Abstract
The conversion of prothrombin (FII) to the serine protease, thrombin (FIIa), is a key step in the coagulation cascade because FIIa triggers platelet activation, converts fibrinogen to fibrin, and activates regulatory pathways that both promote and ultimately suppress coagulation. However, several observations suggest that FII may serve a broader physiological role than simply stemming blood loss, including the identification of multiple G protein-coupled, thrombin-activated receptors, and the well-documented mitogenic activity of FIIa in in vitro test systems. To explore in greater detail the physiological roles of FII in vivo, FII-deficient (FII−/−) mice were generated. Inactivation of the FII gene leads to partial embryonic lethality with more than one-half of the FII−/− embryos dying between embryonic days 9.5 and 11.5. Bleeding into the yolk sac cavity and varying degrees of tissue necrosis were observed in many FII−/− embryos within this gestational time frame. However, at least one-quarter of the FII−/− mice survived to term, but ultimately they, too, developed fatal hemorrhagic events and died within a few days of birth. This study directly demonstrates that FII is important in maintaining vascular integrity during development as well as postnatal life.
Prothrombin (FII), a vitamin K-dependent zymogen synthesized by hepatocytes, is activated to form thrombin (FIIa) by factor Xa in the presence of factor Va (FVa), calcium, and a phospholipid surface. FIIa plays a central role in the blood coagulation system by triggering the activation of platelets, converting soluble fibrinogen into insoluble fibrin polymer, and activating regulatory pathways that control the rate of further thrombin formation (1). In the presence of thrombomodulin, FIIa functions as an anticoagulant by activating protein C and protein S, which in turn inactivates factors Va and VIIIa. FIIa is thought to serve a broader biological role than merely controlling blood loss, based on the fact that there are at least two G protein-coupled receptors (i.e., PAR-1 and PAR-3) that are proteolytically activated by thrombin, and these receptors are present on a variety of cell types (2–4). FIIa has been proposed to influence a variety of physiological and pathological processes, including inflammation, tissue repair, neurite outgrowth, atherosclerosis, and tumor cell metastasis (5–9). The expression of both thrombin receptor and FII during organogenesis in the mouse suggests that FIIa may play an important role in development (3), a hypothesis that is supported further by the finding of partial embryonic lethality in mice deficient in tissue factor (TF), factor V (FV), and PAR-1 (10–16). To understand in greater detail the diverse biological roles of FII in vivo, and to directly establish the importance of FII in development, the FII gene was disrupted in mice. We report that FII deficiency results in a loss of vascular integrity and death around the tenth day of gestation in a high percentage of FII−/− embryos. Partial embryonic lethality in FII−/− mice has also been observed by Xue and coworkers (17). These results, together with similar findings in TF-, FV-, and PAR-1-deficient mice, strongly imply that thrombin-mediated proteolysis plays at least one, and conceivably several, important roles in mouse development.
MATERIALS AND METHODS
Disruption of the FII Gene and Generation of FII−/− Mice.
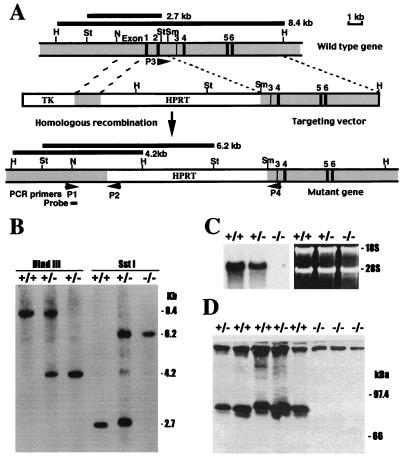
An 8.2-kb region of the 5′-end of the mouse FII gene, including 3.2 kb of the 5′-flanking region and the first six exons, was isolated from a 129/Ola genomic library (18). In the construction of the targeting vector, the first two exons of the gene were replaced by a 6-kb hypoxanthine phosphoribosyltransferase (HPRT) gene (19). Two regions of the FII gene, a 711-bp fragment immediately upstream of exon 1 obtained by PCR and a 4.2-kb SmaI–HindIII fragment containing exons 3–6, were used as the short and long arms of the targeting vector, respectively (Fig. 1A). The short arm was flanked by a 2 kb gene coding for herpes simplex virus thymidine kinase (20). The 711-bp fragment was cloned into the SstI and BamHI sites, and the 4.2-kb fragment was cloned into the SmaI and HindIII sites of Bluescript. The HPRT gene was cloned into the BamHI site of the plasmid vector between the two mouse FII gene fragments in the opposite transcriptional orientation of the FII gene. Disruption of the FII gene was accomplished in the HPRT-deficient embryonic stem (ES) cell line, E14TG2a, which is derived from strain 129/Ola mice (21). The targeting vector was linearized with NotI and introduced into ES cells by electroporation. ES cell clones that survived in selection medium containing hypoxanthine, aminopterin, thymidine, and gancyclovir were collected, expanded, and tested for the presence of a targeted FII allele as described below. Two independent ES cell lines carrying a targeted FII allele were used for blastocyst injection (22) and both yielded chimeric male founders that, when bred to NIH Black Swiss females, transmitted the mutant FII gene to their offspring. FII−/− mice were generated by establishing brother–sister matings between first generation FII+/− mice with an exactly 50:50 129/Black Swiss genetic background. The phenotypes of FII−/− mice were found to be essentially identical regardless of the ES cell clone used to generate the mice.
Figure 1.
Strategy for disrupting the FII gene and analysis of FII expression in gene-targeted mice. (A) Schematic representation and partial restriction map of a portion of the endogenous mouse FII gene, the FII targeting vector, and the expected form of the mutant FII gene following homologous recombination. Exons are represented by solid bars and are numbered 1–6. Shaded boxes represent elements of the FII gene and open boxes represent the HPRT and herpes simplex virus thymidine kinase minigenes used as selectable markers. The region containing exons 1 and 2 was replaced by the HPRT cassette in the targeted FII gene. The relative position of PCR primers (P1, P2, P3, and P4) and a probe (250 bp) used for Southern blot hybridization analysis are indicated. The location and expected sizes of the fragments generated by HindIII and SstI digestion of the endogenous and targeted alleles are indicated. Restriction enzymes: St, SstI; H, HindIII; Sm, SmaI; N, NcoI. (B) Genotype determination in term mice by Southern blot hybridization analysis of tail biopsy DNA. Genomic DNA from mice was digested with HindIII or SstI and hybridized with a 250-bp probe complementary to a region of the FII gene outside the segment used in the targeting vector. Fragments corresponding to the wild-type allele (8.4-kb HindIII and 2.7-kb SstI fragments) and those corresponding to the targeted allele (4.2-kb HindIII and 6.2 kb SstI fragments) are identified. (C) Northern blot hybridization analysis of hepatic FII mRNA in total RNA extracts prepared from newborn FII+/+, FII+/−, and FII−/− mice. Note that FII mRNA levels are about one-half that of wild-type animals in FII+/− mice and undetectable in FII−/− mice. (Right) Ethidium bromide stained gel before RNA transfer; the intensity of the 28S and 18S ribosomal RNAs indicates nearly equivalent loading of total RNA. (D) Immunoblot analysis of FII in plasma samples collected from FII+/+, FII+/−, and FII−/− newborns and fractionated on a 10% reducing SDS/polyacrylamide gel. The migration of the lower band is at the expected position of mouse FII. The protein detected near the top of the gel is caused by nonspecific reaction with the secondary antibody, because it was observed in the absence of primary antibody against human FII (not shown).
Detection of the Targeted Allele of the FII Gene.
Genotypes were determined by either PCR or Southern blot hybridization analysis. For PCR analyses of ES cells, yolk sac membranes, and tissues collected from paraffin sections (see below), tissues were incubated overnight at 60°C in PCR lysis buffer [10 mM Tris⋅HCl, pH 8.0/50 mM KCl/0.5% (vol/vol) Tween 20/0.5% (vol/vol) Nonidet P-40/1 mM EDTA/100 μg/ml proteinase K], heated to 100°C for 15 min, and used directly as templates in PCR mixtures. For Southern blot analyses, genomic DNA was purified from ES cells and tail biopsy samples before restriction enyzme digestion. Deparaffinized embryonic tissue was collected from unstained tissue sections mounted on microscope slides by using a 26-gauge needle and a dissecting microscope; particular care was taken to avoid touching surrounding maternal tissue. PCR analysis of the genotypes was carried out using two pairs of primers; P1 and P2, which generate an 885-bp product from the targeted allele, and P3 and P4, which amplify a 572-bp region of the endogenous allele (Fig. 1). P1 (5′-GCTCTTTGTCCCTCTGTTCTATTTAGACCC-3′) is complementary to the region of the FII gene lying outside the segment used in the targeting vector and P2 (5′-TATTACCAGTGAATCTTTGTCAGCAGTTCCC-3′) is complementary to a sequence in the HPRT cassette. P3 (5′-TCAGACTTTCACTTCTATTCGGACT-3′) is complementary to a sequence within the deleted region of the FII gene and P4 (5′-GGAGTGCTTTATGCAACAGCAACAA-3′) is complementary to a region present in the long arm of the targeting vector. For Southern blot hybridization analysis, genomic DNA was digested with HindIII or SstI, separated electrophoretically, blotted onto nylon membrane (Genescreen Plus) and hybridized with a 32P-radiolabeled 250-bp probe, prepared by PCR, that is complementary to a region of the FII gene outside that used in targeting vector (Fig. 1B).
Developmental Analysis.
Gross evaluation of embryos, with and without yolk sac membranes intact, was done by stereomicroscopy after careful dissection from the decidua. To avoid potential bleeding artifacts that could result from the surgical removal of unfixed embryos from the decidua, for histological studies the embryos were fixed within the uterine horns for up to 24 hr in 10% neutral-buffered formalin (23). Uterine horn segments were subsequently processed into paraffin, sectioned, and deparaffinized for either PCR analysis of genotypes or for staining with hematoxylin/eosin before microscopic analysis. For in situ hybridization experiments, a 35S-labeled RNA probe complementary to mouse FII mRNA (24) was synthesized and hybridized to cryosections of fixed tissues as described (25). For analysis by electron microscopy, a small segment of yolk sac membrane was removed and fixed with 3% gluteraldehyde for 2 hr, followed by secondary fixation in 1% osmic acid, and processed for electron microscopy. Genotyping was performed on the paraffin-processed embryo as described above.
Detection of FII mRNA and Protein.
Total RNA was isolated from the livers of neonates by using Trizol LS reagent (GIBCO/BRL). Total RNA (20 μg) was separated on a 1% denaturing agarose gel and blotted onto a nylon membrane (Micron Separations, Westboro, MA). A 1,018-bp mouse FII cDNA fragment was labeled with [32P-α]dCTP and used as a probe to detect FII mRNA. This cDNA probe contains sequence coding for the kringle domains and part of the serine protease domain of mouse FII (24). Plasma (0.5 μl) was separated on a 10% SDS/polyacrylamide gel under denaturing conditions and transferred to an Immobilon-P transfer membrane (Millipore). Immobilized mouse FII was detected by indirect immunostaining by using a rabbit polyclonal antibody raised against human FII (Nordic Immunological, San Clemente, CA), biotinylated goat anti-rabbit antibody, and the Vectastain ABC horseradish peroxidase staining system (Vector Laboratories). Membrane-bound biotin-peroxidase complex was detected by using the enhanced chemiluminescence system (Amersham).
Prothrombin and Thrombin Time Assays.
Blood was collected from embryonic day (E)18.5 embryos and immediately combined with one-tenth vol of 129 mM sodium citrate. Plasma was isolated by centrifugation for 10 min at 3,000 rpm. Five microliters of plasma was mixed with either 10 μl of rabbit brain thromboplastin (Biopool, Ventura, CA) for determination of prothrombin times or 2 μl of bovine thrombin (Pacific Hemostasis, Huntersville, NC, 0.1 unit/μl) for determination of thrombin times. Plasma and thromboplastin were prewarmed at 37°C before assay. The time of first appearance of a clot was recorded.
RESULTS
The gene-targeting strategy used (Fig. 1A) was expected to result in a FII-null allele as a consequence of the deletion of the first two exons of the FII gene (encoding the signal peptide, the propeptide required for gamma-carboxylation, and the domain that is gamma-carboxylated and responsible for binding of FII to phospholipid membranes). Fifteen of 120 (12.5%) ES cell transfectants that had stably incorporated the targeting vector into their genomes carried the disrupted FII allele based on PCR and Southern hybridization analyses (data not shown). Two independent ES cell lines were used to develop chimeric mice that transmitted the mutant FII allele to their offspring (FII+/−). FII+/− mice were subsequently intercrossed to obtain FII−/− progeny (Fig. 1B). FII−/− mice were observed in term offspring (see below) and, consistent with the expected presence of only disrupted FII alleles, these mice carried no detectable hepatic FII mRNA based on Northern blot hybridization analysis (Fig. 1C) or plasma FII based on Western blot analysis with a specific antibody raised against human FII that crossreacts with mouse FII (Fig. 1D). In addition, there was no detectable prothrombin activity in blood isolated from E18.5 FII−/− embryos as determined by prothrombin time clotting assay [FII+/+, 16 +/− 2 sec (n = 35); FII−/−, uniform failure of clot formation throughout a 240 sec observation period (n = 16)]. However, the addition of thrombin to plasma from E18.5 FII−/− embryos resulted in normal clotting times [FII+/+, 7 +/− 1 sec (n = 5); FII−/−, 6 +/− 1 (n = 7)].
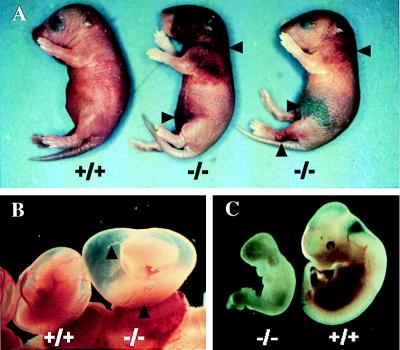
The transmission of the mutant FII allele in term offspring did not follow a Mendelian pattern of inheritance, suggesting intrauterine lethality in a subset of FII−/− embryos, and those that survived to term uniformly developed fatal bleeding events early within the neonatal period. This pattern of partial embryonic lethality and perinatal hemorrhaging was observed with FII−/− mice generated from both ES cell lines used to raise gene-targeted mice. Among 134 mice obtained from 21 litters born after the mating of FII+/− mice derived from one ES cell line, 47 (35%) were wild type, 77 (57.5%) were FII+/−, and 10 (7.5%) were FII−/− mice (Table 1), indicating that only about one-quarter of the expected number of FII−/− animals were present in term offspring (P < 0.001, χ2 test). FII−/− neonates uniformly developed a fatal bleeding phenotype and either died within a few hours of birth or within 2 days. However, in separate studies of FII−/− mice with a slightly more enriched Black Swiss genetic background, 5 of 42 (12%) FII−/− neonates lived as long as 5 days and one FII−/− mouse lived for 7 days. Therefore, like fibrinogen-deficient mice (26), genetic background in FII−/− mice may influence survival, although this has not been formally explored. The FII−/− neonates developed massive bleeding into the abdominal cavity, purpura around the head, back, abdomen, and around joints, as well as bleeding into the intestinal lumen (Fig. 2A), sites observed previously in mice lacking fibrinogen, factor VII (FVII), and FV (14, 26, 27). However, newborn FII−/− mice were otherwise normal in size and showed no obvious congenital malformations by gross or microscopic inspection. Unlike FII−/− animals, the growth, reproduction, and prothrombin times of FII+/− mice were found to be indistinguishable from their wild-type littermates (data not shown). These data are consistent with the general good health and low hemorrhagic risk previously documented in heterozygous mice with a single disrupted TF, FVII, FV, or fibrinogen gene (10–14, 26, 27).
Table 1.
Genotypes of the progeny from hemizygous matings
| Stage | Total | Genotyped | FII genotype
|
||||
|---|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | %* | ||||
| E8.5 | 37 | 34 | 10 | 16 | 8 | 23.5 | P = 0.82† |
| E9.5 | 76 | 73 | 15 | 39 | 18 (1)‡ | 25.4 | P = 1.0 |
| E10.5 | 104 | 103 | 29 | 58 (3) | 7 (6) | 6.8 | P < 0.001 |
| E11.5 | 36 | 32 | 6 (2) | 19 | 3 (2) | 9.4 | P < 0.05 |
| E12.5 | 99 | 86 | 26 (1) | 46 (2) | 8 (3) | 9.3 | P < 0.001 |
| E15.5 | 53 | 48 | 19 | 24 | 5 | 10.4 | P < 0.02 |
| E18.5 | 96 | 95 | 34 | 51 | 10 | 10.5 | P < 0.001 |
| Term | 135 | 134 | 47 | 77 | 10 | 7.4 | P < 0.001 |
Viable FII−/− mice calculated as the percentage of all genotyped mice in the same age group.
Calculated using χ2 test comparing the expected and observed frequency of viable FII−/− mice.
Number of grossly nonviable mice are listed in parentheses.
Figure 2.
Spontaneous bleeding events in FII−/− mice. (A) Severe bleeding in the abdominal cavity, beneath the skin, and around the leg joint of FII−/− neonates is indicated. (B) Gross appearance of E12.5 FII−/− and FII+/+ embryos within their yolk sacs. Note that in the FII−/− embryo the blood vessels in the yolk sac membrane are empty, whereas blood cells are pooled in the yolk sac cavity. (C) Gross appearance of the same embryos shown in B. Note the obvious developmental arrest and pale appearance in the FII−/− embryo.
To rigorously determine whether FII deficiency results in a partial embryonic-lethal phenotype, and to establish the point in gestation in which FII−/− mice suffer developmental arrest, embryos were phenotypically (see below) and genetically (Table 1) evaluated at various time points during gestation. At E8.5, FII−/− embryos were present in the expected numbers (Table 1) and were grossly and microscopically indistinguishable from their littermates (data not shown). However, overt abnormalities were observed during gross examination of many, but not all, E9.5–E12.5 FII−/− embryos. Frequently, the yolk sac membranes of these embryos were pale, the vessel appeared largely empty of blood, and blood pools were grossly visible within the yolk sac cavity (Fig. 2B), phenotypes similar to those described previously in embryos lacking TF and tissue factor pathway inhibitor (TFPI; refs. 10–13 and 28). Among 13 FII−/− embryos examined at E10.5, 6 were obviously developmentally arrested, with sizes as low as 15% of littermate controls. An additional 2 of 13 E10.5 FII−/− embryos also appeared notably smaller than FII+/− and FII+/+ littermates, but appeared viable, and the remaining 5 E10.5 FII−/− embryos were of normal size and appearance. Nonspecific pathologies previously associated with a variety of circulatory failures were often noted in affected E9.5–E11.5 FII−/− embryos, including enlarged pericardial sacs and distended hearts (Fig. 2C and data not shown). Interestingly, the fraction of total embryos that were FII−/− remained nearly constant (≈10%) beyond E11.5 (Table 1) and developmental failures were never recognized in embryos surviving into the latter part of gestation. Thus, as recognized earlier for TF−/− mice (13), the combined phenotype and genotype data suggests that there are two populations of FII−/− embryos, a subset that suffers a developmental failure around the tenth day of gestation and a subset that succeeds in traversing this midgestational risk window and develops to term.
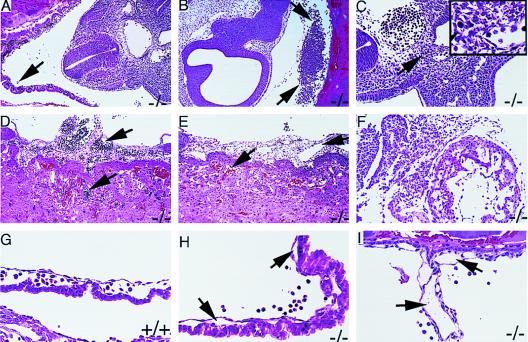
To avoid any bleeding artifacts that might result from tissue manipulation during dissection, and to avoid the possibility of missing the evidence of very subtle blood leakage into the yolk sac cavity, subsequent embryos were fixed and paraffin-embedded intact within the uterine tissue. Genotyping was then performed by using embryonic tissue removed from paraffin sections mounted on glass slides. Histological analysis of embryos shows that variable degrees of bleeding occurred in many of the FII−/− embryos examined between E9.5 and E11.5 (Fig. 3). Nine of 18 E10.5 FII−/− embryos showed some evidence of bleeding, and in 6 of 18 blood loss was judged to be extensive. This bleeding typically occurred into the yolk sac cavity of FII−/− embryos in amounts ranging from minimal (Fig. 3A) to large pools of fetal blood cells (Fig. 3B). Free blood was also occasionally observed in the exocoelomic cavity and pericardial sac (data not shown), but only rarely observed in other sites in the embryo proper. The extraembryonic vessels, including those in the yolk sac membrane, allantois, vitelline, chorionic villi, and umbilical vessels, were well formed in affected FII−/− embryos without significant necrosis, although the lumen of these vessels were largely empty in those embryos with substantial free blood pooled within the yolk sac cavity (Fig. 3 E, H, and I). Similarly, the vascular system in the embryos, including the heart, was well developed and showed no structural abnormalities based on light microscopic analyses of complete serial sections of all the embryos. In the less severely affected embryos, the vessels were generally filled with fetal blood cells, indicating that the extraembryonic and embryonic vasculature had undergone appropriate fusion (Fig. 3 A and D compared with FII+/+ in Fig. 3G). The embryonic vessels in the most severely affected embryos were largely empty but showed no structural changes that could be appreciated, with the exception of those reflecting severe widespread tissue necrosis.
Figure 3.
Microscopic analysis of FII−/− embryos fixed and sectioned in situ within the uterine horns. (A) Section through a generally normal appearing E10.5 FII−/− embryo showing well-developed yolk sac vessels with blood filled lumens. However, there are occasional clusters of fetal blood cells (arrow) lying free in the yolk sac cavity. (B) Section from a FII−/− littermate of the embryo shown in A. This embryo shows a large pool of fetal blood cells in the yolk sac cavity (arrows). (C) Section from an E10.5 FII−/− embryo with extensive bleeding into the yolk sac cavity, but only small foci of tissue necrosis. (Inset) Small area of necrosis, indicated by arrow, at a higher magnification. (D) Section through the placenta from the embryo illustrated in A. The allantoic blood vessels and fetal vessels of the placenta are well developed and filled with fetal blood cells (arrows). (E) Section through the placenta from the embryo illustrated in B. Arrows indicate the allantoic and maternal sinusoids in the placenta. There are no fetal blood cells in the allantoic vessels and only maternal red blood cells are seen in the placenta. (F) Section from an E10.5 FII−/− embryo with widespread necrosis evident in the embryo. (G) Section showing the yolk sac membrane of an E10.5 FII+/+ embryo. Vessels are well developed, patent, and blood filled. (H) Section showing the yolk sac membrane from an E10.5 FII−/− embryo. Yolk sac vessels (arrows) are patent, but slightly collapsed and empty. Note fetal blood cells lying free in the yolk sac cavity. (I) Section through the vitelline vessel of an E10.5 FII−/− embryo that is well developed but empty. (A, B, D–F, 100×; C, G–I, 200×.)
Microscopic evidence of bleeding was frequently associated with varying degrees of necrosis within the E10.5 embryos. Those FII−/− embryos with minimal evidence of bleeding into the yolk sac cavity showed little or no evidence of necrosis (Fig. 3A). In some embryos with appreciable bleeding into the yolk sac cavity, necrosis was limited to small foci of cells (Fig. 3 B and C). In other embryos the necrosis was more widespread and extensive (Fig. 3F). Embryos that showed diffuse severe necrosis in all tissue types were frequently associated with virtually empty fetal blood vessels and large pools of blood in the yolk sac cavity. No necrosis or bleeding was ever observed in the trophectoderm component of the placenta, the decidual tissue, or the underlying uterine tissue.
To determine whether the loss of vascular integrity observed in the yolk sac vasculature in midgestation FII−/− embryos was related to a fundamental structural abnormality, ultrathin sections of yolk sac tissue were prepared from five E9.5 FII−/− embryos and three E9.5 FII+/+ littermates and then examined by transmission electron microscopy. All three of the cell layers that comprise the yolk sac, the visceral endoderm, mesenchymal, and endothelial cell layers, were both present and normal in appearance in every microscopic field examined, regardless of embryo genotype (representative data presented in Fig. 4). By ultrastructural analysis, the yolk sac vessels were well developed in all FII−/− embryos and no obvious differences in cellular structure or densities were noted. Although focal abnormalities cannot be completely ruled out, these analyses suggest that the structural development of these vessels is largely normal in E9.5 FII−/− embryos.
Figure 4.
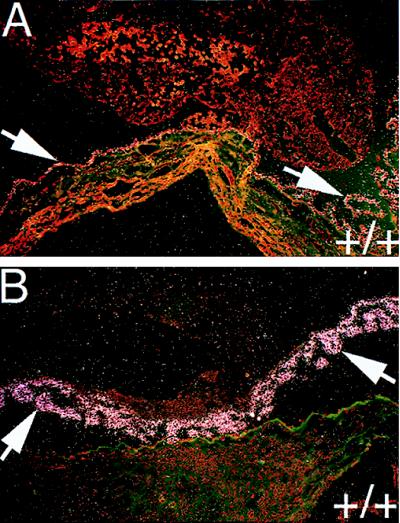
Transmission electron micrographs of yolk sac membranes from E9.5 FII+/+ (A) and FII−/− embryos (B). (A) A yolk sac vessel including the endothelial lining of the yolk sac vessel (el), a pervascular mesenchymal cell (pmc), an endodermal cell (ec), and hematopoietic cells (hc). (B) The same cellular components and vessel structures as in A, in addition to the mesothelial lining of the extraembryonic coelomic cavity (ml). (3,200×.)
Because embryonic lethality of FII−/− mice occurred as early as E10, we examined whether FII was expressed within either embryonic or extraembryonic tissues at this time in development. Indeed, in situ hybridization analysis using an antisense mouse FII probe revealed that FII mRNA was expressed within the visceral endoderm of the yolk sac at E9.5 (Fig. 5A), cells that are known to produce common embryonic plasma components such as α-fetoprotein and transferrin. This signal persisted and intensified in the yolk sac at E13.5 (Fig. 5B), a period when expression was also found in the liver (data not shown).
Figure 5.
FII mRNA in embryonic yolk sac. (A) Detection of FII mRNA by in situ hybridization in a tissue section prepared from an E9.5 FII+/+ embryo. Note that FII mRNA is detectable at low levels (white grains in this darkfield image) exclusively within the yolk sac membrane (arrows). (B) In situ hybridization analysis showing intense signal for FII mRNA in the yolk sac membrane (arrows) of an E13.5 FII+/+ embryo. (100×.)
DISCUSSION
Consistent with the well-established role of FII in directing the local activation of platelets and the conversion of soluble fibrinogen to fibrin polymer at sites of vascular rupture, these studies demonstrate that a total lack of FII in mice invariably leads to fatal hemorrhagic events early in life. These studies also directly show that developmental failures are frequent in midgestation embryos unable to express FII, although embryo-derived FII is clearly not strictly required for development to term. At least one-quarter of FII−/− embryos succeed in traversing the high-risk period in development that occurs around E10. Because FII−/− mice can presently only be raised by breeding FII+/− mothers, maternally derived FII cannot be formally excluded as contributing to the (partial) developmental success of some FII−/− embryos. However, if early embryos do acquire any maternal FII, it is clear that the presence of a maternal reservoir is insufficient to maintain the development of all FII−/− embryos. A similar early lethality may be associated with human FII deficiency, because no individuals have ever been identified that completely lack FII (29).
Although a loss of vascular integrity in extraembryonic vessels that results in hemorrhaging into the yolk sac cavity is a common theme in midgestation FII−/− embryos reported here, the primary mechanism(s) underlying these developmental failures is still uncertain. Remarkably, similar failures of vascular integrity within yolk sac vessels leading to hemorrhage have been reported by three independent groups studying mice deficient in TF, the primary cell-associated initiator of the coagulation cascade (10–13). Indeed, the only obvious phenotypic distinction between TF−/− and FII−/− mice appears to be the penetrance of embryonic failure, which is 85–100% in TF-deficient mice depending on the genetic background. Given this strong similarity, and the established role of TF in triggering FIIa generation, one hypothesis that emerges is that embryonic failure in at least some TF−/− and FII−/− mice mechanistically involves a common pathway, presumably a “downstream” failure of FIIa-mediated proteolysis. Compelling evidence supporting the view that FIIa-mediated proteolysis is important in early development is the finding that deficiencies in either a critical cofactor for FIIa generation, FV, or the proteolytically activated thrombin receptor, PAR-1, also results in developmental arrest and partial embryonic lethality (14, 15). However, it should be noted that in the case of PAR-1−/− mice, a hemorrhagic phenotype was neither observed nor expected based on the absence of any deficit in the coagulation system and the preserved PAR-3-mediated platelet activation in these mice (15). Indeed, presuming that thrombin is the sole physiologically relevant activator of PAR-1, the distinct phenotypic features of FII−/− and PAR-1−/− embryos suggest that multiple pathways may be compromised in FII-deficient mice, which are individually important for developmental success. Crosses between FII and PAR-1 knockout mice will be helpful in determining whether both shared and distinct developmental abnormalities occur in FII−/− and PAR-1−/− embryos.
The view that at least a portion of the developmental failures observed in TF−/−, FV−/−, and FII−/− embryos involve a common failure at the level of FIIa-mediated proteolysis, implies that deficits in other extrinsic pathway factors controlling FIIa generation might also compromise mouse development. In this regard, TFPI gene disruption also leads to partial embryonic lethality, with failing embryos appearing remarkably similar to failing TF−/− and FII−/− embryos (e.g., signs of yolk sac hemorrhage after E9.5). Although the mechanisms leading to failure of TFPI−/− embryos are unclear, it has been proposed that TF/FVIIa activity unrestricted by TFPI in midgestation embryos may lead to consumptive loss of FII and other extrinsic pathway components (28). Whatever the mechanism, FVII is clearly present in E9.5–E10.5 mice and contributes to the demise of TFPI−/− embryos based on the recent finding that TFPI−/− embryos uniformly complete embryonic development if they are simultaneously FVII deficient (30). One fact that seems to challenge the notion of developmental failure because of a failure of TF-mediated FIIa generation is that FVII−/− mice apparently do not suffer from significant embryonic lethality (27). Based on the position of FVII in the amplification cascade, it is conceivable that very low amounts of maternally derived FVII may be sufficient to sustain embryos at a critical embryonic stage. Given that plasma FVII levels that are less than 0.2% of adult levels in E9.5–E11.5 FVII+/+ embryos (27) are apparently sufficient to mediate the death of most TFPI−/− embryos it should perhaps not be surprising that trace levels of FVII may be biologically relevant and adequate to sustain life in a normal embryo. Detailed studies of mice expressing mutant forms of extrinsic system factors, including mice expressing mutant forms of FII with altered procoagulant and anticoagulant activity, and mice expressing mutant forms of TF lacking either its intracellular domain or FVII binding activity, should prove instructive in defining the precise roles of this system of proteins in development.
Xue et al (17) have observed a similar partial embryonic lethality and early neonatal death of FII−/− mice. Furthermore, Xue et al. also suggest that FII deficiency results in a vascular failure. However, there seem to be differences in comparing their findings with our own, most notably, differences in the detection of free blood within yolk sac cavities and structural abnormalities in the yolk sac membranes of non-necrotic embryos. These apparent discrepancies may be related to differences in genetic background, experimental approach (e.g., histological analyses of dissected vs. undissected embryos), or the embryonic ages emphasized in the detailed analyses. Nevertheless, the leading hypothesis from both studies is that thrombin-mediated proteolysis is crucial for vascular development. However, this must still be rigorously explored and ultimately reconciled with both the developmental success of a fraction of TF−/−, FV−/−, and FII−/− embryos and the presence of seemingly normal vascular beds within early-stage and uncompromised late-stage mutant embryos.
Acknowledgments
We thank Susan MacDowell and Jing Peng for cloning and sequencing part of the mouse prothrombin gene; John Eckman, Lisa Artmayer, Kathy Saalfeld, and Pam Groen for technical support; Alicia Emley for photography support; Keith Kombrinck for statistical analyses; and Dr. Mary Jo S. Danton for critically reading the manuscript. This work was funded in part by U.S. Public Health Service Grant HL 38232 and Grant HL58103 from the National Institutes of Health, National Heart, Lung, and Blood Institute (S.J.F.D.), a grant-in-aid from the American Heart Association National Center (S.J.F.D.), and a grant-in-aid (D.P.W.) and postdoctoral fellowship (W.Y.S.) from the American Heart Association, Ohio–West Virginia Affiliate.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: FII, prothrombin; FIIa, thrombin; TF, tissue factor; TFPI, tissue factor pathway inhibitor; FV, factor V; FVII, factor VII; ES, embryonic stem; HPRT, hypoxanthine phosphoribosyltransferase; E, embryonic day.
References
- 1.Davie E W, Fujikawa K, Kisiel W. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Vu T-K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 3.Soifer S J, Peters K G, O’Keefe J, Coughlin S R. Am J Pathol. 1994;144:60–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 5.Morris R, Winyard P G, Blake D R, Morris C J. Ann Rheum Dis. 1994;53:72–79. doi: 10.1136/ard.53.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiernberg J, Redin W R, Warner W S, Carney D H. Thromb Haemostasis. 1993;70:158–162. [PubMed] [Google Scholar]
- 7.Festoff B W, Smirnova I V, Ma J, Citron B A. Semin Thromb Hemostasis. 1996;22:267–271. doi: 10.1055/s-2007-999018. [DOI] [PubMed] [Google Scholar]
- 8.Walz D A, Fenton J W. Invasion Metastasis. 1994;14:303–308. [PubMed] [Google Scholar]
- 9.Baykal D, Schmedtje J F J, Jr, Runge M S. Am J Cardiol. 1995;75:82B–87B. doi: 10.1016/0002-9149(95)80019-o. [DOI] [PubMed] [Google Scholar]
- 10.Bugge T H, Xiao Q, Kombrinck K W, Flick M J, Holmbäck K, Danton M J S, Colbert M C, Witte D P, Fujikawa K, Davie E W, Degen J L. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, van Vlaenderen I W, Demunck H, Kasper M, Breier G, Evrard P, et al. Nature (London) 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 12.Toomey J R, Kratzer K E, Lasky N M, Stanton J J, Broze G J., Jr Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 13.Toomey J R, Kratzer K E, Lasky N M, Broze G J., Jr Proc Natl Acad Sci USA. 1997;94:6922–6926. doi: 10.1073/pnas.94.13.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, O’Shea K S, Purkayastha A, Saunders T L, Ginsburg D. Nature (London) 1996;384:66–68. doi: 10.1038/384066a0. [DOI] [PubMed] [Google Scholar]
- 15.Connolly A J, Ishihara H, Kahn M L, Farese R V, Jr, Coughlin S R. Nature (London) 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 16.Darrow A L, Fung-Leung W-P, Ye R D, Santulli R J, Cheung W-M, Derian C K, Burns C L, Damiano B P, Zhou L, Keenan C M, Peterson P A, Andrade-Gordon P. Thromb Haemostasis. 1996;76:860–866. [PubMed] [Google Scholar]
- 17.Xue J, Wu Q, Westfield L A, Tuley E A, Lu D, Zhang Q, Shim K, Zheng X, Sadler J E. Proc Natl Acad Sci USA. 1998;95:7603–7607. doi: 10.1073/pnas.95.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Nature (London) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid L H, Gregg R G, Smithies O, Koller B H. Proc Natl Acad Sci USA. 1990;87:4299–4303. doi: 10.1073/pnas.87.11.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 21.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. Nature (London) 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 22.Bugge T H, Flick M J, Daugherty C C, Degen J L. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 23.Greer C E, Peterson S L, Kiviat N B, Manos M M. Am J Clin Pathol. 1991;95:117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- 24.Degen S J F, Schaefer L A, Jamison C S, Grant S G, Fitzgibbon J J, Pai J-A, Chapman V M, Elliott R W. DNA Cell Biol. 1990;9:487–498. doi: 10.1089/dna.1990.9.487. [DOI] [PubMed] [Google Scholar]
- 25.Witte D P, Aronow B J, Stauderman M L, Stuart W D, Clay M A, Gruppo R A, Jenkins S H, Harmony J A. Am J Pathol. 1993;143:763–773. [PMC free article] [PubMed] [Google Scholar]
- 26.Suh T T, Holmbäck K, Jensen N J, Daugherty C C, Small K, Simon D I, Potter S S, Degen J L. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 27.Rosen E D, Chan J C Y, Idusogie E, Clotman F, Vlasuk G, Luther T, Jalbert L R, Albrecht S, Zhong L, Lissens A, et al. Nature (London) 1997;390:290–294. doi: 10.1038/36862. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z-F, Higuchi D, Lasky N, Broze G J., Jr Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 29.Degen S J F. In: Molecular Basis of Thrombosis and Hemostasis. High K A, Roberts H R, editors. New York: Dekker; 1995. pp. 75–99. [Google Scholar]
- 30.Chan J C Y, Rosen E D, Carmeliet P, Huang Z-F, Broze G J, Jr, Castellino F J, Collen D. Blood. 1997;90:399a. (abstr.). [Google Scholar]