Abstract
We investigated the effects of sympathetic nerve stimulation within ascending and descending reflex pathways underlying the peristaltic reflex in the guinea-pig distal colon.
A three-chambered partitioned bath was used to divide a segment of distal colon into stimulation, recording and intermediate regions. The effects of lumbar colonic nerves (LCN) could be localized to the intermediate region by surgical lesions of the mesentery and by application of guanethidine (3 μm) to the stimulation and recording chambers.
Brush stroking the mucosa in the anal and oral stimulation chambers elicited a synchronous contraction of the longitudinal muscle (LM) and circular muscle (CM) oral to, and transient relaxation of the LM and CM anal to, the stimulus, respectively.
After Nω-nitro-L-arginine (L-NA; 100 μm) in the oral and intermediate chambers, mucosal stimulation in the oral chamber elicited a prolonged descending inhibitory and excitatory complex in both the LM and CM in the anal recording chamber. This was blocked by hexamethonium (300 μm), which did not affect the transient relaxation response recorded in control conditions.
Stimulation of the LCN (1200 pulses, 20 Hz), delivered to the intermediate region, abolished the oral contraction and the L-NA-induced anal complex in both the LM and CM, but was without effect on the transient hexamethonium-resistant anal relaxation. These effects of LCN stimulation were reversed by phentolamine (3 μm) or yohimbine (100 nM), but not propranolol (10 μm), when added to the intermediate chamber.
LCN stimuli (2-20 Hz, 600 μs pulses) directed to the recording chamber elicited synchronous relaxations in the LM and CM that were unaffected by hexamethonium (300 μm), but were reduced by yohimbine and usually blocked by the further addition of propranolol (10 μm).
In conclusion, sympathetic nerve stimulation inhibits orally and anally projecting cholinergic interneurones underlying the peristaltic reflex in the distal colon. In addition, the LM and CM relax synchronously following release of sympathetic neurotransmitter, over a range of stimulus frequencies.
It is generally believed that stimulation of the sympathetic nervous system generates an inhibitory action on the movements of the large intestine (Bayliss & Starling, 1900; Garry & Gillespie, 1955; Gillespie, 1962; Bennett et al. 1966; Mackenna & McKirdy, 1972; Furness & Costa, 1987; Szurszewski & Miller, 1994). There is considerable evidence that this inhibition is mediated largely via an indirect action on the enteric nervous system, rather than directly on the smooth muscle. This has been supported, in many species, by immunohistochemical studies that have revealed nerve terminals with intense immunoreactivity for tyrosine hydroxylase surrounding neurones within the myenteric plexus, and a relatively sparser innervation in the smooth muscle (Norberg, 1964; Furness, 1970; Furness & Costa, 1973; Gabella, 1979; Burnstock & Wong, 1981; Spencer et al. 1998a). In addition, during direct intracellular recordings from myenteric neurones it has been shown that stimulation of sympathetic fibres inhibits spontaneous and evoked fast synaptic transmission within the myenteric plexus (Hirst & McKirdy, 1974), and there is considerable evidence that this is likely to occur via presynaptic inhibition of the release of acetylcholine (Hirst & McKirdy, 1974; Tack & Wood, 1992). However, it is unclear which myenteric neurones receive sympathetic inputs in the colon and, moreover, the role of this innervation during the peristaltic reflex in the large bowel is unclear.
In addition to a lack of knowledge of the classes of enteric neurone under sympathetic neuronal control, the effects of sympathetic stimulation on the relative movements of the circular muscle (CM) and longitudinal muscle (LM) layers have received little attention. Since the late 1960s, there has been some controversy associated with the relative movements of the CM and LM layers in the gastrointestinal tract (see the introductions in McKirdy, 1972 and Smith & Robertson, 1998). Kottegoda (1969, 1970) put forward the once generally accepted idea of reciprocal innervation, i.e. when the CM layer contracts, the LM relaxes during peristalsis and vice versa. However, he appears to have overlooked any possibility of passive mechanical interactions that may occur between the movements of the LM and CM, which can lead to false interpretations of their relative movements (see Gregory & Bentley, 1968; Wood & Perkins, 1970; McKirdy, 1972). When dissection techniques are employed to prevent such mechanical interactions between the muscle layers, they are found to contract and relax synchronously in response to a peristaltic wave (Smith & Robertson, 1998) and reflex stimulation of the mucosa (colon: Smith & McCarron, 1998; ileum: Spencer et al. 1999). However, in response to stimulation of extrinsic nerves, the relative movements of the LM and CM layers is less clear. Some investigators observed that both muscle layers of the large intestine relaxed in response to sympathetic nerve stimulation (canine large intestine: Bayliss & Starling, 1900; rabbit large intestine: Garry & Gillespie, 1955; Mackenna & McKirdy, 1972) but contracted in response to pelvic nerve stimulation (McKirdy, 1972). In some studies, however, it has been reported that sympathetic nerve stimulation elicits contractions in the CM layer (Lee, 1960; Venkova & Krier, 1993; Luckensmeyer & Keast, 1998) but relaxation of the LM (Lee, 1960; Luckensmeyer & Keast, 1998) of the proximal colon. In these latter studies, it is unclear whether the two muscle layers were sufficiently mechanically isolated from each other, so that the actual movements of the circular and longitudinal muscles could be studied unambiguously following extrinsic nerve stimulation.
In the current study, we have used our previously described dissection techniques (Smith & Robertson, 1998; Smith & McCarron, 1998; Spencer et al. 1999) to unambiguously investigate the effects of sympathetic nerve stimulation on the relative movements of the LM and CM layers of the colon. Furthermore, we have used the partitioned organ bath technique to localize the effects of sympathetic nerve stimulation to particular neural elements within ascending and descending reflex pathways.
METHODS
Guinea-pigs weighing approximately 250-350 g were killed by CO2 inhalation overdose, in accordance with the regulations of the animal ethics committee of the University of Nevada School of Medicine. The abdominal cavity was incised in the longitudinal axis and the terminal 6-8 cm of colon were removed, while preserving extrinsic neural continuity with the lumbar colonic nerves (LCN). The inferior mesenteric ganglion (IMG) was not excised from the animal. Faecal pellets were expelled from the colon by gentle purging of the lumen with fresh modified Krebs solution (composition below).
Dissection of the circular muscle
To avoid mechanical interactions between the movements of the LM and CM layers, the CM was dissected away from the myenteric plexus and LM using similar dissection techniques to those recently employed by Smith & Robertson (1998) and Smith & McCarron (1998). In brief, approximately 15-20 mm of the oral or anal extremity of the distal colon was opened along the mesenteric attachment (while carefully preserving extrinsic neural pathways to the LCN) and this region pinned to the base of a Sylgard (Dow Corning)-lined Petri dish, so that the mucosal surface faced uppermost. The mucosa and submucosa were then removed from this exposed region to expose the underlying CM. Strips of CM were then removed to expose the underlying myenteric plexus (see Fig. 2 of Smith & Robertson, 1998). Preparations therefore consisted of a flap of LM and associated myenteric plexus that remained in neural continuity with the enteric plexuses of the remaining preparation of colon (Fig. 1). For preparations in which the oral reflexes were investigated, the dissection techniques were identical, except that the orientation of the preparation was reversed.
Figure 1. Partitioned chamber used to localize the effects of lumbar colonic nerve stimulation.
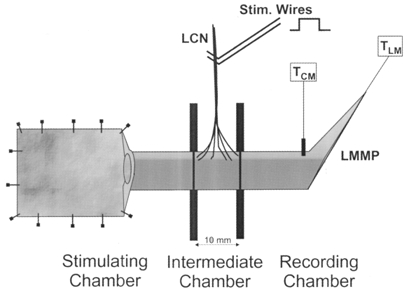
A segment of distal colon with lumbar colonic nerves (LCN) intact in a particular region was threaded through a greased hole in each of 2 rubber diaphragms. The diaphragms separated the bath into stimulation, recording and intermediate chambers (10 mm = length of intermediate chamber). Tension of the longitudinal muscle (LM) with attached myenteric plexus (LMMP) was monitored in the recording chamber by connecting the oral or anal dissected end of the segment to an isometric tension transducer (TLM). Tension of the circular muscle (TCM) was measured using a clip attached orthogonal to the length of the segment. Mechanical stimulation was applied to the mucosa in the opened portion of the segment lying in the stimulation chamber. The segment of colon that received the major distribution of perivascular arcades from the LCN was positioned into the intermediate chamber (or recording chamber), and the mesentery along the colon in the stimulation and recording chamber was removed. Furthermore, addition of guanethidine (3 μm) to the stimulation and recording (but not intermediate) chambers was used to ensure that the effects of sympathetic stimulation were in fact localized to the intermediate chamber. In some experiments the LCN were left intact in the recording chamber and removed from the colon in the other chambers. In this instance guanethidine was added to the stimulation and intermediate chambers. Fine transmural stimulating electrodes were then mounted on the LCN.
Reflex stimulation and mechanical recording of longitudinal and circular muscle activity
At the oral or anal extremity of the colon, changes in LM tension were recorded using silk threads that were connected to an isometric tension transducer (Kent Scientific Corporation) (see Smith & McCarron, 1998). The mechanical activity of the CM was recorded using a small clip (Micro-serrefines No 18055-04; Fine Science Tools Inc., Foster City, CA, USA) attached to the serosal surface. Both the muscles were mounted under an initial resting tone of 1 g, so that we could directly compare our results with a previous study of the distal colon (Smith & McCarron, 1998), where 1 g resting tension was also used.
Oral and anal reflex responses of the LM and CM were elicited by brush stroking the mucosa in the oral or anal stimulation chamber, using five brush strokes, delivered via a fine artists’ paint brush (see Smith & Furness, 1988; Smith & McCarron, 1998).
Electrical nerve stimulation of the lumbar colonic nerves
Fine silver wires were used to transmurally stimulate the lumbar colonic nerves using stimulus trains (100-1200 pulses, 600 μs pulse width) at frequencies ranging from 1-20 Hz with a Grass S44 stimulator (Quincy, MA, USA) that was triggered by a timer (Master-8-cp, AMPI, Jerusalem, Israel).
Partitioned bath
For the selective addition of drugs to certain regions of colon, or the selective activation of a region of colon by the sympathetic nerves, a partitioned organ bath was used. An identical procedure to that in the study of Smith & McCarron (1998) was used, with the major difference being that in the present study, the LCN in continuity with the distal colon was preserved and carefully positioned into a particular chamber (see Fig. 1). To test the effects of sympathetic nerve stimulation on interneurones, the region of colon with intact LCN was mounted between the partitions so that the inferior mesenteric artery and accompanying sympathetic fibres were located in the intermediate chamber. The action of sympathetic nerve stimulation was localized to the intermediate chamber by (1) severing the mesenteric attachment in the oral and anal chambers and (2) by application of guanethidine (3 μm) to the oral and anal, but not to the intermediate, chambers. To examine the direct effects of sympathetic nerve stimulation on the relative movements of the LM and CM, we mounted the segment so that the LCN were positioned in the anal or oral recording chamber.
All three chambers were constantly oxygenated with modified Krebs solution at 36°C. After mounting the preparation in the organ bath, an equilibration period of approximately 1-1.5 h was allowed prior to eliciting mucosal reflexes.
Drugs and solutions
The composition of the modified Krebs solution was (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.5. The solution was gassed continuously with a mixture containing 3 % CO2-97 % O2 (v/v), pH 7.3-7.4. In some experiments, the composition of the Krebs solution was modified to block all synaptic transmission in the colon (see Bywater, 1994). This was performed by a 10-fold reduction in the Ca2+ concentration and by raising the Mg2+ concentration 10-fold to stabilize the extracellular ionic composition across the cell membrane. This low Ca2+/high Mg2+ solution contained (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 12.0; CaCl2, 0.25; and glucose, 11.5.
The following drugs were used throughout the course of these experiments: atropine sulphate, clonidine, hexamethonium bromide, guanethidine, Nω-nitro-L-arginine (L-NA), propranolol and tetrodotoxin (TTX) (all from Sigma Chemical Co.) and yohimbine (from RBI).
Analysis of data and statistics
To facilitate comparison of responses between animals, measurements were made of the responses obtained from five brush strokes, as this stimulus has been shown to elicit near-maximal responses in this tissue (Smith & McCarron, 1998). Statistical assessment of the responses of the LM and CM was made by comparing the mean of responses obtained before and after equilibration of the drug treatment. The amplitude and area under stimulated responses were measured using Acqknowledge 3.2.6 (BIOPAC Systems, Inc., Santa Barbara, CA, USA) and tests for statistical significance were made using SigmaPlot 5.0 (Jandel Scientific, San Rafael, CA, USA). The area under the mechanical responses was used for comparison of drug treatments (or responses to nerve stimulation), as this sensitive parameter reflects the force generated by the tissue over time, expressed as millinewton seconds (mN s). In Results, the use of n refers to the number of animals on which experiments were performed. All data are presented as the mean ±s.e.m. Statistical comparison of data was performed using Student's paired t tests and a minimum level of significance was reached at P < 0.05.
RESULTS
Effects of sympathetic nerve stimulation on ascending transmission to the longitudinal and circular muscles
We examined the effects of localized sympathetic stimulation on ascending neurotransmission associated with the ascending excitatory reflex (Fig. 2). To do this, we recorded the mechanical activity of the LM and CM in the oral chamber and applied five brush strokes to the mucosa in the anal chamber. LCN stimulation was confined to the intermediate chamber.
Figure 2. Effects of localized sympathetic stimulation on ascending neurotransmission.
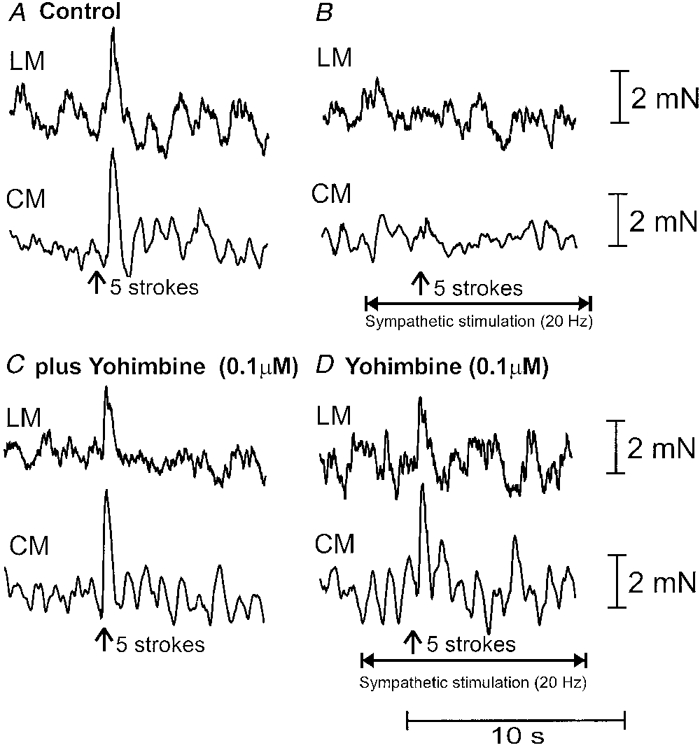
A, brush-stroking the mucosa (5 strokes) in the anal stimulating chamber elicited ascending excitation, whereby a synchronous contraction was elicited in the longitudinal muscle (LM) and circular muscle (CM) in the oral recording chamber. B, prior to mucosal stroking (10 s), a train of stimuli was delivered to the LCN (200 pulses, 20 Hz, 600 μs; see horizontal arrow) and 5 brush strokes were delivered to the mucosa at the vertical arrow. It can be seen that LCN stimulation blocked the orally migrating reflex contraction of the LM and CM in the oral recording chamber. C, the addition of yohimbine (100 nM) to the intermediate chamber did not affect the control oral reflex. D, yohimbine (100 nM) blocked the inhibitory actions of LCN stimulation.
Mucosal stimulation applied in the anal chamber elicited a synchronous contraction of the LM (amplitude: 2.1 ± 0.2 mN; area: 6.0 ± 1.2 mN s; n = 8) and CM (amplitude: 6.7 ± 2.1 mN; area: 21.5 ± 6.6 mN s; n = 8) in the oral chamber (Fig. 2A). Hexamethonium to either the stimulation, intermediate or recording chambers has been shown to substantially reduce the oral reflex contraction (Smith & McCarron, 1998), suggesting that fast cholinergic synaptic potentials in ascending interneurones largely mediate transmission during these oral reflexes.
When a train of stimuli was delivered to the LCN (200- 300 pulses, 20 Hz, 600 μs pulse width) (in the intermediate chamber), 10 s prior to brush stroking in the anal chamber, it was found that the contraction of the LM and CM was abolished in the oral recording chamber (Fig. 2B; P < 0.05; n = 5). No change was noted in the background mechanical activity of either the LM or CM in the oral recording chamber during LCN stimulation, suggesting that sympathetic transmitter action had been blocked by guanethidine in this region of colon. Also, the effects of sympathetic nerve stimulation were specific and not due to the spread of current to the colon, since responses were abolished upon severing the LCN or by adding guanethidine (3 μm) to the intermediate chamber.
We investigated whether blockade of α2-adrenoceptors in the intermediate chamber would reverse the effects of LCN stimulation. It was found that application of yohimbine (100 nM) to the intermediate chamber reversed the effects of LCN stimulation on the ascending mucosal reflex recorded in the oral chamber (Fig. 2C and D); the mean contractile responses obtained during LCN stimulation in the presence of yohimbine (100 nM) (LM: 4.0 ± 0.6 mN s and CM 9.1 ± 1.5 mN s; n = 5) were 68.2 ± 9.8 and 78.4 ± 17.2 % of the control responses of the LM and CM, respectively. These values were significantly different from reflex responses obtained during LCN stimulation prior to yohimbine application to the intermediate chamber (P < 0.05; n = 5).
Effects of sympathetic stimulation on descending transmission to the longitudinal and circular muscle layer
Brush stroking the mucosa (1-5 strokes) in the oral chamber consistently elicited a transient relaxation, synchronously in the LM and CM, in the anal recording chamber (Fig. 3A). Relaxations of the LM and CM had peak amplitudes of 2.1 ± 0.2 mN (area: 8.2 ± 0.7 mN s) and 3.1 ± 0.3 mN (area: 12.0 ± 1.2 mN s; n = 29), respectively. To test the involvement of nicotinic transmission in the descending transmission of these transient relaxations, hexamethonium (300 μm) was added to the intermediate chamber. It was found that hexamethonium (300 μm) was without effect on the transient relaxations of either the LM (control: 7.3 ± 0.6 mN s; hexamethonium: 7.3 ± 1.1 mN s; P > 0.05; n = 8) or the CM (control: 12.6 ± 1.8 mN s; hexamethonium: 14.0 ± 2.1 mN s; P > 0.05; n = 8) in the anal recording chamber. The further addition of atropine (2 μm) to the intermediate chamber (while in the presence of hexamethonium) was also without effect on the relaxations of either the LM (hexamethonium: 7.3 ± 1.1 mN s; atropine: 8.3 ± 1.4 mN s; P > 0.05; n = 7) or CM (hexamethonium: 14.0 ± 2.1 mN s; atropine: 11.9 ± 2.3 mN s; P > 0.05; n = 7) (Fig. 3C), suggesting the possibility that non-cholinergic descending interneurones may be involved in transmission of descending inhibition to the LM and CM. To further test the presence of non-cholinergic synapses in the intermediate chamber, the composition of the Krebs solution was changed to a low Ca2+/high Mg2+ solution to block all synaptic transmission in this chamber (see Bywater, 1994). It was found that after approximately 30-45 min of the low Ca2+/high Mg2+ solution in the intermediate chamber, the relaxation responses were attenuated by 73.9 ± 5.9 % in the LM (to 1.7 ± 0.3 mN s; P < 0.05; n = 6) and 71.6 ± 5.2 % in the CM (to 3.8 ± 1.5 mN s; P < 0.05; n = 6). Upon replacement of the normal Krebs solution into the intermediate chamber, the transient relaxations of both muscles were restored to similar responses obtained prior to application of the low Ca2+ solution (Fig. 3E). Tetrodotoxin (1 μm) in the intermediate chamber blocked the transient relaxations of the LM and CM in the anal recording chamber (n = 2) (Fig. 3F).
Figure 3. Effects of lumbar colonic nerve stimulation on non-nicotinic descending transmission.
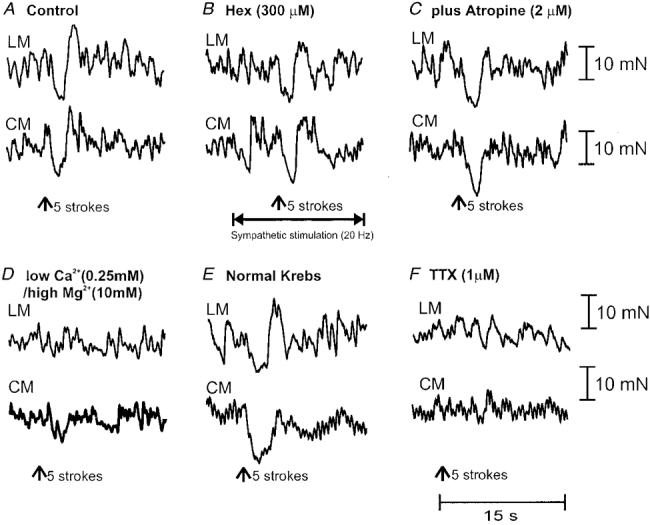
A, 5 brush strokes delivered to the oral stimulating chamber elicited a synchronous relaxation of the LM and CM in the anal recording chamber. B, the addition of hexamethonium (Hex; 300 μm) to the intermediate chamber was without effect on the amplitude of the relaxations of the LM and CM in the anal recording chamber. Furthermore, in hexamethonium (300 μm), a train of stimuli (300 pulses, 20 Hz, 600 μs, 70 V) delivered to the LCN (in the intermediate chamber; 10 s prior to mucosal stroking) was without effect on descending transmission to the LM or CM in the anal recording chamber. C, the further addition of atropine (2 μm) did not reduce the hexamethonium-resistant descending transmission, suggesting an involvement of non-cholinergic descending transmission. D, in hexamethonium and atropine (intermediate chamber), changing the Krebs solution to a low Ca2+/high Mg2+ solution (in the intermediate chamber only) almost blocked the relaxations of the LM and CM in the anal recording chamber. E, replacement of the low Ca2+/high Mg2+ solution with normal Krebs solution (in the intermediate chamber) restored the amplitude of the relaxations of the LM and CM in the recording chamber. F, the further application of TTX (1 μm) to the intermediate chamber abolished the relaxations in the anal recording chamber.
We then tested the possibility that hexamethonium-resistant transmission may be affected by localized LCN stimulation in the intermediate chamber. A train of LCN stimuli (300 pulses, 10-20 Hz, 600 μs pulses) was delivered (selectively to the intermediate chamber) 10-20 s prior to mucosal stroking in the oral chamber. It was found that sympathetic stimulation was without effect on the transient relaxation responses of the LM (prior to stimulation: 7.3 ± 1.1 mN s; following LCN stimulation: 7.6 ± 1.0 mN s; P > 0.05; n = 7) and CM (prior to stimulation: 14.0 ± 0.6 mN s; following stimulation: 9.5 ± 2.2 mN s; P > 0.05; n = 7) in the anal recording chamber (Fig. 3B). This was further tested by applying clonidine (2 μm) to the intermediate chamber, where it was also found that the transient relaxations of the LM and CM were still recorded in the anal recording chamber, following five brush strokes to the oral stimulating chamber (n = 4).
Effects of sympathetic nerve stimulation on descending cholinergic transmission
It has been shown that following inhibition of nitric oxide (NO) synthesis in the oral stimulation chamber and/or intermediate chamber of the distal colon, stroking the mucosa elicits a prolonged inhibitory and excitatory complex which occurs synchronously in both the LM and CM anal to the point of stimulation (see Fig. 5 of Smith & McCarron, 1998). We examined the effects of sympathetic nerve stimulation on these complexes, which are blocked by hexamethonium, since they appear to result from enhanced descending cholinergic transmission that is normally suppressed by ongoing NO release (see Smith & McCarron, 1998). We therefore selectively applied the NO synthesis inhibitor L-NA (100 μm) to the oral and intermediate chambers to induce a prolonged relaxation (LM: 46.9 ± 5.1 mN s; n = 17; CM: 67.9 ± 10.2 mN s; n = 17) and contraction of both muscles (LM: 84.1 ± 9.4 mN s; n = 17; CM: 157.0 ± 17.0 mN s; n = 17) (Fig. 4B). In 9 out of 17 of these animals, L-NA also induced spontaneous complexes, which appeared indistinguishable from evoked complexes and occurred irregularly. These events may be similar to the myoelectric complexes that propagate spontaneously along the isolated whole mouse colon (Bywater et al. 1998; Spencer et al. 1998b,c).
Figure 4. Effects of sympathetic nerve stimulation on descending nicotinic transmission.
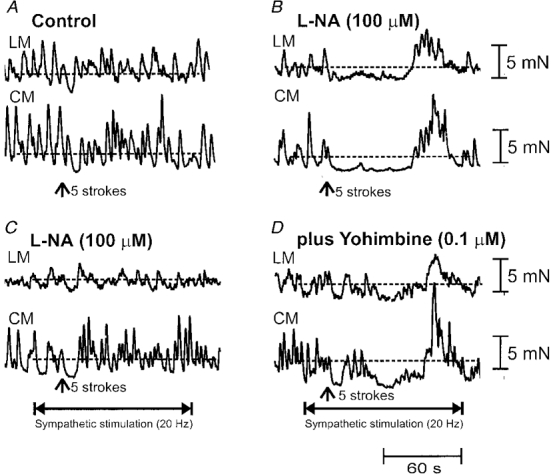
A, 5 mucosal brush strokes (see arrow) applied to the oral stimulating chamber elicited a transient relaxation of the LM and CM in the anal recording chamber. B, following application of the NO synthesis inhibitor L-NA (10−4 M) to the oral and intermediate chambers, 5 brush strokes applied to the oral chamber induced a prolonged slow relaxation and rebound contraction of the LM and CM in the anal recording chamber. C, 10 s prior to mucosal stroking in the oral chamber, a train of stimuli (1200 pulses, 20 Hz, 70 V, 600 μs) delivered to the LCN in the intermediate chamber blocked both the L-NA-induced inhibitory and excitatory complex in the anal recording chamber. The transient relaxation recorded in control solution (A) was unaffected by LCN stimulation. D, the addition of yohimbine (100 nM) to the intermediate chamber, reversed the effects of LCN stimulation on descending transmission underlying the anally migrating complex.
To examine the effects of localized sympathetic nerve stimulation on descending cholinergic transmission of these complexes, stimuli were delivered to the LCN in the intermediate chamber. It was found that when prolonged trains of LCN stimuli (1200 pulses, 5-20 Hz, 600 μs pulses, 70 V) were delivered (to the intermediate chamber) 10 s prior to mucosal stroking in the oral stimulating chamber, both the inhibitory and excitatory phases of the complex in the LM and CM were abolished in the anal recording chamber (n = 16; Fig. 4C). In four preparations, the addition of the α2-adrenoceptor antagonist yohimbine (100 nM) to the intermediate chamber was found to prevent sympathetic blockade of the L-NA-induced complex into the anal recording chamber, so that both the relaxation and after-contraction phases in the LM and CM were restored (Fig. 4D). In the presence of yohimbine (100 nM), the relaxation (LM: 43.8 ± 15.2 mN s; CM: 83.1 ± 12.4 mN s) and after-contraction (LM: 49.0 ± 19.7 mN s, CM: 100.9 ± 29.5 mN s) phases of the complex were significantly different from control responses (P < 0.05; n = 4). In two separate animals, the addition of phentolamine (3 μm) to the intermediate chamber was also found to reverse the actions of LCN stimulation. To further test that sympathetic stimulation was acting via the α2-adrenoceptor, clonidine (2 μm) was added to the intermediate chamber. In four preparations, we found that clonidine (2 μm) also abolished the slow relaxation and ‘off’ contraction of the LM and CM in the anal recording chamber. We further tested for the possibility that adrenergic transmission was responsible for suppression of descending transmission by selectively applying guanethidine (3 μm) to the intermediate chamber. Guanethidine was also found to prevent sympathetic blockade of descending transmission (n = 2). This was unlikely to be due to a local anaesthetic action of guanethidine, since transmission of the descending complex still occurred into the anal recording chamber.
To test whether blockade of β-adrenoceptors on the smooth muscles would reverse the effects of LCN stimulation, propranolol was added to the intermediate chamber. It was found that in four animals tested, propranolol (10 μm) did not reverse the inhibitory actions of LCN stimulation on L-NA-induced descending transmission.
Effects of sympathetic nerve stimulation on the relative movements of the longitudinal and circular muscle layers
To examine the direct effects of sympathetic nerve stimulation on the LM and CM we left the LCNs intact in only that portion of the colon lying within the recording chamber, and added guanethidine (3 μm) to both the intermediate and stimulation chambers. When trains of stimuli (2-20 Hz, 100 pulses, 70 V, 600 μs) were delivered to the LCN in the anal or oral recording chamber (in the absence of guanethidine), synchronous relaxations were recorded in the LM and CM (n = 9; Fig. 5). Increases in stimulus frequency elicited graded increases in the amplitude of relaxations, until a maximal inhibitory response was typically elicited following a 5-10 Hz stimulus (Fig. 5). No contractile responses were observed immediately following LCN stimulation although, on occasions, relaxations were followed by rebound contraction of both muscles. Clearly, reciprocal movements of the two muscles were not detected when the muscles were mechanically isolated from one another. In five preparations, it was found that a stimulus frequency of 1 Hz to the LCN was insufficient to elicit a resolvable inhibitory response in either the LM or CM. However, when the stimulus frequency was increased to 5 Hz in these animals, synchronous relaxations of the LM and CM were elicited. To test whether sympathetic relaxations of the muscle were mediated via suppression of enteric nicotinic synapses, hexamethonium was applied to the colon. No significant difference was noted in the relaxation responses of the LM or CM before or after application of hexamethonium (300 μm) following 5, 10 or 20 Hz stimuli, delivering 100 pulses to the LCN (P > 0.05; n = 5). In hexamethonium, further addition of yohimbine (2 μm) to the recording chamber consistently increased the resting tone of the LM (control: 8.7 ± 0.8 mN; yohimbine: 12.2 ± 1.0 mN; P < 0.05; n = 5) and CM (control: 8.3 ± 0.6 mN; yohimbine: 11.2 ± 0.7 mN; P < 0.05; n = 5) (Fig. 6A). The effects of yohimbine on the LCN-induced relaxation responses of the LM and CM were variable. From five out of seven animals tested, relaxations were attenuated in the LM (by 38.7 ± 9.2 %; P > 0.05) and in the CM (by 22.2 ± 17.6 %; P > 0.05), while in one animal, yohimbine abolished sympathetic relaxations of both muscles. However, in another animal, yohimbine was without effect on relaxations of either the LM or CM. Since yohimbine did not completely block sympathetic relaxations in six animals, propranolol was further added to these tissues. In five out of six of these animals, propranolol (10 μm) abolished yohimbine-resistant relaxations in both the LM and CM (Fig. 5G-I). In one animal, a combination of propranolol (10 μm) and yohimbine (2 μm) was ineffective in blocking sympathetic relaxations, but relaxations ceased upon severing of the LCN, confirming that these inhibitory responses were of extrinsic neural origin.
Figure 5. Direct effects of sympathetic nerve stimulation on the longitudinal and circular muscle.
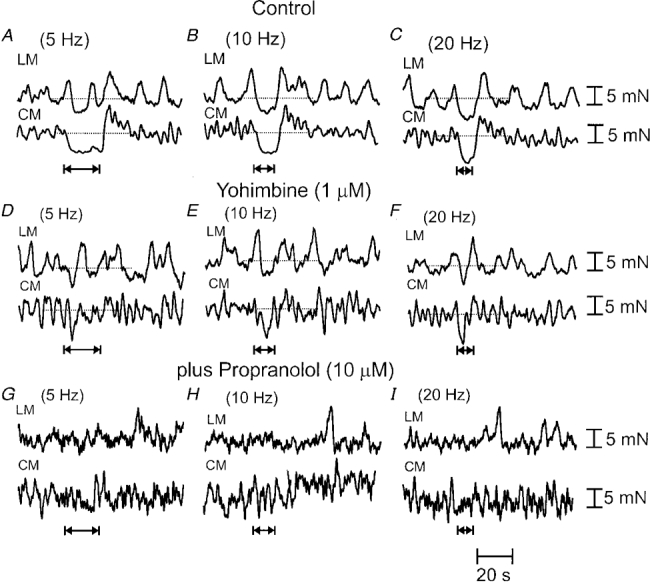
A-C, in the presence of hexamethonium (to the recording chamber), graded increases in the frequency (5 Hz, 10 Hz, 20 Hz, 100 pulses, 600 μs pulse width) of LCN stimulation applied to the recording chamber elicited graded increases in the amplitude of the relaxations in both muscle layers. It can be seen that the LM and CM relax synchronously following release of sympathetic neurotransmitter. Rebound contractions occur following relaxations, particularly in the CM. D-F, the addition of yohimbine (1 μm) to the recording chamber attenuated, but did not block, relaxations induced by LCN stimulation. G-I, in the combined presence of hexamethonium and yohimbine, the further addition of propranolol (10 μm) blocked yohimbine-resistant relaxations.
Figure 6. Effects of an α-adrenoceptor agonist and antagonist on the cholinergic tone of longitudinal and circular muscle.
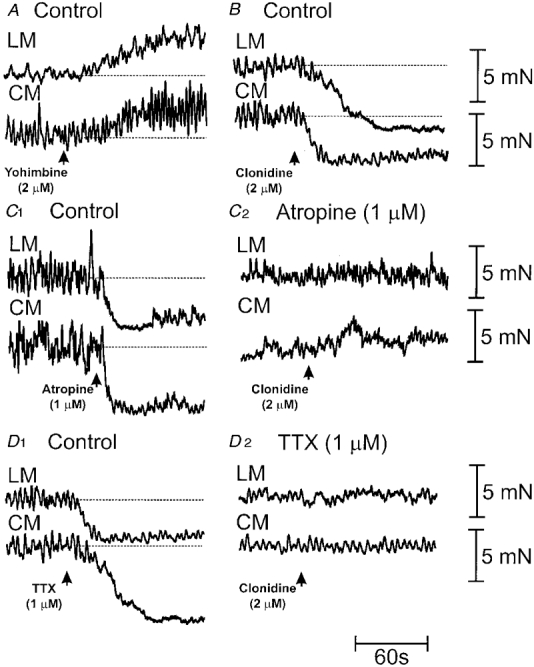
A, addition of yohimbine (2 μm) to the recording chamber enhanced the tone of the LM and CM. B, in contrast, addition of clonidine (2 μm) to the recording chamber induced a rapid relaxation of the LM and CM. C1, addition of atropine (1 μm) to normal Krebs solution reduced the tone of the LM and CM, suggesting the presence of ongoing cholinergic tone in the muscles. C2, while in the presence of atropine (1 μm) (and once the resting tone had been restored to 1 g), the further addition of clonidine (2 μm) (see arrow) was without effect on the tone of either muscle layer. D1, the addition of TTX (1 μm) to control solution in a different preparation also induced a relaxation of the LM and CM, further supporting a neural basis for cholinergic tone in the LM and CM. D2, upon restoration of the resting tone (in the maintained presence of TTX), the further addition of clonidine was also without effect on the tone of the LM or CM.
Is cholinergic tone in the longitudinal and circular muscles modulated by α2-adrenoceptor stimulation?
One of the ways in which LCN stimulation may have relaxed the LM and CM is by inhibiting spontaneous release of acetylcholine, to remove cholinergic tone in the muscles. Therefore, we tested the possibility that α2-adrenoceptor stimulation may remove cholinergic tone in the LM and CM. It was found that addition of the α2-adrenoceptor agonist clonidine (2 μm) to the recording chamber induced an immediate reduction in the resting tone of the LM (control: 11.8 ± 2.6 mN; clonidine: 9.3 ± 2.4 mN; P < 0.05; n = 4) and CM (control: 10.1 ± 2.6 mN; clonidine: 6.6 ± 1.9 mN; P < 0.05; n = 4) (Fig. 6B). To test the presence of cholinergic tone in the LM and CM, TTX and atropine were also applied to the recording chamber. In all animals tested (n = 4), TTX (1-1.6 μm) in the recording chamber also reduced the resting tone of the LM (control: 11.5 ± 0.9 mN; TTX: 8.5 ± 1.1 mN; P < 0.05) and CM (control: 9.8 ± 1.0 mN; TTX: 5.2 ± 1.1 mN; P < 0.05) (Fig. 6D 1). Similarly, in 12 out of 17 animals, the addition of atropine (1-2 μm) to the recording chamber also reduced the resting tone of both the LM (control: 9.4 ± 1.5 mN; atropine: 8.3 ± 0.8 mN; P < 0.05; n = 12) and CM (control: 12.2 ± 2.1 mN; atropine: 9.4 ± 1.5 mN; P < 0.05; n = 12) (Fig. 6C 1). In the remaining five animals, the LM and CM did not appear to be under ongoing cholinergic tone, since atropine (1-2 μm) did not affect resting tone in the LM or CM. In these particular animals, clonidine (2 μm) was also without effect on the tone of the LM or CM. Moreover, following the addition of TTX to the recording chamber to remove tone in these muscles, the further addition of clonidine (2 μm) did not affect the resting tone of either the LM (TTX: 10.7 ± 1.6 mN; clonidine: 11.0 ± 1.6 mN; P > 0.05; n = 4) and CM (TTX: 7.3 ± 1.9 mN; clonidine: 7.1 ± 1.7 mN; P > 0.05; n = 4) (after the resting tone of the LM and CM had been re-established) (cf. Fig. 6D1 and 2). Similarly, in the presence of atropine, the further addition of clonidine (2 μm) to the recording chamber did not affect the tone of the LM (atropine: 11.0 ± 1.4 mN; clonidine: 10.9 ± 1.4 mN; P > 0.05; n = 9) or CM (atropine: 13.6 ± 2.4 mN; clonidine: 13.9 ± 2.6 mN; P > 0.05; n = 9) (cf. Fig. 6C1 and 2), suggesting that α2-adrenoceptor stimulation only relaxes the LM and CM provided these muscles exhibit cholinergic tone.
DISCUSSION
The findings of the current study demonstrate that stimulation of the sympathetic neural supply to the guinea-pig distal colon inhibits orally and anally directed transmission that underlies the peristaltic reflex, most probably by suppressing neuro-neuronal transmission in ascending and descending enteric interneurones. In addition, we have shown that sympathetic stimulation synchronously relaxes the LM and CM over a range of stimulus frequencies, that is likely to involve suppression of ongoing cholinergic tone to both muscle layers.
Effects of sympathetic nerve stimulation on ascending and descending transmission in the colon
The partitioned organ bath technique was used to localize the effects of sympathetic nerve stimulation on enteric interneurones involved in the transmission of orally and anally directed peristaltic reflex activity. Trains of lumbar colonic nerve stimulation (delivered selectively to the intermediate chamber) abolished the ascending excitatory reflex of both muscles in the oral recording chamber. Since hexamethonium substantially attenuated the ascending contraction of both the CM and LM (Smith & McCarron, 1998), it is most likely that these ascending interneurones are largely of cholinergic origin.
Mucosal stroking in the oral chamber was found to elicit a transient relaxation of the LM and CM in the anal recording chamber. Unlike the ascending excitatory reflex, these relaxations persisted when hexamethonium was added to the intermediate chamber, suggesting an involvement of non-nicotinic pathways in anally directed transmission to the LM and CM (Smith & McCarron, 1998). The combined presence of hexamethonium and atropine in the intermediate chamber did not affect these relaxations in the anal chamber, which were however attenuated by low Ca2+/high Mg2+ solution in the intermediate chamber, implying an involvement of non-cholinergic descending transmission to the LM and CM. Localized sympathetic stimulation to the intermediate chamber was ineffective in reducing this non-cholinergic transmission, suggesting that descending interneurones, which transmit non-cholinergic potentials, may receive relatively sparse sympathetic inputs, although additional immunohistochemical studies would be required to substantiate this.
The inhibition of NO synthesis in the oral and intermediate chambers evoked a prolonged descending relaxation followed by an ‘off’ contraction synchronously in both muscles in the anal chamber, which was abolished by the addition of hexamethonium to any chamber (Smith & McCarron, 1998). These evoked complexes were suggested to be induced by the facilitation of cholinergic transmission in descending interneurones, which are normally suppressed by ongoing release of endogenous NO (Smith & McCarron, 1998). In the current study, we found that sympathetic nerve stimulation applied to the segment of colon lying in the intermediate chamber abolished these evoked complexes. The observation that LCN stimulation selectively prevented the hexamethonium-sensitive descending complex, but not hexamethonium-resistant transmission, suggests that at least two different kinds of descending interneurones exist in the distal colon and that sympathetic stimulation may selectively impede descending cholinergic interneurones (see above).
Our observation that yohimbine applied to the intermediate chamber reversed the inhibitory effects of LCN stimulation suggests that α2-adrenoceptors are located on both ascending and descending cholinergic interneurones to both the LM and CM in the distal colon. Our results support the conclusions of Frigo et al. (1972), where it was suggested that ‘….similarly to other adrenergic nerve-mediated effects, inhibition of peristalsis is completely removed by α receptor blockade alone.‘
Relative movements of the longitudinal and circular muscle layers during sympathetic nerve stimulation
A major finding of our study was that the two muscle layers of the colon relaxed synchronously over a range of stimulus frequencies (2-20 Hz) when LCN stimulation was applied to the dissected segment in the anal or oral recording chamber; on occasions these relaxations were followed by ‘rebound’ contractions in both the LM and CM. However, we were surprised to find that these relaxations of the LM and CM were unaffected by hexamethonium, since we had assumed that these inhibitory responses might have been mediated by suppression of fast cholinergic potentials in the enteric plexuses (Hirst & McKirdy, 1974), which may have been responsible for generating cholinergic tone in the LM and CM. We did find, however, that in the presence of hexamethonium, yohimbine usually attenuated sympathetic relaxations of the LM and CM, suggesting the possibility that α2-adrenoceptors are more likely to be located on the terminals of cholinergic motorneurones within the LM and CM (see Paton & Vizi, 1969). These relaxations are likely to be due to the removal of cholinergic tone caused by sympathetic stimulation inhibiting the release of acetylcholine from the terminals of spontaneously active motor neurones (see Smith et al. 1999). That the relaxation responses may involve inhibition of tonic ACh release is further supported by the observation that clonidine caused a similar reduction in tone of the LM and CM as atropine and TTX. We also found that yohimbine-resistant relaxations to sympathetic stimulation were usually blocked by the further addition of propranolol (5 out of 6 animals), supporting the existence of inhibitory β-adrenoceptors on the LM and CM.
Our results are consistent with the work of Bayliss & Starling (1899, 1900) who observed that in the canine large intestine both ‘….circular and longitudinal muscles layers appear to be equally inhibited’ by sympathetic nerve stimulation, without ‘….any trace of a motor effect of these nerves on either muscle coat’. What motor effect we did observe may be due to a myogenic ‘rebound’ of the muscles or, alternatively, may be due to a transient overshoot in cholinergic tone. However, our observations contrast with the recent mechanical observations of Luckensmeyer & Keast (1998), who showed that in the rat proximal colon, lumbar colonic nerve stimulation appeared to elicit reciprocal movements, that is the LM relaxed and the CM contracted following LCN stimulation. Furthermore, their mechanical responses differed in each muscle layer (excitatory or inhibitory) and were dependent on the stimulus frequency. Although simultaneous recordings of LM and CM activity were not made in their study, they did find that in the distal colon, the two muscles contracted in response to sympathetic stimulation. Many factors may account for the differences between their work and our findings in the current study. Firstly, our responses were obtained in control (drug-free) conditions. Luckensmeyer & Keast (1998) obtained their results after precontracting the colon with carbachol, which stimulates both the enteric plexus (via a nicotinic action) and musculature (via a muscarinic action). Furthermore, simultaneous mechanical recordings were not made in their study and no attempt appears to have been made to remove the possibility of passive mechanical interactions between the movements of the two muscle layers. Wood & Perkins (1970) unequivocally showed that CM contraction causes an artifactual lengthening (passive relaxation) of the LM (also see Gregory & Bentley, 1968). This explanation could also account for the results obtained by Lee (1960), who showed that sympathetic stimulation of the guinea-pig intestine caused a relaxation of the LM, which was associated with ‘….an increase in the rate of circular contractions, or a sustained contraction.’ In our study, we took particular care to mechanically isolate the movements of the LM and CM by using similar dissection techniques to those we have recently employed for the guinea-pig colon (Smith & Robertson, 1998; Smith & McCarron, 1998). The findings of this study have shown that the release of sympathetic transmitter causes synchronous relaxation of both muscle layers of the colon. These responses appear to be mediated, at least partly, via stimulation of α2-adrenoceptors within the enteric plexuses (to remove cholinergic tone), and partly via direct stimulation of β-adrenoceptors in the LM and CM.
Conclusions
It is suggested that in the guinea-pig distal colon, sympathetic nerve stimulation inhibits orally and anally directed transmission underlying the peristaltic reflex, most probably by suppressing neuro-neuronal transmission in ascending and descending enteric cholinergic interneurones. In addition we report that following sympathetic stimulation, the LM and CM do not exhibit reciprocal movements, but rather relax synchronously over a range of stimulus frequencies.
Acknowledgments
Sarah McCarron was a visiting research scholar from the University of Ulster, Coleraine, UK. We would like to thank Michelle Walsh for technical assistance. This work was supported by an RO1 grant NIDDK 45713 from the National Institutes of Health, USA.
References
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. The Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the large intestine. The Journal of Physiology. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Burnstock G, Holman ME. Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. The Journal of Physiology. 1966;182:527–540. doi: 10.1113/jphysiol.1966.sp007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wong H. Systemic pharmacology of adrenergic agonists and antagonists: effects on the digestive system. In: Szekeres L, editor. Handbook of Experimental Pharmacology. New York: Springer-Verlag; 1981. pp. 129–159. [Google Scholar]
- Bywater RA. Activity following colonic distension in enteric sensory fibres projecting to the inferior mesenteric ganglion in the guinea-pig. Journal of the Autonomic Nervous System. 1994;46:19–26. doi: 10.1016/0165-1838(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Bywater RAR, Spencer NJ, Fida R, Taylor GS. Second-, minute- and hour metronomes of intestinal pacemakers. Clinical and Experimental Pharmacology and Physiology. 1998;25:857–861. doi: 10.1111/j.1440-1681.1998.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Torsoli A, Lecchini S, Falaschi CF, Crema A. Recent advances in the pharmacology of peristalsis. Archives Internationales de Pharmacodynamie et de Thérapie Supplementum. 1972;196:9–22. [PubMed] [Google Scholar]
- Furness JB. The origin and distribution of adrenergic nerve fibres in the guinea-pig colon. Histochemie. 1970;21:295–306. doi: 10.1007/BF00280899. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The ramifications of adrenergic nerve terminals in the rectum, anal sphincter and anal accessory muscles of the guinea-pig. Zeitschrift für Anatomie und Entwicklungsgeschichte. 1973;140:109–128. doi: 10.1007/BF00520721. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Gabella G. Innervation of the gastrointestinal tract. International Review of Cytology. 1979;59:129–193. doi: 10.1016/s0074-7696(08)61662-9. [DOI] [PubMed] [Google Scholar]
- Garry RC, Gillespie JS. The responses of the musculature of the colon of the rabbit to stimulation, in vitro, of the parasympathetic and of the sympathetic outflows. The Journal of Physiology. 1955;128:557–576. doi: 10.1113/jphysiol.1955.sp005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JS. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic nerve stimulation. The Journal of Physiology. 1962;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Bentley GA. The peristaltic reflex in the isolated guinea-pig ileum during drug-induced spasm of the longitudinal muscle. Australian Journal of Experimental Biology and Medical Science. 1968;46:1–16. doi: 10.1038/icb.1968.1. [DOI] [PubMed] [Google Scholar]
- Hirst GD, McKirdy HC. Presynaptic inhibition at mammalian peripheral synapse? Nature. 1974;250:430–431. doi: 10.1038/250430a0. [DOI] [PubMed] [Google Scholar]
- Kottegoda SR. An analysis of the possible nervous mechanisms involved in the peristaltic reflex. The Journal of Physiology. 1969;200:687–712. doi: 10.1113/jphysiol.1969.sp008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottegoda SR. Peristalsis in the small intestine. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. London: Edward Arnold Ltd; 1970. pp. 525–541. chap. 17. [Google Scholar]
- Lee CY. The effect of stimulation of extrinsic nerves on peristalsis and on the release of 5-hydroxytryptamine in the large intestine of the guinea-pig and of the rabbit. The Journal of Physiology. 1960;152:405–418. doi: 10.1113/jphysiol.1960.sp006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckensmeyer G, Keast JR. Activation of α- and β-adrenoceptors by sympathetic nerve stimulation in the large intestine of the rat. The Journal of Physiology. 1998;510:549–561. doi: 10.1111/j.1469-7793.1998.549bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenna BR, McKirdy HC. Peristalsis in the rabbit distal colon. The Journal of Physiology. 1972;220:33–54. doi: 10.1113/jphysiol.1972.sp009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKirdy HC. Functional relationship of longitudinal and circular layers of the muscularis externa of the rabbit large intestine. The Journal of Physiology. 1972;227:839–853. doi: 10.1113/jphysiol.1972.sp010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg KA. Adrenergic innervation of the intestinal wall studied by fluorescence microscopy. International Journal of Neuropharmacology. 1964;3:379–382. doi: 10.1016/0028-3908(64)90067-x. [DOI] [PubMed] [Google Scholar]
- Paton WD, Vizi ES. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. British Journal of Pharmacology. 1969;35:10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CW. Topographical and electrophysiological characteristics of highly excitable S neurons in the myenteric plexus of the guinea-pig ileum. The Journal of Physiology. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological study in the isolated guinea-pig ileum. Journal of the Autonomic Nervous System. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, McCarron SL. Nitric oxide modulates cholinergic reflex pathways in the guinea-pig distal colon. The Journal of Physiology. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. The Journal of Physiology. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR, Klemm MF. Effects of sympathetic nerve stimulation on membrane potential in the circular muscle layer of mouse distal colon. Neurogastroenterology and Motility. 1998a;10:543–552. doi: 10.1046/j.1365-2982.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR, Taylor GS. Evidence that myoelectric complexes in the isolated mouse colon may not be of myogenic origin. Neuroscience Letters. 1998b;250:153–156. doi: 10.1016/s0304-3940(98)00461-3. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. Journal of the Autonomic Nervous System. 1998c;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? The Journal of Physiology. 1999;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH, Miller SM. Physiology of prevertebral ganglia. In: Johnson LL, editor. The Physiology of the Gastrointestinal Tract. I. New York: Raven Press; 1994. pp. 795–877. chap. 19. [Google Scholar]
- Tack JF, Wood JD. Actions of noradrenaline on myenteric neurons in the guinea-pig gastric antrum. Journal of the Autonomic Nervous System. 1992;41:67–77. doi: 10.1016/0165-1838(92)90128-4. [DOI] [PubMed] [Google Scholar]
- Venkova K, Krier J. Stimulation of lumbar sympathetic nerves evokes contractions of cat colon circular muscle mediated by ATP and noradrenaline. British Journal of Pharmacology. 1993;110:1260–1270. doi: 10.1111/j.1476-5381.1993.tb13951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, Perkins WE. Mechanical interactions between the longitudinal and circular axes of small intestine. Journal of Neurophysiology. 1970;42:582–593. doi: 10.1152/ajplegacy.1970.218.3.762. [DOI] [PubMed] [Google Scholar]


