent refractoriness, rather than to the presence of noise per se. The rising phase of the peak in plots such as those of Figs 2 and 3 then became a step function, occurring as the background depolarization reached the level at which the stimulus could just lead to threshold crossing. As before, the response then fell progressively (and non-linearly) to an approximate plateau as the increase in firing rate and the exponentially decaying AHP left the MN refractory to the particular stimulus for a progressively greater proportion of the time.
Effect of changing the AHP
Duration of the AHP
When the model motoneurone is already firing its post-spike after-hyperpolarization (AHP) plays a major part in setting its excitability, since it determines how long the MN remains unresponsive to a stimulus of a given magnitude. Increasing the duration of the AHP, corresponding to studying a slower MN, reduces the firing rate for a given synaptic drive, and can be expected to reduce the response elicited by a given stimulus applied during firing (with the drive held constant). This was confirmed for a range of synaptic drives using a single strength of stimulus (7 units) on increasing the time constant of the underlying conductance of the AHP by either 50 or 150 %. It is, however, of more physiological interest to compare the responses with those for the standard AHP at the same firing rate rather than at the same drive, and this also simplifies understanding. The response at a given firing rate is still reduced, but plotted in this way the change was simply one of temporal scaling; as shown in Fig. 6, the new curve (long dash) superimposed itself upon the original when its firing rate was normalized (the firing rate for the prolonged AHP was scaled up by multiplying it by the ratio of the two time constants). This is to be expected; both firing rate and responsiveness are set by the time course of the various AHP voltage trajectories, and these can be normalized by expressing them relative to the time constant of the AHP. The increase of the time constant of the model from the standard 30 ms to 45 ms corresponds approximately to switching from a biceps brachii to a soleus MN (Matthews, 1996). Thus, for many purposes such as testing for other differences between them, the behaviour of fast and slow motoneurones is best compared by choosing a lower absolute rate of firing for the slow MNs.
Size of the AHP
The response to the same fixed stimulus (7 units) was also determined on increasing the initial value of the conductance responsible for the AHP by both 50 and 150 %. This also reduced both the firing rate and the response, irrespective of whether the latter was plotted against the drive or the firing rate, but the curves retained their original overall form with a maximum and subsequent plateau. The reduction was largely insignificant at the lowest firing rates (up to 2-3 Hz), since spike initiation then depends largely upon the noise statistics rather than recovery from post-spike refractoriness. Normalization of these curves is more complex and less important physiologically. It is, however, described below to help deepen understanding of the factors that set the behaviour of the model and the form of the curves in Fig. 4.
The known properties of the purely voltage model of Matthews (1996) suggests that amplitude scaling should now permit the response pattern to be standardized. In this model, the voltage trajectory and all other variables were scaled in terms of ‘Noise Units’, equivalent to the standard deviation of the membrane noise. Its excitability and pattern of firing depend, as usual, upon the way in which the trajectory comes up to threshold. On changing the size of the AHP, the firing rate for a given drive depended upon the initial size of the AHP expressed in the normalized Noise Units, rather than upon its absolute size in millivolts. Likewise, the size of any stimulus needs to be normalized in Noise Units. Thus, for that model, normalization for a given increase in the absolute size of the AHP can be achieved by increasing the stimulus size and noise amplitude by the same proportion.
The present model has sufficient in common with the simpler model to expect the same procedure to work, provided that the size of the response is plotted against the firing rate rather than the mean synaptic drive. This is because in the simpler model the drive is also expressed in Noise Units, and this cannot be done for the present conductance drive. However, standardizing the firing rate is equivalent to standardizing the drive. Using the present model, this line of reasoning was confirmed by observing that the plot of response vs. firing rate for a 50 % increase in AHP superimposed with the original when the noise and stimulus strength were also increased by 50 % (Fig. 6 short dash). The same procedures would therefore also permit normalization of the effect of increasing the noise. On doubling the noise, as in Fig. 5, a plot of response vs. firing rate should be restored to its original shape by also doubling both the stimulus and the AHP.
Variation of threshold
Consideration was given to extending the model to allow the threshold to be variably reduced during the course of the interspike interval; this was rejected as the results can be predicted without actually running the model. The change in MN threshold to a sudden test depolarization is known to mirror the change in potential during the recovery cycle (Calvin, 1974; Powers & Binder, 1996); this is probably due to the hyperpolarization of the AHP reducing sodium carrier inactivation, thereby allowing a spike to be initiated with less absolute depolarization. Assuming approximate linearity, this simply scales down the separation between any point on the trajectory and the on-going threshold. This is equivalent to running the model with a smaller AHP conductance; this does not affect the conclusions, as shown above (Fig. 6).
Interaction of conditioning and testing responses to simultaneous stimuli
The modelling was next extended to examine the reliability of using classical monosynaptic testing to assess the effect of a conditioning stimulus on the excitability of motoneurones. The limitations of such testing are too easily overlooked as its use in man is expanded, as in analysing the effects of cortical stimulation. The principle of the testing is simple for silent MNs and will be outlined to help explain the modelling. A fixed testing stimulus (here S1) is given to an appropriate nerve to elicit a I a volley that excites the chosen MNs to give the test monosynaptic response. The test stimulus (S1) is then combined with an excitatory conditioning stimulus (here S2) whose facilitatory action is to be assessed and which would not normally, on its own, excite the MNs to discharge. S2 then facilitates the action of S1 and the resulting increase in the size of the monosynaptic discharge provides a quantitative measure of the excitatory action of S2. A classical difficulty is the selection of an appropriate strength for S1, since this will influence the amount of facilitation of S1 with a constant S2 (Crone et al. 1990; Capaday, 1997). With the application of the technique to more complex situations, especially in man, the further problem has arisen as to whether S1 should be adjusted to allow for the appreciable changes in the test response that may occur when the effect of a conditioning volley is studied under different conditions, as in the presence and absence of voluntary contraction. On the basis of their experiments, Crone et al. (1990) concluded that comparability could be best preserved by adjusting the strength of S1 to keep the initial size of the testing response the same, for each condition studied. As described in Methods a minor transformation of the present input- output curves allowed such matters to be modelled for the individual MN (Fig. 2). This showed that comparability cannot be achieved for widely different conditions, whether by keeping S1 constant or by adjusting it to give the same test response; a particular example is illustrated in Fig. 7 and explained below.
Figure 7A plots the absolute amount of facilitation produced by a fixed conditioning (S2) stimulus against the strength of the test (S1) stimulus, in the presence and absence of background firing. The curve for the silent MN has a sharp maximum while that during firing is much flatter and crosses the former at two places. Thus for a given S1 stimulus, depending upon its particular value, the transition from silence to firing may either increase or decrease the facilitation due to the fixed S2 stimulus, thus potentially creating confusion. For example, with S1 = 5 the onset of firing reduced the S2-induced facilitation thereby potentially suggesting that voluntary contraction increases the level of tonic presynaptic inhibition of the S2 afferents; in contrast, with S1 = 2 the facilitation is increased, now apparently suggesting that contraction reduces pre-existing tonic presynaptic inhibition. Figure 7B shows that the situation is somewhat simplified when the strength of S1 was adjusted to keep the test response constant in the presence and absence of firing; on plotting the S2-induced facilitation against the S1 response the facilitatory effect of the constant conditioning S2 stimulus then always decreased with the onset of background firing. Related inverse plots would have been obtained if S2 had produced inhibition rather than excitation. Similar curves, differing in detail, were obtained on altering the various remaining parameters (background drive to MN and consequent firing rate, noise level, strength of S2).
It may be concluded that, as with single stimuli (Figs 2 and 3), the use of a monosynaptic conditioning-testing paradigm to compare MN excitability in the presence and absence of firing is fraught with hazard. In contrast, comparisons between different levels of sub-threshold excitatory drive are entirely valid, provided that the test stimulus is adjusted to give a constant unfacilitated test response; similar analysis readily shows that the plot for the silent MN in Fig. 7B is then invariant. With higher firing rates and adjustment of S1 reasonably reliable comparisons may be made for different levels of maintained firing, at above about 10 Hz for the present model since the curves of Fig. 4 then become approximately parallel. At low firing frequencies, however, the problems recur. Thus reliance on the massed response of a population of MNs of varying threshold is potentially unreliable whenever some are firing while others remain silent; any change in background drive will alter their relative proportions and thus their overall responsiveness.
DISCUSSION
Excitability does not mirror the background activation of the MN
The main lesson from the present model MN is that the excitability of a real motoneurone, as assessed by its response to a brief test input, can be expected to change drastically as it commences firing in response to a separate tonic input. This is a simple consequence of the relative refractory period produced by its AHP, and occasions no surprise. The special new feature is that the nature of the effect depends upon the size of the testing stimulus, and more particularly on its relation to the on-going level of synaptic noise whose crucial role is often neglected. With weak testing stimuli, comparable in size to the noise, the response rises smoothly to a plateau as the background input increases and the MN starts and then increases its firing. In contrast, with strong stimuli the response of the MN rises to a maximum as it starts firing only to fall back by as much as 50 % to a lower plateau as its firing rate increases. In man, a reduction has been clearly seen for H reflex testing of single units on increasing their firing rate by voluntary action (Kudina, 1988; Piotrkiewicz et al. 1992; Jones & Bawa, 1995), but without the effect of stimulus strength being explored as would now seem desirable. Piotrkiewicz et al. stated quite explicitly for their five motor units, as for the present model, that ‘in the lower range of firing rates’ the response ‘decreased when increasing the firing rate, but it remained constant in the higher rate range’. The approximate constancy of the response found by others can now be attributed to the use of smaller stimuli and/or higher firing rates, in relation to the intrinsic properties of the MNs studied (Ashby & Zilm, 1982; Brouwer et al. 1989; Miles et al. 1989; even in these studies, the response tended to increase when very low firing rates were studied). Such supposed constancy has hitherto been taken to justify the use of a model with a strictly linear trajectory whose slope is proportional to the firing rate (i.e. always starting from a fixed point; Ashby & Zilm, 1982; Miles et al. 1989; Nordstrom et al. 1992); geometry shows that the response is then independent of both the drive and the firing rate. Given the simplicity of the present model, the present conclusions should be applicable to other types of neurone provided that they have a single pacemaker and that their discharge is controlled by a prolonged post-spike after-hyperpolarization dependent upon an exponentially decaying conductance.
For comparison with the behaviour of real MNs the curves of Fig. 4 can be usefully divided into three sections, depending upon the level of drive; hitherto these have tended to be studied independently. The first is before firing starts and is well studied classically (Hunt, 1955). The second is from the inception of firing to about 10 Hz (for the present model of a biceps brachii MN), which is where all the complications occur. This is essentially due to the fact that the mean voltage trajectory of the MN has its final equilibrium close to threshold. In consequence, the trajectory spends an appreciable time just below threshold thereby emphasizing its curvature and invalidating a linear approximation (Piotrkiewicz et al. 1992; Jones & Bawa, 1995, 1997; Matthews, 1996). This also allows the noise transients to play a major part in excitation, and this underlies the marked change in the shape of the curves of Fig. 4 with stimulus strength. The third section is for firing rates above about 10 Hz (again for the present biceps MN model) when the mean equilibrium trajectory first reaches threshold, with the equilibrium for higher firing rates lying well beyond threshold. The trajectory remains exponential, but finally only a short segment is involved in excitation (see Fig. 11, Matthews, 1996) so that a linear approximation becomes acceptable. The slope of the final segment involved then varies slightly with the firing rate so that the responses for an exponential trajectory converge towards those for a linear trajectory with its slope dependent of firing rate (Ashby & Zilm, 1982).
It may be concluded that once a motoneurone begins to fire the measurement of its ‘excitability’ ceases to provide a valid indication of its background synaptic drive, as works reasonably well for the silent MN. At high firing rates, the excitability shows little or no change with the drive. At low firing rates, contrasting with the silent MN, the excitability falls rather than rises with the drive. Moreover, the modelling showed that the terms ‘high’ and ‘low’ applied to the firing rate have to be interpreted in relation to the duration of the AHP of a motoneurone. A fast motoneurone has to be studied at higher absolute firing rate than a slow MN to give a comparable response; this applies equally to their interspike interval histograms (Matthews, 1996). Careful measurements of firing rate will, of course, always indicate whether or not an unknown steady input is tonically exciting or inhibiting a particular MN. However, an understanding how the ‘excitability’ of a unit varies with firing remains essential both for using the response of the MN to deduce its response to a brief input and for understanding population responses.
Effect of non-linearities on behaviour
The stimulus- response curve also changed markedly with the onset of firing. Both relations were inherently non-linear: that for the silent MN was a simple sigmoid displaced along the abscissa, while that during firing was nearly linear, starting from the origin. When it is firing, the MN responds to the weakest stimuli and the detection of a threshold response is set by the stimulus/noise ratio. When it is at rest then it has a threshold for all practical purposes, given approximately by extrapolating the central pseudo-linear portion of the stimulus-response relation. This all helps explain why the effect of contraction on the monosynaptic reflex differs so much from muscle to muscle. For MNs with a large I a input (or low threshold) a response is readily elicited at rest but then shows little change with contraction, as with the ankle jerk (Rüegg et al. 1990). In contrast, MNs with a weak monosynaptic input fail to give a jerk at rest, but one can be detected in the EMG when the muscle contracts, as with the intrinsic hand muscles (Burke et al. 1989). Likewise, the ability of a corticofugal volley to evoke a monosynaptic response from different resting MNs will depend upon the density of their corticospinal innervation. Thus, in life, because of a limit on the size of the inputs, the maximum response elicitable from either an individual MN or in the EMG may often be less during relaxation than during contraction, in spite of the theoretical maximum being greater for relaxation when there is no refractoriness to prevent 100 % firing.
The monosynaptic conditioning-testing paradigm was also affected by these various non-linearities. The facilitation of the test ‘monosynaptic reflex’ produced by a fixed conditioning then varied between silence and activity, including when the strength of the test stimulus was adjusted to keep the size of the test reflex constant. Likewise, even for a single unit, the isolated observation of the failure of a pair of suprathreshold responses to sum arithmetically provides equivocal evidence for the occurrence of mutual ‘facilitation’ or ‘inhibition’, though less severely so for the firing MN. Precision can only be achieved through knowledge of the stimulus-response relation of the unit.
The non-linearity of real MNs is of course well known and in a recent intracellular study was rather fully described mathematically by computing the first three Wiener kernels for the input-output transform (including its temporal relations), together with their dependence upon firing rate (Poliakov et al. 1997). Poliakov et al. considered, however, that their kernels varied with the noise level and were restricted to applications in which the input signals were comparable in magnitude to the noise. The present stimuli covered a much wider range, favouring modelling to generate a series of ad hoc plots to obtain a qualitative feel for the situation. They provide a template against which the behaviour of real motoneurones can be tested; while awaiting this, it would be foolhardy to presume that they behave more simply.
Multi-unit summed responses
When gross EMG responses are studied, then further well-recognized complications are introduced by the distribution of the thresholds and synaptic inputs of the various units involved (cf. Crone et al. 1990; Capaday, 1997). The present anomolous unitary behaviour with the onset of firing is then more easily neglected, since there is no information on firing rates. Moreover, there can be no guarantee that equating the background EMG under two sets of conditions standardizes the firing rates of the same constant population of active MNs. Muscles for which the majority of MNs are recruited early are more likely to show anomolous inverse effects (with saturation or reduction of the summed population response with increased mean activity) than those whose MN recruitment thresholds are widely staggered and show only small changes in firing rate. The present modelling is thus entirely in line with the suggestion that the known differences in recruitment pattern are responsible for the weaker facilitatory effect of increasing the strength of voluntary contraction on the cortically evoked responses of adductor pollicis (uniform early recruitment) in comparion with biceps brachii (widely spread recruitment; Taylor et al. 1997).
Relevance for cortical stimulation
MN contribution
The most direct point has long been obvious, namely that changes in the excitability of the spinal motoneurone pool can greatly affect the size of the EMG response evoked by a given cortical stimulus. The modelling, however, extends appreciation of the complex behaviour of the MNs as their mean level of activity varies. In particular, it emphasizes the role of stimulus size and this may already have led to confusion. Brouwer et al. (1989), for example, suggested that fine movements generating little force engage the motor cortex more strongly than cruder forcible contractions of the same muscle, because the cortical response in a unitary PSTH decreased when the contraction increased. Their control H reflex remained the same, but it unfortunately happened to be much smaller than the cortical responses; thus the MNs may have behaved in the way illustrated in Fig. 4 and responded differentially to different sized stimuli.
Much, however, can still be done using the gross EMG especially when an input-output plot of EMG response against cortical stimulus strength is determined for each condition studied, rather than relying upon a single observation (Devanne et al. 1997; Ridding & Rothwell, 1997). Unlike the unitary model (Fig. 3) the experimentally observed overall input-output curve shows a striking increase in slope with voluntary contraction, which can thus be attributed to population effects, presumably for the cortical as well as the motoneuronal pools. Imagine, for example, a population of MNs with regularly spaced thresholds, extending to infinity, all with the same stimulus and background drive. With the onset of firing the number of silent MNs responding to the stimulus will remain unchanged and give the same response as the quiescent pool, as fresh MNs are recruited within range of the stimulus; but to this must be added the response of the newly firing MNs, thereby producing an increase of slope. Different muscles will vary in the distribution of thresholds etc. of their constituent motor units so if population properties are responsible, then the increase of slope with contraction could be expected to vary between muscles as is indeed found, with elbow flexors showing much larger increases than hand muscles (Taylor et al. 1997; Abbruzzese et al. 1999). Devanne et al. (1997) likewise attributed the slope change to population effects, and surveyed the factors involved.
Cortical excitability
Next, given the behaviour of the MN, it becomes quite unjustifiable to assume that the overall descending response evoked by a given cortical stimulus (i.e. ‘excitability’) will always increase monotonically as the corticospinal neurones are brought by some manoeuvre (such as making a voluntary contraction) first to threshold and then to increase their firing rate. Any response evoked from an individual corticospinal neurone by excitation of its pacemaker, whether by presynaptic activity or extrinsic current, must be suspected to remain constant over part of the range or decrease, and for the effect of firing rate to vary with stimulus size. Like the motoneurone, the tonic firing of corticospinal neurones is regulated by a moderately prolonged post-spike recovery process (Takahashi, 1965; Reyes & Fetz, 1993a) so the present modelling of neuronal behaviour is potentially applicable. As discussed for the MN, the overall descending response evoked by transcranial stimulation will also depend upon the distribution of firing thresholds etc. of the population of excited neurones; given the difference in their ‘targets’, rate coding rather than recruitment may be suspected to be the relatively more important for corticospinal neurones which would stabilize the ‘excitability’ of the motor cortex as its activity increased.
It is, of course, already widely recognized that the level of cortical activity does not affect D wave responses evoked by electrical stimulation, since these normally depend upon the excitation of axons (Rothwell et al. 1991). However, most workers appear to assume that magnetically determined ‘excitability’ normally increases monotonically with increasing activation of corticospinal neurones, including when they are firing (Datta et al. 1989). Exceptionally, Baker et al. (1995) cautioned that their findings with transcranial magnetic stimulation (TMS) ‘implied that the period of maximum cortical susceptibility to TMS may not coincide with the period of maximum corticospinal cell activity’, since during precision grip the maximum size of the directly recorded D wave in the monkey failed to coincide with the highest rate of corticospinal firing as known from earlier recordings.
There are, however, two major complications about the behaviour of the individual corticospinal neurone. First, magnetic stimulation evokes a repetitive corticospinal discharge with an initial D wave followed by one or more sharply timed I waves. The I waves are probably due to subsequent synaptic excitation following direct excitation of interneurones and/or presynaptic terminals; they are notably absent on intracellular stimulation of a single neurone (Reyes & Fetz, 1993a), and so do not affect the modelling of the corticospinal neurone per se. Second, an intracellular stimulus delivered during the middle third of the interspike interval can initiate a slow regenerative process which may then trigger a delayed spike (Reyes & Fetz, 1993a), totally distinguishable from the I wave by its long and variable latency and contrasting with the synchronized spikes elicited by stimulation later in the cycle. Such delayed spikes will thus make no contribution to the initial peak in the PSTH as currently modelled or to the corresponding cortically evoked EMG response in life, though the underlying regenerative process might sensitize the neurone to subsequent I wave excitation. Thus detailed modelling of the overall response to cortical stimulation is not currently feasible. However, the view that the ‘excitability’ of corticospinal neurones does not increase with their firing rate is validated by findings of Reyes & Fetz (1993b) with intracellular stimulation of single neurones in cortical slices. This should apply to both D and I responses elicited by transcranial stimulation, presuming that any cortical interneurones involved with the I wave behave like those studied; I waves dependent upon stimulation of axons (as discussed below) simply re-test the excitability of neurones to a synaptic input shortly after conditioning them with a direct ‘magnetic’ stimulus.
The important work of Reyes & Fetz (1993a, b) requires further detailed comment as it seems to have been overlooked by those involved in cortical stimulation and its applicability is not immediately obvious. They themselves were unconcerned with such issues, never presented a PSTH, and tailored their analysis to their particular needs by computing an average ‘shortening-delay plot’. This clouds the situation for present purposes, particularly because with weak stimuli delivered near the end of the interspike interval (ISI) there can then be ‘an artificial lengthening of the ISI where there may have actually been an ISI shortening’ (1993a). Nonetheless, their Fig. 5 (1993b) clearly indicates that with a large stimulus the synchronized response remained the same for firing rates of 12, 28 and 49 Hz, while their Fig. 6D shows that the overall response (predominantly consisting of synchronized rather than delayed spikes) was likewise invariant. They specifically concluded that the average stimulus-induced increase in firing rate (synchronized + delayed spikes) ‘did not vary with the baseline firing rate’ for frequencies of 8-70 Hz.
Finite cortical threshold suggests magnetic stimulation excites axons
The final conclusion from the modelling is that the rather high threshold for magnetic stimulation applied during voluntary contraction has clear implications for its site of action. First, however, it is necessary to strip away any uncertainties about the relation between the output dial reading on a standard stimulator (such as those made by Magstim, UK) and the stimulus received by any individual element within the cortex, such as a corticospinal neurone. The dial reading simply gives the voltage applied to the element on a linear scale starting from zero (confirmed with Dr M. Polson of Magstim). The scaling factor will depend upon a variety of factors and will differ for every individual neurone and axon. This is physics, leaving the occurence of a threshold for excitation to physiology; the current does not spread deeper to reach new structures with increase of stimulus strength, it simply becomes larger everywhere.
Since this does not seem to be always recognized it becomes desirable to explain the underlying basis, so that the force of the present argument can be appreciated. The stimulator acts by discharging a capacitor through a coil to create a changing magnetic field which then induces a voltage gradient in the underlying brain tissue. The spatial distribution of the voltage field, and likewise the resulting current flow, is remarkably complex, largely because the brain is so electrically inhomogeneous. This makes it impossible to predict the absolute value of the stimulus at any point. However, the waveform of the discharge current that induces the magnetic field remains fixed when the output of the stimulator is changed (Barker et al. 1991), so that the waveform of the magnetic flux change and of the resulting induced voltage also remain the same. The maximum value of each is directly proportional to the output reading of the stimulator, which gives the initial voltage on the discharge capacitor as a percentage of the maximum available (Dr M. Polson, Magstim, personal communication). Thus any individual cortical neurone or axon receives an electrical stimulus of constant pulse shape and fixed spatial distribution; its magnitude is directly proportional to the stimulator reading, starting from zero. Of course, every cell body and axon will have its own constant of proportionality, relating degree of excitation to dial reading, and this will depend upon its shape, where it lies, how it is orientated and so on, as well as its chronaxie. Thus predicting the behaviour of a population is currently impossible, although the behaviour of every individual within it is basically simple.
The appreciable cortical threshold required to evoke a response from a non-contracting muscle tells one nothing about what is happening in the cortex, since the silent MN pool has a threshold and an appreciable descending volley is required to evoke a discharge; repetitive descending activity may be particularly effective. However, firing MNs should give a response to the weakest input (see Fig. 3), so even a weak stimulus should affect their discharge the moment they start firing, with the background noise level limiting the EMG detectability of the occurrence of an evoked corticospinal volley. Likewise, if the stimulus were to directly excite discharging corticospinal neurones which acted monosynaptically on the discharging motoneurones, then both should respond with the weakest input and the threshold should be close to zero. In fact, as numerous recordings testify, the threshold for cortical stimulation remains high during voluntary contraction, only slightly reduced from the value obtained with relaxation.
In the curves of Devanne et al. (1997), for example, the EMG response rises from zero to maximum on increasing the stimulus from 45 to 60 for tibialis anterior and from 30 to 40 for first dorsal interosseus (values = percentage maximum output during 40 % MVC; a similar threshold is observed for sample discharging single motor units). This mirrors the behaviour of the silent MN in Fig. 3 and strongly suggests that the stimulus is first acting on a cortical element which itself has a definite threshold. There seems little doubt that many of the relevant corticospinal neurones do discharge during voluntary contraction (Porter & Lemon, 1993), thereby excluding their pacemakers as the site of action of the stimulus. Different corticospinal neurones will, of course, have different scaling factors in relation to stimulator setting, but the input-output relation for their summed discharge should still start from zero and increase approximately linearly; recruitment of non-firing corticospinal neurones with an increase of stimulus strength would give an upward inflection to the plot. Likewise, the stimulus is unlikely to act via the cell bodies of nearby neurones whose terminals then excite the corticospinal neurones; many of these can also be expected to be firing during voluntary contraction. However, a variety of axons terminate on corticospinal and other cortical neurones and would have a definite threshold under all conditions, and one remaining unchanged during voluntary contraction. Thus the present modelling firmly suggests that transcranial magnetic stimulation acts on axons and their terminals rather than on the various pacemakers of the neurone.
It is notable that the originators of magnetic stimulation drew essentially the same conclusion (Barker et al. 1991), without particularly emphasizing its significance, when they investigated the effect of varying the time course of the magnetic stimulus. They used a complex variant of the classical strength-duration curve to estimate the ‘cortical membrane time constant’ for activation of the relaxed abductor digiti minimi. The particular value obtained (150 μs) may well be open to question. The important thing is that the value was the same as that obtained when the ulnar nerve was stimulated in the arm, evoking an EMG in the same muscle. If cortical stimulation directly activated neurones rather than axon terminals then the cortical value should have been appreciably larger, assuming that the pacemaker of the cortical neurone (presumably its initial segment) is sufficiently well-coupled electrically to the cell body for the neuronal rather than the axonal chronaxie (time constant) to apply.
In accordance with all this Di Lazarro et al. (1998) recently suggested that the I waves elicited by magnetic stimulation depend upon the activation of axons rather than cell bodies, when they found little or no change during contraction in I wave threshold in their epidural recordings of the massed descending I wave activity. However, the site of origin of any magnetically evoked D wave remains controversial. Its continued high threshold during contraction suggests that in conscious man it also arises from excitation of axons (whether pre- or post-synaptic). Likewise, the threshold for D wave induced facilitation of the H reflex also remains high during contraction, unchanged from its resting value (Mazzocchio et al. 1994), and there is no change with contraction in the small high-threshold D waves recorded epidurally (Di Lazarro et al. 1998). In the conscious monkey, however, the magnetically evoked and directly recorded D wave varies with the level of anaesthesia and during the course of a precision grip (Baker et al. 1994, 1995), and in unconscious humans reduction of anaesthesia can increase the D waves produced by magnetic stimulation (Burke et al. 1993). Thus it seems premature to generalize, since what happens may depend upon variants in the stimulating conditions and how the D wave is characterized.
In conclusion, the present model neglects the detailed complexity of the MN but has sufficed to emphasize that the apparently simple concept of ‘excitability’ needs to treated with extreme care when it is extrapolated from quiescent neurones to those that are already firing. The modelling was performed for spinal motoneurones, but corticospinal neurones can be expected to show related non-linearities. Study of real neurones under a comparably wide range of conditions would now seem highly desirable to check their actual behaviour and confirm the applicability of the modelling.
References
- Abbruzzese G, Assini A, Buccolieri A, Schieppati M, Trompetto C. Comparison of intracortical inhibition and facilitation in distal and proximal arm muscles in humans. The Journal of Physiology. 1999;514:895–903. doi: 10.1111/j.1469-7793.1999.895ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Zilm D. Characteristics of postsynaptic potentials produced in single human motoneurons by homonymous group I volleys. Experimental Brain Research. 1982;47:41–48. doi: 10.1007/BF00235884. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Recording an identified pyramidal volley evoked by transcranial magnetic stimulation in a conscious macaque monkey. Experimental Brain Research. 1994;99:529–532. doi: 10.1007/BF00228989. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Task-related variation in corticospinal output evoked by transcranial magnetic stimulation in the macaque monkey. The Journal of Physiology. 1995;488:795–801. doi: 10.1113/jphysiol.1995.sp021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarisation time course. First interval firing. Acta Physiologica Scandinavica. 1974;91:528–544. doi: 10.1111/j.1748-1716.1974.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Barker AT, Garnham CW, Freeston IL. Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. In: Levy WJ, Cracco RQ, Barker AT, Rothwell JC, editors. Magnetic Motor Stimulation: Basic Principles and Clinical Experience. suppl. 43. Amsterdam: Elsevier; 1991. pp. 227–237. EEG. [PubMed] [Google Scholar]
- Bawa P, Lemon RN. Recruitment of motor units in response to transcranial magnetic stimulation in man. The Journal of Physiology. 1993;471:445–464. doi: 10.1113/jphysiol.1993.sp019909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 3–53. [Google Scholar]
- Brouwer B, Ashby P, Midroni G. Excitability of corticospinal neurones during tonic muscle contraction in man. Experimental Brain Research. 1989;74:649–652. doi: 10.1007/BF00247369. [DOI] [PubMed] [Google Scholar]
- Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. 1989;112:417–433. doi: 10.1093/brain/112.2.417. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. The Journal of Physiology. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin WH. Three modes of repetitive firing and the role of threshold time course between spikes. Brain Research. 1974;69:341–346. doi: 10.1016/0006-8993(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. Journal of Neuroscience Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Maziéres L, Morin C, Nielsen J, Pierrot-DeSeilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of test reflex size: a study in man and the cat. Experimental Brain Research. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Datta AK, Harrsion LM, Stephens JA. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseus muscle. The Journal of Physiology. 1989;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Experimental Brain Research. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. The Journal of Physiology. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. The variability in the discharge of fusimotor neurones in the decerebrated cat. Experimental Brain Research. 1972;14:105–117. doi: 10.1007/BF00234794. [DOI] [PubMed] [Google Scholar]
- Hunt CC. Monosynaptic reflex response of spinal motoneurons to graded afferent stimulation. Journal of General Physiology. 1955;38:813–852. doi: 10.1085/jgp.38.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Bawa P. Responses of human motoneurones to Ia inputs: effects of background firing rate. Canadian Journal of Physiology and Pharmacology. 1995;73:1224–1234. doi: 10.1139/y95-174. [DOI] [PubMed] [Google Scholar]
- Jones KE, Bawa P. Computer simulation of the responses of human motoneurons to composite Ia EPSPs: Effects of background firing rate. Journal of Neurophysiology. 1997;77:405–420. doi: 10.1152/jn.1997.77.1.405. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TS. Cross-correlation analyses of motoneuron inputs in a coordinated motor act. In: Kruger J, editor. Neuronal Cooperativity. Berlin: Springer-Verlag; 1991. pp. 225–248. [Google Scholar]
- Kudina LP. Excitability of firing motoneurones tested by Ia afferent volleys in human triceps surae. Electroencephalography and Clinical Neurophysiology. 1988;69:576–580. doi: 10.1016/0013-4694(88)90170-8. [DOI] [PubMed] [Google Scholar]
- MacGregor RJ. Neural and Brain Modelling. San Diego: Academic Press; 1987. [Google Scholar]
- Matthews PBC. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. The Journal of Physiology. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Spindle and motoneuronal contributions to the phase advance of the human stretch reflex and the reduction of tremor. The Journal of Physiology. 1997;498:249–275. doi: 10.1113/jphysiol.1997.sp021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. The Journal of Physiology. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles TS, Türker KS, Le TH. Ia reflexes and EPSPs in human soleus motorneurones. Experimental Brain Research. 1989;77:628–636. doi: 10.1007/BF00249616. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. The Journal of Physiology. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier E, Bawa P, Lemon RN. Excitability of human upper limb motoneurones during rhythmic discharge tested with transcranial magnetic stimulation. The Journal of Physiology. 1995;485:257–269. doi: 10.1113/jphysiol.1995.sp020728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrkiewicz M, Churikova L, Person R. Excitability of single firing human motoneurones to single and repetitive stimulation (experiment and model) Biological Cybernetics. 1992;66:253–259. doi: 10.1007/BF00198478. [DOI] [PubMed] [Google Scholar]
- Poliakov AV, Powers RK, Binder MD. Functional identification of the input-output transforms of motoneurones in the rat and cat. The Journal of Physiology. 1997;504:401–424. doi: 10.1111/j.1469-7793.1997.401be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Powers RK, Sawczuk A, Binder MD. Effects of background noise on the response of rat and cat motoneurones to excitatory current transients. The Journal of Physiology. 1996;495:143–147. doi: 10.1113/jphysiol.1996.sp021580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Oxford University Press; 1993. [Google Scholar]
- Powers RK, Binder MD. Experimental evaluation of input-output models of motoneuron discharge. Journal of Neurophysiology. 1996;79:367–379. doi: 10.1152/jn.1996.75.1.367. [DOI] [PubMed] [Google Scholar]
- Reyes AD, Fetz EE. Two modes of interspike interval shortening by brief transient depolarizations in cat neocortical neurons. Journal of Neurophysiology. 1993a;69:1661–1672. doi: 10.1152/jn.1993.69.5.1661. [DOI] [PubMed] [Google Scholar]
- Reyes AD, Fetz EE. Effects of transient depolarizing potentials on the firing rate of cat neocortical neurons. Journal of Neurophysiology. 1993b;69:1673–1683. doi: 10.1152/jn.1993.69.5.1673. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalography and Clinical Neurophysiology. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Experimental Physiology. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Rüegg DG, Krauer R, Drews H. Superposition of H reflexes on steady contractions in man. The Journal of Physiology. 1990;427:1–18. doi: 10.1113/jphysiol.1990.sp018157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB. The information capacity of nerve cells using a frequency code. Biophysical Journal. 1967;7:797–826. doi: 10.1016/S0006-3495(67)86623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. Journal of Neurophysiology. 1965;28:908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Experimental Brain Research. 1997;117:472–478. doi: 10.1007/s002210050243. [DOI] [PubMed] [Google Scholar]


