Abstract
Single fibres isolated from the anterior tibialis muscle of Rana temporaria were tetanized (0.9-1.8 °C) while a marked (≈1 mm) segment was held at constant length by feedback control. Force enhancement was produced by applying a controlled stretch ramp to the fibre segment during the tetanus plateau, the steady force reached during stretch being used as a measure of the maximum force that the myosin cross-bridges can hold before they detach.
The amplitude of force enhancement during stretch did not vary in proportion to the isometric force as the sarcomere length was changed, maximum force enhancement being attained near 2.4 μm sarcomere length compared with 2.0 μm for the isometric force.
The influence of fibre width on the force enhancement-sarcomere length relationship was evaluated by normalizing force enhancement to the tetanic (pre-stretch) force in this way allowing for the differences in myofilament overlap at the various lengths. The amplitude of force enhancement (normalized to the tetanic force) increased by approximately 70 % as the relative width of the myofilament lattice was reduced from a nominal value of 1.05 at a sarcomere length of 1.8 μm to 0.85 at a sarcomere length of 2.8 μm.
Changes in fibre width equivalent to those produced by altering the sarcomere length were produced by varying the tonicity of the extracellular medium. Force enhancement, normalized to the control isometric force at each tonicity, exhibited a width dependence that agreed well with that described in the previous point. Stretch ramps applied to frog skinned muscle fibres during calcium-induced contracture likewise resulted in a greater force enhancement during stretch after reducing the fibre width by osmotic compression.
The results suggest that the strength of binding of the myosin cross-bridges, unlike the isometric force, varies with the lateral distance between the myofilaments.
Skeletal muscle that is stretched during activity responds with a rapid rise of force that reaches a maximum after which the force remains essentially constant or, if the muscle is stretched beyond its optimal length, increases slowly during the remainder of the stretch period. This phenomenon, which is referred to as ‘force enhancement during stretch’ (Edman et al. 1978) has been investigated in several studies in the past, both in whole muscle (Fenn, 1924; Abbott & Aubert, 1952; Hill & Howarth, 1959; Cavagna & Citterio, 1974) and in single fibre preparations (Sugi, 1972; Edman et al. 1978; Julian & Morgan, 1979). The amplitude of the force recruited during stretch has been shown to increase with the velocity of stretch, and a plot of the enhanced force against the stretch velocity shows the well-known ‘negative’ branch of the force-velocity relationship in skeletal muscle (Katz, 1939; Aubert, 1956; Edman, 1988). Force enhancement during stretch most probably represents the increased strain of attached cross-bridges as the sarcomeres are forcibly extended (Edman et al. 1978, 1981; Lombardi & Piazzesi, 1990) and the recruited extra force disappears within 2-3 s after the end the stretch ramp at low (∼2°C) temperature. This component of force enhancement should be clearly distinguished from the long-lasting increment of force that appears when the muscle is stretched beyond its optimal length during stimulation. This increment of force, referred to as ‘residual force enhancement after stretch’ (Edman et al. 1978), is velocity independent. It increases with the amplitude of stretch and remains throughout the stimulation period. Recent evidence would seem to show that the residual force enhancement after stretch is attributable to ‘tension creep’ due to redistribution of sarcomere length that occurs as the muscle is stretched above optimal length (Edman & Tsuchiya, 1996).
The present investigation has been aimed at further characterizing the force enhancement during stretch, i.e. the force held by the muscle after the steep rise of force during stretch. Previous evidence suggests that the attainment of maximum force during stretch marks the point at which the cross-bridges are no longer able to withstand the pull and therefore start to slide along the thin filament (Edman et al. 1978, 1981; Flitney & Hirst, 1978; Lombardi & Piazzesi, 1990). The amplitude of force enhancement during stretch can thus be presumed to be proportional to the number of attached cross-bridges and the strength by which the bridges are attached to the thin filament. The following report quantifies the relationship between force enhancement during stretch and sarcomere length in frog muscle based on experiments in which a marked segment of an isolated muscle fibre was kept under strict length control. The results provide evidence that the width of the myofilament lattice is an important determinant of the force recruited during stretch.
METHODS
Intact muscle fibres
Preparation and mounting
Experiments were carried out according to procedures approved by the Animal Ethics Committee of the University of Lund. Single fibres were isolated from the anterior tibialis muscle of Rana temporaria. The frogs had been stored at +4°C for at least 7 days before use and were killed by decapitation followed by destruction of the spinal cord. The fibres were mounted horizontally in a temperature-controlled Perspex chamber between two electromagnetic pullers of the type previously described (Edman & Reggiani, 1984). One of the pullers (motor 1) was used for segment-length control, the other (motor 2) served to assist in shortening the segment below slack length (see further below) so as to keep the selected segment within the domain of the photodiode array during the shortening. The moving arm of motor 2 was provided with a force transducer (AE801, Akjsjeselskapet Mikroelektronikk, Horten, Norway) to which one end of the fibre was attached. The tendons were held in aluminium clips of the type described previously (Edman & Reggiani, 1984), and the fibre was continuously superfused with solution. The temperature of the bathing solution was constant to within 0.2°C in a given experiment but ranged from 0.9 to 1.8°C within the whole series of experiments. The Ringer solution had the following composition (mM): NaCl, 115.5; KCl, 2.0; CaCl2, 1.8; Na2HPO4+ NaH2PO4 (total concentration), 2.0; pH 7.0. The fibres were normally dissected the afternoon before the experiment and were kept in Ringer solution at +4°C overnight.
In some experiments the tonicity of the extracellular medium was varied using the following solutions. The osmotic strength of these solutions (given in parentheses) is expressed as a multiple of the value for the normal Ringer (R) (for calculation see Edman & Hwang, 1977). (i) Normal Ringer solution (1.00R): for composition see above. (ii) Hypotonic Ringer solutions (0.62R and 0.81R): the same composition as (i) except for NaCl which was reduced to 69.3 and 92.4 mM, respectively. (iii) Hypertonic Ringer solutions (1.22R and 1.44R): the same composition as (i) plus 50 and 98 mM sucrose, respectively. Fibre length, sarcomere length and cross-sectional area were determined as described by Edman & Reggiani (1984).
For the evaluation of the present results it was of interest to determine the changes in relative fibre width that occurred in association with altered sarcomere length and altered tonicity of the extracellular medium. Based on this information it was possible to estimate the corresponding changes in the side distance between the thick and thin filaments by using data from X-ray diffraction studies concerning the 1,0 lattice spacing and the thickness of the actin and myosin filaments. Since an intact muscle fibre maintains a constant volume as its length is changed the relative width of the fibre could be readily determined at the different sarcomere lengths using the width at 2.0 μm sarcomere length as unity (Elliott et al. 1963; Huxley, 1969; Millman, 1998). Changes in relative fibre width induced by altering the tonicity within the range 0.81R-1.22R were previously determined in frog intact muscle fibres (Edman & Hwang, 1977). Two perpendicular diameters were measured at ten equally spaced sites along the fibre at × 500 magnification and a mean value of the cross-sectional area was derived for each fibre after equilibration in the different osmotic media. The solutions used by Edman & Hwang (1977) had the same composition as the corresponding solutions in the present study. The earlier measurements of the relative fibre width are therefore applicable also to the present experiments.
Stimulation
The fibre was stimulated transversely by rectangular current pulses of 0.2 ms duration delivered via two platinum plate electrodes that were placed symmetrically, approximately 4 mm apart, on either side of the preparation. The stimulus strength was 15-20 % above the threshold. A train of pulses of appropriate frequency (usually within the range 16-22 Hz) was used to produce a fused tetanus of 0.5-1.0 s duration. The pulse frequency was adjusted to be just sufficient to give complete mechanical fusion in the individual fibre at the sarcomere length considered. The fibre was tetanized at regular, 2 min intervals throughout the experiment.
Segment length recording
The length of a discrete segment of an intact muscle fibre was recorded during tetanic stimulation using the surface marker technique previously described (Edman & Reggiani, 1984). The segment was delineated by two opaque markers of letterpress (Edman & Lou, 1990) that were attached to the upper surface of the fibre in the muscle chamber, the relative position of the markers being recorded by means of a photodiode array (Fairchild CCD133, Fairchild Corporation, Mountain View, CA, USA). An analog circuit provided a signal that was proportional to the change in distance between the two markers, i.e. to the change in length of the marked segment. The accuracy of this measurement was better than 0.2 % of the segment length. The time resolution was 40 μs. A digital display (3½ digit voltmeter) continuously indicated the actual length of the segment.
The segment length signal was used for feedback control of the electromagnetic puller (motor 1). By this approach the overall length of the fibre was continuously adjusted to keep the segment at a predetermined length during contraction (segment length clamp). The switch-over to segment length control of the puller was made just before the start of stimulation and the puller was kept in the ‘segment length control’ mode until the end of the linear phase of relaxation. A controlled length change of the segment was achieved by altering the reference level in the feedback loop for the puller by a voltage ramp of selected speed and amplitude. This approach was used in the present study for applying a stretch ramp during the tetanus plateau and also, in studies below slack fibre length, for allowing the segment to pre-shorten actively during the early phase of the tetanus. During the latter manoeuvre a second puller (motor 2), operating from the opposite end of the fibre, was employed to assist in the shortening as described earlier.
Stretch ramps
The stretch ramp was applied at the beginning of the plateau of an isometric tetanus. The amplitude of the stretch ramp was approximately 20 nm per half sarcomere (HS) which was sufficient to delineate the transition between the initial steep phase of tension rise and the subsequent ‘plateau’ phase of force enhancement during stretching (Fig. 1). The velocity of the stretch ramp was approximately 210 nm HS−1 s−1, i.e. high enough to cause maximal force enhancement (see Fig. 7 in Edman, 1988). The force recorded after completion of the initial tension rise was used as a measure of the force enhancement during stretch (b, Fig. 1). In cases of unevenness of the force record (due to underdamping and minute jerkiness of the puller movement during length clamping in combination with high fibre stiffness) the mean force during the short plateau phase of force enhancement was measured.
Figure 1. Force and displacement records from a length-controlled segment of frog muscle fibre subjected to stretch on the plateau of a fused tetanus.
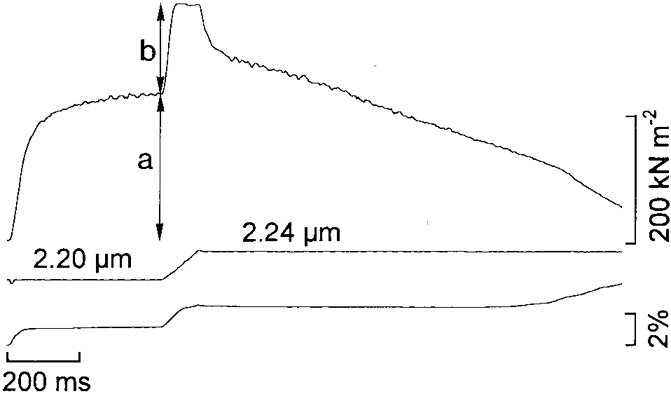
Upper trace, force; middle trace, segment length calibrated in μm sarcomere−1; lower trace, change of puller position as required to adjust the segment length. Note the rapid rise of force after the onset of stretch ending at a breakpoint of the force myogram after which the force remains quite constant during the rest of the stretch ramp. Stretch amplitude at the breakpoint of the force myogram is approximately 8 nm HS−1. a, isometric force; b, measured force enhancement.
Skinned muscle fibres
Segments of single fibres isolated from the semitendinosus muscle of the frog were chemically skinned following essentially the same procedure as previously described (Ekelund & Edman, 1982). Segments of single fibres were isolated while the muscle was immersed in a high-potassium solution containing 100 mM KCl, 2 mM EGTA and 10 mM NaH2PO4+ Na2HPO4, pH 7.0. The isolated fibre segments were first treated for approximately 1 h at +4°C in an extraction solution containing 30 % (v/v) glycerol, 2 mM EGTA and 10 mM NaH2PO4+ Na2HPO4, pH 7.0. After a brief rinse in the high-potassium solution the fibres were placed in a relaxing solution (see below for composition) containing 0.5 % (w/v) Brij 58 for 15-20 min. The fibre segments were then stored in relaxing solution at 0°C until used for experimentation later on the same day. Before use small aluminium foil clips were attached to each end of the segment as described in detail by Ekelund & Edman (1982).
The fibres, aluminium clips attached, were mounted horizontally in a Perspex chamber between two stainless-steel hooks attached to a force transducer and an electromagnetic puller, respectively. The length of the fibre segment was adjusted to give a resting sarcomere length of approximately 2.2 μm as measured by the laser diffraction technique (Ekelund & Edman, 1982). The force transducer and puller were both similar to those used for intact muscle fibres (see above). To ensure that the fibre did not alter its position during the experiment the side parts of the aluminium foil clips were folded tightly around the hooks. Furthermore, two thin nylon threads were tied around the aluminium foil on the puller side. Four identical, jacketed muscle chambers, each having a volume of 4 ml, were located in a Perspex block that could be lowered and translated so that any of the chambers could be used for immersion of the fibre. A rapid and complete exchange of solution around the fibre could be achieved in this way. The temperature was maintained constant to within ± 0.2°C for any given experiment and varied between 2.0 and 3.0°C for different experiments. Measurements of fibre diameter were performed to the nearest 2.5 μm using a Zeiss stereo microscope while the fibre was mounted in the experimental chamber and submerged in relaxing solution (see below).
Solutions
Relaxing solution had the following composition (mM): KCl, 120; MgCl2, 6.05; EGTA, 2; ATP, 5.5; creatine phosphate, 20; imidazole, 10; pH 7.0.
Contracture solution had the same composition as relaxing solution except that EGTA was increased to 4 mM and 3.93 mM CaCl2 was added to provide 10 μm calcium ion concentration. For estimation of [Ca2+] the stability constants given in Table 1 of Edman & Ekelund (1982) were used. A calcium conditioning solution was used to raise the free calcium concentration in the fibre to a sub-threshold level (1-2 μm in different experiments) immediately before the contracture solution was introduced. Apart from the low Ca2+ concentration of the calcium conditioning solution the composition of the latter solution was identical to that of the contracture solution. Polyvinylpyrrolidine (PVP) K30 (40 000 molecular weight) was added (with no other adjustment) to the relaxing, calcium conditioning and contracture solutions in order to change the osmotic pressure and to reduce the myofilament lattice width. The concentration of the polymer in the bathing solution was 6 % (w/v).
Statistics
Student's t test was used for determination of statistical significance. All statistics are given as means ±s.e.m.
RESULTS
Intact muscle fibres
Variation of sarcomere length and fibre width
Single muscle fibres of the frog were stimulated to produce a 1 s fused tetanus while a marked segment was held at constant length throughout the contraction as described in Methods. When the tetanic force had reached its plateau value the length-controlled segment was slowly stretched, the stretch ramp having an amplitude of ∼20 nm HS−1 and a velocity of 210 nm HS−1 s−1. Figure 1 shows typical records from such an experiment carried out at a resting sarcomere length of 2.20 μm. In accordance with previous results (e.g. Edman et al. 1978, 1982; Julian & Morgan 1979; Lombardi & Piazzesi, 1990; Edman & Tsuchiya, 1996), stretch of the length controlled segment led to a rapid rise of tension to a breakpoint after which the tension remained high during the remainder of the stretch period. After the end of the stretch there was a biphasic fall in tension: an initial steep drop in force that reduced the enhanced force to about half its maximal value followed by a slow decay phase.
The increase in force during the stretch ramp is most likely attributable to strain of myosin cross-bridges that are in contact with the thin filaments. The following experiments were performed to investigate if this rise of force (b, Fig. 1) bears the same relationship to sarcomere length and filament overlap as does the isometric tetanic force (a, Fig. 1). Measurements like those shown in Fig. 1 were therefore carried out in individual fibres at different sarcomere lengths within the range 1.7-3.1 μm with five or six recordings at each length. Figure 2 illustrates records from a given fibre derived at 1.8, 2.2 and 2.8 μm sarcomere length. For measurements below slack length (Fig. 2A) the contraction was initiated, under segment length control, at 2.00 μm sarcomere length. Soon after the onset of tension rise the reference level for the segment length clamp was altered to allow the segment to shorten and develop maximum tetanic force at a pre-set length. A stretch ramp was thereafter applied to the segment in the same way as performed above slack fibre length. As is readily seen in Fig. 2 the force enhancement during stretch did not change in proportion to the isometric force as the sarcomere length was altered. For example, increasing the sarcomere length from 1.80 to 2.20 μm (Fig. 2A and B) caused only a small rise of the tetanic force whereas the force enhancement during stretch was markedly potentiated. Conversely, an increase in sarcomere length from 2.20 to 2.80 μm (Fig. 2B and C) led to a marked decrease in isometric force but only to a moderate reduction of the force enhancement during stretch.
Figure 2. Records from a length-controlled segment of a muscle fibre illustrating force enhancement during stretch at three different sarcomere lengths.
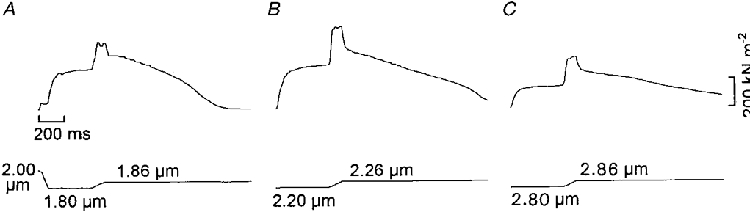
Upper traces, force. Lower traces, segment length calibrated in μm sarcomere−1.
Figure 3A shows the amplitude of tetanic force and the amplitude of force enhancement during stretch in relation to sarcomere length between 1.7 and 3.1 μm based on data from one muscle fibre. In accordance with previous results (Edman & Reggiani, 1987) the relationship between tetanic force and sarcomere length recorded in a short length-clamped segment had a smooth rounded shape with a maximum close to 2.00 μm sarcomere length. The length dependence of the force enhancement during stretch, on the other hand, differed markedly from that of the plain isometric force. The force enhancement during stretch increased with sarcomere length above 1.8 μm and reached maximum amplitude at approximately 2.4 μm sarcomere length. At this point the force enhancement during stretch can be seen to equal the isometric force (Fig. 3A), i.e. the stretch led to a doubling of the force held by the fibre during the tetanus plateau. With further increase in sarcomere length the force enhancement during stretch gradually decreased in size.
Figure 3. Tetanic force and force enhancement during stretch in relation to sarcomere length.
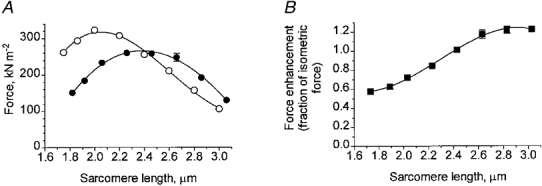
A, measurements of tetanic force (○) and force enhancement during stretch (•) recorded from the same length-controlled segment of a single muscle fibre. Mean values of five or six repeated recordings at each sarcomere length; error bars (if exceeding symbols) indicate s.e.m.B, force enhancement during stretch normalized to the tetanic force recorded at respective sarcomere length.
In Figs 3B and 4 the force enhancement during stretch is expressed as a fraction of the control isometric force recorded at the various sarcomere lengths in this way normalizing the force output to the amount of filament overlap at each sarcomere length (see further Discussion). Figures 3B and 4 demonstrate that there was a steady increase of force enhancement during stretch, relative to the isometric force, as the sarcomere length was changed from approximately 1.8 to 2.8 μm. This finding strongly suggests that the width of the myofilament lattice is an important determinant of the force enhancement during stretch. The values given above the abscissa in Fig. 4 indicate the estimated changes in fibre width as the sarcomere length is altered, the width existing at 2.0 μm sarcomere length being used as a standard. In calculating the change in fibre width the myofilament lattice was assumed to maintain a constant volume within the range of sarcomere lengths considered (see Elliott et al. 1963; Huxley, 1969; Millman, 1998). The results show that a decrease in relative fibre width below the value (1.05) existing at 1.8 μm sarcomere length led to a steady increase of the normalized force enhancement during stretch. Maximum force enhancement was attained near 2.8 μm sarcomere length at which point the relative fibre diameter was estimated to be 0.85 of the value at 2.0 μm sarcomere length. The mean force enhancement during stretch calculated for data points between 2.60 and 2.84 μm sarcomere length in Fig. 4 was 65 % greater (P < 0.001) than the mean value derived between 1.70 and 2.05 μm sarcomere length.
Figure 4. Force enhancement during stretch (normalized to tetanic force) related to sarcomere length and fibre width.
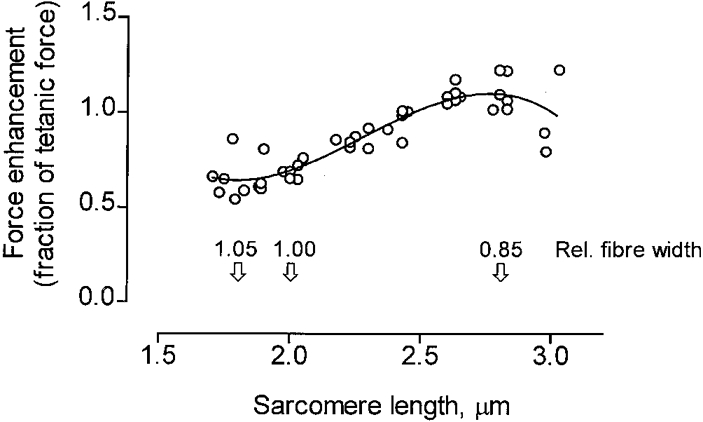
Pooled data from seven experiments similar to that illustrated in Fig. 3B. The relative fibre width at different sarcomere lengths is indicated above the abscissa (for calculation, see text).
Varied tonicity
In an attempt to further explore the width dependence of the force enhancement during stretch, experiments were performed in which the diameter of the fibre was altered by varying the tonicity of the bathing medium while the sarcomere length was kept constant at 2.00 or 2.10 μm. The osmotic strength was varied within the range 0.62-1.44 of the osmolality of normal Ringer solution (1.00R), hypotonicity being produced by reducing the sodium chloride concentration of the Ringer solution and hypertonicity by addition of sucrose (see Methods).
An increase in tonicity from 0.62R to 1.44R led to a steady reduction of the tetanic force (Fig. 5A) and to a corresponding augmentation of the force enhancement during stretch (Fig. 5B). The two effects matched each other remarkably well such that the total force held by the fibre during stretch, i.e. the sum of tetanic force and force enhancement, remained nearly constant over the whole range of tonicities studied (Fig. 5C).
Figure 5. Influence of tonicity on tetanic force and force enhancement during stretch.
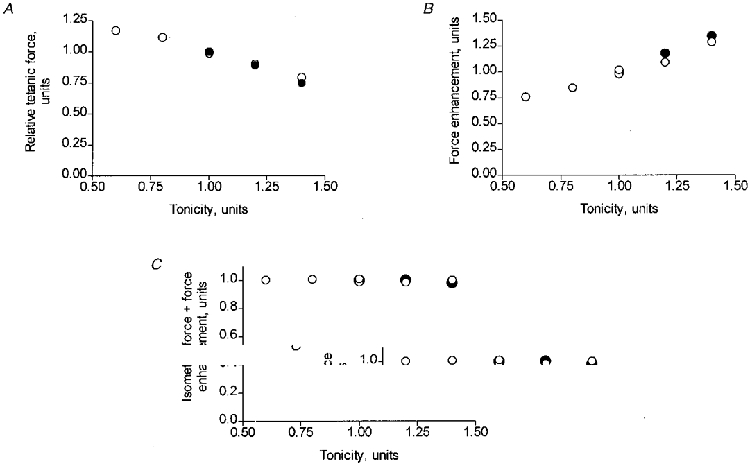
A, relationship between tetanic force and tonicity of the extracellular medium. B, force enhancement during stretch vs. tonicity. C, total force (the sum of a and b in Fig. 1) held by the length-controlled fibre segment during stretch at different tonicities. Force in diagrams A-C is expressed as a fraction of the value derived in normal (tonicity 1.0) Ringer solution. Data points in the three diagrams are mean values of five or six recordings from a given fibre segment at each tonicity. Open and filled symbols represent data from two experiments.
The amplitude of force enhancement during stretch, expressed as a fraction of the isometric force, is plotted against tonicity in Fig. 6 using the data shown in Fig. 5. The values given above the abscissa show, for comparison, the relative width of the fibre at the different tonicities. For this calculation the following information was used. The relative width of the fibre in 0.81R and 1.22R solutions has previously been measured to be 1.05 and 0.93, respectively, the measurements being normalized to the value derived in normal Ringer (Edman & Hwang, 1977). It has previously been demonstrated that the width of a frog muscle fibre varies like an osmometer over a wide range of osmotic strength (Millman et al. 1981; Takemori, 1990). Thus assuming a linear relationship between fibre width and tonicity within the entire range studied the relative fibre width in the 0.62R and 1.44R solutions can be estimated to be 1.11 and 0.86, respectively.
Figure 6. Force enhancement during stretch (normalized to tetanic force) related to tonicity and fibre width.
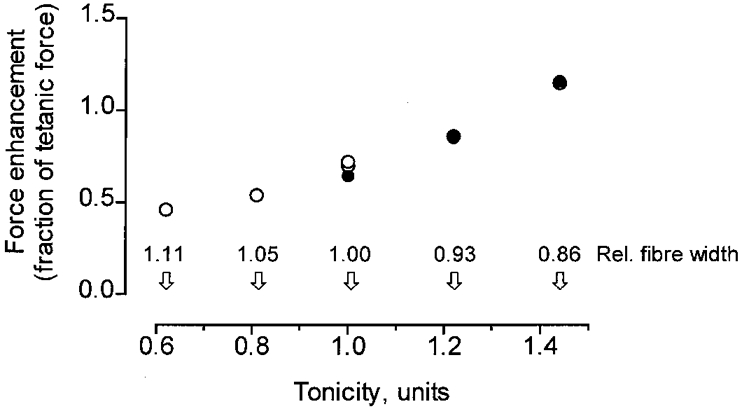
Tonicity and relative fibre width (indicated above the abscissa) are expressed as a fraction of the values in normal Ringer solution (see further text). Data refer to the same experiments as in Fig. 5.
The influence of tonicity on the force enhancement during stretch, as displayed in Fig. 6, bears a striking resemblance to the effect of altered sarcomere length (cf. Fig. 4). In common for these two interventions is the change in fibre width indicated above the abscissa in the two diagrams. As can be seen, the force enhancement during stretch (normalized to the control isometric force) increased by a factor of 1.7-2.0 as the relative fibre width was reduced from 1.05 to 0.85 either by changing the tonicity from 0.81R to 1.44R (Fig. 6) or by increasing the sarcomere length from 1.8 to 2.8 μm (Fig. 4). These results, taken together, strongly suggest that the width of the myofilament lattice is an important determinant of the force that is recruited over and above the isometric tension when a muscle fibre is stretched during activity.
Skinned muscle fibres
The above conclusion that the amplitude of force enhancement during stretch is related to the myofilament lattice width was further tested in experiments on skinned muscle fibres. Segments of frog semitendinosus muscle fibres, chemically skinned as described in Methods, were contracted isometrically (fixed-fibre ends) by exposure to 10 μm Ca2+. When maximum force had been attained (‘active’ sarcomere length approximately 2.0 μm), a stretch ramp (amplitude, ∼50 nm HS−1; speed, 130-170 nm HS−1 s−1) was applied to the fibre segment in a manner similar to that used on the intact fibres. Two contractures with stretch were first performed in standard solutions and another two contractures with stretch were thereafter carried out in the same fibre segment in the presence of 6 % PVP (see Methods). A time period of 5 min was allotted to allow the fibre to equilibrate in the PVP-containing medium before a contracture was initiated (cf. Tsuchiya, 1988). Immersion of the fibre in the polymer solution reduced the fibre diameter to 0.70 ± 0.02 (n = 19, P < 0.001) of the control value. Reimmersion of the fibre in the PVP-free control solution fully restored the fibre width. Figure 7 shows records from a representative experiment. In accordance with earlier reports (Metzger & Moss, 1987; Tsuchiya, 1988), the rate of rise of force and the total amplitude of the contracture (Fig. 7C) were both increased in the presence of 6 % PVP, the mean increase of the contracture amplitude being 1.18 ± 0.05 (n = 19, P < 0.001) of the control. The measured isometric force per fibre cross-sectional area in the PVP solution was 95 ± 7 kN m−2 (n = 19).
Figure 7. Example records illustrating force enhancement during stretch in skinned muscle fibre contracted in the presence and absence of PVP.
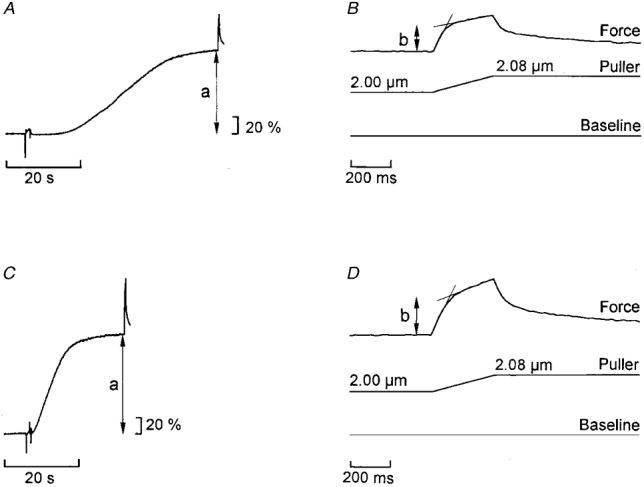
Calcium-induced contracture in standard solution with no PVP (A and B) and in the presence of 6 % (w/v) PVP (C and D). A and C, force records on a slow time base. B and D, force enhancement during stretch (end portions of traces in A and C) shown on faster time base. a, measured isometric force; b, force enhancement. Force scales: percentage of isometric force recorded in A.
Similar to the situation in the intact fibres, stretch of the skinned fibre during contracture caused an initial steep rise of force followed by a second, less steep phase during the remainder of the stretch ramp (Fig. 7B and D). Compared to the intact fibres the transition between the two phases of tension rise during stretch was less distinct in the skinned preparation and there was a more pronounced rise of force during the second phase in this preparation. Both these features of the force response to stretch in the skinned fibre are most likely attributable to a less uniform distribution of the length change compared with the intact muscle fibre (see further Edman & Tsuchiya, 1996). The amplitude of force enhancement during stretch (b, Fig. 7) amounted to 0.45 ± 0.05 (n = 19) of the isometric force (a, Fig. 7) in the control solution. This response to stretch accords well with that observed in intact muscle fibres at short sarcomere lengths and in hypotonic solution, i.e. under conditions where the width of the myofilament lattice is relatively large (compare Figs 3B and 6). The amplitude of stretch required to complete phase b was not significantly different in the absence and presence of PVP, the measured amplitudes being 14.8 ± 0.8 and 14.9 ± 1.0 nm HS−1, respectively.
In accordance with the results obtained with the intact fibres the force enhancement during stretch, measured at the end of the steep phase (b, Fig. 7B and D), increased proportionally more than the isometric force as the fibre was compressed in the presence of 6 % PVP. The force enhancement during stretch, normalized to the isometric force in the same contraction, was 23.1 ± 3.6 % (P < 0.001, n = 19) greater than the corresponding value measured in the absence of PVP. The total force measured at the end of the steep phase, i.e. the sum of isometric (pre-stretch) force and force enhancement, was 19.1 ± 3.2 % (P < 0.001, n = 19) greater in the PVP solution than in the control.
The changes in fibre width induced in the skinned fibres only partly fell within the range of changes studied in the intact fibres. The skinning procedure can be presumed to increase the fibre diameter by a factor of 1.3 (Maughan & Godt, 1979). The relative width of the fibres in the control solution was thus considerably larger than the largest width studied in the intact fibres (see Figs 4 and 6). The 30 % reduction in fibre width in the PVP solution (see earlier) would restore the relative width to approximately 0.9, i.e. to a point mid-way on the curve relating force enhancement during stretch and fibre width in Fig. 4.
DISCUSSION
When skeletal muscle is stretched during a tetanus there is an initial rapid rise of force ending at a breakpoint after which the tension remains constant or, if the stretch is applied above slack length, continues to rise slowly during the remainder of the stretch period. Evidence has recently been presented to show that the second, slow phase of tension rise represents ‘tension creep’ resulting from sarcomere non-uniformity that develops during the course of the stretch (Edman & Tsuchiya, 1996). In the present study the amplitude of force enhancement was measured soon after the end of the rising phase, i.e. at a point during the stretch ramp where the development of sarcomere non-uniformity is negligible (see further Edman & Tsuchiya, 1996). The velocity of stretch was high enough to cause maximal, or near-maximal, force enhancement. The force recorded under these conditions may therefore be regarded as the force-bearing capacity of the contractile system, i.e. the maximum force that the bridges can hold before they detach and start to slide along the thin filament during continued stretch. During the stretch ramp the bridges are likely to undergo repeated detachment-reattachment cycles (Lombardi & Piazzesi, 1990) and, if the stretch is sufficiently fast, the bridges will reach a point where the attachment to the thin filament is forcibly disrupted. According to this view the force enhancement recorded here may reflect the binding strength of the cross-bridges. It should be pointed out in this connection that the force measured during stretch also includes strain of ‘new’ cross-bridges that are recruited during the stretch phase. This additional force is likely to be small, however, as may be inferred from the fact that a stretch ramp like that used in the present study results in only a small (∼10 %) increment of fibre stiffness (Sugi & Tsuchiya, 1988; Lombardi & Piazzesi, 1990). Furthermore, with the small amplitude of stretch used in the present study, the recruitment of passive force during stretch is insignificant within the range of sarcomere lengths studied.
Recent evidence suggests that the thick and thin filaments have a finite stiffness that is comparable to that residing in the cross-bridges (Huxley et al. 1994; Kojima et al. 1994; Wakabayashi et al. 1994; Higuchi et al. 1995). The nature of the filament elasticity is still incompletely known, i.e. whether the elasticity is Hookean and whether it is uniformly distributed along the filaments or merely involves the free portions of the filaments outside the overlap region. The filament compliance is small and may increase the overlap zone by no more than ∼2 nm HS−1 as a length-clamped fibre segment develops maximal tetanic force. No account of filament compliance has therefore been made in evaluating the force-length relations in this study. The existence of filament compliance does, however, impose some uncertainty in measurements of cross-bridge stiffness. If the filament compliance has the characteristics of a Hookean spring acting in series with the the cross-bridges, a change in cross-bridge stiffness will be underestimated by using the total sarcomere stiffness as an index. For example, if the filament compliance were to account for 30 % of the sarcomere compliance (assuming that the myofilament compliance is linear), a 10 % increase in sarcomere stiffness would in reality correspond to a 15 % increase in cross-bridge stiffness. Further information on the mechanical properties of the myofilament elasticity is required to settle this point.
The force enhancement during stretch exhibits a sarcomere length dependence that is markedly different from that of the isometric force. The results show that, whereas the tetanic force is steadily reduced as the sarcomere length is increased above 2.0 μm, the force enhancement during stretch increases to a maximum at 2.4-2.5 μm and thereafter diminishes in amplitude with further increase in sarcomere length. The nature of the isometric force-length relationship needs to be considered first.
The relationship between tetanic force and sarcomere length, based on measurements in short (ca 0.5 mm) length-controlled segments (Fig. 3A) has a smooth shape without formation of a distinct plateau: the isometric force increases continuously as the sarcomere length is reduced from an extended length towards 2.0 μm, below which point force declines again. These features of the length-tension relation have been described and evaluated in considerable detail before (Edman & Reggiani, 1987). It was demonstrated that the length-tension relationship, including the features described above, can be fitted well with a model in which maximum force is proportional to the length of overlap between the thick and thin filaments if allowance is made for some variation in filament overlap within the myofibrillar sarcomeres (for details, see Edman & Reggiani, 1987). The shape of the length-tension relationship has furthermore been found to be only slightly (Bagni et al. 1990), or not at all (Edman & Andersson, 1968; April & Brandt, 1973), affected by osmotic interventions that alter the myofilament lattice width. There is thus good reason to believe that the length of filament overlap, rather than the width of the myofilament lattice, is the main determinant of the isometric force. This is in line with the view (see later) that the bridge arm (the myosin S2 fragment) runs essentially parallel with the fibre axis at the fibre widths encountered above optimal length.
The present results provide evidence that the force recruited during stretch, contrary to the plain isometric force, is sensitive to changes in myofilament lattice width. This was demonstrated by normalizing the force enhancement during stretch to the control (pre-stretch) isometric force. By this procedure the force enhancement during stretch becomes, in effect, normalized to the length of filament overlap according to the foregoing discussion, i.e. to the number of active cross-bridges at each sarcomere length. Any influence of sarcomere length on the normalized value of the force enhancement is therefore attributable to the concomitant change in fibre width or to some other variable that changes with sarcomere length. Stienen et al. (1992) have demonstrated that the amplitude of force enhancement during stretch varies with the calcium ion concentration at submaximal activation levels in skinned muscle fibres. It is unlikely, however, that altered calcium sensitivity would underlie the width dependence of the force enhancement by stretch observed in the present study on intact fibres. The present measurements were made during the plateau phase of a fused tetanus, i.e. under conditions where the contractile system can be presumed to be maximally activated (see Caputo et al. 1994).
Since a muscle fibre maintains a virtually constant volume as the sarcomere length is changed (Elliott et al. 1963; Huxley, 1969; Millman, 1998), the relative fibre width could be estimated at each sarcomere length investigated. The results show (Fig. 4) that, within the range of fibre widths existing between 1.8 and 2.8 μm sarcomere length, there is an inverse relationship between the normalized force enhancement during stretch and fibre width. Assuming that the 1,0 lattice spacing in frog muscle is 38 nm at 2.0 μm sarcomere length (Matsubara & Elliott, 1972; Millman, 1998) and the diameter of the thick and thin filaments are 12 and 7 nm (Huxley, 1963), respectively, the side spacing between the thick and thin filaments can be estimated to decrease by 5.3 nm, from 17.2 to 11.9 nm, as the sarcomere length is increased from 1.8 to 2.8 μm. This decrease in lattice spacing was associated with a 65 % increase in the normalized value of force enhancement during stretch (Fig. 4).
The conclusion that force enhancement during stretch is dependent on the myofilament lattice width within the range studied in the intact muscle fibres was corroborated by similar measurements on skinned muscle fibres. In these experiments the sarcomere length, and therefore the number of active cross-bridges, was kept constant while the width of the fibre was reduced by osmotic compression in the presence of polyvinylpyrrolidine (PVP). It is pertinent that the total force, i.e. the sum of isometric (pre-stretch) force and force enhancement, increased as the fibre width was reduced. This strengthens the view (see further below) that the force by which the cross-bridge is attached to the thin filament during stretching becomes greater as the lateral spacing between the filaments is reduced within the range covered in this study. The width dependence of the force enhancement during stretch improves the bridges’ ability to resist stretch at greater sarcomere lengths. In this way the ability of muscle to withstand stretch will remain fairly high even when the muscle is elongated beyond its optimal length where the number of attached cross-bridges is reduced.
Force enhancement during stretch related to cross-bridge function
It is generally assumed that myosin cross-bridges go through several states of attachment during a working cycle and that each new step leads to progressively stronger binding of the bridge to the actin unit and to further production of force (Huxley & Simmons, 1971). As pointed out above, it is reasonable to assume that the tension recorded after the breakpoint of the force myogram during stretch represents the force by which the myosin bridge is held by the actin filament. The bridge attachment exhibits a viscous behaviour in that the amplitude of force enhancement, i.e. the force required to disrupt the bridge connection, increases with the stretch velocity approaching a maximal value at velocities greater than 200 nm HS−1 s−1 (Edman, 1988; Lombardi & Piazzesi, 1990).
The viscous behaviour of the contractile system during stretch has been simulated by Lombardi & Piazzesi (1990) using a kinetic cross-bridge model. In their program the bridges become ‘forcibly’ detached in a strained (force bearing) state by making the dissociation rate constant(s) progressively larger as the extension of the cross-bridges is increased at higher speeds. The number of attached cross-bridges in the model could be kept constant at different velocities of stretch, in accordance with actual stiffness measurements in intact muscle fibres (see earlier), by increasing the association rate constant appropriately. The general shape of the stretch velocity-force relationship could be simulated well by the model used by Lombardi & Piazzesi (1990), but the stretch velocity required to produce maximal force enhancement was greater than that recorded in an intact fibre. Related models for simulating the enhancement of force during stretch were later presented by Månsson (1994) and Burmeister Getz et al. (1998). However, the molecular basis of the viscous behaviour of the contractile system during stretch still remains obscure.
Recent progress in the study of the molecular structure of actin and myosin (Rayment et al. 1993) has opened up the possibility of elucidating the interaction between the myosin head and the actin filament in considerable detail. Based on information on the 3D structure of the two proteins, models of the actin-myosin interaction have been proposed to explain the structural changes of the cross-bridge during a working cycle. Positively charged residues of the S1 portion of the myosin molecule are thought to make sequential attachments to negatively charged actin sites, and two or more interaction sites between the myosin head and the actin moieties may be formed in this way. According to molecular dynamic calculations presented by Diaz Baños et al. (1996) each new binding site will lead to a stronger attachment between the two molecules and to a greater force output of the bridge thus supporting the basic assumptions of the multi-step cross-bridge model that was originally advanced by Huxley & Simmons (1971) and later used in modified versions.
The present results suggest that the active force produced by a cross-bridge is not a simple function of the strength by which the bridge is attached to the thin filament. This is indicated by the finding that the two properties are affected differentially by a change in lattice width. Thus, whereas the force producing capability of the bridge is found to be largely independent of the lateral distance between the thick and thin filaments (see earlier), the apparent binding strength, measured from the axial force required to disconnect the bridge, is markedly sensitive to changes of the myofilament lattice width within the range of fibre widths encountered between 1.8 and 2.8 μm sarcomere length. The molecular mechanism underlying the width dependence of the cross-bridge binding force is unknown at the present time and requires further work for its clarification. It is of interest to note that the myosin head (approximately 17 nm long and 6 nm wide, Elliott & Offer, 1978; Rayment et al. 1993) is large enough to span the entire gap between the thick and thin filaments near slack fibre length (side-to-side distance 17.2 nm at 1.8 μm sarcomere length, see earlier), and it is reasonable to presume that the attitude of the myosin head will change as the spacing between the filaments becomes narrower at longer sarcomere lengths. The available space for the myosin head will indeed become drastically reduced as the sarcomere length is increased. For example, at 2.8 μm sarcomere length the side-to-side distance between the thick and thin filaments can be estimated to be merely 11.9 nm (see earlier). The tighter packing of the filaments at greater lengths can be presumed to increase the effective area over which the actin and myosin molecules are able to interact. This may increase the opportunities for bond formation between the two structures and thus lead to a firmer attachment of the bridge.
A contractile change that has features in common with the force enhancement during stretch is the potentiation of force development induced by phosphorylation of the myosin regulatory light chains (Metzger et al. 1989; Levine et al. 1996). Myosin light chain phosphorylation leads to a conformational change of the S1 unit that increases the mobility of the myosin heads making them more easily available for interaction with the actin moities than when unphosphorylated. Interestingly, the potentiation of force resulting from myosin light chain phosphorylation can be substituted by decreasing the the lateral distance between the thick and thin filaments, either by increasing the sarcomere length or by osmotically compressing of the myofilament lattice (Levine et al. 1996). It remains to be explored, however, whether phosphorylation of the myosin regulatory light chains also leads to increased force enhancement during stretch that would indicate a stronger attachment of the myosin bridges to the thin filament as discussed above.
Effects of tonicity on isometric force and force enhancement during stretch
The present study provides further insight into the effects of altered tonicity on isometric force and force enhancement during stretch in intact muscle fibres. In accordance with previous observations (Månsson, 1989, 1994; Piazzesi et al. 1994) hypertonicity of the extracellular medium leads to a decrease of the tetanic force and to an increase of the force enhancement during stretch while the opposite is true for hypotonicity which increases tetanic force and reduces force enhancement during stretch. The change in contractile force induced by tonicity may be attributed to altered force output of the individual cross-bridge. The latter change is most probably effectuated by the altered intracellular ionic strength, since the active force produced by the bridge is little affected by the fibre width (see earlier). In accordance with this view earlier experiments have demonstrated that altered tonicity does not change the instantaneous stiffness of the muscle fibre to any significant degree (Månsson, 1994; Piazzesi et al. 1994) suggesting that the number of attached cross-bridges remains essentially constant.
It is of particular interest to note that the total force during stretch, which most probably reflects the strength of binding of the myosin cross-bridge, remains virtually constant over the wide range of tonicities studied (Fig. 5C). A change in tonicity leads both to a change in fibre width and to altered intracellular ionic strength and these two interventions affect the binding strength of the myosin cross-bridge in opposite ways. A decrease in fibre width may, according to the foregoing, be expected to increase the binding strength, whereas the concomitant increase in intracellular ionic strength will have the opposite effect (e.g. Brenner, 1990). The present results suggest that the two effects cancel one another out resulting in a nearly unchanged total force during stretch as the tonicity is varied.
Acknowledgments
This work was supported by a grant from the Swedish Medical Research Council (project No. 14X-184).
References
- Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. The Journal of Physiology. 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- April EW, Brandt PW. The myofilament lattice: Studies on isolated fibers. III. The effect of myofilament spacing upon tension. Journal of General Physiology. 1973;61:490–508. doi: 10.1085/jgp.61.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert X. Le couplage Énergétique de la Contraction Musculaire. Brussels: Arscia; 1956. [Google Scholar]
- Bagni MA, Cecchi G, Colomo F. Myofilament spacing and force generation in intact frog muscle fibres. The Journal of Physiology. 1990;430:61–75. doi: 10.1113/jphysiol.1990.sp018281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Muscle mechanics and biological kinetics. In: Squire JM, editor. Topics in Molecular and Structural Biology, Molecular Mechanisms in Muscular Contraction. Vol. 13. London: MacMillan Press; 1990. pp. 77–149. [Google Scholar]
- Burmeister Getz E, Cooke R, Lehman SL. Phase transition in force during ramp stretches of skeletal muscle. Biophysical Journal. 1998;75:2971–2983. doi: 10.1016/S0006-3495(98)77738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Edman KAP, Lou F, Sun Y-B. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. The Journal of Physiology. 1994;478:137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Citterio G. Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. The Journal of Physiology. 1974;239:1–14. doi: 10.1113/jphysiol.1974.sp010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Baños FG, Bordas J, Lowy J, Svensson A. Small segmental rearrangements in the myosin head can explain force generation in muscle. Biophysical Journal. 1996;71:576–589. doi: 10.1016/S0006-3495(96)79292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP. Double-hyperbolic force-velocity relation in frog muscle fibres. The Journal of Physiology. 1988;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Andersson K-E. The variation in active tension with sarcomere length in vertebrate skeletal muscle and its relation to fibre width. Experientia. 1968;24:134–136. doi: 10.1007/BF02146942. [DOI] [PubMed] [Google Scholar]
- Edman KAP, Elzinga G, Noble MIM. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. The Journal of Physiology. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Elzinga G, Noble MIM. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. Journal of General Physiology. 1981;78:365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Elzinga G, Noble MIM. Residual force enhancement after stretch of contracting frog single muscle fibers. Journal of General Physiology. 1982;80:769–784. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Hwang JC. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. The Journal of Physiology. 1977;269:255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Lou F. Changes in force and stiffness induced by fatigue and intracellular acidification in frog muscle fibres. The Journal of Physiology. 1990;424:133–149. doi: 10.1113/jphysiol.1990.sp018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. The Journal of Physiology. 1984;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Reggiani C. The sarcomere length-tension relation determined in short segments of intact muscle fibres of the frog. The Journal of Physiology. 1987;385:709–732. doi: 10.1113/jphysiol.1987.sp016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Tsuchiya T. Strain of passive elements during force enhancement by stretch in frog muscle fibres. The Journal of Physiology. 1996;490:191–205. doi: 10.1113/jphysiol.1996.sp021135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund M, Edman KAP. Shortening induced deactivation of skinned fibres of frog and mouse striated muscle. Acta Physiologica Scandinavica. 1982;116:189–199. doi: 10.1111/j.1748-1716.1982.tb07129.x. [DOI] [PubMed] [Google Scholar]
- Elliott A, Offer G. Shape and flexibility of the myosin molecule. Journal of Molecular Biology. 1978;123:505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Elliott GF, Lowy J, Worthington CR. An X-ray and light-diffraction study of the filament lattice of striated muscle in the living state and in rigor. Journal of Molecular Biology. 1963;6:295–305. [Google Scholar]
- Fenn WO. The relation between work performed and the energy liberated in muscular contraction. The Journal of Physiology. 1924;58:373–395. doi: 10.1113/jphysiol.1924.sp002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney FW, Hirst DG. Cross-bridge detachment and sarcomere ‘give’ during stretch of active frog's muscle. The Journal of Physiology. 1978;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Yanagida T, Goldman Y. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophysical Journal. 1995;69:1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV, Howarth JV. The reversal of chemical reactions in contracting muscle during an applied stretch. Proceedings of the Royal Society. 1959;B 151:169–193. [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. Journal of Molecular Biology. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- Huxley HE, Stewart A, Sosa H, Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophysical Journal. 1994;67:2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Morgan DL. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. The Journal of Physiology. 1979;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. The Journal of Physiology. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Ishijima A, Yanagida T. Direct measurement of stiffness of single actin filaments with and without tropomyosin by an in vitro nanomanipulation. Proceedings of the National Academy of Sciences of the USA. 1994;91:12962–12966. doi: 10.1073/pnas.91.26.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophysical Journal. 1996;71:898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V, Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. The Journal of Physiology. 1990;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson A. Changes in force and stiffness during stretch of skeletal muscle fibers, effects of hypertonicity. Biophysical Journal. 1989;56:429–433. doi: 10.1016/S0006-3495(89)82689-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson A. The tension response to stretch of intact skeletal muscle fibres of the frog at varied tonicity of the extracellular medium. Journal of Muscle Research and Cell Motility. 1994;15:145–157. doi: 10.1007/BF00130425. [DOI] [PubMed] [Google Scholar]
- Matsubara I, Elliott GF. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. Journal of Molecular Biology. 1972;72:657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Maughan DW, Godt RE. Stretch and radial compression studies on relaxed skinned muscle fibers of the frog. Biophysical Journal. 1979;28:391–402. doi: 10.1016/S0006-3495(79)85188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. Journal of General Physiology. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Shortening velocity in skinned single muscle fibers, influence of filament lattice spacing. Biophysical Journal. 1987;52:127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM. The filament lattice of striated muscle. Physiological Reviews. 1998;78:359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- Millman BM, Racey TJ, Matsubara I. Effects of hyperosmotic solutions on the filament lattice of intact frog skeletal muscle. Biophysical Journal. 1981;33:189–202. doi: 10.1016/S0006-3495(81)84880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Linari M, Lombardi V. The effect of hypertonicity on force generation in tetanized single fibres from frog skeletal muscle. The Journal of Physiology. 1994;476:531–546. doi: 10.1113/jphysiol.1994.sp020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewsky WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Stienen GJM, Versteeg PGA, Papp Z, Elzinga G. Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: effects of phosphate and Ca2+ The Journal of Physiology. 1992;451:503–523. doi: 10.1113/jphysiol.1992.sp019176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. The Journal of Physiology. 1972;225:237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. The Journal of Physiology. 1988;407:215–229. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori S. Influence of osmotic swelling on cross section and resting tension in isolated skeletal muscle fibers. Japanese The Journal of Physiology. 1990;40:595–611. doi: 10.2170/jjphysiol.40.595. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T. Passive interaction between sliding filaments in the osmotically compressed skinned muscle fibers of the frog. Biophysical Journal. 1988;53:415–423. doi: 10.1016/S0006-3495(88)83118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophysical Journal. 1994;67:2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


