Abstract
Paired intracellular recordings were performed in slices of adult rat neocortex and hippocampus to examine presynaptic depression. A novel form of depression that occurs even in the absence of transmitter release during conditioning activity was observed at a subset of synaptic connections.
In each pair studied, a pyramidal neurone was presynaptic and inputs onto a range of morphologically identified postsynaptic target cells were analysed; high probability connections exhibiting the more traditional forms of release-dependent depression, as well as low probability connections exhibiting facilitation, were tested (n = 35).
Connections were tested with presynaptic spike pairs and trains of spikes with a range of interspike intervals. Sweeps in which the first action potential elicited no detectable response (apparent failures of transmission) and sweeps in which the first action potential elicited large EPSPs were selected. Second EPSPs that followed apparent failures were then compared with second EPSPs that followed large first EPSPs.
Release-independent depression was apparent when second EPSPs at brief interspike intervals (< 10–15 ms) were on average smaller than second EPSPs at longer interspike intervals, even following apparent failures and when the second EPSP amplitude at these short intervals was independent of the amplitude of the first EPSP.
Release-independent depression appeared selectively expressed. Depressing inputs onto some interneurones, such as CA1 basket-like and bistratified cells, and facilitating inputs onto others, such as some fast spiking neocortical interneurones, exhibited this phenomenon. In contrast, depressing inputs onto 10/10 neocortical pyramids and facilitating inputs onto 7/7 oriens-lacunosum moleculare and 5/5 burst firing, sparsely spiny neocortical interneurones did not.
Postsynaptic target neurones somehow select the temporal pattern of neurotransmitter release they will receive, even though presynaptic mechanisms control these patterns. This has been most thoroughly documented at synapses involving presynaptic cortical pyramidal axons. Their ‘depressing connections’ onto other pyramids act as ‘low-pass’ filters. Low frequency presynaptic firing produces the most efficient information transfer and increasing rates bring diminishing returns (Thomson & West, 1993; Thomson et al. 1993a; Thomson & Deuchars, 1994, 1997; Thomson, 1997; Markram, 1997; Markram et al. 1997, 1998; Ali et al. 1998). In contrast, ‘facilitating connections’ onto specific classes of interneurones act as ‘high-pass’ filters. High frequency burst firing maximizes transfer, slow tonic discharge generating little or no output (Thomson et al. 1993b, 1995; Markram et al. 1998; Ali & Thomson, 1998).
Strongly depressing connections display a high probability of transmitter release at low firing rates, but release sites appear to become refractory (Betz, 1970) after each release and fewer remain available to contribute to the next event. Here, the amplitude of a second EPSP is inversely proportional to that of the first at intervals up to 30–50 ms (Thomson et al. 1993a; Thomson, 1997). In contrast, strongly facilitating connections display very low release probabilities at low firing rates (Deuchars & Thomson, 1995), but the Ca2+ that enters during the first action potential (AP) is proposed to prime the release machinery, increasing the probability that subsequent APs will reach release threshold (Katz & Miledi, 1970; Stanley, 1985, 1986, 1995; Fisher et al. 1997). The dynamic properties of synaptic connections therefore select both the targets activated by a particular discharge pattern and the order in which they will be recruited.
The Ca2+ channels mediating transmitter release display intriguing activity-dependent properties. Recombinant N-type channels expressed in cell lines (McNaughton & Randall, 1997; McNaughton et al. 1998) inactivate rapidly, especially when activated repetitively by brief, AP-like depolarizations at physiological temperatures. Native presynaptic P-type channels also exhibit inactivation, but this is not immediately apparent with brief spike trains, (Forsythe et al. 1998), which result first in a transient Ca2+-dependent facilitation (Cuttle et al. 1998). If P-type channels dominated release, therefore, spike pairs and brief trains would be expected to elicit facilitating Ca2+ influx. If, on the other hand, N-type channels dominated, these channels would be partially inactivated following the first spike. Release in response to a second AP could be depressed, even if the first AP had released no transmitter. If this type of depression is indeed expressed, it would differ strikingly from other well-documented forms of synaptic depression in being independent of the recent release history of the connection.
To determine, therefore, whether release-independent depression can occur in mature brain, slices of adult rat neocortex or hippocampus were used. To ensure that APs in a single identifiable presynaptic cell initiated all recorded postsynaptic responses, dual intracellular recordings from synaptically connected pairs of neurones were performed with biocytin-filled electrodes. In each case a pyramidal cell was the presynaptic neurone and a range of postsynaptic targets, including other pyramidal cells and several classes of morphologically identified interneurones, was studied.
METHODS
Preparation
Young adult male Sprague-Dawley rats (120–180 g) were anaesthetized with halothane (Fluothane), then intraperitoneal sodium pentobarbitone (Sagatal, Rhone Merieux; 60 mg kg−1) and perfused transcardially with 50–100 ml ice-cold artificial cerebrospinal fluid (ACSF) with added sodium pentobarbitone (60 mg l−1) containing (mM): 248 sucrose, 25.5 NaHCO3, 3.3 KCl, 1.2 KH2PO4, 1.0 MgSO4, 2.5 CaCl2 and 15 D-glucose equilibrated with 95 % O2-5 % CO2. The animals were decapitated and 450–500 μm coronal sections of brain including neocortex and/or hippocampus cut (Vibroslice, Camden Instruments, UK). Slices were maintained at the interface between the sucrose-containing ACSF and warm, humidified 95 % O2-5 % CO2 at 35–36°C for 1 h. The sucrose-containing medium was then replaced with an ACSF in which 124 mM NaCl replaced the sucrose. This ACSF was used for all recordings, which commenced after another hour.
Electrophysiological recordings
Paired intracellular recordings were performed using conventional sharp electrodes (80–160 MΩ) containing 2 M potassium methylsulphate and 2 % biocytin (w/v) under current clamp (Axoprobe, Axon Instruments). Sharp electrodes were used to avoid any possibility of presynaptic ‘run-down’ during these long recordings and because sharp electrodes are more efficient for paired recordings in relatively thick slices of adult tissue. Presynaptic firing was initiated by current injection at 1 spike, 1 spike pair, or 1 spike train per 3 s. Since dual sharp recordings are typically confounded by capacitance coupling artifacts, large brief current pulses cannot be used to initiate presynaptic spikes at very specific interspike intervals without obscuring the details of small postsynaptic responses. A range of presynaptic interspike intervals was therefore elicited by driving the presynaptic pyramidal cell with a range of square-wave and/or ramp-shaped current pulses delivered from a range of sub-threshold membrane potentials. Continuous analog recordings from both neurones were made on analog tape (Racal, Store 4, Southampton, UK). For powerfully depressing connections (e.g. Fig. 3) with few first spike failures, up to 2000 sweeps were required for the necessary comparisons. Of 61 long paired recordings tested, 35 included enough first spike failures and an adequate range of second spike intervals for the required analysis.
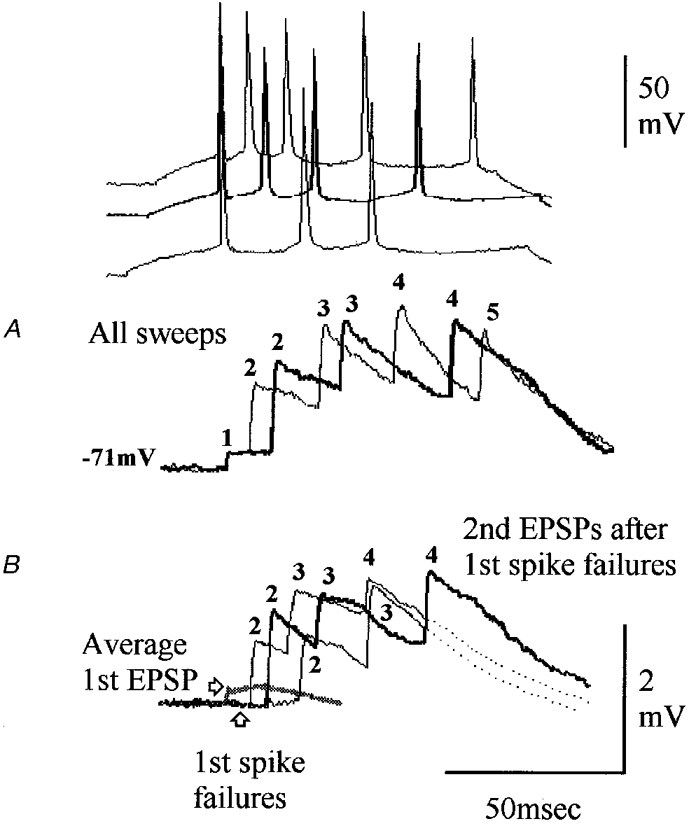
Figure 3. A depressing connection onto a stratum oriens basket-like cell without release-independent depression.
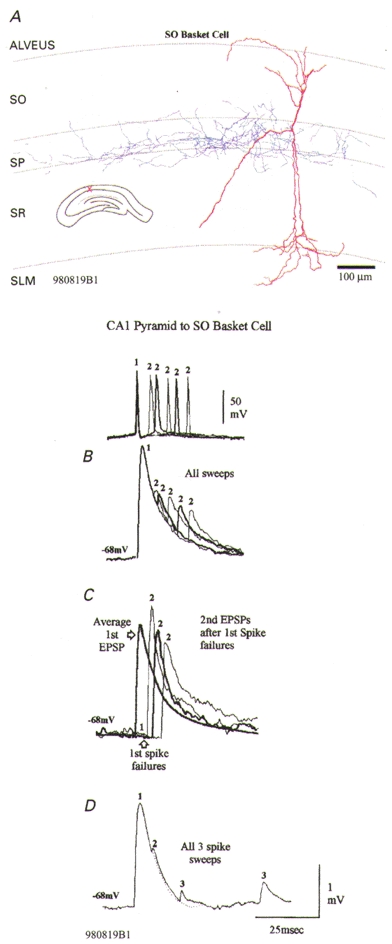
A, postsynaptic stratum oriens (SO) basket-like cell. The soma of this interneurone was in SO, its dendrites (red) extended through SO and through stratum radiatum (SR) into stratum lacunosum moleculare (SLM). Its broad axonal arbor (blue) was largely confined to stratum pyramidale (SP). B, the second EPSP in this interneurone is very powerfully depressed, with some recovery at longer intervals (normal and thick traces alternating, representative spike pairs shown above). Recovery from third EPSP depression is slow (D). This depression is not, however, independent of release. Following first spike failures, second EPSPs are slightly facilitated relative to average first EPSPs (grey trace in C). These responses can be explained by release-dependent depression with some facilitation.
Data analysis
Data were digitized (5–10 kHz, voltage resolution 0.005–0.01 mV) and analysed off-line (using in-house software). Individual sweeps were observed and the trigger points associated with the rising phase of each presynaptic AP checked and/or edited. Sweeps including large spontaneous events or artifacts were rejected. Data sets that included total apparent first AP failures and data sets including only large first EPSPs were generated. These were then divided into subsets, each with a narrow range of second (third …) spike intervals (e.g. 5–8 ms, or 20–25 ms). Averaging of EPSPs was triggered by the rising phase of single presynaptic spikes for the average first EPSP, the rising phase of the second presynaptic spike for the average second EPSP, and so on. Illustrated EPSPs are therefore composite averages with single spike responses and second (third …) EPSPs at several specific interspike intervals superimposed (individual averages in these composites include at least 30 and up to 900 sweeps). Average first EPSP amplitudes were measured as the difference between an average of the voltage preceding the presynaptic spike and an average around the peak of the EPSP. Where second EPSPs were well separated in time the same procedure was used. Where second EPSPs summed with first EPSPs, an averaged single spike EPSP was scaled to match the peak of the first EPSP and the second EPSP was measured between its peak and the appropriate point on the falling phase of the first EPSP.
Data from 15 of the 35 paired recordings included here have been published previously. However, all the analysis of events that follow first EPSP failures is novel. Data from two pairs included in previously published studies are illustrated here: cell 920618A1 in Fig. 4 (Thomson et al. 1997) and 950531A1 in Fig. 1 (Ali et al. 1998).
Figure 4. A depressing connection onto a layer III pyramidal cell without release-independent depression.
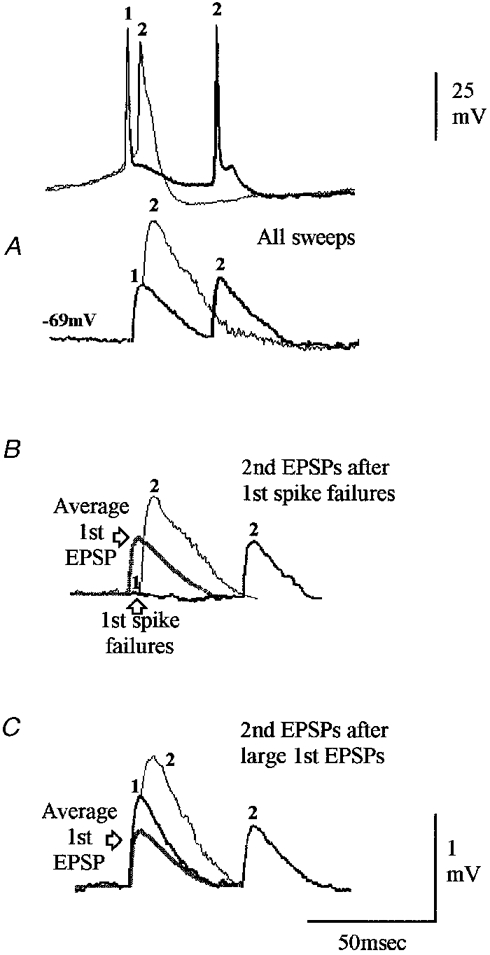
Although there is slight second EPSP facilitation in this connection, third and fourth EPSPs in trains were depressed (not illustrated). A, facilitation of the second EPSP relative to the average first EPSP, with recovery at longer intervals. This depression is not, however, independent of release. Following first spike failures second EPSPs are further facilitated (B), but after large first EPSPs, second EPSPs are depressed (C) relative to average first EPSPs (grey traces). These responses can be explained by release-dependent depression with some facilitation.
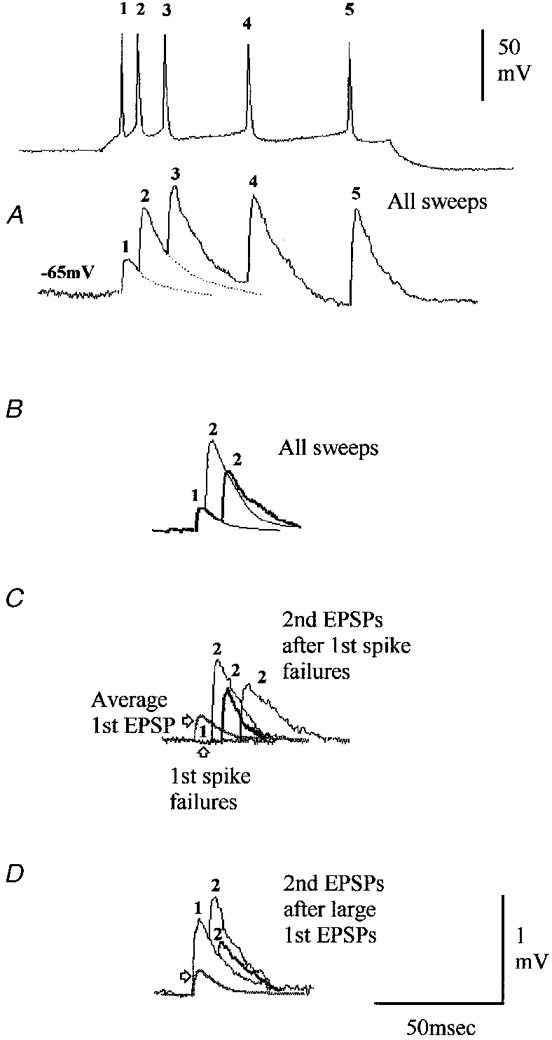
Figure 1. A depressing connection onto a CA1 stratum pyramidale basket cell exhibiting release-independent depression.
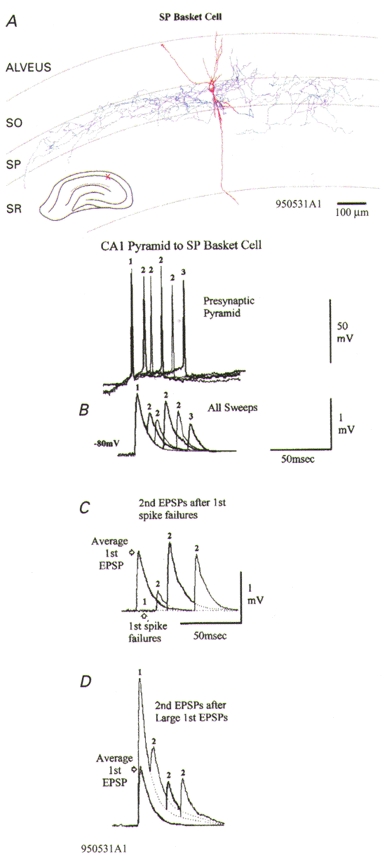
A, the dendrites of this basket cell are drawn in red (partial recovery) and the axon, which ramifies extensively within stratum pyramidale (SP) is drawn in blue. SO, stratum oriens; SR, stratum radiatum. B-D, averaged responses to single presynaptic spikes and spike pairs with different interspike intervals are superimposed, with thin and thick traces alternating. B, averaged responses to all spike pairs at the intervals illustrated and to spike triplets (see spike and EPSP labelled 3). Second and third EPSPs are, on average, depressed. Representative presynaptic spike pairs and 1 spike triplet are shown above (relative positions of baselines represent relative membrane potentials, with different holding potentials and rates of depolarization to threshold used to elicited spike pairs/triplets with different intervals). C, even following first spike failures, second EPSPs are depressed. D, second EPSPs following large first EPSPs are depressed at 3 interspike intervals relative to the average first EPSP (grey trace), showing some time-dependent recovery. At short intervals, depression is similar following failures and following large EPSPs (compare C with D). However, at intervals > 20 ms, there is either release-dependent depression (D) or facilitation (C), depending on the size of the first EPSP. Release-independent and release-dependent depression with facilitation would explain these data.
Data from other paired recordings included, but not illustrated, here were reported in the following studies. Thomson et al. (1995): three neocortical pyramid to burst firing interneurone pairs; Ali & Thomson (1998): two CA1 pyramid to oriens-lacunosum moleculare (OLM) cell pairs; Thomson et al. 1993b: two neocortical pyramid to fast spiking interneurone pairs; Thomson, 1997: one neocortical pyramid to burst firing interneurone pair; Thomson & West, 1993: two layer III neocortical pyramid-pyramid pairs; Thomson et al. 1993a: three layer V neocortical pyramid-pyramid pairs.
Histological processing
Cell pairs were filled with biocytin. Slices were fixed overnight in 0.1 M phosphate buffer containing 1.25 % glutaraldehyde, 2.5 % paraformaldehyde and 15 % saturated picric acid, then washed in 0.1 M phosphate buffer, embedded in gelatin, sectioned at 60 μm (Vibratome), cryoprotected in 20 % sucrose and permeabilized by freeze thawing in liquid nitrogen. Biocytin was localized using the Elite ABC kit (Vector) overnight and visualized using 3′,3′-tetra-aminodiaminobenzidine (DAB; Sigma). Sections were then dehydrated and embedded in Durcupan resin (Fluka) on slides. Filled interneurones were fully reconstructed using a drawing tube (×100 objective).
RESULTS
Thirty-five paired recordings in which there was no change in average EPSP amplitude with time and in which a significant proportion of first spikes elicited no detectable postsynaptic response (first spike failures, or apparent failures of transmission) were studied in detail. These paired recordings were first divided into two groups, those in which presynaptic spike trains elicited trains of steadily increasing EPSPs (facilitating connections, n = 16) and those in which steadily declining EPSPs were elicited (depressing connections, n = 19). Three pyramid-pyramid connections with slight second EPSP facilitation (e.g. Fig. 4) were included in the depressing group since the maintained facilitation of third and fourth EPSPs typical of truly facilitating mature connections (e.g. Figs 5–8) was absent. To distinguish synaptic failures from small first EPSPs that might fall below the level of detection on single sweeps, all sweeps, including apparent failures in response to the first spike were selected and averaged. Only where there was no detectable first spike response in that average was analysis continued.
Figure 5. A facilitating connection onto a stratum pyramidale interneurone exhibiting reduced facilitation at short interspike intervals.
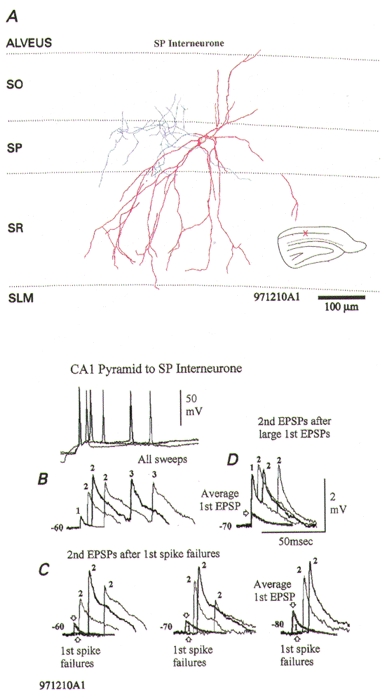
A, postsynaptic stratum pyramidale (SP) interneurone. This SP interneurone had an unusually sparse axonal arbor (blue), despite intense staining (facilitating EPSPs are also not typical of classical CA1 SP basket cells; Ali et al. 1998, and see Fig. 1). B, average third EPSPs are also strongly facilitated (see averaged EPSPs labelled 3). C and D, average second EPSPs, although larger than average first EPSPs (grey traces) are of similar amplitude at the shortest intervals, whether they follow large first EPSPs (D) or first spike failures (C). At slightly longer intervals, however, larger second EPSPs follow failures (C) than follow large first EPSPs (D). The reduced facilitation at short intervals following failures can be seen at several membrane potentials (C). Strong facilitation with release-dependent and release-independent depression would explain these data.
Figure 8. A facilitating connection onto a sparsely spiny, burst firing neocortical interneurone without release-independent depression.
The postsynaptic interneurone was similar to those described previously (Thomson et al. 1995; Deuchars & Thomson, 1995). In this pyramid-interneurone connection, facilitation is maintained throughout a 5 spike train (A). B, facilitation of the average second EPSP relative to the average first EPSP (grey traces) is greatest at the shortest interspike intervals. However, larger first EPSPs are followed by less facilitated second EPSPs (D) than are first spike failures (C). Both facilitation and release-dependent depression are therefore involved.
The resting membrane potentials for the postsynaptic neurones ranged from −57 to −84 mV (−71.5 ± 7.0 mV, mean ± s.d.). As a group, the neocortical pyramidal cells had the most negative resting potentials (78.1 ± 5.8 mV, range 65–84 mV) and the OLM interneurones in CA1 the least negative (−64 ± 4.8 mV, range −57 to −70 mV). The analysis was performed for more than one membrane potential at least 10 mV and up to 30 mV apart, in 10 pairs, including all broad classes reported here (see Fig. 5C for an example) and similar findings were obtained at all potentials.
Release-independent depression at depressing synapses
Six of nineteen depressing connections displayed release-independent depression. Even following total apparent failures of transmission, averaged second EPSPs were smaller than averaged first EPSPs at very short interspike intervals (Figs 1C, 2B and C). This depression recovered or, following failures, was masked within 10–15 ms by a facilitation which decayed within 50 ms. Following larger first EPSPs, release-dependent depression that also decayed within 50–60 ms was apparent. Release-independent depression can therefore account for a significant component of the paired pulse depression at very short interspike intervals. It cannot, however, account for the depression that is related to the amplitude of the first EPSP and apparent up to 50 ms. In Table 1, some of the characteristics of these EPSPs are summarized. From the rate of change of second EPSP amplitude with increasing interspike intervals, it can be seen that an early (up to 10–12 ms), relatively rapid phase of recovery from depression is followed by a slower phase (10–20 ms).
Figure 2. A depressing connection onto a CA1 pyramidal cell exhibiting release-independent depression.
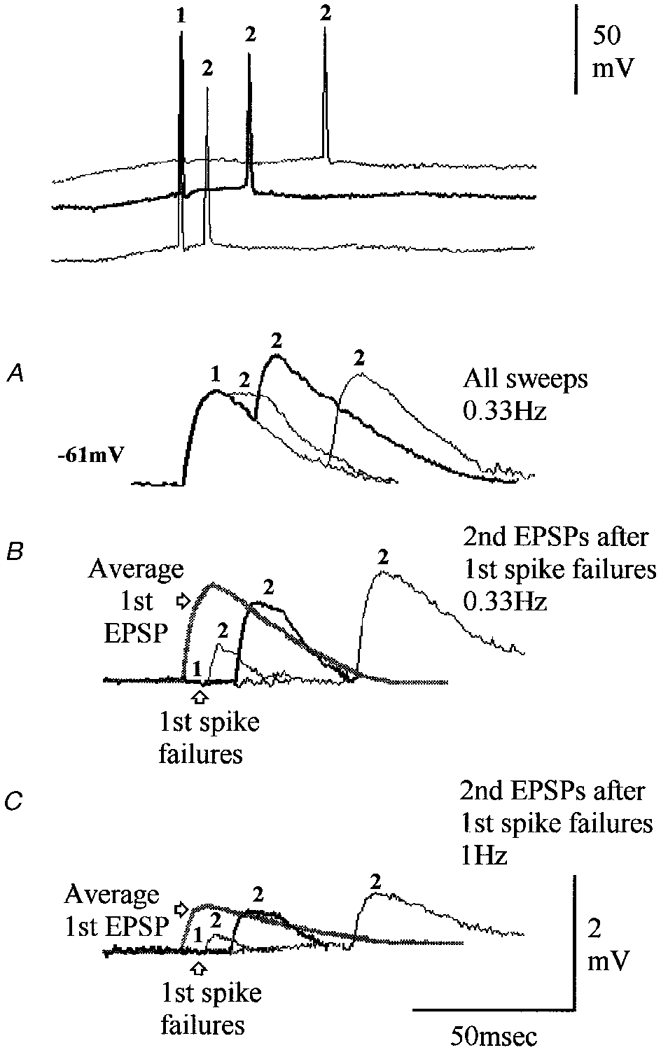
A-C, averaged responses to single presynaptic spikes and spike pairs with different interspike intervals are superimposed, with thin and thick traces alternating. A, averaged responses to all spike pairs at the intervals illustrated. Second EPSPs are on average depressed, recovering with longer intervals. Representative presynaptic spike pairs are shown above (baselines shifted for clarity, all holding potentials in this example similar). B, at short intervals, second EPSPs following first spike failures are depressed relative to the average first EPSP (grey trace). C, second EPSPs are similarly depressed (relative to average first EPSPs) at 0.33 Hz (B) and at 1 Hz (C), when the readily releasible transmitter pool is reversibly exhausted, or ‘fatigued’. Release-independent and release-dependent depression, with facilitation and fatigue would explain these data.
Table 1.
Characteristics of EPSPs that exhibited release-independent depression and EPSPs that did not
| 1st EPSP amplitude, all sweeps (mV) | 2nd EPSP at 10 ms as % of 1st EPSP amplitude, all sweeps | 2nd EPSP at 10 ms as % of 1st EPSP amplitude, after failures | Rate of change of 2nd EPSP with interval (μV ms−1) 4–12 ms | Rate of change of 2nd EPSP with interval (μV ms−1) 10–20 ms | |
|---|---|---|---|---|---|
| Depressing EPSPs | 1.45 ± 1.39 | 61 ± 12 | 72 ± 13 | +43 ± 16 | +26 ± 12 |
| with RID (n = 6) | (0.55–5.17) | (45–81) | (58–92) | (+23 to +75) | (+7 to +52) |
| Depressing EPSPs | 0.68 ± 0.56 | 111 ± 50 | 169 ± 35 | −43 ± 50 | −23 ± 20 |
| without RID (n = 8) | (0.21–2.05) | (71–154) | (121–233) | (−160 to 0) | (−5 to −76) |
| Facilitating EPSPs | 0.27 ± 0.09 | 407 ± 133 | 461 ± 147 | +88 ± 50 | −32 ± 39 |
| with RID (n = 5) | (0.19–0.42) | (244–650) | (250–650) | (+36 to +110) | (−97 to +38) |
| Facilitating EPSPs | 0.92 ± 0.67 | 354 ± 154 | 396 ± 145 | −125 ± 230 | −36 ± 22 |
| without RID (n = 10) | (0.12–2.64) | (155–758) | (244–650) | (−220 to +18) | (−2 to −93) |
| Cell 980819B1 | |||||
| All sweeps | 2.05 | 20 | +49 | +30 | |
| 1st spike failures | 121 | −150 | |||
EPSPs were separated into 4 broad classes: depressing EPSPs that exhibited release-independent depression (RID); depressing EPSPs that did not exhibit RID; facilitating EPSPs that exhibited RID; and facilitating EPSPs that did not. Average first EPSP amplitudes (± s.d.) and ranges are given. Average second EPSP amplitudes at an interspike interval of 10–12 ms are normalized and expressed as a percentage of average first EPSP amplitudes. Two values for normalized second EPSPs are given: for all second EPSPs (at an interval of 10–12 ms), and for those second EPSPs that followed first spike failures. The change in second EPSP average amplitude with increasing interspike intervals is expressed as a rate of change in amplitude between the shortest intervals studied (4–6 ms) and 10–12 ms, and again as a rate of change between 10 and 20 ms. All paired recordings for which second EPSP average amplitude could be measured at short interspike intervals, at 10–12 ms, and between 10 and 20 ms, are included in the means except for cell 980819B1 (Fig. 3), which displayed an unusually powerful depression.
All these connections involved presynaptic CA1 pyramidal cells and 5/6 involved interneuronal targets: 3/3 classical stratum pyramidale (SP) putative basket cells with dense axonal arbors confined to SP (Fig. 1), 1/1 bistratified interneurone (Buhl et al. 1996; Freund & Buzsáki, 1996; Ali et al. 1998) with axonal ramification in stratum oriens (SO) and proximal stratum radiatum (SR), and one fast spiking SO interneurone with no sag in its response to hyperpolarizing current injection. The final target was the only postsynaptic pyramid to display this phenomenon (1/2 CA1 pyramids tested, Fig. 2).
Release-independent depression is not apparent at all depressing synapses
Thirteen of nineteen depressing connections did not exhibit release-independent depression. Second EPSP amplitude was inversely related to first EPSP amplitude at all interspike intervals below 30–60 ms, with the strongest depression at intervals < 10 ms (Fig. 3). In eight of these connections second EPSPs were slightly facilitated at the shortest intervals after first spike failures. At these connections, therefore, release-independent depression is either absent, or is only weakly expressed and masked by facilitation (Fig. 4).
Two of these thirteen connections were in CA1, one involving a postsynaptic pyramid and one an atypical SO basket-like cell sparsely innervating SP (Fig. 3). Interestingly, this connection displayed the most profound depression in the present study and yet there was no sign of release-independent depression (Fig. 3C). There was a strong negative correlation between first and second EPSP amplitude at short intervals with second EPSP amplitude largest following failures and showing a profound depression of the second EPSP following large first EPSPs. This depression recovers only slowly with increasing interspike intervals. The other 11 connections were in neocortex. Ten of these involved all the postsynaptic neocortical pyramids studied (Fig. 4) and one an incompletely recovered fast spiking layer III interneurone. In Table 1, average second EPSP amplitude can be seen to decrease with increasing interspike interval up to 10–20 ms, contrasting with the increase over this range of intervals at depressing connections displaying release-independent depression. This probably represents the decay of facilitation and typically reverses between 10 and 20 ms with EPSPs recovering from release-dependent depression over the next 30–50 ms.
Selected facilitating connections may exhibit release-independent depression
In facilitating connections, second EPSPs were on average larger than first EPSPs at all interspike intervals of less than 50–60 ms. However, in 5/16 facilitating connections, second EPSPs were smaller at intervals < 10–15 ms than between 15 and 25 ms, even following failures (Figs 5 and 6). The similarity in the time course of this reduced facilitation to the release-independent depression described above suggests that both paired pulse facilitation and release-independent depression may be strongly expressed at these connections. In Table 1, second EPSPs in these connections can be seen to increase in average amplitude with increasing interspike interval up to 10–12 ms, like those at depressing connections expressing release-independent depression. However, after this interval, these facilitating EPSPs decreased in amplitude as facilitation declined.
Figure 6. A facilitating connection onto a regular spiking CA1 interneurone (soma in proximal stratum oriens) exhibiting reduced facilitation at short interspike intervals.
Following all first EPSPs (A) and following first spike failures (B), second EPSPs at the shortest intervals are less facilitated than at slightly longer intervals. With these summing EPSPs the peak amplitude attained during the train is independent of frequency or of first/second EPSP amplitude. Release-dependent depression may thus be limiting peak responses. Facilitation with release-dependent and release-independent depression would therefore explain these data.
Two of these connections were in CA1. One was a pyramidal input onto an unusual fast spiking SP interneurone with a meagre axonal arbor confined to SP (Fig. 5). Facilitating inputs onto SP basket cells are atypical (Ali et al. 1998, and see Fig. 1 for a more typical example) indicating that this cell may not, as its sparse axonal arbor also indicates, belong to one of the major classes of SP basket cells. There was little or no correlation between first and second EPSP amplitude at the shortest interspike intervals, but a strong correlation at intervals between 15 and 25 ms. Second EPSP amplitude was largest following first EPSP failures, with a gradual decline in second EPSP facilitation with increasing interspike intervals. The other postsynaptic CA1 neurone in this group was a partially recovered SO interneurone (Fig. 6) whose axonal arbor was too poorly filled for full identification. The other three connections in this group involved powerfully facilitating inputs to fast spiking aspiny postsynaptic neocortical interneurones similar to those described previously (Thomson et al. 1993b; Kawaguchi & Kubota, 1993; Thomson & Deuchars, 1997), two in layer III and one in layer V.
Facilitating connections without release-independent depression
In 11/16 facilitating connections, facilitation dominated at all intervals with no evidence for release-independent depression. The greatest facilitation was observed at the shortest interspike intervals. Release-independent depression was therefore absent or weakly expressed and masked by strong facilitation. There was a weak negative correlation between first and second EPSP amplitude at the shortest interspike intervals and a gradual decline in second EPSP facilitation with increasing interval. In low probability, strongly facilitating connections, correlations between first and second EPSP amplitudes are often weak under control conditions because first EPSPs typically involve only a small proportion of the available release sites. In Table 1, average second EPSPs can be seen to decrease relatively rapidly in amplitude up to 10–12 ms and thereafter to decline more gradually and in parallel with the decline over these intervals at facilitating connections that did express release-independent depression.
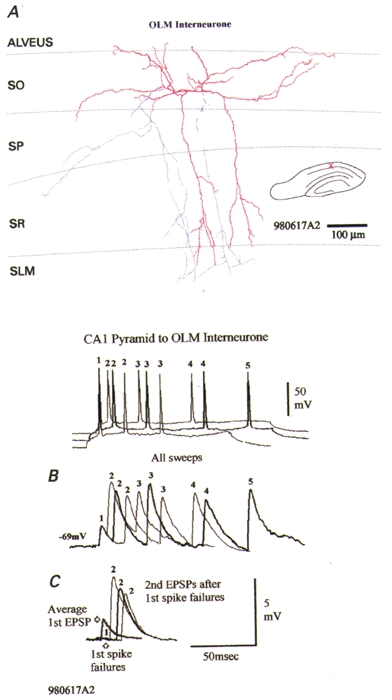
Six of these connections were in CA1 and one at the CA1/3 border (Fig. 7) and included all of the postsynaptic OLM interneurones studied (7/7). These cells were identified by their typical pronounced sag in response to hyperpolarizing current injection, their thorny, horizontally oriented dendrites in SO (and in SR in CA3 OLM cells) and by their axonal arbor, which innervated SLM almost exclusively (Lacaille & Williams, 1990; McBain et al. 1994; Blasco-Ibanez & Freund, 1995; Freund & Buzsáki, 1996; Ali & Thomson, 1998). The five postsynaptic cells in neocortex were all burst firing, sparsely spiny (n = 4 recovered morphologically) interneurones (Kawaguchi & Kubota, 1993; Deuchars & Thomson, 1995) (Fig. 8).
Figure 7. A facilitating connection onto a thorny oriens-lacunosum moleculare interneurone at the CA1/CA3 border exhibits only enhanced facilitation at short interspike intervals.
A, the postsynaptic thorny oriens-lacunosum moleculare (OLM) cell at the CA1/CA3 border is morphologically intermediate between OLM cells typical of CA1 and CA3 (Freund & Buzsáki, 1996), since its dendrites (red) extend horizontally in both subfields, but also extend into stratum radiatum in CA3. Its axons (blue) descend to stratum lacunosum moleculare (SLM) but were cut in this slice. Strong facilitation of second and subsequent EPSPs in trains is apparent following all first EPSPs (B) and following first spike failures (C). This facilitation is greatest at the shortest interspike intervals. Under these conditions, therefore, facilitation alone accounts for these data.
DISCUSSION
We report a novel type of presynaptic synaptic depression that is selectively expressed at pyramidal axon terminals onto different classes of postsynaptic target. Certain readily identifiable classes of connections consistently displayed this phenomenon (e.g. inputs onto classical SP CA1 basket-like cells) while some consistently did not (inputs onto neocortical pyramidal cells, OLM cells and burst firing, sparsely spiny neocortical interneurones). Unlike previously well-documented forms of presynaptic depression, this depression is independent of the transmitter release probability and of the recent release history of the connections.
Traditional forms of presynaptic depression and facilitation
Several previous studies document forms of short term synaptic plasticity whose selective expression at different terminals of pyramidal axons correlates with postsynaptic target cell class (Thomson, 1997; Markram et al. 1998). However, all these traditional forms are directly related to the probability of transmitter release and to the recent release history of the synapse. Moreover, they were described many years ago at the neuromuscular junction, or at invertebrate synapses (e.g. Betz, 1970; and see McLachlin, 1978 for review). In brief, where release probability is high, many of the available release sites are used in the response to the first AP and appear refractory for tens of milliseconds afterwards. Fewer are available to respond to a second AP and second EPSP amplitude is inversely related to first EPSP amplitude. Facilitation is not absent. It is seen following first spike failures or if probability is reduced by lowering external [Ca2+], but is typically masked by depression. In contrast, where release probability is very low, facilitation dominates. Ca2+ entering during the first AP primes the release machinery, lowering the threshold for Ca2+ entering during subsequent spikes to cause release. Since few of the available release sites were utilized by the first AP, most remain available for subsequent spikes. Again, release site refractoriness is not absent. It is revealed by raising release probability (e.g. Thomson et al. 1995). Simple models of release could therefore be built using time-dependent decays for facilitation and depression and modified to generate facilitating or depressing connections by altering probability (Markram et al. 1998). Postsynaptic receptor desensitization, non-linear summation, or presynaptic autoreceptor activation can be treated similarly as they also rely on the preceding release history. The mechanism described here is unusual in being independent of release history.
Depressing connections that display release-independent depression
Here release-independent depression accounts for a significant component of paired pulse depression at intervals up to 10–15 ms, but not the depression that is related to first EPSP amplitude and apparent up to 50–60 ms. These connections therefore have two independent ‘low-pass’ filters, release-dependent depression (or refractoriness) and release-independent depression. Facilitation is only apparent within a narrow range of intervals as release-independent depression decays and only following small first spike responses.
Depressing connections apparently devoid of release-independent depression
Here second EPSP amplitude was inversely related to first EPSP amplitude at all intervals < 30–50 ms. Release-independent depression is either absent, therefore, or only weakly expressed and masked by facilitation. A single ‘low-pass’ filter (release site refractoriness) is therefore functionally expressed at most neocortical pyramid-pyramid connections.
Facilitating connections that appear devoid of release-independent depression
Two major classes of interneurones received facilitating inputs that also consistently lacked this phenomenon. These were the OLM cells in CA1 (7/7) and the sparsely spiny, burst firing interneurones in neocortex (5/5). Interestingly, both are classes of interneurones that inhibit relatively distal dendrites of pyramidal cells. With brief presynaptic spike trains, therefore, only ‘high-pass’ filtering is apparent at these connections. This filter is, however, dynamic, its range increasing during repetitive firing (Thomson, 1997).
Facilitating connections that appear to display release-independent depression
At the very low probability, strongly facilitating inputs onto other classes of interneurones, facilitation was reduced at very short interspike intervals. The similarity in the time course of this reduced facilitation to the release-independent depression seen at some depressing connections suggests that both facilitation and release-independent depression are strongly expressed. With a low release probability at low frequencies and this depression at very high frequencies, these synapses would act as ‘band-pass’ filters.
Spike propagation failure as a mechanism underlying release-independent depression
The reduced facilitation at short intervals cannot be ascribed to first presynaptic spike propagation failure. First spikes preceding short interval second spikes were no more likely to fail than those preceding longer intervals. A contribution to second EPSP depression at short intervals, cannot, however, be ruled out. However, most connections exhibited some facilitation, suggesting that both spikes had propagated successfully through most of the axonal arbor. If propagation failure does contribute to release-independent depression, it would be necessary to propose that APs fail only in certain, selected regions of the axonal arbor. Most pyramidal axon terminals are made en passage and there is no evidence to date to suggest that distinct axonal branches innervate different classes of targets. We therefore have no anatomical substrate for selective AP failure. However, fluctuation analysis of EPSPs elicited in fast spiking neocortical interneurones has provided some evidence for spike propagation failure (Thomson et al. 1993b). Activation of K+ channels may likewise play a role here if the properties of these channels are different at different terminals.
Activity-dependent inactivation of N-type Ca2+ channels
An interesting possibility is that ‘release-independent depression’ is due to rapid inactivation of Ca2+ channels which are differentially expressed at the axon terminals of mature pyramidal cells. This mechanism would modulate the degree of presynaptic facilitation and determine optimal activation frequencies in an energy-efficient manner. It would help to maintain longer-term repetitive transmission at connections where other forms of depression are powerfully expressed. Human N-type channels (encoded by α1B-a, β1b, α2bδ) stably expressed in a cell line and activated by trains of AP-like waveforms depressed in a frequency, pulse-width, Ca2+ and temperature dependent manner (McNaughton et al. 1998). Although the degree of inactivation depends on subunit composition, inactivation of N- and P/Q-type channels occurs preferentially from a closed state so that brief, AP-like activations are effective (Patil et al. 1998). McNaughton et al. (1998) predicted that a single preceding AP at an interval of 10 ms could suppress release by > 20 % (at 22°C, if a 4th power relation between Ca2+ influx and release is assumed). This depression would, moreover, be cumulative, and could achieve almost total depression of release at 36°C with eight APs at 100 Hz. The present data suggest that release-independent depression declines rapidly, contributing little to paired pulse depression at intervals > 10–15 ms. In contrast, significant depression of recombinant channel currents occurred at 0.3 Hz (pulse half-width, 1.7 ms). However, the subunit compositions of N-type channels at pyramidal axon terminals are unknown and channel kinetics might be affected by the β-subunits (Stea et al. 1993). The channel density at the active zone and therefore the localized change in [Ca2+]i would be an important factor in determining the efficacy of a Ca2+-dependent inactivation process, as would localized Ca2+ buffering affinity and capacity.
In contrast, repetitive activation of P/Q-type channels results in a transient facilitation. Relief of G-protein-mediated suppression of P/Q-type Ca2+ channels expressed in HEK 293 cells (Brody et al. 1997) was achieved with AP-shaped depolarizations (2–4 times longer than APs in the calyx of Held; Borst & Sakmann, 1995). In the presence of modulators that suppress P/Q-type channels via G-proteins, therefore, repetitive firing could facilitate Ca2+ influx. Without G-protein activation, these Ca2+ currents remained stable during brief AP bursts at 100 Hz. In contrast, native P-type channels in the calyx of Held facilitated in a G-protein-independent manner. Brief, 1 ms depolarizing steps facilitated currents in a Ca2+-dependent manner (Cuttle et al. 1998) maximally at intervals < 5–10 ms and significantly up to 50–100 ms. Again differences between reconstituted and native channels might result from subunit composition, associated cellular proteins and/or intracellular milieu. With more prolonged repetitive stimulation, however, these channels expressed accumulating inactivation (Forsythe et al. 1998), which might contribute to the more slowly developing frequency-dependent synaptic depression (Fig. 2 and Thomson et al. 1993a). Whether the kinetics of P/Q-type channels at identified cortical synaptic sites display G-protein dependent or independent facilitation remains to be determined. P/Q-type channels might, however, contribute to facilitation and post-tetanic potentiation at intervals up to 50–100 ms (at 24–26°C).
To determine whether N-type channel inactivation does indeed underlie this form of depression requires pharmacological investigation. N- and P-type Ca2+ channels are differentially expressed at the terminals of different interneurones in hippocampal slice cultures (Poncer et al. 1997). Whether differential expression of channels at different terminals of a single axon occurs remains unknown, but some other presynaptic proteins have been shown to be selectively expressed, e.g. metabotropic glutamate receptors (Shigemoto et al. 1996).
Functional implications
If N-type Ca2+ channels are responsible, they must dominate only at specific synapses and require subcellular compartmentalization (Doughty et al. 1998). Calcium channels are an ideal target for selective synaptic modification. With a 3rd-4th power relation between Ca2+ entry and transmitter release probability, small changes in Ca2+ influx exert a dramatic influence on release (Dodge & Rahamimoff, 1967; Augustine & Charlton, 1986; Stanley, 1985, 1986, 1995). This novel mechanism will filter only very high frequency transmission and only at synapses onto selected targets. Moreover, ‘slow transmitters’ have selective actions on Ca2+ channels. N-type channels are modulated by muscarinic and metabotropic glutamate receptor (mGluR) agonists and by adenosine (Vazquez et al. 1995; Qian & Saggau, 1997; Vazquez & Sanchez-Prieto, 1997), R-type by mGluR and GABAB agonists (Wu et al. 1998) and P-type Ca2+ channels by noradrenergic agonists (Huang et al. 1998). Slow transmitters may therefore also affect the frequency-filtering properties of specific synaptic connections.
Acknowledgments
This work was supported by The Wellcome Trust, the MRC and Novartis Pharma.
References
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. The Journal of Physiology. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. The Journal of Physiology. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and postsynaptic response at the squid giant synapse. The Journal of Physiology. 1986;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. The Journal of Physiology. 1970;206:620–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurones in stratum oriens of the hippocampal CA1 subfield: structural basis of feedback activation. European Journal of Neuroscience. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Brody DL, Patil PG, Mulle JG, Snutch TP, Yue DT. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. The Journal of Physiology. 1997;499:637–644. doi: 10.1113/jphysiol.1997.sp021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Szilagyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus. 1996;6:294–305. doi: 10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in the rat brainstem. The Journal of Physiology. 1998;512:723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex: Paired intracellular recordings with biocytin filling. Neuroscience. 1995;69:739–755. doi: 10.1016/0306-4522(95)00288-t. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: Dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Cooperative action of calcium ions in transmitter release at the neuromuscular junction. The Journal of Physiology. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty J, Barnes-Davis M, Rusznak Z, Tarasztosi C, Forsythe ID. Contrasting calcium channel subtypes at cell bodies and synaptic terminals of rat anterior cochlear bushy neurones. The Journal of Physiology. 1998;512:365–376. doi: 10.1111/j.1469-7793.1998.365be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends in Neurosciences. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurones of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Huang CC, Wang SJ, Gean PW. Selective enhancement of P-type calcium currents by isoproterenol in the rat amygdala. Journal of Neuroscience. 1998;18:2276–2282. doi: 10.1523/JNEUROSCI.18-06-02276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Further study of the role of calcium in synaptic transmission. The Journal of Physiology. 1970;207:789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindin D28k-immunoreactive neurons in layer V of rat frontal cortex. Journal of Neurophysiology. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Lacaille J-C, Williams S. Membrane properties of interneurones in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience. 1990;36:349–359. doi: 10.1016/0306-4522(90)90431-3. [DOI] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects 2 classes of hippocampal interneurons and potentiates excitatory synaptic transmission. Journal of Neuroscience. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlin EM. The statistics of transmitter release at chemical synapses. In: Parker R, editor. Neurophysiology III, (International Review of Physiology Series) Vol. 17. Baltimore: University Park; 1978. pp. 49–117. [PubMed] [Google Scholar]
- McNaughton NCL, Bleakman D, Randall AD. Electrophysiological characterisation of the human N-type Ca2+ channel II. Activation and inactivation by physiological patterns of activity. Neuropharmacology. 1998;37:67–81. doi: 10.1016/s0028-3908(97)00153-6. [DOI] [PubMed] [Google Scholar]
- McNaughton NCL, Randall AD. Electrophysiological properties of human N-type Ca2+ channel I. Channel gating in Ca2+, Ba2+ and Sr2+ containing solutions. Neuropharmacology. 1997;36:895–915. doi: 10.1016/s0028-3908(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Markram H. Network of tufted layer 5 pyramidal neurons. Cerebral Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. The Journal of Physiology. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signalling via the same axon of neocortical pyramidal neurons. Proceedings of the National Academy of Sciences of the USA. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gähwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18:463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Presynaptic inhibition of synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx. British Journal of Pharmacology. 1997;122:511. doi: 10.1038/sj.bjp.0701400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JDB, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Evidence for 4 calcium activated sites involved in transmitter release and facilitation at the squid giant synapse. Biological Bulletin. 1985;167:532. [Google Scholar]
- Stanley EF. Decline in calcium cooperativity as the basis of facilitation at the squid giant synapse. Journal of Neuroscience. 1986;6:782–789. doi: 10.1523/JNEUROSCI.06-03-00782.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. Calcium entry and the functional organization of the presynaptic transmitter release site. In: Wheal HV, Thomson AM, editors. Excitatory Amino Acids and Synaptic Transmission. UK: Academic Press; 1995. pp. 17–27. [Google Scholar]
- Stea A, Dubel SJ, Pragnell M, Leonard JP, Campbell KP, Snutch TP. A β-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel α1-subunit. Neuropharmacology. 1993;32:1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. The Journal of Physiology. 1997;502:131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends in Neurosciences. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: Dual intracellular recordings in vitro. Cerebral Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. Journal of Neurophysiology. 1993a;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993b;54:347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC. Fluctuations in pyramid-pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993;54:329–346. doi: 10.1016/0306-4522(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Deuchars J. Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurons in slices of rat neocortex. Neuroscience. 1995;69:727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- Vazquez E, Herrero I, Miras-Portugal MT, Sanchez-Prieto J. Developmental change from inhibition to facilitation in the presynaptic control of glutamate exocytosis by metabotropic glutamate receptors. Neuroscience. 1995;68:117–124. doi: 10.1016/0306-4522(95)00121-x. [DOI] [PubMed] [Google Scholar]
- Vazquez E, Sanchez-Prieto J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. European Journal of Neuroscience. 1997;9:2009–2018. doi: 10.1111/j.1460-9568.1997.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proceedings of the National Academy of Sciences of the USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]


