Abstract
The coeliac plexus can organize a gastroduodenal inhibitory reflex without action potentials. The involvement of the nitric oxide-cGMP pathway in this reflex was investigated in the rabbit on an in vitro preparation of the coeliac plexus connected to the stomach and duodenum. Intraluminal duodenal pressures were measured with water-filled balloons. Gastric distension inhibited duodenal motility, thus characterizing a gastroduodenal inhibitory reflex organized by the coeliac plexus.
l-Arginine, superfused at the coeliac plexus level, enhanced this reflex, whereas Nω-nitro-l-arginine (l-NOARG) or 2-(4-carboxyphenyl)-4,4,5,5 tetramethylimidazoline-1-oxyl-3-oxide (carboxy PTIO) reduced or abolished it. Moreover, diethylamine/nitric oxide complex superfused at the coeliac plexus level inhibited duodenal motility in the absence of gastric distension.
The effects of nitric oxide were mediated through the activation of guanylyl cyclase, as 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) reduced or abolished the gastroduodenal inhibitory reflex, whereas zaprinast enhanced it. Moreover, 8-bromo-cGMP and cGMP, superfused at the coeliac plexus level, inhibited duodenal motility in the absence of gastric distension.
On the other hand, when perfused at the visceral level, l-NOARG, propranolol plus phentolamine, and guanethidine did not affect the reflex. Thus, neither nitric oxide nor noradrenaline could be the transmitters released at the muscular level to induce this reflex.
Our study demonstrates that the gastroduodenal inhibitory reflex, which is organized by the coeliac plexus without action potentials, is induced by the release within the plexus of nitric oxide acting on the cGMP pathway. These results provide new insights into the control of digestive motility by the prevertebral ganglia.
The autonomic nervous system is involved in the regulation of visceral function, including the regulation of digestive tract motility. One characteristic of this system is that the nervous regulatory structures are not localized exclusively in the cerebrospinal axis. From a classic point of view, it could be described as having an intrinsic level of regulation located in the viscera and an extrinsic level of regulation in the prevertebral ganglia and central nervous structures, with each level of regulation having its own integrative properties.
The prevertebral ganglia, which are part of the sympathetic system, have long been considered as a simple relay on the efferent central pathway. During the last 20 years, numerous studies devoted to the prevertebral ganglia have shown that they behave as true integrative nervous structures able to organize intra- and interorgan regulation.
Experiments performed on in vitro preparations consisting of prevertebral ganglia connected to part of the digestive tract have revealed the involvement of these ganglia in the organization of gastrointestinal reflexes. Kreulen & Szurszewski (1979) showed that an inhibitory reflex occurred between two segments of the colon connected only via the coeliac ganglion and the inferior mesenteric ganglion by the mesenteric nerves. This reflex disappeared after sectioning of the intermesenteric nerves. Inhibitory gastrointestinal reflexes organized by the coeliac plexus have also been demonstrated in in vitro models consisting of isolated stomach and duodenum connected only to the coeliac plexus by nervous rami (Kreulen et al. 1983; Mazet et al. 1993b).
In a previous study (Mazet et al. 1993b), we demonstrated that a delayed and long-lasting gastrointestinal inhibitory reflex was organized by the coeliac plexus. This nervous reflex involved an unconventional and complex mechanism. The reflex was, in fact, still present when the coeliac plexus was superfused with TTX or when the nervous rami connecting the coeliac plexus to the viscera were superfused with a Na+-free solution or a Ca2+ blocker. These results would exclude any involvement of action potentials in the organization of this reflex. Moreover, the fact that this gastrointestinal reflex could not be induced when the coeliac plexus was superfused with a low Ca2+-high Mg2+ solution indicated that Ca2+ was needed in its organization. These results strongly suggested that a synaptic relay was involved at the coeliac level during the genesis of the reflex.
The striking features of this nervous reflex led us to investigate the possible involvement of an unconventional transmitter, such as nitric oxide, in the organization of the gastrointestinal inhibitory reflex at the coeliac level.
METHODS
Experimental animals and in vitro preparation
Forty-seven rabbits (Marras, Lambesc, France) of either sex ranging from 1.5 to 2.0 kg in weight were used. The animals were stunned and killed by exsanguination. All procedures were approved by the French Ministry of Agriculture and were in agreement with the European Communities Council Directive (86/609/EEC). The stomach and duodenum were dissected out still connected to the coeliac plexus (coeliac and superior mesenteric ganglia). A myotomy was performed in the pyloric region to interrupt the enteric nervous pathways between the stomach and duodenum. This preparation was fixed in an organ bath with two adjacent compartments: one received the viscera (stomach and duodenum), and the other the coeliac plexus (Mazet et al. 1989, 1993b). These compartments could be perfused separately with drugs. In some experiments, in addition to the portion of duodenum connected to the coeliac plexus, an isolated segment of duodenum was also placed in the organ bath receiving the viscera. The aim of this was to compare the effects of gastric distension between innervated and isolated segments of duodenum. The organ bath was superfused with a modified Krebs solution with the following composition (mM): 120 NaCl, 5 KCl, 1 NaH2PO4, 2.5 CaCl2, 1 MgSO4, 25 NaHCO3 and 11 glucose (osmotic pressure, 320 mosmol kg−1). The solution was aerated with 95 % O2-5 % CO2 to a final pH of 7.4. Experiments were carried out at 30–32°C.
Pressure measurements
Intraluminal duodenal pressure was measured with a water-filled balloon ligated around the tip of a catheter. The balloon (1–3 ml) was placed in the duodenum. Duodenal pressure was measured with a piezoresistive Gould pressure transducer (DTRX-Spectramed) connected to the free tip of the catheter.
Gastric distension
A water-filled balloon (20–30 ml) was placed in the whole stomach. Gastric distensions were performed by adding 50 ml of water in the balloon for 5 min.
Drugs used
The drugs used were: L-arginine, Nω-nitro-L-arginine (l-NOARG), diethylamine/nitric oxide complex sodium (DEA/NO), 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), guanosine 3′,5′-cyclic monophosphate (cGMP), guanethidine, phentolamine, propranolol (all from Sigma) and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy PTIO), 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) and 2-(2-propyloxyphenyl)-8-azapurin-6-one (zaprinast) (all from Tocris Cookson). Drugs were dissolved in Krebs solution except: zaprinast, which was first dissolved in 0.1 N NaOH then diluted in Krebs solution readjusted to pH 7.4; ODQ, which was dissolved in dimethyl sulphoxide (DMSO); and cGMP, which was dissolved in 9 % NaCl. Superfusion of the coeliac plexus with DMSO alone at 0.2 % (final concentration) or NaCl alone did not affect duodenal motility.
Statistical analysis
The effects of gastric distension were analysed from the mean amplitude of the duodenal contractions. For statistical analysis, the mean values of this parameter before and after distension were then compared using Student's bilateral unpaired t test, including variance analysis (ANOVA). Values were taken to be statistically different if P < 0.05.
RESULTS
Gastroduodenal inhibitory reflex
At 30–32°C, the duodenum still connected to the coeliac plexus showed spontaneous phasic contractions with a frequency of 10–20 min−1 and an amplitude of 2–5 mmHg. The isolated duodenum also showed spontaneous phasic contractions with a frequency of 8–13 min−1 and an amplitude of 1–4 mmHg; these phasic contractions were more regular in amplitude than those of the innervated duodenum. Under our experimental conditions (superfusion with warm, oxygenated Krebs solution), the preparation was maintained in a satisfactory state for 6–7 h, during which the amplitude of the contractions decreased slowly. This slight diminution with time in the amplitude of the duodenal contractions was taken into account when comparing the successive effects of gastric distensions. For this purpose, the mean amplitude of the duodenal contractions preceding each distension was taken as 100 % and no more than three distensions were performed on the same preparation. After 7 h, the amplitude of duodenal phasic contractions decreased rapidly, indicating a deterioration of the preparation.
Gastric distension (50 ml for 5 min) triggered a decrease of between 14.6 and 55.1 % (mean, 33.2 ± 2.6 %) in the amplitude of the phasic contractions of the duodenum still connected to the coeliac plexus (n = 16). This phenomenon had a latency of 1–7 min (mean, 3.2 ± 0.5 min) and lasted between 4 and 44 min (mean, 16.9 ± 3.5 min); it was named the gastroduodenal inhibitory reflex and was organized by the coeliac plexus (Fig. 1B). In contrast, the same gastric distension induced increases of 24.6 and 84.7 % in the phasic contraction amplitudes of the isolated duodenum (n = 2), with latencies of 2 and 4 min and lasting 17 and 23 min (Fig. 1A). Such an increase in duodenal motility could only be explained by the release of a hormonal substance into the organ bath. It was also recorded in some innervated segments of duodenum, in which case it always occurred prior to the inhibitory reflex (see Figs 2A and 6B).
Figure 1. Effect of gastric distension on contractions of isolated (A) and innervated (B) duodenum from the same preparation.
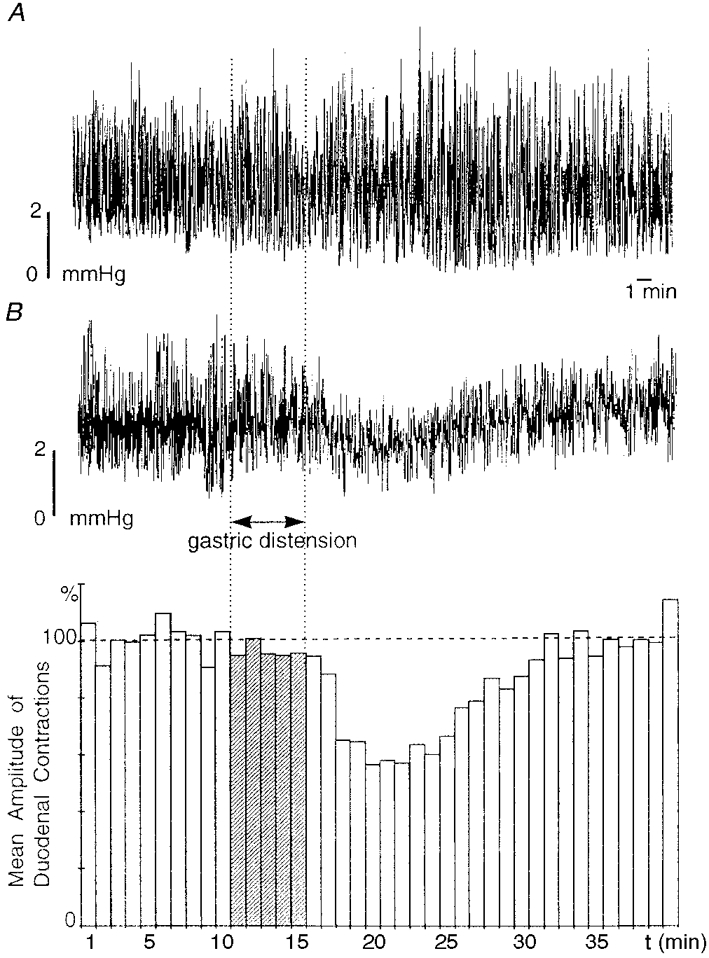
A and B (top) show intraluminal duodenal pressures for isolated and innervated duodenum, respectively. Each column of the histogram in B represents the mean amplitude of duodenal contractions of the innervated duodenum over a 1 min period and the dashed horizontal line indicates the mean amplitude of duodenal contractions over the period preceding the distension shown in the histogram. The vertical dotted lines and the hatched bars of the histogram indicate the period of gastric distension. In A, gastric distension (50 ml, 5 min) triggered an increase in the amplitude of duodenal contractions after a 2 min latency period; this effect could only be explained by the release of a hormonal substance. In B, gastric distension triggered a significant (P ≤ 0.001), long-lasting (19 min) decrease in the amplitude of duodenal contractions after a 3 min latency period; this decrease characterized a gastroduodenal inhibitory reflex.
Figure 2. Effect of superfusion of the coeliac plexus with L-NOARG on the gastroduodenal inhibitory reflex.
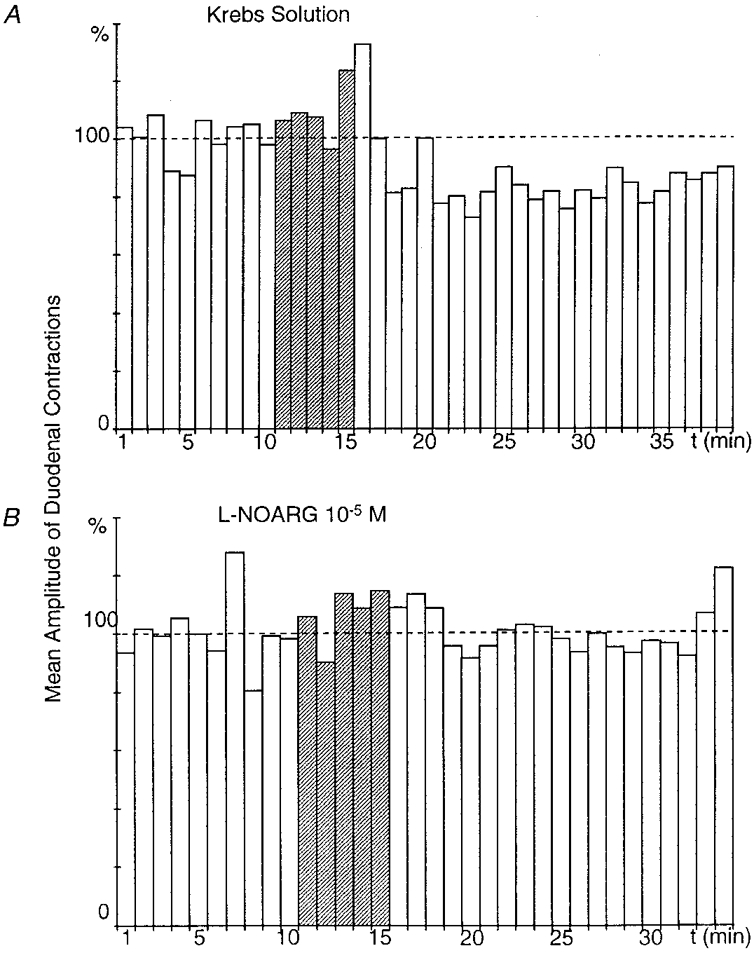
A and B were obtained from the same preparation. Gastric distension (here and in the following figures, 50 ml for 5 min, indicated by the hatched columns) triggered a significant (P ≤ 0.001), long-lasting (22 min) decrease in the amplitude of duodenal contractions after a 7 min latency period (A). When the coeliac plexus was superfused with L-NOARG (10−5 M), the same gastric distension failed to produce the inhibitory response (B).
Figure 6. Effect of superfusion of the coeliac plexus with ODQ on the gastroduodenal inhibitory reflex.
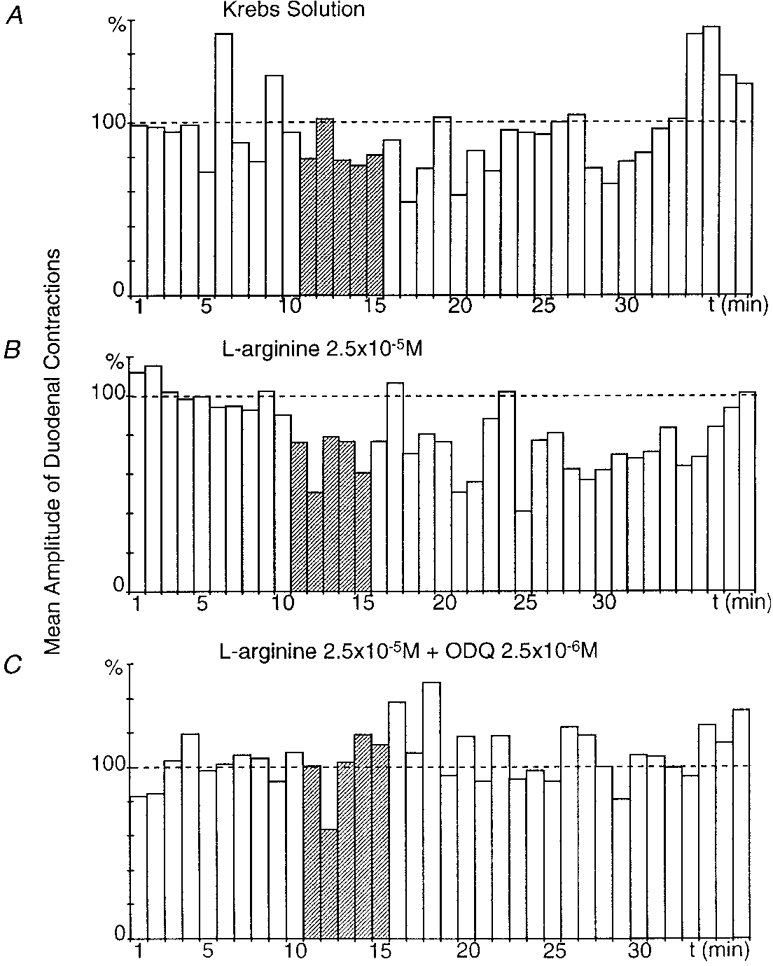
All recordings were obtained from the same preparation. In the presence of Krebs solution, gastric distension produced a slight gastroduodenal inhibitory reflex (P ≤ 0.05) (A). When the coeliac plexus was superfused with L-arginine (2.5 × 10−5 M), gastric distension elicited a significant (P ≤ 0.001), long-lasting (28 min) decrease in the amplitude of duodenal contractions after a 1 min latency period (B). When the coeliac plexus was superfused with L-arginine (2.5 × 10−5 M) + ODQ (2.5 × 10−6 M), gastric distension failed to produce a gastroduodenal inhibitory reflex (C).
Involvement of NO at the coeliac plexus level during organization of the gastroduodenal inhibitory reflex
Effect of L-NOARG
L-NOARG (10−5 M), an inhibitor of NO synthase, was tested in five preparations. In all of these, gastric distension (50 ml, 5 min) during superfusion of the coeliac plexus with normal Krebs solution triggered a decrease in amplitude of the duodenal contractions of between 27.3 and 52 % (mean, 38.3 ± 4.3 %), which characterized the gastroduodenal inhibitory reflex. The same gastric distension performed while the coeliac plexus was superfused for at least 15 min with L-NOARG triggered a decrease of only 0–44.3 % (mean, 17.8 ± 8.3 %), which revealed an inhibition of the gastroduodenal inhibitory reflex of 58.4 ± 17.5 % (Fig. 2).
Effect of L-arginine
L-Arginine (5 × 10−4 M), a precursor of NO, was tested on eight preparations. In five, gastric distension (50 ml, 5 min) performed while the coeliac plexus was superfused with normal Krebs solution decreased the amplitude of duodenal contractions by between 20.5 and 46.2 % (mean, 29.6 ± 4.6 %). The same gastric distension after 15 min superfusion of the coeliac plexus with L-arginine reduced the contractions by between 34.4 and 71.1 % (mean, 48 ± 6.4 %) and increased the gastroduodenal inhibitory reflex by 77.8 ± 39.3 % (Fig. 3).
Figure 3. Effect of superfusion of the coeliac plexus with L-arginine on the gastroduodenal inhibitory reflex.
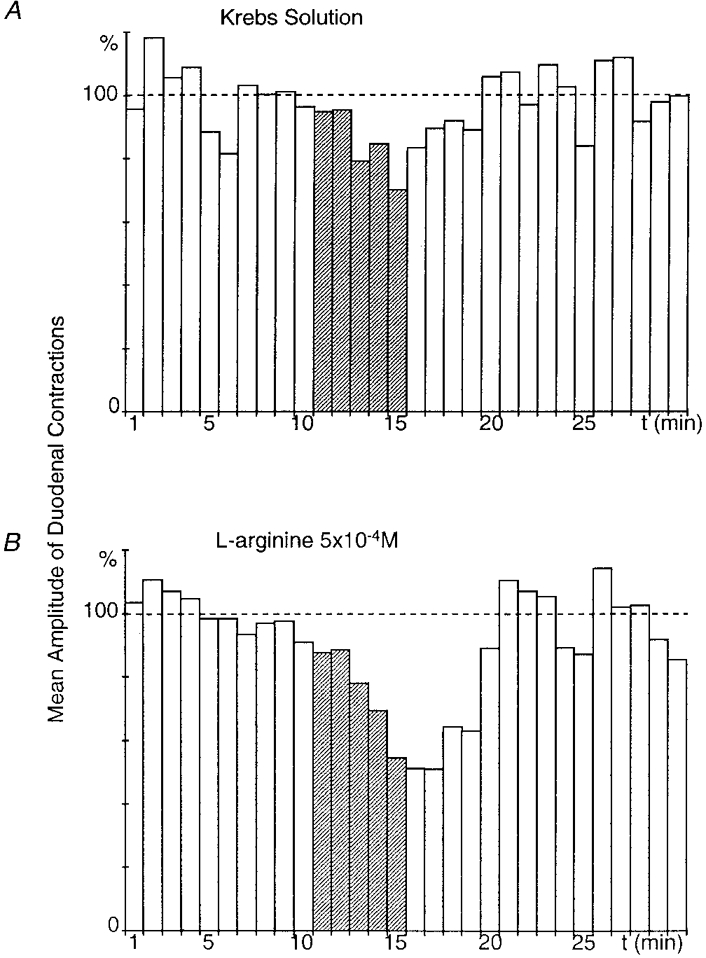
A and B were obtained from the same preparation. Gastric distension triggered a significant (P ≤ 0.01), long-lasting (7 min) decrease in the amplitude of duodenal contractions after a 3 min latency period (A). When the coeliac plexus was superfused with L-arginine (5 × 10−4 M), the amplitude of the inhibitory response was enhanced (P≤ 0.001) (B).
In three preparations, gastric distension (50 ml, 5 min) in the presence of normal Krebs solution at the coeliac plexus level failed to produce the reflex. In these cases, after superfusion of the coeliac plexus with L-arginine for 15 min, the same gastric distension triggered a decrease in the duodenal contraction amplitude of between 20 and 46 % (mean, 33 ± 7.5 %) and elicited the occurrence of the gastroduodenal inhibitory reflex.
The effect of L-arginine makes it clear that the variability in the occurrence of the reflex depends on the preparation and is also linked directly to the availability of NO in the coeliac plexus. When the required level of NO is reached, a marked reflex reaction is triggered when the coeliac plexus is superfused with normal Krebs solution. In contrast, a weak reflex or no reflex indicates that the level of endogenous NO inside the coeliac plexus is too low. In such cases, superfusion of the coeliac plexus with L-arginine enhances or allows the occurrence of the reflex.
Effect of carboxy PTIO
Carboxy PTIO (1.5 × 10−6 M), a NO scavenger, was tested in three preparations. After superfusion of the coeliac plexus with L-arginine (5 × 10−4 M) for 15 min, gastric distension (50 ml, 5 min) reduced the amplitude of duodenal contractions by between 37.9 and 54.5 % (mean, 47 ± 4.7 %) in all these preparations (Fig. 4A).
Figure 4. Effect of superfusion of the coeliac plexus with carboxy PTIO on the gastroduodenal inhibitory reflex.
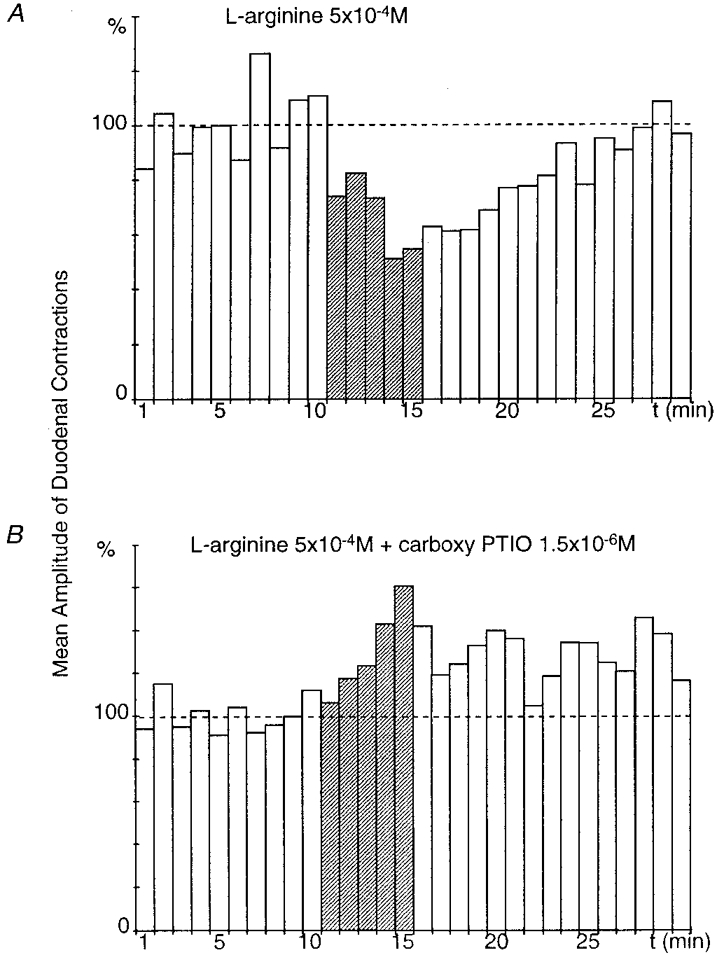
A and B were obtained from the same preparation. Gastric distension performed while the coeliac plexus was superfused with L-arginine (5 × 10−4 M) triggered a significant (P≤ 0.001), long-lasting (16 min) decrease in the amplitude of duodenal contractions after a 1 min latency period (A). When the coeliac plexus was superfused with L-arginine (5 × 10−4 M) + carboxy PTIO (1.5 × 10−6 M), the inhibitory response was abolished and gastric distension elicited an excitatory response (B).
The coeliac plexus was then superfused with L-arginine (5 × 10−4 M) and carboxy PTIO (1.5 × 10−6 M). In this case, after gastric distension the decrease in amplitude of duodenal contractions was only by between 0 and 25.5 % (mean, 8.3 ± 8.3 %), which shows an inhibition of the gastroduodenal inhibitory reflex of 84.3 ± 15.7 % (Fig. 4B). In two out of the three preparations, abolition of the inhibitory reflex revealed a facilitation of duodenal motility after gastric distension.
These results demonstrate that NO is involved at the level of the coeliac plexus during the organization of the gastroduodenal inhibitory reflex.
Effect of DEA/NO
DEA/NO (4 × 10−5 M), a NO donor, was tested in three preparations. In all these, DEA/NO superfused for 10 min at the coeliac plexus level induced, in the absence of gastric distension, a decrease in the amplitude of duodenal contractions (range, 51.4–66.9 %; mean, 60.7 ± 4.9 %). This phenomenon had a latency of 1–6 min (mean, 3.3 ± 1.4 min), lasted 24–27 min (mean, 25.3 ± 0.9 min) and mimicked the gastroduodenal inhibitory reflex (Fig. 5).
Figure 5. Effect of superfusion of the coeliac plexus with DEA/NO.
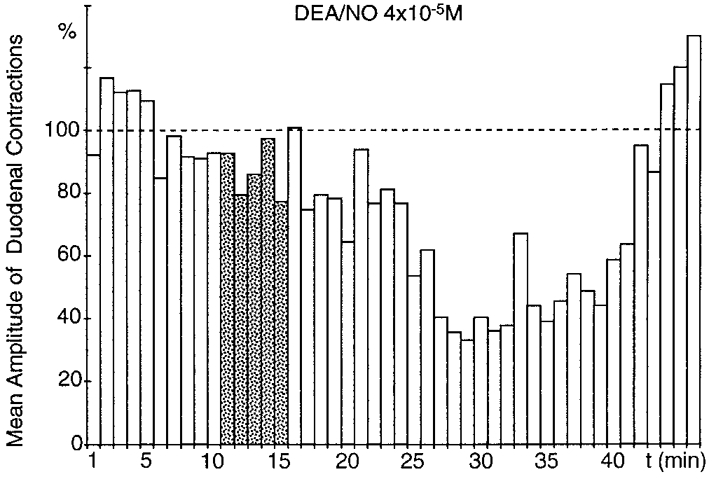
In the absence of gastric distension, superfusion of the coeliac plexus with DEA/NO (4 × 10−5 M; ) triggered a significant (P≤ 0.001), long-lasting (27 min) decrease in the amplitude of duodenal contractions, with a 6 min latency period.
) triggered a significant (P≤ 0.001), long-lasting (27 min) decrease in the amplitude of duodenal contractions, with a 6 min latency period.
This result confirms the involvement of NO within the coeliac plexus during the organization of the gastroduodenal inhibitory reflex.
Involvement of cGMP at the coeliac plexus level during organization of the gastroduodenal inhibitory reflex
Effect of ODQ
ODQ (2.5 × 10−6 M), a selective inhibitor of the NO-activated soluble guanylyl cyclase, was tested in three preparations. Gastric distension (50 ml, 5 min) performed while the coeliac plexus was superfused with normal Krebs solution triggered an inhibition of duodenal contractions ranging from 20.5 to 46.2 % (mean, 29.3 ± 8.4 %) (Fig. 6A). A gastric distension performed after superfusion of the coeliac plexus with L-arginine (2.5 × 10−5 M) for 15 min triggered a decrease in the amplitude of duodenal contractions from 34.4 to 71.1 % (mean, 51.7 ± 10.7 %) in all these preparations, which revealed a facilitation of the gastroduodenal inhibitory reflex (Fig. 6B). Lastly, gastric distensions were performed during superfusion of the coeliac plexus by L-arginine (2.5 × 10−5 M) + ODQ (2.5 × 10−6 M) for 15 min. In all these preparations, gastric distension only produced a decrease in the amplitude of duodenal contractions from 0 to 23.2 % (mean, 7.7 ± 7.7 %), which revealed inhibition of the gastroduodenal inhibitory reflex of 90.7 ± 9.3 % (Fig. 6C).
This leads to the conclusion that within the coeliac plexus, the organization of the gastroduodenal inhibitory reflex involves the NO-cGMP pathway.
Effect of zaprinast
Zaprinast (5 × 10−5 M), a selective inhibitor of the phosphodiesterases involved in the cGMP pathway, was tested on six preparations. In two, gastric distension performed while the coeliac plexus was superfused with normal Krebs solution did not elicit the gastroduodenal inhibitory reflex (Fig. 7A). Therefore gastric distension was performed again after superfusion of the coeliac plexus with L-arginine (5 × 10−4 M) for 10 min; this distension decreased the amplitude of the duodenal contractions in the two preparations by 36.3 % for 8 min and 42 % for 9 min (Fig. 7B). After superfusion of the coeliac plexus with L-arginine (5 × 10−4 M) + zaprinast (5 × 10−5 M) for 10 min, the decrease in amplitude of the duodenal contractions after gastric distension in the two preparations was 39.2 % for 16 min and 46 % for 14 min (Fig. 7C).
Figure 7. Effect of superfusion of the coeliac plexus with zaprinast on the gastroduodenal inhibitory reflex.
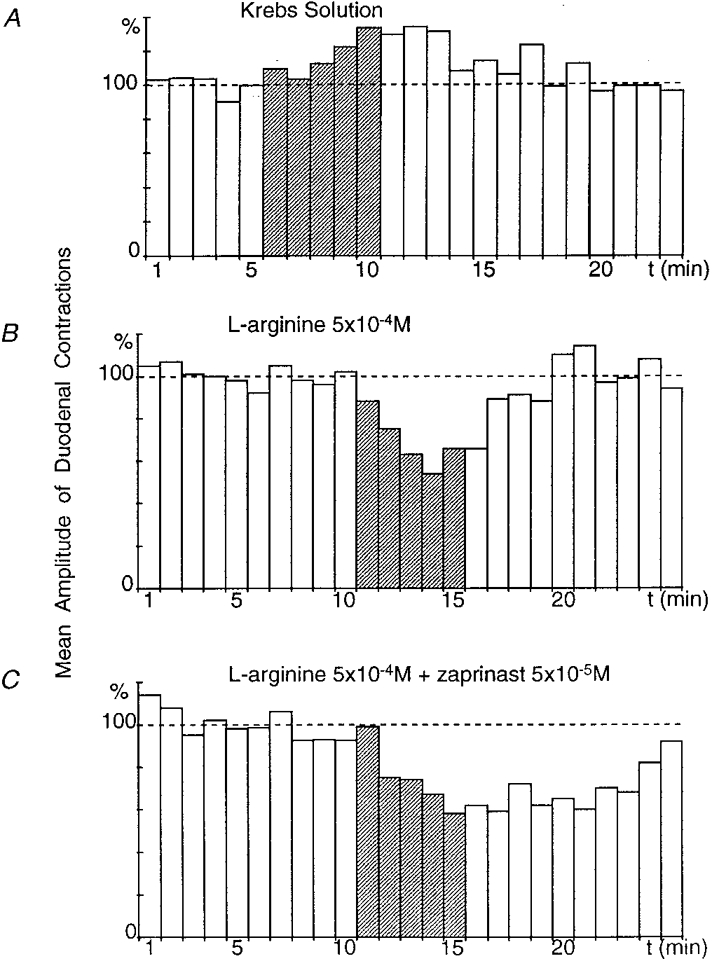
All recordings were obtained from the same preparation. In the presence of Krebs solution, gastric distension (50 ml, 5 min) induced an increase in the amplitude of duodenal contractions over 10 min (A). When the coeliac plexus was superfused with L-arginine (5 × 10−4 M), gastric distension elicited a marked (P≤ 0.001) inhibitory reflex (latency, 1 min and duration, 9 min) (B). In the presence of L-arginine (5 × 10−4 M) + zaprinast (5 × 10−5 M), the duration of the inhibitory reflex was significantly enhanced (14 min) (C).
In four preparations, while the coeliac plexus was superfused by normal Krebs solution gastric distension inhibited the amplitude of duodenal contractions by 20.5 ± 8.1 % over a period of 11.6 ± 4 min. Gastric distensions were then performed after superfusion of the coeliac plexus with zaprinast (5 × 10−5 M) for 10 min; under these conditions, gastric distensions decreased the amplitude of duodenal contractions by 27.8 ± 4.1 % for 14.3 ± 3.3 min. Finally, in three out of these four preparations, gastric distensions were performed when the coeliac plexus was superfused with zaprinast (5 × 10−5 M) + L-NOARG (5 × 10−5 M). This led to abolition of the gastroduodenal inhibitory reflex (not shown). These results demonstrate that zaprinast enhances the amplitude and duration of the gastroduodenal inhibitory reflex and confirms the involvement of the cGMP pathway in the coeliac plexus during organization of the reflex.
Effects of 8-Br-cGMP and cGMP
8-Br-cGMP (2 × 10−4 M) was tested in four preparations. In all of these, 8-Br-cGMP superfused for 10 min at coeliac plexus level triggered, in the absence of gastric distension, a decrease in amplitude of the duodenal contractions (range, 35.4 to 46.3 %; mean, 39 ± 2.5 %). This phenomenon had a latency of 8–13 min (mean, 10.25 ± 1.1 min), lasted from 6–20 min (mean, 16 ± 3.4 min) and mimicked the decrease in the duodenal motility occurring during gastric distension (Fig. 8).
Figure 8. Effect of superfusion of the coeliac plexus with 8-Br-cGMP.
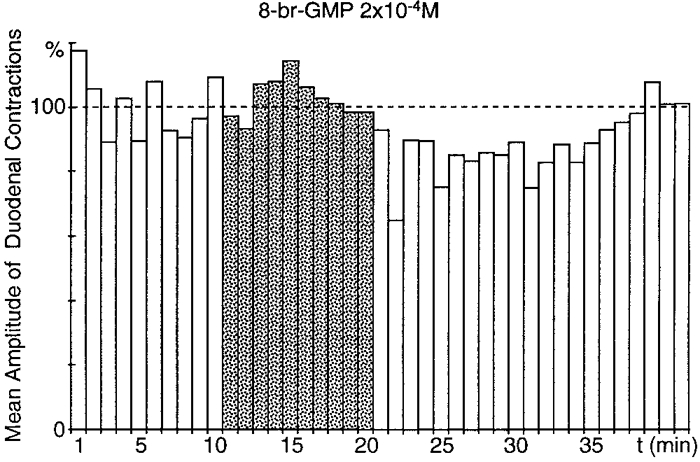
In the absence of gastric distension, superfusion of the coeliac plexus with 8-Br-cGMP (2 × 10−4 M; ) triggered a significant (P≤ 0.001), long-lasting (20 min) decrease in the amplitude of duodenal contractions, with an 8 min latency period.
) triggered a significant (P≤ 0.001), long-lasting (20 min) decrease in the amplitude of duodenal contractions, with an 8 min latency period.
cGMP (2 × 10−4 M) was also tested in the same way on four preparations, where it triggered a decrease in amplitude of the duodenal contractions. The characteristics of this decrease were as follows: amplitude, range 33.5–50.8 % (mean, 40.3 ± 3.8 %); latency, 1–8 min (mean, 3.25 ± 1.6 min) and duration, 9–21 min (mean, 13.3 ± 2.7 min).
These results strengthen our conclusions concerning the involvement of the cGMP pathway within the coeliac plexus during organization of the gastroduodenal inhibitory reflex. The fact that cGMP itself, when superfused at the coeliac plexus level, triggered inhibition of duodenal motility is puzzling since this compound is not generally known to be membrane permeable. However, we found the action of cGMP easily reproducible, which could indicate that it had some degree of permeability. This intriguing result may be useful for a better understanding of the action of this molecule.
Lack of involvement of NO or noradrenaline at duodenal level during the gastroduodenal inhibitory reflex
Effect of L-NOARG
In four preparations, L-NOARG (10−4 M) was superfused at the visceral level 25 min before gastric distension. Under these conditions, gastric distension decreased the amplitude of the duodenal contractions by 23.8–64.6 % (mean, 45.5 ± 8.4 %) (Fig. 9A). This reflex had a latency of 1–5 min (mean, 3.25 ± 0.9 min) and lasted from 9–20 min (mean, 14.8 ± 2.4 min).
Figure 9. Effect of superfusion of the stomach and duodenum with a NO synthase inhibitor (L-NOARG), with adrenoceptor antagonists (phentolamine + propranolol) and with an inhibitor of noradrenaline release (guanethidine).
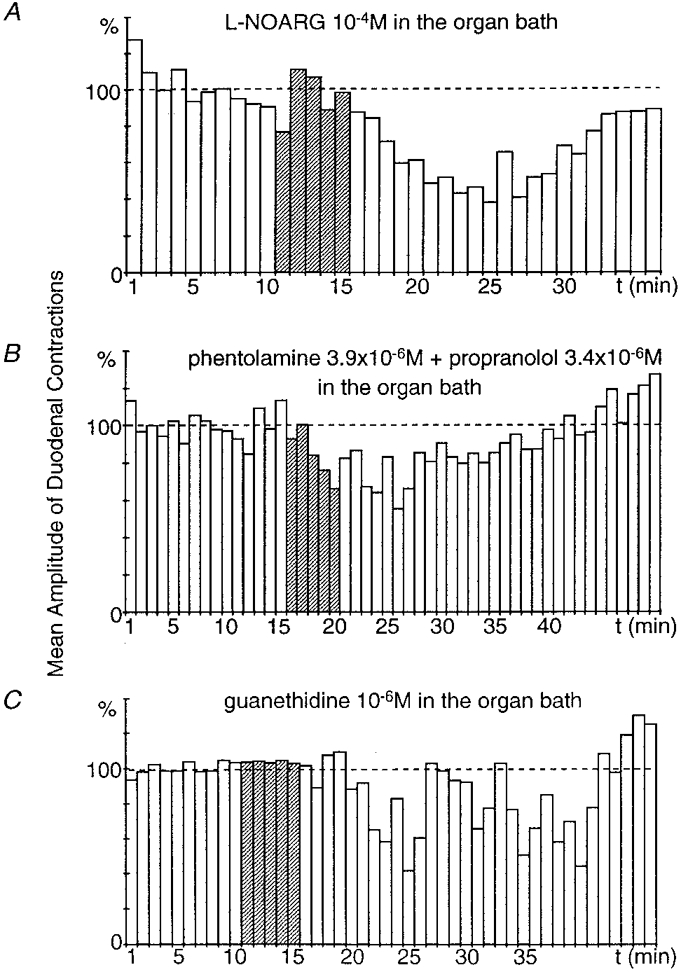
The recordings were obtained from 3 different preparations. When the viscera were superfused with L-NOARG (1 × 10−4 M), gastric distension elicited a significant (P ≤ 0.001), long-lasting (19 min) inhibitory reflex (A). In the presence of phentolamine (3.9 × 10−6 M) + propranolol (3.4 × 10−6 M) at the visceral level, gastric distension still produced a significant (P ≤ 0.001), long-lasting (23 min) inhibitory reflex (B). When the viscera were superfused with guanethidine (10−6 M), gastric distension still produced a significant (P≤ 0.001), long-lasting (22 min) inhibitory reflex (C).
This leads to the conclusion that NO is not the transmitter released at muscular level that induces the inhibition of duodenal motility.
Effects of phentolamine + propranolol and guanethidine
In two preparations, phentolamine (3.9 × 10−6 M; an α-adrenoceptor antagonist) and propranolol (3.4 × 10−6 M; a β-adrenoceptor antagonist) were superfused concomitantly at the visceral level 30 min before gastric distension. Under these conditions, gastric distension decreased the amplitude of the duodenal contractions by 36.2 and 44.4 % (Fig. 9B). These reflexes lasted 22 and 25 min and had a latency of 1 and 2 min.
In two preparations, guanethidine (10−6 M; an inhibitor of noradrenaline release) was superfused at the visceral level 1 h before gastric distension. Despite this superfusion, gastric distension decreased the amplitude of the duodenal contractions by 53.4 and 58.5 % (Fig. 9C). These reflexes lasted 13 and 22 min and had a latency of 1 and 9 min.
These results allow us to rule out the involvement of noradrenergic neurotransmission, and indicate that noradrenaline is not the transmitter released at duodenal level and inducing the inhibitory reflex.
DISCUSSION
The present study has demonstrated that within the coeliac plexus, the release of NO is involved in the organization of a gastroduodenal inhibitory reflex by activation of the cGMP pathway. However, NO is not the neurotransmitter released at muscular level that induces inhibition of duodenal motility during this reflex.
Characteristics of the gastroduodenal inhibitory reflex
This reflex presented wide variations in amplitude, latency and duration from one preparation to another. This may be related to the fact that the reflex was characterized by a modification of intestinal motility. Under our in vitro experimental conditions, this motility reflects the myogenic, myenteric and coeliac ganglionic activity. The intensity of activity of each of these levels may differ depending on the preparations, as well as the level of activity of NO synthase and the available amount of L-arginine. Under these conditions, it is not surprising that the same stimulus (gastric distension, 50 ml for 5 min) applied to different preparations affected intestinal motility with some variability. However, despite this variability, the occurrence of the reflex in most of the preparations made it possible to perform the present pharmacological study and underlines the importance of the mechanism sustaining this reflex in the control of intestinal motility.
The coeliac ganglia are considered to exert an inhibitory drive on intestinal activity. The gastroduodenal reflex described in this paper could represent an increase in this tonic inhibitory drive, which would be in accordance with the classic inhibitory effects exerted on intestinal motility by the sympathetic motor fibres (Roman & Gonella, 1987).
Release of nitric oxide within the coeliac plexus during organization of the gastroduodenal inhibitory reflex
In a previous study, we demonstrated that the gastroduodenal inhibitory reflex is abolished when the coeliac plexus is selectively superfused with a low Ca2+-high Mg2+ solution (Mazet et al. 1993b). We interpreted this result as demonstrating the involvement of a synaptic mechanism. We now offer evidence that NO is the neurotransmitter released at ganglionic level during organization of the reflex. This result is of importance because, so far, only modulatory effects of NO at neuro-neural synapses have been described in central and peripheral nervous structures (Schuman & Madison, 1994; Szabo, 1996; Quinson et al. 1998). Until now, the only known role of NO as a neurotransmitter was at the neuromuscular synapses of vascular or digestive tract smooth muscle cells. The blockade of the gastrointestinal inhibitory reflex when the extracellular calcium concentration was lowered at ganglionic level can be ascribed to the fact that NO released within the coeliac plexus is synthesized by a constitutive neuronal NO synthase. These NO synthase isoforms are activated by calcium and bind calmodulin as an enzyme cofactor (Bredt & Snyder, 1992).
Origin of nitric oxide released in the coeliac plexus
Since the gastroduodenal inhibitory reflex is triggered by mechanical activation of gastric afferent fibres, our results demonstrate that NO is released by these fibres projecting onto the coeliac plexus. The presence of NO-containing nerve fibres in the prevertebral ganglia has been demonstrated by numerous immuno- and histochemical studies (Anderson et al. 1993; Furness & Anderson, 1993; Mazet et al. 1993a; Santer & Symons, 1993; Schmidt et al. 1993). The cell bodies of these neurones are located in the myenteric plexus or dorsal root ganglia. This hypothesis is supported by the presence of nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase-positive neuronal cell bodies in the myenteric plexus and dorsal root ganglia (Aimi et al. 1991; Grozdanovic et al. 1992; Llewellyn-Smith et al. 1992; Aoki et al. 1993; Cracco & Filogamo, 1994). Moreover, studies have been performed using intracellular injections of a fluorescent dye in the coeliac plexus which led to the detection of NO synthase (NOS)-positive and dye-labelled neurones in the nerve plexus and dorsal root ganglia. However, the results of these studies only demonstrate the projection of NOS-containing neurones onto the coeliac plexus from the dorsal root ganglia in the rat (Domoto et al. 1995). There are no data on the projection to the coeliac ganglion of NOS-containing neurones located in the myenteric plexus of the stomach. Studies performed at the visceral level demonstrate only the projection of such neurones from the colon in the rat (Domoto et al. 1995), guinea-pig (Anderson et al. 1995; Mann et al. 1995) and pig (Barbiers et al. 1994). So the hypothesis of a dorsal root localization seems more likely for the cell bodies of the NOS-containing neurones projecting on the coeliac plexus.
Activation of the cGMP pathway in the coeliac ganglionic neurones
Our study shows that at the ganglionic level, NO triggers activation of the cGMP pathway during the organization of the gastroduodenal inhibitory reflex. This result can be explained by the activation of guanylyl cyclase by NO (Vincent, 1995). Although there are no available data on the presence of cGMP and guanylyl cyclase in the coeliac plexus, the present neuropharmacological study strongly suggests that these molecules are present and active. In a previous study on the modulation of nicotinic activation of coeliac ganglionic neurones by NO, we also offered neuropharmacological evidence of the presence and activity of cGMP and guanylyl cyclase in the rabbit coeliac plexus (Quinson et al. 1998). Activation of guanylyl cyclase by NO is frequently reported in studies of the action of NO on neuronal effectors. However, this effect must not be taken as the rule since there is an increasing body of evidence that NO can exert an action on the activity of neurones that is independent of the activation of the cGMP pathway (Hebeiß & Kiebinger, 1996; Ientile et al. 1996; Mothet et al. 1996; Suzuki et al. 1997; Quinson et al. 1998).
Transmitter release at the muscular level during the reflex
Our study has shown that the transmitter released at the muscular level and inducing inhibition of intestinal motility is neither noradrenaline nor NO. The fact that noradrenaline is not involved in inhibition of duodenal motility is surprising, since it is the classic inhibitory transmitter released along the non-sphincteric area of the digestive tract by the sympathetic efferent fibres (Burks, 1994). We have also tested the involvement of NO, which is well known to exert inhibitory effects along the gastrointestinal tract (Stark & Szurszewski, 1992; Burks, 1994; Lefebvre, 1995). So far, the nature of the transmitter triggering muscular inhibition during the gastrointestinal inhibitory reflex has not been determined.
Nature of the activity along the afferent and efferent fibres during organization of the reflex
In a previous study, we have shown that the gastrointestinal inhibitory reflex is organized by the coeliac plexus without action potentials along the afferent and efferent fibres (Mazet et al. 1993b). Moreover, the delay between gastric distension and the occurrence of intestinal inhibition (range, 1–10 min; mean, 3.6 ± 0.3 min) excludes any modification of axonal transport along these fibres in the genesis of this reflex. Thus, the mechanism which occurs along these fibres and which triggers a release of NO within the coeliac plexus and the release of an inhibitory transmitter at muscular level remains unknown, although it may be an enzymatic cascade throughout the neuronal processes. Whatever this mechanism might be, the fact that it is a non-classic one adds to the originality of the involvement of NO as a neurotransmitter at ganglionic level in the organization of this reflex. To our knowledge, the above characteristics of a regulatory reflex have never been described before. Further investigation of the mechanism sustaining the activity of afferent and efferent fibres during organization of the reflex will probably provide new insights into the activity of autonomic neurones.
Functional implications
In a previous study (Quinson et al. 1998), we demonstrated that nerve-induced release of NO could enhance or decrease nicotinic transmission of central origin within the coeliac plexus. In this study, endogenous NO was released after repetitive stimulation of the splanchnic nerves. The existence of this mechanism, considered in the light of the present results, raises the important question of the modulation of central input to the ganglionic neurones by NO released within the coeliac plexus during the organization of the gastroduodenal inhibitory reflex. If the existence of such modulation is proven by further study, it could be a very important step towards the better understanding of the integrative properties of the prevertebral ganglia.
It is well known that prevertebral ganglionic neurones and visceral afferents fire Na+ action potentials, as their spiking activity is abolished by tetrodotoxin or Na+-free solution. However, these fibres also have the property of organizing at the prevertebral level, and without action potentials (Mazet et al. 1993b), the gastroduodenal inhibitory reflex analysed in the present paper. Taken together, these results suggest that visceral afferents and prevertebral neurones have two levels of activity, one involving the genesis of action potentials and probably involved in fast regulation, and the other without action potentials and probably involved in slow regulation. This dual level of activity strengthens the integrative properties of the prevertebral ganglia and opens new perspectives on the study of autonomic neurones.
Acknowledgments
The authors wish to thank G. Ivaldi for the construction of the in vitro set-up, M. Maneville for construction of the electronic devices and M. Paul for revising the English. This work was supported by CNRS and by a grant from Jouveinal, Parke-Davies Laboratories.
References
- Aimi Y, Fujimura M, Vincent SR, Kimura H. Localization of NADPH-diaphorase-containing neurons in sensory ganglia of the rat. Journal of Comparative Neurology. 1991;306:382–392. doi: 10.1002/cne.903060303. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Edwards SL, Furness JB, Bredt DS, Snyder SH. The distribution of nitric oxide synthase-containing autonomic preganglionic terminals in the rat. Brain Research. 1993;614:78–85. doi: 10.1016/0006-8993(93)91020-s. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Furness JB, Woodman HL, Edwards SL, Crack PJ, Smith AI. Characterisation of neurons with nitric oxide synthase immunoreactivity that project to prevertebral ganglia. Journal of the Autonomic Nervous System. 1995;52:107–116. doi: 10.1016/0165-1838(94)00150-i. [DOI] [PubMed] [Google Scholar]
- Aoki E, Takeuchi IK, Shoji R, Semba R. Localization of nitric oxide-related substances in the peripheral nervous tissues. Brain Research. 1993;620:142–145. doi: 10.1016/0006-8993(93)90281-q. [DOI] [PubMed] [Google Scholar]
- Barbiers M, Timmermans JP, Scheuermann DW, Adriaensen D, Mayer B, De Groodt Lassel MH. Nitric oxide synthase-containing neurons in the pig large intestine: topography, morphology, and viscerofugal projections. Microscopy Research and Technique. 1994;29:72–78. doi: 10.1002/jemt.1070290203. [DOI] [PubMed] [Google Scholar]
- Bredt D, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Burks TF. Neurotransmission and neurotransmitters. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3. New York: Raven Press; 1994. pp. 211–242. [Google Scholar]
- Cracco C, Filogamo G. Quantitative study of the NADPH-diaphorase-positive myenteric neurons of the rat ileum. Neuroscience. 1994;61:351–359. doi: 10.1016/0306-4522(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Domoto T, Teramoto M, Tanigawa K, Tamura K, Yasui Y. Origins of nerves fibers containing nitric oxide synthase in the rat coeliac-superior mesenteric ganglion. Cell and Tissue Research. 1995;281:215–221. doi: 10.1007/BF00583390. [DOI] [PubMed] [Google Scholar]
- Furness JB, Anderson CR. Origins of nerve terminals containing nitric oxide synthase in the guinea-pig coeliac ganglion. Journal of the Autonomic Nervous System. 1993;46:47–54. doi: 10.1016/0165-1838(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Baumgarten HG, Bruning G. Histochemistry of NADPH-diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic nervous system of the mouse. Neuroscience. 1992;48:225–235. doi: 10.1016/0306-4522(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Hebeiß K, Kiebinger H. Differential effects of nitric oxide donors on basal and electrically evoked release of acetylcholine from guinea-pig myenteric neurones. British Journal of Pharmacology. 1996;118:2073–2078. doi: 10.1111/j.1476-5381.1996.tb15646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ientile R, Picciurro V, Pedale S, Nucci C, Malecka B, Nistico G, Macaione S. Nitric oxide enhances amino acid release from immature chick embryo retina. Neuroscience Letters. 1996;219:79–82. doi: 10.1016/s0304-3940(96)13163-3. [DOI] [PubMed] [Google Scholar]
- Kreulen DL, Muir TC, Szurszewski JH. Peripheral sympathetic pathways to gastroduodenal region of the guinea pig. American Journal of Physiology. 1983;245:G369–375. doi: 10.1152/ajpgi.1983.245.3.G369. [DOI] [PubMed] [Google Scholar]
- Kreulen DL, Szurszewski JH. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. The Journal of Physiology. 1979;295:21–32. doi: 10.1113/jphysiol.1979.sp012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre RA. Nitric oxide in the peripheral nervous system. Annals of Medicine. 1995;27:379–388. doi: 10.3109/07853899509002591. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJL, Song ZM, Costa M, Bredt DS, Snyder SH. Ultrastructural localization of nitric oxide synthase immunoreactivity in guinea-pig enteric neurons. Brain Research. 1992;577:337–342. doi: 10.1016/0006-8993(92)90294-j. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB, Pompolo S, Mader M. Chemical coding of neurons that project from different regions of intestine to the coeliac ganglion of the guinea pig. Journal of the Autonomic Nervous System. 1995;56:15–25. doi: 10.1016/0165-1838(95)00053-1. [DOI] [PubMed] [Google Scholar]
- Mazet B, Miller S, Ermilou L, Szurszewski JH. Distribution of NADPH-diaphorase in abdominal prevertebral ganglia. Journal of Gastrointestinal Motility. 1993a;5:204. [Google Scholar]
- Mazet B, Miolan JP, Niel JP, Julé Y, Roman C. Modulation of synaptic transmission in the rabbit coeliac ganglia by gastric and duodenal mechanoreceptors. Neuroscience. 1989;32:235–243. doi: 10.1016/0306-4522(89)90122-x. [DOI] [PubMed] [Google Scholar]
- Mazet B, Miolan JP, Niel JP, Roman C. New insights into the organization of a gastroduodenal inhibitory reflex by the coeliac plexus. Journal of the Autonomic Nervous System. 1993b;46:135–146. doi: 10.1016/0165-1838(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Fossier P, Tauc L, Baux G. Opposite actions of nitric oxide on cholinergic synapses: Which pathways? Proceedings of the National Academy of Sciences of the USA. 1996;93:8721–8726. doi: 10.1073/pnas.93.16.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinson N, Catalin D, Miolan JP, Niel JP. Nerve-induced release of NO exerts dual effects on nicotinic transmission within coeliac ganglion in the rabbit. Neuroscience. 1998;84:229–240. doi: 10.1016/s0306-4522(97)00508-3. [DOI] [PubMed] [Google Scholar]
- Roman C, Gonella J. Extrinsic control of digestive tract motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 507–553. [Google Scholar]
- Santer RM, Symons D. Distribution of NADPH-diaphorase activity in rat paravertebral, prevertebral and pelvic sympathetic ganglia. Cell and Tissue Research. 1993;271:115–121. doi: 10.1007/BF00297549. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Dorsey DA, McDaniel ML, Corbett JA. Characterization of NADPH diaphorase activity in rat sympathetic autonomic ganglia - effect of diabetes and aging. Brain Research. 1993;617:343–348. doi: 10.1016/0006-8993(93)91103-y. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Nitric oxide and synaptic function. Annual Reviews of Neuroscience. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Stark ME, Szurszewski JH. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakajima K, Fujimoto K, Fujii T, Kawashima K. Nitric oxide increases stimulation-evoked acetylcholine release from rat hippocampal slices by a cyclic GMP-independent mechanism. Brain Research. 1997;760:158–162. doi: 10.1016/s0006-8993(97)00291-6. [DOI] [PubMed] [Google Scholar]
- Szabo C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Research Bulletin. 1996;41:131–141. doi: 10.1016/0361-9230(96)00159-1. [DOI] [PubMed] [Google Scholar]
- Vincent SR. Nitric Oxide in the Nervous System. London: Academic Press; 1995. [Google Scholar]


