Abstract
The role of adrenaline in regulating hepatic glucose production and muscle glucose uptake during exercise was examined in six adrenaline-deficient, bilaterally adrenalectomised humans. Six sex- and age-matched healthy individuals served as controls (CON).
Adrenalectomised subjects cycled for 45 min at 68 ± 1 % maximum pulmonary O2 uptake (V̇O2,max), followed by 15 min at 84 ± 2 %V̇O2,max without (–ADR) or with (+ADR) adrenaline infusion, which elevated plasma adrenaline levels (45 min, 4.49 ± 0.69 nmol l−1; 60 min, 12.41 ± 1.80 nmol l−1; means ± s.e.m.). Glucose kinetics were measured using [3-3H]glucose.
Euglycaemia was maintained during exercise in CON and –ADR, whilst in +ADR plasma glucose was elevated. The exercise-induced increase in hepatic glucose production was similar in +ADR and –ADR; however, adrenaline infusion augmented the rise in hepatic glucose production early in exercise. Glucose uptake increased during exercise in +ADR and –ADR, but was lower and metabolic clearance rate was reduced in +ADR.
During exercise noradrenaline and glucagon concentrations increased, and insulin and cortisol concentrations decreased, but plasma levels were similar between trials. Adrenaline infusion suppressed growth hormone and elevated plasma free fatty acids, glycerol and lactate. Alanine and β-hydroxybutyrate levels were similar between trials.
The results demonstrate that glucose homeostasis was maintained during exercise in adrenalectomised subjects. Adrenaline does not appear to play a major role in matching hepatic glucose production to the increase in glucose clearance. In contrast, adrenaline infusion results in a mismatch by simultaneously enhancing hepatic glucose production and inhibiting glucose clearance.
The rise in plasma adrenaline that occurs during exercise has been suggested to play an important role in mediating the increase in hepatic glucose production. In accordance with this, studies have demonstrated that liver glycogen breakdown was attenuated during exercise in adrenodemedullated animals when compared with that of control animals with normal adrenomedullary secretion (Richter et al. 1981a; Sonne et al. 1985). Somewhat in contrast to this, other studies in adrenodemedullated animals have shown that hepatic glucose production and liver glycogen breakdown were not affected during exercise (Carlson et al. 1985; Arnall et al. 1986; Marker et al. 1986; Winder et al. 1987). However, since adrenaline stimulates glucose production late in prolonged exercise when gluconeogenesis is accelerated (Moates et al. 1988), it has been suggested that the role of adrenaline during exercise is to facilitate gluconeogenic substrate mobilisation from peripheral sites (Richter et al. 1981b; Winder et al. 1985).
Studies in adrenaline-deficient bilaterally adrenalectomised humans during moderate intensity exercise have shown that euglycaemia was maintained (Järhult & Holst, 1979) and that the exercise-induced increase in hepatic glucose output was similar when compared with that in normal subjects (Hoelzer et al. 1986b). It should be noted that results from the latter paper were partly retracted (Cryer, 1989); however, this was only in reference to the experiments performed with an islet clamp. Although these studies suggest that adrenaline does not play an essential role in mediating the increase in hepatic glucose output during moderate intensity exercise, control experiments in which adrenaline was replaced were not performed. Thus, it cannot be ruled out that the apparent normal exercise-induced increase in glucose production in adrenalectomised subjects could be due either to compensatory changes in other hormonal systems influencing glucoregulation, or to development of adrenergic receptor hypersensitivity in combination with other remaining adrenaline-secreting tissue. It is possible that the tissue could secrete adrenaline into the portal area and changes in circulating adrenaline may be difficult to detect due to clearance by the liver. Therefore, it is important to determine the role of glucoregulatory hormones, other than catecholamines, during exercise. In addition, it is important to infuse adrenaline at rest in both adrenalectomised and control subjects, and during exercise in adrenalectomised individuals. In a previous study in exercising humans, pharmacological blockade of the coeliac ganglion was employed to inhibit sympathetic neural activity to the liver, adrenal medulla and pancreas (Kjær et al. 1993). The effects of liver nerve blockade were separated from the effects of impaired adrenaline secretion by infusion of adrenaline during exercise. It was concluded that although high physiological concentrations of adrenaline could enhance glucose production in exercising humans, normally adrenaline was not a major stimulus (Kjær et al. 1993). However, coeliac ganglion blockade does not completely inhibit adrenaline secretion, and although somatostatin, insulin and glucagon were infused to compensate for the sympathetic neural blockade to the pancreas, this experimental approach does not yield optimal conditions for evaluation of the physiological role of adrenaline.
In addition to regulation of hepatic glucose production, it has been suggested that adrenaline may affect the exercise-induced increase in muscle glucose uptake; however, the results are conflicting. Indeed, in animal studies infusion of adrenaline resulted in both a decrease (Issekutz, 1985) and an increase (Richter et al. 1982) in glucose uptake. In humans, infusion of adrenaline into one leg reduced muscle glucose uptake compared with the contralateral leg during two-legged cycling (Jansson et al. 1986). Furthermore, β–ADRenergic receptor blockade during intense exercise in humans resulted in an increase in glucose disappearance (Sigal et al. 1994). In line with these findings, glucose clearance was higher and plasma glucose concentration decreased more rapidly during exercise in experiments in which adrenaline secretion was inhibited by pharmacological coeliac ganglion blockade than when compared with control experiments with a normal adrenaline response (Kjær et al. 1993). Taken together these findings support the hypothesis that adrenaline can inhibit glucose uptake during exercise.
The aim of the present study, therefore, was to examine the effect of adrenaline on hepatic glucose production and muscle glucose uptake during exercise. Bilaterally adrenalectomised humans were examined during both moderate and high intensity exercise by infusing adrenaline in an attempt to obtain plasma levels similar to those observed during exercise in normal humans with intact adrenal glands. Results were compared with healthy, sex- and age-matched control individuals. Finally, all individuals underwent adrenaline infusion at rest to determine whether adrenalectomised individuals have altered adrenergic receptor sensitivity.
METHODS
Subjects
Six adrenaline-deficient bilaterally adrenalectomised subjects (5 females, 1 male; age, 45.8 ± 5.8 years; weight, 75.2 ± 8.0 kg; means ±s.e.m.) volunteered to participate in the experiment. Subjects were in good health and taking adrenocortical replacement medications (hydrocortisone and aldosterone), which were not altered for the purpose of this study. In addition, six normal healthy untrained individuals with intact adrenal glands who were sex and age matched (47.0 ± 4.7 years, 69.3 ± 2.4 kg) to the adrenalectomised subjects also volunteered to participate in the experiment. The experimental procedures and possible risks of the study were explained to each subject verbally and in writing. All subjects gave their informed, written consent to the experiment, which was approved by the Ethical Committee of Copenhagen.
Pre-experimental protocol
In order to determine maximum pulmonary oxygen uptake (VO2,max) and to establish the relationship between workload and oxygen uptake (VO2) all subjects performed an incremental workload test to exhaustion on a modified electromagnetically braked Krogh cycle ergometer. The subjects lay behind the cycle ergometer on a couch in a semi-recumbent position with the upper part of the body forming an angle of ∼45 deg with the couch. Maximum VO2 was the highest VO2 attained during the latter stages of the test and was accompanied by a respiratory exchange ratio (RER) that was greater than 1.1 and a heart rate (in beats per minute, bpm) that was close to the age-predicted maximum (220 bpm - age). Mean VO2,max was 1.46 ± 0.19 and 2.30 ± 0.31 l min−1 for the adrenalectomised and normal subjects, respectively. For the day preceding each trial the subjects were instructed to abstain from strenuous exercise, tobacco, caffeine and alcohol. The subjects reported to the exercise laboratory in the morning after a 10-12 h overnight fast.
Experimental protocol
Adrenalectomised subjects
The adrenalectomised subjects were studied during two exercise periods, separated by at least 14 days. During exercise either isotonic saline (–ADR) or adrenaline (+ADR) was infused. The exercise trials were performed on the same modified cycle ergometer as used for VO2,max determination.
On arrival at the exercise laboratory, the adrenalectomised subjects rested quietly on the modified cycle ergometer. Following local anaesthesia (0.5 ml lidocaine, 10 mg ml−1) a catheter was introduced percutaneously into the radial artery for blood sampling and continuous measurement of blood pressure. The catheter for blood sampling was kept patent by regular flushing with saline. In addition, a catheter was inserted into a contralateral forearm vein and a three-way stop cock was attached to allow for simultaneous infusion of tracer and isotonic saline or adrenaline. Following a priming dose of 24 μCi, D-[3-3H]glucose (pharmaceutically prepared and dissolved in isotonic saline; Isotopapoteket, Amersham, Denmark) was infused continuously at a rate of 0.24 μCi min−1 by a syringe pump (P4000 anaesthesia syringe pump, IVAC, Damca) for the duration of the 2 h rest period and exercise. On completion of the rest period the adrenalectomised subjects commenced exercise at a workload that elicited an exercise intensity of 68 ± 1 %VO2,max for 45 min, immediately followed by a 15 min bout of exercise at 84 ± 2 %VO2,max. At the onset of exercise either isotonic saline or adrenaline was infused continuously via a volumetric infusion pump (IMED 960, San Diego, CA, USA). In the first trial (–ADR), isotonic saline was infused at a similar rate to +ADR. In the subsequent trial (+ADR), an adrenaline solution (2 μg ml−1) was infused at a rate of 0.15 nmol kg−1 min−1 for 45 min and the rate was then increased to 0.40 nmol kg−1 min−1 for the final 15 min of exercise. The infusion rates were based on expected increases in plasma concentrations obtained in previous experiments with adrenaline infusion (Kjær et al. 1993). Arterial blood was sampled at 5 min intervals for the last 15 min of the rest period and throughout exercise for later analysis of plasma glucose, lactate and [3-3H]glucose specific activity. Arterial samples were also obtained immediately prior to the commencement of exercise, at 10, 20, 30 and 45 min of exercise, and at the completion of the exercise period for analysis of catecholamines. Additional samples were taken at 0, 20, 45 and 60 min for analysis of insulin, glucagon, cortisol, growth hormone, free fatty acids (FFA), glycerol, β-hydroxybutyrate, alanine, haematocrit and oxygen saturation. Blood for analysis was collected in chilled tubes containing EGTA and glutathione for catecholamines, in heparinised tubes for the metabolites and in tubes containing aprotinin and EDTA for the other hormones. The blood samples were centrifuged immediately and the plasma removed and stored at -20°C for later analysis. Plasma for catecholamine and FFA analysis was stored at -80°C. In preparation for hormone analysis the plasma was deproteinised in perchloric acid, centrifuged again and the supernatant removed and stored at -20°C. Expired gases were sampled during exercise for measurement of oxygen consumption and carbon dioxide production by an Oxycon chamber system (Jaeger Instruments). Heart rate was measured continuously using electrocardiography. During exercise the intensity of effort was quantified by a rating of perceived exertion on a scale from 6 (minimum effort) to 20 (maximum effort).
Control subjects
The sex- and age-matched normal subjects with intact adrenal glands were studied during one exercise period (CON). On arrival at the exercise laboratory, the subjects rested quietly on the cycle ergometer and a catheter was inserted into the radial artery of one arm for blood sampling. The subjects exercised at a workload that required 73 ± 3 %VO2,max for 45 min, immediately followed by a 15 min bout of exercise at 90 ± 4 %VO2,max. The exercise intensity and duration were not significantly different from those of the adrenalectomised subjects. Arterial blood samples were taken at 0, 10, 20, 30, 45 and 60 min of exercise for analysis of catecholamines, glucose and lactate. Haematocrit and oxygen saturation were measured at 0, 20, 45 and 60 min of exercise. Expired gases were sampled during exercise. Heart rate and blood pressure were measured continuously.
Sensitivity test
Each subject (both controls and adrenalectomised subjects) was infused with adrenaline in the resting state to test for adrenergic receptor sensitivity. After completion of the exercise trial (CON and –ADR for adrenalectomised subjects) subjects rested for 30 min and then adrenaline was infused at a rate of 0.15 nmol kg−1 min−1 for 15 min followed by 0.40 nmol kg−1 min−1 for another 15 min to simulate adrenaline levels during exercise. Arterial blood was sampled before infusion, and at 15 and 30 min for analysis of catecholamines, glucose and lactate. Heart rate and blood pressure responses were recorded every minute.
Analytical techniques
Plasma glucose and lactate were measured using an automated glucose-lactate analyser (YSI 23 AM; Yellow Springs Instruments, Yellow Springs, OH, USA). Catecholamine concentrations were determined by a single-isotope radioenzymatic method (Ben-Jonathan & Porter, 1976). The concentrations of insulin, glucagon, cortisol and growth hormone were determined by radioimmunoasssay as previously described (Galbo et al. 1979). Plasma FFA, glycerol, β-hydroxybutyrate and alanine concentrations were measured by enzymatic fluorometric methods. Haematocrit was determined by the microhaematocrit method. Blood oxygen saturation was measured by an automated blood gas microanalyser (OSM 3; Acid-Base Laboratory, Radiometer, Denmark) immediately after blood samples had been drawn. Plasma [3-3H]glucose radioactivity was determined as described by Kjær et al. (1984). Specific activity was calculated from the [3-3H]glucose activity divided by the corresponding plasma glucose concentration. The measured variables were smoothed by a sliding fit technique that employed three consecutive glucose or specific activity values (Cherrington & Vranic, 1973). From measurement of plasma glucose concentration and determination of specific activity in the pre-exercise period the individual distribution volumes for glucose were calculated according to Hentenyi & Norwich (1974). The mean distribution volume for glucose was determined to be 19.9 ± 1.7 % and was not different between –ADR and +ADR. The non-steady state equations of Steele et al. (1956) were used to calculate the rate of glucose appearance (Ra: hepatic glucose production) and disappearance (Rd). The metabolic clearance rate for glucose was calculated as glucose Rd divided by the corresponding plasma glucose concentration. Results from the adrenalectomised (–ADR and +ADR) subjects were compared using a two-way analysis of variance (ANOVA) for repeated measures (cross-over design); results from the normal (CON) and adrenalectomised (–ADR) subjects were compared using a two-way ANOVA for repeated measures (parallel design). The level of significance was set at P < 0.05 in two-tailed tests. Specific differences were determined using the Student-Newman-Keuls post hoc test. All data are reported as means ±s.e.m.
RESULTS
Adrenalectomised subjects
Plasma adrenaline concentrations were similar at rest between –ADR (0.04 ± 0.02 nmol l−1) and +ADR (0.03 ± 0.01 nmol l−1). During exercise in –ADR, plasma adrenaline did not change from resting levels. In +ADR, infusion of adrenaline during exercise resulted in a marked increase (P < 0.05) in plasma arterial levels to 4.49 ± 0.69 and 12.41 ± 1.80 nmol l−1 at 45 and 60 min, respectively (Fig. 1). Plasma noradrenaline concentrations were similar at rest between –ADR and +ADR, and increased during exercise (P < 0.05, time effect), but were not different between trials (Fig. 1).
Figure 1. Catecholamines and exercise.
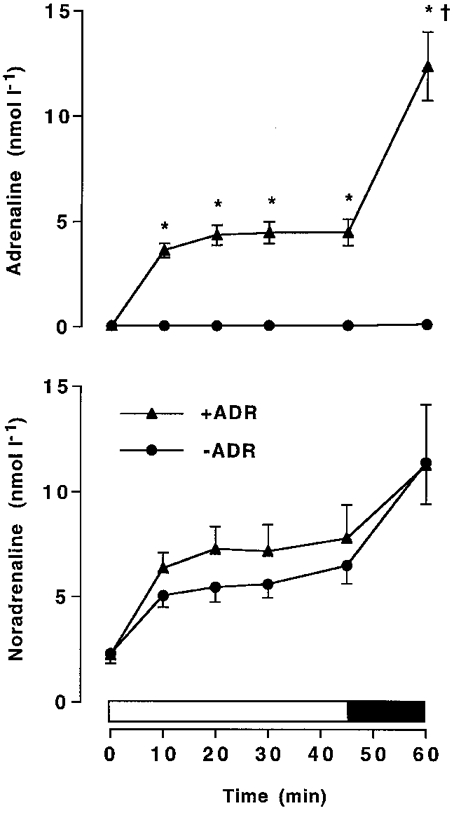
Plasma adrenaline and noradrenaline during cycling exercise at 68 %VO2,max for 45 min, followed by 15 min at 84 %VO2,max in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise and increased after 45 min. Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from –ADR; † significantly different (P < 0.05) from 45 min.
During exercise oxygen uptake, RER and heart rate were similar between –ADR and +ADR. However, there was an increase (P < 0.05, time effect) in these variables when the exercise intensity was raised from 68 to 84 %VO2,max (Table 1). Perceived exertion increased (P < 0.05, time effect) similarly during exercise in –ADR and +ADR (Table 1). Mean arterial pressure (Table 1), haematocrit and oxygen saturation (data not shown) remained constant throughout exercise and were similar between the two trials.
Table 1.
Oxygen uptake, RER, heart rate, MAP and perceived exertion during 60 min of cycling exercise in normal (CON) and adrenalectomised subjects with saline (−ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise
| 0–45 min | 45–60 min | |
|---|---|---|
| Oxygen uptake (l min−1) | ||
| −ADR | 0.97 ± 0.13 | 1.28 ± 0.20 |
| +ADR | 1.02 ± 0.12 | 1.17 ± 0.13 |
| CON | 1.68 ± 0.23 | 2.15 ± 0.27 |
| RER | ||
| −ADR | 0.82 ± 0.02 | 0.86 ± 0.02 |
| +ADR | 0.87 ± 0.02 | 0.87 ± 0.02 |
| CON | 0.86 ± 0.01 | 0.94 ± 0.02 |
| Heart rate (bpm) | ||
| −ADR | 113 ± 12 | 141 ± 13 |
| +ADR | 124 ± 14 | 144 ± 11 |
| CON | 136 ± 13* | 166 ± 6* |
| MAP (mmHg) | ||
| −ADR | 108 ± 6 | 107 ± 7 |
| +ADR | 107 ± 6 | 104 ± 5 |
| CON | 112 ± 4 | 109 ± 4 |
| Perceived exertion | ||
| −ADR | 12 ± 1 | 18 ± 1 |
| +ADR | 13 ± 1 | 17 ± 1 |
| CON | 12 ± 1 | 16 ± 1 |
Values are means ± s.e.m. (n = 6). RER, respiratory exchange ratio; MAP, mean arterial pressure.
Significantly different (P < 0.05) from –ADR.
During exercise in –ADR the plasma glucose concentration did not change significantly from resting levels, whilst in +ADR there was a marked increase (P < 0.05) in plasma glucose which was significantly higher than that in –ADR, except for the first 5 min (Fig. 2). Glucose concentration in +ADR reached steady state after 30 min of moderate intensity exercise, reflecting a match between hepatic glucose production and glucose uptake. When the adrenaline infusion rate and intensity of exercise were further increased, there was a tendency for the glucose concentration to increase further, reflecting a more rapid rise in glucose production than in glucose uptake. Hepatic glucose production increased (P < 0.05) during exercise in both –ADR and +ADR; however, infusion of adrenaline augmented the rise (P < 0.05) in hepatic glucose production during the first 10 min of exercise (Fig. 2). When the exercise intensity was raised at 45 min, there was a tendency for hepatic glucose production to increase more so in +ADR than in –ADR (Fig. 2). Glucose Rd increased (P < 0.05) during exercise in both trials, but was lower (P < 0.05, main effect) in +ADR compared with that in –ADR (Fig. 3). There was an increase (P < 0.05) in metabolic clearance rate during exercise in –ADR, although this was significantly reduced (P < 0.05) with infusion of adrenaline (Fig. 3).
Figure 2. Glucose concentration and production.
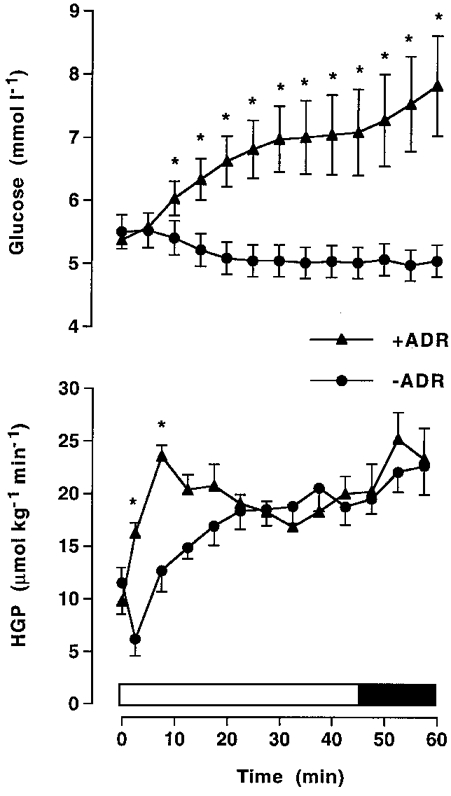
Plasma glucose and hepatic glucose production (HGP) during cycling exercise at 68 %VO2,max for 45 min, followed by 15 min at 84 %VO2,max in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise and increased after 45 min. Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from –ADR.
Figure 3. Glucose uptake and clearance.
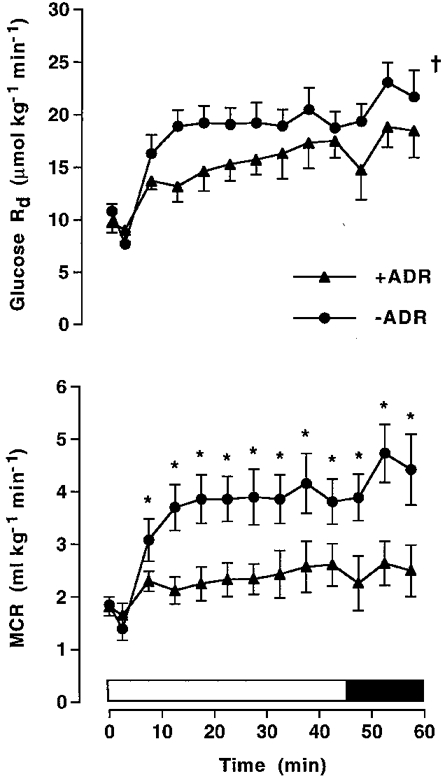
Whole-body glucose uptake (Glucose Rd) and metabolic clearance rate (MCR) during cycling exercise at 68 %VO2,max for 45 min, followed by 15 min at 84 %VO2,max in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise and increased after 45 min. Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from +ADR; † significantly different (P < 0.05, main effect) from +ADR.
Plasma insulin and glucagon concentrations, and the glucagon:insulin molar ratio were similar between –ADR and +ADR at rest and during exercise. However, during exercise there was an increase (P < 0.05, time effect) and a decrease (P < 0.05, time effect) in plasma glucagon and insulin, respectively, which resulted in an elevated (P < 0.05, time effect) glucagon:insulin molar ratio (Table 2). Plasma cortisol was not different between –ADR and +ADR trials at rest and during exercise. However, during exercise there was a decrease (P < 0.05, time effect) in the cortisol concentration (Table 2). During exercise infusion of adrenaline suppressed the increase in plasma growth hormone (Table 2).
Table 2.
Plasma hormones during 60 min of cycling exercise in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise
| Rest | 20 min | 45 min | 60 min | |
|---|---|---|---|---|
| Insulin (pmol l−1) | ||||
| −ADR | 87.8 ± 37.1 | 57.9 ± 22.8 | 43.0 ± 16.3 | 36.6 ± 10.6 |
| +ADR | 66.8 ± 23.1 | 62.2 ± 15.6 | 61.6 ± 14.5 | 32.1 ± 6.9 |
| Glucagon (ng l−1) | ||||
| −ADR | 62.6 ± 10.7 | 64.5 ± 11.8 | 69.4 ± 12.9 | 70.8 ± 11.8 |
| +ADR | 65.1 ± 10.7 | 75.0 ± 14.6 | 71.4 ± 15.4 | 78.1 ± 16.4 |
| G:I molar ratio | ||||
| −ADR | 0.35 ± 0.08 | 0.47 ± 0.09 | 0.63 ± 0.10 | 0.76 ± 0.15 |
| +ADR | 0.39 ± 0.08 | 0.46 ± 0.13 | 0.41 ± 0.11 | 0.90 ± 0.25 |
| Cortisol (nmol l−1) | ||||
| −ADR | 441 ± 120 | 411 ± 123 | 336 ± 102 | 298 ± 91 |
| +ADR | 428 ± 58 | 391 ± 57 | 333 ± 50 | 308 ± 50 |
| GH (ng ml−1) | ||||
| −ADR | 1.02 ± 0.44 | 5.32 ± 2.65 | 6.63 ± 2.53* | 6.59 ± 2.89* |
| +ADR | 1.18 ± 0.44 | 2.99 ± 1.40 | 2.22 ± 1.16 | 1.75 ± 0.76 |
Values are means ±s.e.m. (n = 6; except for growth hormone (GH), n = 5). NA, noradrenaline; G:I, glucagon:insulin.
Significantly different (P < 0.05) from +ADR.
Plasma FFA and glycerol concentrations were similar at rest between the trials. During exercise, infusion of adrenaline increased (P < 0.05) plasma levels of FFA and glycerol (Table 3). β-Hydroxybutyrate and alanine concentrations were similar between –ADR and +ADR at rest and during exercise; however, alanine increased (P < 0.05, time effect) during exercise (Table 3). Lactate increased (P < 0.05) during exercise in both trials, but was significantly higher with adrenaline infusion from 10 min (Fig. 4).
Table 3.
Plasma substrates during 60 min of cycling exercise in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise
| Rest | 20 min | 45 min | 60 min | |
|---|---|---|---|---|
| FFA (mmol l−1) | ||||
| −ADR | 0.67 ± 0.06 | 0.53 ± 0.07* | 0.76 ± 0.08* | 0.68 ± 0.08* |
| +ADR | 0.63 ± 0.07 | 0.92 ± 0.11 | 1.04 ± 0.06 | 1.05 ± 0.09 |
| Glycerol (μmol l−1) | ||||
| −ADR | 107 ± 21 | 152 ± 17* | 250 ± 23* | 272 ± 35* |
| +ADR | 98 ± 10 | 237 ± 12 | 296 ± 36 | 333 ± 42 |
| βHydroxybutyrate (mmol l−1) | ||||
| −ADR | 0.23 ± 0.09 | 0.17 ± 0.05 | 0.21 ± 0.07 | 0.22 ± 0.08 |
| +ADR | 0.35 ± 0.11 | 0.25 ± 0.06 | 0.33 ± 0.09 | 0.38 ± 0.11 |
| Alanine (μmol l−1) | ||||
| −ADR | 122 ± 22 | 156 ± 11 | 172 ± 17 | 178 ± 11 |
| +ADR | 113 ± 19 | 166 ± 18 | 167 ± 17 | 173 ± 18 |
Values are means ±s.e.m. (n = 6).
Significantly different (P < 0.05) from +ADR.
Figure 4. Lactate in exercising adrenalectomised humans.
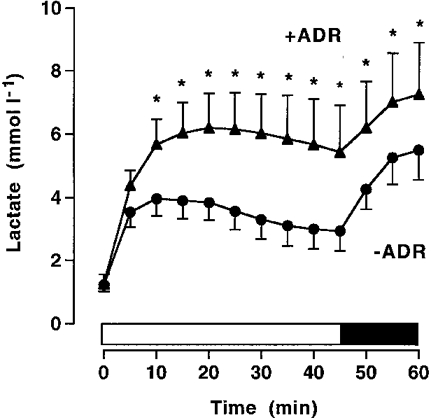
Plasma lactate during cycling exercise at 68 %VO2,max for 45 min, followed by 15 min at 84 %VO2,max in adrenalectomised subjects with saline (–ADR) or adrenaline (+ADR) infusion commencing at the onset of exercise and increased after 45 min. Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from –ADR.
Control subjects
At rest the plasma adrenaline concentration was ∼10-fold lower in bilaterally adrenalectomised subjects (0.04 ± 0.02 nmol l−1) when compared with that in normal subjects (0.35 ± 0.03 nmol l−1). During exercise in CON, plasma adrenaline levels increased (P < 0.05) to a peak of 2.20 ± 0.58 nmol l−1 at 60 min. Plasma adrenaline levels were significantly greater in CON when compared with those in –ADR, but were somewhat lower than levels obtained in +ADR during exercise (Figs 1 and 5). Plasma noradrenaline concentration increased (P < 0.05, time effect) during exercise in CON, but was not significantly different from that in –ADR (Fig. 5).
Figure 5. Catecholamines in adrenalectomised humans and controls.
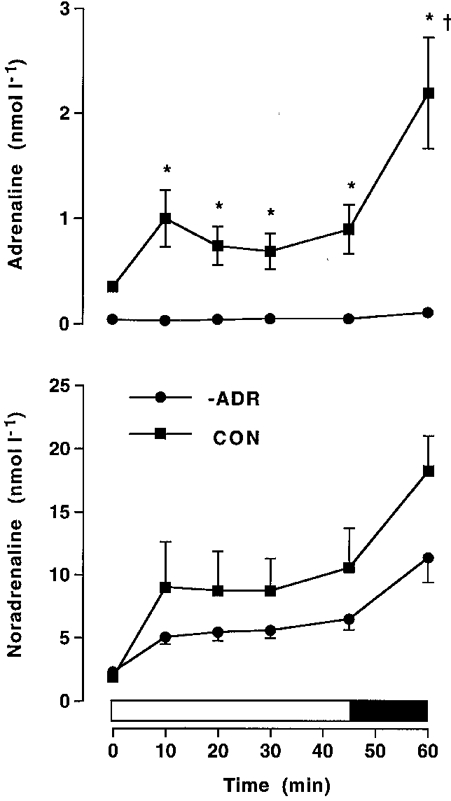
Plasma adrenaline and noradrenaline during cycling exercise at ≈70 %VO2,max for 45 min, followed by 15 min at ≈86 %VO2,max in normal control (CON) or adrenalectomised subjects with saline infusion (–ADR). Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from –ADR; † significantly different (P < 0.05) from 45 min.
Relative oxygen uptake (percentage VO2,max) was not different between CON and adrenalectomised subjects during exercise, whereas absolute VO2, RER and heart rate were all higher (P < 0.05) in CON compared with both –ADR and +ADR (Table 1). Mean arterial pressure, perceived exertion (Table 1), haematocrit and oxygen saturation (data not shown) were similar between the two subject groups.
Euglycaemia was maintained throughout exercise and plasma glucose was not different between CON and –ADR (Fig. 6). Plasma lactate increased (P < 0.05) during exercise and was similar between the two subject groups, except at 60 min when lactate was higher (P < 0.05) in CON (7.68 ± 0.97 mmol l−1) than in –ADR (5.49 ± 0.94 mmol l−1) (Fig. 6).
Figure 6. Glucose and lactate in adrenalectomised humans.
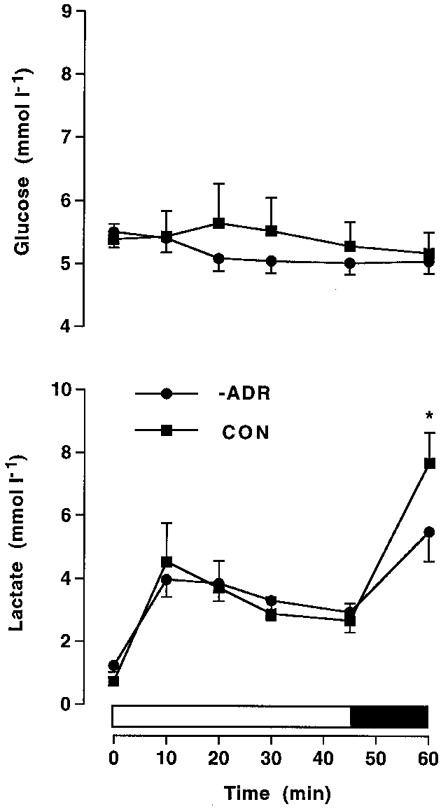
Plasma glucose and lactate during cycling exercise at ≈70 %VO2,max for 45 min, followed by 15 min at ≈86 %VO2,max in normal control (CON) or adrenalectomised subjects with saline infusion (–ADR). Values are means ±s.e.m. (n = 6). * Significantly different (P < 0.05) from –ADR.
Sensitivity test
Infusion of adrenaline at a rate of 0.15 nmol kg−1 min−1 increased (P < 0.05) the plasma adrenaline concentration to a similar level in the two subject groups (Table 4). When the adrenaline infusion rate was increased to 0.40 nmol kg−1 min−1 the plasma adrenaline concentration was further elevated (P < 0.05), but was higher (P < 0.05) in adrenalectomised subjects (11.9 ± 1.12 nmol l−1) compared with CON (9.17 ± 0.83 nmol l−1). In adrenalectomised subjects plasma adrenaline concentrations were similar regardless of whether adrenaline was infused at rest (Table 4) or during exercise (Fig. 1). Plasma noradrenaline was not different between subject groups prior to and during adrenaline infusion (Table 4). Infusion of adrenaline increased (P < 0.05, time effect) plasma glucose, but this was not different between subject groups (Table 4). Adrenaline infusion at rest had no significant effect on plasma lactate concentration, heart rate and mean arterial blood pressure (Table 4).
Table 4.
Sensitivity test: response to adrenaline infusion at rest in normal (CON) and adrenalectomised (–ADR) subjects
| 0 min | 15 min (0.15 nmol kg−1 min−1) | 30 min (0.40 nmol kg−1 min−1) | |
|---|---|---|---|
| Adrenaline (nmol l−1) | |||
| CON | 0.85 ± 0.61 | 3.73 ± 0.29† | 9.17 ± 0.83*‡ |
| −ADR | 0.07 ± 0.02 | 4.57 ± 0.37† | 11.90 ± 1.12‡ |
| Nordrenaline (nmol l−1) | |||
| CON | 1.85 ± 0.30 | 1.77 ± 0.20 | 1.74 ± 0.24 |
| −ADR | 2.52 ± 0.44 | 2.70 ± 0.45 | 2.50 ± 0.40 |
| Glucose (mmol l−1) | |||
| CON | 5.07 ± 0.33 | 5.83 ± 0.33 | 7.24 ± 0.42 |
| −ADR | 5.27 ± 0.27 | 6.16 ± 0.23 | 7.57 ± 0.33 |
| Lactate (mmol l−1) | |||
| CON | 1.73 ± 0.24 | 1.42 ± 0.21 | 1.64 ± 0.26 |
| −ADR | 1.97 ± 0.47 | 1.79 ± 0.34 | 2.16 ± 0.33 |
| Heart rate (bpm) | |||
| CON | 84 ± 6 | 89 ± 6 | 96 ± 7 |
| −ADR | 84 ± 5 | 87 ± 6 | 91 ± 7 |
| MAP (mmHg) | |||
| CON | 78 ± 4 | 72 ± 3 | 74 ± 3 |
| −ADR | 82 ± 2 | 75 ± 3 | 76 ± 3 |
Values are means ±s.e.m. (n = 6). MAP, mean arterial pressure.
Significantly different (P < 0.05) from –ADR
significantly different (P < 0.05) from 0 min
significantly different (P < 0.05) from 15 min.
DISCUSSION
The results from the present study demonstrate that in adrenaline-deficient bilaterally adrenalectomised humans, infusion of adrenaline that resulted in plasma levels within the physiological range augmented the rise in hepatic glucose output early during exercise. However, the overall magnitude of the exercise-induced increase in hepatic glucose production was not significantly different regardless of whether or not adrenaline was replaced. Elevated plasma adrenaline levels also attenuated the exercise-induced increase in peripheral glucose uptake. Furthermore, glucose homeostasis was maintained during exercise in adrenalectomised subjects infused with saline. Taken together, the results indicate that normally adrenaline does not play a major role in matching glucose production to the increase in glucose clearance during exercise. Rather, adrenaline infusion results in a mismatch by simultaneously enhancing glucose production and inhibiting glucose clearance.
Comparisons between experiments with and without adrenaline infusion in adrenalectomised subjects suggest that increases in adrenaline concentration, as seen during transition from rest to exercise and from moderate to intense exercise, may enhance glucose production (Figs 1 and 2). The increase in glucose production during exercise in +ADR was accompanied by increases in plasma glucose concentration (Fig. 2) which suggests that adrenaline may cause a mismatch between glucose production and clearance. Indeed, the findings indicate that the increase in plasma glucose reflects both an increase in glucose production (Fig. 2) and a reduction in glucose clearance (Fig. 3). During steady-state periods of exercise, glucose production was similar in +ADR and –ADR. However, given that elevated plasma glucose (Fig. 2), per se, can inhibit glucose production (Jenkins et al. 1985), this suggests that during the steady state, adrenaline may also enhance glucose production.
The observed differences in glucose kinetics between –ADR and +ADR during exercise are unlikely to be elicited by glucoregulatory hormones other than adrenaline, as plasma levels of glucagon, insulin and cortisol were not different between trials (Table 2). Furthermore, during exercise the growth hormone concentration was lower in +ADR than in –ADR, a difference which per se would tend to cause changes in glucose kinetics opposite to those observed (that is, lower glucose production and higher glucose clearance). The fact that the exercise-induced increase in growth hormone was suppressed by adrenaline infusion probably reflects reduced secretion as a result of increased plasma glucose levels (Galbo, 1983). The finding that plasma cortisol concentration always decreased during exercise in hydrocortisone-substituted, adrenalectomised subjects (Table 2) is in accordance with the fact that cortisol clearance is increased by exercise (Galbo, 1983).
The finding that adrenaline can stimulate hepatic glucose production during exercise has previously been demonstrated for other experimental conditions. Indeed, when adrenaline was infused at rates that resulted in high physiological concentrations hepatic glucose production was enhanced in healthy subjects (Kjær et al. 1993; Howlett et al. 1999). However, when adrenaline was accurately replaced by infusion during exercise in coeliac ganglion-blocked humans there was no effect on hepatic glucose output (Kjær et al. 1993). Although in some studies adrenodemedullated animals have been shown to display reduced hepatic glycogenolysis (Richter et al. 1981a) and tracer-determined glucose production (Sonne et al. 1985) during exercise, other studies find no effect on the decrease in liver glycogen content during exercise (Winder et al. 1985; Arnall et al. 1986). In other animal studies, adrenaline has been shown to indirectly stimulate hepatic glucose production by increased gluconeogenesis as a result of accelerated muscle glycogenolysis and subsequent lactate release (Sonne et al. 1987; Wasserman et al. 1987). In the present study, adrenaline infusion increased plasma lactate levels (Fig. 4); however, the time course of the increase in lactate during exercise differed from that of the increase in glucose production. This suggests that adrenaline increased glucose production primarily by stimulating hepatic glycogenolysis.
Despite the fact that elevated levels of adrenaline can stimulate hepatic glucose production, it appears that in the absence of adrenaline, glucose production was not impaired during moderate and high intensity exercise. In the present study, euglycaemia was maintained during exercise in adrenalectomised humans and the plasma glucose response was similar when compared with that of normal subjects (Fig. 6). Similar findings have also been demonstrated during moderate exercise in adrenalectomised humans (Järhult & Holst, 1979; Hoelzer et al. 1986b), and in normal subjects following anaesthetic blockade of the coeliac ganglion (Kjaer et al. 1993) and adrenergic receptor blockade (Simonson et al. 1984; Hoelzer et al. 1986a). This study also provides further support for the suggestion that adrenaline is not essential for regulating hepatic glucose production even at high exercise intensities (Kjær et al. 1993; Coker et al. 1997b; Howlett et al. 1999). However, these findings do not exclude the possibility that adrenaline may play a role during more prolonged exercise in humans. Indeed, in adrenalectomised dogs the effect of adrenaline on hepatic glucose production was not seen until late during prolonged moderate-intensity exercise (Moates et al. 1988).
The exercise-induced increase in hepatic glucose production in the adrenalectomised humans was probably stimulated by changes in the pancreatic hormones. Indeed, during exercise there was a decrease and an increase in insulin and glucagon, respectively (P < 0.05). Sympathetic neural activity may also have contributed to the increase in glucose production; however, previous studies in exercising humans (Kjær et al. 1993, 1995) and dogs (Coker et al. 1997b) suggest that this is not the case. The changes in plasma cortisol and growth hormone concentration are probably too slow to account for glucose production during exercise in the present study. Furthermore, glucose production increased despite a reduction in cortisol during exercise (Table 2).
Although extra–ADRenomedullary adrenaline secretion occurs in bilaterally adrenalectomised humans (Shah et al. 1984; Hoelzer et al. 1986b), this is likely to be minimal as plasma adrenaline levels were ∼10-fold lower in adrenalectomised subjects than in healthy control subjects (Figs 1 and 5). However, it cannot be completely ruled out that adrenaline secreted from other tissues into the portal area is cleared by the liver, and subsequently does not result in a notable increase in peripheral circulating levels of adrenaline during exercise (Coker et al. 1997a). Plasma adrenaline did not rise above resting levels during exercise in adrenalectomised subjects (–ADR), whereas infusion of adrenaline elevated plasma levels to concentrations that are normally observed during intense exercise. However, the adrenaline levels in +ADR were somewhat higher than those measured during exercise in the control subjects who exercised at a comparable relative workload. As adrenaline infusion rates were calculated based on findings in healthy young individuals (Kjær et al. 1993) it is likely that the rate of adrenaline clearance was lower in the adrenalectomised subjects. This could be due to the fact that the individuals in the present study were older than those in the study by Kjær et al. (1993), as it has recently been found that adrenaline clearance decreases with ageing (Marker et al. 1998). In the present study, adrenaline infusion resulted in a comparable rise in plasma levels at rest and during exercise (Table 4 and Fig. 1). This agrees with our previous observation that adrenaline clearance is only minimally affected by exercise (Kjær et al. 1985). In the present study there was no indication that adrenalectomised subjects had developed adrenergic receptor hypersensitivity, similar to that seen in spinal cord injured subjects with impaired sympathetic activity (Mathias et al. 1976), as adrenaline infusion at rest resulted in metabolic and circulatory responses that were similar in adrenalectomised and control subjects.
The exercise-induced increase in glucose uptake was significantly reduced in adrenalectomised subjects when adrenaline was infused (Fig. 3). Furthermore, as plasma glucose levels increased markedly during exercise with adrenaline infusion, glucose clearance was even more diminished than glucose uptake (Fig. 3). The conclusion that adrenaline inhibits glucose clearance during exercise is in agreement with a previous study in which plasma adrenaline levels were manipulated by coeliac ganglion blockade and adrenaline infusion (Kjær et al. 1993). The mechanism responsible for this reduction in glucose clearance may be related to reduced glucose utilisation in muscle secondary to an increase in muscle glycogenolysis, as a result of adrenergic receptor stimulation (Raz et al. 1991). Adrenaline-mediated muscle glycogen breakdown is supported by some (Richter et al. 1981a, b, 1982; Katz et al. 1986; Jansson et al. 1986; Febbraio et al. 1998), but not all studies (Chesley et al. 1995; Wendling et al. 1996). In the present study higher plasma lactate levels in +ADR compared with those in –ADR are compatible with stimulation of muscle glycogen breakdown during exercise (Fig. 4). Other possible mechanisms that may account for the reduction in muscle glucose uptake with adrenaline infusion include effects on muscle glucose transport and/or fat metabolism. Under resting conditions adrenaline has been shown to decrease muscle glucose transport despite an increase in glucose transporter (GLUT4) translocation to the plasma membrane, which suggests a reduction in GLUT4 intrinsic activity (Bonen et al. 1992). Elevated plasma FFA may contribute to a reduction in leg glucose uptake during exercise (Hargreaves et al. 1991) although this remains controversial (Romijn et al. 1995; Odland et al. 1997). In the present study, lipolysis and the supply of FFA to the muscle were increased by adrenaline infusion as indicated by higher plasma glycerol and FFA levels in +ADR compared with those in –ADR (Table 3). However, it is unlikely that an adrenaline-mediated increase in fat mobilisation influenced glucose uptake because fat oxidation, as determined from oxygen consumption and RER measurements, was not different between trials (Table 1).
In summary, in adrenaline-deficient bilaterally adrenalectomised humans, infusion of adrenaline augmented the rise in hepatic glucose production early in exercise. However, overall levels of glucose production were not different during exercise in adrenalectomised subjects regardless of whether or not adrenaline was infused. Infusion of adrenaline also markedly attenuated the exercise-induced rise in muscle glucose uptake and clearance. Furthermore, glucose homeostasis was maintained during exercise in adrenalectomised subjects infused with saline. The results suggest that during exercise adrenaline does not play an essential role in matching hepatic glucose production to an increase in glucose clearance. In contrast, adrenaline infusion tends to result in a mismatch by simultaneously enhancing glucose production and inhibiting glucose clearance.
Acknowledgments
The authors would like to thank Professor Mark Hargreaves for valuable discussion and advice, and Lisbeth Kall, Regitze Kraunsøe, Inge Rasmussen and Viebeke Staffeldt for their excellent technical assistance.
This study was supported by grants from the Danish National Research Foundation (J.nr. 504-14) and the NOVO Foundation.
References
- Arnall DA, Marker JC, Conlee RK, Winder WW. Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats. American Journal of Physiology. 1986;250:E641–649. doi: 10.1152/ajpendo.1986.250.6.E641. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Porter JC. A sensitive radioenzymatic assay for dopamine, norepinephrine, and epinephrine in plasma and tissue. Endocrinology. 1976;98:1497–1507. doi: 10.1210/endo-98-6-1497. [DOI] [PubMed] [Google Scholar]
- Bonen A, Megeney LA, McCarthy SC, McDermott JC, Tan MH. Epinephrine administration stimulates GLUT4 translocation but reduces glucose transport in muscle. Biochemical and Biophysical Research Communications. 1992;187:685–691. doi: 10.1016/0006-291x(92)91249-p. [DOI] [PubMed] [Google Scholar]
- Carlson KI, Marker JC, Arnall DA, Terry ML, Yang HT, Lindsay LG, Braken ME, Winder WW. Epinephrine is unessential for stimulation of liver glycogenolysis during exercise. Journal of Applied Physiology. 1985;58:544–548. doi: 10.1152/jappl.1985.58.2.544. [DOI] [PubMed] [Google Scholar]
- Cherrington AD, Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973;22:537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Chesley A, Hultman E, Spriet LL. Effects of epinephrine infusion on muscle glycogenolysis during intense aerobic exercise. American Journal of Physiology. 1995;268:E127–134. doi: 10.1152/ajpendo.1995.268.1.E127. [DOI] [PubMed] [Google Scholar]
- Coker RH, Krishna MG, Lacy DB, Allen EJ, Wasserman DH. Sympathetic drive to liver and non-hepatic splanchnic tissue during heavy exercise. Journal of Applied Physiology. 1997a;82:1244–1249. doi: 10.1152/jappl.1997.82.4.1244. [DOI] [PubMed] [Google Scholar]
- Coker RH, Krishna MG, Lacy DB, Bracy DP, Wasserman DH. Role of hepatic α- and β–ADRenergic receptor stimulation on hepatic glucose production during heavy exercise. American Journal of Physiology. 1997b;273:E831–838. doi: 10.1152/ajpendo.1997.273.5.E831. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Letter to the editor. American Journal of Physiology. 1989;257:E338. doi: 10.1152/ajpendo.1989.256.2.E338. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. Journal of Applied Physiology. 1998;84:465–470. doi: 10.1152/jappl.1998.84.2.465. [DOI] [PubMed] [Google Scholar]
- Galbo H. Hormonal and Metabolic Adaptation to Exercise. New York: Thieme; 1983. [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiologica Scandinavica. 1979;107:19–32. doi: 10.1111/j.1748-1716.1979.tb06438.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Kiens B, Richter EA. Effect of increased plasma free fatty acid concentrations on muscle metabolism in exercising men. Journal of Applied Physiology. 1991;70:194–201. doi: 10.1152/jappl.1991.70.1.194. [DOI] [PubMed] [Google Scholar]
- Hetenyi G, Jr, Norwich KH. Validity of the rates of production and utilisation of metabolites as determined by tracer methods in intact animals. Federation Proceedings. 1974;33:1841–1848. [PubMed] [Google Scholar]
- Hoelzer DR, Dalsky GP, Clutter WE, Shah SD, Holloszy JO, Cryer PE. Glucoregulation during exercise: hypoglycemia is prevented by redundent glucoregulatory systems, sympathochromaffin activation and changes in islet hormone secretion. Journal of Clinical Investigation. 1986a;77:212–221. doi: 10.1172/JCI112279. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hoelzer DR, Dalsky GP, Schwartz NS, Clutter WE, Shah SD, Holloszy JO, Cryer PE. Epinephrine is not critical to prevention of hypoglycemia during exercise in humans. American Journal of Physiology. 1986b;251:E104–110. doi: 10.1152/ajpendo.1986.251.1.E104. [DOI] [PubMed] [Google Scholar]
- Howlett K, Febbraio M, Hargreaves M. Glucose production during strenuous exercise in humans: role of epinephrine. American Journal of Physiology. 1999;276:E1130–1135. doi: 10.1152/ajpendo.1999.276.6.E1130. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Effect of epinephrine on carbohydrate metabolism in exercising dogs. Metabolism. 1985;34:457–464. doi: 10.1016/0026-0495(85)90212-4. [DOI] [PubMed] [Google Scholar]
- Jansson E, Hjemdahl P, Kaijser L. Epinephrine-induced changes in muscle carbohydrate metabolism during exercise in male subjects. Journal of Applied Physiology. 1986;60:1466–1470. doi: 10.1152/jappl.1986.60.5.1466. [DOI] [PubMed] [Google Scholar]
- Järhult J, Holst J. The role of the adrenergic innervation to the pancreatic islets in the control of insulin release during exercise in man. Pflügers Archiv. 1979;383:41–45. doi: 10.1007/BF00584473. [DOI] [PubMed] [Google Scholar]
- Jenkins AB, Chisholm DJ, James DE, Ho KY, Kraegen EW. Exercise-induced hepatic glucose output is precisely sensitive to the rate of systemic glucose supply. Metabolism. 1985;34:431–436. doi: 10.1016/0026-0495(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. American Journal of Physiology. 1986;251:E65–70. doi: 10.1152/ajpendo.1986.251.1.E65. [DOI] [PubMed] [Google Scholar]
- Kjær M, Christensen NJ, Sonne B, Richter EA, Galbo H. Effect of exercise on epinephrine turnover in trained and untrained male subjects. Journal of Applied Physiology. 1985;59:1061–1067. doi: 10.1152/jappl.1985.59.4.1061. [DOI] [PubMed] [Google Scholar]
- Kjær M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. American Journal of Physiology. 1993;265:E275–283. doi: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- Kjær M, Keiding S, Engfred K, Rasmussen K, Sonne B, Kirkegård P, Galbo H. Glucose homeostasis during exercise in humans with a liver or kidney transplant. American Journal of Physiology. 1995;268:E636–644. doi: 10.1152/ajpendo.1995.268.4.E636. [DOI] [PubMed] [Google Scholar]
- Kjær M, Mikines KJ, Christensen NJ, Tronier B, Vinten J, Sonne B, Richter EA, Galbo H. Glucose turnover and hormonal changes during insulin-induced hypoglycemia in trained humans. Journal of Applied Physiology. 1984;57:21–27. doi: 10.1152/jappl.1984.57.1.21. [DOI] [PubMed] [Google Scholar]
- Marker JC, Arnall DA, Conlee RK, Winder WW. Effect of adrenodemedullation on metabolic responses to high-intensity exercise. American Journal of Physiology. 1986;251:R552–559. doi: 10.1152/ajpregu.1986.251.3.R552. [DOI] [PubMed] [Google Scholar]
- Marker JC, Clutter WE, Cryer PE. Reduced epinephrine clearance and glycemic sensitivity to epinephrine in older individuals. American Journal of Physiology. 1998;275:E770–776. doi: 10.1152/ajpendo.1998.275.5.E770. [DOI] [PubMed] [Google Scholar]
- Mathias C, Christensen NJ, Corvett JL, Frankl HL, Spalding JMK. Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Clinical Research. 1976;39:204–208. doi: 10.1161/01.res.39.2.204. [DOI] [PubMed] [Google Scholar]
- Moates JM, Lacy DB, Goldstein RE, Cherrington AD, Wasserman DH. Metabolic role of the exercise-induced increment in epinephrine in the dog. American Journal of Physiology. 1988;255:E428–436. doi: 10.1152/ajpendo.1988.255.4.E428. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJF, Wong D, Hollidge-Horvat MG, Spriet LL. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. American Journal of Physiology. 1998;274:R894–902. doi: 10.1152/ajpregu.1998.274.4.R894. [DOI] [PubMed] [Google Scholar]
- Raz I, Katz A, Spencer MK. Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle. American Journal of Physiology. 1991;260:E430–435. doi: 10.1152/ajpendo.1991.260.3.E430. [DOI] [PubMed] [Google Scholar]
- Richter EA, Galbo H, Christensen NJ. Control of exercise-induced muscular glycogenolysis by adrenal medullary hormones in rats. Journal of Applied Physiology. 1981a;50:21–26. doi: 10.1152/jappl.1981.50.1.21. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. American Journal of Physiology. 1982;242:E25–32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Richter EA, Sonne B, Christensen NJ, Galbo H. Role of epinephrine for muscular glycogenolysis and pancreatic hormonal secretion in running rats. American Journal of Physiology. 1981b;240:E526–532. doi: 10.1152/ajpendo.1981.240.5.E526. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Zhang X-J, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. Journal of Applied Physiology. 1995;79:1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- Shah SD, Tse TF, Clutter WE, Cryer PE. The human sympathochromaffin system. American Journal of Physiology. 1984;247:E380–384. doi: 10.1152/ajpendo.1984.247.3.E380. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Purdon C, Bilinski D, Vranic M, Halter JB, Marliss EB. Glucoregulation during and after intense exercise: effects of beta-blockade. Journal of Clinical Endocrinology and Metabolism. 1994;78:359–366. doi: 10.1210/jcem.78.2.7906280. [DOI] [PubMed] [Google Scholar]
- Simonson DC, Koivisto V, Sherwin RS, Ferrannini E, Hendler R, Juhlin-Dannfelt A, Defronzo RA. Adrenergic blockade alters glucose kinetics during exercise in insulin-dependent diabetics. Journal of Clinical Investigation. 1984;73:1648–1658. doi: 10.1172/JCI111371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne B, Mikines KJ, Galbo H. Glucose turnover in 48-hour-fasted running rats. American Journal of Physiology. 1987;252:R587–593. doi: 10.1152/ajpregu.1987.252.3.R587. [DOI] [PubMed] [Google Scholar]
- Sonne B, Mikines KJ, Richter EA, Christensen NJ, Galbo H. Role of liver nerves and adrenal medulla in glucose turnover of running rats. Journal of Applied Physiology. 1985;59:1640–1646. doi: 10.1152/jappl.1985.59.5.1640. [DOI] [PubMed] [Google Scholar]
- Steele R, Wall JS, DeBodo RC, Altszuler N. Measurement of the size and turnover rate of body glucose pool by isotope dilution method. American Journal of Physiology. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Lacy DB, Green DR, Williams PE, Cherrington AD. Dynamics of hepatic lactate and glucose balances during prolonged exercise and recovery in the dog. Journal of Applied Physiology. 1987;63:2411–2417. doi: 10.1152/jappl.1987.63.6.2411. [DOI] [PubMed] [Google Scholar]
- Wendling PS, Peters SJ, Heigenhauser GJF, Spriet LL. Epinephrine infusion does not enhance net muscle glycogenolysis during prolonged aerobic exercise. Canadian Journal of Applied Physiology. 1996;21:271–284. doi: 10.1139/h96-024. [DOI] [PubMed] [Google Scholar]
- Winder WW, Terry ML, Mitchell VM. Role of plasma epinephrine in fasted exercising rats. American Journal of Physiology. 1985;248:R302–307. doi: 10.1152/ajpregu.1985.248.3.R302. [DOI] [PubMed] [Google Scholar]
- Winder WW, Yang HT, Jaussi AW, Hopkins CR. Epinephrine, glucose, and lactate infusion in exercising adrenodemedullated rats. Journal of Applied Physiology. 1987;62:1442–1447. doi: 10.1152/jappl.1987.62.4.1442. [DOI] [PubMed] [Google Scholar]


