Abstract
Whole-cell patch-clamp recordings were made from visually identified hippocampal interneurones in slices of rat brain tissue in vitro. Bath application of the bombesin-like neuropeptides gastrin-releasing peptide (GRP) or neuromedin B (NMB) produced a large membrane depolarization that was blocked by pre-incubation with the subtype 2 bombesin (BB2) receptor antagonist [D-Phe6,Des-Met14]bombesin-(6-14)ethyl amide.
The inward current elicited by NMB or GRP was unaffected by K+ channel blockade with external Ba2+ or by replacement of potassium gluconate in the electrode solution with caesium acetate.
Replacement of external NaCl with Tris-HCl significantly reduced the magnitude of the GRP-induced current at -60 mV. In contrast, replacement of external NaCl with LiCl had no effect on the magnitude of this current.
Photorelease of caged GTPγS inside neurones irreversibly potentiated the GRP-induced current at -60 mV. Similarly, bath application of the phospholipase C (PLC) inhibitor U-73122 significantly reduced the size of the inward current induced by GRP.
Reverse transcription followed by the polymerase chain reaction using cytoplasm from single hippocampal interneurones demonstrated the expression of BB2 receptor mRNA together with glutamate decarboxylase (GAD67).
Although bath application of GRP or NMB had little or no effect on the resting membrane properties of CA1 pyramidal cells per se, these neuropeptides produced a dramatic increase in the number and amplitude of miniature inhibitory postsynaptic currents in these cells in a TTX-sensitive manner.
Bombesin is a 14 amino acid-containing peptide first isolated from the skin of the frog Bombina bombina (Anasti et al. 1971). Since its isolation, two related mammalian neuropeptides, neuromedin B (NMB) and gastrin-releasing peptide (GRP), have been isolated and shown to have a widespread distribution (Wada et al. 1990). Recently two distinct receptor subtypes for mammalian bombesin-like peptides have been cloned: the BB1 receptor is the preferred receptor for NMB whilst the BB2 receptor preferentially binds GRP (Spindel et al. 1990; Wada et al. 1991).
In situ hybridization analysis has shown that mRNAs encoding these two receptor subtypes exhibit a widespread and distinct distribution throughout the CNS (Battey & Wada, 1991). In agreement with this, autoradiographical studies demonstrate that NMB and GRP binding sites within the CNS are differentially distributed (Ladenheim et al. 1990). Areas rich in NMB-preferring binding sites include the ventromedial hypothalamus and dentate gyrus whilst the supraoptic nucleus, arcuate nucleus and outer rim of the hippocampal oriens layer exhibit dense amounts of GRP binding.
Since previous studies have demonstrated that bombesin-like peptides mediate potent biological effects in several of the above nuclei (Piggins & Rusak, 1993; Pinnock et al. 1994) in the present study we have investigated the actions of these peptides on GABAergic interneurones in the stratum oriens of the hippocampus. Although these interneurones represent a relatively small proportion of the hippocampal neurone population (Freund & Buszsáki, 1996) they are believed to exert a powerful tonic inhibition of pyramidal neurones in addition to powerful feedforward and feedback inhibitory postsynaptic potentials (IPSPs) after stimulation (Lacaille et al. 1987). As such, these neurones represent an important site in the regulation of hippocampal excitability.
METHODS
Preparation
Wistar rats (14-28 days old) were killed by cervical dislocation. The brains were removed and 300 μm coronal slices containing the hippocampus prepared in ice-cold physiological saline (see below for composition) using a vibratome. A Zeiss Axioskop microscope (Carl Zeiss Ltd, Welwyn Garden City, UK) fitted with a × 64 water-immersion objective lens was used to view slices using light in the infrared range.
Recording and analysis
Whole-cell recording electrodes were pulled from borosilicate glass capillaries and had resistances of 3-6 MΩ when filled with electrolyte. Electrophysiological signals were detected using an Axopatch-1D patch-clamp amplifier and were recorded onto digital audiotape for later figure production. Following formation of the whole-cell configuration, series resistance was partially compensated using the amplifier and cellular conductance was continually monitored via the injection of hyperpolarizing current (current-clamp mode: -100 pA in amplitude, 300 ms in duration at 0.1 Hz) or voltage (voltage-clamp mode: -10 mV in amplitude, 300 ms duration at 0.1 Hz). Membrane signals were filtered at 1 kHz and digitized at 5 kHz through a Digidata 1200B A/D converter using pCLAMP 6.0 software (Axon instruments). In voltage-clamp recordings, neurones were clamped at -60 mV and current-voltage relationships were examined using a voltage ramp protocol between -140 and -60 mV (20 mV s−1). Analysis of the frequency and amplitude of inhibitory postsynaptic currents (IPSCs) was performed using Jaejin mini analysis software (v3.0.1. Jaejin Software, NJ, USA). All data in the text and figures are presented as means ±s.e.m. unless otherwise stated.
Solutions and drugs
The physiological saline contained (mM): 125.0 NaCl, 25.0 NaHCO3, 20.0 glucose, 2.5 KCl, 1.25 NaH2PO4, 2.0 CaCl2, 1.0 MgCl2 and was bubbled with a 95 % O2-5 % CO2 gas mixture. The intracellular (pipette) solution comprised (mM): 120.0 potassium gluconate, 10.0 NaCl, 2.0 MgCl2, 0.5 K2EGTA, 10.0 Hepes, 4.0 Na2ATP, 0.3 Na2GTP, pH adjusted to 7.2 with KOH. In some experiments, the ammonium salt of 500 μM guanosine 5′-O-(3-thiotriphosphate) P3-(S)-(1-(4,5-dimethoxy-2-nitrophenyl)ethyl ester (caged GTPγS) was added to the pipette solution whilst in others, the potassium salt of Lucifer Yellow (1 mg ml−1) was included. To investigate the effects of NMB or GRP on IPSCs in CA1 pyramidal neurones, the electrode solution contained (mM): 120.0 KCl, 10.0 NaCl, 2.0 MgCl2, 1.0 2(triethylamino)-N-(2,6-dimethylphenyl)acetamide (QX-314), 0.5 K2EGTA, 10.0 Hepes, 4.0 Na2ATP, 0.1 Na2GTP, pH adjusted to 7.2 with KOH.
Throughout the course of an experiment, slices were continuously perfused at a rate of 3-4 ml min−1 with physiological saline by means of a gravity feed system. GRP and other drugs used in this study were applied by changing the solution which superfused the slice to one which contained the drug. In experiments designed to examine the role of various currents in the observed effect, BaCl2 was used to directly replace CaCl2 in the Ba2+-containing saline whilst caesium acetate directly replaced potassium gluconate in the electrode solution. When low Na+ saline was used, NaCl was completely replaced by either LiCl or Tris-HCl. All experiments were conducted at 30-33°C.
The following drugs were used: diazo-2 and caged GTPγS were obtained from Molecular Probes (Eugene, OR, USA). 3,5-Dihydroxyphenylglycine (DHPG), 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(F)quinoxalone (NBQX), D-2-amino-5-phosphovalerate (APV) and (S)-α-methyl-4-carboxyphenylglycine (MCPG) were from Tocris Cookson (Bristol, UK). 1-(6-((17β-3-Methoxyoestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U-73122) and 1-(6-((17β-3-methoxyoestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrolidine-2,5-dione (U-73343) were obtained from Calbiochem-Novabiochem (Nottingham, UK) whilst [D-Phe6, Des-Met14]bombesin-(6-14)ethyl amide was obtained from Peninsula laboratories. TTX and all other chemicals were obtained from Sigma Chemicals. All drug stocks used were made up in distilled water except NBQX, U-73122 and U-73343, which were made up as 10 mM stock solutions in DMSO whilst NMB and GRP were prepared as 1 mM stock solutions in DMSO.
Single cell RT-PCR
The cytoplasm from hippocampal neurones was gently aspirated under visual control into the recording electrode until at least 40 % of the somatic cytoplasm had been collected (Lee et al. 1998). The electrode was then withdrawn from the cell, forced into a microtube and the RNA reverse transcribed using an anchored oligo dT primer and 200 Units of MMLV reverse transcriptase (BRL) according to the manufacturer's recommendations. After 60 min at 37°C the cDNA was stored frozen at -20°C prior to processing. After amplification of the cDNA using Taq polymerase (Dixon et al. 1998), the expression of specific genes was measured using primers designed to amplify products of between 150 and 250 base pairs in length, close to the 3′ ends of the mRNA transcripts.
The primers used were as follows. For cyclophilin (accession number M19533): forward primer (bases 388-407), ACTGCCAAGACTGAGTGGCT; reverse primer (bases 575-554), AATGGTTTGATGGGTAAAATGC. For BB2 receptor (accession number X56661): forward primer (3′ untranslated), GGGGGAAAAGAGCTAAGCC; reverse primer (3′ untranslated), TATTCAAAGCACCGAAAGCC; For glutamate decarboxylase (GAD67, accession number X57573): forward primer (bases 2912-2933), ATCTTGCTTCAGTAGCCTTTGC; reverse primer (bases 3131-3110), TGTCTTCAAAAACACTTGTGGG. These PCR reactions were run for 50 cycles at 92°C (denaturing, 2.5 min), 55°C (annealing, 1.5 min) and 72°C (extension, 1 min), followed by a final extension of 10 min at 72°C. The PCR products were separated on 2.5 % agarose gels and the product sizes were as predicted from the sequences. Confirmation of the nature of the bands was achieved using restriction enzyme analysis after reamplification with the appropriate gene specific primers.
RESULTS
Identification of stratum oriens interneurones
This study was performed on a total of 102 visually identified interneurones present in hippocampal slices taken from 76 animals. Only neurones located in the stratum oriens of the hippocampus were chosen for this study. These neurones were typically present in low density and were found to exhibit a heterogeneous morphology. Following formation of the whole-cell configuration, these neurones displayed electrophysiological characteristics similar to those previously attributed to GABAergic interneurones in this area (Zhang & McBain, 1995). For example, in current-clamp recordings, these neurones had a resting membrane potential of -53.6 ± 4.4 mV (n = 34) and often fired spontaneous action potentials at a rate of 2-10 Hz which were of short duration (1.1 ± 0.1 ms, n = 34, measured at half-amplitude) and which were followed by a rapid after-hyperpolarization (16.1 ± 1.3 mV in amplitude, 151.4 ± 11.3 ms in duration). Immediately after membrane breakthrough these neurones had an input resistance of 486.9 ± 23.2 MΩ (n = 34) and exhibited a characteristic reduction in apparent input resistance in response to hyperpolarizing current injection, which has previously been attributed to the non-selective cation channel current IH (Maccaferri & McBain, 1996).
To confirm the GABAergic nature of these neurones, in five cells, following electrophysiological identification, cytoplasm was harvested and processed for reverse transcription and cDNA amplification. The resultant cDNA was subjected to PCR analysis with primers specific for the enzyme glutamate decarboxylase (GAD67). Each neurone examined was positive for this enzyme (Fig. 9).
Figure 9. Molecular identity of the GRP-sensitive interneurones.
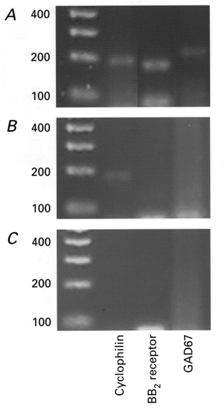
A, using the cytoplasmic contents harvested from a single GRP-sensitive interneurone, the expression of GAD67 mRNA together with the housekeeping gene cyclophilin, and the BB2 receptor was detectable. B, using the cytoplasmic contents harvested from a single CA1 neurone only the housekeeping gene cyclophilin was detectable. C, under the same conditions, replacement of cytoplasmic contents with the same volume of ACSF from the bath solution failed to yield any products. Left-hand column is the DNA ladder, units are base pairs (bp).
Effect of bombesin-like peptides on resting membrane properties
In current-clamp recordings, bath application of GRP or NMB at concentrations previously shown to excite neurones (Pinnock & Woodruff, 1991; Pinnock et al. 1994) was found to depolarize interneurones within the stratum oriens. Thus, 100-200 nM GRP produced a 21.2 ± 3.2 mV depolarization (n = 5 of 8 neurones; Fig. 1A and B) whilst 100-200 nM NMB induced a 21.4 ± 4.2 mV depolarization (n = 16 of 22 cells). In each case these effects were usually associated with pronounced oscillations in membrane potential. These large oscillations in membrane potential were unaffected by the subsequent addition of 1 μM TTX together with the glutamate receptor antagonists NBQX (10 μM) and D-APV (50 μM) and the GABAA receptor antagonist bicuculline (10 μM, n = 3 for each; Fig. 1B). In the remaining neurones these peptides induced either a small depolarization (n = 3 of 9 cells) or had no apparent effect on resting membrane properties (n = 6 of 9) (not shown).
Figure 1. Bombesin-like peptides depolarize rat hippocampal stratum oriens interneurones.
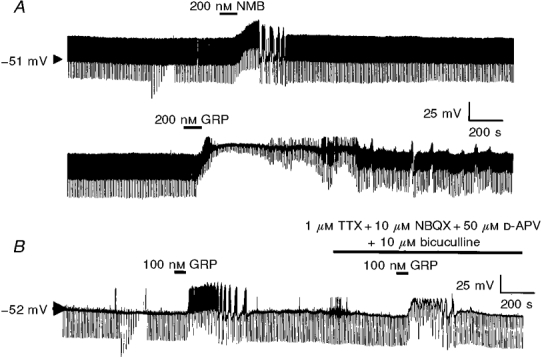
A, continuous whole-cell current-clamp recording trace illustrating the effect of NMB or GRP on resting membrane properties of a stratum oriens interneurone. B, the effect of GRP is retained in the presence of TTX and blockers of ionotropic glutamate and GABA receptors.
To determine the nature of neurones sensitive to these neuropeptides, some cells were filled with Lucifer Yellow via the recording electrode and after fixation subjected to epifluorescence examination of cell morphology (Grace & Llinás, 1985). Five of the seven cells examined had a morphology similar to the horizontal cells described by McBain and coworkers (McBain et al. 1994). Thus each neurone possessed a large cell body (30-50 μm) that was horizontally orientated with respect to the pyramidal cell layer. Each interneurone had several dendrites which extended through the stratum oriens whilst the axons of these cells crossed through the stratum pyramidale and into the stratum lacunosum. The remaining two GRP-sensitive neurones displayed a morphology reminiscent of vertically orientated neurones. These cells possessed dendrites which projected both horizontally and vertically through the stratum oriens. The axons of these cells formed a branched network through the stratum oriens and stratum pyramidale.
Nature of the current induced by bombesin-like peptides
When neurones were voltage clamped at -60 mV, GRP and NMB were found to induce an inward current of similar magnitude and with comparable concentration dependence to each other. Thus 1 nM of either ligand had no effect on the magnitude of the holding current (n = 5), 50 nM NMB or GRP induced an inward current of 65.5 ± 12.5 pA (n = 6) whilst 100 and 200 nM NMB or GRP induced a noisy inward current 125.2 ± 8.5 pA (n = 28) in magnitude. In each case, the inward current displayed a similar time course to the depolarization observed under current-clamp conditions.
To investigate the nature of this inward current, voltage ramps from -140 to -60 mV were applied in the absence and presence of the induced current (see Fig. 2B). Using this approach, it was possible to extrapolate the current plots using linear regression and thus determine the point at which the currents intersect. According to this protocol, the extrapolated reversal potential of the NMB- or GRP-induced current was -5.2 ± 4.1 mV (Fig. 2B; n = 6).
Figure 2.
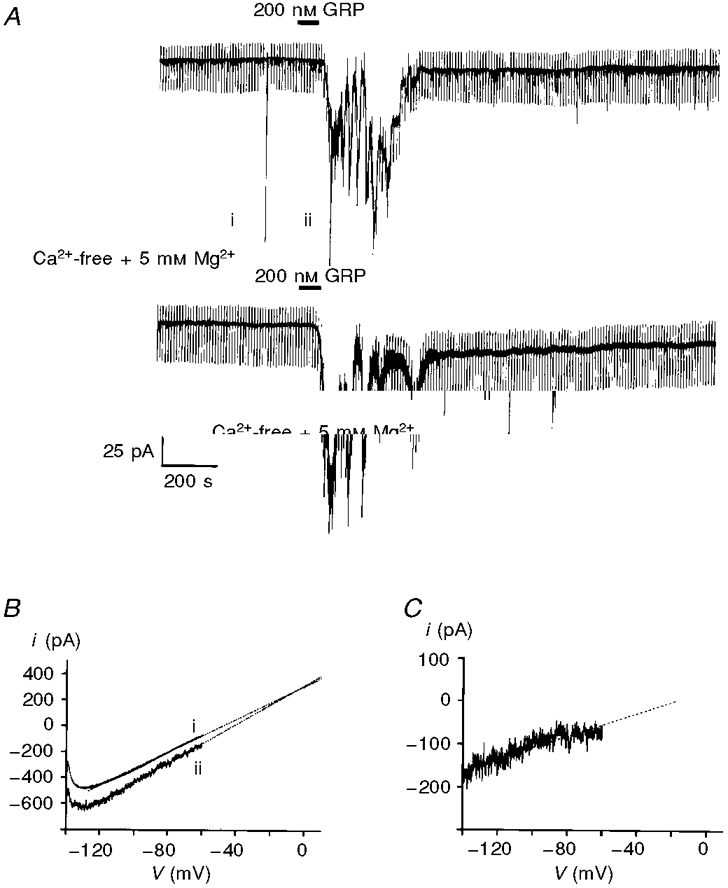
A, GRP activates an inward current in neurones voltage clamped at -60 mV. The magnitude of this inward current was unaffected by removal of extracellular Ca2+ and addition of 5 mM MgCl2. The numerals beside the upper trace correspond to depolarizing ramps (from -140 to -60 mV at 20 mV s−1). B, expanded traces of the current responses to the voltage ramps shown in A. The numbers beside these currents correspond to those shown in A. The dotted lines are the lines of best fit by linear regression. C, subtraction of the control current from the current observed in the presence of GRP yields the GRP-induced current.
To discount the possibility that NMB or GRP might be acting presynaptically via the release of other transmitters, Ca2+-free solutions containing 5 mM Mg2+ were used. Under these conditions, although the Ca2+-free solution reduced the level of baseline noise, the size of the NMB- or GRP-induced current, together with associated noise, was unaffected (control, 124.3 ± 17.5 pA; Ca2+-free, 126.1 ± 14.1 pA; n = 4 at -60 mV; Fig. 2A).
To determine the type of bombesin receptor responsible for these effects, slices were pre-incubated with the BB2 receptor-selective antagonist [D-Phe6,Des-Met14]bombesin-(6-14)ethyl amide (Jensen & Coy, 1991). In each case examined 1 μM [D-Phe6,Des-Met14]bombesin-(6-14)ethyl amide was found to completely block the inward current induced by either 200 nM NMB or 200 nM GRP (n = 3 for each; Fig. 3).
Figure 3.
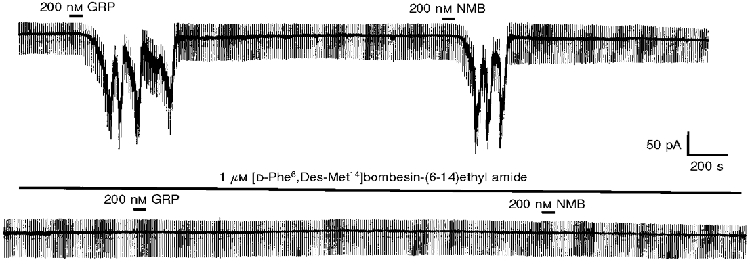
The inward current induced by the bombesin-like peptides GRP and NMB is inhibited by preincubation with the BB2 receptor antagonist [D-Phe6,Des-Met14]bombesin-(6-14)ethyl amide. The recording is a continuous trace made at -60 mV.
Since previous studies have shown that stimulation of group I metabotropic glutamate receptors (mGluRs) elicits a similar response in stratum oriens interneurones to that observed with GRP (McBain et al. 1994; Woodhall et al. 1999), we examined the effects of group I mGluR agonists and antagonists on these GRP-sensitive interneurones. The group I mGluR agonist DHPG (50 μM; Schoepp et al. 1994) was found to elicit an inward current 143.4 ± 11.1 pA in magnitude in eight of the nine neurones on which it was tested. In five of these neurones, 200 nM GRP induced an inward current of 119.5 ± 14.5 pA, whilst the remaining four were insensitive to this peptide. Preincubation of slices with the group I mGluR antagonist MCPG (500 μM; Watkins & Collingridge, 1994) was found to inhibit the response to DHPG (by 82.3 ± 5.4 %, n = 7) without affecting the magnitude of the response to GRP (n = 5, Fig. 4).
Figure 4. The GRP-induced current is unaffected by application of the mGluR antagonist MCPG.
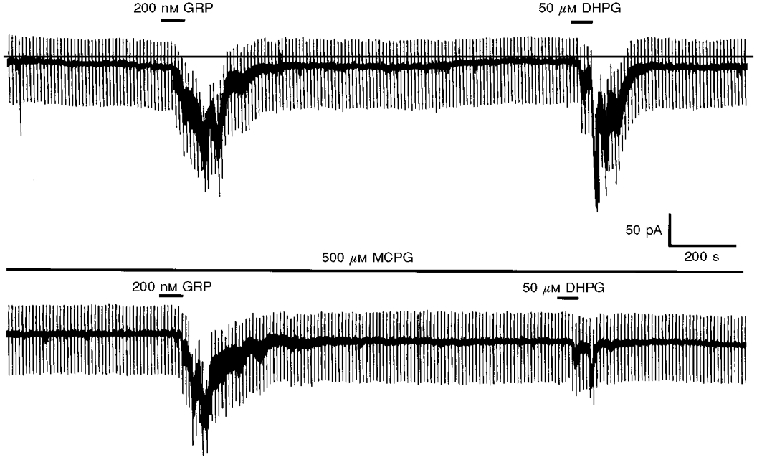
Continuous recording performed at -60 mV. In the upper panel, bath application of GRP or DHPG are shown to induce a marked oscillatory inward current. In the lower panel, preincubation with MCPG is shown to have no effect on the magnitude of the GRP-induced current whilst causing a large reduction in the size of the DHPG-induced current.
The inward current elicited by 200 nM GRP was not affected by K+ channel blockade with either extracellular Ba2+ (control, 144.3 ± 15.5 pA; 2 mM Ba2+, 138.1 ± 15.1 pA; n = 4 at -60 mV) or by the inclusion of caesium acetate in the electrode solution (105.3 ± 11.1 pA at -60 mV; n = 4), which indicates that a K+ conductance is not involved in the generation of the GRP-induced current.
To determine whether Na+ ions are involved in the generation of this current, the external NaCl was replaced with Tris-HCl. Under these conditions, the amplitude of the GRP-induced current was reduced to 46.2 ± 6.3 % of its control value (n = 4, Fig. 5A). In contrast replacement of external NaCl with LiCl failed to affect the magnitude of the GRP-induced current at -60 mV (control, 98.3 ± 12.5 pA; LiCl, 97.4 ± 14.6 pA; n = 4; see Fig. 5B).
Figure 5. The GRP-induced inward current is dependent on monovalent cations in the extracellular solution.
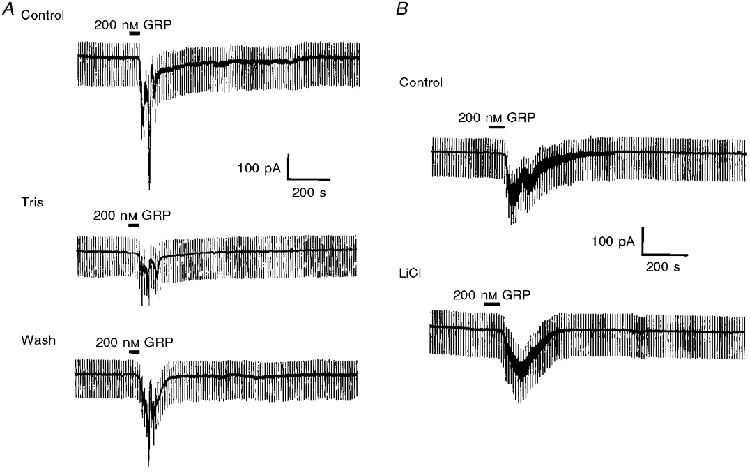
A, top trace shows the control response to GRP at -60 mV. Middle trace shows the diminished response to the same concentration of GRP 10 min after replacing all the extracellular NaCl with Tris-HCl. Bottom trace, 30 min after returning to normal physiological saline, the response to GRP had partially returned. B, replacement of extracellular NaCl with LiCl had no effect on the magnitude of the GRP-induced current at -60 mV.
Sequence of intracellular events
Molecular cloning studies have shown that bombesin receptors belong to the G-protein-coupled receptor superfamily (Battey & Wada, 1991). To address the possible involvement of G-proteins in the generation of this inward current by GRP, 500 μM caged GTPγS was incorporated into the electrode solution. Once the control response to 200 nM GRP was established, the release of the free GTPγS was initiated by exposure to UV light. Using this system, the inward current induced by 200 nM GRP at -60 mV was increased (to 154.2 ± 7.2 %, n = 4, P < 0.05) and was not readily reversed following washout of GRP (Fig. 6). In contrast, in the absence of caged compounds, exposure to UV light for a similar length of time had no effect on the current induced by GRP at -60 mV (n = 4, not shown).
Figure 6. Continuous whole-cell voltage-clamp recording trace showing that caged GTPγS irreversibly potentiates the GRP-induced inward current at -60 mV when photolysed by UV irradiation.
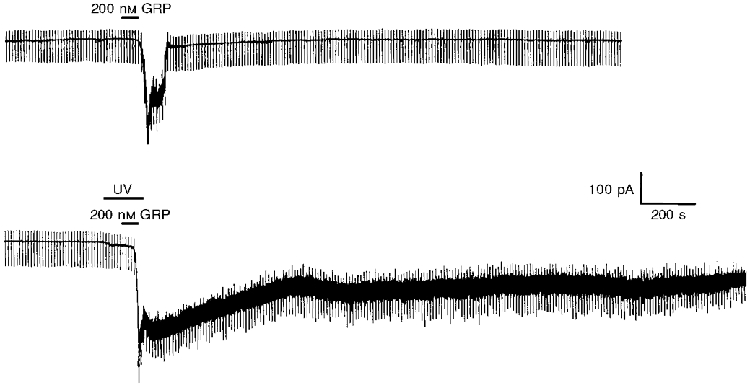
In this continuous recording 500 μM GTPγS was included in the electrode solution at the start of the experiment. From this caged precursor, free GTPγS was liberated by the application of UV light onto the neurone for the period indicated.
The involvement of PLC in the present study was demonstrated using the PLC inhibitor U-73122 (Bleasdale et al. 1990). Application of 10 μM U-73122 was found to significantly reduce the GRP-induced current by 78.3 ± 8.3 % at -60 mV (n = 5, P < 0.05, Fig. 7). These effects of U-73122 were poorly reversible on washout (not shown). In contrast, 10 μM U-73343, an inactive analogue of U-73122, did not affect the magnitude of this current (n = 6, Fig. 7).
Figure 7. PLC is important in the generation of the GRP-induced inward current at -60 mV.
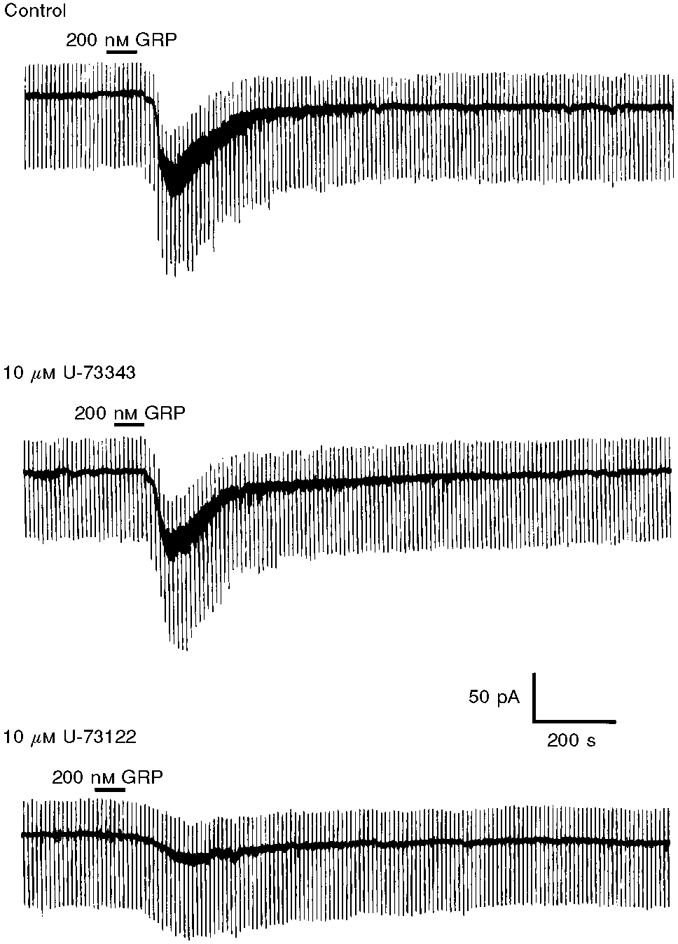
The inward current induced by GRP was diminished by bath application of the PLC inhibitor U-73122. In contrast, this effect is not mimicked by the inactive analogue U-73343.
To establish that an increase in intracellular calcium was a prerequisite in the generation of the GRP-induced current, 10 mM diazo-2, a caged Ca2+ chelator was incorporated into the electrode solution. Once the control reponse to GRP was established, UV light was directed onto the neurone to produce photolysis of the calcium chelator. Using this protocol, it was possible to reduce the magnitude of the GRP-induced current by 66.5 ± 8.7 % at -60 mV (n = 4, P < 0.05, Fig. 8).
Figure 8. UV photolysis of diazo-2 inside a hippocampal interneurone reduces the magnitude of the GRP-induced current.
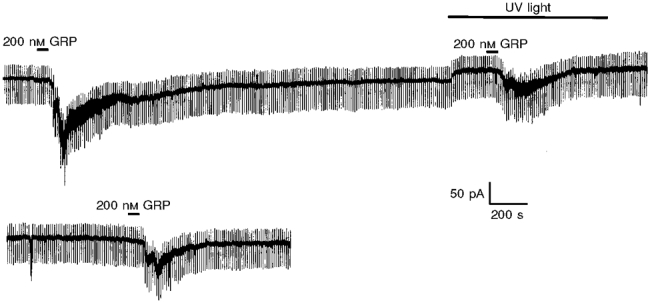
In this continuous recording 10 mM diazo-2 was included in the electrode solution at the start of the experiment. From this caged precursor, free diazo-2 was liberated by the application of UV light onto the neurone for the period indicated. The release of free diazo-2 was associated with a reduction in the magnitude of the GRP-induced current which was partially reversible on removal of the UV light source.
Molecular identification of the bombesin receptor involved
To confirm the identity of these GRP-sensitive cells the cytoplasm of five neurones sensitive to GRP or NMB was harvested and subjected to single cell RT-PCR analysis using primer oligonucleotides specific for the cytoplasmic marker gene, cyclophilin and the enzyme GAD67. In each case, mRNA encoding the enzyme GAD67 was detected to indicate the GABAergic nature of these neurones (n = 5). In addition to detecting GAD67, mRNA for the BB2 receptor was also detected (Fig. 9A). In contrast, in five CA1 pyramidal neurones, although mRNA for the housekeeping gene was detected, no expression of GAD67 and BB2 receptors was found (Fig. 9B).
Effect of bombesin-like peptides on membrane properties of CA1 pyramidal neurones
NMB or GRP (100 nM to 300 nM) were tested on 14 visually identified CA1 pyramidal neurones obtained from eight separate animals. These neurones were easily distinguished from hippocampal interneurones both morphologically and electrophysiologically. For example, these neurones exhibited a more negative resting membrane potential (-64.3 ± 1.2 mV, n = 11) and had a lower input resistance (150.1 ± 5.2 MΩ, n = 11). In two of the nine neurones on which it was tested, 300 nM NMB was found to produce a small depolarization (2.1 and 2.3 mV) whilst in the remaining seven neurones, NMB was without apparent effect (not shown). Similarly 300 nM GRP had no effect on the resting membrane potential of the CA1 neurones on which they were tested (n = 5). In contrast, carbachol, which has previously been shown to excite CA1 pyramidal neurones (Bernado & Prince, 1982) produced a 22.1 ± 3.2 mV depolarization following bath application at a concentration of 10 μM (n = 4; not shown).
Since previous studies have demonstrated that axons of stratum oriens interneurones arborize with CA1 pyramidal neurones (Lacaille et al. 1987; McBain et al. 1994), we hypothesized that bombesin-related neuropeptides should increase the number of spontaneous IPSCs recorded from CA1 pyramidal neurones. In the presence of 50 μM D-APV and 10 μM NBQX to block glutamatergic transmission, spontaneous IPSCs were observed under control conditions at a rate of 3.7 ± 1.2 Hz with a mean amplitude of 64.3 ± 15.6 pA (n = 6). In three neurones, IPSCs were abolished by bath application of 10 μM bicuculline to indicate that they were elicited via the GABAA receptor. Under these conditions bath application of 100-200 nM NMB or GRP produced a significant increase in both the frequency of these IPSCs (to 24.3 ± 4.3 Hz, n = 6, P < 0.05) and their amplitude (to 135.5 ± 38.5 pA, n = 6; Fig. 10).
Figure 10.
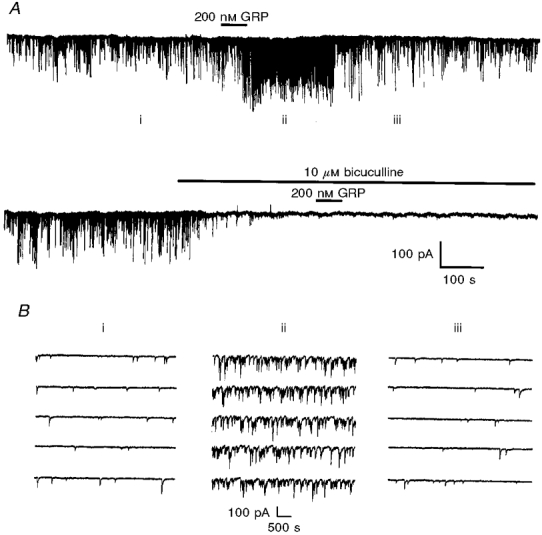
A, bath application of GRP increases the frequency and amplitude of spontaneous IPSCs in a CA1 pyramidal neurone held at -60 mV. In the lower part of the recording, these effects of GRP are shown to be bicuculline sensitive. B, expanded traces of the currents in A. The numbers above these currents correspond to those shown in A.
These effects were abolished by the addition of 10 μM bicuculline (Fig. 10A) which indicates that these effects were again mediated by the GABAA receptor. Furthermore pre-incubation with 1 μM TTX was also found to prevent these effects indicating that these actions were not due to a direct action at the nerve terminal or due to some change in responsiveness to the released GABA (n = 3).
DISCUSSION
Bombesin-like peptides depolarize hippocampal interneurones
In the present study, we demonstrate that the bombesin-like peptides NMB and GRP depolarize interneurones in the stratum oriens of the hippocampus by the induction of an inward current at -60 mV. This inward current has an estimated reversal potential of -5.2 ± 4.1 mV and is often associated with large oscillations in the current amplitude. These findings support and extend the study of Dreifuss & Raggenbass (1986) who used extracellular recording techniques to show that bombesin and related ligands excite non-pyramidal neurones in hippocampus.
The reversal potential of this current suggests the possible involvement of a non-selective cation current in the generation of this response. This hypothesis is supported by the Na+ dependence of the current. Replacement of extracellular Na+ ions with Tris-HCl reduced the size of the GRP-induced current, which indicates that Na+ ions are involved in this effect. However, it is unlikely that TTX-sensitive Na+ channels participate in the generation of this current since TTX was present in the external solution. Another possibility is that this inward current is mediated through activation of a non-selective cation channel although any contribution from ionotropic glutamate receptors can be ruled out since neither NBQX nor D-APV affects the magnitude of the GRP-induced inward current.
The inward current was also shown to be independent of Ca2+ influx since it was unchanged in Ca2+-free physiological saline. In contrast, the cytoplasmic concentration of Ca2+ was shown to be important in the generation of this inward current using the caged Ca2+chelator diazo-2 (Adams et al. 1989). In the present study when diazo-2 was photolysed inside the neurone, the size of the GRP-induced response was significantly reduced. These findings indicate that a significant component of this response is dependent on a rise in the concentration of cytoplasmic Ca2+.
The profile of this GRP-activated inward current exhibits characteristics similar to that of the current induced by (1S,3R)-aminocyclopentane dicarboxylic acid (ACPD) in the same neurones (McBain et al. 1994). In this former study, the authors suggest that the ACPD-induced current could arise through a Na+-Ca2+ exchange mechanism. To investigate whether such a mechanism could be involved in the present study we replaced the extracellular NaCl with LiCl. Li+ is a small monovalent cation which would be expected to pass through any putative non-selective cation channel (Partridge & Swandulla, 1988) but which is not utilized by the Na+-Ca2+ exchanger (Ledvora & Hegyvary, 1983). Consequently, the lack of effect Li+ substitution on the magnitude of the GRP-induced current suggests that the present effect is likely to be due to the activation of a calcium-activated non-selective cation channel rather than any Na+-Ca2+ exchange mechanism.
In a recent study, the oscillatory nature of the mGluR-induced current was shown to coincide with rises in intracellular Ca2+ through a coupling of mGluR receptors to voltage-gated Ca2+ channels and Ca2+ release from intracellular stores (Woodhall et al. 1999). Given the similarity of the mGluR response to that seen with BB2 receptor stimulation, it is possible to envisage that the two receptors may converge upon a common signalling pathway akin to that described for mGluR5 receptors (Kawabata et al. 1996). However, although it appears that these transmitters evoke a similar response in certain interneurones, differences seem to exist in the populations of neurones sensitive to these agents since only five of the eight DHPG-sensitive neurones examined were sensitive to GRP.
Pharmacology of the current induced by bombesin-like peptides
Previous studies have demonstrated the presence of mRNA for both BB1 and BB2 receptors in the mammalian hippocampus (Wada et al. 1991). In the present study, the effects of both NMB and GRP were inhibited by pre-incubation with the BB2-selective antagonist [D-Phe6,Des-Met14]bombesin-(6-14)ethyl amide to suggest that the observed response was mediated solely via the BB2 receptor (Jensen & Coy, 1991). This finding agrees with the results obtained from single cell RT-PCR analysis demonstrating the expression of BB2 receptor mRNA in these neurones and previous autoradiographical studies where a predominance of GRP binding was observed in this region of the hippocampus (Ladenheim et al. 1990). The ability of NMB to interact with the BB2 receptor with a similar efficacy to GRP is reminiscent of previous studies performed in both the suprachiasmatic nucleus and periventricular nucleus (Pinnock et al. 1994).
Sequence of intracellular events leading to activation of the GRP-induced current
The involvement of a G-protein in the present sequence of events was demonstrated using caged GTPγS. When exposed to light of the appropriate wavelength, this compound undergoes a photochemical reaction with the liberation of free GTPγS (Lee & Boden, 1997). Using this technique, it is possible to demonstrate that when free GTPγS was generated inside the cell from its caged precursor the magnitude of the GRP-induced current was greater and more prolonged than the control current.
In previous studies, bombesin-like peptides have been reported to be coupled to phosphoinositide turnover resulting from the activation of PLC (Hari-Prasad & Moody, 1988; Hollingsworth, 1989). PLC was shown to play a role in the generation of the GRP-induced current using the PLC inhibitor U-73122. This compound has been used in a number of preparations to inhibit PLC activity and thus inositol 1,4,5-trisphosphate (IP3) formation (Bleasdale et al. 1990). In the present study, U-73122 inhibited the GRP response indicating the involvement of PLC in the generation of this current. Furthermore the related compound U-73343 was unable to mimic this inhibitory effect discounting the possibility that U-73122 mediates this inhibition through some non-specific mechanism.
Physiological significance
In contrast to their dramatic effects upon stratum oriens interneurones, NMB and GRP were found to exert relatively little or no effect on the resting membrane properties of CA1 pyramidal neurones under these conditions (i.e. potassium gluconate-filled electrodes with resting potential close to the chloride equilibrium potential). However, under conditions optimized for the detection of IPSCs, these neuropeptides were shown to elicit a marked increase in the numbers of GABAA-mediated IPSCs received by these neurones in a TTX-sensitive manner. These findings imply that the GABAergic interneurones depolarized by bombesin-like peptides in the stratum oriens make synaptic connections with CA1 pyramidal neurones. This scenario is similar to that recently reported by others both for the metabotropic glutamate agonist ACPD (McBain et al. 1994) and also for adrenergic agonists (Bergles et al. 1996). However, whilst we demonstrate that bombesin-like peptides have virtually no effect on the resting membrane properties of CA1 neurones themselves, the excitatory actions of both ACPD (e.g. Stratton et al. 1989) and noradrenaline (Nicoll et al. 1990) upon these neurones are well established.
The role performed by local interneurones in the modulation of neuronal excitability in the hippocampus as well as other regions of the CNS is now well documented. For example, agents which hyperpolarize interneurones can increase the excitability of their target neurones through a reduction in the frequency and amplitude of IPSCs (Zieglgansberger et al. 1979; Johnson & North, 1992). Thus it is tempting to suggest that agents which excite interneurones such as NMB or GRP may exert potent inhibitory effects within the hippocampus in vivo. In support of this, it is noteworthy that noradrenaline has been shown to reduce the firing rate of CA1 neurones in vivo despite its ability to directly excite CA1 neurones in vitro (e.g. Segal & Bloom, 1974).
At present, little is known regarding the exact role performed by bombesin-like peptides within the hippocampal formation. Previous studies have revealed that substantial amounts of immunoreactivity for bombesin-like peptides reside in the hippocampus where it appears to be localized in synaptosomes (Moody et al. 1986). Further studies using in situ hybridization have revealed many layers of the hippocampus exhibit considerable GRP mRNA (Zoeller et al. 1989; Wada et al. 1990). However, little is known as to the site of action of these peptides although it is possible to speculate that the release of GRP may act upon these interneurones to provide a powerful form of feedback inhibition under normal conditions and may ultimately be neuroprotective under excitotoxic conditions (Freund & Buzsáki, 1996).
In view of these findings, in future studies it will be important to characterize the nature of these GRP-sensitive neurones more fully using morphological and neurochemical techniques in addition to examining the effects of such peptides in other groups of hippocampal neurones.
References
- Adams SR, Kao JPY, Tsien RY. Biologically useful chelators that take up Ca2+ upon illumination. Journal of the American Chemical Society. 1989;111:7957–7968. [Google Scholar]
- Anasti A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, two analogues of active peptides from the skin of the European amphibians bombina and alytes. Experentia. 1971;27:166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends in Neurosciences. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. Journal of Neuroscience. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernado LS, Prince DA. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Research. 1982;249:315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. Journal of Pharmacology and Experimental Therapeutics. 1990;256:756–768. [PubMed] [Google Scholar]
- Dixon AK, Richardson PJ, Lee K, Carter NC, Freeman TC. Expression profiling of single cells using 3 prime end amplification (TPEA) PCR. Nucleic Acids Research. 1998;26:4426–4431. doi: 10.1093/nar/26.19.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss JJ, Raggenbass M. Tachykinins and bombesin excite non-pyramidal neurones in the rat hippocampus. The Journal of Physiology. 1986;379:417–428. doi: 10.1113/jphysiol.1986.sp016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Grace AA, Llinás R. Morphological artifacts induced in intracellularly stained neurons by dehydration; circumvention using rapid dimethyl sulfoxide clearing. Neuroscience. 1985;16:461–475. doi: 10.1016/0306-4522(85)90018-1. [DOI] [PubMed] [Google Scholar]
- Hari-Prasad VN, Moody TW. Bombesin-like peptides stimulate phosphatidylinositol turnover in rat brain slices. Peptides. 1988;9:1345–1349. doi: 10.1016/0196-9781(88)90201-x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth EB. Gastrin-releasing peptide receptor in rat brain membranes: specific binding and stimulation of phosphoinositide breakdown. Molecular Pharmacology. 1989;35:689–694. [PubMed] [Google Scholar]
- Jensen RT, Coy DH. Progress in the development of potent bombesin receptor antagonists. Trends in Neurosciences. 1991;12:13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. Journal of Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kahara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- Lacaille J-C, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens alveus interneurons and CA1 pyramidal cells in hippocampal slices: Electrophysiology and morphology. Journal of Neuroscience. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, McHugh PR, Moran TH. Receptor heterogeneity for bombesin-like peptides in the rat central nervous system. Brain Research. 1990;537:233–240. doi: 10.1016/0006-8993(90)90363-g. [DOI] [PubMed] [Google Scholar]
- Ledvora RF, Hegyvary C. Dependence of Na+-Ca2+ exchange and Ca2+-Ca2+ exchange on monovalent cations. Biochimica et Biophysica Acta. 1983;729:123–136. doi: 10.1016/0005-2736(83)90463-7. [DOI] [PubMed] [Google Scholar]
- Lee K, Boden PR. Characterisation of the inward current induced by metabotropic glutamate receptor stimulation in rat ventromedial hypothalamic neurones. The Journal of Physiology. 1997;504:649–663. doi: 10.1111/j.1469-7793.1997.649bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Dixon AK, Freeman TC, Richardson PJ. Characterization of the KATP channel current present in striatal cholinergic interneurones. The Journal of Physiology. 1998;510:441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Dichiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. Journal of Neuroscience. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (IH) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. The Journal of Physiology. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Korman LY, O'Donohue TL. Neuromedin B-like peptides in rat brain: Biochemical characterisation, mechanism of release and localization in synaptosomes. Peptides. 1986;7:815–820. doi: 10.1016/0196-9781(86)90100-2. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiological Reviews. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Swandulla D. Calcium-activated non-specific cation channels. Trends in Pharmacological Sciences. 1988;11:69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Rusak B. Electrophysiological effects of pressure-ejected bombesin-like peptides on the hamster suprachiasmatic nucleus neurons in vitro. Journal of Neuroendocrinology. 1993;5:575–581. doi: 10.1111/j.1365-2826.1993.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Pinnock RD, Reynolds T, Woodruff GN. Different types of bombesin receptors on neurons in the dorsal raphe nucleus and rostral hypothalamus in rat brain slices in vitro. Brain Research. 1994;653:119–124. doi: 10.1016/0006-8993(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Pinnock RD, Woodruff GN. Bombesin excites a subpopulation of 5-hydroxytryptamine-sensitive neurones in the rat dorsal raphe nucleus in vitro. The Journal of Physiology. 1991;440:55–65. doi: 10.1113/jphysiol.1991.sp018695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-Dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. Journal of Neurochemistry. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Research. 1974;72:79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and functional expression of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Molecular Endocrinology. 1990;4:1956–1963. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- Stratton KD, Worley PF, Baraban JM. Excitation of hippocampal neurons by stimulation of glutamate Qp receptors. European Journal of Pharmacology. 1989;173:235–237. doi: 10.1016/0014-2999(89)90529-3. [DOI] [PubMed] [Google Scholar]
- Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. Journal of Neuroscience. 1990;10:2917–2930. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battey JF. cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron. 1991;6:421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Collingridge GL. Phenylglycine derivatives as antagonists of metabotropic glutamate receptors. Trends in Pharmacological Sciences. 1994;15:333–342. doi: 10.1016/0165-6147(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille J-C. Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. Journal of Neurophysiology. 1999;81:371–382. doi: 10.1152/jn.1999.81.1.371. [DOI] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. The Journal of Physiology. 1995;488:661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Lebacq-Verheyden AM, Battey J. Distribution of two distinct messenger ribonucleic acids encoding gastrin-releasing peptide in rat brain. Peptides. 1989;10:415–422. doi: 10.1016/0196-9781(89)90052-1. [DOI] [PubMed] [Google Scholar]


