Abstract
Growing evidence points toward involvement of the human motor cortex in the control of the ipsilateral hand. We used focal transcranial magnetic stimulation (TMS) to examine the pathways of these ipsilateral motor effects.
Ipsilateral motor-evoked potentials (MEPs) were obtained in hand and arm muscles of all 10 healthy adult subjects tested. They occurred in the finger and wrist extensors and the biceps, but no response or inhibitory responses were observed in the opponens pollicis, finger and wrist flexors and the triceps.
The production of ipsilateral MEPs required contraction of the target muscle. The threshold TMS intensity for ipsilateral MEPs was on average 1.8 times higher, and the onset was 5.7 ms later (in the wrist extensor muscles) compared with size-matched contralateral MEPs.
The corticofugal pathways of ipsilateral and contralateral MEPs could be dissociated through differences in cortical map location and preferred stimulating current direction.
Both ipsi- and contralateral MEPs in the wrist extensors increased with lateral head rotation toward, and decreased with head rotation away from, the side of the TMS, suggesting a privileged input of the asymmetrical tonic neck reflex to the pathway of the ipsilateral MEP.
Large ipsilateral MEPs were obtained in a patient with complete agenesis of the corpus callosum.
The dissociation of the pathways for ipsilateral and contralateral MEPs indicates that corticofugal motor fibres other than the fast-conducting crossed corticomotoneuronal system can be activated by TMS. Our data suggest an ipsilateral oligosynaptic pathway, such as a corticoreticulospinal or a corticopropriospinal projection as the route for the ipsilateral MEP. Other pathways, such as branching of corticomotoneuronal axons, a transcallosal projection or a slow-conducting monosynaptic ipsilateral pathway are very unlikely or can be excluded.
Humans are distinct in their ability to perform dexterous, independent finger movements. These depend on a highly developed corticomotoneuronal system (for review, see Porter & Lemon, 1993). It is thought that transcranial magnetic stimulation (TMS) of the motor cortex produces motor-evoked potentials (MEPs) in contralateral hand muscles primarily, if not exclusively, via the fastest-conducting crossed corticomotoneuronal fibres (for review, see Rothwell, 1991). MEPs in muscles ipsilateral to the stimulated motor cortex have been reported in only a few studies (Wassermann et al. 1991, 1994; Basu et al. 1994). In fact, their existence in hand and forearm muscles of healthy adult humans was doubted by some investigators (Carr et al. 1994; Müller et al. 1997), while others found ipsilateral MEPs in only a small percentage of subjects (Netz et al. 1997). In contrast, ipsilateral MEPs in upper limb muscles were reported to be common in children up to the age of 10 years (Müller et al. 1997).
Ipsilateral MEPs may also occur in congenital conditions such as cerebral palsy (Farmer et al. 1991; Carr et al. 1993; Maegaki et al. 1997), and congenital mirror movements (Farmer et al. 1990; Konagaya et al. 1990; Britton et al. 1991; Capaday et al. 1991; Cohen et al. 1991; Danek et al. 1992; Cincotta et al. 1994; Kanouchi et al. 1997; Mayston et al. 1997), and in postnatally acquired lesions such as cerebrovascular stroke (Benecke et al. 1991; Hömberg et al. 1991; Turton et al. 1996; Netz et al. 1997), or after hemispherectomy (Benecke et al. 1991). Probably a number of different pathophysiological mechanisms exist for these ipsilateral MEPs. In patients with congenital conditions, ipsilateral MEPs have the same onset latency as the contralateral MEP, and single-unit electromyogram (EMG) recordings from a pair of homologous muscles show central peaks of short duration in the cross-correlogram, indicating axonal branching of crossed corticospinal fibres (Farmer et al. 1990, 1991; Carr et al. 1993). In contrast, the latency of ipsilateral MEPs in patients with acquired brain damage later in life (Benecke et al. 1991; Carr et al. 1993; Netz et al. 1997) and in healthy subjects (Wassermann et al. 1991, 1994; Basu et al. 1994; Netz et al. 1997) was greater than the contralateral MEPs by some 5-14 ms. It was concluded that these delayed ipsilateral MEPs were mediated through unmasking of a ‘slow’ ipsilateral corticospinal pathway from the unaffected hemisphere (Netz et al. 1997), for instance a corticoreticulospinal projection (Benecke et al. 1991; Wassermann et al. 1994).
The purpose of the present TMS study was to clarify how commonly ipsilateral MEPs are obtainable in hand and arm muscles of adult healthy subjects, and to examine some of the properties of the pathways of these ipsilateral MEPs.
METHODS
Subjects and patients
Fourteen healthy subjects (mean age, 30.9 ± 8.0 years, range 17-44 years; 4 women, 10 men) and one 20-year-old man with complete agenesis of the corpus callosum (Fig. 7A) were tested. All subjects were right-handed according to the Edinburgh Inventory (Oldfield, 1971). Written informed consent was obtained from all subjects. The study was performed according to the declaration of Helsinki and was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board.
Figure 7. Magnetic resonance imaging (MRI) scans and ipsilateral and contralateral MEPs of one patient with complete agenesis of the corpus callosum.
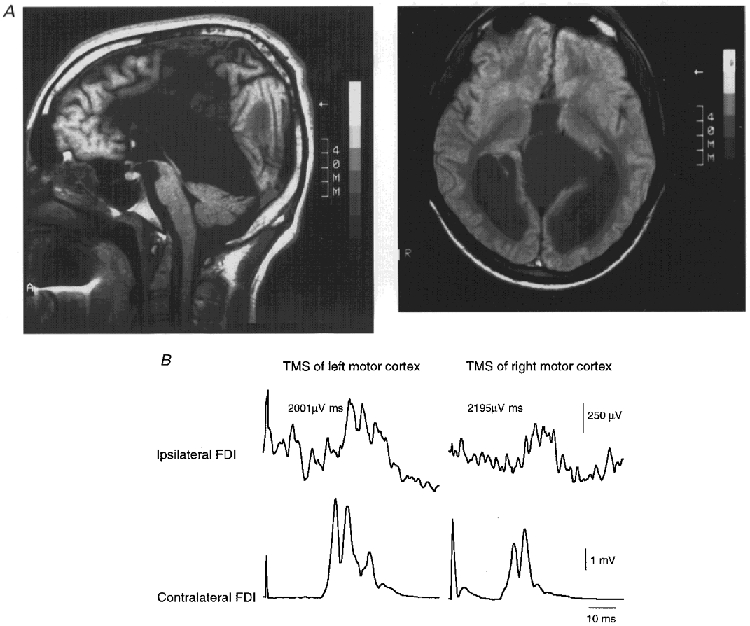
A, T1-weighted mid-sagittal (left) and proton density-weighted axial MRI (right) of the patient with agenesis of the corpus callosum and septum pellucidum cyst. The anterior commissure is preserved. B, in this patient, large ipsilateral MEPs (top traces) and normal contralateral MEPs (bottom traces) were elicited in the contracting FDI by focal TMS of the left (left panels) and right motor cortex (right panels). The delay in onset latency between the ipsilateral and contralateral MEP of 9.6 ms on both sides was within the normal range.
Recording and stimulation procedures
Subjects were seated comfortably in a reclining chair. Surface EMG was recorded from various target muscles (see below), using Ag-AgCl cup electrodes in a belly-tendon montage. After amplification and 10 Hz-2 kHz bandpass filtering (Counterpoint Electromyograph, Dantec Electronics, Skovlunde, Denmark) the EMG signal was fed into an IBM 486 AT-compatible laboratory computer (A/D rate, 5 kHz) for off-line analysis. Focal transcranial magnetic stimulation (TMS) was performed with a figure-of-eight-shaped coil (diameter of each wing, 5 cm) connected to a Cadwell rapid-rate magnetic stimulator (Cadwell Laboratories Inc., Kennewick, WA, USA).
Ipsilateral MEPs in different target muscles and effects of coil orientation
In 10 subjects, ipsilateral MEPs were studied in separate blocks of trials in the FDI, abductor digiti minimi, opponens pollicis, wrist extensors, wrist flexors, biceps brachii and triceps brachii muscles of the left arm. In all muscles, the recordings were made during approximately 30 % of maximum voluntary isometric contraction. The level of contraction was controlled by auditory feedback of the EMG signal. Fatigue was avoided by allowing for breaks between trials.
Experiments started with recordings in the ipsilateral first dorsal interosseus (FDI). The stimulating coil was placed over the left motor cortex at a position optimal for eliciting MEPs in the contralateral FDI. In eight blocks of 20 trials, eight different coil orientations spaced by 45 deg were tested (i.e. 0, 45, 90, 135, 180, 225, 270 and 315 deg) by rotating the coil around the centre of the junction of the wings, which was kept at the optimal position. Throughout this paper, the angles refer to the direction of the first phase of the damped cosine current induced in the brain, with 0 deg being lateral to medial and 90 deg posterior to anterior (see also inset to Fig. 5B). The order of coil orientations was randomized across subjects. For the testing of ipsilateral MEPs in the other muscles, the coil location on the head was adjusted so as to produce a maximum MEP in the contralateral homologous muscle, while the coil orientation was the same as the one producing the largest ipsilateral MEPs in the FDI. Stimulus intensity was always 100 % of maximum stimulator output.
Figure 5. Preferred current direction for ipsilateral and contralateral MEPs in the FDI.
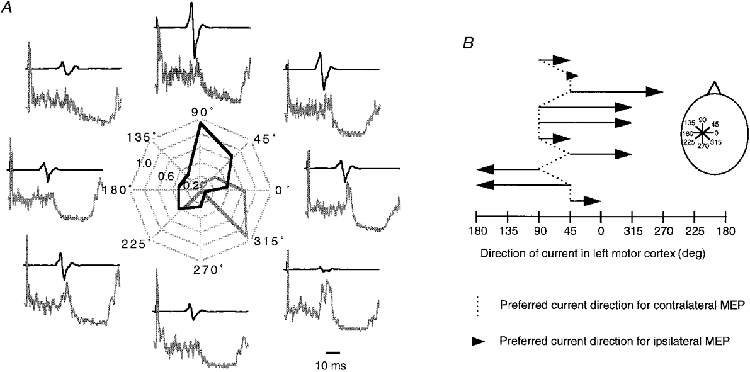
A, polar plot of ipsilateral (grey) and contralateral (black) MEPs in 1 subject. The angles around the perimeter indicate the direction of the current induced in the left hemisphere. Ipsilateral and contralateral MEP sizes are normalized to the maximum ipsilateral or contralateral MEP (= 1), respectively. Pairs of contralateral (top) and ipsilateral MEP recordings (bottom) are displayed around the polar plot at the positions corresponding to current direction. All traces are averages of 20 trials. The EMG recordings for the ipsilateral MEP are full-wave rectified. Note that the preferred direction for activation of the contralateral MEP in this subject was approximately orthogonal to the line of the central sulcus, while it was in parallel for the ipsilateral MEP. B, preferred direction of current induced in the left motor cortex for activation of contralateral MEPs (dotted line) and ipsilateral MEPs (arrows) in all 10 subjects tested. The inset illustrates the different current directions in the left hemisphere. Note that the preferred current direction for contralateral MEPs was 45-90 deg in all subjects, while it was different by 45-135 deg in all but 1 subject for the ipsilateral MEPs.
The effect of coil orientation was also investigated in the contralateral FDI at rest (5 trials for each orientation), using a stimulus intensity set to 110 % of resting motor threshold. Motor threshold was determined to the nearest 1 % of maximum stimulator output and defined as the minimum stimulus intensity resulting in a contralateral MEP of ≥ 50 μV in at least 5 out of 10 consecutive trials. Muscle relaxation was monitored by auditory feedback of the EMG signal at high gain. The size of the contralateral MEP was measured peak-to-peak in the single trials and averages were calculated.
For quantitative analysis of the ipsilateral MEP, single-trial rectification and averaging of the EMG from 20 trials was performed. The level of prestimulus EMG was integrated over a period of 50 ms immediately prior to the magnetic stimulus. The presence of an ipsilateral MEP was accepted if the poststimulus EMG exceeded the prestimulus EMG by at least 1 standard deviation (s.d.) for at least 5 ms. This EMG peak (ΔEMG, in μV ms) was expressed as:
where duration is the length of the period during which the poststimulus EMG exceeded the prestimulus EMG. The onset latency of the ipsilateral MEP was defined as the left border of this period. For comparison, the first negative deflection of the contralateral MEP was determined (only in the active wrist extensors) using just-above-threshold stimuli in order to match the sizes of the contralateral and ipsilateral MEPs.
Some target muscles showed no ipsilateral MEPs but rather inhibition of the EMG at the expected time of the ipsilateral MEPs. This inhibition was quantified in a similar way as above. The time window for quantification was set arbitrarily to the same limits as for an ipsilateral MEP in a different target muscle supplied by the same or a neighbouring segmental spinal level.
Other properties of the ipsilateral MEP in the FDI
Further experiments were conducted to characterize the ipsilateral MEP in the FDI in more detail. All of these experiments used the optimal coil orientation for producing ipsilateral MEPs in this muscle, and were performed at 100 % of stimulator output with the FDI activated at 30 % of maximum voluntary contraction, unless specified otherwise (see below). Twenty trials were run for all conditions. In order to determine the threshold for ipsilateral MEPs, different stimulus intensities (1.0, 1.5, 1.75, 2.0 and 2.25 × the active motor threshold for the contralateral MEP) were tested in four subjects. To determine the effects of the level of contraction of the FDI, the hand was immobilized in a pronated position in a hand rest and the subject was requested to abduct the index finger against a strain gauge (5 subjects). The level of contraction was fed back to the subject on a digital display. Rest and 10, 20, 30 and 50 % of maximum voluntary contraction were tested. In another experiment, the size of the ipsilateral MEP was compared between the left and the right FDI, in order to detect a possible left-hemispheric preference, as suggested by previous clinical, neuroimaging and electrophysiological studies (for review, see Chen et al. 1997). For this experiment, a coil rotation experiment equivalent to the one described above for the left FDI was also conducted for the right FDI (10 subjects). The maximum ipsilateral MEPs from both sides were selected for the between-sides comparison. Finally, a mapping study of the the cortical source of the ipsilateral MEP was performed over the left motor cortex, testing 15-25 positions on a 2 cm × 2 cm grid. Starting at the optimal position for the contralateral MEP, the grid was extended until the map for the contralateral resting FDI was surrounded by ineffective stimulation sites. The maps for the ipsilateral and the contralateral MEP were compared by calculating the centres of gravity (for methods, see Wassermann et al. 1992).
Effects of lateral head rotation on the size of ipsilateral and contralateral MEPs
In order to dissociate the pathways of ipsilateral and contralateral MEPs by afferent input, the effects of lateral head rotation on MEP size were investigated in eight subjects. Head rotation produces sensory input, mainly from neck afferents, which feeds into propriospinal and reticulo- and vestibulospinal neurones (for review, see Wilson & Peterson, 1981). If it were true that the ipsilateral MEP is mediated via one of these systems (see Introduction), then the effects of lateral head rotation on the ipsilateral MEP should be more pronounced or even qualitatively different from those on the contralateral MEP. The experiments were conducted in the contracting wrist extensors. Ipsilateral MEPs were elicited by TMS of the left motor cortex using a stimulus intensity of 100 % of maximum stimulator output. In separate blocks of trials, contralateral MEPs were elicited using either the same coil location and orientation over the left motor cortex or the optimal position to elicit contralateral MEPs over the right motor cortex. The stimulus intensity was scaled down to match the size of the contralateral MEP to the size of the ipsilateral MEP when measured in the head straight position. For both ipsilateral and contralateral MEPs, three different head rotations (head straight, head turned 90 deg to the left or 90 deg to the right; gaze always parallel to the nose) were compared in blocks of 20 trials each. MEP size was quantified as above. The head turn conditions were classified as away from or toward the target muscle. Differences in MEP size between the head turn conditions and the head straight condition were then expressed as increments (for instance, for the head turned away from the target muscle condition, where
MEP onset latency was expressed as the difference between the head turned away from or toward the target muscle conditions and the head straight condition).
Ipsilateral MEPs in a patient with complete agenesis of the corpus callosum
This patient has suffered from sporadic nocturnal generalized tonic seizures since early childhood. The neurological examination showed slight mental retardation and right-sided hyper-reflexia, but was otherwise normal. In particular, there were no mirror movements. MRI scanning revealed complete agenesis of the corpus callosum and a large interhemispheric cyst (Fig. 7A). The anterior commissure was preserved and its cross-sectional area of 13 mm2, as determined by MR volumetry, was enlarged compared with the normal range of 3-5 mm2 (Meyer et al. 1998). Furthermore, the MRI revealed a focal cortical heterotopia in the left frontal lobe outside the primary motor cortex (not shown). A routine TMS investigation confirmed the integrity of both crossed corticospinal tracts (that is normal central motor conduction times and normal ratios of the contralateral MEP over the maximum M wave). At the time of the examination, the patient was treated with carbamazepine (300 mg day−1, plasma level 3.3 μg ml−1) and had been seizure free for 4 weeks.
Statistics
The distributions of ipsilateral MEP sizes for the different target muscles (cf. Fig. 2C) were tested for difference from zero by a one-sample signed ranks test. The comparison of ipsilateral MEP size in the left versus the right FDI was performed by Student's paired t test. The effects of MEP side (ipsilateral MEP in left wrist extensors, contralateral MEP in left or right wrist extensors) and head rotation (away or toward the target muscle) on MEP size and MEP onset latency were tested in a two-way ANOVA for repeated measures. Conditional on significant F values, post hoc paired t tests were performed. For all tests, the significance level was set at P < 0.05.
Figure 2. Ipsilateral MEPs in various muscles of the hand and arm.
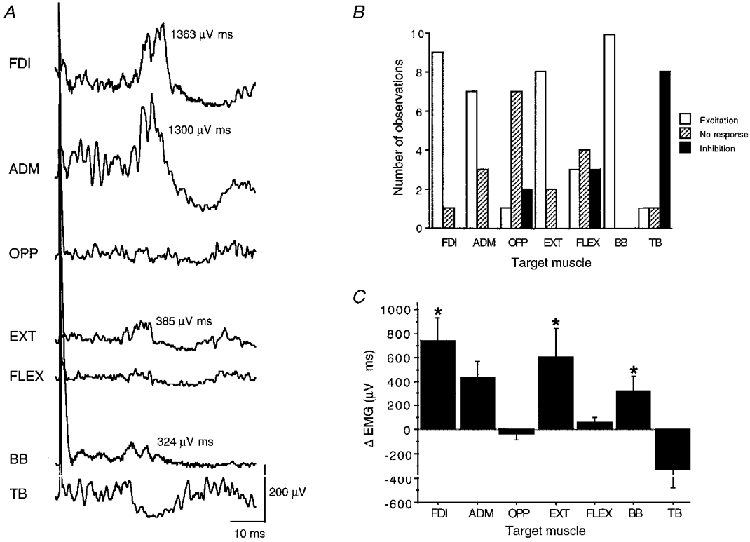
A, single trial rectified and averaged (n = 20) EMG recordings of various muscles ipsilateral to high-intensity TMS of the left motor cortex in 1 representative subject (FDI, first dorsal interosseus; ADM, abductor digiti minimi; OPP, opponens pollicis; EXT, wrist and finger extensors; FLEX, wrist and finger flexors; BB, biceps brachii; TB, triceps brachii). Each muscle was recorded in a separate block of trials while being activated isometrically at 30 % of maximum voluntary contraction. Note that ipsilateral MEPs are clearly visible in the FDI, ADM, EXT and BB, while OPP and FLEX did not show a significant ipsilateral response. The response in the TB was purely inhibitory. The size of the ipsilateral MEPs is given as the area under the EMG curve exceeding the level of the prestimulus EMG. B, frequency distribution of excitatory responses, no responses and inhibitory responses in the different target muscles after TMS of the ipsilateral motor cortex across all 10 subjects tested and C, mean size (+1 s.e.m.) of these responses given as the area under the EMG curve exceeding the level of the prestimulus EMG (μV ms). * Significant difference from zero (P < 0.01).
RESULTS
Ipsilateral MEPs in various muscles of the upper limb
Ipsilateral MEPs were obtained in the FDI muscle in 9 out of 10 subjects. Ten consecutive EMG trials in Fig. 1 show that the ipsilateral MEP displays a high trial-to-trial variability in amplitude and negative peak latency (range, 4.5 ms). Ipsilateral MEPs were also present in the abductor digiti minimi, wrist extensors and biceps brachii muscles in most or all of the subjects (Fig. 2). The size of the ipsilateral MEP was on average 736 ± 618 μV ms in the FDI, 426 ± 451 μV ms in the abductor digiti minimi, 597 ± 775 μV ms in the wrist extensors and 321 ± 364 μV ms in the biceps (Fig. 2C). In contrast, a small ipsilateral MEP was seen in only one subject in the opponens pollicis while all other subjects showed either no response or an ipsilateral inhibition (Fig. 2). A similar pattern was obtained in the wrist flexors (Fig. 2). The triceps brachii muscle was dissimilar from all other muscles because this muscle exhibited inhibition in most subjects (Fig. 2). The difference in onset latency between size-matched ipsilateral and contralateral MEPs in the contracting wrist extensors was 5.7 ± 1.1 ms (range, 4.1-7.0 ms).
Figure 1. EMG recordings of ipsilateral MEPs.
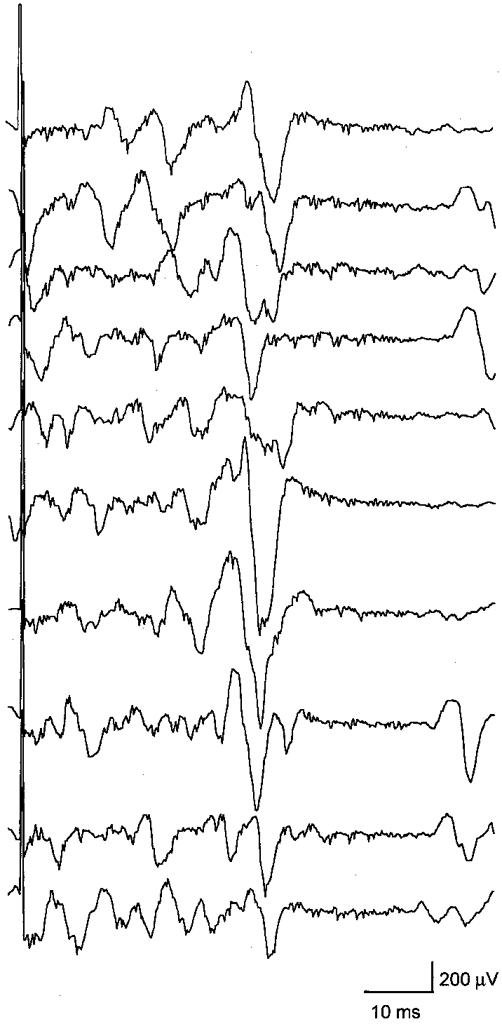
Ten consecutive EMG recordings of ipsilateral MEPs in the tonically active left FDI of 1 representative subject. TMS was delivered to the left motor cortex at maximum stimulator output. Note the trial-to-trial variability in MEP amplitude and latency.
Properties of ipsilateral MEPs in the FDI
Ipsilateral MEPs had a higher threshold compared with contralateral MEPs. Significant ipsilateral MEPs were obtained at 1.75 × the active motor threshold of the contralateral FDI in two subjects, at 2.0 × in one subject, and at 2.25 × in another subject.
When tested at maximum stimulator output, the size of the ipsilateral MEP depended in an approximately linear fashion on the level of contraction in the ipsilateral FDI (Fig. 3).Ipsilateral MEPs were never seen when the target muscle was at rest, and usually did not become significant with activation of less than 20 % of the maximum voluntary contraction (Fig. 3).
Figure 3. Effect of the level of contraction on the size of the ipsilateral MEP in the FDI.
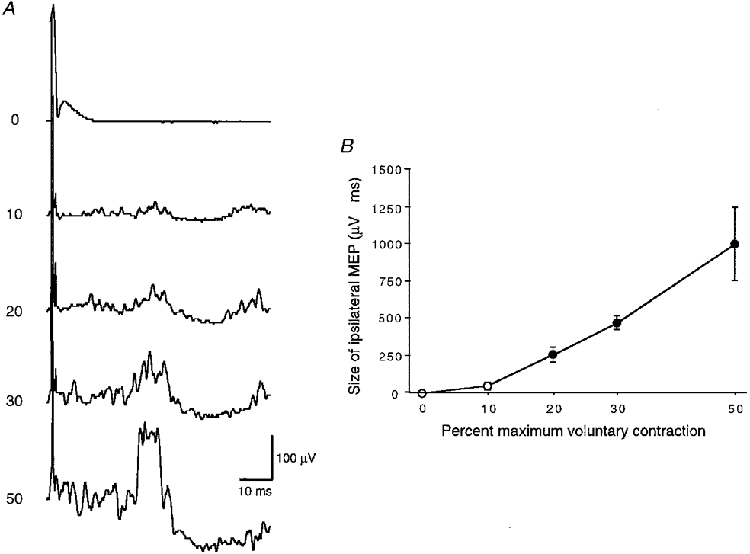
A, EMG recordings from 1 representative subject at increasing levels of contraction given as the percentage of maximum voluntary contraction on the left. All traces are averages of 20 rectified trials. TMS was given at an intensity of 100 % of maximum stimulator output to the left motor cortex. B, mean (± 1 s.e.m.) size of the ipsilateral MEP in 5 subjects plotted against the level of voluntary contraction. The filled symbols indicate that the distribution of ipsilateral MEPs was significantly different from zero (P < 0.05).
A comparison between stimulation of the left and right motor cortex revealed an asymmetry in the size of ipsilateral MEPs in the FDI muscle in most subjects. Six subjects had larger ipsilateral MEPs when the left motor cortex was stimulated, two subjects had larger MEPs with right motor cortex stimulation, and two subjects showed no side difference. On average, ipsilateral MEP size across subjects was not different between the two hemispheres (736 ± 618 μV ms for the left FDI versus 918 ± 1610 μV ms for the right FDI; P = 0.70).
Maps and preferred current direction of ipsilateral and contralateral MEPs in the FDI
Four of the seven subjects tested showed a centre of gravity clearly located more laterally for the ipsilateral compared with the contralateral map (subjects a-d in Fig. 4). A medial shift of the ipsilateral compared with the contralateral map was not observed in any of the subjects. The average centre of gravity was located more lateral by 0.59 cm and more anterior by 0.27 cm for the ipsilateral compared with the contralateral map (Fig. 4).
Figure 4. Mapping of ipsilateral and contralateral MEPs in the FDI.
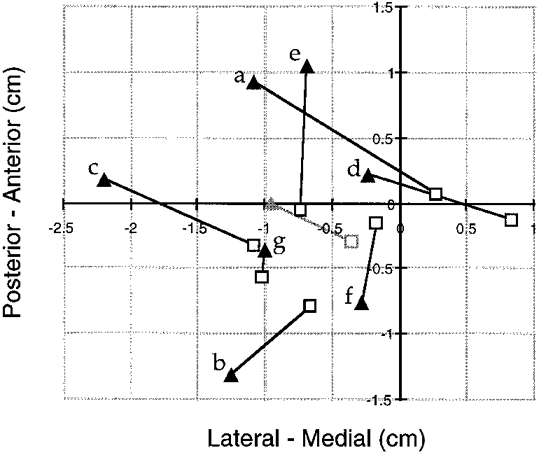
Comparison of the centres of gravity (COG) of the maps for the ipsilateral (filled triangles) and the contralateral MEP (□) in 7 subjects (a-g). The grey symbols show the means of the COG across subjects. In four subjects (a-d), the COG of the ipsilateral map was clearly more lateral compared with the contralateral map, while a medial shift was not observed in any of the subjects. The point 0/0 refers to the coil location, which was considered the optimal position for eliciting contralateral MEPs during the experiment.
The preferred current for contralateral MEPs was either 45 or 90 deg in all subjects (Fig. 5), which is approximately orthogonal to the central sulcus. In contrast, ipsilateral MEPs were best elicited in all but one subject when the inducing current was rotated away by 45-135 deg from the preferred current direction for the contralateral MEP (Fig. 5).
Effects of lateral head rotation on ipsilateral and contralateral MEPs in the wrist extensors
The ANOVA showed a significant interaction between the effects of head rotation and side of MEP for both MEP size (F2,14 = 7.41; P = 0.0064) and MEP onset latency (F2,14 = 27.96; P < 0.0001). The interactive effect on MEP size was explained by a significant facilitation of ipsilateral MEPs (P = 0.011) by head rotation toward the (left) ipsilateral wrist extensors compared with the condition when the head was turned away from the ipsilateral muscle. In contrast, contralateral MEPs in both the right and left wrist extensors showed a non-significant trend toward facilitation when the head was turned away compared with when the head was turned toward the target muscle (Fig. 6A and B). The interactive effect on MEP onset latency was explained by a significant (P < 0.0001) shortening of ipsilateral MEPs if the head was turned toward the ipsilateral wrist extensors (Fig. 6A and C). For the ipsilateral MEPs, the difference in onset latencies between the head turned toward and away conditions was on average 2.1 ms (range, 0.5-4.5 ms). In contrast, the weak facilitation of the contralateral MEPs in the head turned away condition was not associated with a significant decrease in onset latency (Fig. 6A and C).
Figure 6. Effects of lateral head rotation on ipsilateral and contralateral MEPs in wrist extensor muscles.
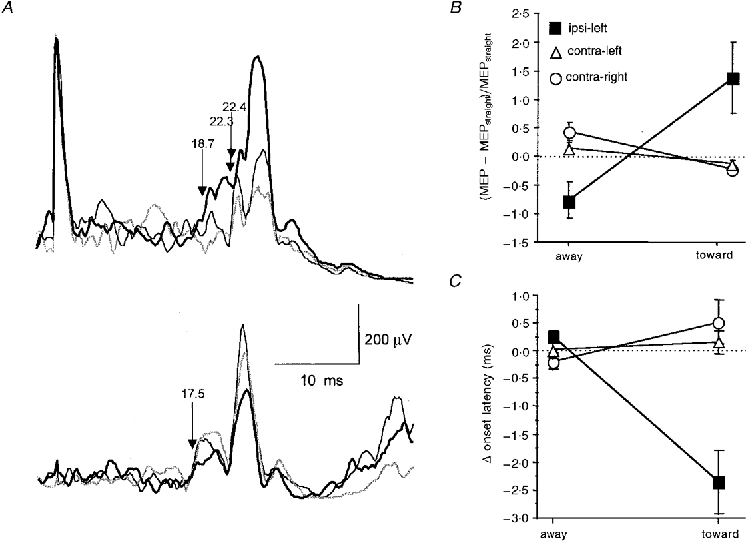
A, EMG recordings of ipsilateral MEPs (top) and size-matched contralateral MEPs (bottom) from the voluntarily activated wrist extensors of 1 representative subject. All traces are single-trial rectified averages of 20 trials. The thin black lines refer to the head straight condition, thick black lines are the head turn toward the target muscle conditions, and grey lines are the head turn away from the target muscle conditions. The arrows and numbers indicate the MEP onset latencies. Intensity of TMS was 100 % of maximum stimulator output for the ipsilateral MEP and 34 % for the contralateral MEP. It was always the left motor cortex that was stimulated. Note that for the ipsilateral MEP, turning the head toward the ipsilateral muscle was associated with a marked increase in MEP amplitude and a decrease in MEP onset latency. In contrast, for the contralateral MEP, turning the head toward the contralateral muscle was associated with a decrease in MEP amplitude but no concomitant change in MEP onset latency (17.5 ms for all 3 directions of the head). B and C, group data (8 subjects) on the effects of head rotation on ipsilateral (▪, recording from left wrist extensors and TMS of left motor cortex) and contralateral MEPs (^, recording from right wrist extensors and TMS of left motor cortex; ▵, recording from left wrist extensors and TMS of right motor cortex). Increments (MEP size) or differences (MEP onset latency) between the head turn conditions (away from or toward the ipsi- or contralateral target muscle) and the head straight condition are displayed. Error bars are ± 1 s.e.m.
Ipsilateral MEPs in the patient with complete agenesis of the corpus callosum
In this patient, it was possible to elicit ipsilateral MEPs in the FDI on both sides (Fig. 7B). The delays in onset latency of the ipsilateral MEP with respect to the MEP in the active contralateral FDI (9.2 ms on both sides) were within the range that we found in normal subjects, while the amplitudes of the ipsilateral MEP (2001 μV ms for the left and 2195 μV ms for the right FDI) were large compared with the normal mean (on the left side, more than 2 s.d. above the mean).
DISCUSSION
The principal new findings of this TMS study are that (1) ipsilateral MEPs were commonly elicited in hand and arm muscles of healthy adults; (2) ipsilateral MEPs occurred preferentially in finger abductors, finger and wrist extensors and elbow flexors, but not or to a lesser extent in finger and wrist flexors and elbow extensors; (3) ipsilateral MEPs had a different preferred current direction for activation when compared with contralateral MEPs; (4) lateral head rotation exerted a crossed modulation of ipsilateral and contralateral MEP size; (5) large ipsilateral MEPs were obtainable in a patient with complete agenesis of the corpus callosum.
Several points may explain why some previous investigators have failed to demonstrate ipsilateral MEPs. We have shown that some target muscles, like the thenar muscles, exhibit ipsilateral MEPs much less frequently than other muscles such as the FDI or abductor digiti minimi. Furthermore, ipsilateral MEPs have a higher threshold than contralateral MEPs and occur only if the ipsilateral target muscle is active at least at 20-30 % of maximum voluntary contraction (see also Taylor et al. 1997). Previously unsuccessful studies (Carr et al. 1994; Müller et al. 1997; Netz et al. 1997) used an insufficient TMS intensity, an insufficiently strong contraction of the target muscle or a target muscle with a low yield of ipsilateral MEPs.
Exclusion of various pathways as routes for the ipsilateral MEP in hand muscles
Most of our analysis of the ipsilateral MEP is based on rectified and averaged EMG recordings. Peaks in rectified EMG averages can be caused by inhibition of motoneurones (Widmer & Lund, 1989). However, we have shown here and previously (Wassermann et al. 1994) that ipsilateral MEPs can be detected consistently in the single unrectified trial EMG (Fig. 1). These ipsilateral MEPs had the same polarity (first phase negative) as large contralateral MEPs recorded under identical conditions in the same target muscle, indicating the true excitatory nature of the ipsilateral MEP.
Current spread
Another concern is that the observed ipsilateral MEPs could have been caused by current spreadinto the opposite motor cortex. Three lines of evidence exclude this possibility. First, if current spread had contributed, ipsilateral MEPs should have been more prominent in the medial part of the map (toward the opposite hemisphere), but the opposite was true in this and a previous study (Wassermann et al. 1994). Second, contralateral MEPs of different hand muscles do not differ significantly with respect to MEP threshold or MEP amplitude (Hess et al. 1987). Therefore, current spread would not explain why ipsilateral MEPs occurred in some hand muscles but not in others. Third, ipsilateral MEPs were delayed by 5-13 ms compared with the contralateral MEPs, which is incompatible with current spread. Although the onset latency of a contralateral MEP becomes longer the further the stimulating coil moves away from the optimal position, latency shifts of more than 4 ms have not been reported (Fuhr et al. 1991).
Branching of corticomotoneuronal axons
This can be excluded as a possible pathway of the ipsilateral MEP because one would expect a delay equal or close to zero between the ipsilateral and contralateral MEP (Farmer et al. 1990; Carr et al. 1994). However, other left and right homologous muscle pairs, in particular axial and bulbar muscles, seem to have, at least in part, a common drive through the corticospinal tract from one or both hemispheres (Carr et al. 1994).
Fast-conducting uncrossed corticomotoneuronal (i.e. monosynaptic) pathway
The existence of such a pathway was put forward as an explanation in some patients with persistent mirror movements where the ipsilateral MEP was of the same latency but larger in amplitude compared with the contralateral MEP (Mayston et al. 1997). Again, our results are incompatible with the expected delay of close to zero between the ipsi- and contralateral MEPs. We took the most conservative measures, i.e. voluntary activation of the target muscles and matched amplitudes of the ipsilateral and contralateral MEPs, in order to determine differences in onset latency. Voluntary activation should largely eliminate differences in the summation time necessary to bring spinal motoneurones above firing threshold at MEP onset because some motoneurones are always at firing threshold when the first wave of the descending corticofugal volley arrives. Matched MEP amplitudes eliminate the problem that would arise if the ipsilateral MEP was due to a weaker projection than the contralateral MEP.
Crossed corticospinal tract and recrossing through commissural interneurones at the segmental level
This has been invoked to explain the rapid recovery of patients with surgical incisions into the spinal cord interrupting the lateral corticospinal tract on one side (Nathan, 1994). However, no evidence has been found for recrossing at the segmental level in healthy subjects. Reflex studies, using muscle stretch, or mixed or cutaneous nerve stimulation, have shown consistently that in normal subjects the early spinal as well as the late, supposedly transcortical, components of the reflex occur strictly unilaterally in muscles on the side of the stimulation (Caccia et al. 1973; Farmer et al. 1990; Capaday et al. 1991).
Transcallosal (interhemispheric) pathway
Another possibility is that the ipsilateral MEP is mediated through a transcallosal (interhemispheric) pathway. Neuroanatomical data from the monkey show that the hand areas of the two motor cortices are interconnected, although sparsely, by callosal fibres (Rouiller et al. 1994). However, our data are incompatible with a significant contribution of interhemispheric connections to the ipsilateral MEP. First, we obtained large ipsilateral MEPs with a normal delay in onset latency in a patient with complete agenesis of the corpus callosum and an enlarged anterior commissure (Fig. 7A and B). Compensatory enlargement of the anterior commissure in acallosal patients can serve for interhemispheric transfer, primarily of visual, auditory and olfactory information (Fischer et al. 1992), but none of the available anatomical or behavioural evidence supports a role of the anterior commissure in the interhemispheric transfer of somatosensory information or intermanual motor commands (Pandya & Seltzer, 1986). In contrast, our data support the hypothesis of an enhanced ipsilateral motor projection in acallosal patients (Jeeves, 1986), while weaker or absent ipsilateral MEPs would be expected if they were mediated through an enlarged extracallosal pathway which is not used for motor interhemispheric transfer under normal circumstances. Second, we showed that the difference in the onset latency of size-matched ipsilateral and contralateral MEPs was 5.7 ms. This difference decreased to 3.1 ± 1.9 ms (range, 0.6-6.4 ms) when the ipsilateral MEP was facilitated by lateral head rotation (Fig. 6A). These differences are too small to be explained by a transcallosal pathway because measurements of the minimum interhemispheric conduction time between the motor cortices in humans resulted in values of no less than 7.8-8.0 ms (Cracco et al. 1989).
Dissociation of the ipsilateral and contralateral MEPs and candidate routes for the ipsilateral MEP
The corticofugal fibres of the ipsilateral and contralateral MEPs can be dissociated by virtue of preferred stimulating current direction and map location. We can only speculate about the nature of these differences. The preferred current direction for the contralateral MEP (anteromedial, approximately perpendicular to the central sulcus) has previously been shown by others (Brasil-Neto et al. 1992) and is the preferred direction for the production of I1 waves (Sakai et al. 1997). The preferred current direction for the ipsilateral MEP (in most cases anterolateral or posteromedial, approximately parallel to the central sulcus) preferentially elicits I3 waves (Sakai et al. 1997). If I3 waves contributed to the production of the ipsilateral MEP, then this may explain in part the delay in onset latency between the ipsilateral and contralateral MEP.
This study and an earlier report (Wassermann et al. 1994) showed that ipsilateral MEPs were elicited more prominently from sites lateral to the optimal position for MEPs in the contralateral hand (Fig. 4). Relevant to this might be the identification of a small percentage of neurones in the monkey (Tanji et al. 1988; Aizawa et al. 1990) and human motor cortex (Goldring & Ratcheson, 1972), which are active with arm or hand movements on the ipsilateral or both sides. These neurones were located lateral to the representation of the contralateral hand (Aizawa et al. 1990). For the following reasons, it might be speculated that these neurones are the origin of an ipsilateral oligo- or polysynaptic projection, such as a corticoreticulospinal (Nathan et al. 1996) or corticopropriospinal pathway (Pierrot-Deseilligny, 1996). First, within the precentral gyrus, corticobulbar neurones are located in its lateral one-third (Keizer & Kuypers, 1989). Second, at least in the cat, finger and wrist extensors and elbow flexors receive more monosynaptic excitatory input from the reticulospinal system than do elbow extensor muscles (Peterson et al. 1979), similar to the distribution of ipsilateral MEPs in various muscles of the hand and arm in this study. Third, neck afferents project onto reticulospinal, vestibulospinal and propriospinal neurones (for review, see Wilson & Peterson, 1981). Typically, head rotation toward one side elicits activation of arm extensor muscles on the same side and activation of arm flexor muscles on the other (Wilson & Peterson, 1981). In humans, however, this asymmetrical tonic neck reflex becomes clinically overt only under pathological conditions (Wilson & Peterson, 1981). The observed facilitation or reduction of the ipsilateral MEP in the wrist extensors with the head turned respectively toward or away from the ipsilateral arm (Fig. 6A and B) is in accord with the asymmetrical tonic neck reflex. In contrast, the weaker opposite modulation of the contralateral MEP (Fig. 6A and B) is inconsistent with the tonic neck reflex. Discordant results were also obtained when the effects of head rotation on spinal motoneurone excitability were probed with H-reflexes (Aiello et al. 1988). Therefore, the modulation of the contralateral MEP by lateral head rotation can be explained by changes in motoneurone excitability. The opposite modulation of ipsilateral MEPs, concordant with the tonic neck reflex, indicates a privileged input of tonic neck afferents to the pathways of the ipsilateral MEP. It is very likely that this input from neck receptors converges with the pathways of the ipsilateral MEP upstream from the spinal motoneurone because the facilitation of ipsilateral MEPs induced by lateral head rotation was associated with a significant shortening (on average 2.1 ms) of its onset latency (Fig. 6A and C). Such a shortening should be impossible at the level of the spinal motoneurone, since all measurements were performed in the voluntarily contracting muscle, largely eliminating the summation time at the spinal motoneurone at MEP onset (see above). This argument renders a monosynaptic slow-conducting ipsilateral pathway very unlikely. Consequently, the facilitation of the contralateral MEP (mediated by the corticomotoneuronal projection) induced by lateral head rotation was not associated with a shortening of MEP onset latency (Fig. 6A and C).
In conclusion, the dissociation of the ipsilateral and contralateral MEPs at the cortical level through differences in map location and preferred current direction indicates that corticofugal motor fibres other than the fast-conducting crossed corticomotoneuronal system can be activated by TMS in healthy adults. The properties of the ipsilateral MEP demonstrated in this study are most compatible with an oligosynaptic ipsilateral pathway, such as a corticoreticulospinal or corticopropriospinal projection.
Acknowledgments
We thank Dr Mirko Cosottini for the neuroradiological investigation of the acallosal patient and Dr Leonardo G. Cohen for helpful discussions on the manuscript. Dr Ziemann was supported by Grant Zi 542/1-1 from the Deutsche Forschungsgemeinschaft. This manuscript has been presented in preliminary form (Ziemann et al. 1998) during the International TMS Symposium (October 1-4, 1998, Göttingen, Germany) where it won the first prize for posters.
References
- Aiello I, Rosati G, Sau GF, Patraskakis S, Bissakou M, Traccis S. Tonic neck reflexes on upper limb flexor tone in man. Experimental Neurology. 1988;101:41–49. doi: 10.1016/0014-4886(88)90063-5. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Experimental Brain Research. 1990;82:219–221. doi: 10.1007/BF00230856. [DOI] [PubMed] [Google Scholar]
- Basu AP, Turton A, Lemon RN. Activation of ipsilateral upper limb muscles by transcranial magnetic stimulation. The Journal of Physiology. 1994;479.P:144P. [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Experimental Brain Research. 1991;83:419–426. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. Journal of Clinical Neurophysiology. 1992;9:132–136. [PubMed] [Google Scholar]
- Britton TC, Meyer BU, Benecke R. Central motor pathways in patients with mirror movements. Journal of Neurology, Neurosurgery and Psychiatry. 1991;54:505–510. doi: 10.1136/jnnp.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia MR, McComas AJ, Upton AR, Blogg T. Cutaneous reflexes in small muscles of the hand. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. The Journal of Physiology. 1991;440:243–255. doi: 10.1113/jphysiol.1991.sp018706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. The Journal of Physiology. 1994;475:217–227. doi: 10.1113/jphysiol.1994.sp020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Role of the ipsilateral motor cortex in voluntary movement. Canadian Journal of the Neurological Sciences. 1997;24:284–291. doi: 10.1017/s0317167100032947. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ragazzoni A, de Scisciolo G, Pinto F, Maurri S, Barontini F. Abnormal projection of corticospinal tracts in a patient with congenital mirror movements. Neurophysiologie Clinique. 1994;24:427–434. doi: 10.1016/s0987-7053(05)80075-9. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, Sanes JN, Jabbari B, Branscum B, Hallett M. Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain. 1991;114:381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalography and Clinical Neurophysiology. 1989;74:417–424. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Danek A, Heye B, Schroedter R. Cortically evoked motor responses in patients with Xp22.3-linked Kallmann's syndrome and in female gene carriers. Annals of Neurology. 1992;31:299–304. doi: 10.1002/ana.410310312. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. The Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–1510. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. The Journal of Physiology. 1990;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Archives of Neurology. 1992;49:271–277. doi: 10.1001/archneur.1992.00530270085023. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Cohen LG, Roth BJ, Hallett M. Latency of motor evoked potentials to focal transcranial stimulation varies as a function of scalp positions stimulated. Electroencephalography and Clinical Neurophysiology. 1991;81:81–89. doi: 10.1016/0168-5597(91)90001-e. [DOI] [PubMed] [Google Scholar]
- Goldring S, Ratcheson R. Human motor cortex: sensory input data from single neuron recordings. Science. 1972;175:1493–1495. doi: 10.1126/science.175.4029.1493. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. The Journal of Physiology. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hömberg V, Stephan KM, Netz J. Transcranial stimulation of motor cortex in upper motor neurone syndrome: its relation to the motor deficit. Electroencephalography and Clinical Neurophysiology. 1991;81:377–388. doi: 10.1016/0168-5597(91)90027-u. [DOI] [PubMed] [Google Scholar]
- Jeeves MA. Callosal agenesis: neuronal and developmental adaptations. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres - One Brain: Functions of the Corpus Callosum. New York: Alan Liss, Inc.; 1986. pp. 403–421. [Google Scholar]
- Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. Journal of Neurology, Neurosurgery and Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Experimental Brain Research. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Konagaya Y, Mano Y, Konagaya M. Magnetic stimulation study in mirror movements. Journal of Neurology. 1990;237:107–109. doi: 10.1007/BF00314672. [DOI] [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Ishii S, Shiota M, Takeuchi A, Yoshino K, Takeshita K. Mechanisms of central motor reorganization in pediatric hemiplegic patients. Neuropediatrics. 1997;28:168–174. doi: 10.1055/s-2007-973695. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain. 1997;120:1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Niehaus L. Morphology of acallosal brains as assessed by MRI in six patients leading a normal daily life. Journal of Neurology. 1998;245:106–110. doi: 10.1007/s004150050187. [DOI] [PubMed] [Google Scholar]
- Müller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Annals of Neurology. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117:337–346. doi: 10.1093/brain/117.2.337. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119:1809–1833. doi: 10.1093/brain/119.6.1809. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, Hömberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres - One Brain: Functions of the Corpus Callosum. New York: Alan Liss, Inc.; 1986. pp. 47–73. [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Experimental Brain Research. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. [Google Scholar]
- Rothwell JC. Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalography and Clinical Neurophysiology. 1991;(suppl. 43):29–35. [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Experimental Brain Research. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. Journal of Neurophysiology. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia S. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Experimental Brain Research. 1997;117:472–478. doi: 10.1007/s002210050243. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalography and Clinical Neurophysiology. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, Hallett M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 1991;41:1795–1799. doi: 10.1212/wnl.41.11.1795. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalography and Clinical Neurophysiology. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Experimental Brain Research. 1994;100:121–132. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Lund JP. Evidence that peaks in EMG averages can sometimes be caused by inhibition of motoneurons. Journal of Neurophysiology. 1989;62:212–219. doi: 10.1152/jn.1989.62.1.212. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Peterson BW. Vestibulospinal and reticulospinal systems. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, The Nervous System. Vol. 2. Bethesda: The American Physiological Society; 1981. pp. 667–702. [Google Scholar]
- Ziemann U, Wassermann EW, Ishii K. Ipsilateral motor evoked potentials. How to get them in normal adults. Electroencephalography and Clinical Neurophysiology. 1998;107:77–78P. [Google Scholar]


