Abstract
Oscillatory electro-encephalographic activity at theta frequencies (4-15 Hz) can be recorded from the hippocampus in vivo and depends on intact septal projections. The hypothesis that these oscillations are imposed on the hippocampus by rhythmically active septal inputs was tested using dual intracellular recordings from CA1 and CA3 pyramidal cells in septo-hippocampal cocultures.
Septo-hippocampal cocultures displayed spontaneous oscillatory synaptic activity at theta frequencies. In CA3 cells, EPSP/IPSP sequences predominated, whereas only EPSPs were apparent in CA1 cells. Synaptic potentials in CA3 cells preceded those in CA1 cells by 5-10 ms.
Oscillatory synaptic activity was blocked in cocultures by the muscarinic antagonist atropine (0.1 μm), facilitated but unchanged in frequency upon application of the acetylcholinesterase inhibitor neostigmine (1 μm), and not seen in hippocampal monocultures.
The muscarinic agonist methacholine (5-20 nM) induced oscillatory synaptic activity at 4-15 Hz in hippocampal monocultures, which was identical to that occurring spontaneously in septo-hippocampal cocultures.
Synaptic theta activity was observed in cocultures of septal tissue with subdissected hippocampal slices containing area CA3 alone, but not in septo-CA1 cocultures.
We conclude that oscillatory synaptic activity at theta frequencies, with similar characteristics to theta activity in vivo, can be generated by the hippocampal network in response to activation of muscarinic receptors by synaptically released acetylcholine from septal afferents. Furthermore, the oscillatory activity is determined by mechanisms intrinsic to the hippocampal circuitry, particularly area CA3. Rhythmic septal input is not required.
During behaviour associated with learning and memory formation, the rodent hippocampus displays rhythmic oscillatory electro-encephalographic activity whose dominant frequency is 4-15 Hz (Green & Arduini, 1954; Vanderwolf, 1969; Winson, 1978). These so-called theta oscillations facilitate the induction of synaptic plasticity (Buzsáki, 1989; Huerta & Lisman, 1993), the presumptive basis of memory formation. The mechanisms underlying generation of theta oscillations are controversial.
There is substantial evidence supporting an essential role for septal cholinergic fibres in the generation of hippocampal theta oscillations in vivo (Gray, 1971; Andersen et al. 1979). Hippocampal theta activity in both anaesthetized and freely moving rats can be triggered by stimulation in the medial septum (Wetzel et al. 1977), as well as by intrahippocampal administration of acetylcholine (ACh) or muscarinic cholinergic agonists (Malisch & Ott, 1982; Bland & Colom, 1993). In addition, theta discharge in anaesthetized animals can be largely abolished by local infusion of muscarinic antagonists (Karmis et al. 1975), transection of the fornix/fimbria (Gray, 1971; Winson, 1978; Andersen et al. 1979), or selective destruction of septo-hippocampal cholinergic cells with an antibody-bound neurotoxin which targets the nerve growth factor (NGF) receptor (Lee et al. 1994).
It is widely believed that the characteristic frequency of this oscillatory activity is imposed upon the hippocampus by the rhythmic activity of the septal afferent cells (Stewart & Fox, 1990; Bland & Colom, 1993; Buzsáki & Chrobak, 1995). In several studies, application of ACh or muscarinic agonists to acutely isolated hippocampal brain slices has been shown to induce epileptiform discharge of which only a small transient component occurs at theta (MacVicar & Tse, 1989; Williams & Kauer, 1997) or gamma (Fisahn et al. 1998) frequencies. As this activity bears little resemblance to theta discharge in vivo, it has been suggested that the prominent septo-hippocampal GABAergic afferent system may play a critical role in determining the frequency of theta activity in the hippocampus (Buzsáki & Chrobak, 1995; Cobb et al. 1995; Toth et al. 1997).
We have re-examined this issue using electrophysiological techniques to record from a simplified in vitro analogue of the septo-hippocampal system: cocultures of tissue slices from the hippocampus and medial septum (Gähwiler et al. 1987). Our results indicate that the intrinsic properties of the hippocampal synaptic circuitry are more crucial for the determination of the frequency of theta oscillations than is currently appreciated.
METHODS
Hippocampal slice cultures and septo-hippocampal cocultures were prepared from 6-day-old rats killed by decapitation, and maintained for 3-6 weeks in vitro using the roller-tube technique (Gähwiler, 1981; Gähwiler & Brown, 1985; Gähwiler et al. 1987). All experiments were carried out according to guidelines laid down by the Swiss Federal Department for Veterinary Affairs. All isolated CA1 and CA3 cocultures were maintained in the presence of 50 ng ml−1 NGF, and full septo-hippocampal cocultures were cultured with either 0, 50 or 75 ng ml−1 NGF. Natural growth factor (Genentech Inc., San Francisco, CA, USA) was added continuously from the time of explantation. For electrophysiological recordings, cultures were transferred to a recording chamber mounted on an inverted microscope and continuously superfused with warmed (32°C) saline containing (mM): Na+, 145; Cl−, 149; K+, 2.7; Ca2+, 2.8; Mg2+, 2; HCO3−, 7.7; H2PO4−, 0.4; glucose, 5.6; and Phenol Red (10 mg l−1) at pH 7.4. Acetylcholinesterase staining was performed as described previously (Gähwiler et al. 1987). Methacholine (MCh), atropine and neostigmine were purchased from Sigma-Aldrich).
Paired recordings were made from pyramidal cells using sharp microelectrodes filled with 1 M potassium methylsulphate (30-60 MΩ). The analog signal was digitized at 22 kHz and recorded on a videotape recorder. For analysis, data were digitized at 1 kHz, without further filtering. Spectrograms were created using a windowed Fourier analysis which calculated the fast Fourier transform (FFT) in a sliding window of 500 ms. The frequencies of the Fourier analysis (2-500 Hz) were then plotted as a function of the time, using a false colour scale to provide the power of the spectrum in decibels (dB) (warm colours represent dominant frequencies). All analysis was done using Matlab (The MathWorks, Inc., Natick, MA, USA). In addition to standard techniques of calculating the windowed FFT, the routine used wavelet methods (Hubbard, 1996) to reduce white noise and improve the signal-to-noise ratio.
RESULTS
Synaptic theta activity in septo-hippocampal cocultures
Septal cholinergic cells were shown to provide numerous projection fibres to the hippocampus in septo-hippocampal cocultures by using acetylcholinesterase (AChE) histochemistry (Fig. 1), but intrinsic cholinergic neurons were not seen in cultures of hippocampus alone. Cholinergic septal fibres were shown previously to release acetylcholine and produce cholinergic synaptic responses in these cultures (Gähwiler & Brown, 1985).
Figure 1. Septo-hippocampal cocultures.
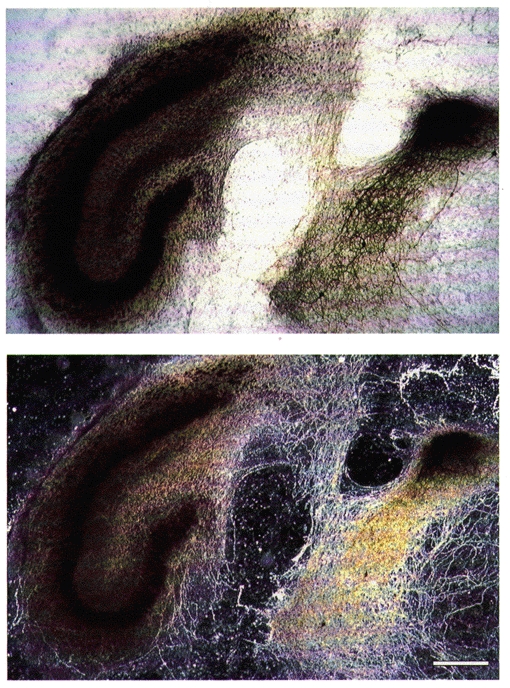
Septo-hippocampal coculture stained for the presence of AChE, viewed with bright- (upper) and dark-field optics (lower). Cholinergic neurons are observed within the septal slice (dark cell, bright-field optics). They send AChE-positive fibres (white fibres, dark-field optics) to innervate the hippocampal slice. Scale bar for both: 400 μm.
In the absence of stimulation, spontaneous synchronous oscillatory synaptic potentials were observed with simultaneous intracellular recordings from CA3 and CA1 pyramidal cells. At their resting membrane potential (-61.9 ± 4.9 mV (mean ±s.d.), n = 42), CA3 pyramidal cells primarily displayed depolarizing and/or hyperpolarizing sequences of excitatory and inhibitory postsynaptic potentials (EPSPs/IPSPs), whereas CA1 pyramidal cells at similar membrane potentials (-62.3 ± 3.9 mV (mean ±s.d.), n = 42) displayed only depolarizing EPSPs (Fig. 2A). The EPSPs in CA1 cells ranged in amplitude from 0.5-10 mV. These potentials are thus slightly larger than the mean amplitude of unitary EPSPs in CA1 cells in response to action potentials in single CA3 cells (Debanne et al. 1995), indicating that relatively few CA3 cells are discharging synchronously. Indeed, in 80 % (34/42) of the cocultures, the frequency of action potentials in CA3 cells never exceeded 0.5 Hz during periods of sustained synaptic theta activity.
Figure 2. Synaptic theta oscillations in septo-hippocampal cocultures.
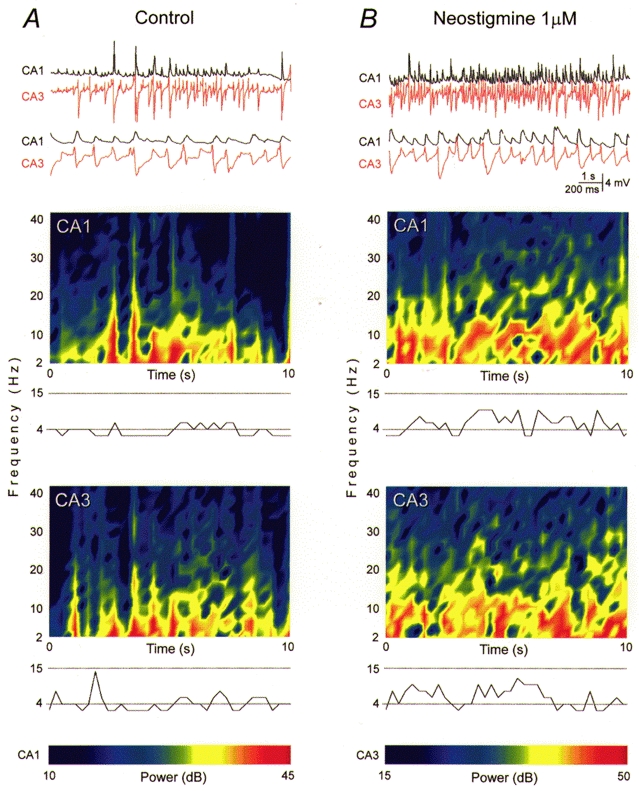
Synchronous oscillatory synaptic activity in a septo-hippocampal coculture. A, top: simultaneous intracellular recordings of membrane potential in CA1 and CA3 pyramidal cells are illustrated at two time scales (upper traces, 10 s; lower traces, 2 s from the upper segment). Resting membrane potential -65 mV for both cells. Bottom, spectrograms of the 10 s recordings shown above reveal the occurrence of oscillations at theta frequencies. The spectrogram shows the changes in FFT profile as a function of time. For each time window (500 ms), frequencies with higher power are depicted in warm colours (calibration scale for both cells at bottom). A plot of the frequency with the highest power in each window is provided below. Dominant frequencies were consistently within the so-called theta band of 4-15 Hz. B, membrane potential changes at two time scales recorded during application of the AChE inhibitor neostigmine (1 μm) to the same cell pair as in A, accompanied by the corresponding spectrograms and dominant frequency plots. Note that the synaptic potential amplitudes and the dominant frequencies were comparable with those in A, but that this synaptic theta activity became more continuous.
Spectrographic analysis indicated that the dominant frequencies of the synaptic activity in CA3 and CA1 cells were primarily within the theta range (4-15 Hz; Fig. 2A). A significant contribution of synaptic signals within the gamma range of frequencies (20-80 Hz) was never seen (power > 30 dB less than the dominant frequency) (cf. Fisahn et al. 1998). We shall refer to this oscillatory discharge as synaptic theta activity.
Spontaneous synaptic theta activity in septo-hippocampal cocultures was abolished by the muscarinic antagonist atropine (0.1 μm; n = 11, not shown), indicating that it requires release of ACh from septal neurons.
Synaptic theta activity in CA3 and CA1 pyramidal cells was tightly coupled and nearly in phase (Fig. 2), as is theta discharge in situ (Winson, 1974; Buzsáki et al. 1986). Cross-correlation analysis indicated that the EPSPs in the CA1 cells were delayed by 5-10 ms relative to the simultaneously recorded synaptic potentials in CA3 cells. This delay corresponds to monosynaptic excitation in paired CA3-CA1 intracellular recordings (Debanne et al. 1995).
We next asked how synaptic theta activity would be affected if the action of the endogenous ACh was facilitated by inhibition of the AChE activity with neostigmine. In control saline, periods of synaptic theta activity typically lasted from several seconds to minutes, and were interrupted by periods of non-rhythmic activity (Fig. 2A). Neostigmine (1 μm) prolonged the periods of oscillatory synaptic activity until they occurred continuously for periods > 1 h in duration (Fig. 2B). The amplitude of the largest synaptic potentials did not change notably, but their amplitudes became more consistently large. Application of neostigmine also revealed synaptic theta activity in two of seven cocultures not displaying such behaviour in control saline.
Synaptic theta oscillations could be observed in 22 % (2/9) of recordings from hippocampus and septum grown in normal culture medium. Application of NGF during the entire period of tissue cultivation enhances the density of the functional cholinergic connections between septal and hippocampal slices (Gähwiler et al. 1987). Consistent with this denser innervation, oscillatory synaptic activity at theta frequencies was observed in 64 % (21/33) of cocultures treated during their entire growth with 50 or 75 ng ml−1 NGF (significantly greater than untreated, P < 0.05, Mann-Whitney U test). There was no qualitative difference in the frequency distribution of the oscillatory responses recorded from pyramidal cells in NGF-treated cultures, but the periods of steady theta activity were more prolonged.
Cholinergic induction of theta activity in hippocampal monocultures
How is this synaptic theta activity generated? Although cholinergic inputs from the septum are required for generation of theta activity in vivo, attempts to establish an in vitro model for the generation of theta activity by applying exogenous cholinergic agonists (at the micromolar concentration range) to ex vivo hippocampal slices have been disappointing. Only oscillatory activity at gamma frequencies (Fisahn et al. 1998) or brief periods of theta activity accompanied by strong epileptiform discharge (MacVicar & Tse, 1989; Williams & Kauer, 1997) have been observed. Similar epileptiform discharge was obtained in hippocampal slice cultures upon application of the muscarinic agonist methacholine at concentrations of 2-10 μm. The synchronous paroxysmal activity, characterized by prolonged bursts of action potentials lasting for 10-100 s punctuated by periods of synchronous inactivity, was observed (not shown; n = 5), and was identical to activity elicited by the convulsant bicuculline. Although instantaneous firing rates were between 4-15 Hz during these bursts, this discharge resembles neither synaptic theta activity in septo-hippocampal cocultures nor theta activity in vivo (Ylinen et al. 1995) in which the oscillations are continuous and only low rates of action potential discharge occur.
Application of MCh at 1000-fold lower concentrations (5-20 nM), in contrast, readily induced synaptic theta activity in hippocampal monocultures, and never generated epileptiform burst activity (all of the 96 cultures). As in septo-hippocampal cocultures, the intracellularly recorded activity of CA1 pyramidal cells consisted primarily of synchronous EPSPs, whereas synchronous EPSP/IPSP sequences predominated in CA3 pyramidal cells (Fig. 3A). The rhythmic activity induced by MCh was shown by spectral analysis to display dominant frequencies in the theta range (4-15 Hz; Fig. 3B). MCh-induced synaptic theta activity was phase locked in dual CA3-CA1 cell recordings (Fig. 3A). Oscillations in area CA1 were delayed by 5-10 ms relative to oscillations in CA3 cells (Fig. 3C). Oscillatory synaptic activity was reversed within 10 min of wash out of the agonist, and never occurred spontaneously in control cultures. MCh-induced synaptic theta activity was mediated by muscarinic receptors, as it was abolished by 0.1 μm atropine (n = 12).
Figure 3. Cholinergic induction of synaptic theta oscillations in hippocampal slice cultures.
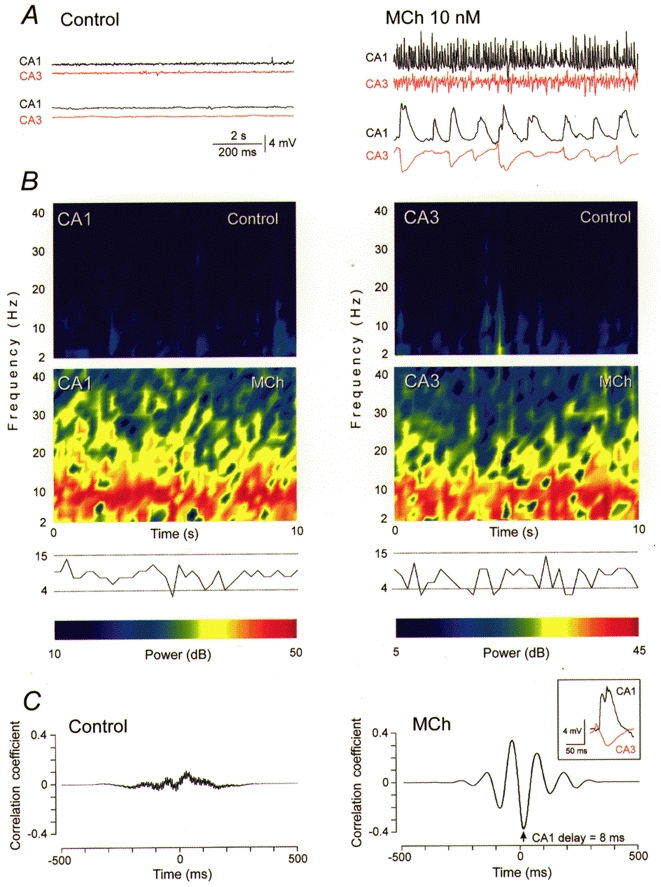
Synaptic theta activity induced by 10 nM MCh in a hippocampal monoculture. A, membrane potential changes were simultaneously recorded intracellularly from CA1 and CA3 pyramidal cells before (left) and during (right) MCh application. Data are illustrated at two time scales (upper traces, 10 s recording; lower traces, 1 s from the upper segment). Resting potentials in CA1 and CA3 cells were -62 and -60 mV, respectively. B, spectrograms of the 10 s recordings in A reveal the occurrence of oscillatory synaptic activity at theta frequencies (dominant frequencies consistently 4-15 Hz). C, averaged cross-correlations for the 10 s recordings illustrated in A, showing that synaptic activity is not synchronized in control saline, but that strongly correlated synaptic activity is induced by MCh. The activity in area CA3 precedes that in area CA1 by a mean of 8 ms during MCh application. Individual events are illustrated in the inset and show the synaptic potential in the CA3 cell preceding the EPSP in the CA1 cell. Note the similarity of the MCh-induced synaptic events in hippocampal monocultures to those seen in septo-hippocampal cocultures (Fig. 2).
Cross-correlation analysis in septo-hippocampal cocultures and hippocampal monocultures suggested that synaptic theta activity in area CA1 is driven by synaptic excitation originating in area CA3. To test this hypothesis, we cocultured medial septal slices with either isolated CA3 or CA1 sections, subdissected from the hippocampal slices at the time of explantation (Fig. 4). Synaptic theta activity was observed spontaneously in 75 % (n = 9/12) of the isolated CA3 cocultures in control saline. The percentage was increased to 89 % (n = 8/9) by neostigmine (1 μm), and all cultures (n = 11) responded with synaptic theta activity when MCh (10 nM) was applied (Fig. 4). All oscillatory activity was blocked by atropine (0.1 μm; n = 8). In contrast, in isolated CA1 cocultures lacking CA3 cells, synaptic theta activity was observed spontaneously or with neostigmine in only 1/12 experiments. Likewise, only 1/12 isolated CA1 cocultures displayed MCh-induced synaptic theta activity (Fig. 4). We conclude that area CA1 is incapable of responding to cholinergic afferents with the generation of rhythmic synaptic theta activity.
Figure 4. Synaptic theta oscillations in septal slices cocultured with isolated CA1 or CA3.
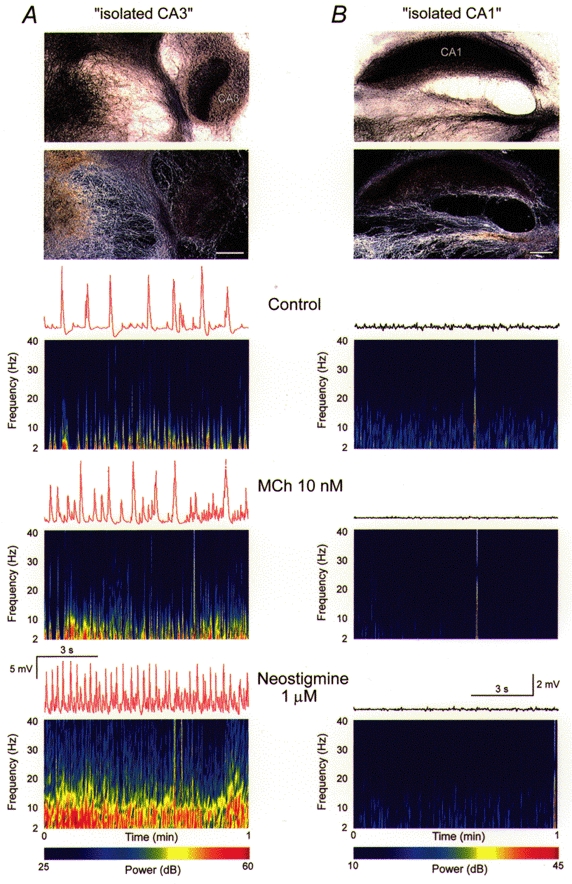
Synaptic theta activity in isolated CA3, but not CA1 septo-hippocampal cocultures. A, top: coculture of septal and isolated CA3 slices, stained for the presence of AChE and viewed with bright- (upper) and dark-field optics (lower). Area CA3 can be seen on the right, whereas cholinergic cells are visible in the septal tissue (left). Note the cholinergic fibre ingrowth to the isolated CA3. Scale bar = 400 μm. Bottom, intracellular recordings (10 s) and spectrograms of activity (1 min each) for a CA3 cell in control saline (upper), in 10 nM MCh (middle), and in 1 μm neostigmine without MCh (lower). Note that oscillatory synaptic activity at 4-15 Hz occurs under all three conditions. Resting potential -73 mV. B, top: coculture of septal and isolated CA1 slices, stained for AChE and visualized as in A. The CA1 slice occupies the upper part of the image. The coculture contained septal cholinergic cells projecting to the isolated CA1 tissue. Bottom, intracellular recordings (10 s) and spectrograms of activity (1 min) in control saline (upper), in 10 nM MCh (middle), and in 1 μm neostigmine alone (lower). No oscillatory synaptic activity occurred under any condition. Resting potential -63 mV. We conclude that oscillatory synaptic theta activity can be generated in area CA3, but not area CA1.
DISCUSSION
In summary, we have shown that synaptic theta activity in septo-hippocampal cocultures depends upon the synaptic release of acetylcholine which then activates muscarinic receptors. Prolongation of the oscillations by neostigmine indicates that the extent of ACh release and its rate of hydrolysis are important in determining the duration of oscillatory activity.
How ACh released from septal fibres induces theta activity in the hippocampus remains controversial. It has been suggested that oscillatory activity at theta frequencies is imposed on a relatively passive hippocampus by a rhythmic septal pacemaker through cholinergic (Bland & Colom, 1993) and/or non-cholinergic (Stewart & Fox, 1990) septal activity. A direct synchronizing action of cholinergic septal fibres on pyramidal cell discharge appears unlikely in view of the slow time course of muscarinic EPSPs (Gähwiler & Brown, 1985).
Non-cholinergic septal pacemaker activity, thought to be mediated by septal GABAergic fibres that innervate hippocampal interneurons (Freund & Antal, 1988; Buzsáki & Chrobak, 1995; Toth et al. 1997), might indirectly induce oscillatory hippocampal activity. In this model, the action potential discharge in hippocampal interneurons would be synchronized by the rhythmic inhibition produced by septal GABAergic inputs. The IPSP resulting from synchronous activity in hippocampal interneurons would then allow pyramidal cells to fire only during the repolarizing phase, and their discharge would thus be phase locked to the rhythmicity of the septal input. Synchronized septal GABAergic activity at theta frequencies would thus entrain pyramidal cell discharge (Cobb et al. 1995; Toth et al. 1997). Indeed, some theta activity persists in the hippocampus of unanaesthetized animals after block of cholinergic receptors (Karmis et al. 1975) or destruction of septal cholinergic neurons (Lee et al. 1994). However, such a mechanism requires that septal GABAergic cells discharge in synchrony at theta frequencies.
The evidence for such a septal theta pacemaker activity remains ambiguous. Rhythmic septal discharge occurs in only a subset of cells and displays variable coherence with the hippocampal theta cycle (Brazhnik & Fox, 1997; King et al. 1998). It is not known whether these cells are GABAergic or cholinergic projection cells, or whether their phasic activity is intrinsic. The role of septal pacemaker activity in relation to the mechanisms underlying the generation of hippocampal theta oscillations thus remains uncertain.
We have demonstrated that the hippocampal network can respond to septal cholinergic inputs by generating a high level of spontaneous synaptic activity that oscillates synchronously at theta frequencies. The frequency and amplitude of synaptic theta oscillations were identical whether they occurred spontaneously in septo-hippocampal cocultures or were elicited by application of nanomolar concentrations of MCh to hippocampal monocultures. The ability of a bath-applied muscarinic agonist to produce the identical pattern of complex ensemble behaviour suggests that no additional rhythmically active non-cholinergic inputs to the hippocampus are required to elicit synaptic theta activity. Nevertheless, our preparation may not mimic the in vivo situation perfectly, where additional mechanisms may also contribute to the generation of hippocampal theta oscillations. The occurrence of synaptic theta activity in isolated CA3, but not CA1, cultures demonstrates that the essential determinants of intrinsic hippocampal theta oscillations are located within area CA3.
We conclude therefore that hippocampal theta discharge can be triggered solely by global muscarinic receptor activation, and that the timing and synchronization of this activity is determined by the excitatory and inhibitory synaptic circuitry intrinsic to the hippocampus, particularly area CA3. This oscillatory input from area CA3, together with direct oscillatory input from the entorhinal cortex (Buzsáki et al. 1986; Ylinen et al. 1995), is then responsible for driving rhythmic theta activity in area CA1. It is interesting to note that pyramidal cells in hippocampal slice cultures adopt an oscillatory mode of synaptic activity at theta frequencies upon MCh application, despite the fact that this purely pharmacological trigger conveys no temporal information.
Acknowledgments
We gratefully acknowledge the technical assistance of Dr R. Dürr, L. Heeb, E. Hochreutener, R. Kägi, H. J. Kasper, L. Rietschin, H. P. Rothenbühler and R. Schöb. This work was supported by the Dr Eric Slack-Gyr and Swiss National Science Foundations.
References
- Andersen P, Bland HB, Myhrer T, Schwartzkroin PA. Septo-hippocampal pathway necessary for dentate theta production. Brain Research. 1979;165:13–22. doi: 10.1016/0006-8993(79)90040-4. [DOI] [PubMed] [Google Scholar]
- Bland BH, Colom LV. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Progress in Neurobiology. 1993;41:157–208. doi: 10.1016/0301-0082(93)90007-f. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Intracellular recording from medial septal neurons during hippocampal theta rhythm. Experimental Brain Research. 1997;114:442–453. doi: 10.1007/pl00005653. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Czopf J, Kondákor I, Kellényi L. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Research. 1986;365:125–137. doi: 10.1016/0006-8993(86)90729-8. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. Journal of Neurophysiology. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. Journal of Neuroscience Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA. Functional innervation of cultured hippocampal neurones by cholinergic afferents from cocultured septal explants. Nature. 1985;313:577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Enz A, Hefti F. Nerve growth factor promotes development of the rat septo-hippocampal cholinergic projection in vitro. Neuroscience Letters. 1987;75:6–10. doi: 10.1016/0304-3940(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Gray JA. Medial septal lesions, hippocampal theta rhythm and control of vibrissal movement in freely moving rat. Electroencephalography and Clinical Neurophysiology. 1971;30:189–197. doi: 10.1016/0013-4694(71)90053-8. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. Journal of Neurophysiology. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Hubbard BB. The World According to Wavelets. Wellesley, MA, USA: A. K. Peters; 1996. [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Karmis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relation to behavior and effects of atropine, diethyl ester, urethane and pentobarbital. Experimental Neurology. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in awake freely moving rat: relationships with behaviour and hippocampal theta. European Journal of Neuroscience. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsáki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1074. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FWY. Local neuronal circuitry underlying cholinergic rhythmical slow activity in area CA3 of rat hippocampal slices. The Journal of Physiology. 1989;417:197–212. doi: 10.1113/jphysiol.1989.sp017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch R, Ott T. Rhythmical slow wave electroencephalographic activity elicited by hippocampal injection of muscarinic agents in the rat. Neuroscience Letters. 1982;28:113–118. doi: 10.1016/0304-3940(82)90217-8. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends in Neurosciences. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. The Journal of Physiology. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Ott T, Matthies H. Hippocampal rhythmic slow activity (‘theta’) and behavior elicited by medial septal stimulation in rats. Behavioral Biology. 1977;19:534–542. doi: 10.1016/s0091-6773(77)92038-7. [DOI] [PubMed] [Google Scholar]
- Williams JH, Kauer JA. Properties of carbachol-induced oscillatory activity in rat hippocampus. Journal of Neurophysiology. 1997;78:2631–2640. doi: 10.1152/jn.1997.78.5.2631. [DOI] [PubMed] [Google Scholar]
- Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalography and Clinical Neurophysiology. 1974;36:291–301. doi: 10.1016/0013-4694(74)90171-0. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Soltész I, Bragin A, Penttonen M, Sik A, Buzsáki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cell, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]


