Abstract
Receptor current and spiking responses were recorded simultaneously from isolated frog olfactory receptor cells using the suction pipette technique. Cells were stimulated with the odour cineole by rapid exchange of the solution bathing the olfactory cilia.
The receptor current response to a 1 s odour stimulus increased in a graded manner over a 300-fold range of odour concentration without clear saturation, and was accompanied by a train of action potentials. As the concentration of the odour stimulus increased, the frequency of firing increased also, until it saturated at the highest concentrations. The number of spikes evoked by the stimulus first increased and then decreased with increasing concentration, reaching a maximum at intermediate odour concentrations. The dose-response relation for spike firing rose at lower odour concentrations than the dose-response relation for the receptor current response.
Adaptation to steady odour stimuli was investigated by exposing the cilia to a 4 s odour pre-pulse and then to a 1 s odour test pulse. As the pre-pulse concentration was increased the dose-response relations derived from the receptor current and spiking responses shifted to higher absolute test pulse concentrations. However the number of spikes fired in response to a given test pulse was little affected by the pre-pulse until, at the highest pre-pulse concentrations spike firing was abolished despite the continued presence of a receptor current response.
The sensitivity of the receptor-current response to incremental stimuli fell with increasing pre-pulse concentration, declining with a limiting slope of 2.4 in double logarithmic co-ordinates. The sensitivity determined from the spiking responses declined to zero at a lower pre-pulse concentration, reflecting the abolition of spike firing at pre-pulse concentrations which still evoked a graded receptor-current response.
Amphibian olfactory receptor cells respond to stimulation with an inward-receptor current (Firestein & Werblin, 1989; Kurahashi, 1989), which leads to depolarisation of the cell (Trotier & MacLeod, 1983; Anderson & Hamilton, 1987; Firestein et al. 1990) and the firing of action potentials (O'Connell & Mozell, 1969; Mathews, 1972; Getchell & Shepherd, 1978a) which are conveyed to the olfactory bulb. Stimulation with high odour concentrations leads to a progressive and sometimes complete decline in action potential amplitude during the odour-induced spike train (Shibuya & Shibuya, 1963; Mathews, 1972; van Drongelen, 1978; Trotier & MacLeod, 1983; Trotier, 1994), which has been suggested to reflect progressive inactivation of voltage-gated Na+ channels during the odour-induced depolarisation (Trotier, 1994). While whole-cell patch clamp recordings from olfactory receptor cells have demonstrated that the odour-induced receptor current follows a steep dose-response relation (Firestein et al. 1993), the relationship between the receptor current and action potential firing has hitherto received relatively little attention (Trotier, 1994).
The adaptation of the response to a steady stimulus is a common feature of sensory systems (for review see Shapley & Enroth-Cugell, 1984; Torre et al. 1995). Classically, adaptation is often regarded as the effect of steady stimulation on the stimulus-response relation for superimposed brief stimuli. However, previous studies of olfactory adaptation have concentrated instead on the related phenomena of the relaxation in the response to steady stimulation, and the recovery of the odour response after stimulation (Baylin & Moulton, 1979; Kurahashi & Shibuya, 1990; Kurahashi & Menini, 1997; Zufall & Leinders-Zufall, 1997; Reisert & Matthews, 1998b). We have therefore investigated the effect of adaptation on the dose-response relation to brief stimuli.
The suction pipette technique (Baylor et al. 1979; Lowe & Gold, 1991) was chosen to investigate these unresolved questions for two reasons. First, because the cell's intracellular potential is free to vary, the suction pipette technique simultaneously collects not only the receptor current, but also the biphasic action currents which underlie the action potential. Second, this technique allows recordings of much greater duration than can normally be achieved in the whole-cell patch configuration (Frings & Lindemann, 1988; Dionne, 1992; Zhainazarov & Ache, 1995). We have therefore used the suction pipette technique in combination with multiple rapid solution changes to examine adaptation in olfactory cells systematically, and to relate the action potential responses to odour stimuli to the receptor currents which evoke them. Preliminary results of this study have been communicated to the Physiological Society (Reisert & Matthews, 1998a).
METHODS
Frogs (Rana temporaria) were killed by rostral and caudal pithing. The basal olfactory epithelia were dissected and placed receptor side up on a layer of cured silicone rubber (Sylgard 184, Dow Corning, Wiesbaden, Germany) in a Petri dish filled with Ringer solution. Olfactory receptor cells were isolated mechanically by lightly cutting the olfactory epithelium with a piece of razor blade. The dissociated cells were collected with a 200 μl pipette and transferred to the recording chamber on the stage of an inverted microscope with phase contrast optics (Nikon TMS; Nikon Ltd, Kingston, UK). Cells were allowed to settle on the floor of the recording chamber for 30 min before bath perfusion commenced.
Electrical recording
The suction pipette technique was used to record odour-induced electrical responses (Baylor et al. 1979; Lowe & Gold, 1991). The cell body of an isolated olfactory receptor cell was drawn into a suction pipette leaving the cilia exposed to the superfusing solution. Following their isolation, olfactory receptor cells rounded progressively and the dendrite retracted, as has also been observed by others (Dubin & Dionne, 1994). Consequently, virtually the entire cell could be drawn into the suction pipette, so that only the cilia were accessible to solution changes in the bath. The suction pipette current was recorded with a patch clamp amplifier (Warner PC-501A, Warner Instruments, Hamden, CT, USA) and digitised over a relatively low bandwidth (filtered DC-20 Hz, sampled at 100 Hz) by an IBM PC-compatible microcomputer equipped with an intelligent interface card (Cambridge Research Systems, Rochester, UK) in order to analyse the receptor current. In addition, the current signal was recorded over a wider bandwidth by a modified digital audio tape recorder (DTC-1000, Sony, Tokyo, Japan; modified for DC-8 kHz bandwidth) and subsequently digitised at a higher sampling rate (filtered DC-500 Hz, sampled at 1000 Hz) to resolve the action currents accompanying action potential firing. The seal resistance between the cell body and the suction pipette was between 2 and 7 MΩ, which corresponds to a Johnson noise of 1-2 pA root mean square over this bandwidth. Traces are plotted according to the convention that current flowing into the suction pipette is of negative sign. Since the suction pipette collects current from the cell body, this sign convention has the consequence that the inward receptor current flowing across the ciliary membrane is represented correctly as a negative (or inward) current, but that the action currents across the membrane of the soma are inverted.
External solutions and solution changes
Ringer solution contained (mM): 111 NaCl, 2.5 KCl, 1.6 MgCl2, 1 CaCl2, 0.01 EDTA, 3 Hepes, 10 glucose, pH adjusted to 7.7 with NaOH. Odour solutions were made daily by a single dilution from a stock solution containing 1 mM cineole in Ringer solution. Successive odour concentrations normally increased in a 1-2-5 sequence, which was chosen to meet the conflicting demands of adequately resolving the dose-response relation and keeping the total number of solutions, and hence the duration of each experiment, within reasonable bounds. Cineole concentrations above 300 μm were not used, due to concerns about possible adsorption to the walls of the perfusion system.
Rapid solution changes for odour stimulation were carried out by stepping the interface between parallel streams of flowing solution across the tip of the suction pipette (Hodgkin et al. 1985). Four streams of solution emerged from grooves cut into the back of the recording chamber. The chamber was stepped sideways under computer control using a miniature stepper motor (Matthews, 1995) allowing multiple solution changes to be carried out. The time course of the solution change was typically around 70 ms, measured from the junction current evoked by stepping the cell between solutions of different ionic composition. Solutions were delivered by gravity, and selected by inert 6-way rotary valves (Rheodyne, Cotati, CA, USA).
Data reduction and analysis
The times of arrival of individual action potentials were extracted from wide-bandwidth suction pipette current recordings low-pass filtered at 500 Hz and sampled at 1000 Hz. These digitised current traces were imported into Matlab (The Mathworks Inc., Natick, MA, USA) and digitally high-pass filtered at corner frequencies ranging from 5 to 20 Hz to isolate action potentials from the slower underlying receptor-current response. All events which exceeded a threshold level set just above the baseline noise were tentatively assigned as spikes; individual traces were subsequently re-examined to ensure that no spurious events were included and that no spikes with small amplitudes were omitted. The instantaneous firing frequency was calculated for each spike as the reciprocal of the mean of the two time intervals between that spike, its predecessor, and its successor. This time-symmetrical procedure is equivalent to smoothing the instantaneous firing frequency over two inter-spike intervals. For the first and last action potentials in each sweep, the initial and final inter-spike intervals were set relative to the start and end of the imported sweep respectively.
RESULTS
Responses to brief odour stimuli
Figure 1 shows responses collected by the suction pipette from an isolated olfactory receptor cell stimulated by a 1 s odour exposure of progressively increasing concentration. When the cell was exposed to 1 μm cineole, no response was generated, while 2 μm cineole elicited a short train of action potentials after a considerable delay. When the cineole concentration was increased to 5 μm a longer spike train of higher frequency was evoked after a shorter latency, and the underlying receptor current became clearly visible. The rising receptor current was accompanied by a rapid decline in spike amplitude, probably reflecting progressive inactivation of the voltage-gated Na+ conductance (Trotier, 1994). As the odour concentration was raised further the spike firing frequency progressively increased, and the latency steadily declined. The number of spikes evoked by the stimulus reached a maximum at intermediate odour concentrations but then declined again to only two or three spikes at the highest stimulus concentrations. We also observed a similar development of the response with increasing odour concentration when recording spike firing from individual olfactory cilia in the intact olfactory epithelium using a sucked cilium technique (Frings & Lindemann, 1990). The basal spike rate was 0.3 Hz (mean of 31 cells; maximum basal spike rate 3 Hz). Inhibitory responses in the form of a reduction in the basal spike rate or an outward receptor current (Gesteland et al. 1965; Getchell & Shepherd, 1978b; Morales et al. 1994) were never observed (> 180 cineole-responsive cells tested).
Figure 1. Responses of an olfactory receptor cell to odour stimuli of increasing concentration recorded with the suction pipette technique.
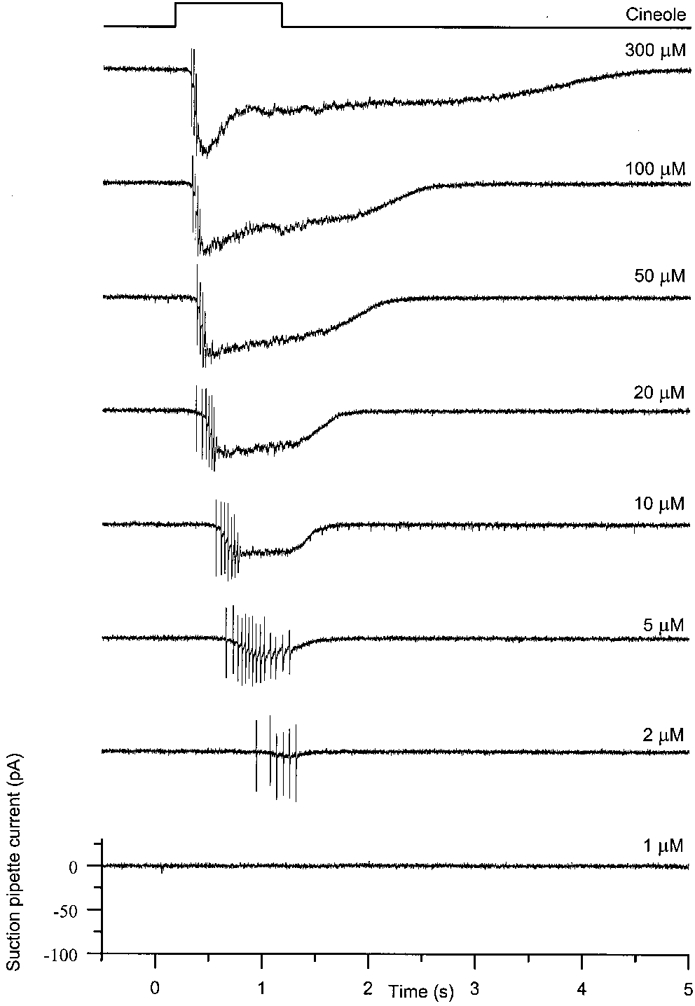
An isolated olfactory receptor cell was stimulated for 1 s by rapid superfusion with Ringer solution containing the odour cineole at concentrations ranging from 1-300 μm, as indicated beside each trace. The suction pipette current was recorded over a wide bandwidth (D/C 500 Hz). Top trace represents the timing of the solution change.
The dose-dependent development of the receptor current was investigated by low-pass filtering the suction pipette current at 20 Hz to exclude the fast current spikes which correspond to action potentials. Figure 2A shows a superimposed family of such filtered and averaged records obtained from the cell of Fig. 1. At low odour concentrations the receptor current had a relatively symmetrical waveform, but as the odour concentration was increased further, the response developed more complex kinetics exhibiting an initial peak followed by a maintained plateau which outlasted the end of stimulation by several seconds. These responses have been analysed in Fig. 2B by plotting as functions of odour concentration the magnitude of the receptor current measured at the response peak (□), and also at a fixed time of 1.05 s after stimulus onset which corresponds to the plateau phase of the response (▪). Over the 300-fold range of odour concentration used in these experiments, the peak current rose monotonically without a clear sign of saturation. In contrast, the plateau current saturated at the highest odour concentrations, yielding a maximum current of around 50 pA in this cell. In early experiments three highly sensitive cells displayed saturation of the peak receptor current when exposed to 300 μm cineole. However, since these cells still generated 50-80 % of their maximal current at the lowest available odour concentrations (2 or 5 μm), full dose-response relationships could not be constructed.
Figure 2. Receptor current responses of an olfactory receptor cell.
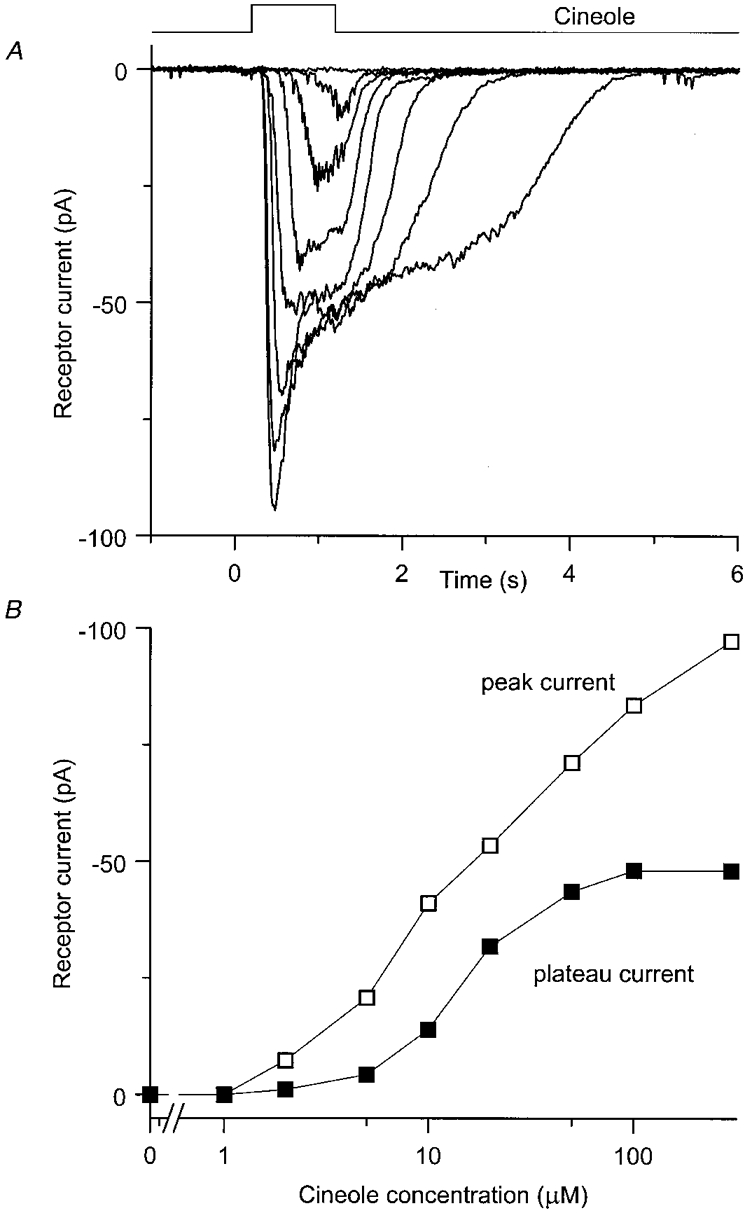
A, superimposed suction pipette current responses to 1 s stimulation with cineole at concentrations of 1, 2, 5, 10, 20, 50, 100 and 300 μm, low-pass filtered at 20 Hz so as to display only the receptor current. Each trace is the mean of two trials; same cell as Fig. 1. Top trace represents the timing of the solution change. B, dose-response relations derived from the data of A.□, measured at response peak; ▪, represent plateau current, measured at 1.05 s after stimulus onset.
Results similar to those shown in Figs 1 and 2 were obtained from a total of six cells. From these raw data were derived values for each cell of the peak receptor current and spike firing frequency, the number of spikes fired, the latency to the first spike, and the time to the response peak as a function of odour concentration. In order to allow comparison between individual cells, the data have been normalised in odour concentration using the following procedure. First, the relationship between spike firing frequency F, and odour concentration C, for each cell was fitted by the Hill equation:
| (1) |
Then the dose-dependent data from each cell were normalised by dividing each odour concentration by kfreq, the concentration required to elicit a half-maximal spiking response in that cell, and by dividing the spike firing frequency by Fmax.
Data which have been normalised in odour concentration using this procedure are collected in Fig. 3. Figure 3A compares the dependence of the peak receptor current (▪) and the maximal spike firing frequency (○) on odour concentration. The dose-response relationship for spike firing was normalised according to the Fmax of the individual cell, while the receptor current response was normalised in amplitude according to the maximum current response in each cell. The pooled dose-response relation for spike firing has been fitted with the Hill equation. It can be seen that spike firing began at odour concentrations too low to evoke a receptor current sufficiently large to be detected in the presence of the noise associated with the suction pipette technique. Spike frequency then rose steeply with a Hill coefficient of 1.8 (nH; range 1.2-2.8), to saturate within a 15-fold concentration range at a mean frequency of 44 Hz (range 39-51 Hz). In contrast, the dose-response relation for the peak receptor current was displaced to higher odour concentrations, and did not show a clear saturation within the concentration range employed. It was therefore necessary to normalise the receptor current data for each cell at a still-unsaturated level, which will have tended to reduce the apparent difference between the position of these two relations. However, it seems likely that the error introduced by this procedure was relatively small, since all the cells had a broadly comparable sensitivity, as assessed from their spiking responses (kfreq 2.4 to 9.8 μm).
Figure 3. Collected dose-response data from six cells.
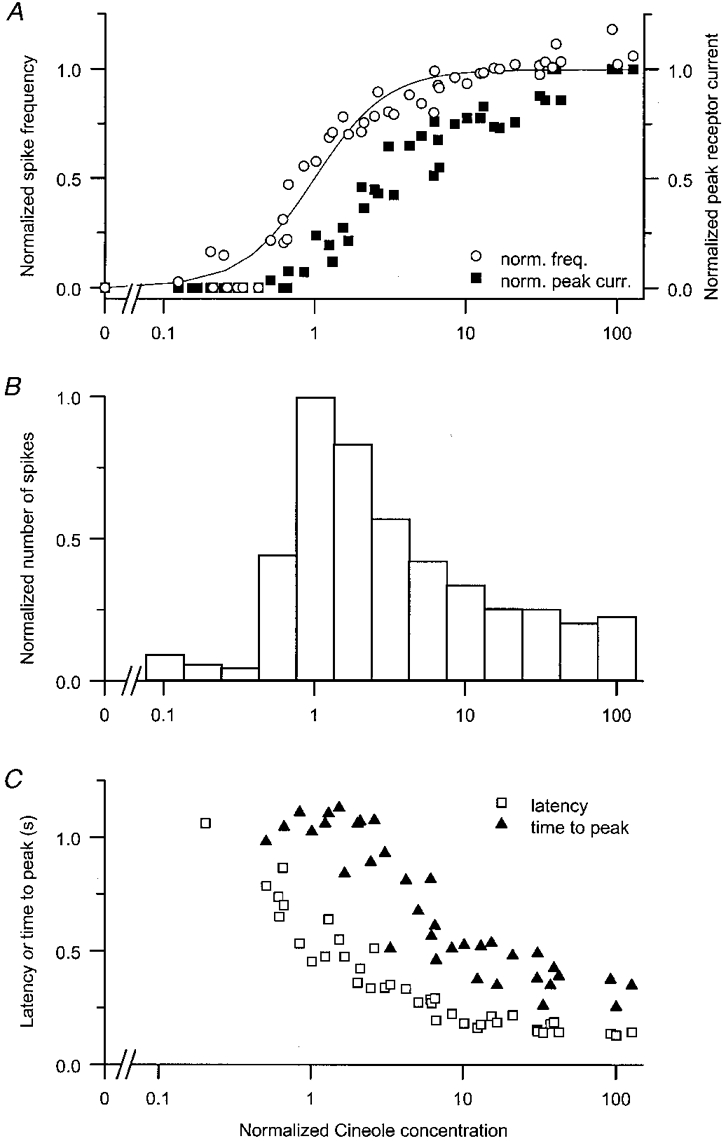
A, comparison of the dose-response relations derived from the peak receptor current (▪) and spike frequency (○). The receptor-current data have been normalised according to the maximum response at high odour concentrations, while the frequency data have been normalised by dividing by the maximum frequency derived from the fitted Hill curve. Cineole concentrations have been normalised according to the stimulus required to elicit a half-maximal spike frequency kfreq, derived by fitting the Hill equation individually to the spike firing data from each cell. Continuous line is the Hill equation (eqn (1)) fitted to the pooled spike frequency data (unity amplitude and half-saturating frequency; Hill coefficient of 1.8). B, number of spikes fired in response to the odour stimulus. Data have been normalised for each cell according to the maximum number of spikes fired, and binned in 0.25 log10 units of normalised concentration. Concentration normalised as in A. C, latency to the first spike (□) and time to peak of the receptor-current response (▴). Values have been corrected for the delay before the solution change, estimated from the junction currents between dissimilar ionic solutions in suction pipette and bath. Concentration normalised as in A. Each panel shows pooled data from six cells.
Figure 3B presents data for the normalised number of spikes fired in response to each odour stimulus. To minimise the effects of scatter between cells, the normalised data have been grouped into bins whose widths vary logarithmically in odour concentration. It can be seen that the number of spikes fired rose rapidly with increasing odour concentration reaching a mean peak number of 15 spikes (range 8-20) at the concentration kfreq, which evoked half the maximum firing frequency. Consequently the longest and most stable spike trains were evoked in the steepest part of the dose- response relation for spike firing, where small alterations in concentration caused large changes in firing frequency. Even higher odour concentrations led to a progressive decrease in the number of spikes fired until, at concentrations which evoked the highest firing frequency, only a few spikes were elicited, corresponding to about 20 % of the maximum number.
The time to peak of the receptor current evoked by 1 s stimulation, shown as the filled symbols in Fig. 3C, initially changed relatively little at low to moderate odour concentrations, but declined steeply as the odour concentration was raised further. In contrast, the pooled latency from the onset of the stimulus to the first spike, shown as the open symbols in Fig. 3C, declined progressively at even the lowest stimulus concentrations, falling steeply over a 10-fold range of odour concentration. This observation reflects the progressive shift of the spike train to earlier times in the rising phase of the receptor current response (see Fig. 1). Still higher odour concentrations led to only a modest further decrease in response latency, which stabilised at around 170 ms, a value which was relatively consistent for the six cells studied.
Adaptation of the odour response
To investigate adaptation of the odour response to a prolonged stimulus, olfactory receptor cells were exposed to a 4 s adapting pre-pulse of fixed concentration and then stimulated by a 1 s test pulse of varying concentration. An example of such an experiment is shown in Fig. 4. In each panel the response to a test pulse of a given odour concentration is compared in the presence (thick trace) and absence (thin trace) of a pre-pulse of a fixed odour concentration of 5 μm. The response to the 5 μm pre-pulse itself was transient, the receptor current relaxing back to baseline levels before the onset of the test pulse. Not surprisingly, a further 1 s of stimulation by a 5 μm test pulse did not evoke any additional response (thick trace), but when presented alone (thin trace) yielded a receptor current response of amplitude nearly equal to that of the pre-pulse itself. When the test pulse concentration was increased to 10 μm, only a minimal receptor current response was now evoked following adaptation to the pre-pulse, representing a considerable reduction in its amplitude when compared with the unadapted control. In most cases a test pulse concentration of twice the adapting pre-pulse was sufficient to yield a discernible receptor current response. Increasing the test pulse concentration still further evoked a progressively larger receptor current response. However, comparison with the traces in the absence of the pre-pulse revealed that the peak receptor current was reduced and the rising phase of the response slowed by adaptation to the pre-pulse over the whole range of test pulse concentrations studied. In contrast, the effects of adaptation on the plateau current response were less marked, especially at higher test pulse concentrations (Fig. 4, 100 and 300 μm). Only at lower test pulse concentrations, before the clear separation of the peak and plateau phases of the response, could a clear reduction be seen in the receptor current response at these late times.
Figure 4. The effect of adaptation on the odour-induced receptor current.
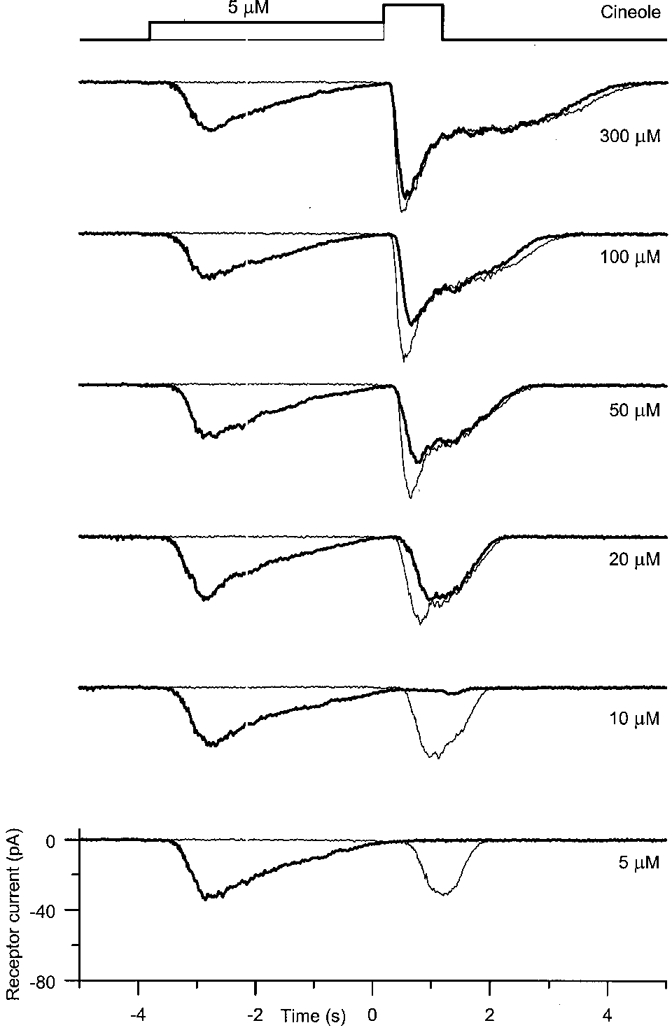
An olfactory receptor cell was exposed to a 4 s adapting pre-pulse at a fixed concentration of 5 μm cineole and immediately thereafter exposed to a 1 s test pulse of increasing concentration as indicated beside each trace. Thick traces represent the responses recorded in the presence of the pre-pulse, while thin traces are control recordings in the absence of the pre-pulse. Current traces were low-pass filtered at 20 Hz and are the means of two trials. Top trace represents the timing of the solution change.
Responses to a range of pre-pulse odour concentrations are illustrated for the same cell in Fig. 5 over a wider bandwidth. As before, the receptor cell was exposed to a 4 s adapting pre-pulse and then stimulated with a 1 s test pulse, but in this case the test pulse concentration was kept constant at 20 μm and the pre-pulse concentration varied. In the absence of the pre-pulse, the fixed 20 μm test pulse induced a substantial receptor current which evoked a brisk burst of action potentials (Fig. 5A). The lowest pre-pulse concentration of 2 μm stimulated a slow rise in the receptor current, which elicited a train of action potentials lasting several seconds (Fig. 5B). As the pre-pulse concentration was raised further, the receptor current response and the peak spike frequency which it evoked progressively increased, and the number of spikes and the duration of the spike train progressively decreased. At the lower two concentrations (2 and 5 μm) both the receptor current and the accompanying spike train had ended by the time at which the test pulse was delivered. In contrast, for the highest pre-pulse concentration of 10 μm, the receptor current remained elevated throughout the stimulus. As the pre-pulse concentration was increased, the peak amplitude of the receptor current response to the fixed 20 μm test pulse progressively decreased, as did the plateau component of the response at later times. In addition, the spike frequency evoked by the test pulse was reduced in comparison with the unadapted control (see also Fig. 6B). However, for all but the highest pre-pulse concentration the total number of spikes fired was virtually unchanged (see also Fig. 6C). In contrast, at the highest pre-pulse concentration of 10 μm, although the test pulse elicited a siseable receptor current response, it failed to evoke any spike discharge, perhaps reflecting the fact that the receptor-current response to the pre-pulse itself had not recovered by that time. Recovery of the receptor current back to baseline levels after termination of the test pulse was either similar or slightly faster under adapted conditions than that in the absence of the pre-pulse.
Figure 5. The effect of adaptation on the odour-induced spike firing response.
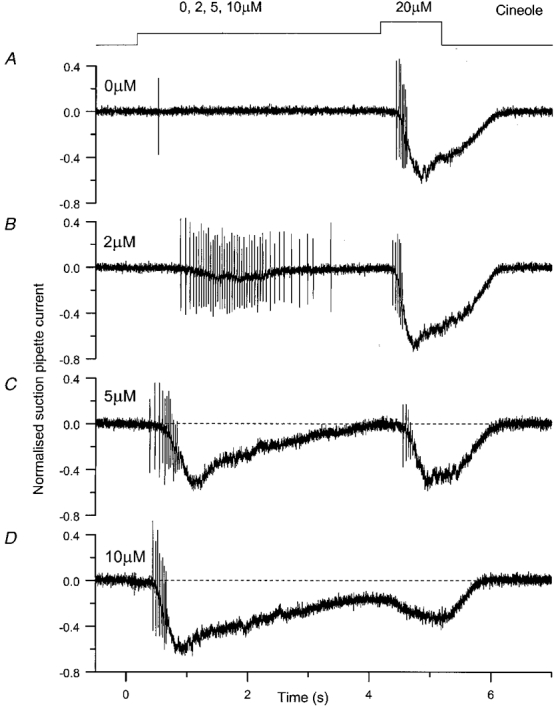
Suction current response family to a 4 s pre-pulse of increasing concentration as indicated beside each trace, followed by a test pulse at a fixed concentration of 20 μm cineole. Current traces low-pass filtered at 500 Hz, and are individual responses. Top trace represents the timing of the solution change. Same cell as Fig. 4. During the experiment the cell was repositioned in the tip of the suction pipette, leading to the collection of an increased proportion of the receptor current from the cell body. Therefore all current traces have been normalised to the maximal current elicited by a 300 μm test pulse in the absence of an adapting pre-pulse.
Figure 6. Effect of adaptation on the dose-response relations.
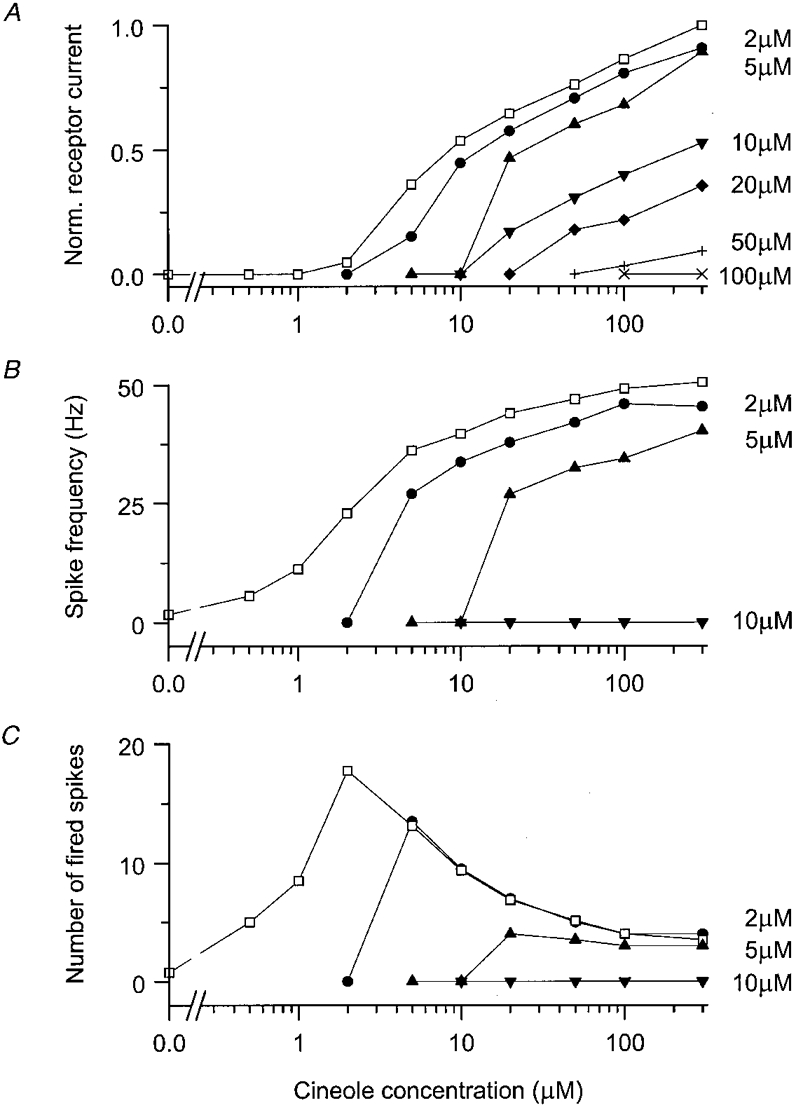
Stimulus-dependent data from the cell of Figs 4 and 5, in response to a 4 s pre-pulse followed by a 1 s test pulse. A, peak receptor current response plotted as a function of test pulse concentration following pre-pulse concentrations of 2 (•), 5 (▴), 10 (▾), 20 (♦), 50 (+) and 100 μm (×), normalised according to the peak response in the absence of the pre-pulse. □, dose-response relation under control conditions in the absence of the pre-pulse. B, peak spike firing frequency plotted as a function of test pulse concentration; pre-pulse concentrations as in A. Data not shown for pre-pulse concentrations above 10 μm, which completely abolished the spiking response. C, number of spikes fired in response to the test pulse plotted as a function of test pulse concentration; pre-pulse concentrations as in A. Same cell as Figs 4 and 5; each data point is the mean of 2-6 measurements.
Results from this experiment are collected in Fig. 6, which plots peak receptor current, spike frequency, and the number of spikes fired as functions of test pulse concentration both under unadapted conditions (open symbols) and after adaptation to six different pre-pulse concentrations (filled symbols). As the pre-pulse concentration was increased, the peak receptor current response to any given test pulse decreased, corresponding to a shift in the dose-response relation to higher test pulse concentrations. As was the case for the unadapted dose-response relation, no clear saturation of the response amplitude could be seen even at the highest test pulse concentrations. Similar results were obtained from a further seven cells, each of which was exposed to 2-4 different pre-pulse concentrations, depending on the duration of the recording.
As well as these changes in receptor current, adaptation to the pre-pulse resulted in a progressive shift of the dose- response relationship for spike firing to higher test pulse concentrations, accompanied by a reduction in the maximum spike firing frequency. However, the number of spikes evoked by a test pulse concentration which succeeded in stimulating a response was little affected by exposure to the pre-pulse, until the pre-pulse concentration became sufficiently high to abolish spike firing altogether (see also Fig. 5D). Similar results were obtained from a total of seven cells at low to moderate pre-pulse concentrations. Five cells were exposed to pre-pulse concentrations sufficiently high to abolish spike firing; two of these recovered only partially thereafter. The pre-pulse concentration required to abolish spike firing was around eight times the concentration kfreq, which evoked half the maximum firing frequency in the absence of the pre-pulse; the large range of 4-12 prepulse concentrations probably representing the considerable variation in the ability of individual cells to recover during the pre-pulse.
The data presented in Fig. 6 are plotted as a function of the absolute test pulse concentration. In order to determine how the sensitivity of the olfactory receptor cell varies during adaptation, it is necessary instead to express the odour stimulus as an increment above the concentration of the pre-pulse. Sensitivity was estimated by interpolating linearly between individual points on the dose-response relation and dividing by the increment in the test pulse stimulus which would have been required to evoke 20 % of the peak response elicited by a 300 μm test pulse in the absence of the pre-pulse. Values for sensitivity S, were obtained in this way from the receptor current and the spike frequency dose- response relations in three cells, for which an especially wide range of pre-pulse concentrations were available. These data were normalised for each cell with respect to the sensitivity S0, in the absence of the pre-pulse and fitted with a modified Weber relation:
| (2) |
where C is the concentration of the pre-pulse, C½ is the pre-pulse concentration which halves the sensitivity relative to its unadapted value, and n represents the limiting exponent of the relation at high pre-pulse concentrations. The data have been normalised in pre-pulse concentration according to the value of C½ in each cell, and plotted in Fig. 7 as a function of the normalised pre-pulse concentration in double logarithmic co-ordinates. It can be seen that this relation fitted the normalised receptor current sensitivity data with a limiting slope n, of 2.4. This value for n of greater than unity indicates a steeper decline in sensitivity than would be anticipated from the simple inverse relationship of Weber's law.
Figure 7. Sensitivity as a function of adapting pre-pulse concentration.
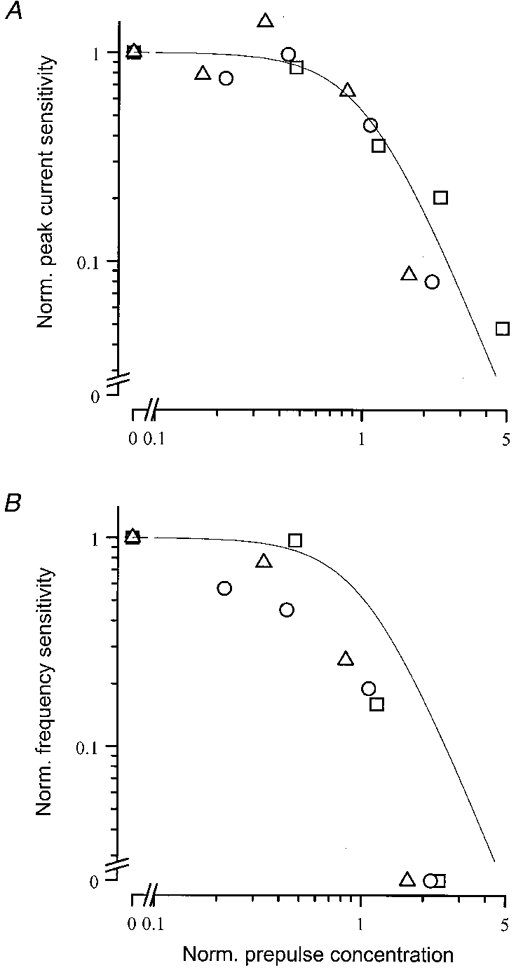
Sensitivity derived from the peak receptor current response (A) and from the spiking response (B) Sensitivity has been calculated from the dose-response relation for each cell by dividing a 20 % response criterion by the increment above the pre-pulse concentration of the test pulse required to evoke it. Sensitivity has been normalised for each cell to that in the absence of the pre-pulse. Pre-pulse concentration has been normalised in both panels according to the pre-pulse concentration which reduced current sensitivity by a factor of two. Continuous curve fitted to the peak current sensitivity data in A is a modified Weber relation (eqn (2)) with an exponent of 2.4 and normalised to unity; this curve is replotted in B for ease of comparison. Cell of Figs 4–6 is represented by □.
Figure 7B shows the sensitivity derived from the dose- response relations for spike firing, plotted as a function of pre-pulse concentration for the same three cells. In each case pre-pulse concentration has been normalised according to the value for C½ obtained from the receptor- current responses; the curve fitted to the data of Fig. 7A is replotted for comparison. It can be seen that the sensitivity of the spiking response declined to zero when spiking was abolished at a pre-pulse concentration for which the receptor current still varied in a graded manner with test pulse concentration.
DISCUSSION
The odour dose-response relationship
The odour-induced spike responses recorded in this study with the suction pipette technique are in broad agreement with previous results from amphibian and reptile olfactory receptor cells (O'Connell & Mozell, 1969; Mathews, 1972; Getchell & Shepherd, 1978a; van Drongelen, 1978; Trotier, 1994). In these early reports higher stimulus concentrations generally achieved an increase in the rate of spike firing, and when stimulus onset was well controlled a decrease in latency was observed. However, the responses to high odour concentrations were typically not investigated, and hence the saturation of the relationship between spike frequency and odour concentration and the decline in spike amplitude which we see under these conditions were not consistently observed.
The Hill coefficient of 1.8 obtained from the pooled spike frequency dose-response relation (Fig. 3A, ○) illustrates the steep dependence of action potential firing on the stimulus concentration and reflects the narrow dynamic range over which olfactory receptor cells are able to encode odour concentration. This limitation may be partly counteracted by the increase in the number of spikes fired which we observed at intermediate odour concentrations, for which spike firing is most sensitive to changes in stimulus concentrations. At high odour concentrations not only did the spike firing frequency saturate, but only two to three spikes were generated in response to the stimulus. Consequently any further increases in stimulus concentrations could no longer be coded reliably. However, the latency between stimulus onset and the first spike decreased monotonically in all the cells studied over the whole concentration range, falling to around 170 ms at the highest odour concentrations (Fig. 3C, □). So, at high odour concentrations although the concentration of the stimulus was only poorly encoded by the brief high-frequency burst of action potentials which it evoked, the time of onset of the stimulus was accurately preserved. This observation is of particular interest in light of the recent observation that olfactory receptor cells which express a given receptor protein project onto perhaps as few as two glomeruli in the olfactory bulb (Ressler et al. 1994; Vassar et al. 1994; Mombaerts et al. 1996), corresponding to the convergence of this highly synchronised input onto only a relatively small number of second order cells. Furthermore, some features of the patterns of spike firing exhibited by olfactory receptors appear to be preserved in the mitral and tufted cells within the olfactory bulb. The increase in spike firing frequency, the reduction in the number of spikes fired and the reduction in latency to the first spike evoked from an isolated olfactory receptor by increasing odour concentration closely resemble the excitatory responses of mitral and tufted cells (Døving, 1964; Kauer, 1974; Kauer & Shephard, 1977; Harrison & Scott, 1986; Meredith, 1986). Intriguingly, similar response properties have also been observed for individual cells within the olfactory cortex (Duchamp-Viret et al. 1996).
In contrast to these spiking responses, which have previously been investigated in some detail, the receptor current of the isolated olfactory receptor cell has hitherto been little studied under conditions in which the intracellular voltage is free to vary during the odour response. An unexpected finding of our study was the relatively gradual approach of the peak receptor current to saturation with increasing odour concentration, which contrasts with the abrupt saturation which is observed when the receptor current is recorded under whole-cell voltage clamp (Firestein et al. 1993). One factor which might explain these differences in the current responses is the variation of intracellular voltage which takes place during the odour response when the receptor current is recorded by the suction pipette technique. The olfactory cyclic nucleotide-gated channel is known to be blocked by external Ca2+ in a voltage-dependent manner (Kleene, 1993; Zufall & Firestein, 1993). Furthermore, it has been demonstrated by whole-cell patch clamp recording that small depolarising voltage clamp steps can reveal or enlarge previously unrecorded odour-induced responses, an effect which may have arisen either from the voltage-dependent block of the cyclic-nucleotide gated channel by Ca2+ or a voltage-dependent gating of the Ca2+-activated Cl− channel (Firestein & Shepherd, 1995). It therefore seems possible that the strong depolarisation which accompanies the responses to higher odour concentrations may have caused a progressive relief of this Ca2+ block and increased the flux of Na+ through the cyclic-nucleotide gated channel. It is interesting to note that during the plateau phase which constitutes the latter part of the response, the greater part of which appears to be carried by the Ca2+-activated Cl− conductance (Reisert & Matthews, 1998b), saturation was observed with increasing odour concentration, in contrast to the situation at the response peak.
The displacement of the dose-response relationship for spike frequency to lower odour concentrations in comparison with that for the receptor-current response is consistent with previous, but more restricted, observations made using the loose patch clamp technique. (Trotier, 1994). As noted in that study, a contributing factor is likely to be the high input impedance of the olfactory receptor cell, which leads to spike firing in response to even the smallest depolarising currents. Such small currents cannot readily be resolved in the suction pipette recording due to the noise associated with the loose seal formed between the pipette and the cell body. This difference between the dose-response relationships is also exacerbated further by the wider dynamic range of the receptor current response.
Olfactory receptor cell adaptation
Exposure of olfactory receptor cells to an adapting pre-pulse resulted in a progressive shift of the dose-response relationships for the spike firing and receptor current responses to higher test pulse odour concentrations. Such odour pre-exposure potently reduced the peak receptor current evoked by a given test pulse, and slowed the rising phase of this response. In contrast, the plateau current was much less affected by the pre-pulse, and response termination was only marginally faster under adapted conditions.
In a similar manner, the spiking frequency dose-response relationship was progressively shifted to higher test pulse concentrations under adapted conditions and the peak firing frequency saturated at lower levels. Consequently, although olfactory receptor cells still generated a train of spikes in response to the test pulse following adaptation to low and intermediate pre-pulse concentrations, they did so only at the expense of a reduced range of firing frequencies. Taken together with the observation that the number of spikes fired in response to a given test pulse remained similar regardless of the adapting pre-pulse concentration, this reduction in spike frequency caused the duration of the spike train elicited by a given test pulse to become longer in comparison with the unadapted response. It is important to note that a proportion of the prepulse-induced shift which we observed in these dose-response relationships will have been the result of plotting these data against the absolute as opposed to the incremental odour concentration. This issue is addressed below when we consider the effect of pre-pulse exposure on odour sensitivity.
Exposure to the highest pre-pulse concentrations resulted in the complete abolition of action potential discharge in response to the test pulse, despite the continued presence of a graded and desensitised receptor-current response. This inability of the adapted cell to fire spikes may have resulted from the failure of the receptor current to recover completely to the baseline by the time at which the test pulse was delivered, in contrast to the complete recovery which was observed during exposure to lower pre-pulse concentrations. It therefore seems possible that the intracellular voltage may still have been sufficiently depolarised at the time at which the test pulse was delivered largely to inactivate voltage-gated Na+ channels, thereby rendering the membrane of the cell electrically inexcitable, and preventing the receptor current evoked by the test pulse from eliciting action potentials.
When the test pulse concentration is translated into an increment above the pre-existing concentration of the pre-pulse, it was found that the apparent sensitivity of the receptor current response fell steeply as a function of pre-pulse concentration. This contrasts with the situation in other sensory receptors, which appear to adapt in a more graded manner during continuous stimulation, exhibiting a less steep decline in sensitivity as the adapting stimulus is increased. For example, toad rod photoreceptors adapt by decreasing their sensitivity inversely with steady light intensity according to Weber's law (Fain, 1976; Baylor et al. 1980), while in turtle hair cells adaptation simply displaces the stimulus-response relation to higher stimulus levels without a change in form (Crawford et al. 1989). Even more profound were the decreases in sensitivity estimated from the peak spike firing frequency evoked by the test pulse following adaptation to the pre-pulse. This contrasts with the ‘curve shifting’ which occurs during adaptation in retinal ganglion cells where the dynamic range and the maximal firing rate are preserved (Sakmann & Creutzfeldt, 1969). The inability of olfactory receptor cells to fire spikes at the highest odour concentrations resulted in a precipitous decline in sensitivity, and will have prevented them from sending any information to the olfactory bulb under these conditions. It could be argued that the rather limited adaptation which we observed may have resulted from the relatively early time of 4 s after the onset of the adapting odour pre-pulse at which we measured sensitivity. However, at later times the responses of frog olfactory receptors become complex, in most cases exhibiting oscillations of the receptor current and repeated bursts of spike firing (Frings & Lindemann, 1988; Reisert & Matthews, 1996), which makes characterisation of sensitivity difficult. At these late times it seems possible that the absolute rate of spike firing may be of less importance to the olfactory bulb than the temporal pattern of afferent activity.
Acknowledgments
This work was supported by The Wellcome Trust, and by a MRC Research Studentship (J.R.).
References
- Anderson PAV, Hamilton KA. Intracellular recordings from isolated salamander olfactory receptor neurons. Neuroscience. 1987;21:167–173. doi: 10.1016/0306-4522(87)90330-7. [DOI] [PubMed] [Google Scholar]
- Baylin F, Moulton DG. Adaptation and cross-adaptation to odor stimulation of olfactory receptors in the tiger salamander. Journal of General Physiology. 1979;74:37–55. doi: 10.1085/jgp.74.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-Y. The membrane current of single rod outer segments. The Journal of Physiology. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. The Journal of Physiology. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. The Journal of Physiology. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne VE. Chemosensory responses in isolated olfactory receptor neurons from Necturus maculosus. Journal of General Physiology. 1992;99:415–433. doi: 10.1085/jgp.99.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Døving KB. Studies of the relation between the frog's electro-olfactogram (EOG) and single unit activity in the olfactory bulb. Acta Physiologica Scandinavica. 1964;60:150–163. doi: 10.1111/j.1748-1716.1964.tb02878.x. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Dionne VE. Action potentials and chemosensitive conductances in the dendrites of olfactory neurons suggest new features for odor transduction. Journal of General Physiology. 1994;103:181–201. doi: 10.1085/jgp.103.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Palouzier-Paulignan B, Duchamp A. Odor coding properties of frog olfactory cortical neurons. Neuroscience. 1996;74:885–895. doi: 10.1016/0306-4522(96)00194-7. [DOI] [PubMed] [Google Scholar]
- Fain GL. Sensitivity in toad roads: dependence on wave-length and background illumination. The Journal of Physiology. 1976;261:71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Picco C, Menini A. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. The Journal of Physiology. 1993;468:1–10. doi: 10.1113/jphysiol.1993.sp019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Shepherd GM. Interaction of anionic and cationic currents leads to a voltage dependence in the odor response of olfactory receptor neurons. Journal of Neurophysiology. 1995;73:562–567. doi: 10.1152/jn.1995.73.2.562. [DOI] [PubMed] [Google Scholar]
- Firestein S, Shepherd GM, Werblin FS. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurons. The Journal of Physiology. 1990;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Werblin F. Odor-induced membrane currents in vertebrate olfactory receptor neurons. Science. 1989;244:79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Odorant response of isolated olfactory receptor cells is blocked by amiloride. Journal of Membrane Biology. 1988;105:233–243. doi: 10.1007/BF01871000. [DOI] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Single unit recording from olfactory cilia. Biophysical Journal. 1990;57:1091–1094. doi: 10.1016/S0006-3495(90)82627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland RC, Lettvin JY, Pitts WH. Chemical transmission in the nose of the frog. The Journal of Physiology. 1965;181:525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Responses of olfactory receptor cells to step pulses of odour at different concentration in the salamander. The Journal of Physiology. 1978a;282:521–540. doi: 10.1113/jphysiol.1978.sp012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. The Journal of Physiology. 1978b;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TA, Scott JW. Olfactory bulb responses to odor stimulation: analysis of response pattern and intensity relationships. Journal of Neuorscience. 1986;56:1571–1589. doi: 10.1152/jn.1986.56.6.1571. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. The Journal of Physiology. 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JS. Response patterns of amphibian olfactory bulb neurons to odour stimulation. The Journal of Physiology. 1974;243:695–715. doi: 10.1113/jphysiol.1974.sp010772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JS, Shephard GM. Analysis of the onset phase of olfactory bulb unit responses to odour pulses in the salamander. The Journal of Physiology. 1977;272:495–516. doi: 10.1113/jphysiol.1977.sp012056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. The cyclic nucleotide-activated conductance in olfactory cilia: effects of cytoplasmic Mg2+ and Ca2+ Journal of Membrane Biology. 1993;131:237–243. doi: 10.1007/BF02260112. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. The Journal of Physiology. 1989;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Research. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. The Journal of Physiology. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DF. Response pattern of single neurons in the tortoise olfactory epithelium and olfactory bulb. Journal of General Physiology. 1972;60:166–180. doi: 10.1085/jgp.60.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. The Journal of Physiology. 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. Journal of Neuroscience. 1986;56:572–597. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualising an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morales B, Ugarte G, Labarca P, Bacigalupo J. Inhibitory K+ current activated by odorants in toad olfactory neurons. Proceedings of the Royal Society of London. 1994;257:235–242. doi: 10.1098/rspb.1994.0120. B. [DOI] [PubMed] [Google Scholar]
- O'Connell RJ, Mozell MM. Quantitative stimulation of frog olfactory receptors. Journal of Neurophysiology. 1969;32:51–63. doi: 10.1152/jn.1969.32.1.51. [DOI] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Oscillating responses of olfactory receptor cells during prolonged stimulation. Goettingen Neurobiology Report. 1996;2:299. [Google Scholar]
- Reisert J, Matthews HR. Adaptation of odour-induced responses in frog olfactory receptor cells. The Journal of Physiology. 1998a;506.P:83–83. doi: 10.1111/j.1469-7793.1999.0801n.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. Journal of General Physiology. 1998b;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organised epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Creutzfeldt OD. Scotopic and mesopic light adaptation in the cat's retina. Pfügers Archiv. 1969;313:168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain control. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon; 1984. pp. 263–346. [Google Scholar]
- Shibuya T, Shibuya S. Olfactory epithelium: unitary responses in the tortoise. Science. 1963;140:495–496. doi: 10.1126/science.140.3566.495. [DOI] [PubMed] [Google Scholar]
- Torre V, Ashmore JF, Lamb TD, Menini A. Transduction and adaptation in sensory receptor cells. Journal of Neuroscience. 1995;15:7757–7768. doi: 10.1523/JNEUROSCI.15-12-07757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D. Intensity coding in olfactory receptor cells. Seminars in Cell Biology. 1994;5:47–54. doi: 10.1006/scel.1994.1007. [DOI] [PubMed] [Google Scholar]
- Trotier D, MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Research. 1983;268:225–237. doi: 10.1016/0006-8993(83)90488-2. [DOI] [PubMed] [Google Scholar]
- van Drongelen W. Unitary responses of near threshold responses of receptor cells in the olfactory mucosa of the frog. The Journal of Physiology. 1978;277:423–435. doi: 10.1113/jphysiol.1978.sp012282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organisation of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Ache BW. Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. Journal of Neurophysiology. 1995;74:479–483. doi: 10.1152/jn.1995.74.1.479. [DOI] [PubMed] [Google Scholar]
- Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. Journal of Neurophysiology. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. Identification of a long-lasting form of odor adaptation that depends on the carbon monoxide/cGMP second-messenger system. Journal of Neuroscience. 1997;17:2703–2712. doi: 10.1523/JNEUROSCI.17-08-02703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


