Abstract
Responses to capsaicin in isolated sensory neurones have been shown to desensitize in a Ca2+- and voltage-dependent manner. We have studied desensitization of capsaicin-activated currents in cultured adult rat dorsal root ganglion (DRG) neurones over a range of membrane potentials using whole-cell patch-clamp techniques.
Acute desensitization of responses to capsaicin (0.5 μM) was significantly less when the holding potential (Vh) was +40 mV rather than -60 mV. This was not due only to reduced Ca2+ entry as the response to capsaicin was desensitized by the same amount whether prior exposure to capsaicin was at -60 or +40 mV. The I-V relationship for capsaicin-induced current, determined using a voltage step protocol, was outwardly rectifying and during the acute phase of desensitization the degree of outward rectification increased.
Acute desensitization and the increase in outward rectification that accompanied desensitization were inhibited when cells were dialysed with the rapid Ca2+ chelator BAPTA. Addition of a pseudosubstrate inhibitor of the Ca2+-calmodulin-dependent enzyme calcineurin (CI, 100 μM) prevented the increase in outward rectification although it did not cause a significant decrease of acute desensitization.
Removal of external Ca2+ or Mg2+ did not reverse the increase in outward rectification of capsaicin-activated current after Ca2+-dependent desensitization had occurred. This indicates that a voltage-dependent block of the capsaicin-activated ion channel by Ca2+ or Mg2+ was not responsible for the observed changes in the properties of the capsaicin-activated conductance.
Capsaicin is a chemical activator of peripheral sensory neurones (Wood & Docherty, 1997) that causes acute pain, secondary hyperalgesia and local erythema when it is injected into the skin (LaMotte et al. 1991). It opens vanilloid-gated non-selective cation channels in the plasma membrane of some C- and Aδ-fibre sensory neurones (Bevan & Szolcsányi, 1992), depolarizes and excites them and thereby causes pain and neurogenic inflammation (Holzer, 1991). Several studies of the action of capsaicin and related vanilloid compounds on isolated sensory neurones have been published recently (Liu & Simon, 1996; Petersen et al. 1996; Liu et al. 1997, 1998; Koplas et al. 1997; Zeilhofer et al. 1997; for earlier references see Wood & Docherty, 1997). There is convincing evidence that vanilloids evoke multiple currents in neurones due to the existence of receptor/ion channel subtypes that display different properties (see Szallasi, 1994; Liu & Simon, 1996; Petersen et al. 1996; Liu et al. 1997). All these authors agree that there is a wide cell-to-cell variation in the size of capsaicin responses in individual cells and the extent to which they desensitize. Further, all the authors agree that the vanilloid-gated ion channel is permeable to Ca2+ and that Ca2+ plays an important role in the development of desensitization. The cDNA encoding a capsaicin-activated ion channel has been identified and cloned (Caterina et al. 1997). This capsaicin receptor, named VR1, is exclusively expressed in sensory neurones. Experiments where the cloned VR1 receptor has been expressed heterologously in either Xenopus oocytes or human embryonic kidney (HEK) cells have confirmed most of the data obtained from studies of the native receptor in sensory neurones and have shown in addition that the receptor responds to a noxious heat stimulus (Caterina et al. 1997).
Paradoxically capsaicin and related vanilloid compounds display analgesic and anti-inflammatory properties (Szallasi & Blumberg, 1996). The mechanisms of the analgesic and anti-inflammatory effects of capsaicin are poorly understood but could be due to cross-desensitization of vanilloid receptors with other noxious chemical or environmental stimuli. Studies of the cellular mechanisms of vanilloid receptor desensitization are therefore of considerable importance in understanding the analgesic and anti-inflammatory actions of vanilloid drugs. Desensitization of vanilloid-gated ion channels is composed of at least two separate cellular mechanisms. A fast process that depends on an increase in intracellular Ca2+ (Yeats et al. 1992a, b; Cholewinski et al. 1993; Bevan & Docherty, 1993; Docherty et al. 1996; Liu & Simon, 1996; Koplas et al. 1997) accounts for most of the acute desensitization. There is also a slower process of desensitization that can occur in the absence of extracellular Ca2+ (Yeats et al. 1992a; Docherty et al. 1996; Liu & Simon, 1996; Koplas et al. 1997). Vanilloid-gated ion channels are permeable to Ca2+ (Wood et al. 1988; Bevan & Szolcsányi, 1990; Vlachová & Vyklický, 1993; Oh et al. 1996; Caterina et al. 1997) and Ca2+-dependent desensitization normally relies on entry of Ca2+ through active ion channels (Cholewinski et al. 1993; Docherty et al. 1996; Koplas et al. 1997). Desensitization of active channels may also be triggered by the release of intracellular Ca2+ caused by agents such as caffeine (Koplas et al. 1997). There is good evidence that the rate at which acute Ca2+-dependent desensitization develops is increased by dephosphorylation of the ion channels by the Ca2+- and calmodulin-dependent enzyme calcineurin (Yeats et al. 1992a; Docherty et al. 1996).
A feature of desensitization of vanilloid-gated ion channels is that it is reduced or slowed at positive membrane potentials (Yeats et al. 1992b; Bevan & Docherty, 1993; Liu & Simon, 1996). Our main objective in the present study was to quantify the desensitization that occurs at positive and negative membrane potentials and to determine whether the voltage dependence occurs due to differences in the electrochemical gradient for Ca2+ as suggested previously (Yeats et al. 1992b) or to another mechanism. Some of the data have already been published in abstract form (Piper et al. 1997; Piper & Docherty, 1997).
METHODS
Preparation of rat dorsal root ganglion neurone cultures
Experiments were performed on cultured dorsal root ganglion (DRG) neurones taken from adult Wistar, Sprague-Dawley or Hooded rats (> 200 g, either sex). There were no noticeable differences in responses from neurones from the different strains of rat or from either sex. Animals were killed by asphyxiation in a chamber filled with a slowly rising concentration of CO2 gas. Details of culture methods and recording conditions have previously been described (Docherty et al. 1991). Briefly, neurones were maintained in culture in the presence of either 50 ng ml−1 (2.5S) or 200 ng ml−1 (7S) of nerve growth factor (NGF) for 1-7 days. Cells were brushed from the base of the culture dish with a jet of medium from a glass Pasteur pipette 2-6 h prior to an experiment and then replated on polyornithine- and laminin-coated glass coverslips. The replating procedure provided a preparation of spherical neuronal somata that were free of processes.
Electrophysiological recordings
All recordings were made from neuronal somata at room temperature using the whole-cell patch-clamp technique with an Axopatch 2B amplifier and pCLAMP software (Axon Instruments). For determination of I-V relationships for capsaicin-induced current, series resistance was compensated by 50-90 %. The residual uncompensated series resistance was 2.2 ± 0.2 MΩ (n = 55), which would produce an expected error of 2.2 mV for a capsaicin-induced current of 1 nA at a holding potential (Vh) of -60 mV. The membrane patch was ruptured and a waiting period of at least 10 min was allowed for equilibration of the pipette solution with the contents of the cell. Capsaicin (at a concentration of 0.5 μM throughout) was applied to cells using a U-tube. This allowed a complete solution change in the vicinity of the cell in 0.70 ± 0.10 s. Only one recording was performed on any coverslip of cells to ensure that recordings were not made from cells which had been inadvertently exposed to capsaicin.
Solutions
Cells were normally perfused with a solution of the following composition (mM): NaCl, 130; CaCl2, 1; MgCl2, 1; KCl, 3; Hepes, 5; and glucose, 11; with 0.5 or 1 % DMSO, pH 7.4. Ultrapure water was used in the preparation of all solutions. For certain experiments CaCl2 and/or MgCl2 were omitted from the external solution to produce nominally Ca2+-free or nominally Ca2+- and Mg2+-free solutions. In some cases 100 μM EGTA was added to the external solution to buffer any residual free Ca2+ remaining. The pipette solution was made up as follows (mM): NaCl, 130; CaCl2, 1; MgCl2, 1; EGTA or BAPTA (see text), 10; and Hepes, 10; pH 7.4. Final adjustment of pH to 7.4 was achieved by adding NaOH to the extracellular and pipette solutions (thereby increasing the sodium concentration in the extracellular solution by 2-3 mM and in the pipette solution by 8-10 mM).
Using the internal and external solutions decribed above the holding current at a Vh of -60 mV was -380 ± 40 pA (n = 48). The rather high value for holding current was probably due to activation of a Na+-activated K+ conductance (Bischoff et al. 1998) by the high Na+ concentration (130 mM) of the internal solution. Data were only included for analysis if the maximum capsaicin-activated current in a cell was more than twice the holding current at a Vh of -60 mV.
Chemicals were prepared as concentrated stock solutions in either distilled water or DMSO and diluted to the final concentration using superfusate solution or pipette solution as appropriate. EGTA, BAPTA and polyornithine were supplied by Sigma, NGF and laminin by Promega or Alomone Laboratories, and calcineurin autoinhibitory peptide by Calbiochem-Novabiochem (UK) Ltd.
Voltage dependence of desensitization of responses to capsaicin
Capsaicin (0.5 μM) was applied to neurones as a continuous 2 min application or as a series of 5 s pulses repeated at 2 min intervals. This was carried out at a Vh of either -60 or +40 mV. In a related set of experiments, 5 s pulses of capsaicin (0.5 μM) were applied while the Vh was alternated between these values, i.e. capsaicin was applied first at a Vh of +40 mV, then the Vh was changed to -60 mV and capsaicin was applied again. After the second response to capsaicin, Vh was returned to +40 mV and capsaicin was applied for the third time.
To generate I-V curves for capsaicin-induced current, capsaicin (0.5 μM) was applied for 2 min at a Vh of -60 mV and I-V curves were constructed by applying a voltage step protocol (Fig. 1A). Vh was stepped for 20 ms to potentials from -100 to +40 mV in 10 mV increments at intervals of 1 s. The I-V relationship for capsaicin-activated current was determined at about 30, 60 and 120 s from the start of the application. After capsaicin had been washed off, the I-V curve was repeated to measure the background cell conductance and this was subtracted from the records obtained during capsaicin application to obtain the I-V curve for the capsaicin-activated conductance. The background conductance was measured after capsaicin had been washed off to minimize contamination of the data with voltage-dependent calcium currents that are blocked during and after application of capsaicin (in capsaicin-sensitive cells) but are usually present before (Docherty et al. 1991). Figure 1 shows data from capsaicin-insensitive cells that acted as controls for the background current subtraction protocol. Although these data show that the protocol was effective there was nevertheless a small but consistent error in the subtracted capsaicin-sensitive current. This appears as a small outward bump in the I-V curve at potentials between -20 and +10 mV. The reason for this error is not known but it is suggestive of a small reversible block of TTX-resistant sodium currents. For this reason the data points between -20 and +10 mV were excluded from curve fitting procedures (see below) although even when these data were included there was no significant effect on the results. To calculate the reversal potential (Vrev) for individual cells a second-order polynomial function was fitted to the I-V data (omitting points between -20 and +10 mV) to produce a smooth curve. Vrev was calculated as the point at which the fitted curve crossed the X-axis.
Figure 1. Protocol used to determine I-V relationship for capsaicin-activated current.
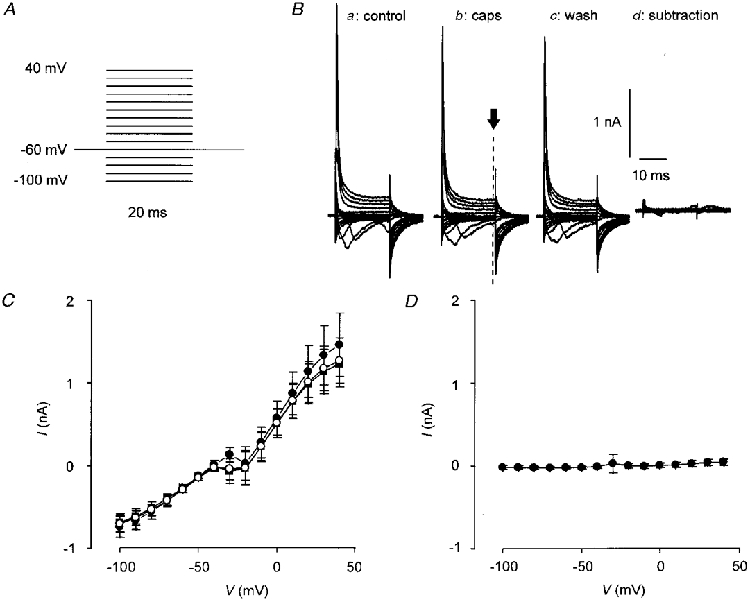
A, voltage step protocol used to generate I-V curves. Vh was stepped from -100 to +40 mV in 10 mV increments for 20 ms at 1 s intervals from a Vh of -60 mV. B, an example of an I-V trace from a capsaicin-insensitive cell. Capsaicin (0.5 μM) was applied for 2 min. Panel a shows current before capsaicin was applied; b, current in the presence of capsaicin (caps); c, current after 2-3 min wash; and d, current in the presence of capsaicin from which the current after capsaicin washout has been subtracted (see Methods). The dashed line marked with an arrow in panel b indicates the time point at which current was measured. C, I-V curves constructed from current before (•) and in the presence of capsaicin (^), and after 2-3 min wash (▪) in capsaicin-insensitive cells. D, I-V plot of subtracted currents. In both C and D points are means ±s.e.m., n = 17.
In some experiments the I-V relationship for capsaicin-activated current was determined in cells that had been pre-exposed to a conditioning pulse of capsaicin that was applied to induce desensitization. The conditioning pulse (5 s, 0.5 μM capsaicin in normal Ca2+- and Mg2+-containing solution) was applied 2-3 min prior to a test pulse (30-120 s, 0.5 μM capsaicin in Ca2+-containing, nominally Ca2+-free or nominally Ca2+- and Mg2+-free external solution). The I-V relationship was determined 30-60 s after the onset of the test pulse.
Statistical analysis
Data are expressed as means ±s.e.m. The absolute amplitude of capsaicin-activated current varied widely between cells so in most cases current amplitude was normalized to the inward current induced by capsaicin at a Vh of -60 mV. Data were compared by Student's two-tailed paired or unpaired t test or single factor analysis of variance as appropriate using Microsoft Excel version 4.0 software (Microsoft Corp., USA). Curve fitting was carried out with Origin version 3.73 software (Microcal, Northampton, MA, USA).
RESULTS
Characteristics of capsaicin-activated current
Figure 2A shows a typical trace from a single voltage-clamped DRG neurone to which capsaicin (0.5 μM) had been applied twice for 2 min in each case. During the first application of capsaicin the inward current in response to capsaicin reached a peak of 2.0 ± 0.5 nA after 22.9 ± 4.3 s (n = 10) and then decayed to a steady level of 0.5 ± 0.1 nA. The extent to which desensitization occurred varied between cells but on average the current at the end of a 2 min application of capsaicin was reduced by 65.2 ± 7.5 % (n = 10) with respect to the peak current achieved during the application. Current trajectories which had more than one clear peak or which reached a peak value in less than 10 s were observed only rarely. Re-application of capsaicin produced a smaller current that reached a maximum of 0.4 ± 0.1 nA (n = 11, P = 0.009) in 55.6 ± 19.7 s (n = 10). There was significantly less desensitization during the second 2 min application of capsaicin (27.1 ± 7.7 %; n = 10, P = 0.016).
Figure 2. Desensitization of capsaicin-activated current in voltage-clamped DRG neurones.
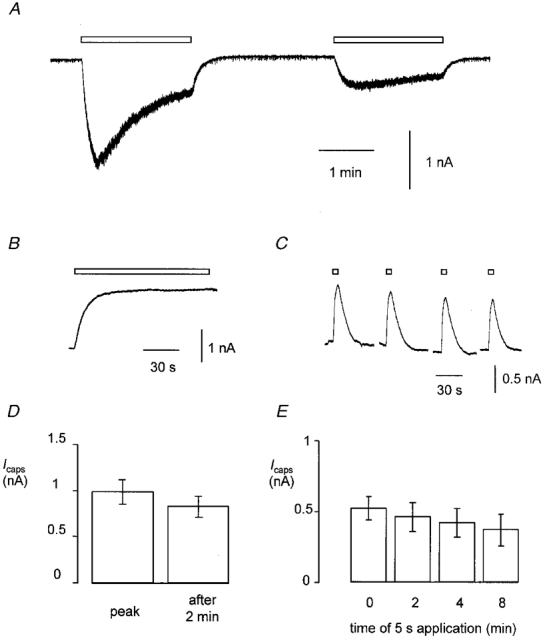
A, current recording from a typical cell. Capsaicin (0.5 μM) was applied for 2 min twice as indicated by the open bars. Vh was -60 mV. B, capsaicin-induced current in a different cell from that shown in A. Capsaicin (0.5 μM) was applied for 2 min once at a Vh of +40 mV. C, capsaicin-activated current in a third cell. Capsaicin (0.5 μM) was applied as 5 s pulses at 2 min intervals at a Vh of +40 mV. D, bar chart showing percentage reduction in current during a continuous 2 min application of capsaicin (as shown in B, Vh=+40 mV, n = 27). E, bar chart showing peak outward current in response to 5 s pulses of capsaicin (0.5 μM) applied at 2 min intervals (as shown in C, Vh=+40 mV, n = 15). Data in both D and E represent means ±s.e.m.
When capsaicin was applied to cells at a positive membrane potential, the capsaicin-activated current was outward as expected for a non-specific cation conductance recorded under the present experimental conditions (Fig. 2B). During a single 2 min application of capsaicin at a Vh of +40 mV the capsaicin-activated outward current reached a maximum of 1.0 ± 0.1 nA in 50.6 ± 3.5 s (n = 27). This is significantly slower than the time to peak of the inward current evoked at -60 mV (P < 0.001, unpaired t test). The current decayed to an amplitude of 0.8 ± 0.1 nA (P = 0.002, paired t test, compared with peak) by the end of the 2 min. The mean decay in amplitude, i.e. the degree of desensitization, was 15.4 ± 3.5 %, which was significantly less than that which occurred at -60 mV (Fig. 2D; P < 0.001, unpaired t test). Figure 2C shows traces from a DRG neurone to which 5 s pulses of capsaicin (0.5 μM) had been applied at 2 min intervals at a Vh of +40 mV. There was a small reduction in the amplitude of responses to 5 s pulses of capsaicin applied 2 min apart (from 0.71 ± 0.14 to 0.62 ± 0.14 nA; n = 17; Fig. 2E) but the reduction was not statistically significant. In agreement with previous data that were obtained using a similar protocol at a Vh of -60 mV (Docherty et al. 1996) the desensitization seen during continuous application of capsaicin and the tachyphylaxis that occurred during repeated pulses of capsaicin at +40 mV were quantitatively similar.
Desensitization of capsaicin-evoked current is both Ca2+ and voltage dependent
There was no significant reduction in the amplitude of responses to 5 s pulses of capsaicin (0.5 μM) applied at 2 min intervals at +40 mV when cells were dialysed with BAPTA (equimolar substitution of EGTA in the pipette solution with BAPTA, data not shown). On the contrary, there was a 22.6 ± 15.4 % (n = 7, P = 0.007) increase in the second compared with the first response to capsaicin. A similar potentiation of responses in BAPTA-loaded cells has been observed at negative membrane potentials (Docherty et al. 1996) especially when extracellular Ca2+ was replaced with Ba2+ (Koplas et al. 1997). These data suggest that the relatively small amount of Ca2+ that would be expected to enter the cells at positive potentials is sufficient to cause some desensitization of the response.
The reduced desensitization at positive membrane potentials could be due to the reduced electrochemical gradient for Ca2+ entry (Yeats et al. 1992b). In order to test this hypothesis further, capsaicin (0.5 μM) was applied in 5 s pulses at 2 min intervals first at +40 mV, then at -60 mV and again at +40 mV. Data obtained using this protocol were then compared with the data obtained when the Vh was maintained at either +40 or -60 mV throughout (Fig. 3). Since very little reduction in the amplitude of responses occurs when cells are maintained at +40 mV (see above and Fig. 2) we anticipated that the response to the second application of capsaicin at -60 mV would be large - equivalent to the response of cells that were not desensitized. Surprisingly, the responses were not significantly different from the responses of desensitized neurones (i.e. where Vh was maintained at -60 mV throughout): -0.21 ± 0.05 nA (n = 11) compared with -0.36 ± 0.06 nA (n = 25, P = 0.14, unpaired t test). Also, when the Vh was returned to +40 mV responses were not significantly different from responses in cells that had been maintained at +40 mV throughout (0.46 ± 0.12 nA, n = 11; compared with 0.56 ± 0.13 nA, n = 25; P = 0.61, unpaired t test; Fig. 3B).
Figure 3. Effect of switching Vh on desensitization of the response to 5 s pulses of capsaicin applied at 2 min intervals.
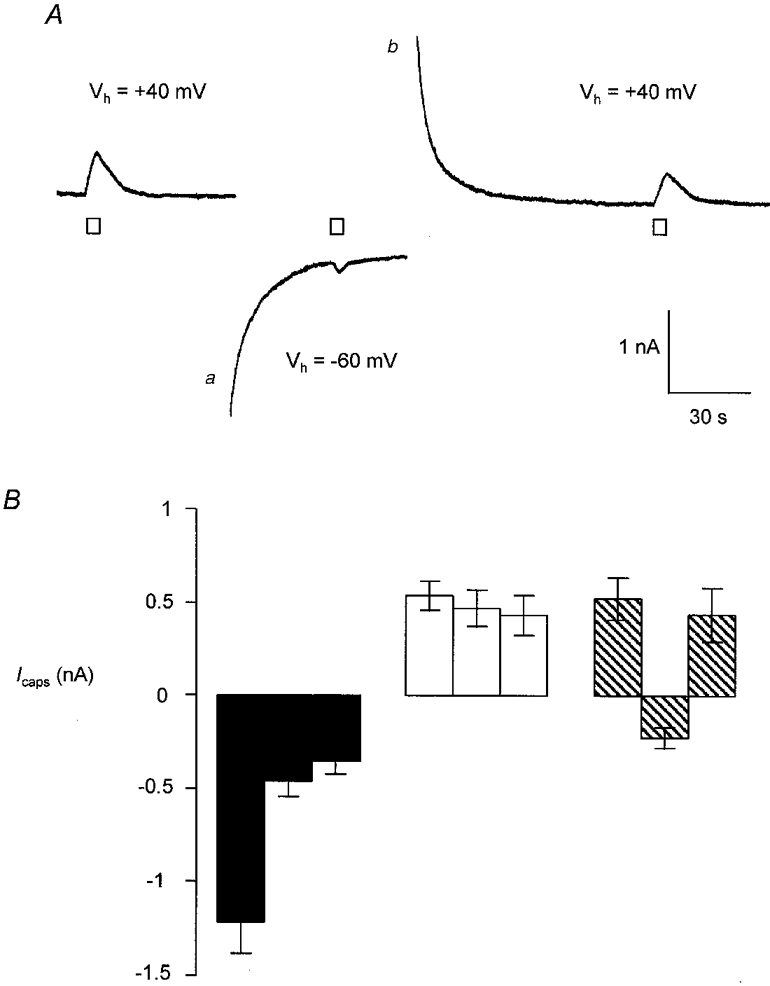
A, recording of current from a voltage-clamped DRG neurone. Capsaicin (0.5 μM) was applied in 5 s pulses at 2 min intervals as indicated, at a Vh of +40 or -60 mV. Large transient inward and outward currents (marked a and b) were often observed and were a consequence of changing Vh from +40 to -60 mV and vice versa. These currents did not affect the capsaicin-induced current. B, plot of peak current in response to 5 s pulses of capsaicin applied at time t = 0, 2 and 4 min. Data shown are from cells held at -60 mV throughout (▪, n = 47), +40 mV throughout (□, n = 15) or at alternating holding potentials ( , n = 9). Each bar represents the mean ±s.e.m.
, n = 9). Each bar represents the mean ±s.e.m.
The I-V relationship for capsaicin-activated current changes as desensitization progresses
I-V curves for the vanilloid-gated current were obtained at various times during a 2 min application of capsaicin. Figure 4A shows a typical trace from a single DRG neurone to which capsaicin (0.5 μM) had been applied for 2 min. Breaks in the trace indicate the points at which the I-V protocol was applied. Figure 4B shows the I-V trace on an expanded scale. It can be seen that there was a relatively large decrease in inward current at negative membrane potentials compared with the outward current evoked at positive potentials. Interestingly, in most cells, including the one illustrated in Fig. 4A and B, the change in capsaicin-induced current during a voltage step showed clear time dependence (see below). The first I-V curve in the series was constructed near the peak of the inward current. From the averaged normalized data (Fig. 4C) it may be seen that the relationship between membrane potential and current was outwardly rectifying and the current reversal potential was 8.4 ± 1.8 mV (n = 9). At later times in the response the I-V relationship became progressively more curved showing a significant loss of inward current at negative potentials and a small but significant negative shift in the reversal potential to -3.0 ± 4.4 mV (n = 9, P = 0.01, paired t test). The percentage reduction in capsaicin-activated current over the range of voltages studied is plotted in Fig. 4D. Data for -10 to +10 mV were omitted as current at these potentials was close to zero. One-way analysis of variance comparing percentage reduction in capsaicin-activated current at potentials between -100 and -20 mV indicated that there was no significant difference between any of these points (P = 0.93). Similarly, there was no significant difference in percentage reduction of capsaicin-activated current at potentials between +10 and +40 mV (P = 0.73). However, if percentage reduction at any point between -100 and -20 mV was compared with data from +30 and +40 mV the values were significantly different (P = 0.003-0.05, two-tailed paired t test). Repeating these statistical tests using the log10 of percentage reduction to ensure normal distribution of the data yielded similar results. These data show that desensitization of capsaicin-activated current is accompanied by a profound change in the voltage dependence of the current.
Figure 4. I-V relationship for capsaicin-activated current in voltage-clamped DRG neurones.
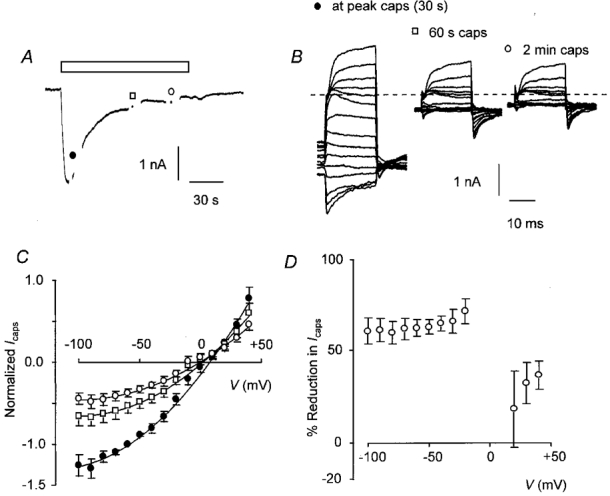
A, inward current in response to a 2 min application of capsaicin (0.5 μM). I-V curves were constructed close to the peak of the inward current, then 60 and 120 s from the start of the application as indicated by the breaks in the trace. B, I-V trace for the cell shown in A at the peak of the capsaicin response and after 60 and 120 s on an expanded scale. The dashed line indicates zero current and the I-V relationship for background conductance has been subtracted. C, I-V curves for capsaicin-activated current. The curves shown were constructed after 30 s (•), 1 min (□) and 2 min (^). Points are joined by a polynomial curve fitted after omitting data from potentials between -20 and +10 mV although all points have been plotted. The I-V relationship for background conductance was subtracted and all current was normalized to the inward current after 30 s at a Vh of -60 mV. Points represent means ±s.e.m., n = 9. D, plot of percentage reduction in capsaicin-activated current after 120 s against Vh. Points are means ±s.e.m., n = 9.
Although the majority of cells tested behaved as described above, a proportion (3/12) of cells did not desensitize even at -60 mV (0.6 ± 0.1 nA maximum and 0.6 ± 0.1 nA at the end of 2 min). There was no change in the I-V relationship during application of capsaicin in these cells. This is illustrated in Fig. 5. The capsaicin response was qualitatively and quantitatively the same as the second of the two responses shown in Fig. 2A, i.e. the same as the response to capsaicin after acute desensitization. A time-dependent increase in conductance at positive membrane potentials was evident but there was little indication of a similar inactivation of current after hyperpolarizing voltage steps (Fig. 5B). The averaged normalized I-V curve of the capsaicin-evoked current showed as much rectification at the earliest time point (i.e. 30 s) as at later times during the capsaicin application (Fig. 5C). In this case there was no change in the voltage dependence of the current during drug application. There was no obvious correlation between the peak amplitude of the current at the Vh of -60 mV and the amount of desensitization that occurred in any given cell (data not shown).
Figure 5. Some DRG neurones exhibit pronounced outward rectification of capsaicin-induced current initially and do not undergo acute desensitization.
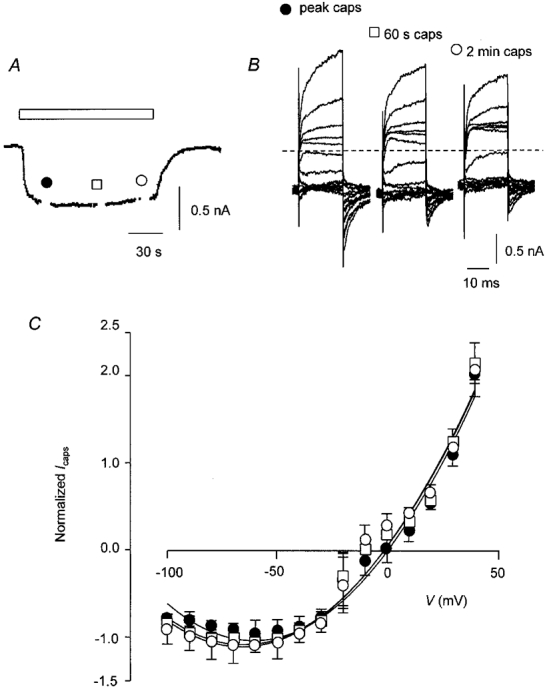
A, trace from a single DRG neurone exposed to capsaicin (0.5 μM) as indicated by the open bar. I-V curves were constructed at 30 s (•), 60 s (□) and 120 s (^) from the start of the application. B, I-V trace recorded from the cell shown in A on an expanded scale. The dashed line indicates zero current; background current has been subtracted. C, I-V relationship for capsaicin-activated current in non-desensitizing cells after 30 s (•), 60 s (□) and 120 s (^). Points are joined by a fitted polynomial curve omitting data from potentials between -20 and +10 mV although all points have been plotted. Current was normalized to the inward current after 30 s at a Vh of -60 mV. Points are means ±s.e.m., n = 3.
Inhibition of calcineurin can prevent the progressive change in the I-V relationship for capsaicin-activated current
The I-V curve for capsaicin-activated current was determined in cells which were dialysed with BAPTA (10 mM) instead of EGTA or when cells were loaded with calcineurin autoinhibitory peptide (CI), a 25 amino acid peptide that is a pseudosubstrate inhibitor of calcineurin. Data are shown in Fig. 6. Replacement of EGTA by BAPTA had no significant effect on the size of the peak response (Ipeak) to capsaicin (EGTA: Ipeak= 1.86 ± 0.49 nA, n = 9; BAPTA: Ipeak= 1.51 ± 0.52 nA, n = 7; P > 0.05, unpaired t test; Fig. 6A). The peak occurred more slowly when cells were dialysed with BAPTA instead of EGTA (EGTA: time to peak = 16.3 ± 2.5 s, n = 9; BAPTA: time to peak = 40.6 ± 10.5 s, n = 7; P = 0.02, unpaired t test). Desensitization was significantly reduced in the BAPTA-loaded cells. After 120 s, inward current in response to capsaicin was reduced (desensitized) by only 22.5 ± 16.9 % (n = 7) in BAPTA-loaded cells as compared with 65.2 ± 5.4 % (n = 9, P = 0.02, unpaired t test) in EGTA-loaded cells (see above). There was no change in the degree of rectification of the I-V relationship for capsaicin-activated current with time (Fig. 6C). A plot of percentage reduction in capsaicin-activated current in the range of voltages studied (omitting points between -10 and +10 mV) is shown in Fig. 6E. There was no significant difference in the percentage reduction in capsaicin-activated current at any voltage when compared using one-way analysis of variance (P = 0.73). Data from CI-loaded cells are shown in Fig. 6B, D and F. At a Vh of -60 mV the peak inward current in response to capsaicin in the CI-loaded cells was not significantly different from control EGTA-loaded cells (Ipeak= 1.08 ± 0.11 nA; n = 3, P = 0.39, unpaired t test) but the time to peak was significantly longer (31.7 ± 6.7 s; n = 3, P = 0.02, unpaired t test, Fig. 6B). There was no significant change in the degree of rectification of the I-V relationship for capsaicin-activated current in CI-loaded cells (Fig. 6D). Some desensitization did take place but there was no significant difference in the reduction in capsaicin-activated current at any membrane potential examined, either negative or positive (Fig. 6F; one-way analysis of variance, P = 0.92). Overall, although some loss of capsaicin-activated current was evident, the distinctive change in voltage dependence that accompanied desensitization in control cells did not occur when cells were dialysed with either CI or BAPTA. Interestingly, addition of CI (100 μM) to the pipette solution significantly reduced the probability that a cell would be sensitive to capsaicin (P < 0.001, chi-squared test). Only 6/78 cells tested responded to capsaicin, while over the same period of time, 30/56 cells dialysed with either EGTA or BAPTA were capsaicin sensitive. Addition of CI to the internal solution had a similar effect on the probability that DRG neurones would respond to ATP (10 μM). Of the 37 cells treated with CI (100 μM), 10 responded to ATP while recordings from the same cell cultures showed that 15/30 cells dialysed with EGTA were ATP sensitive (P = 0.047, chi-squared test).
Figure 6. Effect of dialysing DRG neurones with BAPTA or CI on desensitization of capsaicin-activated current.
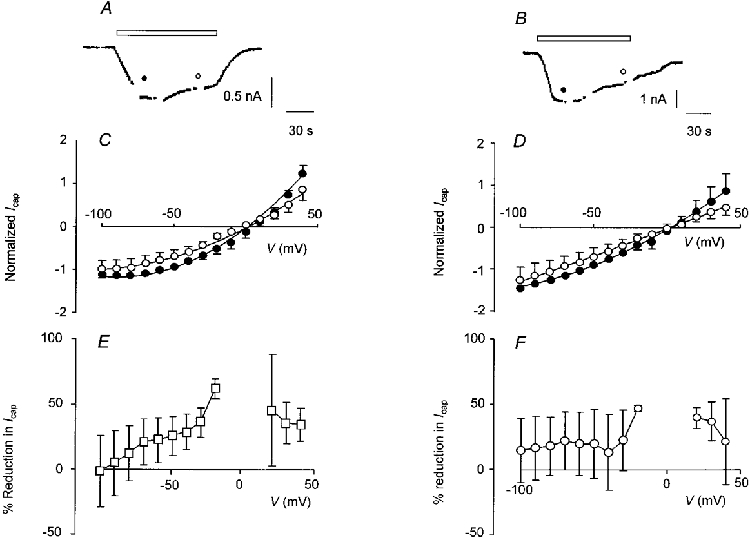
Typical recordings of capsaicin-activated current from a BAPTA-loaded cell (A) and from a CI-loaded cell (B). Capsaicin (0.5 μM) was applied as indicated by the open bars. In both A and B, I-V curves were constructed about 30 s, 1 min and 2 min from the start of the application as indicated by the breaks in the trace; Vh was -60 mV. C, I-V relationship for capsaicin-activated current recorded in cells dialysed with BAPTA (10 mM, n = 7). D, I-V curves for capsaicin-activated current recorded in CI (100 μM)-loaded cells (n = 3). In both C and D, I-V curves constructed after 30 s (•) and 2 min (^) are shown; points are joined by a curve fitted after omitting data from potentials between -20 and +10 mV, although in each case all points have been plotted. Current was normalized to the inward current after 30 s at a Vh of -60 mV; points are means ±s.e.m.E, plot of percentage reduction in capsaicin-activated current after 120 s against Vh for cells dialysed with BAPTA (10 mM, n = 7). F, plot of percentage reduction in capsaicin-activated current after 120 s for CI (100 μM)-loaded cells (n = 3). In both E and F points are means ±s.e.m.
Is outward rectification due to block of the channel by extracellular Ca2+ or Mg2+?
As shown above, the I-V curves in non-desensitizing cells and the I-V curve in cells after desensitization had occurred often displayed a region at negative potentials where the slope conductance was zero or negative. This is suggestive of voltage-dependent gating behaviour or more probably a voltage-dependent channel-blocking mechanism. If this were so we would expect to reverse the increase in outward rectification by removing Ca2+ and/or Mg2+ from the extracellular medium. To test this we exposed cells to a conditioning pulse of capsaicin for 5 s in the presence of normal extracellular Ca2+ and Mg2+ concentrations to produce desensitization and then reapplied capsaicin for 60-120 s either in normal medium or in the absence of divalent cations (Fig. 7). The I-V curve was measured 30-60 s after applying the second pulse of capsaicin. When normal medium was used throughout then prior exposure of neurones to capsaicin resulted in ‘conditioned’ currents that appeared very much like currents recorded in non-desensitizing cells (mean amplitude of conditioned current = 1.1 ± 0.3 nA, n = 5; Fig. 7A). There was no overt desensitization when capsaicin was reapplied. The I-V relationship exhibited pronounced outward rectification (Fig. 7A, right-hand panel) and was similar to the I-V relationship observed in normal cells after desensitization had occurred (Fig. 4) or in non-desensitizing cells (Fig. 5). Reapplication of capsaicin in nominally Ca2+-free external solution or with EGTA (100 μM) added (mean amplitude of conditioned current = 0.4 ± 0.2 nA, n = 8; Fig. 7B) did not reverse the increase in outward rectification that had developed (Fig. 7B, right-hand panel). Similar results were obtained when both Ca2+ and Mg2+ were removed from the external solution (mean amplitude of conditioned current = 0.4 ± 0.2 nA, n = 5; Fig. 7C). Again, the I-V relationship showed pronounced outward rectification (Fig. 7C, right-hand panel). The reversal potentials for the capsaicin-induced currents obtained using this protocol were 4.6 ± 2.6 mV (n = 5), -4.0 ± 6.5 mV (n = 7) and -18.9 ± 4.1 mV (n = 5) for data obtained in normal medium, Ca2+-free medium and Ca2+- and Mg2+-free medium, respectively.
Figure 7. Removal of external Ca2+ or Mg2+ does not affect capsaicin-induced inward current after prior desensitization of the response to capsaicin.
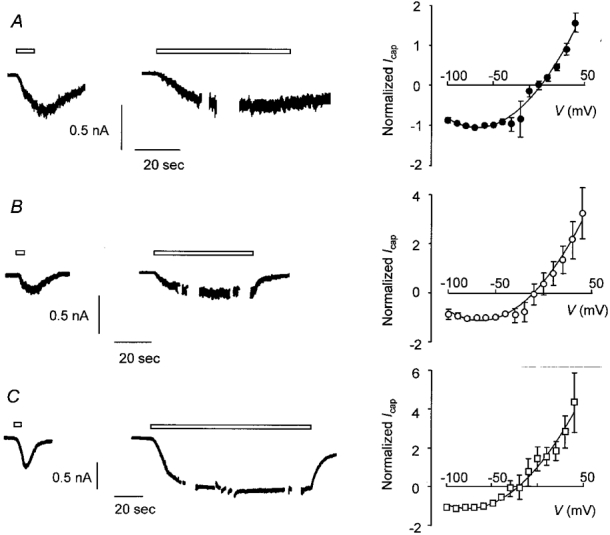
A, trace from a voltage-clamped DRG neurone exposed to Ca2+ (1 mM)- and Mg2+ (1 mM)-containing external solution throughout. Capsaicin (0.5 μM) was applied as a conditioning pulse for 5 s to produce desensitization, then re-applied as a test pulse for approximately 60 s during which time the I-V curve was determined. The right-hand panel shows the I-V relationship for capsaicin-activated current obtained during the test pulse (means ±s.e.m., n = 5). B, current recorded from a second DRG neurone. Data were obtained using the same protocol as in A except that the test pulse was applied in the absence of Ca2+ (no added Ca2+, 100 μM EGTA). The right-hand panel shows the I-V relationship obtained during the test pulse (means ±s.e.m., n = 8). C, trace obtained from a third DRG neurone. The protocol was the same as in A and B except that the test pulse (120 s) was applied in the absence of Ca2+ and Mg2+. The right-hand panel shows the I-V relationship obtained during the test pulse (means ±s.e.m., n = 5). For each trace open bars indicate application of capsaicin and breaks in the trace during capsaicin application show the points at which I-V curves were generated. Vh was -60 mV. The points on each I-V curve are joined by a polynomial curve fitted after omitting data from potentials between -20 and +10 mV although all data points have been plotted. Background current was subtracted and current was normalized to the inward current at the Vh of -60 mV.
DISCUSSION
The results presented above indicate that acute desensitization of responses to capsaicin in adult rat DRG neurones was voltage dependent. During the acute phase of desensitization, outward rectification of the I-V relationship for capsaicin-activated current became more pronounced, meaning that current was reduced more at negative potentials than at positive potentials. These changes in the biophysical properties of the current were inhibited by dialysing cells with BAPTA (10 mM) instead of EGTA (10 mM) and by adding calcineurin autoinhibitory peptide (CI, 100 μM) to the internal solution. The increased outward rectification was not affected by removal of external Ca2+ or Mg2+.
One possible explanation of our data is that the capsaicin-induced currents that we observed were composite currents due to the activity of at least two subtypes of capsaicin-activated ion channel. For example, if one subtype of ion channel activates rapidly, has a fairly linear I-V curve and desensitizes almost completely and the other is more slowly activating, has an I-V curve that shows strong outward rectification and does not desensitize, then composite currents made up of mixtures of these two would be expected to give rise to data similar to those described above. However, we very rarely see capsaicin-evoked currents (in DRG neurones) with more than one peak at negative potentials and never when the compound is applied to cells at positive potentials. While we remain open-minded on the subject of vanilloid channel subtypes (see Introduction) as a possible explanation for some of our findings, we do not believe we have seen convincing evidence for channel subtypes in our experiments. Our data do, however, suggest that activation of the vanilloid receptors in DRG neurones may produce currents with different biophysical characteristics depending on the ‘sensitization’ state of the channels.
In a previous report we showed that, on average, the extent of the desensitization of vanilloid-gated channels that occurs between brief applications of capsaicin is quantitatively similar to the desensitization that occurs when capsaicin is applied continuously for the same period (Docherty et al. 1996). In this study we have repeated these experiments and extended the data to include responses evoked at positive membrane potentials. These data support our earlier conclusion that the two protocols represent two methods of measuring the same phenomenon and cannot be used to distinguish between ‘desensitization’ and ‘tachyphylaxis’ as two separate mechanisms. That is not to say that desensitization of vanilloid-gated channels can be explained entirely by a single mechanism. Vanilloid-evoked responses in isolated neurones undergo a slow inactivation process that is not reduced in the absence of extracellular Ca2+. The mechanism of this slower process is not known but it has been suggested that it may be due to depletion of intracellular ATP stores that are required to maintain the vanilloid receptor/ion channel in a phosphorylated state (Koplas et al. 1997). In this study we confine ourselves to the diminution of responsiveness that occurs within the first few minutes after capsaicin is applied for the first time to a cell. Throughout the paper we have used the term desensitization to describe the phenomenon irrespective of the protocol used to produce it.
The speed and/or the extent of desensitization that occurs when capsaicin is applied to cells at positive potentials is very much less than that which occurs at negative membrane potentials. In other words, acute desensitization of vanilloid-gated ion channels is voltage dependent. The voltage dependence could arise for any of several reasons. Given that desensitization is facilitated by raised intracellular Ca2+ and that vanilloid-gated ion channels are permeable to Ca2+ an obvious hypothesis is that less desensitization occurs at positive potentials because there is a smaller electrochemical gradient for Ca2+ entry (Yeats et al. 1992b). The experiment illustrated in Fig. 3 shows that the amount of desensitization that occurred was about the same irrespective of the potential at which capsaicin was applied but desensitization was apparent only at negative potentials. This suggests that a reduced electrochemical gradient for Ca2+ is not the main mechanism involved. The data in Fig. 3 show in addition that internalization of capsaicin receptors cannot be responsible for desensitization of capsaicin-activated current. We believe that the most likely explanation for the voltage dependence of capsaicin desensitization is given by the data presented in Figs 4 and 5 which show that desensitization of capsaicin-activated current is accompanied by a change in the voltage dependence of the current. We found that, at the peak of the response, the I-V relationship for capsaicin-induced inward current was outwardly rectifying, which is consistent with other published reports (Oh et al. 1996; Liu & Simon, 1996; Caterina et al. 1997; Liu et al. 1997; Wood & Docherty, 1997). However, at later times in the response the I-V relationship became progressively more curved showing a significant loss of inward current at negative potentials but a smaller reduction in current amplitude at positive potentials. This progressive increase in outward rectification causes a change in the voltage dependence of the capsaicin-induced current that must contribute to the voltage dependence of desensitization.
If the development of outward rectification is linked to desensitization then it should be reduced by intracellular BAPTA or by inhibitors of calcineurin (Docherty et al. 1996). This was found to be the case. Intracellular BAPTA reduced desensitization and prevented the acute change in the I-V relationship of the current. The addition of CI to the internal solution also prevented the development of outward rectification, although the amount of capsaicin-activated current underwent a significant reduction at all potentials tested.
A proportion (25 %) of control EGTA-loaded cells displayed capsaicin-induced currents that did not show acute desensitization. The response in these cells was indistinguishable from the capsaicin response in desensitized cells. Great care was taken during experiments to ensure that cells were being exposed to capsaicin for the first time so the vanilloid receptors on these cells cannot have been desensitized in the normal pharmacological sense of the word. This suggests that the channels can enter a state that is equivalent, and perhaps identical to the desensitized state even in the absence of the ligand. This may help to explain the large cell-to-cell variation in the capsaicin responsiveness that is commonly reported (see above). It may be that the phosphorylation state of the channels can determine the degree of outward rectification they exhibit. If the channels are phosphorylated then the I-V relationship is relatively linear and if they are not phosphorylated then outward rectification occurs. The degree of rectification of the whole-cell currents will then depend on the proportion of the channels that are phosphorylated or otherwise. Calcium entry through activated ion channels may promote dephosphorylation (Docherty et al. 1996) and because of the associated outward rectification this will appear as a reduction of the response but only at negative membrane potentials. Presumably any stimulus that promotes dephosphorylation of the vanilloid-gated ion channels will cause a heterologous desensitization. This hypothesis is in agreement with the work of Koplas et al. (1997) who have also suggested that phosphorylation of the vanilloid receptor/ion channel is necessary for maintained capsaicin sensitivity.
The mechanism by which outward rectification of the capsaicin I-V curve develops is unclear. In cells where desensitization had taken place and in non-desensitizing cells the I-V curve displayed regions where the slope was zero or negative. This might indicate a voltage-dependent block of the ion channel. An obvious analogy is the voltage-dependent block of NMDA receptor channels by extracellular Mg2+ (Mayer et al. 1984). A feature of capsaicin-evoked inward current in naive DRG neurones is that it is substantially increased in the absence of extracellular Ca2+ (Docherty et al. 1996; Liu & Simon, 1996). Also we noted that capsaicin-induced current can exhibit time-dependent activation and inactivation (Fig. 5B). We and others (Liu et al. 1997) have noted a small negative shift in the reversal potential for capsaicin-activated current that accompanies desensitization. The increased outward rectification of the I-V relationship and the change in Vrev could occur if the status of Ca2+ and/or Mg2+ changed from permeant to blocking ions perhaps due to an increased affinity for a divalent cation-binding site in the channel pore. However, this does not appear to be the case. Removal of extracellular Ca2+ and Mg2+ had no effect on the shape of the capsaicin I-V relationship after desensitization had occurred. The reversal potential for capsaicin-induced current became progressively more negative as Ca2+ and then both Ca2+ and Mg2+ were omitted from the external solution (4.6 mV in Ca2+- and Mg2+-containing solution, -4.0 mV in Ca2+-free and -18.9 mV in Ca2+- and Mg2+-free solutions). These differences in Vrev were probably due to reduced screening of membrane surface charge when Ca2+ and Mg2+ were absent. Interestingly, removal of Ca2+ and/or Mg2+ tended to decrease the size of the capsaicin-induced current after desensitization, which is the opposite effect to normal (Docherty et al. 1996; Liu & Simon, 1996). Taken together with the apparent change in Vrev for the capsaicin current that accompanies desensitization these data suggest a change in the permeability of the channel to divalent cations may occur when the channel desensitizes but the development of outward rectification is not due to block of the channels by divalent cations. The mechanism of the development of outward rectification and the apparent time dependence of capsaicin-activated current are currently under further study.
To summarize, we have shown that acute desensitization of responses to capsaicin in adult rat DRG neurones is dependent on membrane potential. During the acute phase of desensitization, the voltage dependence of capsaicin-activated ion channels changes and outward rectification develops, meaning that current is reduced at negative potentials more than at positive potentials. The reversal potential of the capsaicin-activated current also appears to become more negative. These changes in the biophysical properties of the current may be due to dephosphorylation of the ion channel/receptor complex. The mechanism that causes the increased outward rectification is not clear; however, it is not due to extracellular block of the vanilloid-gated ion channel by Ca2+ or Mg2+.
Acknowledgments
This work was supported by The Wellcome Trust.
References
- Bevan S, Docherty RJ. Cellular mechanisms of the action of capsaicin. In: Wood J, editor. Capsaicin in the Study of Pain. London: Academic Press; 1993. pp. 27–44. [Google Scholar]
- Bevan S, Szolcányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends in Pharmacological Sciences. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- Bischoff U, Vogel W, Safronov BV. Na+-activated K+ channels in small dorsal root ganglion neurones of rat. The Journal of Physiology. 1998;510:743–754. doi: 10.1111/j.1469-7793.1998.743bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Robertson B, Bevan S. Capsaicin causes prolonged inhibition of voltage-activated calcium currents in adult rat dorsal root ganglion neurons in culture. Neuroscience. 1991;40:513–521. doi: 10.1016/0306-4522(91)90137-d. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HWGM. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacological Reviews. 1991;43:143–201. [PubMed] [Google Scholar]
- Koplas PA, Rosenburg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EFP. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. Journal of Neurophysiology. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Liu L, Lo Y-C, Chen I-J, Simon SA. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. Journal of Neuroscience. 1997;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. Journal of Neurophysiology. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- Liu L, Szallasi A, Simon SA. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13 acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- Mayer MS, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. Journal of Neuroscience. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, LaMotte RH, Klusch A, Kniffki KD. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neuroscience. 1996;75:495–505. doi: 10.1016/0306-4522(96)00259-x. [DOI] [PubMed] [Google Scholar]
- Piper AS, Docherty RJ. Inhibition of calcineurin prevents the development of outward rectification of capsaicin-activated current in adult rat dorsal root ganglion (DRG) neurons in culture. The Journal of Physiology. 1997;505.P:40P. [Google Scholar]
- Piper AS, Yeats JC, Docherty RJ. Use-dependent outward rectification of capsaicin-activated current in adult rat dorsal root ganglion neurons causes apparent desensitization. The Journal of Physiology. 1997;499.P:14P. [Google Scholar]
- Szallasi A. The vanilloid (capsaicin) receptor: receptor types and species differences. General Pharmacology. 1994;2:223–243. doi: 10.1016/0306-3623(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Mechanisms and therapeutic potential of vanilloids (capsaicin-like molecules) Advances in Pharmacology. 1996;24:123–155. doi: 10.1016/s1054-3589(08)60936-9. [DOI] [PubMed] [Google Scholar]
- Vlachová V, Vyklický L. Capsaicin-induced membrane currents in cultured sensory neurons of the rat. Physiological Research. 1993;42:301–311. [PubMed] [Google Scholar]
- Wood JN, Docherty RJ. Chemical activation of sensory neurons. Annual Review of Physiology. 1997;59:457–482. doi: 10.1146/annurev.physiol.59.1.457. [DOI] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. Journal of Neuroscience. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats JC, Boddeke HWGM, Docherty RJ. Capsaicin desensitization in rat dorsal root ganglion neurons is due to activation of calcineurin. British Journal of Pharmacology. 1992a;107:238P. [Google Scholar]
- Yeats JC, Docherty RJ, Bevan S. Calcium-dependent and independent desensitization of capsaicin-evoked responses in voltage-clamped adult rat dorsal root ganglion (DRG) neurons in culture. The Journal of Physiology. 1992b;446:390P. [Google Scholar]
- Zeilhofer HU, Kress M, Swandulla D. Fractional Ca2+ currents through capsaicin- and proton-activated ion channels in rat dorsal root ganglion neurones. The Journal of Physiology. 1997;503:67–78. doi: 10.1111/j.1469-7793.1997.067bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


