Abstract
The behaviour of inspiratory motoneurones is poorly understood in humans and even for limb muscles there are few studies of motoneurone behaviour under concentric conditions. The current study assessed the discharge properties of the human phrenic motoneurones during a range of non-isometric voluntary contractions.
We recorded activity from 60 motor units in the costal diaphragm of four subjects using an intramuscular electrode while subjects performed a set of voluntary inspiratory contractions. These included a range of inspiratory efforts above and below the usual tidal range: breaths of different sizes (5-40% vital capacity, VC) at a constant inspiratory flow (5% VC s−1) and breaths of a constant size (20% VC) at different inspiratory flows (2.5-20% VC s−1).
For all the voluntary tasks, motor units were recruited throughout inspiration. For the various tasks, half-way through inspiration, 61-87% of the sampled motor units had been recruited.
When the inspiratory task was deliberately altered, most single motor units began their discharge at a particular volume even when the rate of contraction had altered.
The initial firing frequency (median, 6.5 Hz) was consistent for tasks with a constant flow regardless of the size of the breath. However, for breaths of a constant size the initial firing frequencies increased as the inspiratory flow increased (range across tasks, 4.8-9.3 Hz). The ‘final’ firing frequency at the end of inspiration increased significantly above the initial frequency for each task (by 0.8-5.2 Hz) and was higher for those tasks with higher final lung volumes and higher inspiratory flows (range across tasks, 7.8-11.0 Hz).
There was no correlation within a task between the time of recruitment and the initial or final firing frequency for each motor unit. However, for each inspiratory task, initial and final firing frequencies were positively correlated.
Because the discharge of three to four units could be recorded simultaneously in a range of tasks, a quantitative ‘shuffle’ index was developed to describe changes in their recruitment order. Recruitment order was invariant in the task with the slowest inspiratory flow, but varied slightly, but significantly, in tasks with higher inspiratory flows.
The discharge rates of single motor units were compared for targeted voluntary breaths and non-targeted involuntary breaths which were matched for size. There were no significant differences in the initial or final firing frequencies, but recruitment order was not always the same in the two types of breath.
In the anaesthetized cat, the resting membrane potential of inspiratory motoneurones fluctuates with the respiratory cycle as they are depolarized during inspiration and hyperpolarized during expiration (Sears, 1964; Smith et al. 1988; for review see Monteau & Hilaire, 1991). In the cat, two separate pools of phrenic motoneurones have been described, those recruited ‘early’ and those recruited ‘late’ in inspiration (Hilaire et al. 1972; Nail et al. 1972; Berger, 1979; Dick et al. 1987). Activation of the diaphragm is achieved both by increases in the discharge frequency of phrenic motoneurones and by recruitment of additional motoneurones (Hilaire et al. 1972, 1983; Nail et al. 1972; Iscoe et al. 1976; Road & Cairns, 1997). In spinal and anaesthetized cats, recruitment of the phrenic motoneurones is believed to follow the ‘size principle’ (Iscoe et al. 1976; Dick et al. 1987; see below).
Much of the knowledge about the properties of single motor units in human limb muscles comes from experiments involving contractions under isometric conditions. Under these conditions, motoneurones increase their discharge rate as drive increases (see Kernell, 1965) and are usually recruited in a stable order (Milner-Brown et al. 1973; Desmedt & Godaux, 1977; cf. Grimby & Hannerz, 1977; Stephens et al. 1978; Nardone et al. 1989; Howell et al. 1995). The stable recruitment order is thought to reflect the ‘size principle’ by which, if all motoneurones in a pool receive the same excitatory and inhibitory drives, then recruitment is determined by factors related to the size of the motoneurones (Henneman, 1957; for review see Henneman & Mendell, 1981; Binder et al. 1996). Recent studies of motor unit recruitment in limb muscles have established the existence of task-dependent heterogeneous activation of the motoneurone pool and subsequent changes in recruitment patterns of motor units within a muscle (ter Haar Romeny et al. 1982, 1984; Chanaud et al. 1991; Riek & Bawa, 1992; Puckree et al. 1998), while in other muscles, such as first dorsal interosseous, recruitment order is stable regardless of the task (e.g. Enoka et al. 1989; Jones et al. 1994). However, for limb muscles there have been few studies of motor units during natural cyclic behaviour or when muscle length changes (Grimby, 1984). Biceps brachii and first dorsal interosseous have been studied during both isometric and isotonic contractions when motor unit recruitment order was not different (Thomas et al. 1987; Tax et al. 1989). The present study examined the behaviour of human diaphragmatic motor units during a range of carefully controlled voluntary breathing tasks, while the muscle shortened.
Data on the firing rates of human inspiratory motoneurones come from recordings of single motor unit activity in the parasternal intercostal muscles (Whitelaw & Watson, 1992; Gandevia et al. 1996), the scalenes (Gandevia et al. 1996) and the diaphragm (De Troyer et al. 1997). During quiet breathing, the discharge frequency of motor units for the parasternal intercostal muscles is ∼11 Hz (Whitelaw & Watson, 1992; Gandevia et al. 1996). For the diaphragm, the discharge frequency is ∼10 Hz in control subjects but increases to ∼18 Hz during quiet breathing in patients with chronic obstructive pulmonary disease (De Troyer et al. 1997) and to ∼18 Hz in control subjects with increased chemical drive to breathe (Gorman et al. 1999). In human subjects, the recruitment order of parasternal intercostal motor units appears to be stable during normal breathing (Watson & Whitelaw, 1987).
The present study was designed (i) to determine whether there are different groups of diaphragmatic motor units based on early and late recruitment; (ii) to measure the increases in discharge frequency of diaphragmatic motor units during voluntary increases in inspired volume and inspiratory flow; and (iii) to devise a method to assess recruitment order of multiple units and to apply it to determine the extent of changes in diaphragmatic motor unit recruitment. Some of the data have been presented as an abstract (Butler et al. 1997).
METHODS
Experiments were performed on four healthy subjects (3 male, 1 female) seated comfortably in a chair. The subjects had no significant history of respiratory or neurological illness. All procedures were approved by the local ethics committee and informed consent was obtained from the subjects.
Experimental set-up
Single motor unit activity was recorded using a Teflon-coated monopolar needle electrode with an exposed tip of 0.15 mm2. The electrode was inserted into the diaphragm through the 7th or 8th intercostal space close to the mid-clavicular line. The approach is below the reflection of the visceral pleura and is close to the origin of the costal fibres (Bolton et al. 1992). This minimizes the risk of pneumothorax and reduces needle movement associated with muscle shortening. The precise location and depth of needle insertion were guided by prior ultrasonography of the diaphragm and chest wall (Acuson, 128 XP14, CA, USA). Constant auditory feedback of EMG activity during needle insertion allowed monitoring of the progression of the electrode through the abdominal and intercostal muscles until it reached the diaphragm. Once positioned within the costal diaphragm, the electrode was manoeuvred to a site where single motor unit activity could be discriminated. Recordings of single diaphragmatic motor units were made from three to five separate needle penetrations of the diaphragm in each subject to enable sampling of 13-21 motor units from each subject. In two subjects, local anaesthetic (lignocaine, 2% with adrenaline, 0.5-1.0 ml) was injected into the intercostal muscles in the space overlying the diaphragm recording site prior to the insertion of the electrode. A surface reference electrode was placed over an adjacent rib 2-3 cm from the monopolar electrode and a large ground electrode was positioned over the right shoulder.
Measurements of inspiratory flow were made with a heated pneumotachometer and integrated to give inspired volume. Respiratory movements of the upper rib cage and the abdomen (at umbilical level) were recorded with inductance plethysmographs (Respitrace, Ambulatory Monitoring, Ardsley, NY, USA). End-tidal levels of CO2 were monitored throughout to ensure that overall levels of ventilation were constant (Ametek, Pittsburgh, PA, USA).
Experimental protocol
Subjects breathed through a mouthpiece during the experimental session and were instructed to follow one of seven different target ramp breaths (presented in random order). Feedback was provided on a monitor which was previously calibrated to the subject's vital capacity (VC). Thus, VC was used to standardize the inspiratory targets for each subject. The end-expiratory lung volume during quiet breathing approximates functional residual capacity (FRC) and is therefore referred to as FRC in the text. For half the target breaths, subjects inspired from their usual end-expiratory level (or FRC) to a constant lung volume (20% VC above FRC) with one of four inspiratory flows (20%, 10%, 5% or 2.5% VC s−1, profiles A-D in Fig. 2B). For the other half of the target breaths, subjects breathed with a constant inspiratory flow (5% VC s−1) to one of four lung volumes (40%, 20%, 10% or 5% VC above FRC, profiles E-H in Fig. 2C). One task (20% VC volume and 5% VC s−1 flow, profiles C and G in Fig. 2B and C) occurred in both groups of target breaths. At all sites at which single motor units were recorded, the subject performed at least three repetitions of each target breath for each task. Between target breaths, subjects were free to breathe quietly at a self-chosen volume and rate.
Figure 2. Rib cage and abdominal expansion in different voluntary target breaths.
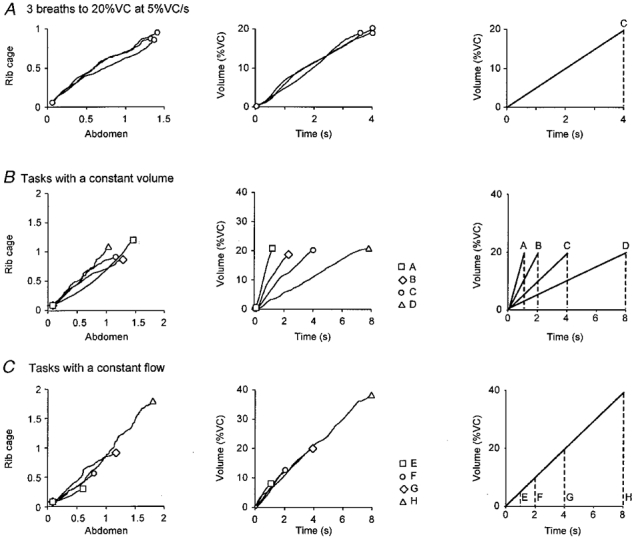
Superimposed plots of the rib cage and abdominal circumference (left panels) and lung volume-time traces (middle panels) for each inspiration: A, during three breaths within a task (20% VC at 5% VC s−1; profile C); B, for target breaths with a constant volume but different inspiratory flows (profiles A-D, see right panel); and C, for target breaths at a constant flow to different volumes (profiles E-H, see right panel). The gradients for the left panels indicate the relative contribution of the expansion of the rib cage and abdomen to the development of lung volume (middle panels). Values for rib cage and abdominal expansion are given in arbitrary units. Right panels show the profiles of target breaths.
Data analysis
Diaphragm EMG activity was sampled at 10 kHz (bandwidth, 16 Hz to 3.2 kHz; amplification, × 5000-10 000). All signals were stored on tape for subsequent analysis with a digital interface and spike analysis software (CED 1401 and Spike2, CED). In addition, we used custom-designed software to examine each single motor unit potential (e.g. see Gandevia et al. 1996; Leeper, 1998). Single motor units were sorted using a manually driven interactive program which allowed the user to label individual unit potentials based on their size and morphology (see Fig. 1B). At each recording site usually three to four single motor units could be distinguished. Figure 1 shows an example of five different single motor units that were recorded simultaneously and subsequently sorted.
Figure 1. Five simultaneously recorded single motor units in the diaphragm.
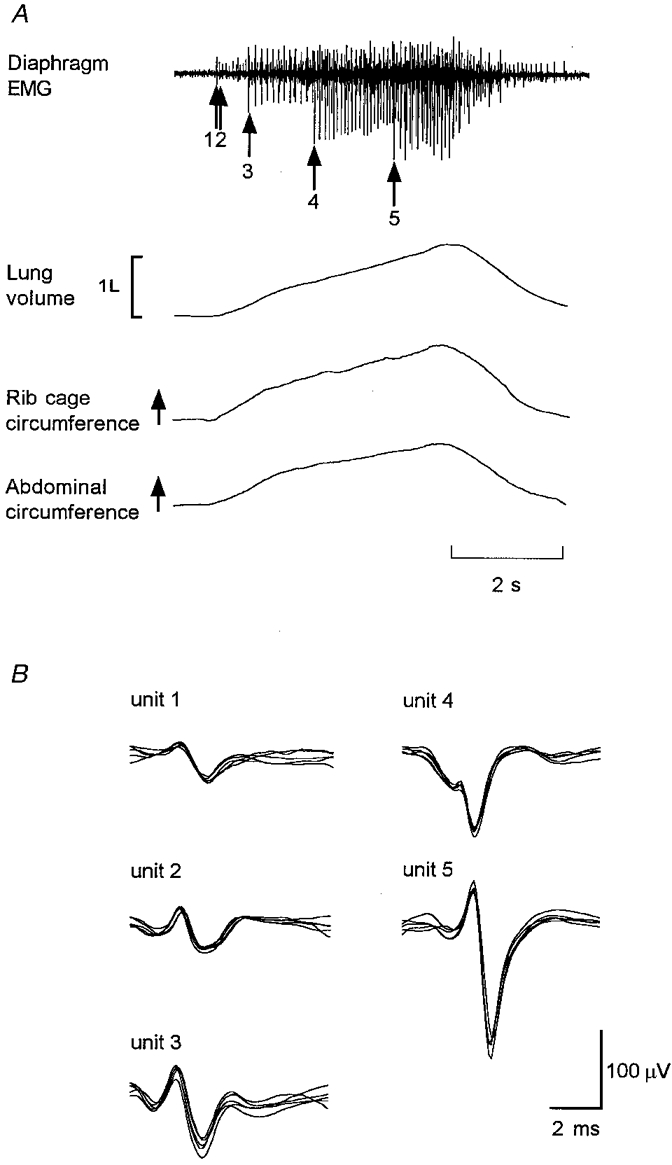
A, costal diaphragm EMG activity recorded during a single breath. Arrows indicate the recruitment time of each motor unit. Note the progressively increasing size of the recruited motor units in this site. Lung volume, rib cage expansion and abdominal expansion during the breath are shown. B, superimpositions of the five different single motor units recorded simultaneously in A at a single site sorted into groups on the basis of their size and shape.
Recruitment times of motor units and discharge frequencies
The recruitment time of each motor unit was measured relative to the actual onset of inspiration based on the volume signal. As diaphragmatic motor units in human subjects rarely discharge tonically (De Troyer et al. 1997), the initial discharge frequency was calculated from the first interspike interval for each individual motor unit. The few units that did discharge tonically (n = 7) were excluded from the analysis of onset time and initial frequency. A frequency histogram of the onset time of discharge (bin width, 100 ms) was used for each task to determine whether there were two separate populations of diaphragmatic single motor units based on onset times. The average onset time for each motor unit was also used to calculate the inspired volume at the time of recruitment and thus to determine the presence of a recruitment ‘threshold’ related to lung volume. In addition, a separate analysis based on the abdominal configuration at the time of recruitment of each motor unit within a task for each subject gave similar results to those when recruitment was based on the time after the onset of inspiration (see Results).
Measurements were also made of the average final discharge frequency for each motor unit during the voluntary inspiratory contractions. This was measured for each motor unit by averaging the instantaneous firing frequencies between two cursors positioned manually around the last 0.5-1.0 s of each inspiration. At this time, firing rates had increased from their initial value and were usually stable.
A sub-population of motor units showed an unusual recruitment pattern in that the volume at which they were recruited changed when the target volume was altered despite a constant flow. These units were termed ‘task-sensitive’ units and were labelled as such if, in tasks with the four inspiratory volumes reached at a constant inspiratory flow (5% VC s−1), the recruitment threshold increased progressively with an increase in volume across three or four of the tasks by greater than 5% of VC (i.e. greater than 1 s difference in recruitment time across tasks with a constant flow).
Data for the single motor units were analysed for the three breaths for each task. For each breath the onset times and the initial firing frequencies of a single motor unit were similar but not identical. Therefore, unless otherwise stated, in the results each unit is represented once for each measured breath.
Rib cage and abdominal expansion during different voluntary inspiratory tasks
Expansions of the rib cage and abdomen were measured throughout the study using calibrated inductance plethysmographs (Sackner et al. 1980; see also Konno & Mead, 1967). The contribution of each compartment to the inspired volume was determined with a plot of rib cage expansion against abdominal expansion, known as a Konno-Mead plot. The gradient of this line reveals the extent to which lung volume was developed by each compartment and gives an indication of the relative contribution of the diaphragm (represented largely by abdominal expansion) to the development of inspiration (Konno & Mead, 1967; see also Mead & Loring, 1982; Loring et al. 1985).
Recruitment order
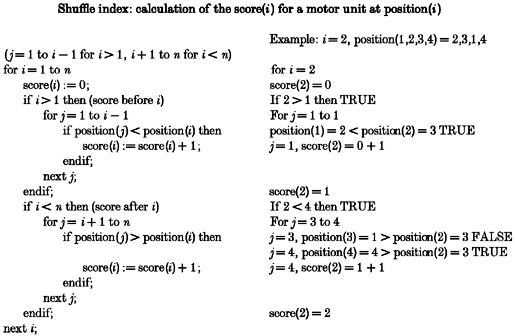
The recruitment order of the motor units was analysed for each task in which simultaneous recordings of three or more single motor units were obtained. The order of recruitment was then compared across three breaths within each task to test its stability and subsequently the stability of recruitment order across inspiratory tasks was assessed. To give a quantitative measurement of the variability of the recruitment order, we developed a measure of changes in the order of recruitment of simultaneously recorded motor units, termed the ‘shuffle’ index (see below). This index measures changes in the recruitment order of each unit, relative to the other units, in repetitions of a single task. It can also be used to compare any observed order of recruitment with a pre-determined or ‘reference’ order of recruitment. A shuffle index of 100% indicates complete reversal of recruitment order while a shuffle index of 0% indicates a stable recruitment order. Each breath in each voluntary task was assigned a calculated shuffle index that allowed statistical comparisons to be made about variability in recruitment order (from the reference order) in a given task. The calculation of the shuffle index within a voluntary task is explained below and given in more detail in the Appendix.
If four units (A, B, C and D) were recorded simultaneously over three breaths in a given task and were recruited in the following order: breath 1 - B,C,A,D; breath 2 - A,B,C,D; and breath 3 - A,B,C,D, the reference order would be A,B,C,D since this is the order which would result in the lowest median shuffle index for this task (see below).
For breath 1 with recruitment order B,C,A,D, we can calculate the shuffle index based on a score for each unit, given that the reference order defines the ‘correct’ order of recruitment for each task. Unit B: score = 2. Here, two of the units (C and D) are still recruited in the correct order relative to unit B; unit A is not (therefore, unit B receives a score of 2). Unit C: score = 2. Here, two of the units (B and D) are recruited in the correct order relative to unit C; unit A is not. Unit A: score = 1. Here, only one unit (D) is recruited in the correct order relative to unit A. Unit D: score = 3. Here, three of the units (B, C and A) are still recruited in the correct order relative to unit D even though they are not in order themselves.
For breath 1 (B,C,A,D) we calculate the shuffle index as follows. Observed total score = 2 + 2 + 1 + 3 = 8; maximal score for four units = 12. The maximal score is n(n - 1) where n is the number of simultaneously recorded motor units. The shuffle index =[1 - (observed total score/maximal score)]× 100, i.e. 33% for this breath 1.
For breaths 2 and 3 the shuffle index would be 0% since recruitment occurred in the reference order. The shuffle index for breath 1 = 33%, breath 2 = 0% and breath 3 = 0%. Therefore, the median shuffle index for this task is 0%. If the recruitment order was reversed (D,C,B,A), the shuffle index would be 100%. If one or more motor units did not discharge in one breath but were present in the reference order, it was assumed that the unrecruited units would have been recruited later in the breath in the correct order so that calculations of the shuffle index were still made on the full set of simultaneously recorded units. The Appendix gives values for the shuffle indices for four simultaneously recorded single motor units and describes the range of indices for two to six simultaneously recorded motor units.
Recruitment order of single motor units within and between voluntary tasks
For the measurement of variability of recruitment order within a task, the reference order was based on the most commonly observed recruitment order for a given set of units in a single task and the median shuffle index was determined for each task. If the motor units were recruited in a different order for each of the three breaths, and the reference order was not clear, it was necessary to calculate the median shuffle index for the task with each of the breaths acting as a temporary ‘reference’ breath. The final reference order was assigned to the breath that resulted in the lowest median shuffle index for the task.
For the measurement of the stability of recruitment order between tasks, the average recruitment time was calculated for each motor unit for the three breaths in each task and, from this, the average recruitment order was determined. The reference order was assigned to the task in which the subject breathed at 2.5% VC s−1 to a volume equivalent to 20% VC (i.e. profile D in Fig. 2B). This task was selected because it had the most stable order of recruitment across breaths (see Results). The shuffle index (between tasks) was then calculated for each task relative to the order in the reference task.
Although the study was not designed to compare voluntary and involuntary drive to breathe, subjects were free to breathe between the target breaths at a self-chosen rate and depth without any instruction, feedback or constraint. These breaths were considered to be non-targeted or involuntary. Measurements of the recruitment of single motor units and discharge properties in the non-target breaths were also made from each recording site in each subject. Results were compared, therefore, with data from the same units recorded in the voluntary inspiratory task that had the most closely matched profile of volume and flow to that in the non-targeted breath. Analyses were performed on 299 voluntary breaths and 28 involuntary breaths and the analyses were repeated up to 7 times depending on the number of units.
Statistics
For the measurements of discharge frequencies for each task, data are expressed as the median and interquartile range (IQ range). A Wilcoxon signed-rank test was used to compare initial and final discharge frequencies for each task. A Kruskal-Wallis one-way analysis of variance on ranks was used to compare data for discharge frequencies and shuffle indices across tasks with an all-pairwise multiple comparison procedure (Dunn's method) used as a post hoc test. Linear regression was used to determine significant correlations between recruitment time and the initial and final discharge frequencies. A one-way analysis of variance (ANOVA) and an all-pairwise multiple comparison procedure (Student-Newman-Keuls method) were used to compare the gradients derived from the Konno-Mead plots of abdominal and rib cage expansion, to compare strategies of performing the different tasks. Statistical significance was set at the 5% level.
RESULTS
Prolonged recordings were made of the activity of 60 single motor units from the right costal diaphragm in four subjects. Data were obtained from a total of 13 sites, with three or four sites studied in each subject. No complications arose from any of the recordings. Forty-seven units were studied as they discharged during four or more of the seven voluntary tasks. In addition, 38 units were studied during the involuntary non-targeted breaths between the target voluntary breaths.
To establish that the subjects performed the tasks reproducibly using a consistent strategy of expanding the rib cage and abdomen, we measured changes in circumference of the rib cage and abdominal compartments. Figure 2 shows data from a typical subject for expansion of the rib cage and expansion of the abdomen. During inspiration, both the abdomen and the rib cage expanded and the gradient of their linear relationship represents the relative contribution of the expansion of the two compartments to inspiratory volume. Within a repeated voluntary task the gradient was similar (see Fig. 2A). In individual subjects, there was no consistent change in the gradient of the Konno-Mead plot across the wide range of voluntary inspiratory tasks (see Fig. 2B and C). However, analysis of data for all subjects and tasks showed that the gradients were statistically similar except for the task with the smallest target volume (5% VC at 5% VC s−1; profile E). For this task, the gradient of the relationship between rib cage and abdominal expansion was significantly reduced. This indicates that, for this task, subjects used a proportionally larger expansion of the abdomen compared with the rib cage to achieve the target volume (thus suggesting an increased contribution from the diaphragm compared with other inspiratory muscles which expand the rib cage).
Recruitment times of motor units
Single motor units began to discharge at different times following the onset of inspiratory airflow (Fig. 3). However, we did not observe a bimodal distribution of recruitment times either for individual subjects or for the group of subjects in any of the voluntary tasks. Figure 3 shows a histogram of recruitment times for 55 units recorded during all breaths for one inspiratory task (20% VC at 5% VC s−1). For this task half of the motor units had been recruited in the first quarter of inspiration (by 25%Ti, where Ti is total inspiratory time), and 82% had been recruited half-way through the breath (by 50%Ti). Thus, the majority of the motor units were recruited early in the target breath and fewer numbers were recruited throughout the remainder of the breath.
Figure 3. Frequency histogram of onset times for diaphragmatic motor units from one task.
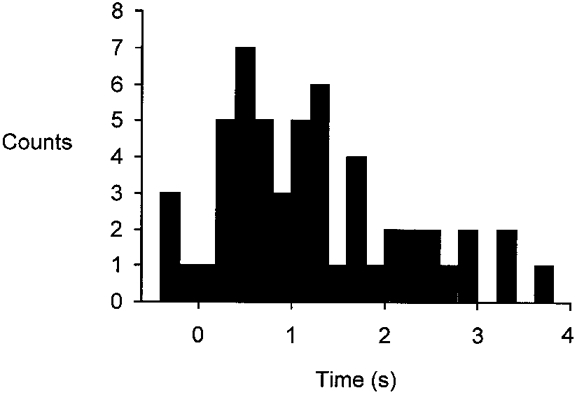
Frequency histogram (bin width, 200 ms) for 55 single motor units recorded from all subjects during a single task (20% VC at 5% VC s−1; profiles C and G). Each unit is represented once by its median onset time. Most units are recruited in the first half of the breath with peak recruitment in less than 25% of inspiratory time, with fewer units recruited thereafter. Time is expressed in relation to the onset time of the increase in lung volume.
There was a systematic difference in the degree of recruitment across tasks. For the shortest breaths (profiles A and E, 1 s duration), fewer motor units were recruited by mid-inspiration (50%Ti). As the duration of the breath increased (see profiles D and H, 8 s duration), more motor units were recruited by 50%Ti (Fig. 4). This implies that, with a constant flow, most motor units have been recruited by 20% VC (Fig. 4B).
Figure 4. Recruitment pattern of diaphragmatic single motor units for each voluntary task.
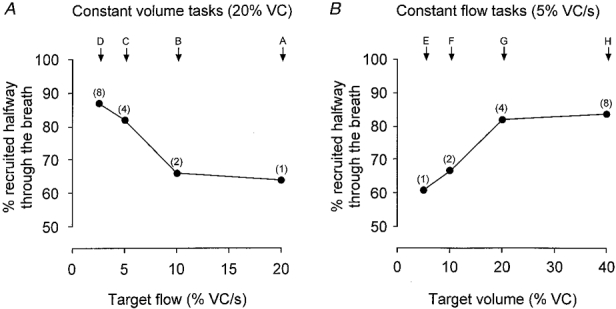
A, data for breaths to a constant volume at different flows. B, data for breaths with a constant flow to different lung volumes. Y-axis represents the percentage of motor units recruited by mid-inspiration (50%Ti). The number of breaths analysed in each task ranged from 118 to 219 for A and 68 to 219 for B. Numbers in parentheses indicate the duration of the inspiratory task in seconds. For the shortest breaths (profiles A and E) fewer motor units were recruited by 50%Ti, while for the longer breaths (profiles D and H) more motor units were recruited by 50%Ti.
Most motor units exhibited a recruitment threshold related to lung volume and this remained relatively constant across the voluntary tasks. Thus, when the breaths to a constant volume required a low inspiratory flow, a unit would begin to discharge at a later time and when the flow was faster, the unit discharged earlier. A typical example of one motor unit is shown in Fig. 5A which gives frequency plots for each voluntary task (A-H). This unit was consistently recruited when the volume was 18-20% VC above the usual end-expiratory level or FRC. However, about 20% of the motor units behaved slightly differently. These were termed ‘task-sensitive’ units. An example is shown in Fig. 5B. When the target volume was fixed (Fig. 5B, left column, tasks A-D), the volume at the time of recruitment of the task-sensitive unit was relatively constant (recruited at a volume about 20% VC above FRC). However, when inspiratory flow was constant, the volume at which this motor unit was recruited depended on the size of the breath (Fig. 5B, right column, tasks E-H). When the target volume was large (40% VC at 5% VC s−1; Fig. 5B, task H), the same unit was recruited later than would be expected on the basis of a volume threshold alone (at about 25% VC above FRC). In addition, when the target volume was small (5% VC at 5% VC s−1; Fig. 5B, task E), these motor units were often recruited earlier than expected on the basis of a volume threshold alone (at as little as 5% VC above FRC). These units were deemed to show task-sensitive recruitment (see Methods). The task-sensitive units were always recruited later in the tasks with the higher target volume and there were no instances where the reverse occurred. This phenomenon was observed for 13 of the 60 motor units. It was observed in recordings from about half the recording sites (range, 0-3 units site−1) and was observed at least once in each subject. The behaviour was repeated on sequential target breaths, and it was observed while simultaneously recorded units did not change the volume at which they were recruited across tasks. Figure 6 shows another example of a task-sensitive unit recorded at the same time as a unit with a threshold related to lung volume. One effect of task-sensitive recruitment is that the recruitment order of some simultaneously recorded motor units could be altered between tasks (Fig. 6; also see below).
Figure 5. Instantaneous frequency plots for two simultaneously recorded diaphragmatic motor units for all the voluntary tasks.
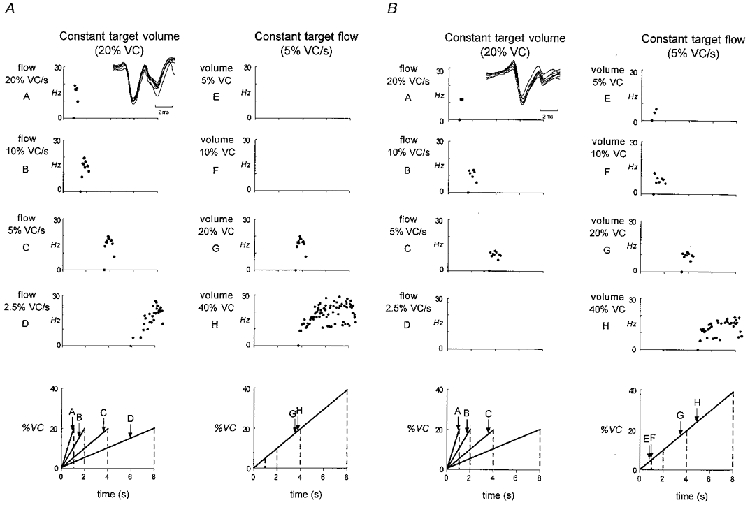
A, instantaneous frequency plots for a single motor unit for each breath profile. Left panels represent target breaths to a constant lung volume at different inspiratory flows (profiles A-D). Right panels represent target breaths with a constant flow to different lung volumes (profiles E-H). The first point on the abscissa (•) represents the onset time of firing for the unit. The next point represents the first interspike interval. Note this single motor unit has a recruitment threshold related to lung volume. Superimposed action potentials from this unit are shown as an inset in the upper left panel. Lower panels represent the different breath profiles (A-H). B, format is the same as for A, showing an example of a single motor unit recorded simultaneously which was not recruited at a constant volume in tasks with a constant flow (profiles E-H). Note the early onset of firing in the task with the small breath (profile E) compared with the late onset of firing in the large breath (profile H). Superimposed action potentials from this ‘task-sensitive’ unit are shown as an inset in the upper left panel.
Figure 6. Volume threshold and task-sensitive recruitment of two simultaneously recorded single motor units from the diaphragm.
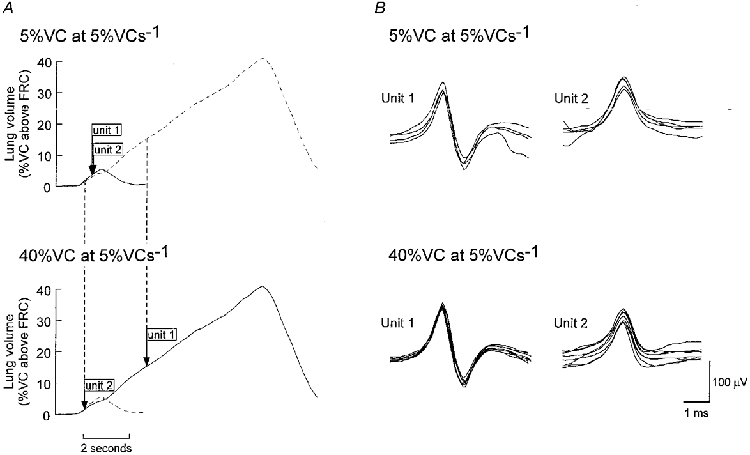
A, lung volume profiles of two voluntary inspiratory tasks with the same target flow but different target volumes (5% VC at 5% VC s−1, profile E (continuous line, upper panel) and 40% VC at 5% VC s−1, profile H (continuous line, lower panel)). Tasks were performed by the same subject while recordings were made from a site in the right costal diaphragm. Arrows indicate the average time of recruitment based on three trials for the two units. B, two different single motor units recorded simultaneously which discharged in each voluntary task (Unit 1 and Unit 2). Upper panels show superimpositions of every discharge of the two units for the smaller breath (5% VC at 5% VC s−1, profile E). Lower panels show superimpositions of the first eight discharges of the two units for the larger breath. Unit 2 was recruited with a threshold related to lung volume at approximately 1-3% of VC above FRC in both tasks. Unit 1 showed task-sensitive behaviour since it was recruited at about 3% of VC above FRC in the breath with the small target volume but was recruited at about 14% of VC above FRC in the breath with the large target volume. Note that the recruitment order of the units was reversed for these breaths.
The median change in time of recruitment for all the task-sensitive units in tasks with a constant flow was 2.0 s (IQ range, 1.6-2.8 s) which resulted in a 10% VC (8-14% VC) change in recruitment volume. For a single task (20% VC at 5% VC s−1), the recruitment times, initial discharge frequencies and final discharge frequencies were not significantly different for the set of task-sensitive units (n = 13) compared with ‘normal’ units which did not show task-sensitive recruitment (n = 42).
Initial and final discharge frequencies
Across all the target breaths, the median firing frequency at the onset of inspiratory discharge for all the motor units was 6.5 Hz (range across tasks, 4.8-9.3 Hz). For the tasks with a constant flow of 5% VC s−1, the median initial firing frequency was 6.5 Hz and, not surprisingly, this was not influenced by the intended size of inspiration (P > 0.05, Kruskal-Wallis test). For the inspiratory target breaths with varying flows, the initial discharge frequencies increased from 4.8 Hz (IQ range, 1.5-6.9 Hz) for low target flows to 9.3 Hz (IQ range, 5.6-13.1 Hz) for high target flows (P < 0.05, Wilcoxon signed-rank test). For the target breath of 20% VC at 5% VC s−1, the median initial firing frequency was 6.4 Hz (IQ range, 3.8-9.0 Hz). Figure 7 shows the median and IQ range for the initial firing frequencies (filled circles) of all motor units for each task.
Figure 7. Initial and final discharge frequencies of diaphragmatic motor units for all voluntary tasks.
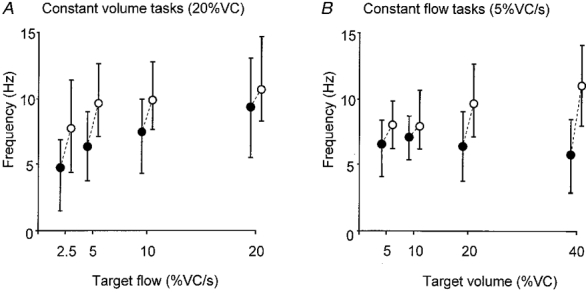
Median values and the IQ range for the initial discharge frequencies (•) and the final discharge frequencies (^) for each task in the same motor units. A, data for breaths to a constant volume at different flows. B, data for breaths with a constant flow to different lung volumes. Dashed lines link the median initial and final discharge frequencies for each task. Note the discharge frequencies are recorded for the same motor units for each task.
For each target breath, the firing frequency increased significantly from the initial rate by 0.8-5.2 Hz to reach the final firing frequency (P < 0.05). Across all target breaths the median for the final firing frequency during inspiration was 9.7 Hz (median frequency range across tasks, 7.8-11.0 Hz). Final discharge frequencies increased from 7.8 to 10.7 Hz as inspiratory flow increased 8-fold (P < 0.05) and increased from 8.0 to 11.0 Hz as target lung volume increased 8-fold (P < 0.05; Fig. 7, open circles). There was no correlation between the time of recruitment and the initial or final firing frequency in any of the target breaths (r2 ranged from 0.009 to 0.0787, all P > 0.05). However, as shown in Fig. 8, there was a significant correlation between the initial and final firing frequencies for each task (r2 ranged from 0.12 to 0.30, all P < 0.05).
Figure 8. Relationship between initial and final discharge frequencies.
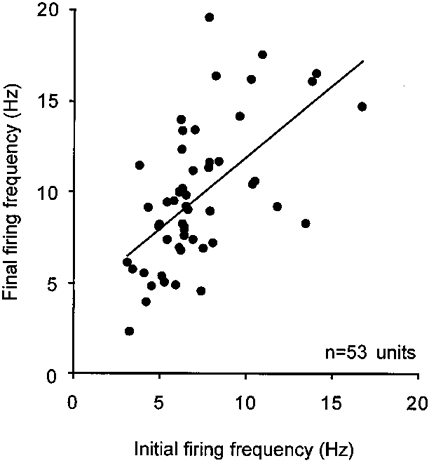
Plot of initial discharge frequency against final frequency for 53 units recorded during a single task (20% VC at 5% VC s−1). Each unit is represented once by the average discharge frequency derived from three breaths. Line fitted to the data shows a significant relationship between the initial and final discharge frequencies (linear regression, r = 0.35, P < 0.05).
Recruitment order
As described above, while the majority of diaphragmatic motor units were recruited at a particular inspiratory target volume, a minority changed their recruitment consistently according to the inspired volume required (Figs 5B and 6). Before comparisons could be made of recruitment order between tasks, it was necessary to measure the variability in recruitment order within a task. This was quantified by the shuffle index which took into account the extent to which the order of recruitment of three or more units recorded simultaneously changed when the same task was repeated (see Methods; see also Appendix).
Median shuffle indices for breaths in the same task ranged from 0% for some tasks (i.e. completely reproducible order of recruitment) to 17% for the task with the fastest flow (profile A; see Table 1). All shuffle indices were relatively low and represent a small degree of change in recruitment order. However, the shuffle index was not the same for all seven inspiratory tasks (Kruskal-Wallis, P < 0.05). Post hoc analysis revealed that the long slow inspiration had the lowest shuffle index (profile D, 20% VC at 2.5% VC s−1; see Figs 2 and 5), while the other tasks were not significantly different from one another.
Table 1.
Summary of shuffle indices (SI) for recruitment order of diaphragmatic motor units within and between the reference task and the other tasks
| Task | Inspiratory time(s) | Volume(%VC) | Flow(%VC s−1) | SI within tasks(%) | IQ range(%) | SI between tasks‡(%) | IQ range(%) |
|---|---|---|---|---|---|---|---|
| A | 1 | 20 | 20 | 17.0 | 16.9–33.0 | 33* | 17–43 |
| B | 2 | 20 | 10 | 0 | 0–16.5 | 7 | 0–17 |
| C | 4 | 20 | 5 | 10.0 | 0–20.0 | 27* | 18–46 |
| D | 8 | 20 | 2.5 | 0† | 0–0 | 0 | ref. task |
| E | 1 | 5 | 5 | 8.5 | 0–18.3 | 30* | 7–46 |
| F | 2 | 10 | 5 | 4.1 | 0–16.8 | 25* | 17–40 |
| G | 4 | 20 | 5 | 10.0 | 0–20.0 | 27* | 18–46 |
| H | 8 | 40 | 5 | 8.5 | 0–17.5 | 12 | 0–33 |
Significant difference from other tasks (P < 0.05)
significant difference from reference (ref.) task (P < 0.05)
overall shuffle indices between tasks greater than those within tasks (P < 0.05).
A shuffle index was also calculated for each inspiratory task at each recording site to quantify the changes in recruitment order between tasks. The ‘reference’ inspiratory task was 20% VC at 2.5% VC s−1 (Fig. 2B, profile D) because its recruitment order was highly reproducible. The shuffle indices between tasks with respect to the reference task ranged from a median of 7 to 33% (Table 1). While there was some variability in the recruitment order within a task, the variability between each task and the reference task was significantly greater (represented by the increased shuffle index, P < 0.05). Post hoc analysis revealed that there was a significant difference between the shuffle indices for the reference task and tasks A, C, E, F and G, but not for tasks B and H (Table 1). In summary, the voluntary tasks contribute to the variability of recruitment order and some of this variability is unaccounted for by the variability within tasks.
Non-targeted breaths
For each subject, the involuntary non-targeted breaths between the target breaths were matched as closely as possible to one of the voluntary tasks. In two subjects this corresponded to breaths with a size of 10% VC with an inspiratory flow of 5% VC s−1, and in the other two subjects it corresponded to breaths with a size of 20% VC and a flow of 10% VC s−1. The initial and final discharge frequencies of the single motor units recorded during involuntary breaths (6.6 Hz; IQ range, 4.9-8.0 Hz; and 8.5 Hz; IQ range, 6.9-10.1 Hz, respectively; n = 40) were not significantly different from those in the matched voluntary inspiratory breaths. This suggests that the same population of units was recruited and discharged similarly in the voluntary targeted breathing and in the involuntary non-targeted breathing.
The recruitment order of motor units in non-targeted breaths was significantly different from the normal variability of recruitment order within a targeted voluntary task. The median shuffle index for the involuntary non-targeted breaths with reference to the recruitment order of the same motor units in the ‘matched’ targeted voluntary breaths was 33% (IQ range, 29-53%). This median is comparable to that between voluntary tasks. Although there were changes in the recruitment order of motor units between voluntary and involuntary breaths, the variability in recruitment order for involuntary non-targeted breaths was no greater than the variability between targeted voluntary tasks and the reference task. All of the units from the involuntary breaths were also recruited in the matched voluntary breaths, although at one site, two extra units were recruited late in the voluntary breaths.
DISCUSSION
This study provides the first data on the recruitment times and discharge properties of human diaphragmatic motor units during voluntary inspiratory tasks and their dependence on inspiratory flow and volume. We have examined the recruitment times and rate modulation of single motor units across a range of different voluntary tasks and have developed a method for quantifying variability in the recruitment order of simultaneously recorded single motor units.
Previous studies of the recruitment of phrenic motoneurones in the cat (e.g. Hilaire et al. 1972; Nail et al. 1972) have developed the concept of two separate populations of phrenic motoneurones whose order and time of recruitment is not due to differences in excitability alone, but also to differences in central respiratory drive. In the current study, while we recorded units with a wide range of onset times and over a wide range of voluntary tasks, we saw no evidence of a bimodal distribution of recruitment times. Most units were recruited early in the breath and the remainder were gradually recruited as inspiration progressed. Although several studies have described ‘early’ and ‘late’ motoneurones (e.g. Hilaire et al. 1972; Nail et al. 1972; Berger, 1979; Dick et al. 1987; Whitelaw & Watson, 1992), the distinction has been somewhat arbitrary and not sufficiently great to produce a completely bimodal distribution of recruitment times. In fact, the pattern of recruitment described in the current study across a range of different sized breaths is similar to that for single motor units in the human parasternal intercostal muscles (Whitelaw & Watson, 1992) and for phrenic motoneurones in the cat and rabbit (Iscoe et al. 1976; Road & Cairns, 1997).
During voluntary inspiratory efforts, the initial firing frequencies of 60 diaphragmatic motor units increased in tasks with higher inspiratory flows (range, 4.8-9.3 Hz), and the final firing frequencies of the diaphragmatic motor units increased as voluntary inspiratory flow and target volume increased (range, 7.9-11.0 Hz). These findings provide support for the use of discharge frequency of a sample of motor units as an index of neural drive to the diaphragm (Gandevia et al. 1996; De Troyer et al. 1997). They are also consistent with observations, during quiet breathing, from human parasternal intercostal motor units, the initial and peak discharge frequencies of which correlated with inspiratory flow (Whitelaw & Watson, 1992). However, we found no correlation between recruitment time and initial or final discharge frequencies, i.e. late-recruited units did not begin to discharge at a higher rate (cf. Nail et al. 1972; Iscoe et al. 1976). The forces produced during tidal breathing and the inspiratory tasks in the current study are only a relatively small percentage (less than ∼25%) of the maximal force that can be produced by the diaphragm (Sieck & Fournier, 1989), and thus the observed discharge frequencies of motor units were probably well below their maxima. The final discharge rate across all the voluntary tasks was about half that observed in patients with severe airflow limitation (18 Hz) breathing quietly (De Troyer et al. 1997) and about half that observed in control subjects breathing with ventilation increased 2- to 3-fold due to elevated chemical drive (Gorman et al. 1999).
Most diaphragmatic motor units were recruited with a volume threshold, but some units (13 out of 60) were recruited with a task-sensitive threshold. These single units (more obvious in tasks with a constant inspiratory flow) discharged later than expected when the target volume was high and earlier than expected when the target volume was small. This change in recruitment behaviour of the ‘task-sensitive’ units could result in subtle changes in recruitment order of other diaphragmatic motor units activated during the breath. It was the potential for slight changes in recruitment order in certain voluntary inspiratory tasks that prompted development of a ‘shuffle’ index to measure the stability of recruitment order for three or more motor units.
Previous studies have suggested that there are different strategies for taking breaths to low or high lung volumes. For example, for large breaths induced by exercise, expansion of the rib cage contributes proportionally more to the increased lung volume than expansion of the abdomen (Chapman et al. 1985). An alteration in the relationship between rib cage and abdominal expansion, such as this, could explain the apparent changes in recruitment thresholds. However, when we assessed the expansion of the rib cage and abdomen, overall there was no significant difference in the strategy for all the tasks except the smallest volume task in which there was proportionally greater abdominal expansion. In addition, this suggestion cannot explain why other units recorded simultaneously did not also discharge later than expected in larger breaths. While the present study has revealed a task-dependent contribution to the recruitment of human diaphragmatic motor units, it is impossible to determine whether it is caused by changes in the descending drives to the motoneurones (either cortical or brainstem in origin) or changes in reflex inputs from the lungs, upper airway or thoracic cage. These inputs may act at spinal or supraspinal sites.
Changes in recruitment order of single motor units can occur in human limb muscles when reflex input changes or the task performed is changed (see Grimby & Hannerz, 1977; Stephens et al. 1978; Desmedt, 1981; Nardone et al. 1989; Howell et al. 1995). Such changes in recruitment order may be a result of coactivation of different motor unit ‘groups’ within each muscle (ter Haar Romeny et al. 1982, 1984; van Zuylen et al. 1988; Chanaud et al. 1991; Riek & Bawa, 1992; Puckree et al. 1998). In addition, the effect of synaptic noise on fluctuations in the membrane potential of human motoneurones (e.g. Matthews, 1996) may contribute to the variability in recruitment threshold or recruitment order within a given task.
Furthermore, some changes in recruitment order have been reported for the human parasternal intercostal muscles during sleep and voluntary hyperventilation (Watson & Whitelaw, 1987; Whitelaw et al. 1993). However, marked increases in chemical drive do not change the recruitment order of phrenic motoneurones in the anaesthetized cat (Iscoe et al. 1976). In conscious human subjects, the inspiratory motoneurones will presumably receive descending input from respiratory ponto-medullary centres, and motor cortical and other areas. Voluntary interventions to change breathing even slightly alter the motor cortical output to inspiratory muscles (Macefield & Gandevia, 1991).
Comparisons between motor unit discharges in voluntary and involuntary non-targeted breaths in the current study can be used to compare two presumably different descending ‘inputs’ to inspiratory motoneurones. While there were no differences in the initial or final discharge frequencies of the motor units, the recruitment order for voluntary and involuntary breaths was not identical despite the fact that the involuntary breaths were matched as closely as possible to the voluntary ones. This suggests that there may be subtle differences in the descending (or other) drives reaching the motoneurones during voluntary and involuntary breathing.
In conclusion, human diaphragmatic motor units increase their discharge frequency with increased drive to breathe. Thus, the initial and final discharge frequencies of these units can be used as an index of inspiratory drive. Secondly, they are recruited throughout each task with no evidence for bimodal patterns of motor unit recruitment, and thus appear to behave similarly to motor units in limb muscles. Finally, recruitment order for the diaphragm is stable but not invariant across a range of voluntary and involuntary tasks.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia and the Asthma Foundation of New South Wales. We are grateful to Mr J. B. Leeper for assistance with the experiments, and to Dr J. L. Taylor for suggestions about the analysis of recruitment order. We thank Professor U. Proske and Mr R. Gorman for their comments on the manuscript.
Appendix
A ‘shuffle’ index was developed to give a quantitative measure of the variability of the recruitment order of three or more simultaneously recorded motor units. This index measures changes in the recruitment order of each unit, relative to other units, in repetitions of a task. It can also be used to compare any observed order of recruitment with a pre-determined or ‘reference’ order. The index was designed so that an index of 100% indicates complete reversal of recruitment order while an index of 0% indicates stable recruitment order. The shuffle index can be used when two or more units are studied. An index of 50% would be consistent with a random recruitment of units.
First, a reference order of recruitment is chosen. It can be the commonest order for a particular task, or the order from a task with the most stable recruitment order. Then, for each unit, a ‘score’ relative to the reference order is calculated. The ‘observed total score’ is the sum of the score of each unit. To obtain the shuffle index, the observed total score is expressed relative to the ‘maximal score’ for the number of simultaneously recorded units.
The general formula for calculating the shuffle index for a set of simultaneously recorded motor units is given below:
| (A1) |
The maximal score =n(n - 1), where n is the number of simultaneously recorded motor units.
Algorithm 1.
The algorithm for calculation of the observed total score is more complex and is shown above (Algorithm 1), but it can also be calculated easily from the description in Methods. Each unit is given a numerical value based on its position in the reference recruitment order: value = 1, 2, 3 …n for the 1st, 2nd, 3rd … and nth recruited units.
In the ‘test’ task, the recruitment order is expressed as: position(1), position(2), position(3) … position(n), where position(i) = value (from the reference task) of the ith unit recruited in the test task; i = 1 to n.
Example: for four units with order A,B,C,D in the reference task and order B,C,A,D in the test task then position(1,2,3,4) = 2,3,1,4.
For this task, the score of any unit equals the number of units that are in the correct order relative to it. To calculate a score for position(i)in this task, we count the number of units before position(i)(position(1) to position(i - 1), i > 1) with values less than the value of position(i) and add that number to the number of units after position(i) (position(i+ 1) to position(n), i < n) with values greater than the value of position(i). The scores for each position(i), i = 1 to n, are then added together to give the observed total score, i.e. observed total score =Σ(1 …n), and the final shuffle index is derived with eqn (A1).
The range of shuffle indices depends on the number of simultaneously recorded motor units. As the number of motor units increases the number of gradations in the shuffle index between 0% (ordered) and 100% (reversed order) increases. When there are only two motor units, there are only two possibilities for the shuffle index (0 or 100%; ordered or reversed). For six motor units, there are 15 steps between 0 and 100%. The number of steps between 0 and 100% increases as the number of motor units increases. The method becomes more sensitive at detecting changes in order when more units are recorded. In the current study, a shuffle index was only calculated for three or more simultaneously recorded motor units since there are only two possible recruitment orders when two units are recorded.
Table 2 shows all possible recruitment orders and the corresponding shuffle indices for four simultaneously recorded motor units (units A, B, C and D). A shuffle index can then be assigned to each repetition of a task to indicate the degree of change in the recruitment order from any designated reference order.
Table 2.
Calculated shuffle index for any ordered combination of four simultaneously recorded motor units
| Recruitment order | Observed total score | Maximal score | Shuffle index (%) | |
|---|---|---|---|---|
| 1 | ABCD | 12 | 12 | 0 |
| 2 | ABDC | 10 | 12 | 17 |
| 3 | ACBD | 10 | 12 | 17 |
| 4 | ACDB | 8 | 12 | 33 |
| 5 | ADBC | 8 | 12 | 33 |
| 6 | ADCB | 6 | 12 | 50 |
| 7 | BACD | 10 | 12 | 17 |
| 8 | BADC | 8 | 12 | 33 |
| 9 | BCAD | 8 | 12 | 33 |
| 10 | BCDA | 6 | 12 | 50 |
| 11 | BDAC | 6 | 12 | 50 |
| 12 | BDCA | 4 | 12 | 67 |
| 13 | CABD | 8 | 12 | 33 |
| 14 | CADB | 6 | 12 | 50 |
| 15 | CBAD | 6 | 12 | 50 |
| 16 | CBDA | 4 | 12 | 67 |
| 17 | CDAB | 4 | 12 | 67 |
| 18 | CDBA | 2 | 12 | 83 |
| 19 | ABC | 6 | 12 | 50 |
| 20 | DACB | 4 | 12 | 67 |
| 21 | DBAC | 4 | 12 | 67 |
| 22 | DBCA | 2 | 12 | 83 |
| 23 | DCAB | 2 | 12 | 83 |
| 24 | DCBA | 0 | 12 | 100 |
A shuffle index of 0% indicates a stable recruitment order while a shuffle index of 100% indicates complete reversal of recruitment order. From the table it is possible to see that a random sample of recruitment orders would result in an average or median shuffle index of 50%.
References
- Berger AJ. Phrenic motoneurones in the cat: subpopulations and nature of respiratory drive potentials. Journal of Neurophysiology. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press, American Physiological Society; 1996. pp. 3–53. [Google Scholar]
- Bolton CF, Grand'Maison F, Parkes A, Shkrum M. Needle electromyography of the diaphragm. Muscle and Nerve. 1992;15:678–681. doi: 10.1002/mus.880150608. [DOI] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Leeper JB, Gandevia SC. Behaviour of single motor units in the human diaphragm during voluntary inspiratory tasks. Proceedings of the Congress of the 33rd International Union of Physiological Sciences; 1997; p. P030.05. [Google Scholar]
- Chanaud CM, Pratt CA, Loeb GE. Functionally complex muscles of the cat hindlimb. V. The roles of histo-chemical fibre-type regionalization and mechanical heterogenity in differential muscle activation. Experimental Brain Research. 1991;85:300–313. doi: 10.1007/BF00229408. [DOI] [PubMed] [Google Scholar]
- Chapman KR, Perl A, Zamel N, Rebuck AS. Thoracoabdominal motion during hypercapnia, hypoxia, and exercise. Canadian Journal of Physiology and Pharmacology. 1985;63:188–192. doi: 10.1139/y85-035. [DOI] [PubMed] [Google Scholar]
- Desmedt JE. The size principle of motor unit recruitment in ballistic or ramp voluntary contractions in man. In: Desmedt JE, editor. Progress in Clinical Neurophysiology, Motor Unit Types, Recruitment and Plasticity in Health and Disease. Vol. 9. Basel, Switzerland: Werner Druck AG; 1981. pp. 97–136. [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. The Journal of Physiology. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. American Journal of Respiratory and Critical Care Medicine. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. Journal of Neurophysiology. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. Journal of Neurophysiology. 1989;62:1344–1359. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Leeper JB, McKenzie DK, De Troyer A. Discharge frequencies of parasternal intercostal and scalene motor units during breathing in normal and COPD subjects. American Journal of Respiratory and Critical Care Medicine. 1996;153:622–628. doi: 10.1164/ajrccm.153.2.8564108. [DOI] [PubMed] [Google Scholar]
- Gorman RB, Gandevia SC, McKenzie DK, De Troyer A. Increased ventilatory drive causes non-uniform increases in motor unit firing rate in human inspiratory muscles. Respirology. 1999;4(suppl.):A14. doi: 10.1164/ajrccm.160.5.9904023. [DOI] [PubMed] [Google Scholar]
- Grimby L. Firing properties of single human motor units during locomotion. The Journal of Physiology. 1984;346:195–202. doi: 10.1113/jphysiol.1984.sp015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L, Hannerz J. Firing rate and recruitment order of toe extensor muscles in different modes of voluntary contraction. The Journal of Physiology. 1977;264:865–879. doi: 10.1113/jphysiol.1977.sp011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 423–507. [Google Scholar]
- Hilaire G, Gauthier P, Monteau R. Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respiration Physiology. 1983;51:341–359. doi: 10.1016/0034-5687(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Dussardier M. Modalités du recrutement des motoneurones phréniques. The Journal of Physiology (Paris) 1972;64:457–478. [PubMed] [Google Scholar]
- Howell JN, Fuglevand AJ, Walsh ML, Bigland-Ritchie B. Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseus muscle. Journal of Neurophysiology. 1995;74:901–904. doi: 10.1152/jn.1995.74.2.901. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respiration Physiology. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Jones KE, Lyons M, Bawa P, Lemon RN. Recruitment order of motoneurons during functional tasks. Experimental Brain Research. 1994;100:503–508. doi: 10.1007/BF02738409. [DOI] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiologica Scandinavica. 1965;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. Journal of Applied Physiology. 1967;22:407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- Leeper JB. An interactive routine for sorting motor unit spikes. Sydney: Masters project report, University of New South Wales; 1998. [Google Scholar]
- Loring SH, Mead J, Griscom NT. Dependence of diaphragmatic length on lung volume and thoracoabdominal configuration. Journal of Applied Physiology. 1985;59:1961–1970. doi: 10.1152/jappl.1985.59.6.1961. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. The Journal of Physiology. 1991;439:545–558. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. The Journal of Physiology. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J, Loring SH. Analysis of volume displacement and length changes of diaphragm during breathing. Journal of Applied Physiology. 1982;53:750–755. doi: 10.1152/jappl.1982.53.3.750. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. Changes in the firing rate of human motor units during linearly changing voluntary contractions. The Journal of Physiology. 1973;230:371–390. doi: 10.1113/jphysiol.1973.sp010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurones. Progress in Neurobiology. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Nail BS, Sterling GM, Widdicombe JG. Patterns of spontaneous and reflexly-induced activity in phrenic and intercostal motoneurones. Experimental Brain Research. 1972;15:318–332. doi: 10.1007/BF00235915. [DOI] [PubMed] [Google Scholar]
- Nardone A, Romano C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. The Journal of Physiology. 1989;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckree T, Cerny F, Bishop B. Abdominal motor unit activity during respiratory and nonrespiratory tasks. Journal of Applied Physiology. 1998;84:1707–1715. doi: 10.1152/jappl.1998.84.5.1707. [DOI] [PubMed] [Google Scholar]
- Riek S, Bawa P. Recruitment of motor units in human forearm extensors. Journal of Neurophysiology. 1992;68:100–108. doi: 10.1152/jn.1992.68.1.100. [DOI] [PubMed] [Google Scholar]
- Road JD, Cairns AM. Phrenic motoneuron firing rates before, during, and after prolonged inspiratory resistive loading. Journal of Applied Physiology. 1997;83:776–783. doi: 10.1152/jappl.1997.83.3.776. [DOI] [PubMed] [Google Scholar]
- Sackner JD, Nixon AJ, Davis B, Atkins N, Sackner MA. Non-invasive measurement of ventilation during exercise using a respiratory inductive plethysmograph. I. American Review of Respiratory Disease. 1980;122:867–871. doi: 10.1164/arrd.1980.122.6.867. [DOI] [PubMed] [Google Scholar]
- Sears TA. The slow potentials of thoracic respiratory motoneurones and their relation to breathing. The Journal of Physiology. 1964;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. Journal of Applied Physiology. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Smith JC, Liu G, Feldman J. Intracellular recording from phrenic motoneurons receiving respiratory drive in vitro. Neuroscience Letters. 1988;88:27–32. doi: 10.1016/0304-3940(88)90310-2. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Garnett R, Buller NP. Reversal of recruitment order of single motor units by cutaneous stimulation during voluntary contraction in man. Nature. 1978;272:362–364. doi: 10.1038/272362a0. [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Gielen CCAM, van den Tempel C M. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Experimental Brain Research. 1989;76:55–63. doi: 10.1007/BF00253623. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, Denier van der Gon JJ, Gielen CCAM. Changes in recruitment order of motor units in the human biceps muscle. Experimental Neurology. 1982;78:360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Ter Haar Romeny BM, van der Gon JJ, Gielen CCAM. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Experimental Neurology. 1984;85:631–650. doi: 10.1016/0014-4886(84)90036-0. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH, Calancie B. Human motor-unit recruitment during isometric contractions and repeated dynamic movements. Journal of Neurophysiology. 1987;57:311–324. doi: 10.1152/jn.1987.57.1.311. [DOI] [PubMed] [Google Scholar]
- van Zuylen EJ, Gielen CCAM, Denier van der Gon JJ. Coordination and inhomogeneous activation of human arm muscles during isometric torques. Journal of Neurophysiology. 1988;60:1523–1548. doi: 10.1152/jn.1988.60.5.1523. [DOI] [PubMed] [Google Scholar]
- Watson TWJ, Whitelaw WA. Voluntary hyperventilation changes recruitment order of parasternal intercostal motor units. Journal of Applied Physiology. 1987;62:187–193. doi: 10.1152/jappl.1987.62.1.187. [DOI] [PubMed] [Google Scholar]
- Whitelaw WA, Rimmer KP, Sun HS. Change in recruitment order of motor units in human parasternal intercostal muscles with sleep state. Journal of Applied Physiology. 1993;74:2718–2723. doi: 10.1152/jappl.1993.74.6.2718. [DOI] [PubMed] [Google Scholar]
- Whitelaw WA, Watson TWJ. Spike trains from single motor units in human parasternal intercostal muscles. Respiration Physiology. 1992;88:289–298. doi: 10.1016/0034-5687(92)90003-f. [DOI] [PubMed] [Google Scholar]


