Abstract
In Helix neurones high doses of Phe-Met-Arg-Phe-NH2 (FMRFamide) often evoke biphasic inward whole-cell currents with brief application, and suppression of the current with prolonged application. With outside-out patches, a transient early suppression of the unitary current amplitude was seen following application of high doses of FMRFamide.
Continuous application of a concentration of FMRFamide from 30 μm to 1 mm resulted in a reduction in the amplitude of the unitary currents and an increase in open state noise. There was also an increase in the occurrence of smaller, ‘subconductance’ currents with the higher concentrations of FMRFamide. Similar effects were seen with FMRFamide on FaNaC expressed in oocytes. The FMRFamide analogues FLRFamide and WnLRFamide were more effective in evoking the lower conductance state. These effects of agonist at high concentrations were voltage dependent suggesting channel block.
A similar effect was seen when one of the related peptides FKRFamide, FM(D)RFamide, nLRFamide or N-AcFnLRFamide was co-applied with a low FMRFamide concentration. However, the non-amidated peptides FKRF, FLRF and nLRF and also WMDFamide did not have this effect.
The inhibition of unitary currents induced by amiloride was qualitatively different from that produced by FMRFamide analogues with no obvious occurrence of subconductance levels. FMRFamide-gated channels were also blocked by guanidinium, but only at very high concentrations.
The results strongly suggest a partial inhibition of current flow through the FMRFamide- gated channel by some part of the agonist or the related antagonist peptide molecules.
The sodium channel gated by Phe-Met-Arg-Phe-NH2 (FMRFamide) (FaNaC) was discovered in Helix neurones and is a rare example of an ion channel which is directly activated by a peptide (Cottrell et al. 1990; Green et al. 1994). Early recordings indicated that two types of unitary currents of differing amplitude are activated by FMRFamide in the C2 neurone. These currents appeared to differ in their susceptibility to desensitization, to block by amiloride and also in their time course of activation. At that time it was unclear whether the two types of current represented distinct populations of ion channels, or a single type the properties of which became modified under certain conditions.
The gene FaNaC has since been cloned from Helix brain by homology screening with primers based on a family of ion channels including the epithelial sodium channel (ENaC) and the degenerins of Caenorhabditis elegans, channels which were grouped based on their structure and their high sensitivity to block by amiloride (Lingueglia et al. 1995). When mRNA transcribed from the clone (FaNaC) was expressed in Xenopus oocytes, FMRFamide activated Na+-dependent, amiloride-sensitive, currents similar to those of the C2 neurone.
Here we have analysed in more detail the properties of FMRFamide-gated unitary currents of identified Helix aspersa neurones in relation to the unusual time course of the whole cell response and to the possible presence of more than one type of FMRFamide-gated channel. An unusual concentration-dependent agonist block is described, which partly accounts for behaviour of the whole cell and unitary currents that were tentatively ascribed to the presence of two channel types (see Green et al. 1994). Comparison with recordings of FaNaC expressed in Xenopus oocytes showed identical behaviour at high agonist concentrations, providing further insight into the mechanism of activation. The similarity in the effects observed in the neurone and oocyte patches underlines the similarity of the native and cloned channels.
METHODS
Experiments were carried out on either the cerebral C2 neurones, or the F2 neurone of the right parietal ganglion, of Helix aspersa (collected locally). Neurones were dissected free from connective tissue and, in most cases for both patch clamp and whole cell recording experiments, exposed to 0.1 % trypsin to facilitate gigaohm seal formation. Some stable patches were also obtained, but with a low success rate, without trypsinization. Currents from perikarya were recorded using microelectrodes filled with 1 M potassium acetate and the discontinuous single electrode voltage clamp method using an Axoclamp-2B amplifier (Axon Instruments). No significant differences were noted between whole cell and unitary currents recorded from the C2 or the F2 neurones. Unitary currents were recorded using an Axopatch 200 integrating amplifier (Axon Instruments). Analog data recordings obtained with physiological concentrations of Na+ were filtered at 500 Hz (Neurolog NL-125 active filter, −40 db decade−1; Digitimer, Welwyn Garden City, UK) and digitized at 400 μs intervals. Data analysis was performed using custom software after further digital Gaussian filtering at 500 Hz (−3 db) giving an effective net bandwidth of approximately 350 Hz (see Colquhoun & Sigworth, 1983). To enhance the time resolution of some recordings, an external solution containing high (260 mM) Na+ and 1 mM Ca2+ was used with isolated patches and the recordings were filtered at 2 kHz (Neurolog as above) with sampling at 100 μs intervals.
The standard physiological solution used for intracellular neuronal recordings had the following composition (mM): NaCl, 100; KCl, 5; MgCl2, 5; CaCl2, 7; Hepes, 10; pH adjusted to 7.4 with NaOH. The external solution used when recording from outside-out patches contained (mM): NaCl, 116 (96 mM for oocyte patches); KCl, 2; MgCl2, 1; CaCl2, 1.8; Hepes, 5; pH adjusted to 7.4 with NaOH. The same external solution was used for neuronal and oocyte patches, but with the Na+ concentration increased by 20 mM for the oocyte-expressed FaNaC. Under these conditions, the conductance of neuronal FMRFamide-activated sodium channels varied from 5 to 9 pS and was similar to that seen for FaNaC expressed in oocytes. All recordings shown are from neuronal patches except where otherwise stated. The standard intracellular type solution for patch recording experiments contained (mM): NaCl, 3; KF or NaF, 100; MgCl2, 1; EGTA, 5; Hepes, 10; pH adjusted to 7.4 with KOH or NaOH. Use of fluoride as the predominant anion in the recording pipette greatly increased the stability of patches. Stock solutions of all peptides (5 mM) and guanidinium HCl (100 mM) were prepared in distilled deionized water and frozen.
The peptides used were as follows: Phe-Met-Arg-Phe-NH2 (FMRFamide), Phe-Leu-Arg-Phe-NH2 (FLRFamide), nLeu-Arg-Phe-NH2 (nLRFamide), Trp-nLeu-Arg-Phe-NH2 (WnLRFamide), N-acetyl-Phe-nLeu-Arg-Phe-NH2 (N-AcFnLRFamide), Trp-Met-Asp-Phe-NH2 (WMDFamide) and Phe-Leu-D-Arg-Phe-NH2 (FM(D)RFamide), all from Sigma; Phe-Lys-Arg-Phe-NH2 (FKRFamide), Phe-Lys-Arg-Phe (FKRF), Phe-Leu-Arg-Phe (FLRF) and Phe-nLeu-Arg (FnLR) synthesized locally within the biochemistry department; and Phe-nLeu-Arg-Phe-NH2 (FnLRFamide) obtained from Cambridge Research Biochemicals Ltd (Northwich, UK).
FMRFamide agonists were transiently applied by pressure ejection (150–200 kPa). More prolonged applications were also made using a Warner SF-77A Perfusion Fast-Step perfusion system (Warner Instrument Corporation, Hamden, CT, USA), or by local leakage from a separate blunt tipped micropipette. The latter technique was most suitable for small volumes of the highest concentrations of the peptides used. The recording chamber was perfused throughout each experiment with physiological solution. Antagonists were applied simultaneously by inclusion in the same application solution as the agonist.
Fragments of ovary were dissected from mature adult female Xenopus laevis, anaesthetized by immersion in 0.2 % MS222. After collagenase treatment (Sigma type 1A) for 2 h in calcium-free saline, defolliculated stage VI oocytes were injected with 0.5–5 ng of cRNA prepared from the clone p-BSK-SP6-Globin-FaNaCh (a gift from Drs M. Lazdunski and E. Lingueglia, Institut de Pharmacologie Moléculaire et Cellulaire Valbonne, France) using a T7 mMessage mMachine kit (Ambion, Austin, TX, USA; see Zhainazarov & Cottrell, 1998). Recordings were made from oocytes 1–8 days after injection.
RESULTS
Time course of the response to FMRFamide
When FMRFamide (10 μM) was transiently applied by pressure ejection to voltage clamped C2 or F2 neurones, a small inward current was seen that was fast to rise and showed an exponential decay as FMRFamide was washed away. Higher doses of FMRFamide activated inward current responses with a different time course. After the initial fast rising phase of activation, the current continued to rise, but much more slowly, to reach a peak at up to 2 s after the application. Example traces from an F2 neurone are shown in Fig. 1A.
Figure 1. The inhibitory effect of high FMRFamide concentration on whole cell and isolated patch responses to FMRFamide.
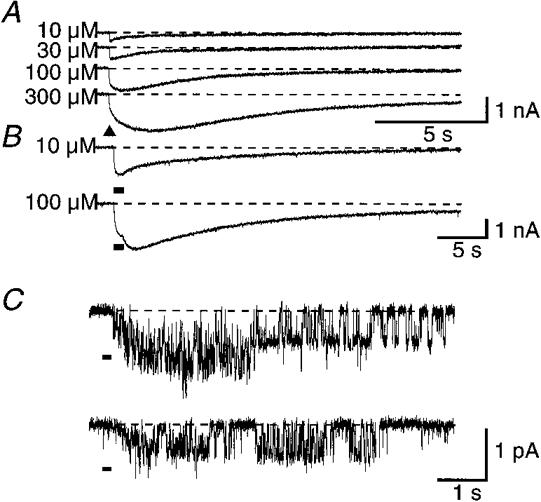
A, whole cell current responses to short 10 ms pressure ejection pulses of 10, 30, 100, or 300 μM FMRFamide applied to the same F2 neurone. The biphasic activation phase was not as pronounced in all recordings. B, whole cell responses to long (1 s) applications of 10 μM, or 100 μM FMRFamide in the same F2 neurone. C, unitary current responses to 100 ms pressure applications of 1 mM FMRFamide to an isolated outside-out patch from the C2 neurone. The holding potential was −60 mV for A and B, and −100 mV for C. ▴ or black bars under the traces denote applications of FMRFamide. Dashed lines indicate the base current level in each case.
With more prolonged application (1 s) of 10 μM FMRFamide to the neurone, the response had a simple time course, rising throughout the application and progressively decaying thereafter. Prolonged application of 100 μM FMRFamide, however, gave a response which reached a peak during the application but thereafter started to rise again as the FMRFamide concentration fell with washing (Fig. 1B). Responses to transient doses of high concentrations of FMRFamide (≥ 100 μM) to outside-out patches also showed a similar attenuation of the initial part of the response (Fig. 1C).
Effects of high agonist concentrations
Steady-state applications of FMRFamide to isolated patches from both the C2 and F2 neurones evoked unitary current activity in many patches. As previously described, the amplitude of the unitary currents activated, and their tendency to desensitize was variable. Such desensitization will not be considered further here. For both the native and cloned channels, the activity gradually ran down, but in many patches at least one channel remained active for significant periods of time even at very high FMRFamide concentrations (> 100 μM). Clustering of events separated by relatively quiet periods was more commonly seen with the native channels; it was most pronounced at the highest (≥ 100 μM) concentration of FMRFamide. Figure 2A shows sections of a recording from one neuronal outside-out patch during application of FMRFamide ranging from 10 μM to 1 mM. As the concentration was increased, the amplitude of the unitary currents decreased and the events became more flickery, with short stays at a subconductance level (30–50 % of the full conductance) being increasingly prevalent. With 1 mM FMRFamide, the channel appeared to spend most of the time at an amplitude equivalent to this subconductance state, with occasional transitions to the fully open state. Similar effects were seen in patches from non-trypsinized cells and from oocytes.
Figure 2. Unitary currents activated by different FMRFamide or WnLRFamide concentrations in outside-out patches.
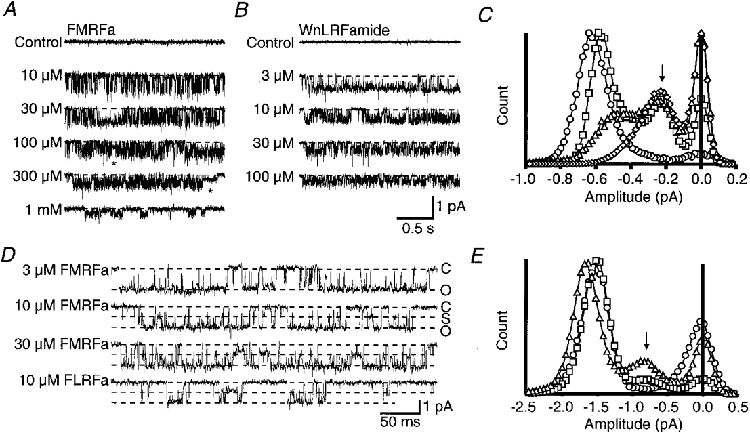
Recordings in A and D are from neuronal patches whilst those in B are from oocyte patches. A, example traces of steady-state activity recorded in a patch from the C2 neurone in the presence of a range of FMRFamide concentrations from 10 μM to 1 mM. The asterisk highlights two examples of unusually long sojourns at a subconductance level. B, example traces of steady-state activity of FaNaC in oocyte patches in the presence of a range of WnLRFamide concentrations from 3 to 100 μM. C, all-point amplitude histograms prepared from the recordings in B. The symbols used are 3 μM (^), 10 μM (□), 30 μM (▵), 100 μM (⋄) WnLRFamide. D, unitary currents activated by 3, 10, or 30 μM FMRFamide, or 10 μM FLRFamide in the presence of external solution containing 260 mM Na+ and 1 mM Ca2+. These records were filtered at 2 kHz. E, amplitude histograms for unitary current recordings under the same conditions as D in the presence of 3 μM (^), 10 μM (□), or 30 μM (▵) FMRFamide. The peaks due to the subconductance level are marked with an arrow in C and E. At least 20 s of recording was used in each case to construct the amplitude histograms. All traces were obtained at a holding potential of −100 mV. The dashed lines superimposed on the traces show the fully closed level (C) in A, B and D, and in D they also mark the fully prominent subconductance level (S) and the fully open level (O).
Unitary currents recorded from the cloned FaNaC in response to a range of concentrations of the FMRFamide analogue WnLRFamide are shown in Fig. 2B. This peptide caused fast flickering, and the appearance of the subconductance state at lower concentrations than FMRFamide, both with the neuronal and cloned channels. All-point amplitude histograms for these patches demonstrated an increase in the occurrence of the subconductance level with concentrations of WnLRFamide greater than 3 μM, and an apparent decrease in the main conductance level (Fig. 2C). At 100 μM WnLRFamide, this subconductance level predominated. Close inspection of the recordings revealed long stays at the subconductance level in both neuronal and oocyte patches. In some cases such sojourns lasted for hundreds of milliseconds (see examples marked by the asterisk in Fig. 2A); they were increasingly frequent at higher agonist concentrations. The single subconductance peak seen in the amplitude histograms strongly supports a partial inhibition of the unitary currents rather than poorly resolved complete closures of the channels. The possible existence of additional subconductance levels cannot be excluded, however, and a few apparent examples of both smaller and larger events were seen on some records. However, they were never sufficiently numerous to contribute a resolvable peak to the amplitude histograms.
These records strongly suggested that in addition to the longer, clearly resolved subconductance events, the fast flickering of the fully open state was also only as far as the same subconductance level and did not involve complete channel closure. To enable a better time resolution of the recordings with less likelihood of low-pass filtering attenuating fast events, some recordings used an external solution containing 260 mM Na+, and Ca2+ lowered to 1 mM, to increase the amplitude of the unitary currents. Figure 2D shows data obtained under such conditions in the presence of various concentrations of FMRFamide. Few subconductance events were seen with 3 μM FMRFamide, but with 10 and 30 μM FMRFamide full openings were increasingly interrupted by transitions to the subconductance level, which showed up as a consistent subsidiary peak in the amplitude histogram at a similar position to that seen with more highly filtered recordings (Fig. 2E). Brief full closures associated with ongoing channel kinetics were also seen at each concentration of FMRFamide. Sojourns at the subconductance level were also obvious with 10 μM FLRFamide.
As previously noted, a characteristic of the FaNaC expressed in oocytes is the occasional presence of prolonged openings (lasting several seconds) that were interrupted by few full rapid closings (Lingueglia et al. 1995; Zhainazarov & Cottrell, 1998). Such prolonged openings were also a notable feature of many recordings from native neuronal FMRFamide-activated channels. During these periods of prolonged high open probability (Popen) activity, the flickery transitions to the subconductance level were effectively revealed in isolation from other ongoing features of the channel's kinetic behaviour. Recordings made during such periods of high Popen activity made possible a comparison of the effect of FMRFamide, FnLRFamide, FLRFamide and WnLRFamide (all of which have been shown to activate the channel) on the occurrence of the subconductance level. All-point amplitude histograms showed that the mean amplitude of the current through the open channel was greatest in the presence 10 μM FMRFamide, and least in the presence of 10 μM WnLRFamide (Fig. 3A and B). The difference appears to be due to variation in the duration of individual sojourns at the subconductance level and also possibly to their frequency. Estimates of the probability of a single open channel residing in the subconductance state rather than the main conductance state were 0.205 for WnLRFamide, 0.157 for FLRFamide, 0.089 for FnLRFamide and 0.058 for FMRFamide. These values were obtained with a single channel from which it was possible to record 10 s periods of long openings, using a concentration of 10 μM for each of the four peptides.
Figure 3. Comparison of flickering induced by different FMRFamide-related agonists in neuronal patches.
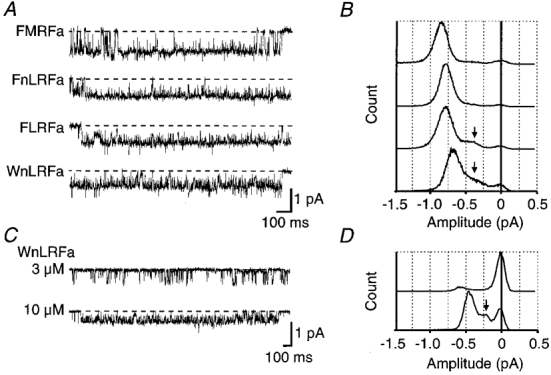
A, traces show flickering during prolonged openings recorded from a single patch in the presence of 10 μM FMRFamide, FnLRFamide, FLRFamide, or WnLRFamide. Dashed lines indicate the fully closed level. Corresponding all-point amplitude histograms are shown opposite in B. C, traces show activation of smaller amplitude unitary currents in a different neuronal patch by either 3 or 10 μM WnLRFamide. The corresponding amplitude histograms are shown in D. In B and D the subconductance level is indicated by an arrow.
Apart from flickery block of FMRFamide-activated unitary currents at high peptide concentrations giving rise to apparently smaller amplitude events, many recordings clearly showed the presence of significantly smaller unitary currents at low (≤ 3 μM) FMRFamide. Often two different amplitudes were active simultaneously within a single patch in the presence of low FMRFamide concentrations. The recordings in this paper including those recordings shown in Fig. 3A are mainly from patches which showed events of the largest amplitude only as far as possible. Figure 3C and D, however, shows that the smaller conductance events were similarly affected by high agonist concentrations, and that the amplitude of the subconductance level seen with these events was a similar fraction (30–40 %) of the main conductance level.
Voltage dependence of the response to FMRFamide
If a physical block of the channel by some part of the FMRFamide molecule is involved in the inhibitory effect at high concentrations, some degree of voltage dependence might be expected. Comparison was made of unitary currents through FaNaC expressed in oocytes at +80 or −80 mV, and under conditions of symmetrical high Na+. With 3 μM FMRFamide as the agonist, the amplitude of the unitary currents was equivalent at positive or negative holding potentials, as seen from the individual traces and the amplitude histograms in Fig. 4A and B. However, when 10 μM WnLRFamide was used as agonist a clear voltage dependence was seen (Fig. 4C and D). At negative holding potentials flickering from the fully open state was seen and significant time was spent at the subconductance level as already described, but at positive holding potentials the phenomenon was less marked or absent and there was no subsidiary peak in the amplitude histogram.
Figure 4. Voltage dependence of the effect of WnLRFamide.
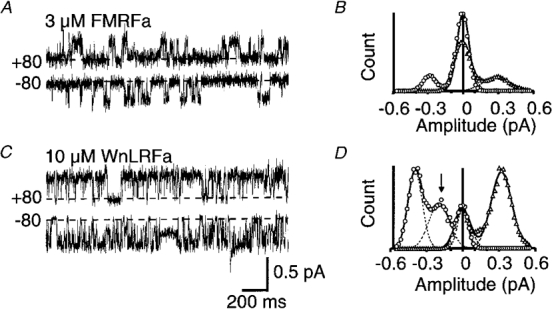
Recordings in this figure were obtained in the presence of symmetrical high sodium from oocytes at holding potentials of +80 mV (▵) or −80 mV (^). The dashed line in each case indicates the fully closed level. A and C, respectively, show traces for 3 μM FMRFamide or 10 μM WnLRFamide applied to two different patches, whilst the corresponding amplitude histograms, constructed from ≥ 5 s of recording in each case, are shown in B and D. An arrow highlights the subconductance seen in the presence of WnLRFamide at negative holding potentials. Individual Gaussian functions and the combined curves fitted to the data are shown by dashed and continuous lines, respectively, in B and D.
Effects of other FMRFamide analogues
Other peptides related to FMRFamide, which have no direct agonist effect on the channels, were tested. FKRFamide had rather weak antagonistic effects on the whole cell neuronal response to FMRFamide (IC50, ∼1 mM). Simultaneous application of 3 μM FMRFamide along with 100 μM FKRFamide produced fast flickering of the open state current level and a reduction in amplitude similar to that seen with high concentrations of FMRFamide. With 260 mM external Na+ (Fig. 5A), it can be seen that the flickering of the open state is predominantly to a level of about 30–40 % of the original control amplitude in the absence of FKRFamide. The occurrence of this level increased with higher doses of FKRFamide.
Figure 5. Inhibitory effects of FKRFamide and FM(D)RFamide (D-Arg).
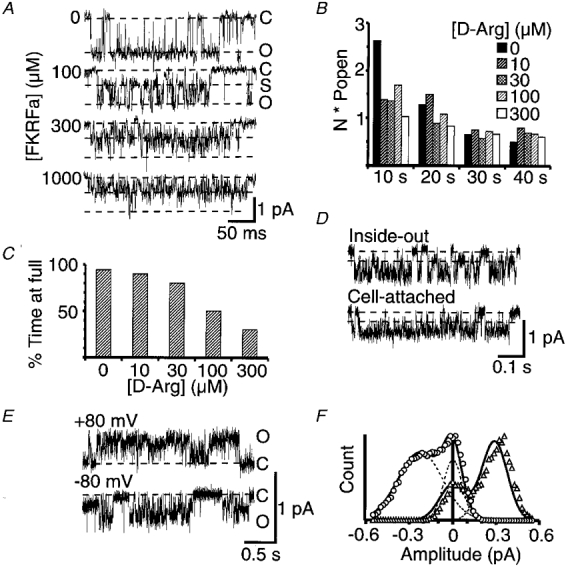
Data in A-D are from neuronal patches, whilst E and F show data from oocyte patches expressing FaNaC. A, unitary currents activated by 3 μM FMRFamide in the presence of 0 μM, 100 μM, 300 μM, or 1 mM FKRFamide. The external solution contained 260 mM Na+ and 1 mM Ca2+ and the recordings were filtered at 2 kHz. Dashed lines indicate the fully closed level (C), the prominent subconductance level (S), and the fully open level (O). The holding potential was −100 mV. B, mean NPopen data from 3 experiments estimated over 10 s time periods after the starting application of each test solution. All solutions contained 3 μM FMRFamide in combination with 0, 10, 30, 100, or 300 μM D-Arg. C, concentration dependence of the blocking effect of D-Arg estimated from the percentage time spent at the full conductance level during long events with only partial closings. D, traces show flickery bursts of openings and the presence of substates also occurring in recordings from inside-out and cell-attached patches from the C2 neurone. The inside-out patch was held at −100 mV membrane potential while the cell-attached patch was held 80 mV hyperpolarized from the cell resting potential. The recording electrode contained 1 μM FMRFamide and 100 μM FKRFamide, in each case dissolved in the standard extracellular solution, and the inside-out patch was perfused with the standard intracellular type solution used in the recording pipette for outside-out patches. Dashed lines indicate the fully closed level, and the prominent subconductance level. Traces in E show unitary currents in an oocyte patch activated by 3 μM FMRFamide in the presence of 300 μM FKRFamide under conditions of symmetrical high sodium, and at holding potentials of +80 or −80 mV (denoted by ▵ or ^, respectively, in F). The corresponding amplitude histograms constructed from ≥ 5 s sections of recording are shown in F, with individual Gaussian functions and the combined curves fitted to the data shown by dashed and continuous lines, respectively.
The persistent activation of the unitary currents by low concentrations of FMRFamide in the presence of high blocker concentrations suggests that blocking peptides bind at a site distinct from the FMRFamide agonist site. This was further tested by monitoring the activity of multiple FMRFamide-activated (3 μM) channels in the presence of increasing concentrations of FM(D)RFamide, a peptide closely related to FMRFamide but with negligible agonist effectiveness. This peptide was as good as FKRFamide in inducing flickery block of FMRFamide-activated channels (Fig. 5B). Apart from the initial higher level of activity with the first application of FMRFamide on its own due to some of the FMRFamide-activated channels rapidly desensitizing, the remaining activity was comparable with all concentrations of FM(D)RFamide tested. Over the same concentration range there was obvious flickery block induced by this peptide (Figs 5C and 6A and C). These data clearly support a non-competitive block by the peptides.
The blocking effect of FKRFamide was not restricted to the outside-out configuration; it could be detected in other modes (Fig. 5D). Inside-out patches with 1 μM FMRFamide and 100 μM FKRFamide in the recording pipette also showed very clearly flickery bursts and the presence of a subconductance level indicative of peptide block of the FMRFamide channel. Cell-attached patches also demonstrated the phenomenon, but less clearly due to the effective filtering by the rest of the cell.
Unitary currents activated by 3 μM FMRFamide in the presence of 300 μM FKRFamide were compared at −80 or +80 mV using symmetrical high Na+ (Fig. 5E and F). As with high concentrations of agonist, the flickering of the open state level was significantly greater at negative than at positive holding potentials.
Of the other peptide analogues tested, N-AcFnLRFamide (100 μM) also induced very fast flickering of the open state level. This was evident mainly as an increased open state noise accompanied by a small decrease in amplitude of the unitary currents (Fig. 6A and B). On the other hand, the non-amidated analogues FKRF and FLRF were completely ineffective in antagonizing unitary currents activated by 3 μM FMRFamide. (It has been known for some time that FMRF and FLRF have no agonist activity - see Cottrell, 1997.) The peptides effective in evoking flickering block incorporate an arginine at position 3. The charged guanidinium group of arginine could be responsible for the channel-blocking effect of such peptides. In accord with this view, the cholecystokinin (CCK) tetrapeptide analogue WMDFamide (ineffective as an agonist) did not cause any flickering of the open state unitary currents evoked by FMRFamide channel or reduction in their amplitude, even at 300 μM. On the other hand, fast flickering block was evoked by nLRFamide (100 μM), although not by FnLR (300 μM or less). Ten millimolar L-arginine alone had no detectable effect. These data, along with the variation in the flickering seen with high concentrations of agonists, indicate that the effect is dependent on some structural features of the peptides and suggest involvement of a specific binding site (see Discussion).
Figure 6. Comparison of the effects of some FMRFamide analogues and other substances.
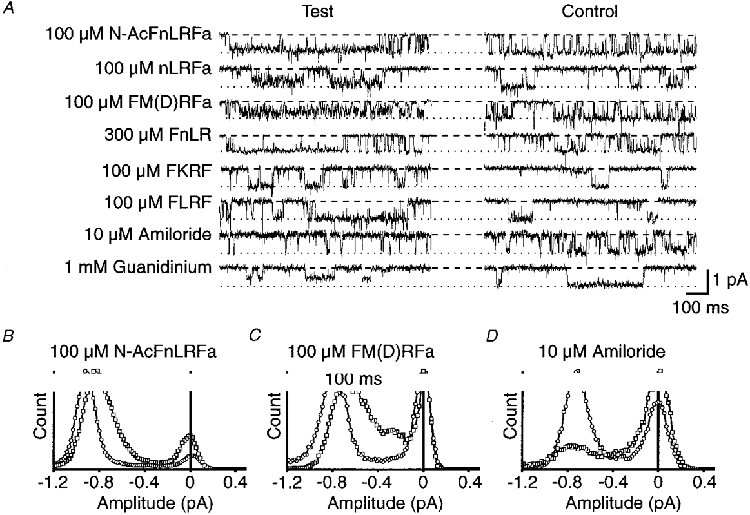
A, in each case control traces are in the presence of 3 μM FMRFamide alone, and test traces are with simultaneous application of N-AcFnLRFamide, nLRFamide, FM(D)RFamide, FKRF, FLRF (all 100 μM), 300 μM FnLR, 10 μM amiloride, or 1 mM guanidinium. The holding potential was −100 mV in each case, and a dashed line and dotted line, respectively, indicate the base and unitary current levels in the presence of 3 μM FMRFamide only. B, C and D show amplitude histograms obtained in three separate patches from ≥ 15 s of recording, in the presence of 3 μM FMRFamide alone (^), or with the additional presence of 100 μM Ac-FnLRFamide, 100 μM FM(D)RFamide, or 10 μM amiloride (□).
Amiloride
Amiloride is diagnostic for the superfamily of ion channels with two membrane spanning domains including FaNaC, ENaC, the Caenohabditis elegans degenerins, the acid-sensing sodium channels, and others (see North, 1996; Cottrell, 1997). On the neuronal FMRFamide-gated channels, 10 μM amiloride decreased open time with no noticeable change in the unitary current amplitude except for very short events attenuated by the limited frequency response of the recording system (Fig. 6A and D). There was also no obvious increase in subconductance levels in the presence of 0.3–10 μM amiloride suggesting the induction of relatively long, fully blocked states. The blocking actions of amiloride on ENaC are related to both the phenamil ring structure of the molecule and the guanidinium group of the side chain; guanidinium itself is a weak blocker (Ki = 10 mM; McNicholas & Canessa, 1997). We therefore tried guanidinium as a blocker of the FMRFamide-gated channel and found it to be slightly more potent (IC50, ∼1 mM) than for the ENaC, producing a consistently decreased unitary current amplitude.
DISCUSSION
Most known cases of channel block involve complete obstruction of the pore resulting in temporary, but complete, abolition of the unitary current. The blocking effect of FMRFamide was different in that the unitary currents were reduced in amplitude, but not completely abolished at any concentration of the peptide. A similar ‘partial block’ has been described for L-type calcium channels (Prod'hom et al. 1987; Colquhoun, 1987). For these channels it was thought that changes to a charged group within the mouth of the channel by protonation/deprotonation led to a change in the effective local ionic gradient that drives the ions through the channel pore, and hence an altered conductance. The observed voltage dependence of the FMRFamide effect suggests movement within the membrane of a charged residue of the peptide for block to occur. Introduction of this charged part of the peptide, perhaps the positively charged guanidinium group of arginine (at neutral pH), within the pore mouth region could interfere with the local sodium gradient and thereby reduce the unitary current amplitude without fully blocking the pore. Such a mechanism would require a specific binding site for the blocking peptide molecule.
The earlier structure-activity experiments showed that even minor alterations to FMRFamide result in loss of agonist activity (Cottrell, 1997). Even FLRFamide, which is formed in neurones containing FMRFamide, is considerably less potent as an agonist on the channel, although not noticeably less potent on other FMRFamide-activated, slower responses (see Cottrell et al. 1984). Amidation of the C-terminal phenylalanine is crucial for agonist and antagonist activity, but the amino group of the N-terminal is less important. Further, tryptophan is a satisfactory substitute of the N-terminal phenylalanine for agonist and antagonist effects. Some other substitutions, which had marked effects on agonist activity, had less influence on antagonist activity. For example, the D-Arg analogue of FMRFamide was a good blocker but a only a poor activator of the receptor and acetylation of the N-terminal phenylalanine, or its complete removal, gave peptides which were ineffective agonists, but blocked the channel. The ineffectiveness of the CCK analogue, WMDFamide, as an antagonist suggests that the arginine moiety is important for blockade, possibly because of the contained, charged guanidinium group. Arginine on its own, however, had no significant antagonist effect. Taken together, these results suggest that the arginine moiety is important for the blocking action, but that it needs to be specifically posititioned to exert maximum effect. The observation that nLRFamide is an effective blocker, whereas FnLR is not, indicates that the carboxy, non-charged phenylalanine is important for providing appropriate spacial alignment of the charged guanidinium to cause blockade.
The properties of the clone FaNaC and the native channel are very similar (Lingueglia et al. 1995; Cottrell, 1997). The neuronal channel is thought to consist of only one type of subunit, FaNaC, four of which probably comprise the active channel (Coscoy et al. 1998). If each subunit possesses a single specific peptide-recognition site, each channel complex may have four such sites. On the other hand, concentration dependence studies of activation conform to a two-binding-site model: activation of the channel by FMRFamide appears to be co-operative, with a Hill coefficient of about 1.5 (Lingueglia et al. 1995; Cottrell, 1997). Further, unitary current activity evoked by low and medium doses of FMRFamide and FLRFamide have been analysed satisfactorily assuming two peptide binding sites per functional channel (Zhainazarov & Cottrell, 1998), and a five-state model similar to that proposed for the nicotinic ACh receptor (see Colquhoun & Sakmann, 1985). There could therefore be spare agonist-binding sites with which blocking peptides could interact. Such sites would, however, have to have changes in binding specificities resulting from channel opening.
The rat epithelial sodium channel, which is related to FaNaC, is also thought to exist as a tetramer (Firsov et al. 1998). When incorporated into lipid bilayers, it behaves as though composed of complexes of three subunits linked by covalent sulfhydryl bonds and ionic interactions (Benos et al. 1997). These normally gate collectively to give a 40 pS main conductance with a 13 pS sublevel, but under certain conditions designed to disrupt intersubunit interactions they can be induced to gate independently to give 13 pS conductance events only. This behaviour of the epithelial channel superficially resembles that reported here for FaNaC. It is, however, unlikely that peptide block of the FMRFamide gated channel could be explained by a similar disruption of subunit interactions by the peptides because the smaller conductance level did not appear to gate independently of the main conductance level, except at the highest blocker concentrations when this state predominated.
The decreased open time, with no reduction in event amplitude, induced by amiloride is quite different from that caused by FMRFamide analogues suggesting a different mechanism of action. Some evidence indicates that the amiloride effect on epithelial sodium channels is due to interaction with an external sodium-binding site in or near the lumen of the channel, possibly involving direct plugging of the pore (McNicholas & Canessa, 1997). The absence of subconductance levels in the presence of amiloride argues against the partial block caused by the peptides being due to block of individual subunits of a multimeric complex, if amiloride and the peptides are simply blocking the pore. A similar decrease in the opening probability with no change in unitary current amplitude has been reported for epithelial sodium channels (Schild et al. 1997).
The data presented here show that some features of the neuronal response to FMRFamide, which were previously interpreted as indicating the presence of two native channel types (Green et al. 1994), are due to peptide block of the channels. Even with low (≤ 3 μM) FMRFamide, however, there was significant variation in the size of unitary FMRFamide-activated currents recorded through native channels. Also the current flow in the subconductance level was proportionate to the full conductance state of the channels suggesting that some other, at present unknown, factors must also affect the channel conductance. The concentration required for the peptide-induced block of FMRFamide-gated channels is less than required for the agonist block of nicotinic acetylcholine receptors (Ogden & Colquhoun, 1985); the phenomenon may occur at physiological concentrations. The blocking effect was noticeably more pronounced with FLRFamide, which is also produced from the FMRFamide gene (Lutz et al. 1992). This probably partly explains the smaller maximal whole cell response exerted by FLRFamide, compared with FMRFamide, on both the neurone and oocyte-expressed FaNaC (Cottrell, 1997).
Acknowledgments
We thank The Wellcome Trust for providing financial support.
References
- Benos DJ, Fuller CM, Shlyonsky VG, Berdiev BK, Ismailov II. Amiloride-sensitive Na+ channels: Insights and outlooks. News in Physiological Sciences. 1997;12:55–61. [Google Scholar]
- Colquhoun D. A new type of ion-channel block. Nature. 1987;329:204–205. doi: 10.1038/329204a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. The Journal of Physiology. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1983. pp. 191–263. [Google Scholar]
- Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. Journal of Biological Chemistry. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- Cottrell GA. The first peptide-gated ion channel. Journal of Experimental Biology. 1997;200:2377–2386. doi: 10.1242/jeb.200.18.2377. [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Davies NW, Green KA. Multiple actions of a molluscan cardio-excitatory neuropeptide and related peptides on identified Helix neurones. The Journal of Physiology. 1984;356:315–333. doi: 10.1113/jphysiol.1984.sp015467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GA, Green KA, Davies NW. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) can activate a ligand-gated ion channel in Helix neurones. Pflügers Archiv. 1990;416:612–614. doi: 10.1007/BF00382698. [DOI] [PubMed] [Google Scholar]
- Firsov D, Gautschi I, Merillat A-M, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO Journal. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Falconer SWP, Cottrell GA. The neuropeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) directly gates two ion channels in an identified Helix neurone. Pflügers Archiv. 1994;428:232–240. doi: 10.1007/BF00724502. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Lutz E, MacDonald M, Hettle S, Price DA, Cottrell GA, Sommerville J. Structure of cDNA clones and genomic DNA encoding FMRFamide-related peptides (FaRPs) in Helix. Molecular and Cellular Neuroscience. 1992;3:373–382. doi: 10.1016/1044-7431(92)90049-8. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. Journal of General Physiology. 1997;109:681–692. doi: 10.1085/jgp.109.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Families of ion channels with two hydrophobic segments. Current Opinion in Cell Biology. 1996;8:474–483. doi: 10.1016/s0955-0674(96)80023-8. [DOI] [PubMed] [Google Scholar]
- Ogden DC, Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proceedings of the Royal Society. 1985;225:329–355. doi: 10.1098/rspb.1985.0065. B. [DOI] [PubMed] [Google Scholar]
- Prod'hom B, Pietrobon D, Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987;329:243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. Journal of General Physiology. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhainazarov AB, Cottrell GA. Single-channel currents of a peptide-gated sodium channel expressed in Xenopus oocytes. The Journal of Physiology. 1998;513:19–31. doi: 10.1111/j.1469-7793.1998.019by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


