Abstract
The short-circuit current (Isc) technique was used to study the role of 5-hydroxytryptamine (5-HT) in the regulation of anion secretion in cultured rat cauda epididymal epithelia.
5-HT, the 5-HT1B-selective agonist 5-nonyloxytryptamine (5-NOT) and the 5-HT2B-selective agonist α-methyl-5-hydroxytryptamine (α-methyl-5-HT) added basolaterally stimulated Isc in a dose-dependent manner with EC50 values of 0.4, 20 and 0.3 μm, respectively. No other agonists for 5-HT receptors had any effect.
The pattern of responses to 5-HT was biphasic. Pretreating the tissues with the 5-HT1B-selective antagonist isamoltane (200 μm) and the 5-HT2B-selective antagonist rauwolscine (200 μm) inhibited the rapid transient phase by 55 and 45 %, whereas the sustained phase could only be blocked by rauwolscine.
Removal of chloride or bicarbonate or both from the normal Krebs-Henseleit solution reduced the responses to 5-HT, 5-NOT and α-methyl-5-HT to varying degrees. The results suggest that 5-HT1B- and 5-HT2B-mediated responses were mainly due to chloride and bicarbonate secretion, respectively.
Manipulation of the cAMP and Ca2+ signal transduction pathways with chemical agents provided evidence that the responses to 5-HT were mediated through cAMP.
Piroxicam pretreatment abolished the Isc response to α-methyl-5-HT but not to 5-NOT, indicating that the 5-HT2B-mediated response, but not the 5-HT1B-mediated response, is dependent on prostaglandin synthesis.
Immunohistochemical studies showed that 5-HT-like immunoreactivity was detected in nerve fibres and in small granular cells surrounding the epididymal tubules.
It is suggested that the 5-HT released from serotonergic nerve endings and/or from mast cells regulates electrolyte and fluid secretion in the epididymis.
The epididymis plays an indispensible role in male reproduction by actively creating a favourable fluid environment in which sperm maturation takes place. This is achieved by the absorptive and secretory functions of the epithelium. Work on cultured epididymal epithelia has shown that under basal conditions, the epididymal cells undergo a net absorption of fluid, which is driven by sodium reabsorption (Wong & Yeung, 1978). Under stimulation conditions, the cells are activated to secrete anions (Cl− and HCO3−) and secondarily water (Wong, 1988a,b). The latter process has been shown to be subject to neurohumoral control (Cuthbert & Wong, 1986; Wong, 1988a,b; Wong et al. 1990; Leung et al. 1992; Lai et al. 1994).
5-Hydroxytryptamine (5-HT) is widely distributed in the vertebrate and is important in controlling gastrointestinal motility, contraction of smooth muscle, vascular constriction and dilatation, microvascular permeability, platelet aggregation, nociception, and excitation and inhibition of neurons in the central nervous system. It has also been reported that 5-HT may affect the secretory processes in the airway (Jung et al. 1997), cornea (Marshall & Klyce, 1984), ileum (Borman & Burleigh, 1993; Franks et al. 1996), jejunum (Hardcastle et al. 1981), colon (Sidhu & Cooke, 1995) and gallbladder (Donowitz et al. 1980).
In the male reproductive system, 5-HT plays an important role in the regulation of testicular blood flow (Collin et al. 1996), secretion of corticotropin-releasing factor from Leydig cells (Dufau et al. 1993; Tinajero et al. 1993) and contraction of vas deferens (Hay & Wadsworth, 1982). Histochemical and biochemical assays revealed high concentrations of 5-HT in the epididymis, probably in mast cells and epithelial cells (Anderson et al. 1979). At the present time, the function of 5-HT in the epididymis remains largely unexplored. The present study was undertaken to characterize the effects of 5-HT and receptor subtypes that may be involved in the anion secretion in cultured rat epididymal epithelium.
METHODS
The experiments described in this paper were approved by the Animal Research Ethics Committee of the Chinese University of Hong Kong.
Tissue culture technique
The procedures of tissue culture have been described previously (Cuthbert & Wong, 1986; Wong, 1988a). In brief, immature male Sprague-Dawley rats weighing 150 g were used as a source of cauda epididymis. Rats were killed by CO2 inhalation. The epididymis was dissected out, finely chopped with scissors, and treated successively with 0.25 % (w/v) trypsin and 0.1 % (w/v) collagenase in Hanks' balanced salt solution (HBSS). The disaggregated cells were suspended in Eagle's minimum essential medium (EMEM) containing non-essential amino acids (0.1 mM), sodium pyruvate (1 mM), glutamine (4 mM), 5α-dihydrotestosterone (1 nM), 10 % fetal bovine serum, penicillin (100 i.u. ml−1) and streptomycin (100 μg ml−1) and seeded into the wells of Millipore filter assemblies with a diameter of 0.4 cm2 (cell concentration, 105 cells ml−1; plating density, 5 × 104 cells cm−2 filter) floating on 15 ml of culture medium. Cultures were incubated in 5 % CO2 for 3 days at 32°C. During this time, the monolayers reached confluency and thereafter were ready for the measurement of short-circuit current (Isc).
Isc measurement
Confluent epididymal monolayers were clamped between the two halves of an Ussing chamber with a 0.6 cm2 window. The tissue was short-circuited by the use of a voltage-clamp amplifier (DVC 1000; World Precision Instruments, New Haven, CT, USA). The Isc was displayed on a pen recorder. Transepithelial resistance was obtained from Ohm's law by clamping the tissue intermittently at a voltage 0.1-0.3 mV displaced from zero. Epithelia with transepithelial resistances of less than 300 Ω cm−2 were discarded. The two channels of the amplifier were mostly used simultaneously on parallel monolayers so that studies could be made under control and experimental conditions. In the majority of cases, the monolayers were bathed on both sides with Krebs-Henseleit solution, gassed with 95 % O2-5 % CO2 and warmed to 32°C.
Measurement of cAMP
Epididymal cell monolayers from rats were grown on 24-well plates (Coster, Cambridge, MA, USA). After reaching confluency, they were washed twice with Krebs-Henseleit solution and then incubated in 0.5 ml of the same buffer containing isobutylmethylxanthine (IBMX; 1 mM) for 10 min at 32°C. 5-HT, 5-nonyloxytryptamine or α-methyl-5-HT was added to the wells and cells were incubated for an additional 10 min. The reaction was terminated by adding 10 μl 60 % (w/v) perchloric acid to each well. The contents of each well were mixed thoroughly and transferred to a 1.5 ml microcentrifuge tube, and were then centrifuged at 10 000 g for 5 s. The supernatant (300 μl) was neutralized by KOH (1 M). The mixture (100 μl) was taken and assayed for cAMP using an assay kit (see ‘Materials’).
Immunohistochemical studies
Adult Sprague-Dawley rats weighing 150-250 g were anaesthetized with urethane (1.2 g kg−1), and injected intraperitoneally and perfused intracardially with 0.1 M phosphate-buffered saline (PBS) followed by freshly prepared 4 % paraformaldehyde in PBS. Epididymis was removed, postfixed for 2 h and cytoprotected in 30 % sucrose-PBS overnight. Longitudinal sections of 30 μm were cut using a cryostat and mounted directly on gel-treated slides. Sections were processed for 5-HT-like immunoreactivity by means of the standard avidin-biotin complex technique as described previously (Dun et al. 1996).
Tissues were first treated with 3 % H2O2 to quench endogenous peroxidase, washed several times and blocked with 10 % normal goat sera. Tissues were then incubated in the primary antisera to 5-HT (rabbit polyconal from DiaSorin, Stillwater, MN, USA; 1:500-1000 dilution with 0.4 % Triton X-100 in PBS) for 48 h at 4°C with gentle agitation. After thorough rinsing, sections were incubated with biotinylated anti-rabbit IgG (1:200, Vector Laboratories). After several rinses in Tris-buffered saline, sections were developed in diaminobenzidine-H2O2 solution and washed for at least 2 h with Tris-buffered saline. Sections were mounted on slides with 0.25 % gel alcohol, air-dried, dehydrated with alcohol followed by xylene and covered with Permount.
Two sets of control experiments were carried out. First, the primary antisera were omitted from the staining procedures. Second, sections were processed with 5-HT antisera pre-absorbed with 5-HT-conjugated bovine serum albumin (10 μg ml−1, DiaSorin) overnight.
Solutions
Krebs-Henseleit solution had the following composition (mM): NaCl, 117; KCl, 4.7; KH2PO4, 1.2; MgSO4·7H2O, 1.2; CaCl2·2H2O, 2.56; NaHCO3, 24.8; and glucose, 11.1. This solution had a pH of 7.4 when bubbled with 95 % O2-5 % CO2. In Cl−-free solution, NaCl, KCl and CaCl2 were replaced by sodium gluconate, potassium gluconate and calcium gluconate, respectively. When HCO3−-free solution was used, NaHCO3 was replaced with NaCl and the solution was buffered with 10 mM Hepes at pH 7.4, gassed with 100 % O2. In Cl−- and HCO3−-free solution, NaCl and NaHCO3 were replaced with sodium gluconate, KCl with potassium gluconate, and CaCl2 with calcium gluconate. The solution was buffered with 10 mM Hepes at pH 7.4, bubbled with 100 % O2. In each solution, the osmolality was adjusted to 290 mosmol kg−1 with D-mannitol if necessary.
Materials
EMEM, fetal bovine serum and non-essential amino acids were purchased from Gibco Laboratories. Penicillin-streptomycin, HBSS, sodium pyruvate, 5α-dihydrotestosterone, trypsin, collagenase Type I, ATP, IBMX, forskolin, piroxicam and prostaglandin E2 (PGE2) were from Sigma. 5-HT and thapsigargin were from Research Biochemicals International. MDL-12330 and H-89 were from Calbiochem. Different kinds of 5-HT agonists and antagonists were bought from Tocris (Bristol, UK). The immunoassay kit for cAMP was purchased from R & B Systems (Minneapolis, MN, USA).
Statistical analysis
Results are expressed as means ±s.e.m. Comparisons between groups of data were made by Student's unpaired t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Effect of 5-HT on the Isc
When bathed in normal Krebs-Henseleit solution, epididymal monolayers exhibited a transepithelial potential difference of 3.0 ± 0.1 mV (n = 392), a basal Isc of 5.4 ± 0.2 μA cm−2 (n = 392) and a transepithelial resistance of about 500 Ω cm−2. The basal current has been shown to be due to anion secretion (Wong, 1988a). It was only partially reduced by piroxicam (Fig. 6) but almost abolished by the adenylate cyclase inhibitor MDL-12330A, indicating that there was a basal level of cAMP formation which could cause some degree of opening of the cystic fibrosis transmembrane conductance regulator (CFTR) even when the tissue was not stimulated (G. P. H. Leung & P. Y. D. Wong, unpublished observation). Apical application of 5-HT had a negligible effect (Fig. 1A). Addition of 5-HT to the basolateral side elicited a biphasic Isc response consisting of an initial spike followed by a sustained response (Fig. 1B). The initial spike and the sustained response to 5-HT were dose dependent (Fig. 1C). The maximal response of the initial spike was 6.72 ± 0.6 μA cm−2 and the EC50 was 0.4 μm. The maximal response of the sustained phase was 1.97 ± 0.31 μA cm−2 and the EC50 was 0.6 μm. In all subsequent experiments, 5-HT was added to the basolateral aspect of the tissue.
Figure 6. Effect of piroxicam pretreatment (added basolaterally) on the Isc response to 5-HT (A), 5-NOT (B) and α-methyl-5-HT (C).
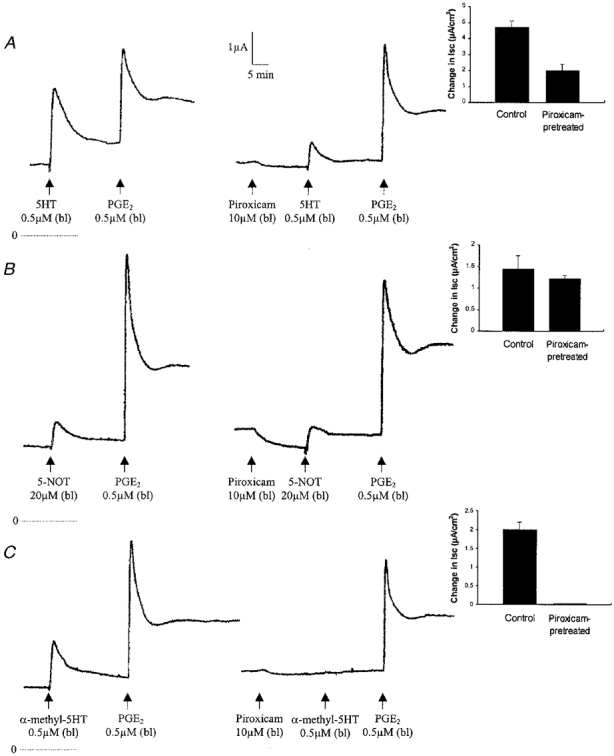
In each panel, PGE2 was used as a control to show the lack of inhibition by piroxicam. The records on the left show the control responses to 5-HT or analogues and PGE2. Each record is representative of 4 different experiments. Insets, responses to 5-HT or analogues before and after treatment with piroxicam. Each column shows the mean and s.e.m. of 4 experiments.
Figure 1. Isc responses in 10 separate epididymal monolayers, area 0.4 cm2.
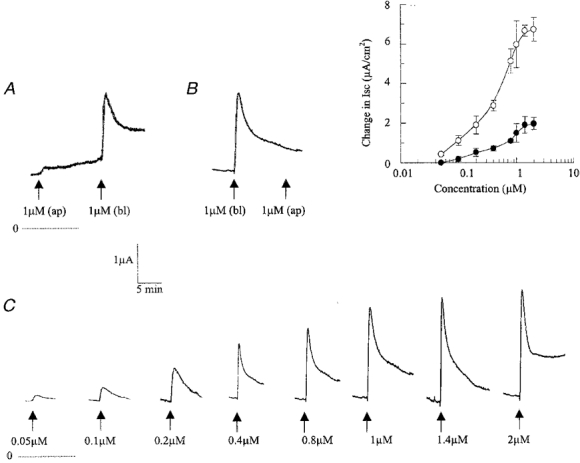
A, 5-HT added to the apical side (ap) followed by addition to the basolateral side (bl). B, 5-HT added to the basolateral side followed by addition to the apical side. C, 5-HT was added to the basolateral side at the concentrations shown. For A-C, each record is representative of 4 different experiments. Inset, concentration-response curves for the effect of 5-HT on the Isc in rat epididymal epithelia. ○, change in current at the peak of the initial spike; •, change in current at the sustained phase of the response. Each point shows the mean ±s.e.m. of 4 experiments. The dotted horizontal lines indicate zero Isc.
Effects of various 5-HT agonists
The pharmacological profile of 5-HT receptors mediating the secretory response in the epididymal epithelium was evaluated by means of selective agonists and antagonists to subtypes of 5-HT receptor. The 5-HT1B agonist 5-NOT induced a dose-dependent increase in Isc. The maximum response was 2.84 ± 0.68 μA cm−2 and the EC50 was 20 μm. The 5-HT2B agonist α-methyl-5-HT also elicited a dose-dependent increase in Isc with an apparent EC50 of 0.3 μm and maximum response of 2.25 ± 0.27 μA cm−2 (Fig. 2).
Figure 2. Concentration-response curves for 5-HT (○), 5-NOT (□) and α-methyl-5-HT (•) stimulation of Isc in cultured rat epididymal epithelia.
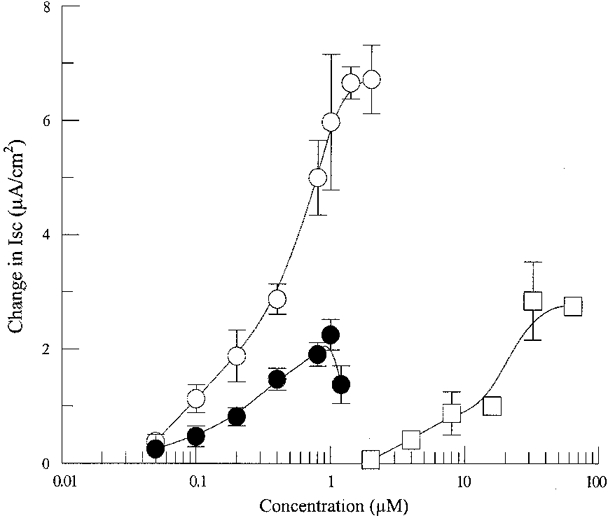
Each point shows the mean ±s.e.m. of 4 experiments.
The 5-HT1A agonist 8-hydroxy-DPAT, the 5-HT1D agonist L-694,247, the 5-HT2C agonist MK 212, the 5-HT3 agonist phenylbiguanide and the 5-HT4 agonist 2-[1-(4-piperonyl) piperazinyl]benzothiazole did not significantly affect the Isc. Methiothepin, a high affinity ligand for 5-HT5, 5-HT6 and 5-HT7 receptors, did not increase Isc when added alone or block the Isc response to 5-HT (results not shown).
Effects of 5-HT1B and 5-HT2B antagonists
In this series of experiments, the hypothesis that the biphasic Isc response to 5-HT may be due to the activation of subtypes of 5-HT receptors was tested (Fig. 3). The 5-HT1B antagonist isamoltane (200 μm) reduced the Isc response to 5-NOT (20 μm) from 2.66 ± 0.45 to 0.94 ± 0.08 μA cm−2 (n = 4), representing a 65 % inhibition. The 5-HT2B antagonist rauwolscine (200 μm) inhibited the Isc response to α-methyl-5-HT (0.5 μm) by 76 %, from 1.98 ± 0.16 to 0.48 ± 0.17 μA cm−2 (n = 4). Pretreating the tissue with isamoltane (200 μm) inhibited the rapid transient phase of the response to 5-HT (0.5 μm) by 55 % with no significant change in the sustained phase. However, pretreatment with rauwolscine (200 μm) reduced the initial spike by 45 % and nearly abolished the sustained phase. The effects of different concentrations of the antagonists on the percentage inhibition are also shown in Fig. 3.
Figure 3. Time course of the Isc response to 5-HT alone and following pretreatment of the tissues with selected 5-HT receptor antagonists.
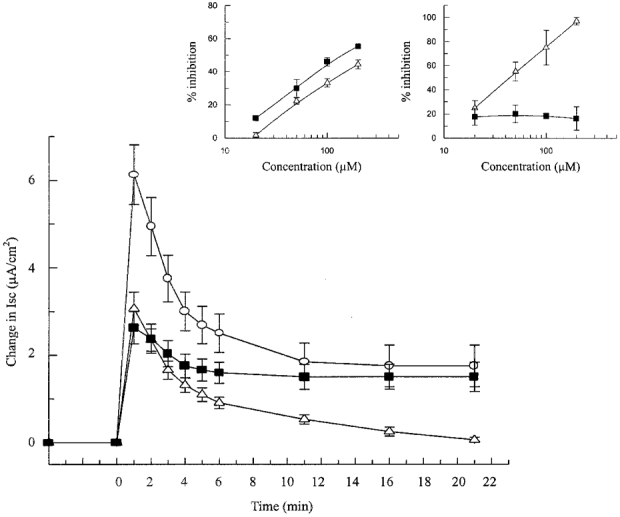
Response to 5-HT (0.5 μm) in the absence (○) and presence of 200 μm isamoltane (▪) or 200 μm rauwolscine (▵). Each point shows the mean ±s.e.m. of 4 experiments. Inset, concentration-inhibition curves for isamoltane (▪) and rauwolscine (▵) for inhibition of the initial phase (left) and the sustained phase (right) of the response to 5-HT.
Effects of ion substitution
Removal of Cl− or HCO3− or both from the bathing solution reduced the Isc response to 5-HT by 80, 82 and 93 %, respectively (Fig. 4). In Cl−-free solution, the Isc responses to 5-NOT and α-methyl-5-HT were reduced by 87 and 56 %, respectively (Fig. 5). In HCO3−-free solution, the Isc responses to 5-NOT and α-methyl-5-HT were reduced by 63 and 85 %, respectively. Omission of both anions from the bathing media resulted in a 96 and 88 % reduction of Isc responses to 5-NOT and α-methyl-5-HT (Fig. 5).
Figure 4. Isc responses to 5-HT in normal and in modified Krebs-Henseleit solution.
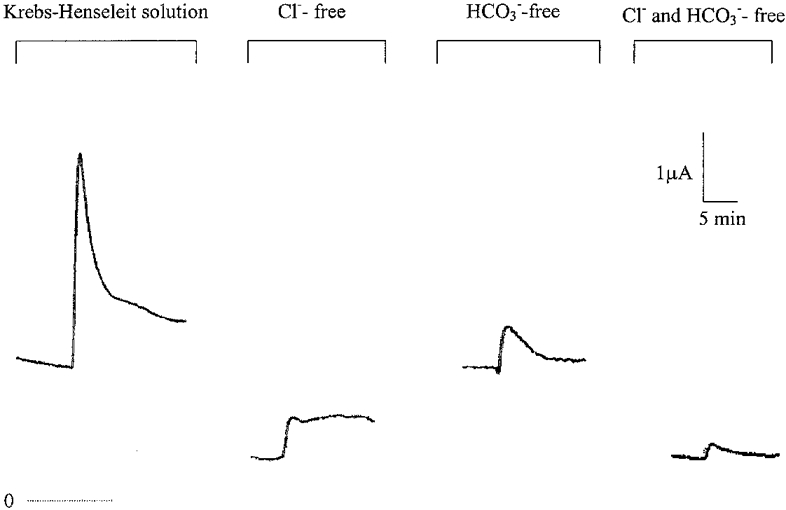
5-HT (1 μm) was applied to the basolateral side and the tissue was bathed in normal Krebs-Henseleit solution or Cl−-free, HCO3−-free, or Cl−- and HCO3−-free solution. Each record is representative of 4 different experiments.
Figure 5. Isc responses to 5-HT receptor agonists in normal and in modified Krebs-Henseleit solutions.
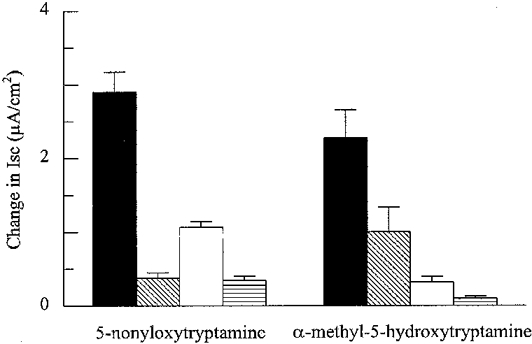
5-NOT (30 μm) and α-methyl-5-HT (1 μm) effects in normal Krebs-Henseleit solution (▪), Cl−-free solution ( ), HCO3−-free solution (□), and Cl−- and HCO3−-free solution (
), HCO3−-free solution (□), and Cl−- and HCO3−-free solution ( ). Each column shows the mean and s.e.m. of 4 experiments.
). Each column shows the mean and s.e.m. of 4 experiments.
Effect of BAPTA AM and thapsigargin
To determine whether the Isc response to 5-HT was mediated through a change in intracellular free Ca2+, the tissue was pretreated with a cell-permeant Ca2+ chelator, BAPTA AM, before stimulation with 5-HT. As shown in Table 1, pretreatment with BAPTA AM (100 μm) did not appreciably affect the response to 5-HT. Similar results were observed when 5-NOT and α-methyl-5-HT were used instead of 5-HT (Table 1).
Table 1.
Effects of BAPTA AM and thapsigargin on the Isc response to 5-HT (1 μm), 5NOT (30 μm) and α-methyl-5-HT (1 μm)
| 5-HT (μA cm−2) | 5NOT (μA cm−2) | αmethyl5HT (μA cm−2) | |
|---|---|---|---|
| Control | 8.28 ± 0.72 | 4.66 ± 0.83 | 3.34 ± 0.63 |
| + BAPTA AM | 7.66 ± 0.48 | 4.19 ± 0.56 | 2.78 ± 0.39 |
| Control | 5.67 ± 0.17 | 4.69 ± 0.19 | 2.79 ± 0.59 |
| + Thapsigargin | 5.04 ± 0.2 | 4.38 ± 0.38 | 2.67 ± 0.6 |
Each ΔIsc value is the mean ±s.e.m. of 4 experiments. BAPTA AM, 100 μm; thapsigargin, 1 μm.
Pretreatment with thapsigargin (1 μm), a microsomal Ca2+-ATPase inhibitor, has been shown to elicit a transient Isc response due to release of Ca2+ from internal calcium stores (Wong, 1988a). When the current had returned to the basal level, 5-HT added to the basolateral side produced a response that was not significantly different from that produced by the same concentration of 5-HT without prior treatment with thapsigargin (Table 1). The Isc response to 5-NOT or α-methyl-5-HT was also unaffected by thapsigargin (Table 1).
Involvement of cAMP
To study the involvement of cAMP in the Isc response to 5-HT, forskolin (10 μm), an adenylate cyclase activator, was used to exhaust the adenylate cyclase so that no further rise in cAMP would be possible upon subsequent addition of cAMP-elevating agents. It has been shown that the basolateral addition of forskolin stimulates a rapid increase followed by a sustained rise in Isc (Wong, 1988a). After a sustained level was reached, basolateral addition of 5-HT, 5-NOT or α-methyl-5-HT caused a Isc response that was much smaller than that of control (Table 2).
Table 2.
Effects of forskolin, MDL-12330A and H-89 on the Isc response to 5-HT (1 μm), 5-NOT (30 μm) and α-methyl-5-HT (1 μm)
| 5-HT (μA cm−2) | 5NOT (μA cm−2) | αmethyl5HT (μA cm−2) | |
|---|---|---|---|
| Control | 5.37 ± 1.36 | 2.19 ± 0.36 | 2.44 ± 0.48 |
| + Forskolin | 0.75 ± 0.18* | 0.63 ± 0.16* | 0.22 ± 0.08* |
| Control | 5.22 ± 0.37 | 1.69 ± 0.36 | 2.56 ± 0.62 |
| + MDL-12330A | 0.78 ± 0.14*** | 0.31 ± 0.06* | 0.44 ± 0.12* |
| Control | 6.09 ± 0.32 | 2 ± 0.44 | .81 ± 0.21 |
| + H-89 | 0.31 ± 0.19*** | 0.03 ± 0.03* | 0** |
Each ΔIsc value is the mean ±s.e.m. of 4 experiments. Forskolin, 10 μm; MDL-12330A, 100 μm; H-89, 75 μm.
P < 0.001
P < 0.01
P < 0.05 compared with the control of the respective group.
To further support the involvement of cAMP in the response to 5-HT, the effects of an adenylate cyclase inhibitor, MDL-12330A, and a protein kinase A (PKA) inhibitor, H-89, were examined. Over 90 % of the Isc responses to 5-HT, 5-NOT and α-methyl-5-HT were inhibited by pretreatment with these agents (Table 2).
Immunoassays were performed to study the effects of 5-HT on intracellular cAMP levels in epididymal epithelium. Under basal conditions, the intracellular cAMP content was 34.76 ± 8.46 pmol well−1 (n = 4). Stimulating the tissue with 5-HT, 5-NOT or α-methyl-5-HT caused a rise in intracellular cAMP to 114.38 ± 15.25 pmol well−1 (n = 4, P < 0.01), 110.37 ± 19.09 pmol well−1 (n = 4, P < 0.05) and 96.37 ± 6.85 pmol well−1 (n = 4, P < 0.01), respectively.
Involvement of prostaglandins in the response to 5-HT
To investigate whether the effect of 5-HT on anion secretion is mediated via synthesis of prostaglandins, the effect of piroxicam (10 μm), a cyclo-oxygenase inhibitor, was studied (Wong et al. 1999). As shown in Fig. 6A, pretreatment with piroxicam (added basolaterally) reduced the response to 5-HT by 57.6 %. It was speculated that the partial inhibition was due to piroxicam selectively blocking either the 5-HT1B or the 5-HT2B receptor. The effect of piroxicam on the response to 5-NOT (the selective 5-HT1B agonist) or α-methyl-5-HT (the selective 5-HT2B agonist) was studied. The results show that piroxicam treatment did not affect the response to 5-NOT (Fig. 6B), but abolished the response to α-methyl-5-HT (Fig. 6C).
Immunohistochemical localization of 5-HT
The pattern of distribution of 5-HT-like immunoreactivity (5-HT-LI) in the epididymis was similar in the five rats examined. 5-HT-LI was present in nerve fibres and in small granular cells, presumably mast cells (Figs 7 and 8). The density of 5-HT-LI nerve fibres was clearly region dependent. For example, the cauda exhibited numerous 5-HT-LI fibres throughout the intertubular and subepithelial space; some of the fibres seemed to penetrate into the epithelium (Fig. 7B and C). 5-HT-LI nerve fibres were less numerous in the corpus and were sparse in the caput (Fig. 8). Neurons immunoreactive to 5-HT were not detected throughout the epididymis. Control experiments in which the primary antisera were either omitted or pre-adsorbed with 5-HT-conjugated bovine serum albumin resulted in no positive labelling (Fig. 7D).
Figure 7. Photomicrographs of a longitudinal cauda section labelled with 5-HT antisera and 5-HT antisera pre-absorbed with 5-HT-conjugated bovine serum albumin.
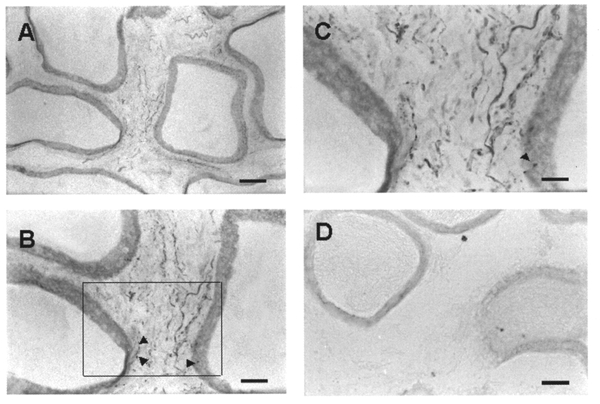
A, low magnification showing numerous 5-HT-LI fibres in the intertubular space. B, higher magnification showing 5-HT-LI fibres between the tubules; some of the fibres seem to penetrate into the epithelium, as indicated by arrowheads. C, higher magnification of the area outlined in B, where bead-like 5-HT-positive fibres are seen beneath the epithelium, some of which seem to penetrate into the epithelium (arrowhead in the lower right-hand corner). D, a cauda section labelled with 5-HT antisera pre-absorbed with 5-HT-conjugated bovine serum albumin (10 μg ml−1); 5-HT-positive fibres are not seen. Scale bars: A, 100 μm; B and D, 50 μm; C, 25 μm.
Figure 8. Photomicrographs of a longitudinal section through corpus and caput labelled with 5-HT antisera.
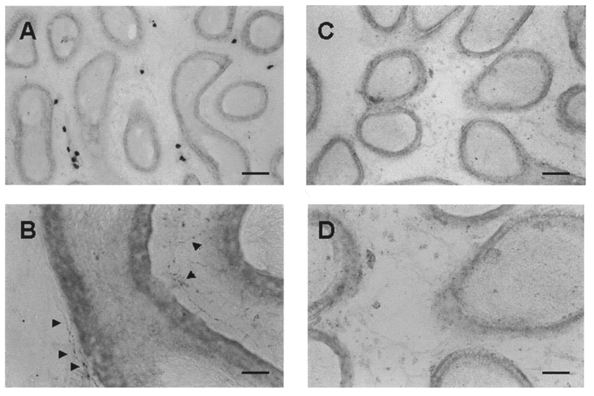
A, low magnification of a section of corpus showing darkly stained, granular cells, presumably mast cells. B, higher magnification of A, where several 5-HT-LI nerve fibres (arrowheads) are seen. C and D, low and higher magnifications of a section of caput where 5-HT-LI fibres are not clearly visible. Scale bars: A and C, 100 μm; B and D, 25 μm.
DISCUSSION
The present study shows that in cultured rat epididymal epithelium, 5-HT stimulated a dose-dependent rise in the Isc, which appears to be mediated by an interaction with basolateral receptors. This is because the stimulation of Isc observed upon the addition of 5-HT to the basolateral side was much greater than that following addition to the apical side. The observation that responses to 5-HT were significantly reduced upon the removal of extracellular Cl− or HCO3− or both (Fig. 4), and by apical addition of diphenylamine-2,2′-dicarboxylic acid (results not shown), suggests that the rise in Isc was due to an increase in anion secretion. The mechanism of anion secretion in rat epididymis involves the interplay of the basolaterally placed Na+-K+ pump, Na+-K+-2Cl− symport, Na+-H+ exchanger and basolateral K+ channel, and apically placed anion channels. As with other anion secretory epithelia, agonists stimulate secretion primarily by raising intracellular cAMP or Ca2+, leading to the opening of apical anion channels (Wong, 1988a; Wong & Huang, 1989).
The pattern of responses to 5-HT was biphasic, consisting of an initial transient phase followed by a sustained phase. This feature has also been observed with noradrenaline, which stimulates different types of adrenergic receptors in the epididymis (Leung et al. 1992). As shown in Fig. 3, the initial spike of the Isc response to 5-HT was reduced by both isamoltane and rauwolscine, whereas only rauwolscine reduced significantly the sustained phase of the response to 5-HT. This finding raises the possibility that the response to 5-HT consists of two or more components mediated by pharmacologically distinct receptors. 5-HT receptors have been divided into seven distinct classes according to their pharmacological profiles, cDNA-deduced primary sequences and signal transduction mechanisms; receptor subtypes can also be found in each class (Hoyer et al. 1994). The present study provides pharmacological evidence for the existence of 5-HT1B and 5-HT2B receptors in the epididymal epithelium. First, the 5-HT1B agonist 5-NOT and the 5-HT2B agonist α-methyl-5-HT stimulated Isc in a dose-dependent manner. Second, the 5-HT1B antagonist isamoltane and the 5-HT2B antagonist rauwolscine inhibited the Isc response to 5-HT. Collectively, our results suggest that the initial spike is 5-HT1B and 5-HT2B mediated and the sustained phase is 5-HT2B mediated only.
Another point of interest concerning the biphasic Isc response is that different ion species may be secreted during the two phases. It has previously been shown that when the epididymal epithelium is stimulated by forskolin or cAMP, the initial phase of the Isc response is Cl− dependent and the sustained phase HCO3− dependent (Chan et al. 1996). In the present study, ion replacement experiments indicated that the Isc response to 5-NOT and α-methyl-5-HT was mainly due to Cl− and HCO3− secretion, respectively. This observation raises the possibility that 5-HT released from nerves and/or mast cells may activate two types of serotonergic receptor on the epididymal epithelium, i.e. 5-HT1B and 5-HT2B receptor activation gives rise to, respectively, a rapid but transient Isc due to Cl− secretion and a sustained phase attributable to HCO3− secretion.
The intracellular signal transduction pathway underlying the 5-HT-induced secretory response was examined here. 5-HT increased the intracellular cAMP concentration in the epididymal epithelial cells, suggesting that 5-HT may stimulate anion secretion through the activation of the cAMP signal transduction pathway. Further, forskolin was employed to maximally stimulate cAMP formation before the addition of 5-HT, 5-NOT and α-methyl-5-HT, and under these conditions, the stimulating effects of forskolin and 5-HT (and also of 5-NOT and α-methyl-5-HT) were not additive, indicating that the two agonists share the same signal transduction pathway. The involvement of cAMP is further corroborated by the effectiveness of the adenylate cyclase inhibitor MDL-12330A and the protein kinase A inhibitor H-89 in abolishing the responses to 5-HT, 5-NOT and α-methyl-5-HT (Table 2).
Unlike cAMP, intracellular Ca2+ appears to play a negligible role in the Isc response to 5-HT. Thus, pretreatment of the tissues with BAPTA AM, a cell-permeant Ca2+ chelator that can chelate intracellular free Ca2+, or thapsigargin, a microsomal Ca2+-ATPase inhibitor that can lead to the depletion of Ca2+ stores, did not significantly change the Isc response to 5-HT, 5-NOT or α-methyl-5-HT (Table 1). BAPTA AM did significantly reduce the responses to ATP (results not shown), whose action is shown to be Ca2+ dependent (Leung et al. 1993).
Various hormones such as bradykinin, angiotensin, endothelin, etc., have been shown to stimulate anion secretion in the epididymis via local formation of prostaglandins, as their effects are abolished by pretreating the tissues with cyclo-oxygenase (COX) inhibitors (see Wong et al. 1999). Our results with piroxicam (Fig. 6) show that the 5-HT1B receptor-mediated secretion is not COX dependent, whereas the 5-HT2B receptor-mediated secretion is. Previous work from our laboratory has shown that the COX-1 isoform is expressed by the basal cells of the epididymis. This isoform is responsible for the formation of PGE2, which mediates the secretory responses to COX-dependent hormones (Wong et al. 1999). On the basis of these findings, it would seem plausible that the 5-HT2B receptors are present on the membrane of the basal cells. These receptors are coupled to phospholipase A2, which hydrolyses membrane phospholipids to arachidonic acid, which, in the presence of COX-1, is converted to PGE2. We hypothesize that 5-HT activates these receptors to form PGE2, which diffuses out to the interstitial fluid and stimulates the carbonic anhydrase (CA)-containing cells to secrete bicarbonate. On the other hand, as the effect of 5-HT1B receptor-mediated secretion is not COX dependent, these receptors may be present on the principal cells lacking carbonic anhydrase. 5-HT stimulates these receptors directly to secrete chloride. In support of this hypothesis, CA has been localized to the narrow and clear cells, the other principal cells being CA negative (Cohen et al. 1976; Kaunisto et al. 1995; Adamali & Hermo, 1996). It is conceivable that the CA-containing cells secrete bicarbonate while the CA-negative cells secrete chloride (Chan et al. 1996).
The involvement of cAMP in the 5-HT1B and 5-HT2B receptor-mediated responses does not seem to reconcile with a large body of evidence that these two receptors are either negatively coupled to cAMP or are transduced by PLC/Ca2+. While the cAMP dependence of the 5-HT2B response could be explained by PGE2 synthesis, the role of cAMP in the 5-HT1B response is less readily explicable. It could be that, in the epididymis, the 5-HT1B receptors are uniquely coupled to adenylate cyclase, or alternatively, that an unidentified Gs protein-coupled receptor (e.g. 5-HT7) with pharmacological properties similar to that of the conventional 5-HT1 receptor exists in the epididymis. In addition, as in the case of 5-HT2B, the involvement of a cAMP-dependent mediator for 5-HT1B cannot be excluded.
The cauda epididymis is densely innervated by autonomic nerves, while the corpus and caput epididymis are sparsely innervated (Ricker, 1998). It has been reported that spinal cord injury leads to infertility (Linsenmeyer & Perkash, 1991). Denervation of the nerves to the epididymis results in a reduction of sperm motility (Billups et al. 1990) and changes in luminal fluid composition (Ricker et al. 1996). While these deficits could be largely attributed to the loss of noradrenergic and cholinergic nerves, the contribution from other putative transmitters cannot be excluded. Our study shows that immunoreactive 5-HT nerve fibres are present in the cauda epididymis. These fibres appear to penetrate into the epithelium (Fig. 7). The distribution of 5-HT-LI fibres shows a rostro-caudal bias in that they were numerous in the cauda but sparse in the caput. The cells of origin of 5-HT-LI fibres in the epididymis are not known nor are the physiological conditions under which the indoleamine is released. 5-HT-LI was also detected in small granular cells, presumably mast cells (Fig. 8), which are distributed widely throughout the male reproductive tract (Gayton et al. 1989). At the present time, the function of mast cells in the epididymis is uncertain. Degranulation of mast cells in the gut has been shown to release numerous mediators such as 5-HT, prostaglandins and histamine, which may influence electrolyte and water transport during inflammation (Crowe et al. 1990; Perdue et al. 1991). A similar role may be ascribed to mast cells in the epididymis.
In conclusion, the present study shows that 5-HT released from serotonergic nerve endings and/or mast cells may act on 5-HT1B and 5-HT2B receptors located on different cell types of the epithelium. Activation of 5-HT1B receptors stimulates Cl− secretion by the principal cells, whereas activation of 5-HT2B receptors stimulates the basal cells to synthesize PGE2 which then causes the CA-containing cells to secrete HCO3−. It may further be hypothesized that these two processes are mediated by the cAMP-PKA pathway terminating on the apical CFTR. In cystic fibrosis (CF), mutation of the gene encoding the CFTR results in defective cAMP-driven Cl− secretion. The ensuing loss of regulation by 5-HT and other cAMP-elevating agents of fluid secretion may be responsible for the obstruction or agenesis of the vas deferens in CF men (Wong, 1998).
Acknowledgments
This work was supported by the Research Grants Council of Hong Kong and the International Consortium on Male Contraception, Population Council, New York.
References
- Adamali HI, Hermo L. Apical and narrow cells are distinct cell types differing in their structure, distribution, and functions in the adult rat epididymis. Journal of Andrology. 1996;17:208–222. [PubMed] [Google Scholar]
- Anderson ME, Paparo AA, Martan J. Paraformaldehyde-induced fluorescence as a histochemical test for 5-hydroxytryptamine in the epididymis of opossum. Journal of Anatomy. 1979;129:141–149. [PMC free article] [PubMed] [Google Scholar]
- Billups KL, Tillman SL, Chang TSK. Reduction of epididymal sperm motility after ablation of the interior mesenteric plexus in the rat. Fertility and Sterility. 1990;53:1076–1082. [PubMed] [Google Scholar]
- Borman RA, Burleigh DE. Evidence for the involvement of a 5-HT4 receptor in the secretory response of human small intestine to 5-HT. British Journal of Pharmacology. 1993;110:927–928. doi: 10.1111/j.1476-5381.1993.tb13901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HC, Ko WK, Zhao W, Fu WO, Wong PYD. Evidence for independent Cl− and HCO3− secretion and involvement of an apical Na+-HCO3− cotransporter in cultured rat epididymal epithelia. Experimental Physiology. 1996;81:515–524. doi: 10.1113/expphysiol.1996.sp003954. [DOI] [PubMed] [Google Scholar]
- Cohen JP, Hoffer AP, Rosen S. Carbonic anhydrase localization in the epididymis and testis of the rat: Histochemical and biochemical analysis. Biology of Reproduction. 1976;14:339–346. doi: 10.1095/biolreprod14.3.339. [DOI] [PubMed] [Google Scholar]
- Collin O, Damber JE, Bergh A. 5-Hydroxytryptamine - a local regulator of testicular blood flow and vasomotion in rats. Journal of Reproduction and Fertility. 1996;106:17–22. doi: 10.1530/jrf.0.1060017. [DOI] [PubMed] [Google Scholar]
- Crowe SE, Sestini P, Perdue MH. Allergic reaction of rat jejunal mucosa. Gastroenterology. 1990;99:74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Wong PYD. Electrogenic anion secretion in cultured rat epididymal epithelium. The Journal of Physiology. 1986;378:335–346. doi: 10.1113/jphysiol.1986.sp016222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M, Tai YH, Asarkof N. Effect of serotonin on active electrolyte transport in rabbit ileum, gallbladder, and colon. American Journal of Physiology. 1980;239:G463–472. doi: 10.1152/ajpgi.1980.239.6.G463. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Tinajero JC, Fabbri A. Corticotropin-releasing factor: an antireproductive hormone of the testis. FASEB Journal. 1993;7:299–307. doi: 10.1096/fasebj.7.2.8382638. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Huang RL, Dun EC, Lai CC, Wong PYD, Forstermann U. Distribution and origin of nitric oxide synthase-immunoreactive nerve fibers in the rat epididymis. Brain Research. 1996;738:292–300. doi: 10.1016/s0006-8993(96)00795-0. [DOI] [PubMed] [Google Scholar]
- Franks CM, Hardcastle J, Hardcastle PT. Neural involvement in 5-hydroxytryptamine-induced net electrogenic ion secretion in the rat intestine in-vivo. Journal of Pharmacy and Pharmacology. 1996;48:411–416. doi: 10.1111/j.2042-7158.1996.tb05943.x. [DOI] [PubMed] [Google Scholar]
- Gayton F, Carrera G, Pinilla L, Aguilar R, Bellido C. Mast cells in the testis, epididymis and accessory glands of the rat: effects of neonatal steroid treatment. Journal of Andrology. 1989;10:351–358. doi: 10.1002/j.1939-4640.1989.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Hardcastle J, Hardcastle PT, Redfern JS. Action of 5-hydroxytryptamine on intestinal ion transport in the rat. The Journal of Physiology. 1981;320:41–55. doi: 10.1113/jphysiol.1981.sp013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DWP, Wadsworth RM. The contractile effects of 5-hydroxytryptamine on the rat isolated vas deferens. British Journal of Pharmacology. 1982;77:605–613. doi: 10.1111/j.1476-5381.1982.tb09338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphery PP. International Union of Pharmacolgy classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacological Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Jung JS, Oh SO, Kim MG, Kang DS, Lee SH. Cl− secretion induced by 5-hydroxytryptamine and calcitonin gene-related peptide in the rat tracheal epithelia. Pflügers Archiv. 1997;435:20–27. doi: 10.1007/s004240050479. [DOI] [PubMed] [Google Scholar]
- Kaunisto K, Parkkila S, Parkkila A-K, Waheed A, Sly WS, Rajaniemi H. Expression of carbonic anhydrase isoenzymes IV and II in rat epididymal duct. Biology of Reproduction. 1995;52:1350–1357. doi: 10.1095/biolreprod52.6.1350. [DOI] [PubMed] [Google Scholar]
- Lai KB, Fu WO, Ko WH, Chan HC, Wong PYD. The effect of [Arg8]-vasopressin on electrogenic chloride secretion in cultured rat epididymal epithelia. American Journal of Physiology. 1994;267:C607–616. doi: 10.1152/ajpcell.1994.267.2.C607. [DOI] [PubMed] [Google Scholar]
- Leung AYH, Leung PY, Cheng-Chew SB, Wong PYD. The role of calcitonin gene-related peptide in the regulation of anion secretion by rat and human epididymis. Journal of Endocrinology. 1992;133:259–268. doi: 10.1677/joe.0.1330259. [DOI] [PubMed] [Google Scholar]
- Leung AYH, Tai HL, Wong PYD. ATP stimulates Ca2+ release from a rapidly exchanging pool in cultured rat epididymal cells. American Journal of Physiology. 1993;264:C1388–1394. doi: 10.1152/ajpcell.1993.264.6.C1388. [DOI] [PubMed] [Google Scholar]
- Leung AYH, Wong PYD. The epididymis as a chloride-secreting organ. News in Physiological Science. 1994;9:31–35. [Google Scholar]
- Leung AYH, Yip WK, Wong PYD. Characterization of adrenoceptors involved in the electrogenic chloride secretion by cultured rat epididymal epithelium. British Journal of Pharmacology. 1992;107:146–151. doi: 10.1111/j.1476-5381.1992.tb14477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeyer TA, Perkash I. Infertility in men with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1991;72:747–754. [PubMed] [Google Scholar]
- Marshall WS, Klyce SD. Cellular mode of serotonin action on Cl− transport in the rabbit corneal epithelium. Biochimica et Biophysica Acta. 1984;778:139–143. doi: 10.1016/0005-2736(84)90457-7. [DOI] [PubMed] [Google Scholar]
- Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Journal of Clinical Investigation. 1991;87:687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker DD. The autonomic innervation of the epididymis: Its effects on epididymal function and fertility. Journal of Andrology. 1998;19:1–4. [PubMed] [Google Scholar]
- Ricker DD, Chamness SL, Hinton BT, Chang TSK. Changes in luminal fluid protein composition in the rat cauda epididymidis following partial sympathetic denervation. Journal of Andrology. 1996;17:117–126. [PubMed] [Google Scholar]
- Sidhu M, Cooke HJ. Role of 5-HT and ACh in submucosal reflexes mediating colonic secretion. American Journal of Physiology. 1995;269:G346–351. doi: 10.1152/ajpgi.1995.269.3.G346. [DOI] [PubMed] [Google Scholar]
- Tinajero JC, Fabbri A, Ciocca DR, Dufau ML. Serotonin secretion from rat leydig cells. Endocrinology. 1993;133:3026–3029. doi: 10.1210/endo.133.6.8243331. [DOI] [PubMed] [Google Scholar]
- Wong PYD. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. American Journal of Physiology. 1988a;254:F121–133. doi: 10.1152/ajprenal.1988.254.1.F121. [DOI] [PubMed] [Google Scholar]
- Wong PYD. Control of anion and fluid secretion by P2-purinoceptor in the rat epididymis. British Journal of Pharmacology. 1988b;95:1315–1321. doi: 10.1111/j.1476-5381.1988.tb11770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PYD. CFTR gene and male fertility. Molecular Human Reproduction. 1998;4:107–110. doi: 10.1093/molehr/4.2.107. [DOI] [PubMed] [Google Scholar]
- Wong PYD, Chan HC, Leung PS, Chung YW, Wong YL, Lee WM, Ng V, Dun NJ. Regulation of anion secretion by cyclo-oxygenase and prostanoids in cultured epididymal epithelia from the rat. The Journal of Physiology. 1999;514:809–820. doi: 10.1111/j.1469-7793.1999.809ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PYD, Fu WO, Huang SJ, Law WK. Effect of angiotensins on electrogenic anion transport in monolayer cultures of rat epididymis. Journal of Endocrinology. 1990;125:449–456. doi: 10.1677/joe.0.1250449. [DOI] [PubMed] [Google Scholar]
- Wong PYD, Huang SJ. Intracellular pH measurement in primary monolayer cultures of rat epididymal cells. Pflügers Archiv. 1989;413:414–421. doi: 10.1007/BF00584492. [DOI] [PubMed] [Google Scholar]
- Wong PYD, Yeung CH. Absorptive and secretory functions of the perfused rat cauda epididymis. The Journal of Physiology. 1978;275:13–26. doi: 10.1113/jphysiol.1978.sp012174. [DOI] [PMC free article] [PubMed] [Google Scholar]


