Abstract
The location of purinoceptors in the pancreatic duct and their role in regulating ductal secretion have been investigated by applying ATP and UTP to basolateral and luminal surfaces of pancreatic ducts isolated from the guinea-pig pancreas.
Changes in intracellular Ca2+ concentration were measured by microfluorometry in microperfused interlobular duct segments. Fluid and HCO3− secretion were estimated by monitoring luminal pH and luminal volume in sealed duct segments microinjected with BCECF-dextran.
Both ATP and UTP (1 μm) caused biphasic increases in intracellular Ca2+ concentration in pancreatic duct cells when applied to either the basolateral or luminal membrane.
Luminal application of both ATP and UTP evoked fluid and HCO3− secretion. The maximum response to 1 μm ATP or UTP was about 75 % of that evoked by secretin. By contrast, basolateral application of ATP or UTP inhibited spontaneous secretion by 52 % and 73 %, respectively, and secretin-evoked secretion by 41 % and 38 %, respectively.
The data suggest that luminal nucleotides may act in an autocrine or paracrine fashion to enhance ductal secretion while basolateral nucleotides, perhaps released from nerve terminals, may have an inhibitory effect. The fact that both apical and basolateral purinoceptors elevate intracellular Ca2+, but that they have opposite effects on secretion, suggests that additional signalling pathways are involved.
Pancreatic duct cells secrete a HCO3−-rich fluid in response to secretin and, to a lesser extent, vasoactive intestinal peptide (VIP), vagal stimulation and perhaps cholecystokinin (CCK) (Case & Argent, 1993). Recently, extracellular ATP has been shown to influence ion transport in a variety of epithelia, including pancreatic duct cells. In a cystic fibrosis pancreatic duct cell line (CFPAC-1), apical application of ATP and UTP increased the intracellular Ca2+ concentration ([Ca2+]i) and activated apical Cl− channels (Chan et al. 1996). In intra- and interlobular ducts isolated from rat pancreas, ATP induced hyperpolarization and raised [Ca2+]i (Hug et al. 1994), and these effects were attributed to P2Y2 and P2X7 receptors (Christoffersen et al. 1998). In non-transformed dog pancreatic duct cells maintained in long-term culture as monolayers, apical or basolateral application of UTP activated an apical Cl− conductance and a basolateral K+ conductance (Nguyen et al. 1998). Taken together, these data suggest that pancreatic duct cells possess a variety of purinoceptors on both membranes and that these probably mediate the activation of apical Ca2+-dependent Cl− channels. Apart from one report suggesting that secretin-evoked secretion by the perfused dog pancreas is enhanced by adenosine and ATP (Yamagishi et al. 1986), the effects of extracellular nucleotides on HCO3− and fluid secretion are not well defined.
Recently we have developed a method for measuring HCO3− and fluid secretion in isolated guinea-pig pancreatic ducts by monitoring luminal pH and luminal volume simultaneously by fluorescence microscopy (Ishiguro et al. 1998b). When stimulated with secretin, these interlobular duct segments secrete a HCO3−-rich fluid (>130 mM) into their closed luminal space at rates as high as 3 nl min−1 mm−2 (with 10 nM secretin). This preparation therefore preserves the in vivo function of the guinea-pig pancreas in which the HCO3− concentration in the secreted fluid can reach 150 mM during secretin stimulation (Padfield et al. 1989).
In this study, to determine the physiological roles of purinoceptors in guinea-pig pancreatic duct cells, we have examined the effects of basolateral or luminal application of ATP and UTP on Ca2+ mobilization and secretion in isolated interlobular duct segments.
Part of this work has been reported to The Physiological Society (Ishiguro et al. 1998a).
METHODS
Isolation and culture of interlobular ducts
Female Hartley guinea-pigs (350-450 g) were killed by cervical dislocation. The body and tail of the pancreas were removed and interlobular ducts (diameter 100-150 μm, length 800-1200 μm) were isolated and cultured overnight as described previously (Ishiguro et al. 1996). During overnight culture both ends of the duct segments sealed spontaneously.
Solutions
Bath solution
The standard HCO3−-buffered solution contained (mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose and 25 NaHCO3, and was equilibrated with 95 % O2-5 % CO2. The Cl−-free HCO3−-buffered solution was made by replacing Cl− with glucuronate. The Cl−-free Hepes-buffered solution contained (mM): 130 sodium glucuronate, 2.5 K2HPO4, 1 CaSO4, 1 MgSO4, 10 D-glucose and 10 Hepes, and was equilibrated with 100 % O2. All solutions were adjusted to pH 7.4 at 37°C.
Luminal injection solution
The Cl−-free solution injected into the duct lumen contained (mM): 139 sodium glucuronate, 2.5 K2HPO4, 1 CaSO4, 1 MgSO4, 10 D-glucose and 1 Hepes and was adjusted to pH 7.2 at 37°C. The luminal injection solution also contained a dextran conjugate of the pH-sensitive fluoroprobe 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-dextran, 70 kDa) at a concentration of 20 μm.
Microperfusion
The lumen of the cultured interlobular ducts was microperfused using a modification of the method originally developed for measuring membrane potentials in ducts isolated from the rat pancreas (Novak & Greger, 1998a,b). Both of the sealed ends of the duct were cut open using sharpened needles and the duct transferred into a 700 μl perfusion chamber mounted on an inverted microscope (Olympus IX). One end of the duct was then cannulated for luminal perfusion as shown in Fig. 1A. The concentric pipette arrangement consisted of (1) an outer, 120 μm diameter holding pipette with a constriction, (2) a perfusion pipette and (3) an inner exchange pipette. The duct was gently aspirated into the holding pipette and the perfusion pipette was advanced into the duct. The tip of the exchange pipette (fused silica capillary, o.d. 440 μm, i.d. 320 μm, Polymicro Technologies Inc., Phoenix, AZ, USA), which allows rapid exchange of the luminal perfusate, was located within the shank of the perfusion pipette. Pressurized nitrogen gas (ca 100 kPa) was applied to the reservoirs supplying the luminal perfusion solutions and the waste line from the back of the perfusion pipette was connected to a reservoir located approximately 30 cm above the chamber. The bath was continuously perfused at 3 ml min−1 at 37°C in the same direction as the flow of luminal perfusate. The luminal solution leaving the open end of the duct was swept away by the flow of the bath solution, thus preventing it from gaining access to the basal surface of the duct epithelium. At the end of some experiments the lumen was perfused with a dye solution (Amido Black, Katayama Chemical, Nagoya, Japan) to judge whether the luminal perfusion was adequate (Fig. 1A). Changes of the luminal perfusate reached the duct lumen within 10 s of switching the reservoir valves.
Figure 1. Effects of basolateral and luminal ATP and UTP on intracellular Ca2+.
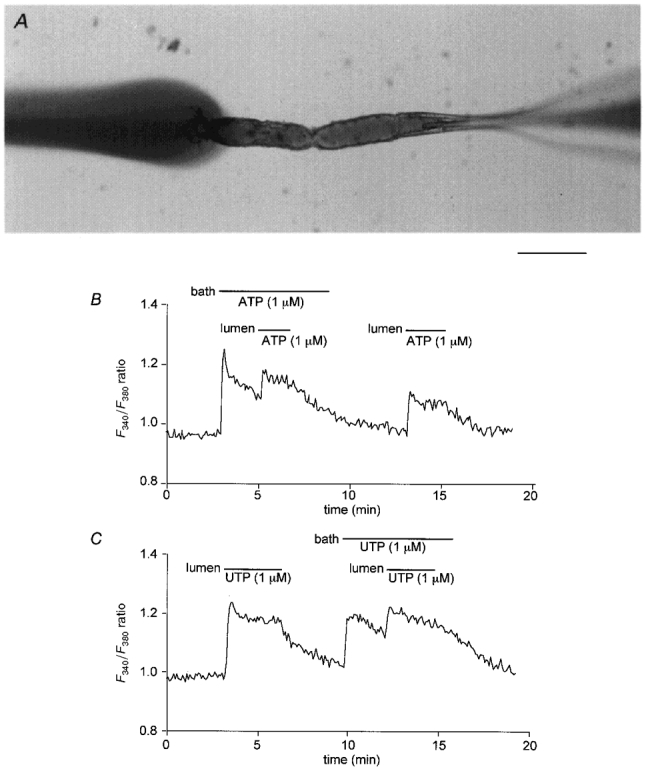
A, interlobular duct segment isolated from guinea-pig pancreas and cannulated (from the right) for luminal microperfusion. In this image the lumen was perfused with a coloured solution which can be seen emerging from the left end of the duct where it is swept away by the flow of the bath solution from right to left. Scale bar represents 200 μm. B and C, changes in fura-2 fluorescence ratio (F340/F380) indicating changes in [Ca2+]i in microperfused ducts stimulated with ATP and UTP. The bath and lumen were perfused separately with the standard HCO3−-buffered solution. During the periods indicated, 1 μm ATP (B) and 1 μm UTP (C) were applied via the bath and/or the lumen. Each trace is representative of four similar experiments.
Measurement of intracellular Ca2+ concentration
Intracellular free Ca2+ concentration ([Ca2+]i) was estimated by microfluorometry in duct cells loaded with fura-2 using a modification of the method previously applied to rat pancreatic ducts (Ashton et al. 1993). The cultured guinea-pig ducts were incubated for 60-90 min at room temperature with the acetoxymethyl ester of fura-2 (fura-2 AM, 3-5 μm) in the standard HCO3−-buffered solution. Microfluorometry was then performed on a small area of the ductal epithelium (ten to twenty cells) in microperfused ducts illuminated alternately at 340 and 380 nm. The fluorescence intensities (F340 and F380) were measured at 510 nm. Changes in [Ca2+]i are presented as changes in the F340/F380 fluorescence ratio.
Measurement of luminal pH and fluid secretory rate
During overnight culture, the ends of the ducts sealed spontaneously thus isolating the luminal space from the bathing medium. Changes in luminal pH (pHL) and luminal volume were measured as described previously (Ishiguro et al. 1998b). Briefly, the duct lumen was first micropunctured and the luminal fluid replaced with a solution containing BCECF-dextran (20 μm). Images of BCECF-dextran fluorescence (at 530 nm) were obtained at 1 min intervals using a CCD camera with the excitation wavelength alternating between 430 and 480 nm. The volume of the duct lumen was calculated from the area of fluorescence in each image assuming cylindrical geometry. The rate of fluid secretion was then calculated from the increment in the luminal volume and expressed as secretory rate per unit area of epithelium (nl min−1 mm−2). Luminal pH was calculated from the fluorescence ratio (F480/F430) using in situ calibration data.
Materials
BCECF-dextran was obtained from Molecular Probes, ATP and UTP from Boehringer Mannheim, fura-2 AM from Dojindo Laboratories (Kumamoto, Japan), and secretin (porcine) from Peptide Institute (Osaka, Japan).
Statistics
Averaged data are presented as the means ±s.e.m. unless otherwise indicated. Tests for statistically significant differences were made with Student's t test for unpaired data.
RESULTS
Effects of ATP and UTP on [Ca2+]i
In order to determine the localization of Ca2+-mobilizing purinoceptors in the guinea-pig pancreatic duct, [Ca2+]i was measured in microperfused interlobular duct segments. The outer and inner surfaces of the ducts were perfused separately with the standard HCO3−-buffered solution. Both basolateral and luminal application of 1 μm ATP (Fig. 1B) caused biphasic increases in [Ca2+]i. The responses comprised an initial peak followed by a slowly declining plateau. When luminal application of ATP was superimposed on the application of basolateral ATP, a significant additional increase in [Ca2+]i was observed (Fig. 1B). This indicates that luminal ATP has access to a pool of receptors that is not accessible to basolateral ATP and vice versa. Furthermore it eliminates the possibility that the effects of luminal ATP were the result of leakage through the paracellular pathway to the basolateral membrane. Similar results were obtained with UTP (Fig. 1C). We therefore conclude that the duct cells of the guinea-pig pancreas possess purinoceptors which are sensitive to ATP and UTP on both the basolateral and luminal membranes.
Effects of luminal ATP and UTP on HCO3− and fluid secretion
We have previously shown that secretin-evoked HCO3− secretion across the luminal membrane can be detected as a rise in luminal pH when the duct is initially filled with a Cl−-free solution (Ishiguro et al. 1998b). Since this also simulates physiological conditions, where the Cl− concentration in the lumen is low, we have examined the effects of extracellular ATP on luminal pH and fluid secretory rate in the nominal absence of Cl−.
Sealed ducts filled with a Cl−-free solution containing BCECF-dextran were first superfused with a Cl−-free Hepes-buffered solution. When the bath solution was switched to a HCO3−/CO2-buffered solution, the lumen initially became acidified as a result of CO2 diffusion into the lumen from the bath. Luminal pH then recovered partially over a period of 4 min as a result of the slow spontaneous secretion of HCO3− (Fig. 2A). During this period, a low level of spontaneous fluid secretion (0.47 ± 0.17 nl min−1 mm−2, n = 5) was also observed (Fig. 2B). When 10 nM secretin was then added to the bath solution, the rise in pHL accelerated (Fig. 2A) and the fluid secretory rate increased to 2.01 ± 0.20 nl min−1 mm−2 (Fig. 2B). The net secretory flux of HCO3−, which is a composite function of both the changes in pHL and the volume flow (Ishiguro et al. 1998b), thus increased significantly.
Figure 2. Effects of luminal ATP on luminal pH and fluid secretion.
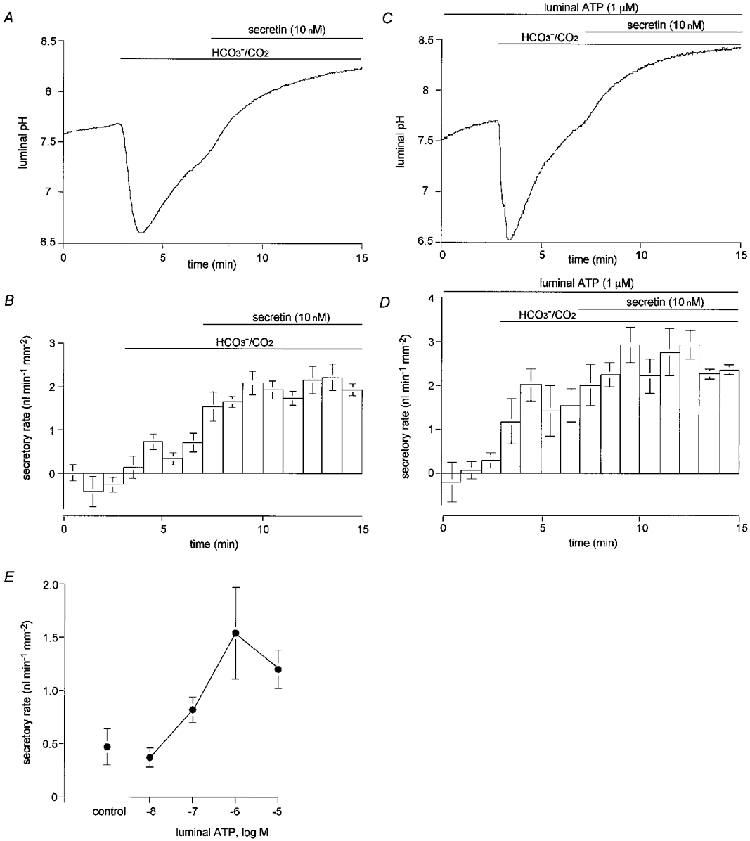
Guinea-pig pancreatic ducts filled by micropuncture with a weakly buffered HCO3−-free, Cl−-free solution containing 20 μm BCECF-dextran were initially superfused with a Cl−-free Hepes-buffered bath solution. After 3 min this was switched to a Cl−-free, HCO3−-buffered solution and, after a further 4 min, 10 nM secretin was applied via the bath. A and B, control experiments: changes in luminal pH (A) and fluid secretory rate (B) in the absence of luminal ATP (A, one of five experiments; B, means ±s.e.m. of all five experiments). C and D, effects of luminal ATP: changes in luminal pH (C) and fluid secretory rate (D) in ducts injected with 1 μm ATP (C, one of four experiments; D, means ±s.e.m. of all four experiments). E, concentration-response curve for the effects of luminal ATP on fluid secretory rate. Data are shown as means ±s.e.m. of at least four experiments.
To examine the effects of luminal ATP on HCO3− secretion and fluid secretory rate, 1 μm ATP was added to the solution injected into the lumen at the beginning of the experiment (Fig. 2C and D). In the absence of HCO3−, luminal ATP had no effect on pHL or on fluid secretory rate. However, when HCO3− was subsequently admitted to the bath solution, the secretory rate increased to 1.54 ± 0.43 nl min−1 mm−2 (n = 4, P < 0.05, Fig. 2D), a value significantly greater than during the equivalent period in the absence of luminal ATP (Fig. 2B, prior to stimulation with secretin). The rise in pHL (3.5-5 min, Fig. 2C) was also markedly accelerated compared with data in Fig. 2A. These results indicate that luminal ATP activates HCO3− secretion and HCO3−-dependent fluid secretion. Figure 2E shows the concentration-response curve for the effect of luminal ATP on fluid secretory rate. The concentration of ATP required for a half-maximal increase in fluid secretion was approximately 0.3 μm.
In the presence of luminal ATP, stimulation with basolateral secretin (10 nM) raised the fluid secretory rate further, but only by an additional 23 % (Fig. 2D). This suggests that fluid secretion was already close to maximal as a result of stimulation with 1 μm luminal ATP.
The same protocol was used to examine the effects of luminal UTP (1 μm) on HCO3− and fluid secretion (Fig. 3A and B). Luminal UTP increased the fluid secretory rate in the presence of HCO3− to 1.67 ± 0.12 nl min−1 mm−2 (n = 4, P < 0.01, Fig. 3B) and, like luminal ATP, also accelerated the rate of pHL increase (Fig. 3A). Luminal UTP therefore appears to stimulate HCO3− and fluid secretion to a similar extent to ATP.
Figure 3. Effects of luminal UTP on luminal pH and fluid secretion.
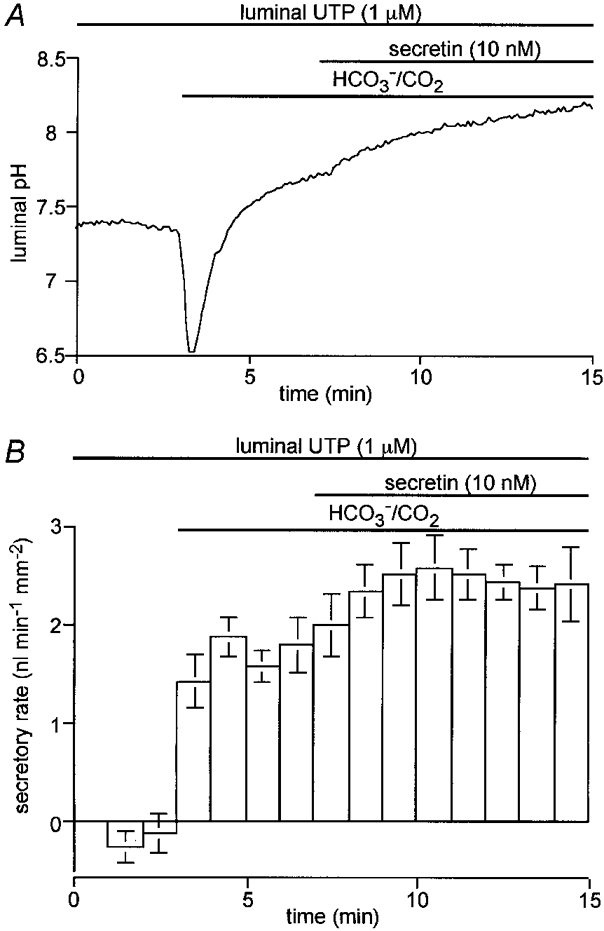
Guinea-pig pancreatic ducts filled by micropuncture with a weakly buffered HCO3−-free, Cl−-free solution containing 1 μm UTP and 20 μm BCECF-dextran were initially superfused with a Cl−-free Hepes-buffered bath solution. After 3 min this was switched to a Cl−-free, HCO3−-buffered solution and, after a further 4 min, 10 nM secretin was applied via the bath. A, changes in luminal pH (one of four experiments); B, changes in fluid secretory rate (means ±s.e.m. of all four experiments).
Effects of basolateral ATP and UTP on HCO3− and fluid secretion
To examine the effects of basolateral ATP on HCO3− and fluid secretion, 1 μm ATP was applied to the bath during experiments similar to those described above. When ATP was applied during the period of spontaneous secretion (i.e. in the presence of HCO3−/CO2 but before the addition of secretin) the secretory rate was reduced by 52 ± 8 % (n = 4, P < 0.05, Fig. 4B). When ATP was applied during secretin stimulation, the secretory rate was reduced by 41 ± 10 % (P < 0.05, Fig. 4B). In both cases the effects of ATP were quickly reversed when ATP was withdrawn from the bath solution. The rate of increase in luminal pH decreased markedly when ATP was applied during secretin stimulation and then recovered when the ATP was withdrawn (Fig. 4A).
Figure 4. Effects of basolateral ATP on luminal pH and fluid secretion.

Guinea-pig pancreatic ducts filled by micropuncture with a weakly buffered HCO3−-free, Cl−-free solution containing 20 μm BCECF-dextran were initially superfused with a Cl−-free, Hepes-buffered bath solution. After 3 min this was switched to a Cl−-free, HCO3−-buffered solution and, after a further 6 min, 10 nM secretin was applied via the bath. During the periods indicated, 1 μm ATP was applied via the bath in the absence or presence of 10 nM secretin. A, changes in luminal pH; B, changes in fluid secretory rate (one of five experiments).C, concentration-dependent effects of basolateral ATP on the fluid secretory rate evoked by 10 nM secretin. Increasing concentrations of ATP (0.1 μm and 1 μm) were applied successively via the bath during stimulation with 10 nM secretin. Data are means ±s.e.m. of four experiments.
When ATP was applied to the bath, first at a concentration of 0.1 μm and then at 1 μm, secretin-evoked fluid secretion was inhibited in a concentration-dependent manner (Fig. 4C). The secretory rate was reduced by 19 ± 3 % (n = 4, P < 0.05) in the presence of 0.1 μm ATP and by 41 ± 4 % (n = 4, P < 0.05) with 1 μm ATP. These data indicate that, in contrast with the stimulatory effects of ATP applied to the luminal membrane, ATP applied to the basolateral membrane inhibits HCO3− and fluid secretion.
Using a similar protocol, basolateral UTP (1 μm) was observed to reversibly reduce spontaneous fluid secretion by 73 ± 9 % (n = 4, P < 0.01, Fig. 5B) and secretin-stimulated fluid secretion by 38 ± 6 % ( P < 0.05, Fig. 5B). It also reduced the rate of increase in luminal pH when applied during secretin stimulation (Fig. 5A). In other words, the effects of basolateral UTP on HCO3− and fluid secretion were similar to those of basolateral ATP (Fig. 4).
Figure 5. Effects of basolateral UTP on luminal pH and fluid secretion.

Guinea-pig pancreatic ducts filled by micropuncture with a weakly buffered HCO3−-free, Cl−-free solution containing 20 μm BCECF-dextran were initially superfused with a Cl−-free, Hepes-buffered bath solution. After 3 min this was switched to a Cl−-free, HCO3−-buffered solution and, after a further 11 min, 10 nM secretin was applied via the bath. During the periods indicated, 1 μm UTP was applied via the bath in the absence or presence of 10 nM secretin. A, changes in luminal pH; B, changes in fluid secretory rate (one of four experiments).
DISCUSSION
In microperfused guinea-pig pancreatic duct segments, application of ATP and UTP to the basolateral and luminal membranes induced similar increases in duct cell [Ca2+]i suggesting the presence of purinoceptors on both membranes. This result is consistent with a previous study of dog pancreatic duct cells where both basolateral and luminal application of UTP activated apical Cl− and basolateral K+ conductances (Nguyen et al. 1998). However, in micropunctured guinea-pig ducts, the effects of basolateral and luminal ATP and UTP on HCO3− and fluid secretion were opposite: luminal nucleotides stimulated secretion while basolateral nucleotides inhibited it.
Differing effects of basolateral and luminal ATP on fluid secretion are not unknown in other epithelia and have, for example, been observed in epididymis and in biliary epithelial cells, which show many similarities to pancreatic duct cells. In rat epididymal cells, luminal but not basolateral ATP stimulated Cl− transport (Wong, 1988). In isolated bile duct units from the rat, basolateral ATP raised [Ca2+]i but did not induce any fluid secretion (Nathanson et al. 1996) while, in cultured cholangiocyte monolayers, luminal ATP raised the secretory short-circuit current (Schlenker et al. 1997).
Since basolateral and luminal nucleotides had apparently identical effects on Ca2+ mobilization in our study, it is difficult to explain their opposite effects on HCO3− and fluid secretion. One possibility is that luminal application activates apical Ca2+-dependent anion channels to induce HCO3− secretion, while basolateral application activates an additional inhibitory pathway which masks the effects of raised [Ca2+]i. Given that secretin acts by raising intracellular cAMP, it is interesting to note that in FRTL-5 thyroid cells extracellular ATP not only stimulates phospholipase C but also inhibits adenylate cyclase (Sato et al. 1992). There are also interesting parallels with the situation in the rat pancreatic duct where carbachol raises [Ca2+]i and depolarizes the basolateral membrane while ATP raises [Ca2+]i and yet hyperpolarizes the basolateral membrane (Hug et al. 1994). Furthermore, secretory studies on rat pancreatic ducts have revealed a dissociation between Ca2+ mobilization and fluid secretion. A high dose (1 μm) of acetylcholine (ACh) by itself stimulates fluid secretion but the same doses inhibit secretin-stimulated fluid secretion (Evans et al. 1996). This inhibition is relieved by staurosporine, an inhibitor of protein kinase C. Substance P, which normally utilizes a Ca2+-mediated transduction pathway, inhibits fluid secretion stimulated by secretin, bombesin, dibutyryl cyclic AMP or forskolin (Ashton et al. 1990).
In human airway epithelial cells (Stutts et al. 1992, 1994), rat proximal tubule cells (Jin & Hopfer, 1997) and human colonic epithelial cells (Guo et al. 1997), the functional effects of extracellular ATP are accomplished without an elevation of [Ca2+]i. These studies suggest that purinoceptors can activate other signalling pathways and/or directly activate ion channels. In our study, the effect of luminal ATP or UTP (1 μm) on fluid secretory rate was unexpectedly potent, evoking approximately 75 % of the secretory rate obtained with maximal secretin stimulation. The responses to ATP (or UTP) and secretin were not, however, additive. Secretin stimulation only increased fluid secretion by about 20 % in ducts already stimulated with luminal ATP or UTP. This result tends to suggest that luminal nucleotides activate the same pathway for HCO3− transport as secretin, although it is possible that it is the rate of HCO3− accumulation across the basolateral membrane that ultimately limits the maximal rate of HCO3− secretion.
In our study, ATP and UTP had essentially identical effects on Ca2+ mobilization and on HCO3− and fluid secretion, which suggests that the receptors in the guinea-pig pancreatic ducts are likely to be of the P2Y2 type. However, the present data can be explained equally well by the presence of both selective purinoceptors (P2Y1) and pyrimidinoceptors (P2Y4 or P2Y6) (Nicholas et al. 1996; Communi & Boeynaems, 1997). Further studies are required to characterize the receptor types.
In summary, our study directly demonstrates that activation of purinoceptors on pancreatic duct cells can regulate pancreatic fluid and HCO3− secretion. Although the identity and origin of the endogenous agonists for these receptors are not known, it is possible that ATP secreted from the duct cell itself may have autocrine actions at the luminal and/or basolateral membranes. Alternatively, ATP secreted with digestive enzymes from the acinar cells may have a paracrine action at the luminal membrane, while ATP released by nerve terminals may act at the basolateral membrane. Despite these uncertainties it is clear that extracellular nucleotides are likely to be potent local regulators of pancreatic duct cell secretion.
Acknowledgments
This study was supported by the Ministry of Health and Welfare (Japan), The Wellcome Trust, the British Council (Tokyo) and a Monbusho International Scientific Research Program from the Ministry of Education, Science, and Culture (Japan). We thank Dr A. C. Elliott for helpful discussions.
References
- Ashton N, Argent BE, Green R. Effect of vasoactive intestinal peptide, bombesin and substance P on fluid secretion by isolated rat pancreatic ducts. The Journal of Physiology. 1990;427:471–482. doi: 10.1113/jphysiol.1990.sp018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, Evans RL, Elliott AC, Green R, Argent BE. Regulation of fluid secretion and intracellular messengers in isolated rat pancreatic ducts by acetylcholine. The Journal of Physiology. 1993;471:549–562. doi: 10.1113/jphysiol.1993.sp019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Argent BE. Pancreatic duct cell secretion: control and mechanisms of transport. In: Go VLW, Dimagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathophysiology, and Disease. 2. New York: Raven Press; 1993. pp. 301–350. [Google Scholar]
- Chan HC, Cheung WT, Leung PY, Wu LJ, Cheng Chew SB, Ko WH, Wong PYD. Purinergic regulation of anion secretion by cystic fibrosis pancreatic duct cells. American Journal of Physiology. 1996;271:C469–477. doi: 10.1152/ajpcell.1996.271.2.C469. [DOI] [PubMed] [Google Scholar]
- Christoffersen BC, Hug MJ, Novak I. Different purinergic receptors lead to intracellular calcium increases in pancreatic ducts. Pflügers Archiv. 1998;436:33–39. doi: 10.1007/s004240050601. [DOI] [PubMed] [Google Scholar]
- Communi D, Boeynaems J-M. Receptors responsive to extracellular pyrimidine nucleotides. Trends in Pharmacological Sciences. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- Evans RL, Ashton N, Elliott AC, Green R, Argent BE. Interactions between secretin and acetylcholine in the regulation of fluid secretion by isolated rat pancreatic ducts. The Journal of Physiology. 1996;496:265–273. doi: 10.1113/jphysiol.1996.sp021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Merlin D, Harvey RD, Laboisse C, Hopfer U. Pharmacological evidence that calcium is not required for P2-receptor stimulated Cl− secretion in HT29-Cl.16E. Journal of Membrane Biology. 1997;155:239–246. doi: 10.1007/s002329900176. [DOI] [PubMed] [Google Scholar]
- Hug MJ, Pahl C, Novak I. Effect of ATP, carbachol and other agonists on intracellular calcium activity and membrane voltage of pancreatic ducts. Pflügers Archiv. 1994;426:412–418. doi: 10.1007/BF00388304. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Case RM, Steward MC. Effects of extracellular ATP on HCO3− secretion in interlobular ducts isolated from guinea-pig pancreas. The Journal of Physiology. 1998a;513.P:54P. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SBH, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. The Journal of Physiology. 1998b;511:407–422. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay ARG, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Hopfer U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: elevated cytosolic Ca2+ is not required. American Journal of Physiology. 1997;272:C1169–1177. doi: 10.1152/ajpcell.1997.272.4.C1169. [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. American Journal of Physiology. 1996;271:G86–96. doi: 10.1152/ajpgi.1996.271.1.G86. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Moody MW, Savard CE, Lee SP. Secretory effects of ATP on nontransformed dog pancreatic duct epithelial cells. American Journal of Physiology. 1998;275:G104–113. doi: 10.1152/ajpgi.1998.275.1.G104. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Molecular Pharmacology. 1996;50:224–229. [PubMed] [Google Scholar]
- Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of basolateral membrane. Pflügers Archiv. 1988a;411:58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- Novak I, Greger R. Properties of luminal membrane of isolated rat pancreatic ducts: effect of cyclic AMP and blockers of chloride transport. Pflügers Archiv. 1988b;411:546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Padfield PJ, Garner A, Case RM. Patterns of pancreatic secretion in the anaesthetised guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 1989;4:204–209. doi: 10.1097/00006676-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Sato K, Okajima F, Kondo Y. Extracellular ATP stimulates three different receptor-signal transduction systems in FRTL-5 thyroid cells. Biochemical Journal. 1992;283:281–287. doi: 10.1042/bj2830281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker T, Romac J M-J, Sharara AI, Roman RM, Kim SJ, LaRusso N, Liddle RA, Fitz JG. Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. American Journal of Physiology. 1997;273:G1108–1117. doi: 10.1152/ajpgi.1997.273.5.G1108. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Chinet TC, Mason SJ, Fullton JM, Clarke LL, Boucher RC. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proceedings of the National Academy of Sciences of the USA. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Fitz JG, Paradiso AM, Boucher RC. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. American Journal of Physiology. 1994;267:C1442–1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- Wong PYD. Control of anion and fluid secretion by apical P2 purinoceptors in the rat epididymis. British Journal of Pharmacology. 1988;95:1315–1321. doi: 10.1111/j.1476-5381.1988.tb11770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi F, Homma N, Haruta K, Iwatsuki K, Chiba S. Effects of three purine-related compounds on pancreatic exocrine secretion in the dog. Clinical and Experimental Pharmacology and Physiology. 1986;13:425–432. doi: 10.1111/j.1440-1681.1986.tb00921.x. [DOI] [PubMed] [Google Scholar]


