Abstract
Mechanical stimuli are thought to modulate the number of sarcomeres in series (sarcomere number) in skeletal muscle fibres. However, the mechanisms by which muscle cells transduce mechanical signals into serial sarcomere addition have not been explored. In this study, we test the hypothesis that nitric oxide positively modulates sarcomere addition.
The soleus muscle was cast-immobilized in a shortened position in 3-week-old female Wistar rats. After 4 weeks, the casts were removed, creating a period of rapid sarcomere addition. During the remobilization period, nitric oxide synthase (NOS) inhibitors or substrate were administered.
Rats treated with the non-isoform-specific NOS inhibitor L-nitro-arginine methyl ester during 3 weeks of remobilization had smaller soleus sarcomere numbers than control rats. Rats treated with 1-(2-trifluoromethyl-phenyl)-imidazole, which has greater specificity for the neuronal isoform than for the endothelial isoform of NOS, also had smaller soleus sarcomere numbers than control rats. These results suggest that inhibition of the neuronal isoform of NOS reduces sarcomere addition during remobilization.
Rats treated with L-arginine, the substrate for NOS, during 1 week of remobilization had soleus sarcomere numbers for the immobilized-remobilized muscle which were closer to that for the contralateral, non-immobilized muscle than did rats that were not treated with L-arginine.
These results support the hypothesis that nitric oxide derived from the neuronal isoform of NOS positively modulates sarcomere addition.
Increases in the number of sarcomeres in series (sarcomere number) in skeletal muscle myofibrils are important for normal muscle development and function. Such sarcomere addition is necessary for longitudinal muscle growth (Williams & Goldspink, 1971). Sarcomere addition influences muscle force-length (Williams & Goldspink, 1978) and force-velocity properties (Spector et al. 1980). Insufficient sarcomere addition is believed to induce complications in cerebral palsy (O'Dwyer et al. 1989) and during bone lengthening (Simpson et al. 1995). Despite the importance of sarcomere addition, little is known about its regulation.
Mechanical stimuli are thought to be involved in regulating sarcomere addition (Herring et al. 1984; Goldspink, 1985). For example, increasing passive tension in adult skeletal muscle by stretch-immobilization results in sarcomere addition (e.g. Tabary et al. 1972). In addition, increasing excursion in growing muscle by increasing the muscle moment arm, or decreasing excursion by immobilization, results in increased or decreased sarcomere addition, respectively (Williams & Goldspink, 1971; Koh & Herzog, 1998). Although mechanical signals appear to modulate sarcomere addition, the mechanisms by which muscle cells transduce mechanical signals into sarcomere addition have not been explored.
A putative mechanotransducer for sarcomere addition should: (1) be localized at the muscle-tendon junction (MTJ), since this is the primary site of sarcomere addition (Williams & Goldspink, 1971), (2) be responsive to mechanical stimuli such as passive stretch and excursion, which have been shown to modulate sarcomere addition, and (3) produce a signal that acts locally at sites of sarcomere addition. The neuronal isoform of nitric oxide synthase (nNOS) fulfills all three criteria. First, nNOS is located in skeletal muscle cells at the sarcolemma through direct interaction with the dystrophin complex, and is particularly concentrated at the MTJ (Chang et al. 1996). Second, nNOS activity is positively regulated by mechanical activity; static stretch of excised muscle and cyclic stretch of cultured myotubes both increase nNOS activity (Tidball et al. 1998). Third, the short half-life of NO (Lowenstein et al. 1994) would limit its actions to targets close to its site of production, which in muscle would be the region of the MTJ. Together, these data are consistent with the possibility that nNOS may be a mechanotransducer for sarcomere addition.
Our hypothesis is that NO derived from nNOS is a positive modulator of sarcomere addition. The experimental model used in this study was the rat soleus muscle exposed to immobilization followed by remobilization. Such remobilization is associated with increased passive stretch and excursion of the soleus muscle, and has been shown to be associated with rapid sarcomere addition (Williams & Goldspink, 1971). We tested the hypothesis by administering NOS inhibitors and substrate during remobilization to determine whether modulation of NOS activity alters sarcomere addition.
METHODS
Immobilization
Three-week-old female Wistar rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) were anaesthetized with an intraperitoneal injection of sodium pentobarbital (35 mg kg−1). A wire mesh-reinforced plaster cast was constructed to immobilize the right ankle joint in full plantarflexion, without hindering motion at the knee joint. The soleus muscle was thus maintained in a shortened position. Casts were replaced 3 and 7 days after the initial casting, then weekly or as needed thereafter to allow for growth. Following four weeks of immobilization, the casts were removed, and the rats were allowed unrestricted cage activity (remobilization). Rats and tissues from all experiments were treated identically except for specific experimental perturbations that were imposed during the remobilization period. All animal procedures were approved by the Animal Research Committee of the University of California, Los Angeles, USA. At the end of experimentation, rats were killed by an intraperitoneal injection of sodium pentobarbital (100 mg kg−1).
Remobilization
For experiment 1, rats (n = 6) were given the non-isoform-specific NOS inhibitor L-nitro-arginine methyl ester (L-NAME) in their drinking water (0.05 %) during 3 weeks of remobilization to determine whether NO influences sarcomere addition during this period. Oral administration of L-NAME has been shown to produce systemic effects consistent with inhibition of NOS (Huang et al. 1995; Boger et al. 1996). Control rats were given the same concentration of the less active enantiomer D-NAME (n = 6) or plain drinking water (n = 7). Water consumption was measured to determine ingested dosages. The resulting mean dose of L-NAME was 79 mg kg−1 day−1, and that of D-NAME was 84 mg kg−1 day−1.
For experiment 2, rats (n = 14) were injected daily with 1-(2-trifluoromethyl-phenyl)-imidazole (TRIM; 10 mg kg−1i.p. in phosphate-buffered saline, PBS) during 3 weeks of remobilization to determine whether results from experiment 1 may have been due to inhibition of the endothelial isoform of NOS (eNOS), and possibly increased blood pressure and decreased blood flow (Hirai et al. 1994). This dose of TRIM has been shown to inhibit nociceptive responses in mice without affecting blood pressure (Handy et al. 1996), suggesting inhibition of nNOS without influencing eNOS. In addition, this dose of TRIM has been shown not to affect behaviour as assessed by a box-maze procedure (Handy et al. 1996). Control rats were injected with PBS (n = 14).
For experiment 3, rats (n = 5) were given L-arginine, the substrate for NOS, in their drinking water (1.25 %) during 1 week of remobilization to determine whether L-arginine supplementation enhances sarcomere addition during this period. Oral L-arginine supplementation has been reported to increase NO production when L-arginine levels limit NO production (Matsuoka et al. 1996; Pieper et al. 1996; Huk et al. 1997). The resulting mean dose of L-arginine was 2.7 g kg−1 day−1. Control rats were given plain drinking water (n = 5).
Muscle architecture
Sarcomere number was determined essentially as described previously (Koh & Herzog, 1998). Briefly, after an overdose of pentobarbital, the lower limbs were immersed in 8 % paraformaldehyde with the soleus muscles attached to the bones and the ankle joints positioned at 90 deg. After fixation for a minimum of 1 week, each soleus was dissected, blotted dry and weighed. The length of each tibia was measured with vernier calipers. The muscles were placed into 30 % nitric acid for approximately 16 h. Ten fascicles were then teased from the central part of each muscle, and fascicle lengths were measured using a video analysis system (Bioquant, R&M Biometrics, Nashville, TN, USA). Sarcomere length was measured using laser diffraction (beam diameter = 0.8 mm) at the centre of each fascicle and 2 mm from each end. Sarcomere number was estimated by dividing the fascicle length by the mean sarcomere length for the fascicle. Sarcomere number was averaged across fascicles to provide a representative value for each muscle. Repeat measurements using this method have been shown previously to vary by only 2 % (Koh & Herzog, 1998).
Electron microscopy
Soleus muscles from three TRIM-injected and two PBS-injected rats (immobilized 4 weeks, remobilized 1 week) were dissected, pinned at resting length, and fixed in 1.4 % gluteraldehyde in 0.2 M sodium cacodylate buffer at pH 7.2 for 1 h at 4°C. Muscles were cut into ∼5 mm3 pieces containing MTJs from the insertion end of the muscles, and fixed for an additional 30 min. The samples were rinsed in buffer and then fixed in 1 % osmium tetroxide for 40 min at 4°C. Samples were dehydrated in a series of ethanols and embedded in epoxy resin. Thick sections were evaluated by light microscopy to select samples that contained longitudinally oriented fibres and included MTJs. Those samples were sectioned at 70 nm thickness, stained with uranyl acetate and lead citrate, and examined by electron microscopy. Sections that were free from sectioning and processing artifacts were photographed and analysed without knowledge of whether the samples were from immobilized-remobilized limbs or non-immobilized contralateral limbs, or whether the samples were from TRIM-treated or PBS-treated animals.
Dry mass and hydroxyproline assay
Gastrocnemius muscles from eight TRIM-injected and eight PBS-injected animals were dissected, blotted dry and weighed. Muscles were then dried to a constant mass under a vacuum. Dry masses were compared with wet masses to determine water concentration of each muscle. Muscles were then hydrolysed in 6 n HCl at 120°C for 16 h. Hydroxyproline concentrations in the hydrolysates were assayed using the colorimetric method of Kivirikko et al. (1967).
Data analysis
For experiment 1, mean values were compared between groups using one-way analysis of variance. The Student-Neuman-Keuls test was used for post hoc comparisons. For experiments 2 and 3, mean non-normalized values were compared using Student's t test. Values normalized to body weight, or to contralateral values, were compared using the non-parametric Mann-Whitney U test. For all statistical tests, the 0.05 level was taken to indicate statistical significance.
RESULTS
Soleus sarcomere number and mass in L-NAME-, D-NAME- and water-treated rats
The mean soleus sarcomere number for rats given plain drinking water during 3 weeks of remobilization was 42 % larger than that for rats killed immediately after immobilization (Table 1), indicating rapid sarcomere addition during remobilization. Rats given L-NAME and D-NAME in their drinking water during remobilization had 8 and 12 % smaller mean soleus sarcomere numbers, respectively, than rats given plain drinking water (Table 1). There was no statistical difference in sarcomere number between L-NAME- and D-NAME-treated rats.
Table 1.
Comparisons of soleus muscle sarcomere number and mass, body mass and tibia length immediately after immobilization, or after 3 weeks of remobilization during which rats were given plain drinking water, l-NAME (0.05% in water) or d-NAME (0.05% in water)
| Group | Soleus sarcomere number | Soleus mass (mg) | Body mass (g) | Tibia length (mm) |
|---|---|---|---|---|
| Immobilization (n = 6) | 3041 (267) | 24 (5) | 189 (12) | 33.2 (0.4) |
| Water (n = 7) | 4325 (235) | 53 (6) | 248 (16) | 36.1 (0.8) |
| l-NAME (n = 6) | 3967 (334)* | 45 (3)* | 229 (25) | 35.3 (0.9) |
| d-NAME (n = 6) | 3798 (335)* | 52 (6) | 237 (12) | 35.1 (0.2) |
Values shown are means (s.d.).
Mean value significantly different from that for rats given plain drinking water during remobilization (P < 0.05).
Percentage differences in sarcomere numbers between remobilized groups underestimate the effects of the experimental treatments on sarcomere addition during remobilization; the ∼3000 sarcomeres present prior to remobilization (Table 1) mask the effects of the experimental treatments. A better measure of the effects of the experimental treatments would be differences in sarcomere addition during remobilization. However, sarcomere addition per se cannot be measured directly with present techniques, because rats must be killed to measure sarcomere number. Differences in soleus sarcomere number between separate groups of rats (1) following remobilization and (2) immediately after immobilization provides an estimate of sarcomere addition during remobilization. Estimated sarcomere addition for rats treated with L-NAME was reduced by 28 % compared with control rats (Fig. 1). Thus, NOS inhibition substantially reduced sarcomere addition during remobilization.
Figure 1. Estimated soleus sarcomere addition for water-, L-NAME- and D-NAME-treated rats.
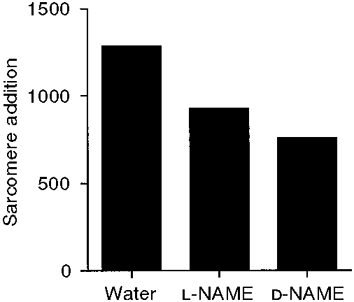
Sarcomere addition was estimated by the difference in mean sarcomere number between (1) each group following remobilization and (2) a group immediately after immobilization.
Mean soleus mass was significantly smaller for L-NAME-treated rats than for control rats (Table 1). Mean soleus mass for D-NAME-treated rats was not statistically different from control, despite the smaller sarcomere number. Mean body mass was not significantly different for any group, suggesting that L-NAME and D-NAME treatments did not affect rat growth (Table 1). Tibia length was not significantly different between groups (Table 1), indicating that NOS inhibition did not reduce longitudinal tibial growth. Daily observation of the rats suggested that none of the experimental treatments in this study (L-NAME, D-NAME, TRIM and L-arginine) influenced rat behaviour; this has been reported previously for the dose of TRIM used in this study (Handy et al. 1996).
Soleus sarcomere number and mass in TRIM- and PBS-treated rats
Rats given daily injections of TRIM during 3 weeks of remobilization had significantly smaller mean soleus sarcomere numbers than PBS-injected rats (Table 2). Estimated sarcomere addition was reduced by 22 % in TRIM- compared with PBS-injected rats (Fig. 2). Thus, nNOS inhibition reduced sarcomere addition during the remobilization period.
Table 2.
Comparisons of soleus muscle sarcomere number and mass, body mass and tibia length for rats given injections of PBS or TRIM (10 mg kg−1 in PBS) during 3 weeks of remobilization
| Group | Soleus sarcomere number | Soleus mass (mg) | Body mass (g) | Tibia length (mm) |
|---|---|---|---|---|
| PBS (n = 14) | 4449 (236) | 59 (8) | 234 (22) | 36.3 (0.5) |
| TRIM (n = 14) | 4142 (337)* | 52 (3)* | 219 (16)* | 36.2 (0.8) |
Values shown are means (s.d.).
Mean value significantly different from that for PBS-injected rats (P < 0.05).
Figure 2. Estimated soleus sarcomere addition for PBS- and TRIM-treated rats.
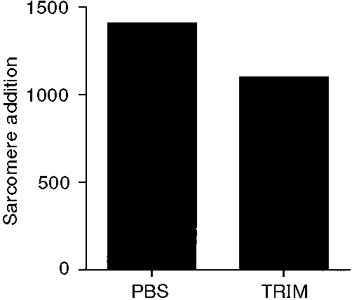
Sarcomere addition was estimated by the difference in mean sarcomere number between (1) each group following remobilization and (2) a group immediately after immobilization.
Mean soleus mass was significantly smaller for TRIM-injected rats than for PBS-injected rats (Table 2). However, body mass was also significantly smaller for TRIM- than for PBS-injected rats. The ratio of soleus mass to body mass was then taken as a measure of soleus mass independent of possible confounding effects of body mass. There was a trend of a smaller ratio for TRIM-injected rats than for PBS-injected rats (0.236 ± 0.019 vs. 0.251 ± 0.031, respectively; P = 0.07). Tibia length was not significantly different between groups (Table 2), indicating that TRIM treatment did not reduce longitudinal tibial growth.
Soleus MTJ ultrastructure in TRIM- and PBS-treated rats
Electron micrographs of longitudinal sections of muscle fibres were analysed blindly, without knowledge of whether the samples were from TRIM- or PBS-treated animals, or from the immobilized-remobilized or non-immobilized contralateral limbs. Micrographs were sorted according to differences in sarcomeric organization and ultrastructural signs of elevated intracellular protein synthesis (e.g. high concentrations of ribosomes and polyribosomes). In all cases, micrographs of MTJs obtained from immobilized- remobilized limbs were distinguished from non-immobilized control limbs by the presence of ultrastructural features that were consistent with new sarcomere synthesis, which included the presence of small diameter myofibrils, sarcomeres that were not in register with those of neighbouring myofibrils, reduced MTJ membrane folding, reduced concentration of MTJ subsarcolemmal densities and high concentrations of polyribosomes (Fig. 3). The sarcomeric and myofibril organization of muscles obtained from immobilized-remobilized or non-immobilized limbs appeared identical at sites within the muscle fibre that were at distances greater than 50 to 100 μm from the MTJ membrane, suggesting that the ends of the myofibrils were the exclusive or primary sites of new sarcomere synthesis in the remobilized muscles. No morphological basis for distinguishing between muscles from TRIM- and PBS-treated animals was identified either through blind analysis or during comparisons made following unblinding of the data.
Figure 3. Electron micrographs of longitudinal section through soleus MTJs.
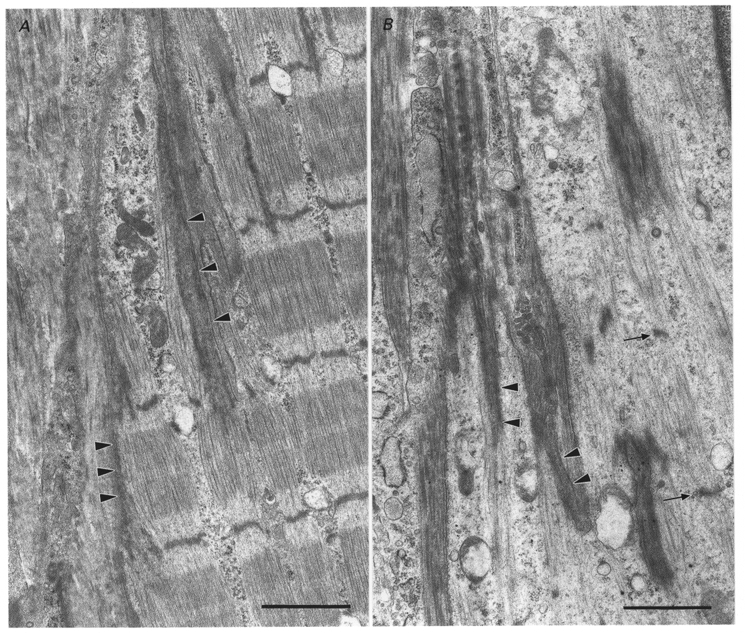
A, muscle from non-immobilized limb of a PBS-treated rat. The tendon lies in the left portion of the micrograph, with the central and right portions showing the MTJ region of a muscle fibre. Thin filaments from the terminal sarcomeres of well-formed myofibrils terminate in subsarcolemmal densities (arrowheads) that are characteristic of MTJs. B, muscle from immobilized-remobilized limb of a PBS-treated rat. The tendon lies along the left edge of micrograph with the remainder of the micrograph showing the MTJ region of a muscle fibre. Sparse thin filaments terminate at subsarcolemmal densities (arrowheads). Dense bodies within the terminal portion of the muscle fibre are incipient Z-disks (arrows) between which lie scant myofilaments. Scale bars, 1.5 μm.
Gastrocnemius mass, water and hydroxyproline concentrations in TRIM- and PBS-treated rats
Gastrocnemius wet mass was 9 % smaller in rats injected with TRIM than in rats injected with PBS (Table 3). Dry mass was 8 % smaller in TRIM-injected than in PBS-injected rats. Water concentration and hydroxyproline concentration were not statistically different between TRIM-injected and PBS-injected rats. Thus, the smaller gastrocnemius mass in the TRIM-treated group was not due to smaller water or connective tissue concentration.
Table 3.
Comparisons of gastrocnemius mass, and water and hydroxyproline concentrations for rats given injections of PBS or TRIM (10 mg kg−1 in PBS) during 3 weeks of remobilization
| Group | Wet mass (mg) | Dry mass (mg) | Water (%) | [Hydroxyproline] (μg mg−1) |
|---|---|---|---|---|
| PBS (n = 8) | 971 (112) | 239 (25) | 75.2 (0.7) | 9.2 (0.6) |
| TRIM (n = 8) | 885 (61)* | 221 (12)* | 75.5 (0.7) | 9.1 (0.7) |
Values shown are means (s.d.).
Mean value significantly different from that for PBS-injected rats (P < 0.05).
Soleus sarcomere number and mass in L-arginine-supplemented rats
Soleus sarcomere number was not significantly different between rats given L-arginine in their drinking water and rats given plain drinking water during 1 week of remobilization (Table 4). Soleus mass, tibia length and body mass were also not significantly different between groups (Table 4). To minimize the variability between rats, sarcomere number and soleus mass were expressed as ratios of values for the right (immobilized/remobilized) leg to those for the left (contralateral, non-immobilized) leg. For sarcomere number, the ratio was significantly larger for L-arginine-supplemented rats than for control rats (Fig. 4). This result suggests that the immobilized-remobilized soleus sarcomere number for L-arginine-supplemented rats had increased closer to the contralateral sarcomere number than had that for control rats. For soleus mass, the ratio was not significantly different between groups, but there was a trend of a larger ratio for L-arginine-treated rats than for control rats (0.50 ± 0.08 vs. 0.42 ± 0.05, respectively; P = 0.08).
Table 4.
Comparisons of soleus muscle sarcomere number and mass, body mass and tibia length for rats given plain drinking water or l-arginine supplementation (1.25% in water) during 1 week of remobilization
| Group | Soleus sarcomere number | Soleus mass (mg) | Body mass (g) | Tibia length (mm) |
|---|---|---|---|---|
| Water (n = 5) | 3378 (235) | 33 (6) | 214 (15) | 33.6 (1.0) |
| l-Arginine (n = 5) | 3438 (227) | 36 (6) | 203 (11) | 33.4 (0.5) |
Values shown are means (s.d.).
Figure 4. Soleus sarcomere number ratio for water- and L-arginine-treated rats.
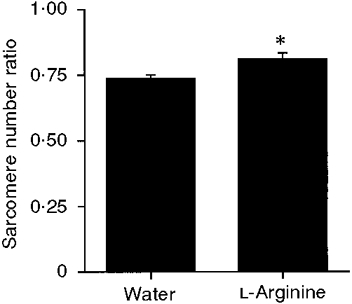
Ratio is immobilized-remobilized soleus sarcomere number divided by contralateral, non-immobilized, soleus sarcomere number. * Ratio for L-arginine-treated rats significantly greater than that for water-treated rats; P < 0.05.
DISCUSSION
The results of the present investigation support the hypothesis that NO derived from nNOS is a positive modulator of sarcomere addition. This conclusion is supported by our findings that NOS inhibitors reduced sarcomere addition, and that NOS substrate supplementation showed evidence of enhanced sarcomere addition. Administration of NOS inhibitors also lessened the increase in muscle mass that normally occurs during remobilization; this may reflect diminished sarcomeric protein synthesis in the NOS-inhibited animals. Finally, electron microscopic data showed that the MTJ was the primary site of sarcomere addition during remobilization, which is also the site where nNOS is most highly concentrated in muscle (Chang et al. 1996). When combined with the previous finding that passive stretching of muscle increases NO production (Tidball et al. 1998), the present data suggest that nNOS may serve as a mechanotransducer for sarcomere addition.
Although the NOS inhibitor-induced reductions in sarcomere addition are consistent with our hypothesis, we had anticipated that D-NAME would have a smaller effect than L-NAME because it is commonly used as a control treatment with the expectation that it is a less active enantiomer than L-NAME. However, there is precedent for similar levels of NOS inhibition in vivo by the two forms of NAME. Oral administration of the same concentration of either L-NAME or D-NAME (∼33 mg kg day−1) to guinea-pigs with induced chronic ileitis produced similar reductions in nitrite levels in the ileal lumen and similar reductions in protein loss and ileal wall thickening (Miller et al. 1993). These findings may be explained if the doses of D-NAME were high enough to cause maximal NOS inhibition in vivo, so that differences in the physiological effects of D-NAME and L-NAME could not be detected.
Though the inhibition of sarcomere addition by L-NAME and D-NAME supports the proposal that NO promotes sarcomere addition, no inferences can be made from those data concerning the source of the NO that modulates sarcomere addition. Potential sites for NO generation would be eNOS located in neighbouring endothelial cells or nNOS present in muscle. Our finding that TRIM administration produced similar inhibition of sarcomere addition as NAME supports the likelihood that muscle nNOS is the primary producer of NO that modulates sarcomere addition, because TRIM is more specific for nNOS than for eNOS (Handy et al. 1996). The specificity of TRIM for nNOS may result from interaction of TRIM with the tetrahydrobiopterin binding site on nNOS, in addition to the L-arginine binding site targeted by arginine analogues such as NAME (Handy & Moore, 1997).
One could argue that the smaller body mass in TRIM-treated rats compared with control rats (Table 2) could have resulted from a reduced food intake, and that this reduced food intake could have reduced sarcomere addition. However, the sarcomere number in the contralateral, non-immobilized soleus of TRIM-treated animals was nearly identical to that of control animals (4422 ± 273 vs. 4425 ± 238, respectively; means ± s.d.). In addition, tibial growth was nearly identical in TRIM-treated and control animals (Table 2). These data suggest that a possibly reduced food intake did not affect longitudinal bone and muscle growth.
L-Arginine supplementation showed evidence of enhancing sarcomere addition (Fig. 4), which would support the proposal that NO promotes sarcomere addition if increased arginine levels augmented NOS activity during remobilization. L-Arginine supplementation has been found to increase NOS activity in muscle ischaemia/reperfusion injury, diabetes and hypertension (Matsuoka et al. 1996; Pieper et al. 1996; Huk et al. 1997). In addition, L-arginine has been found to improve nitrogen balance in animals and patients that had undergone major trauma (Brittenden et al. 1994). However, L-arginine supplementation has also been shown to influence the secretion of a number of hormones (Brittenden et al. 1994), including increasing the secretion of growth hormone via suppression of endogenous somatostatin secretion (Alba-Roth et al. 1988). Thus, although L-arginine may stimulate sarcomere addition, the pathway (e.g. enhanced NO production, increased secretion of growth hormone) remains to be determined.
The inference based on NOS inhibition data that muscle nNOS is the source of NO that modulates sarcomere addition is further supported by the electron microscopic data. MTJs were the only site observed to show structural evidence of new sarcomere formation, and these sites have also been shown previously to contain the highest concentration of nNOS in muscle (Chang et al. 1996). The structural features used to identify sarcomere addition have been described previously in developing, embryonic muscle (Tidball & Lin, 1989), during postnatal growth (Williams & Goldspink, 1971) and during adaptive growth (Dix & Eisenberg, 1990). Our inability to distinguish between the MTJ structures of TRIM-treated, or PBS-treated, remobilized muscles may be attributable to the incomplete inhibition of sarcomere addition by TRIM.
The mechanisms through which NO may influence sarcomere addition remain to be elucidated, but could potentially involve the synthesis of cytoskeletal proteins, and/or satellite cell fusion at the ends of muscle cells. For example, NO can induce the immediate early genes c-fos and c-jun (Morris, 1995; Janssen et al. 1997), the protein products of which can upregulate the expression of cytoskeletal proteins such as skeletal α-actin (Bishopric et al. 1992). In addition, NO donors have been shown to enhance, and NOS inhibitors to reduce, myoblast fusion in vitro (Lee et al. 1994). Thus, NO could enhance satellite cell fusion at the ends of muscle cells, which occurs during sarcomere addition (Williams & Goldspink, 1971; Dix & Eisenberg, 1990).
The role for NO in modulating sarcomere addition is a new function for this pluripotent signalling molecule. This may involve a new potential mechanism through which cytoskeletal assembly can be regulated, in addition to better characterized mechanisms such as receptor binding of specific substratum molecules (Burridge et al. 1992) or receptor binding of soluble messenger molecules, such as growth factors (Herman & Pledger, 1985). Current knowledge indicates that each of these regulatory systems is in place at MTJs, because these sites are enriched in integrins, platelet-derived growth factor receptor and NOS, each of which can activate separate signalling cascades that influence cytoskeletal synthesis and structure (Bozyczko et al. 1989; Tidball & Spencer, 1993; Tidball et al. 1998). Our continuing work is directed towards obtaining mechanistic data concerning how the NO-influenced effects reported here are mediated, so that the interplay of these regulatory systems can be examined.
Acknowledgments
The authors thank Michael Reyes, Yvette Yates and Long-Sheng Hong for technical assistance during the study. This investigation was supported by the National Institutes of Health (AR40343 and AR08479).
References
- Alba-Roth J, Muller OA, Schopohl J, von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. Journal of Clinical Endocrinology and Metabolism. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- Bishopric NH, Jayasena V, Webster KA. Positive regulation of the skeletal α-actin gene by fos and jun in cardiac myocytes. Journal of Biological Chemistry. 1992;267:25535–25540. [PubMed] [Google Scholar]
- Boger RH, Bode-Boger SM, Gerecke U, Gutzki FM, Tsikas D, Frolich JC. Urinary NO3− excretion as an indicator of nitric oxide formation in vivo during oral administration of L-arginine or L-NAME in rats. Clinical and Experimental Pharmacology and Physiology. 1996;23:11–15. [PubMed] [Google Scholar]
- Bozyczko D, Decker C, Muschler J, Horwitz AF. Integrin on developing and adult skeletal muscle. Experimental Cell Research. 1989;183:72–91. doi: 10.1016/0014-4827(89)90419-9. [DOI] [PubMed] [Google Scholar]
- Brittenden J, Heys SD, Ross J, Park KGM, Eremin O. Nutritional pharmacology: effects of L-arginine on host defences, response to trauma and tumour growth. Clinical Science. 1994;86:123–132. doi: 10.1042/cs0860123. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAKaccompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. Journal of Cell Biology. 1992;199:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proceedings of the National Academy of Sciences of the USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DJ, Eisenberg BR. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. Journal of Cell Biology. 1990;111:1885–1894. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. Malleability of the motor system: a comparative approach. Journal of Experimental Biology. 1985;115:375–391. doi: 10.1242/jeb.115.1.375. [DOI] [PubMed] [Google Scholar]
- Handy RLC, Harb HL, Wallace P, Gaffen Z, Whitehead KJ, Moore PK. Inhibition of nitric oxide synthase by 1-(2-trifluoromethylphenyl)-imidazole (TRIM) in vitro: antinociceptive and cardiovascular effects. British Journal of Pharmacology. 1996;119:423–431. doi: 10.1111/j.1476-5381.1996.tb16003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy RLC, Moore PK. Mechanism of inhibition of neuronal nitric oxide synthase by 1-(2-trifluoromethylphenyl) imidazole (TRIM) Life Sciences. 1997;60:389–396. doi: 10.1016/s0024-3205(97)00295-6. [DOI] [PubMed] [Google Scholar]
- Herman B, Pledger WJ. Platelet-derived growth factor-induced alterations in vinculin and actin distribution in BALB/c 3T3 cells. Journal of Cell Biology. 1985;100:1031–1040. doi: 10.1083/jcb.100.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Grimm AF, Grimm BR. Regulation of sarcomere number in skeletal muscle: A comparison of hypotheses. Muscle and Nerve. 1984;7:161–173. doi: 10.1002/mus.880070213. [DOI] [PubMed] [Google Scholar]
- Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. Journal of Applied Physiology. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- Huang M, Manning RD, LeBlanc MH, Hester RL. Overall hemodynamic studies after the chronic inhibition of endothelial-derived nitric oxide in rats. American Journal of Hypertension. 1995;8:358–364. doi: 10.1016/0895-7061(94)00203-n. [DOI] [PubMed] [Google Scholar]
- Huk I, Nonobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittleboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. L-Arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]
- Janssen YMW, Matalon S, Mossman BT. Differential induction of c-fos, c-jun, and apoptosis in lung epithelial cells exposed to ROS or RNS. American Journal of Physiology. 1997;273:L789–796. doi: 10.1152/ajplung.1997.273.4.L789. [DOI] [PubMed] [Google Scholar]
- Kivirikko KI, Laitinen O, Prockop DJ. Modifications of a specific assay for hydroxyproline in urine. Analytical Biochemistry. 1967;19:249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Herzog W. Excursion is important in regulating sarcomere number in the growing rabbit tibialis anterior. The Journal of Physiology. 1998;508:267–280. doi: 10.1111/j.1469-7793.1998.267br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Baek MY, Moon KY, Song WK, Chung CH, Ha D B, Kang MS. Nitric oxide as a messenger molecule in myoblast fusion. Journal of Biological Chemistry. 1994;269:14371–14374. [PubMed] [Google Scholar]
- Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: A physiologic messenger. Annals of Internal Medicine. 1994;124:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Nakata M, Kohno K, Koga Y, Nomura G, Toshima H, Imaizumi T. Chronic L-arginine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 1996;27:14–18. doi: 10.1161/01.hyp.27.1.14. [DOI] [PubMed] [Google Scholar]
- Miller MJS, Sadowska-Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA. Amelioration of chronic ileitis by nitric oxide synthase inhibition. Journal of Pharmacology and Experimental Therapeutics. 1993;264:11–16. [PubMed] [Google Scholar]
- Morris BJ. Stimulation of immediate early gene expression in striatal neurons by nitric oxide. Journal of Biological Chemistry. 1995;270:24740–24744. [PubMed] [Google Scholar]
- O'Dwyer NJ, Neilson PD, Nash J. Mechanisms of muscle growth related to muscle contracture in cerebral palsy. Developmental Medicine and Child Neurology. 1989;31:543–547. doi: 10.1111/j.1469-8749.1989.tb04034.x. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Siebeneich W, Dondlinger LA. Short-term oral administration of L-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. European Journal of Pharmacology. 1996;317:317–320. doi: 10.1016/s0014-2999(96)00831-x. [DOI] [PubMed] [Google Scholar]
- Simpson AHR, Williams PE, Kyberd P, Goldspink G, Kenwright J. The response of muscle to leg lengthening. Journal of Bone and Joint Surgery. 1995;77:630–636. B. [PubMed] [Google Scholar]
- Spector SA, Gardiner PF, Zernicke RF, Roy RR, Edgerton VR. Muscle architecture and force-velocity characteristics of cat soleus and medial gastrocnemius: implications for motor control. Journal of Neurophysiology. 1980;44:951–960. doi: 10.1152/jn.1980.44.5.951. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. The Journal of Physiology. 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. American Journal of Physiology. 1998;275:C260–266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell and Tissue Research. 1989;257:77–84. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Spencer MJ. PDGF stimulation induces phosphorylation of talin and cytoskeletal reorganization in skeletal muscle. Journal of Cell Biology. 1993;123:627–635. doi: 10.1083/jcb.123.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Longitudinal growth of striated muscle fibers. Journal of Cell Science. 1971;9:751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. Journal of Anatomy. 1978;127:459–468. [PMC free article] [PubMed] [Google Scholar]


