Abstract
The majority of primate retinal ganglion cells (RGCs) project to the parvocellular layers of the lateral geniculate nucleus (LGN). These P cells play a central role in early visual processing.
An improved method of systems analysis has allowed us to explore the dynamics of the colour-opponent subregions of P-cell receptive fields with a single chromatic stimulus. The data show that the centre and surround subregions of the P-cell receptive field have similar temporal responses, but the surround is slightly delayed. The centre and surround demonstrate a large degree of chromatic selectivity.
The responses of the centre and surround subregions were fitted with a linear model and the model was used to predict the responses of P cells to new chromatic and achromatic stimuli. Although linear models predict the chromatic responses well, simple linear combinations of centre and surround responses fail to predict P-cell responses to achromatic stimuli.
The temporal responses of the different subpopulations of P cells, such as ON/OFF or L-centre/M-centre were not significantly different.
The P cells of the primate retina form the majority of ganglion cells (∼80 %) (Rodieck, 1988; Kaplan et al. 1990; Lee, 1996). They project to the four parvocellular layers of the primate LGN and have small, colour-opponent receptive fields. Anatomically, these cells correspond to midget ganglion cells (Perry et al. 1984; Lee, 1996). We have investigated the temporal responses of P-cell receptive fields to better understand the role of these chromatically opponent cells in processing visual information. In an earlier study (Benardete & Kaplan, 1997a), we investigated the dynamics of the P-cell receptive field with luminance spots and annuli. In this paper, we report on the temporal responses of P cells measured with chromatic stimuli.
Several previous studies have indirectly measured the temporal responses of the subregions of the P-cell receptive field. Smith et al. (1992) estimated the contributions of the centre and surround chromatic mechanisms to the P-cell response by fitting linear models to the responses. Other investigators have measured the responses of P cells to full-field chromatic stimulation in the frequency domain (e.g. Yeh et al. 1995). Here we measured directly the temporal responses of the colour-opponent subdivisions of the P-cell receptive field to both chromatic and achromatic sine gratings, including cone-isolating gratings, at several spatial frequencies.
The responses of red-green opponent P cells were explored here with sinewave gratings modulated by the multiple m-sequence signal (Benardete & Victor, 1994; Benardete & Kaplan, 1997a). This technique allowed us to stimulate each P cell with two different cone-isolating gratings simultaneously, and to calculate the response to each of the stimulating gratings. The responses of P cells to chromatic gratings that selectively stimulate the centre or the surround were similar, except for an opposite signature and a delay. We quantified the dynamics of the P-cell response to chromatic and achromatic stimuli by fitting the data to a mathematical model composed of linear filters. The dynamical properties of the P cell were independent of the overall contrast in the chromatic or luminance stimulus.
Several groups reported (or suggested) that P cells combine chromatic signals linearly (Ingling & Martinez-Uriegas, 1983; Derrington et al. 1984; Lee et al. 1989). We have tested the hypothesis that P cells simply ‘add’ signals from cones by using a linear model to predict the responses of P cells to novel chromatic and achromatic signals.
Recent anatomical studies have noted subclasses of P cells based on the synaptic organization of bipolar cells in the primate retina (Calkins, 1994; Calkins et al. 1994). Detailed measurements of P-cell dynamics provide an opportunity to determine whether the P-cell population is made up of several distinct physiological subclasses. After fitting the P-cell responses to a dynamic model, the model parameters were used to quantify the differences among the various classes of P cells.
METHODS
Surgical preparation
We studied 13 male monkeys (Macaca fascicularis; 2.5-4.5 kg) (experimental protocol approved by the Rockefeller University Animal Care and Use Committee). The spatial and chromatic vision of this species is known to be similar to that of humans (DeValois et al. 1974a,b). The experimental method has been described elsewhere (Kaplan & Shapley, 1982; Benardete & Kaplan, 1997a,b) and will be summarized here only briefly.
The monkey was initially anaesthetized with an intramuscular (i.m.) injection of xylazine followed by ketamine hydrochloride. Three cannulae (one arterial and two venous) were inserted into the femoral vessels under additional local anaesthesia to monitor blood pressure and to deliver drugs and fluids. An endotracheal tube was inserted for artificial ventilation and to monitor the expired CO2. Penicillin and gentamicin sulfate were administered i.m. to provide antibacterial coverage. Phenylephrine hydrochloride (10 %) and atropine sulfate (1 %) were applied to dilate the pupils and relax accommodation. The corneas were protected with hard, gas-permeable contact lenses. A continuous intravenous flow of lactated Ringer solution with 5 % dextrose was maintained throughout the experiment, while a urinary catheter allowed us to monitor the overall fluid balance.
The animal was placed in a stereotactic apparatus and body temperature, expired CO2, heart rate, blood pressure, ECG and respiration rate were continuously monitored and maintained within the physiological range. Deep anaesthesia was maintained with a continuous infusion of pentothal (3 mg kg−1 h−1). To prevent eye movements, paralysis was maintained by infusion of either gallamine triethiodide (Flaxedil, 5-10 mg kg−1 h−1) or vecuronium bromide (Norcuron, 0.25 mg kg−1 h−1). Artificial pupils (5 mm in diameter) were placed in front of the contact lenses during the experiment, to maintain a constant retinal illumination. Appropriate lenses were used to focus the eyes on the cathode ray tube (CRT) screen, usually placed at 228 cm. The location of the LGN was marked on the skull at Horsley-Clarke coordinates 7 A-11 L, the overlying bone was removed with a trephine and a small well of dental acrylic was formed around the area. A stimulating bipolar electrode of Teflon-coated stainless steel wire was stereotactically guided into the optic chiasm (Horsley-Clarke coordinates: 19.5 A, 0.5 L) and allowed the measurement of propagation latencies when an electrical stimulus (150 μs) was applied. At the termination of the experiment, the animal was killed with an overdose of anaesthetic agent.
Recording
The dura was removed and a glass-coated tungsten electrode (Merrill & Ainsworth, 1972) was lowered to the top of the LGN at a depth of approximately 23 mm below the cortex. The extracellular responses of isolated units were differentially amplified, displayed on an oscilloscope, and routed to an audio monitor. The electrode was advanced with a stepping motor until the extracellular synaptic (S) potentials (Bishop et al. 1958), elicited by the firing of a single RGC, could be detected above the background noise. Two tunable bandpass filters shaped the electrical signal. A level discriminator emitted a pulse when an S potential crossed the threshold, and a second discriminator was used to detect only LGN spikes. The firing times of these pulses were recorded by a PDP 11/73 computer (Digital Equipment Corporation, Boston, MA, USA) to the nearest 0.1 ms.
Visual stimuli
Stimuli were generated by a special-purpose graphics computer (Milkman et al. 1980) on a colour CRT display (Conrac, model 7351) with a mean photopic luminance of 57.2 cd m−2 (1123 trolands) and refresh rate of 135 frames s−1. The display subtended 7 deg × 7 deg at a distance of 228 cm from the animal. The red, green and blue guns of the monitor were modulated independently to produce various spatial and luminance patterns. The output of the three guns was linearized with a lookup table that was generated from photocell readings before each experiment. In the experiments described here, the computer modulated the contrast of sinusoidal gratings. The centre of the cell receptive field was aligned with the centre of the grating to produce the maximum possible modulated response.
Silent substitution
The silent substitution method (Estévez & Spekreijse, 1974, 1982) provides a means for producing a stimulus that activates only a single cone class. The mean luminance of the CRT was set at 50 % of the maximal output of the three guns: red, green, and blue. The appearance of the monitor at this setting is bluish-white (CIE (1931) coordinates:X = 31.29, Y = 34.17, Z = 50.53). For modulation of a single cone class (or any linear combination of cone classes within the physical limits of the CRT), the red, green and blue guns were modulated in a specified ratio that was chosen as follows. The spectral radiance of each of the guns of the CRT was measured with a photometer (Pritchard-1980B, Photo Research (Chatsworth, CA, USA)) at 10 nm intervals in the range 400-700 nm. The spectral sensitivity of each cone class in the macaque was assumed to be similar to that calculated psychophysically by Smith & Pokorny (1975) for human spectral mechanisms. According to the principle of univariance (Rushton et al. 1973), each cone class was stimulated by an amount proportional to its spectral sensitivity at any given wavelength and the energy emitted by a particular gun at that wavelength. Thus, each cone class received a certain amount of activation from each gun (red, green and blue). For example, for the L-cone type, the amount of stimulation due to the red gun, LR, is equal to:
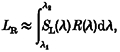 |
(1) |
where SL(λ) is the sensitivity of the L cone at wavelength λ and R(λ) is the spectral radiance of the red gun at λ. Since the spectral radiance of each gun was measured at a discrete set of values, the integral, LR, is replaced by the sum:
| (2) |
The relative stimulation of the L-cone class by each of the three guns was then calculated as the ratio of the cone excitation produced by a given gun to the total excitation. For example, the relative excitation of the L cones by the red gun (∼LR) is given by:
| (3) |
Finally, for a desired set of relative values of stimulation of the L, M and S cones, {l,m,s}, the following set of simultaneous equations was solved:
 |
(4) |
where  is the amplitude of modulation of each gun.
is the amplitude of modulation of each gun.
Because of the colours of the phosphors, the maximum stimulation for isolated L or M cones produced approximately 0.16 of the maximum cone excitation possible with an achromatic stimulus (all three guns modulated equally in synchrony).
For experiments on red-green opponent P cells, we used two sinewave gratings, one to isolate L cones and the other to isolate M cones. A grating that stimulated a chosen L and M combination (L + M grating) was also used, and an isoluminant (‘equiluminant’) grating that stimulates purely chromatic mechanisms. To calculate the proper values for the isoluminant grating, we measured the photopic luminance of each gun of the Conrac monitor (evaluated with the photopic luminous efficacy function, Vλ). The luminance values of the three guns of the Conrac were: red, 11.62 cd m−2; green, 39.99 cd m−2 and blue, 5.61 cd m−2. The luminance of each cone-isolating grating was calculated, and a combination of L- and M-cone-isolating gratings was found that gave a total luminance modulation of 0. Typical values for the relative weightings of the red, green and blue guns for each type of grating are given in Table 1.
Table 1.
Gun intensity values for different grating types
| CRT gun values | Fraction of maximal cone excitation | |||||
|---|---|---|---|---|---|---|
| Type of grating | Red | Green | Blue | L | M | S |
| Achromatic | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| L-cone-isolating | 0.986 | −0.139 | 0.001 | 0.160 | 0.000 | 0.000 |
| M-cone-isolating | −0.868 | 0.337 | −0.027 | 0.000 | 0.160 | 0.000 |
| L + M stimulus | −0.999 | 0.845 | −0.088 | 0.294 | 0.522 | 0.000 |
| Isoluminant stimulus | 0.973 | −0.285 | 0.019 | 0.062 | −0.110 | 0.000 |
CRT gun intensity values for different cone-isolating gratings. The intensity of the red, green and blue guns of the CRT is scaled between 0 and 1 for each type of grating used as a stimulus. The intensity values range from 0 (unmodulated, constant intensity) to 1 (100% modulation). The fraction of maximal cone excitation indicates the amount of cone excitation produced by each stimulus relative to the amount of cone excitation produced by all 3 guns modulated in synchrony. The maximal amount of cone excitation (i.e. 1) is given by the achromatic stimulus. The L and M-cone-isolating gratings each produce 0.16 of the maximal L- or M-cone excitation, respectively.
m-Sequence experiments
In order to investigate the dynamics of P cells, a special signal was used to modulate two sinusoidal gratings simultaneously, while the response of the P cell was being recorded. That signal - the multiple m-sequence signal - is briefly described here, and further details are presented elsewhere (Benardete & Victor, 1994; Benardete & Kaplan, 1997a). An m-sequence, m(t), is a cyclic and binary signal that takes on a new value of +1 or -1 after a predetermined time step, ΔT (Golomb, 1968). A positive value indicates an increment in contrast while a negative value indicates a decrement. The contrast of each grating was temporally modulated by a signal that was a sum of two m-sequences. Appropriately chosen pairs of m-sequences prevent certain kinds of response contamination. In our experiments, the time step was 14.8 ms.
The m-sequence method allows determination of system kernels of successively higher orders. Kernels describe the response of the cell under investigation to its input. The first-order kernel is similar to the impulse response of a linear system. To calculate the first-order m-sequence kernel, h1(t), the response, r(t), of the cell is cross-correlated with the input sequence, m(t):
| (5) |
where < > signifies cross-correlation over full cycles of the m-sequence.
Although ‘kernels’ are useful for formulating detailed mathematical models, in the Results section we present the data in terms of first-order (time domain) responses for convenience. Multiplication by a scale factor yields the response from the kernel calculation. The first-order response shows how the P-cell firing rate changes when an impulse of contrast falls within its receptive field.
The multiple m-sequence signal was generated from one sequence of length 31 steps and another of length 63 steps. The total stimulus cycle was 1953 time steps, which was 28.90 s long. The stimulation program (courtesy of Dr J. D. Victor, Cornell University Medical College) inserted an additional 5 s of stimulation at the beginning of each run. The response during this ‘pre-stimulus’ epoch was discarded to avoid transients in the response. The various stimulus conditions were interleaved, and successive presentations of the same stimulus used alternate m-sequences to avoid artifacts (Benardete & Victor, 1994). The generating polynomials (Golomb, 1968) that determined the individual m-sequences used in successive sets are given in Benardete & Kaplan (1997a). The contrast of one grating was modulated by the sum of one 31- and one 63-length sequence, while the other grating was modulated by long ‘lags’ of the same two m-sequences. Both gratings were modulated simultaneously in the receptive field of the P cell. The program counted the number of events (either LGN spikes or S potentials) in each 3.7 ms ‘bin’, and the response of the cell was converted to spike rate (impulses s−1). The spike rate was used as the measure of the response of the cell. The cross-correlations necessary for kernel calculations were computed using the Fast M-Transform (FMT) described by Sutter (1992). A typical kernel calculation incorporated approximately 3 min (6 ‘runs’) or more of spike data. In order to have a total of four m-sequences modulate these gratings, it was necessary that the maximum output for each red, green and blue gun be reduced by 0.25. During a total cycle of the stimulus, the output of each gun simultaneously incremented the contrast, thus the maximal gun output could be only 0.25 of the maximum, to prevent the gun output from reaching saturation.
m-Sequence kernels and other kernel methods
The multiple m-sequence method is a specific example of more general kernel methods used to study physiological and other dynamical systems. The white-noise method of Wiener (Marmarelis & Marmarelis, 1978) is the most general paradigm. Wiener's method uses a theoretical stimulus of Gaussian white noise as an input to study any system. Any practical implementation of this method has its limitations. While a true ‘white’ signal has infinite bandwidth, the m-sequence pulse used in these experiments has a duration of 14.8 ms, thus the frequency spectrum of the stimulus has less than a 10 % reduction in power out to 16 Hz (past the peak of the P-cell response (see Results)). The width of the m-sequence pulse was chosen to optimize the trade-off between a flat frequency spectrum and the signal-to-noise ratio in the cell response. In addition, the m-sequence signal we used is not Gaussian, but ternary, because it is the sum of two m-sequences. Nevertheless, the low-pass nature of photoreceptors (Schnapf et al. 1990) will greatly smooth out the amplitude distribution of the signals reaching the inner retina (Benardete et al. 1994).
Cell identification
Several tests were used to characterize each cell before it was investigated in detail, and to classify it as a P or an M cell. The depth of the electrode, together with the dominant eye, were usually reliable indications of the target layer of the ganglion cell, as confirmed by histology (see Benardete & Kaplan, 1997a). Each cell was classified as R+G-, R-G+, Y+B-, Y-B+ and ON or OFF according to the changes in its impulse rate in response to full-field stimulation (red, green, yellow, blue or white). We also measured the latency of the S potential of the cell elicited by electrical stimulation of the optic chiasm. The latencies of M cells fall in the range of 1-2.5 ms, and those of P cells between 2.5-4.5 ms (Kaplan & Shapley, 1982). The average latency in our sample of P cells was 3.42 ms. In addition, we used the contrast response function (CRF) to distinguish P from M cells. The CRFs of P cells have a shallow slope that is nearly linear with increasing contrast, while CRFs from M cells have a steep slope at low contrast values (0.01-0.1) and early saturation (Kaplan & Shapley, 1986). The parafoveal P cells in our sample had an average retinal eccentricity of 4.7 deg (range: 2-9.5 deg). Previous measurements on the same population of P cells have shown that the receptive field centres at this eccentricity are approximately 0.06-0.2 deg in diameter (Benardete & Kaplan, 1997a).
Model fits
We used a linear filter model to describe the temporal frequency response of the units that were analysed (Victor, 1987). The model was fitted to the first-order responses from the m-sequence experiments. First, the m-sequence response was transformed into the frequency domain using the fast Fourier transform in a commercial program (Microsoft Excel). The model was then fitted to the log of the (complex) response (log of the amplitude and the phase angle) at the frequency ω by minimizing the sum of squares of the difference between the model and the data. The error at each frequency was weighted according to how much of the total response was accounted for by the response at that frequency (Shapley & Victor, 1981). Finally, the fitted model was transformed back into the time domain. The model consists of two stages: a subtractive high-pass filter and a series of low-pass filters (Benardete et al. 1992):
| (6) |
Five parameters determine the model response, K, at the frequency ω: A, the overall gain; HS, the strength of the subtractive stage; τS, the time constant of the high-pass stage; τL, the time constant of the low-pass stages; and NL, the number of low-pass stages. D is the measured delay of transmission from the optic chiasm to the LGN and i = √-1.
RESULTS
Responses to chromatic and achromatic gratings
LGN cells in the parvocellular layers are known to produce chromatically opponent responses (DeValois et al. 1966; Wiesel & Hubel, 1966; Derrington et al. 1984). The P RGCs, which drive the LGN parvocellular cells, are also chromatically opponent. For example, P ON R+G- cells produce ON responses to a long wavelength (red) stimulus and OFF responses to a middle wavelength (green) stimulus (Gouras, 1968). Later in this paper we show that red-green opponent P cells have L- and M-cone-driven subregions of the receptive field. Using the multiple m-sequence method and cone-isolating gratings, it is possible to elicit from a P cell the first-order responses to two chromatic and/or achromatic gratings simultaneously. This method provides a means to compare directly, using the response to a single stimulus, the dynamics of P-cell responses to these two classes of stimuli. Figures 1–3 show typical results from a P ON and a P OFF cell.
Figure 1. First-order responses of a P ON R-G+ cell (unit 29/14) to low spatial frequency (SF) (0.145 cd−1) chromatic and achromatic gratings.

Each column shows the responses of the cell to 2 grating stimuli presented simultaneously (top and bottom rows). The continuous lines show the responses of the P cell (given in terms of impulses s−1), and the dotted lines show the responses of its target LGN cell. Left, responses of the P cell and its target LGN cell to an L-cone-isolating (top) and an M-cone-isolating (bottom) grating set at 0.25 contrast and presented simultaneously. Notice that the response to the M-cone-isolating grating leads the L-cone-driven response and has the opposite signature. Middle, response of the P cell-LGN cell pair to an isoluminant and an achromatic (black-white) grating set at 0.25 contrast and presented simultaneously. Right, response of the P cell-LGN cell pair to an L + M grating that stimulates a mixture of L and M cones and an achromatic (black-white) grating set at 0.25 and presented simultaneously. Note the complex time course of the P-cell response to stimulation by a low spatial frequency luminance pattern, where the response has two zero crossings.
Figure 3. First-order responses of a P OFF R+G- cell (unit 29/8) to low spatial frequency (0.145 c d−1) chromatic and achromatic gratings.

The continuous lines show the responses of the P cell and the dotted lines show the responses of its target LGN cell. Left, responses of the P cell and the LGN cell to an L-cone-isolating (top) and an M-cone-isolating (bottom) grating set at 0.25 contrast and presented simultaneously. Notice that the response to the M-cone-isolating grating leads the L-cone response and has the opposite signature. Middle, response of the P cell-LGN cell pair to an isoluminant and an achromatic (black-white) grating set at 0.25 contrast and presented simultaneously. Right, response of the P cell-LGN cell pair to a L + M grating and an achromatic (black-white) grating set at 0.25 and presented simultaneously. Note that the overall time course of the P OFF cell responses are similar to those of the P ON cell, although the responses are inverted in polarity.
The responses of a P ON R-G+ cell to low spatial frequency gratings (0.145 cycles deg−1 (c d−1)) of several types are shown in Fig. 1. Each column shows two responses, each calculated from the response of the P cell to a single stimulus, i.e. two gratings that were presented together. For example, the left column shows the first-order responses of the P cell to an L-cone-isolating and an M-cone-isolating grating. These two gratings were presented simultaneously, and the response of the P cell to each grating was calculated from the single experimental record as described above. Notice that the L-cone (top) and M-cone (bottom) responses have opposite signatures in this colour-opponent cell, and that the time course of the L-cone response lags behind that of the M-cone response. In general, the first-order response of the P cell to a stimulus that isolates one class of cones in its receptive field has a peak around 45-55 ms, followed by an undershoot that gradually returns to baseline.
The middle column (top) shows the response of the same cell to an isoluminant grating and to a luminance grating presented simultaneously. In the isoluminant stimulus, the luminance levels of the L-cone-isolating and M-cone-isolating gratings were equal and opposite, so that there was only chromatic contrast and no luminance contrast in this stimulus. This purely chromatic (equiluminant) stimulus produces a more delayed response than the response to the luminance stimulus.
Finally, the right column shows the response of the P cell to an L + M grating and an achromatic (black-white) grating. The response here is noticeably more biphasic than the response to the isoluminance stimulus. The isoluminant and L + M gratings were both presented in combination with an achromatic grating (bottom, middle and right columns). Thus, the responses to the two achromatic gratings can be compared for consistency. Clearly, the responses to the achromatic gratings are similar to each other even when the overall compound stimulus is quite different. In addition, although the gratings in each of these conditions are different, the stimuli are always modulated around the same mean (50 % output of each gun). Thus, the difference in dynamics is not due to different levels of cone adaptation.
Figure 2 shows the responses of the same cell to higher spatial frequency gratings (2.181 c d−1) of the same type as those used to obtain the data in Fig. 1. In this series, the relative amplitudes of the L- and M-cone responses have changed. The L-cone response has become weaker, indicating that the L-cone mechanism, which is driving the surround region of the receptive field, is spatially larger. The response is reduced because the larger surround region averages out the bright and dark portions of the finer grating. The overall response to the achromatic gratings has the same signature (ON) as that of the M-cone response, which comes from the centre region. The second and third columns show that the response to the isoluminant grating has become relatively weaker at this higher spatial frequency compared with the responses to either the L + M or the achromatic stimulus. This relative attenuation of the chromatic response at higher spatial frequencies has been noted both physiologically and psychophysically (Ingling & Martinez-Uriegas, 1983; Mullen, 1985). Also, at this higher spatial frequency, the first-order response to an achromatic grating becomes less biphasic. The responses from P ON R+G- cells were also analysed and demonstrated the same relationships; however, the centre regions of these cells are driven by L cones, and their surround regions are predominantly driven by M cones (data not shown).
Figure 2. First-order responses of a P ON R-G+ cell (unit 29/14) to higher spatial frequency (SF) (2.181 c d−1) chromatic and achromatic gratings as above (same cell as in Fig. 1).

Left, the responses of the P cell (continuous line) and its target LGN cell (dashed line) to a high spatial frequency L-cone-isolating (top) and an M-cone-isolating (bottom) grating set at 0.25 contrast and presented simultaneously. Notice that the response to the L-cone grating is smaller at the higher spatial frequency. Middle, the response of the P cell-LGN cell pair to a high spatial frequency isoluminant and an achromatic (black-white) grating set at 0.25 contrast and presented simultaneously. Right, the response of the P cell-LGN cell pair to a high spatial frequency L + M grating and an achromatic grating set at 0.25 and presented simultaneously.
Figure 3 shows the responses of a P OFF R+G- cell to a low spatial frequency stimulus. For this cell, the signature of the response to the L- and M-cone-isolating stimuli is the opposite of that of the previous ON cell (Figs 1 and 2). The response to the achromatic grating is also inverted. However, the same relationship of the centre and surround responses still holds: the M-cone-driven centre response, which carries the sign of the overall response (OFF), slightly precedes the response of the surround. The response to the isoluminant stimulus is also more delayed than the response to the achromatic or L + M gratings.
These data (Figs 1–3) demonstrate that the dominant signature of a P-cell response is carried by a mechanism that is driven by cone-isolating stimuli, and precedes that of the opponent response that is driven by the opposite cone type. In addition, the data show that the chromatic mechanism that carries the signature of the dominant response tends to have a smaller spatial extent than its opponent mechanism, indicating that the ‘centre’ and ‘surround’ for the typical parafoveal P cell are chromatically opponent. Furthermore, the temporal responses of these two opposing mechanisms are similar except for a brief delay (see also Yeh et al. 1995). In total, we analysed in detail the responses of 20 (RG) P cells: 13 ON and 7 OFF cells, all of which conformed to this general pattern.
LGN responses
By recording in the LGN, we were able to observe S potentials (which reflect the activity of primate RGCs) together with the spikes of parvocellular LGN cells (Bishop et al. 1958; Kaplan & Shapley, 1984). In general, the responses of the LGN cells resembled those of the RGC driving them, except for a slight additional delay and decrease in response amplitude. The responses of the LGN cells are shown in Figs 1–3 as dotted lines. In comparison with RGC responses, the LGN cell responses are delayed and appear less biphasic (see also Benardete & Kaplan, 1997a); however, these cells share the same basic chromatic sensitivity profile.
Linearity of responses
The basic linearity of the responses of a typical P cell to contrast stimuli is illustrated in Fig. 4. The responses to the low spatial frequency L- and M-cone-isolating gratings are shown at three levels: 0.0625, 0.125 and 0.25. For example, the ‘0.25’ stimulus is 25 % of the maximal L-cone-isolating contrast that can be produced on the CRT. In addition, the response to both low and high spatial frequency achromatic gratings are shown at the same contrast levels. Unlike M cells, which modify their temporal frequency responses with increasing contrast (Benardete et al. 1992), P cells maintain the same temporal responses under conditions of low and high chromatic and achromatic contrast. Lee et al. (1994), who had used different techniques, reported similar results. At the highest luminance contrast, the peak of the response is less than one would predict from linearity; this behaviour probably reflects a truncation artifact due to the spike-generating mechanism or possibly saturation of spike generation (Victor, 1987). However, notice that the shape of the response (time-to-peak and zero crossings) remain the same with increasing contrast. In M cells, on the other hand, the shape of the response and time-to-peak of the response change with increasing contrast (Benardete & Kaplan, 1999).
Figure 4. The first-order responses of a P ON R-G+ cell (unit 29/14) to chromatic and achromatic gratings at several contrasts.

The chromatic gratings were L-cone-isolating and M-cone-isolating gratings set at 0.145 c d−1 (left). The achromatic gratings were black-white and set at 0.145 c d−1 (top row, right) and 2.181 c d−1 (bottom row, right). The contrasts were 0.0625 (thinnest line), 0.125 and 0.25 (thickest line). Notice that the time course of the P-cell responses changes depending on the chromatic/achromatic content of the stimulus; however, as contrast increases, the amplitude of the response scales linearly, with no change in the temporal characteristics of the response. At the highest levels of achromatic contrast, there is some response saturation, but there is no change in the time course of the P-cell response.
Model fits
The linear filter model (eqn (6)) was fitted to the first-order responses of P cells to chromatic and achromatic gratings. Typical fits are shown in Fig. 5. First, the model was fitted to the responses to cone-isolating gratings from 20 (RG) P cells. The parameters of the centre and surround responses were tabulated separately (Table 2 and Table 3). We compared the fitted parameters of ON and OFF P cells and P cells with centres driven by L or M cones, and found no significant differences among these various populations.
Figure 5. Typical linear model fits to the first-order responses of the P ON R-G+ cell (unit 29/14).

Top left, first-order response (thin line and •) to an L-cone-isolating grating (0.145 c d−1; 0.25 contrast) compared with the response of the linear model, eqn (6) (thick continuous line). The model parameters here were: A, 199.92 impulses (s unit contrast)−1; NL, 66; τL, 0.74 ms; H, 0.60; NH, 1; τH, 32.25 ms; D, 4.0 ms. Top right, first-order response (•) to an M-cone-isolating grating (0.145 c d−1; 0.25 contrast) along with the response of the linear model (continuous line). The model parameters were: A, 345.24 impulses (s unit contrast)−1; NL, 29; τL, 1.65 ms; H, 0.68; NH, 1; τH, 10.68 ms; D, 4.0 ms. Similarly, the response to an achromatic grating (0.25 contrast) at high spatial frequency (2.181 c d−1) (bottom left) and at low spatial frequency (0.145 c d−1) (bottom right) is shown along with the response of the fitted model (continuous lines). The model parameters at the higher spatial frequency were: A, 601.48 impulses (s unit contrast)−1; NL, 51; τL, 0.87 ms; H, 0.77; NH, 1; τH, 31.73 ms; D, 4.0 ms. For the lower spatial frequency the parameters were: A, 102.28 impulses (s unit contrast)−1; NL, 121; τL, 0.33 ms; H, 0.99; NH, 1; τH, 19.12 ms; D, 4.0 ms. The linear model provides an excellent fit at all stimulus conditions except for the low spatial frequency luminance conditions. The complex shape of this response indicates that a more elaborate (possibly non-linear) model might be needed to describe this response.
Table 2.
P-cell centre parameters (obtained with chromatic stimuli)
| Parameter | Type | Minimum | Maximum | Media | Mean | s.d. |
|---|---|---|---|---|---|---|
| A (impulses s−1) | ALL | 7.48 | 111.42 | 13.98 | 19.86 | 22.04 |
| ON | 7.48 | 111.42 | 13.7 | 122.87 | 27.11 | |
| OFF | 10.15 | 20.29 | 14.20 | 14.27 | 3.34 | |
| L | 7.48 | 27.22 | 13.35 | 14.50 | 5.22 | |
| M | 9.98 | 111.42 | 17.62 | 26.41 | 32.11 | |
| NL | ALL | 16.0 | 36.0 | 26.50 | 26.85 | 4.71 |
| ON | 16.0 | 36.0 | 26.0 | 26.92 | 4.65 | |
| OFF | 19.0 | 36.0 | 27.0 | 26.71 | 5.21 | |
| L | 22.0 | 36.0 | 26.0 | 27.10 | 3.70 | |
| M | 16.0 | 36.0 | 28.0 | 26.56 | 5.96 | |
| NLτL (ms) | ALL | 44.28 | 64.53 | 55.31 | 53.43 | 5.46 |
| ON | 44.28 | 64.53 | 55.33 | 53.80 | 5.25 | |
| OFF | 45.40 | 60.76 | 55.28 | 52.76 | 6.19 | |
| L | 44.28 | 57.62 | 52.25 | 51.52 | 4.68 | |
| M | 46.46 | 64.53 | 56.25 | 55.77 | 5.67 | |
| HS (dimensionless) | ALL | 0.41 | 0.94 | 0.68 | 0.68 | 0.13 |
| ON | 0.41 | 0.94 | 0.64 | 0.67 | 0.15 | |
| OFF | 0.56 | 0.79 | 0.74 | 0.71 | 0.09 | |
| L | 0.41 | 0.81 | 0.71 | 0.69 | 0.12 | |
| M | 0.44 | 0.94 | 0.64 | 0.68 | 0.15 | |
| τS (ms) | ALL | 1.78 | 51.85 | 21.25 | 22.19 | 12.36 |
| ON | 1.78 | 51.85 | 16.31 | 21.10 | 14.91 | |
| OFF | 14.60 | 30.86 | 26.65 | 24.22 | 5.63 | |
| L | 7.97 | 51.85 | 19.74 | 22.88 | 13.00 | |
| M | 1.78 | 39.90 | 22.40 | 21.35 | 12.25 |
Fitted parameters for the P-cell centre response. Responses from the P-cell centre were obtained with cone-isolating gratings (at maximal contrast and 0.145 cd−1) which stimulated the chromatic mechanism that had the signature of the overall response. For example, the centre of a P ON R+G- cell responds to an L-cone-isolating grating. The total number of (RG) P cells in the sample is 20. Thirteen cells were classified as ON and 7 cells were classified as OFF. Eleven cells had centres that were driven by L cones. Nine cells had centres that were driven by M cones. The parameters are given in eqn (6). The model parameters are also subclassified for ON, OFF, L- and M-centre cells. There were no statistical differences in these dynamic parameters among the various subpopulations (see text). Here and elsewhere, s.d. stands for standard deviation.
Table 3.
P-cell surround parameters (obtained with chromatic stimuli)
| Parameter | Type | Minimum | Maximum | Median | Mean | s.d. |
|---|---|---|---|---|---|---|
| A (impulses s−1) | ALL | 2.41 | 65.13 | 10.42 | 14.52 | 14.27 |
| ON | 2.41 | 43.12 | 10.44 | 12.63 | 9.69 | |
| OFF | 8.27 | 65.13 | 10.11 | 18.04 | 20.85 | |
| M | 2.41 | 65.13 | 9.65 | 14.29 | 17.11 | |
| L | 8.76 | 43.12 | 11.41 | 14.81 | 10.84 | |
| NL | ALL | 20.0 | 101.0 | 36.54 | 1.20 | 18.43 |
| ON | 27.0 | 101.0 | 39.0 | 46.15 | 20.64 | |
| OFF | 20.0 | 45.0 | 32.0 | 32.0 | 8.52 | |
| M | 20.0 | 101.0 | 32.0 | 39.45 | 22.78 | |
| L | 28.0 | 66.0 | 39.0 | 43.33 | 12.21 | |
| NLτL (ms) | ALL | 46.82 | 65.31 | 57.13 | 56.85 | 5.71 |
| ON | 46.82 | 65.31 | 55.88 | 56.35 | 5.38 | |
| OFF | 48.66 | 64.71 | 58.70 | 57.78 | 6.65 | |
| M | 46.82 | 61.31 | 55.88 | 55.10 | 4.56 | |
| L | 48.71 | 65.31 | 61.43 | 58.99 | 6.52 | |
| HS (dimensionless) | ALL | 0.48 | 0.90 | 0.61 | 0.65 | 0.13 |
| ON | 0.48 | 0.87 | 0.57 | 0.62 | 0.11 | |
| OFF | 0.51 | 0.90 | 0.72 | 0.72 | 0.15 | |
| M | 0.48 | 0.90 | 0.63 | 0.62 | 0.12 | |
| L | 0.53 | 0.90 | 0.59 | 0.68 | 0.14 | |
| τS (ms) | ALL | 2.04 | 40.92 | 24.41 | 23.88 | 11.99 |
| ON | 2.04 | 40.92 | 29.59 | 25.88 | 10.78 | |
| OFF | 2.94 | 38.21 | 16.33 | 20.16 | 14.07 | |
| M | 2.94 | 40.92 | 22.98 | 22.25 | 11.66 | |
| L | 2.04 | 38.21 | 30.93 | 25.85 | 12.77 |
Fitted parameters for the P-cell surround response. Responses from the chromatic surround were obtained with cone-isolating gratings (at maximal contrast and 0.145 cd−1) which stimulated the chromatic mechanism that carried the signature opposite to the overall response. For example, the surround of a P ON R+G- cell responds to an M-cone-isolating grating. The total number of (RG) P cells in the sample is 20. Thirteen cells were classified as ON and 7 as OFF. Eleven cells had surrounds that were classified as driven primarily by M cones. Nine cells had surrounds that were classified as driven primarily by L cones. The parameters are given in eqn (6). The parameters are also subclassified for ON- OFF, L- and M-centre P cells. The surround parameters were not significantly different among these populations.
The linear filter model was also fitted to the P-cell responses elicited by achromatic gratings whose spatial frequency was near the optimum for the cell. The parameters (data not shown) are similar to those from the fits to the chromatic centre-isolating responses. Figure 6 shows a typical fit of the linear filter model to P-cell response in the frequency domain. Notice that the model conforms well to the data.
Figure 6. The frequency response of a P ON R-G+ cell (unit 29/14) to L-cone isolating grating.

The fit of the linear filter model, eqn (6), is shown as a dashed line, while the Fourier-transformed response of the P cell is shown as a continuous line. The amplitude of the response and the model fit are shown in the top panel. The phase of the cell response and the model fit are shown in the bottom panel. The phase lag of -π radians has been removed for ease of comparison. The model parameters are: A, 199.92 impulses (s unit contrast)−1; NL, 66; τL, 0.74 ms; H, 0.60; NH, 1; τH, 32.25 ms; D, 4.0 ms. Note the good agreement between the actual response and the model fit in the frequency domain.
Figure 7 shows the population average of the fitted linear frequency model for the chromatically stimulated centre and surround. Notice that the gain curves (top) for the centre and for the surround are very similar to each other. The phase data show that the surround lags behind the centre, and that this delay increases with temporal frequency. These data demonstrate that, dynamically, the chromatic surround is similar to the chromatic centre, except for the additional delay of a few (∼3) ms (see also Smith et al. 1992 and Benardete & Kaplan, 1997a). The approximate ‘time-to-peak’ for the chromatic centre and chromatic surround fits were calculated as NLτL, the number of low-pass stages multiplied by the time constant of the low-pass stages, τL. This parameter allowed us to calculate a centre-surround delay for each cell by subtracting the centre time-to-peak from the surround time-to-peak (Table 4). This delay was calculated for each population of cells (ON-centre, OFF-centre, L-centre, M-centre) and the total population. The average delay for the total population was 3.42 ms.
Figure 7. Frequency characteristics of the median centre (continuous line) and surround (dashed line) responses derived from the population parameters in Tables 2 and 3.

Centre parameters: NL, 27; τL, 2.05 ms; H, 0.68; NH, 1; τH, 21.25 ms. Surround parameters: NL, 37; τL, 1.54 ms; H, 0.61; NH, 1; τH, 24.41 ms. The gain, A, was set to 1 for both the centre and the surround for purposes of comparison. Notice that the frequency response of the centre and surround are very similar, except for the slight phase delay of the surround with respect to the centre.
Table 4.
Centre–surround delay (ms)
| Parameter | Type | Minimum | Maximum | Median | Mean | s.d. |
|---|---|---|---|---|---|---|
| Delay (ms) | ALL | −2.59 | 11.27 | 3.26 | 3.42 | 2.99 |
| ON | −2.59 | 5.88 | 2.54 | 2.55 | 2.54 | |
| OFF | 2.24 | 11.27 | 3.26 | 5.02 | 3.29 | |
| L | −2.59 | 11.27 | 3.26 | 3.58 | 3.49 | |
| M | −0.10 | 6.84 | 3.26 | 3.23 | 2.44 |
The total number of (RG) P cells in the sample is 20; 13 ON and 7 OFF cells. Eleven cells had centres that were driven by L cones. Nine cells had centres that were driven by M cones. The centre-surround delay was calculated as the difference between NLτL surround and NLτL centre. In 2 cells, the calculated time-to-peak of the surround response was less than that of the centre. Noise in the responses of these 2 cells probably accounts for the negative values. The median value of the delay was 3.26. There were no significant differences in the ON-, OFF, L- and M-centre populations.
Prediction of P-cell chromatic and achromatic responses
P cells are widely believed to sum their cone inputs linearly, and their linearity is critical for theories about their function and for the interpretation of some physiological experiments (e.g. Ingling & Martinez-Uriegas, 1983; Derrington et al. 1984). The first-order responses of P cells to the L- and M-cone-isolating gratings can be used to try to predict the responses of the same cells to other stimuli, based on simple assumptions that are consistent with linear P-cell models (e.g. Ingling & Martinez-Uriegas, 1983; Derrington et al. 1984; Mullen & Kingdom, 1991). We illustrate here three typical examples of such a prediction (Fig. 8). Consider a P cell that sums linearly the inputs it receives from L cones and from M cones. Stimuli that excite a combination of L and M cones will theoretically produce a response that is a linear combination of the responses to pure L- and M-cone-isolating stimuli. Since each stimulus used in these studies produces a known amount of L- and M-cone stimulation (see Methods), we can compare the linear predictions with the measured responses (Fig. 8).
Figure 8. Comparison of first-order responses.

First-order responses to chromatic and achromatic gratings (continuous lines) and the predictions of the linear model (dashed lines) were compared for P ON R-G+ cell (unit 36/7) (A), P OFF R+G- cell (unit 29/8) (B) and P ON R+G- (unit 27/24) (C). Both stimuli were low spatial frequency (0.145 c d−1). Both the luminance and isoluminant responses are shown at 0.125 (lower continuous curve) and 0.25 contrast (upper continuous curve). The isoluminant prediction for the 0.25 condition (top row, upper dashed curve) is generated by summing 0.387 × model response to an L-cone-isolating grating at 0.25 contrast and the same spatial frequency and -0.6875 × model response to an M-cone-isolating grating at 0.25 contrast. The achromatic prediction for the 0.25 condition (bottom row, upper dashed curve) is obtained by summing 6.25 × model response to an L-cone-isolating grating and 6.25 × model response to an M-cone-isolating grating. Similarly, the predictions for the 0.125 condition were generated by scaling the prediction by 0.5 (top and bottom rows, lower dashed curve). Note that in all 3 cells, the response to the isoluminant (chromatic) stimulus is well predicted by the linear model at both the 0.125 and 0.25 conditions, while the response to the achromatic stimulus is overestimated by the linear prediction at both the 0.125 and 0.25 conditions. This effect occurs at response levels well below saturation and indicates that the P cell does not simply ‘add’ its cone inputs under these conditions.
For a P ON R-G+ cell, the linear prediction for the isoluminant response is in excellent agreement with the data (Fig. 8, top left). This is a typical result for isoluminant stimuli. However, the prediction of responses to achromatic stimuli typically overestimates the size of the actual response (Fig. 8, bottom left). Figure 8 (middle) also shows the same typical results for a P OFF R+ G- cell and for a P ON R+ G- cell (right). The responses of these cells were not saturating under the achromatic condition, and this indicates that the failure of linear prediction is not due to an over-modulation of the response of the cell. Fifteen cells were tested this way, and all showed this general pattern, namely that the responses to achromatic stimuli were overestimated by the linear prediction. The failure of this linear prediction is not due to S-cone input, because the (RG) P cells that we have tested failed to give significant responses to S-cone-isolating stimuli (data not shown; see also Smith et al. 1992). In addition, the predictions are compared at two levels of chromatic and achromatic contrast. The linear model overestimated the response to luminance contrast even at the lower level of contrast, demonstrating that the effect is not due to response saturation.
DISCUSSION
Chromatic and achromatic dynamics
Previous work on the LGN showed that most or all the parvocellular cells are spatially and chromatically opponent (Wiesel & Hubel, 1966; DeValois et al. 1966; Derrington et al. 1984). However, those studies provided little dynamical information about the responses of P retinal ganglion cells to chromatic stimuli. In this paper, we have used a mathematical model to quantify the dynamics of P cells. Using our technique, the mean peak of the centre response of the P-cell population is reached approximately 53 ms after stimulus onset. The peak of the surround response lags the centre response by approximately 3 ms.
Previous work has reported on the dynamics of P cells only indirectly. Gielen et al. (1982), who used white-noise methods, calculated the first-order kernels of parvocellular units in the LGN at different spectral wavelengths. They also used the silent substitution method and calculated the time courses for the L-, M- and S-cone input to parvocellular neurons, but they used only full-field stimuli in their experiments. Although they gave no population statistics, the peak of the centre response appears to have been between 45 and 50 ms. Recently, Maunsell et al. (1999) reported on the visual response latencies of parvocellular LGN neurons; from their graph, the peak appears to be around 50 ms for pure luminance stimuli, a value which is close to our results. Yeh et al. (1995) reported that the peak of the responses averaged for both M and P cells was between 30 and 36 ms. However, our data have shown that the peak of the M-cell response precedes that of P cells by approximately 10 ms (Benardete & Kaplan, 1999).
Reid & Shapley (1992) used an m-sequence modulated checkerboard to examine more fully the spatial extent of the cone-driven inputs to P cells. Their results suggested that the centre and surround of P cells are each driven by a single, different cone type. However, their data lacked fine temporal detail. Yeh et al. (1995) found that the temporal frequency responses of P cells to full-field cone-isolating stimuli were similar for L-, M- and S-cone-isolating inputs. The work we report here strikes a balance between fine temporal and spatial detail, and provides a more complete spatiotemporal characterization of P-cell responses to chromatic stimuli.
Several groups have tried to determine the cone inputs to P cells. Gielen et al. (1982) used a linear regression model, based on known psychophysical spectral sensitivities of human photoreceptors, to predict the responses to other monochromatic stimuli. Derrington et al. (1984) used an elegant technique to quantify the chromatic opponency of P cells, and concluded that the P cell sums its cone inputs linearly. For instance, they reported that (RG) P cells get input almost exclusively from a linear summation of L- and M-cone input. Lee et al. (1989) obtained similar results using linear techniques. The results of these studies showed that simple linear models of the P cell could predict the responses to relatively weak chromatic stimuli. Linearity has been emphasized because of the historical background in human psychophysics on colour perception (Ingling & Martinez-Uriegas, 1983; Mullen & Kingdom, 1991; Lee et al. 1993). Our data, while consistent with the previous experimental work, also indicate that responses to larger achromatic stimuli are not well predicted by linear models. The actual responses are smaller than the linear predictions, and this suggests the involvement of a non-linear control mechanism.
Differences between the P and M streams
Much evidence suggests that the M and P pathways form the substrate for separate luminance and chromatic channels in psychophysics, respectively (Livingstone & Hubel, 1988; reviewed in Kaplan et al. 1990). Although it is clearly an oversimplification, the parallels between the psychophysical and physiological results are quite suggestive. M cell centres are less delayed, more transient, and more sensitive than P cell centres, an observation that agrees with the high sensitivity of the visual system to luminance flicker. The chromatic system is relatively slower and more spatially low-pass than the luminance system, like the responses of P cells to chromatic stimuli. However, it should be noted that in the data shown here, P cells were responsive to achromatic stimuli that have a large amount of luminance contrast. Therefore, P cells probably contribute to luminance detection and discrimination under most conditions (Wassle & Boycott, 1991; Lennie et al. 1993).
Cone inputs to centre and surround
The relationship between the anatomical organization and the physiology of the retina is still incompletely understood. It is clear that, near the fovea, midget bipolar cells that appear to be the anatomical correlates of P cells conduct the signal from a single cone to a midget ganglion cell. This relationship can account for the chromatic specificity of the centre mechanism, but under some experimental conditions, the P-cell surround also seems to carry the signature of a single cone type (e.g. Wiesel & Hubel, 1966; Reid & Shapley, 1992; but compare Cottaris et al. 1996). We investigated the dynamics of P cells with cone-isolating, chromatic and achromatic gratings in order to understand how colour and luminance affect the overall P-cell response. Although we have not performed detailed spatial analysis here, our data show that the peak amplitude of the centre response was always greater than that of the surround response. As the spatial frequency of the stimulus increased, the amplitude of the centre response decreased monotonically, suggesting that there is no spatial tuning of the centre response, i.e. there is minimal input to the surround of the centre cone type.
The average delay between the centre and surround responses, 3.42 ms, is less than we have found with spots and annuli in a previous study (∼7-8 ms) (Benardete & Kaplan, 1997a). This suggests that the delay from regions of the surround that are stimulated by an annulus is greater than the average delay of the entire surround. Low spatial frequency chromatic gratings can stimulate the whole chromatically opponent surround, part of which probably overlaps the centre (see Reid & Shapley, 1992).
It is interesting to speculate about the possible anatomical organization of the P-cell receptive field. Horizontal cells in the outer plexiform layer receive inputs from a mixed group of cones within their dendritic fields (Boycott et al. 1987; Dacheux & Raviola, 1990; Dacey et al. 1996). Furthermore, recent recordings from midget bipolar cells in vitro (D. M. Dacey, personal communication) show them to have a surround mechanism that receives input from both L and M cones. Therefore, if a chromatically pure opponent surround (or surround component) exists, amacrine cells impinging on ganglion cells at the level of the IPL would have to generate it. However, an electron microscopic examination of the synaptic inputs to midget ganglion cells showed that they receive as much non-specific synaptic input from amacrine cells as they do from bipolar cells (Kolb & DeKorver, 1991; Calkins & Sterling, 1996). The cone-horizontal-bipolar circuit may be involved in generating dynamic regulation of P-cell centre sensitivity (see Benardete & Kaplan, 1997b), and this control mechanism is probably broadband, as are the H1 horizontal cells (Dacey et al. 1996). The ‘chromatically pure’ surround seen in some physiological recordings from ganglion cells and LGN cells is probably due to chance connections that the various components of the P-cell receptive field make with the random mosaic of L and M cones. This mosaic includes numerous regions where neighbouring cones are of the same type (Roorda & Williams, 1999).
Sub-classes of P cells?
Calkins et al. (1994) have shown that there are two anatomically distinct subpopulations of bipolars impinging on midget ganglion cells in the primate retina, one with roughly 30 synapses and the other with roughly 50 synapses. They have proposed that there could be two distinct midget ganglion cells populations corresponding, perhaps, to the L- or M-cone-dominated centre types. Based on these results, one might have expected to see two cell groups emerge in our physiological data as well. However, our population data do not suggest any such differences in either contrast gain or in dynamics among (RG) P cells with respect either to centre type or ON/OFF, and the relationship between the anatomical data (Calkins et al. 1994) and our physiological measurements remains unclear.
Non-linear responses
We have previously proposed that the non-linear responses of the P cell may be the signature of a gain control mechanism that is unique to the P cell (Benardete & Kaplan, 1997b). Preliminary results from our laboratory (Kaplan & Shapley, 1989; Shapley & Kaplan, 1990) suggested that the gain of P cells was (at least partly) regulated by surround illumination. Interestingly, M cells, like cat RGCs (Enroth-Cugell et al. 1975), do not show this effect. In P cells, a steadily illuminated annulus reduces the gain of a P ON cell to a flickering spot, and larger annuli cause greater reduction in the gain than smaller annuli (Kaplan & Shapley, 1989; Shapley & Kaplan, 1990; Benardete & Kaplan, 1997b). Under conditions of achromatic stimulation, when the P-cell response is in danger of becoming saturated, the non-linear components of the response are strongest, suggesting that a non-linear mechanism may regulate the P-cell gain in order to prevent response saturation.
Conclusions
In this paper we have investigated the responses of P cells to chromatic stimuli using a novel method of systems analysis - the multiple m-sequence method. We have shown that the P-cell centre and surround mechanisms respond with similar dynamical characteristics to chromatically specific stimuli. The surround response is slightly delayed compared with the centre response. The dynamics of the P-cell response depend strongly on the chromatic and achromatic content of the stimulus. We also show that parvocellular LGN cells share similar chromatic and achromatic responses with their RGC inputs. We demonstrated that the first-order P-cell responses increase linearly with chromatic or achromatic contrast except near saturation, although cone inputs are not summed linearly by the P cell, making the linear prediction of the response to achromatic stimuli inaccurate.
Acknowledgments
This work was supported by NIH grants EY 4888 and EY 1428. E. K. is the Jules & Doris Stein Research to Prevent Blindness Professor at the Ophthalmology Department of the Mount Sinai School of Medicine. The authors would also like to thank Dr J. D. Victor and B. Knight for many useful suggestions and assistance.
References
- Benardete EA, Kaplan E. The dynamics of primate M retinal ganglion cells. Visual Neuroscience. 1999;16:355–368. doi: 10.1017/s0952523899162151. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. The receptive field of the primate P retinal ganglion cell I: Linear dynamics. Visual Neuroscience. 1997a;14:169–185. doi: 10.1017/s0952523800008853. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. The receptive field of the primate P retinal ganglion cell II: Nonlinear dynamics. Visual Neuroscience. 1997b;14:187–205. doi: 10.1017/s0952523800008865. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Visual Neuroscience. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Victor JD. An extension of the m-sequence technique for the analysis of multiple-input nonlinear systems. In: Marmarelis VZ, editor. Advanced Methods of Physiological Systems Modelling. Vol. 3. New York: Plenum Press; 1994. pp. 87–110. [Google Scholar]
- Bishop PO, Burke W, Davis R. Synapse discharge by single fibre in mammalian visual system. Nature. 1958;182:728–730. doi: 10.1038/182728b0. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Hopkins JM, Sperling HG. Cone connections of the horizontal cells of the rhesus monkey's retina. Proceedings of the Royal Society. 1987;229:345–379. doi: 10.1098/rspb.1987.0001. B. [DOI] [PubMed] [Google Scholar]
- Calkins DJ. University of Pennsylvania; 1994. Microcircuitry of M and L cone midget ganglion cell pathways in the primate fovea. Ph.D. thesis. [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in the macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Cottaris NP, DeValois RJ, Elfar SD, Rojers D. Cone inputs to center and surround receptive field regions of monkey LGN neurons. Investigative Ophthalmology and Visual Science. 1996;37:S4855. [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Physiology of H.I. horizontal cells in the primate retina. Proceedings of the Royal Society. 1990;239:213–230. doi: 10.1098/rspb.1990.0014. B. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. The Journal of Physiology. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeValois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. Journal of the Optical Society of America. 1966;56:966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- DeValois RL, Morgan HC, Polson MC, Mead WR, Hull EM. Psychophysical studies of monkey vision - I. Macaque luminosity and color vision tests. Vision Research. 1974a;14:53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- DeValois RL, Morgan HC, Snodderly DM. Psychophysical studies of monkey vision - III. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Research. 1974b;14:75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Lennie P, Shapley RM. Surround contribution to light adaptation in cat retinal ganglion cells. The Journal of Physiology. 1975;247:579–588. doi: 10.1113/jphysiol.1975.sp010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez O, Spekreijse H. Relationship between pattern appearance-disappearance and pattern reversal responses. Experimental Brain Research. 1974;19:233–238. doi: 10.1007/BF00233231. [DOI] [PubMed] [Google Scholar]
- Estévez O, Spekreijse H. The ‘silent substitution’ method in visual research. Vision Research. 1982;22:681–691. doi: 10.1016/0042-6989(82)90104-3. [DOI] [PubMed] [Google Scholar]
- Gielen CCAM, van Gisbergen JAM, Vendrik AJH. Reconstruction of cone-system contributions to responses of colour-opponent neurones in monkey lateral geniculate. Biological Cybernetics. 1982;44:211–221. doi: 10.1007/BF00344277. [DOI] [PubMed] [Google Scholar]
- Golomb SW. Shift Register Sequences. San Francisco: Holden-Day, Inc.; 1968. [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. The Journal of Physiology. 1968;199:533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingling CR, Martinez-Uriegas E. The relationship between spectral sensitivity and spatial sensitivity in the primate r-g X-channel. Vision Research. 1983;23:1495–1500. doi: 10.1016/0042-6989(83)90161-x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Lee BB, Shapley RM. New views of primate retinal function. In: Osborne N, Chader J, editors. Progress in Retinal Research. Vol. 9. New York: Pergamon Press; 1990. pp. 273–336. [Google Scholar]
- Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. The Journal of Physiology. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley R. The origin of the S (slow) potential in the mammalian lateral geniculate nucleus. Experimental Brain Research. 1984;55:111–116. doi: 10.1007/BF00240504. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proceedings of National Academy of Sciences of the USA. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. Illumination of the receptive field surround controls the contrast gain of macaque P retinal ganglion cells. Society for Neuroscience Abstracts. 1989;15(174):75.1. [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. Journal of Computational Neurology. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Lee BB. Receptive field structure in the primate retina. Vision Research. 1996;36:631–644. doi: 10.1016/0042-6989(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. The Journal of Physiology. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A, Kremers J. Physiological mechanisms underlying psychophysical sensitivity to combined luminance and chromatic modulation. Journal of the Optical Society of America A. 1993;10:1403–1412. doi: 10.1364/josaa.10.001403. [DOI] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Kremer J. Responses to pulses and sinusoids in macaque ganglion cells. Vision Research. 1994;34:3081–3096. doi: 10.1016/0042-6989(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Lennie P, Pokorny J, Smith VC. Luminance. Journal of the Optical Society of America A. 1993;10:1283–1293. doi: 10.1364/josaa.10.001283. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Marmarelis PZ, Marmarelis VZ. Analysis of Physiological Systems: The White Noise Approach. New York: Plenum Press; 1978. [Google Scholar]
- Maunsell JHR, Ghose GM, Assad JA, McAdams CJ, Boudreau CE, Noerage BD. Visual response latencies of magnocellular and parvocellular LGN neurons in macaque monkeys. Visual Neuroscience. 1999;16:1–14. doi: 10.1017/s0952523899156177. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Medical and Biological Engineering. 1972;10:662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Milkman N, Schick G, Rossetto M, Ratliff F, Shapley R, Victor J. A two-dimensional computer-controlled visual stimulator. Behavior Research Methods and Instrumentation. 1980;12:283–292. [Google Scholar]
- Mullen KT. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. The Journal of Physiology. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KT, Kingdom FAA. Colour contrast in form perception. In: Gouras P, editor. Vision and Visual Dysfunction, The Perception of Colour. Vol. 6. New York: Macmillan Press; 1991. pp. 198–217. [Google Scholar]
- Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Reid RC, Shapley RM. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992;356:716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The primate retina. In: Horst D, Erwin J, editors. Comparative Primate Biology, Neurosciences. Vol. 4. New York: Alan R. Liss; 1988. pp. 203–278. [Google Scholar]
- Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–52. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- Rushton WAH, Spitzer PD, White KD. The spectral sensitivity of ‘red’ and ‘green’ cone in the normal eye. Vision Research. 1973;13:2017–2031. doi: 10.1016/0042-6989(73)90178-8. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. The Journal of Physiology. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Kaplan E. Tonic suppressive interactions between center and surround in P ganglion cells and parvocellular neurons. Investigative Ophthalmology and Visual Science. 1990;31(88):434. suppl. [Google Scholar]
- Shapley RM, Victor JD. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. The Journal of Physiology. 1981;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Lee BB, Pokorny J, Martin PR, Valberg A. Responses of macaque ganglion cells to the relative phase of heterochromatically modulated lights. The Journal of Physiology. 1992;458:191–221. doi: 10.1113/jphysiol.1992.sp019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Research. 1975;15:161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Sutter EE. A deterministic approach to nonlinear systems analysis. In: Pinter RB, Nabet B, editors. Nonlinear Vision: Determination of Neural Receptive Fields, Function, and Networks. Boca Raton: CRC Press; 1992. pp. 171–220. [Google Scholar]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiological Reviews. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. Journal of Neurophysiology. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Yeh T, Lee BB, Kremers J. Temporal response of ganglion cells of the macaque retina to cone-specific modulation. Journal of the Optical Society of America A. 1995;12:456–464. doi: 10.1364/josaa.12.000456. [DOI] [PubMed] [Google Scholar]
- Victor JD. The dynamics of the cat retinal X cell centre. The Journal of Physiology. 1987;386:219–246. doi: 10.1113/jphysiol.1987.sp016531. [DOI] [PMC free article] [PubMed] [Google Scholar]


