Abstract
Unilateral changes in mammary cell number are elicited when one gland is milked more or less frequently than the contralateral gland in lactating goats. These changes were investigated using histochemical and immunocytochemical markers of mammary cell types, and the degree of mammary apoptosis was determined by end-labelling of fragmented DNA.
Histological analysis confirmed that unilateral cessation of milking initiated involution and cell loss preferentially in the unmilked gland. The presence of fragmented DNA and morphological characteristics consistent with apoptosis demonstrated that these changes in mammary cell number in unmilked glands were, in part, the result of programmed alveolar cell death.
De-differentiation of the remaining secretory cells to ductal epithelial cells occurred with an increase in staining of cytokeratin markers and decreased staining by peanut lectin and casein antisera.
Differential once- and thrice-daily milking of lactating goats was also associated with unilateral changes in mammary cell number and milk yield. Milk yield and alveolar size were reduced after 4 weeks of infrequent milking. The latter was due to the increased loss of secretory cells by apoptosis, as indicated by a higher degree of fragmented DNA laddering.
After 10 weeks of differential milking, a homogeneous secretory morphology, albeit with smaller alveoli, was maintained in thrice-daily milked glands. Once-daily milked glands possessed a heterogeneous composition of terminal structures, resulting in the simultaneous presence of secretory and involuting alveoli as well as resting ductules.
The differences in programmed cell death and mammary morphology between unmilked and twice-daily milked glands, and between once- and thrice-daily milked glands, suggests that mammary apoptosis is subject to modulation by intra-mammary mechanisms sensitive to the frequency of milk removal.
Frequency of milk removal by suckling or milking regulates the rate of milk secretion locally, in each mammary gland, during lactation (Peaker et al. 1998). When the change in milking frequency is prolonged, the milk yield response is sustained by sequential developmental adaptations, initially as an up- or downregulation of cellular differentiation and later as a net change in mammary cell number (Wilde et al. 1987). A higher cell number in ruminant mammary glands milked more frequently can occur against a background of declining cell number after peak lactation (Wilde & Knight, 1990), suggesting that it may result partly from a reduced rate of cell loss.
Mammary cell loss is also observed during tissue involution after cessation of milk removal. In these circumstances, the decline in secretory cell number is due to programmed cell death (Strange et al. 1992; Quarrie et al. 1996; Wilde et al. 1997). This cell death, which occurs by apoptosis, involves a series of ultrastructural changes (Walker et al. 1988), and is associated with the fragmentation of genomic DNA into oligonucleosomal fragments of 180-200 base pair lengths which, on electrophoresis and staining with ethidium bromide, form a characteristic DNA laddering pattern (Arends et al. 1990). Induction of apoptosis on cessation of milk removal is in part a response to local mechanisms operating within the tissue. In both rodents (Quarrie et al. 1996) and ruminants (Quarrie et al. 1994), tissue involution and cell death were induced unilaterally when milk removal from one mammary gland was stopped. That mammary cell loss after termination of lactation is by apoptosis, and subject to local, intra-mammary control, raises the possibility that milking frequency modulates cell number in each lactating mammary gland by a similar mechanism.
Analysis of mammary tissue from animals subjected to different milking regimens was based on biochemical assays of whole mammary tissue extracts (Wilde et al. 1987). It was not possible, therefore, to determine if epithelial differentiation was regulated co-ordinately, or if cell turnover was restricted to a particular cell type. The mammary gland consists of luminal epithelial and myoepithelial cells, and cells intermediate between epithelial and myoepithelial phenotypes (Warburton et al. 1982; Rudland & Hughes, 1989; Li et al. 1999). The relationships of these mammary cell types have been studied using histochemical and immuno-cytochemical markers of cell type. Epithelial cells have been stained with antisera raised to milk fat globule membrane protein (Foster et al. 1982), epithelial membrane antigen (EMA) (Sloane & Ormerod, 1981) and cytokeratins 7, 8, 18 and 19 (Taylor-Papadimitriou & Lane, 1987). Antisera specific to cytokeratins 5 and 14 (Nagle et al. 1986; Taylor-Papadimitriou & Lane, 1987), smooth muscle actin (Bussolati et al. 1980), myosin (Gusterson et al. 1982) and the common acute lymphoblastic leukaemia antigen (CALLA) (Gusterson et al. 1986) stain myoepithelial cells. Intermediate stem cells occur predominantly in budded structures of growing glands and are identified by immunocytochemical staining for both epithelial- and myoepithelial-specific markers (Ormerod & Rudland, 1984; Rudland, 1991). A population of cuboidal epithelial cells, possessing epithelial and myoepithelial characteristics, has been identified by staining with antisera to c-erbB-2 in the alveoli of lactating ruminant mammary glands (Li et al. 1999). Peanut lectin and casein antisera stain secretory alveolar cells, which arise by differentiation of luminal epithelial cells and are responsible for the synthesis and secretion of milk during lactation (Earl & McIlhinney, 1985; Rudland, 1992).
This study was undertaken to define at a cellular level the developmental responses in lactating mammary tissue to changes in milking frequency, using radioisotopic and fluorimetric DNA end-labelling to detect apoptosis (Quarrie et al. 1995) and immunocytochemistry with histochemical and immunocytochemical markers to characterise the mammary cell population (Warburton et al. 1982; Rudland & Hughes, 1989; Li et al. 1999).
METHODS
Unilateral and differential milking of lactating goats
The effects of unilateral twice-daily milking and differential once- and thrice-daily milking were investigated in two separate experiments. Eight non-pregnant British Saanen goats in their sixth lactation were used in the unilateral milking experiment. They were fed 1.8 kg of concentrates daily (Goat Mix-1, Edinburgh School of Agriculture, Edinburgh, UK), with hay and water available ad libitum. Until week 34 of lactation, both glands were milked twice daily at 07.00 and 15.00 h. Thereafter, milking was stopped in one gland, whilst twice-daily milking was continued in the contralateral gland for up to 3 weeks. The milk yield of the glands and the time of milking were recorded. Mammary biopsies from both glands (Knight & Peaker, 1984) were taken at 4 days before the start of unilateral milking, and again post mortem, in animals killed by captive bolt, after 3, 7, 14 or 21 days of unilateral milking. In the differential milking experiment, six non-pregnant British Saanen goats in their fourth to sixth lactation were milked twice daily until week 20-22 of lactation. Thereafter, one gland of each goat was milked thrice daily at 07.00, 15.00 and 23.00 h for 10 weeks; the other gland was milked at 15.00 h only. At each milking, the yield of each gland and time of milking were recorded. Mammary biopsies were taken from both glands before and 4 and 10 weeks after the start of differential milking. The time of biopsy was routinely 30 min after the morning milking. Experiments were performed under Home Office licence, and with local ethical committee approval.
Tissues
One centimetre cubes of goat mammary tissue were removed from goats post mortem, or by biopsy under sodium pentobarbitone anaesthesia (36 mg kg−1i.v.) (Knight & Peaker, 1984) and fixed overnight in modified Methacarn (60 % methanol, 30 % Inhibisol (BDH Chemicals, Liverpool, UK)), 10 % (v/v) acetic acid, or 5 % (v/v) formalin for 2 h at room temperature. The following standard protocol was then used to dehydrate and embed tissues in paraffin wax on a Shandon tissue processor (Life Sciences International, Uxbridge, UK): three 2 h washes of absolute ethanol at room temperature; one 2 h wash with ethanol-1,1,1-trichloroethane (1:1, v/v); one 1 h wash with 1,1,1-trichloroethane; 2 h incubation with two changes of paraffin wax at 60°C, the last stage being in a vacuum incubator; final embedding of the tissue in a block of fresh wax. Blocks were cooled to 4°C and 3 μm sections of tissue were cut on a rotary microtome using disposable steel blades. Sections were floated onto a waterbath at 50°C and mounted onto 3-aminopropyltriethoxysilane (Sigma)-coated slides and dried overnight at 37°C. Every tenth serial section was stained with haematoxylin and eosin so that the glandular system could be traced and different structures readily located.
Antibodies and histochemical reagents
Keratin 7 MAb OVTL 12/30 was purchased from Europath Ltd (Cornwall, UK). Bovine keratin 18 MAb KS.B17.2 and human keratin 18 MAb CY-90 were obtained from Sigma, as were smooth-muscle actin MAb, 1A4, and myosin MAb, hSV-M. MAb CIV22 to type IV collagen, MAb LP34 reactive to keratins 4, 5, 6, 10 and 18, and MAb V9 reactive to vimentin were purchased from DAKO Ltd (Cambridge, UK). MAb PKK3 reactive to keratin 18 and MAb PKK2 specific for keratins 7, 16, 17 and 19 were acquired from Labsystems (Uxbridge, UK). Peroxidase-conjugated peanut lectin was obtained from EY Labs (San Mateo, CA, USA). Horseradish peroxidase (HRP)-conjugated peanut lectin bound preferentially to oligosaccharides containing the terminal sequence Galβ (1′,3)GalNAc, principally terminal Gal (Newman et al. 1979). Gifts of rabbit antisera to rodent laminin and type IV collagen (Warburton et al. 1982) were from M. J. Warburton (St. George's Hospital Medical School, London). Polyclonal antiserum to goat caseins was prepared at the Hannah Research Institute and affinity purified before use.
Non-immune serum (IgG) fraction was used as a control. In addition, the specificities of the antibodies were checked by pre-absorption with the appropriate antigen and the antigen-antibody complex was then used for immunocytochemistry (Rudland & Hughes, 1989). Antisera to laminin and type IV collagen were absorbed with 1 mg ml−1 reconstituted Englebreth-Holm-Swarm (EHS) matrix (Universal Biologicals Ltd, UK). Keratin MAbs CY-90, KS.B17.2, LP34, OVTL 12/30, PKK2 and PKK3 were absorbed with 1 mg ml−1 human callus keratin (Sigma). MAbs V9, 1A4 and hSV-M were absorbed with sonicated 107 Rama 29 myoepithelial-like cells ml−1. The cells and debris were removed by centrifugation before use. The specificity of peanut lectin binding was checked by prior incubation with 4 % (w/v) galactose.
Immunocytochemical and histochemical staining
All sections were dewaxed in xylene for 20 min and rehydrated through a graded series of alcohols to water (Rudland & Hughes, 1989). For staining with antisera to cytokeratins, type IV collagen and laminin, sections were pre-treated with Pronase (Warburton et al. 1982). Sections were incubated in phosphate-buffered saline (PBS) at 37°C for 15 min followed by a second incubation in PBS containing 50 μg ml−1 Pronase (Sigma). Sections were then rinsed in running tap water. All sections were pre-incubated with 0.1 % (w/v) phenylhydrazine-HCl (Sigma) for 5 min to inhibit endogenous peroxidases, and washed with PBS. Sections were incubated with the primary antibody diluted in 0.5 % (w/v) bovine serum albumin (BSA) in PBS for 1.5 h and then washed with PBS. Immunocytochemical staining was carried out with the avidin-biotin detection system (Hsu et al. 1981), using either donkey anti-rabbit or sheep anti-mouse biotinylated Ig, where appropriate, diluted 1:200 with 0.5 % (w/v) BSA in PBS. Histological sections were incubated with the secondary antibodies for 1 h and washed with PBS. Sections were then applied with the avidin-biotin complex, prepared according to the manufacturer's instructions, for 45 min, and washed with PBS. The peroxidase activity was localised by incubating sections with 0.5 mg ml−1 3′,3-diaminobenzidine dihydrate (DAB), 0.0003 % (v/v) H2O2 in 0.05 M Tris-HCl, pH 7.6, for 5 min. Cell nuclei were counterstained with Mayer's haematoxylin, and the sections were dehydrated, cleared and mounted in DPX mountant (BDH).
Peroxidase-conjugated peanut lectin was incubated for 2 h on fixed and paraffin-embedded sections directly or with neuraminidase-treated sections (Newman et al. 1979) as for the first antibody reactions above. Both sets of sections were then incubated directly with DAB/H2O2 before counterstaining and mounting. Sections were photographed on Ilford Pan F film with a Kodak Wratten No. 44 green filter using a Reichert Polyvar microscope.
TUNEL detection of apoptotic cells in tissue sections
Apoptotic cells were visualised in tissue sections using the In Situ Cell Death detection kit from Boehringer Mannheim (East Sussex, UK), according to the manufacturer's instructions. Briefly, formalin-fixed, paraffin-embedded mammary tissue sections were dewaxed, rehydrated, and digested with 50 μg ml−1 Pronase. Endogenous peroxidases were blocked as described in the previous section. Tissue sections were rinsed in PBS and incubated with the TdT-mediated dUTP-X nick-end labelling (TUNEL) reaction mixture for 1 h. After washing with PBS, sections were incubated with HRP-conjugated sheep anti-fluorescein immunoglobulins, diluted 1:20 with BSA/PBS, for 30 min. After washing with PBS, the bound antibody was visualised with DAB, and cell nuclei were counterstained and mounted, as described in the previous section. Apoptotic cell numbers were compared by Student's t test.
DNA analysis
Fragmented DNA was detected by nick-end labelling following by agarose gel electrophoresis using a method adapted from Quarrie et al. (1995). Mammary DNA was isolated using Gentra Systems Puregene DNA isolation kit (Flowgen, Lichfield, UK), according to the manufacturer's instructions, except that frozen mammary tissue was homogenised in liquid nitrogen prior to incubation with the cell lysis solution. Ten micrograms DNA was measured spectrophotometrically and diluted with an equal volume of 100 mM Tris-HCl, pH 8.0, containing 10 mM MgCl2 in an Eppendorf tube and incubated with 100 nCi of [32P]dCTP (ICN Pharmaceuticals Ltd, Oxfordshire, UK) and 2.5 U of DNA polymerase I (Klenow fragment) (Promega, Southampton, UK) for 10 min at room temperature. The reaction was stopped by addition of EDTA (final concentration, 10 mM), and the reaction volume was adjusted to 100 μl with water. DNA was recovered by adding 250 μl of absolute ethanol and 35 μl of 7.5 mM ammonium acetate in the presence of 20 μg of glycogen (Boehringer Mannheim), and resuspended in 10 mM Tris-HCl, pH 8.0, containing 100 mM EDTA.
DNA (1 μg) was separated by electrophoresis in a 1.8 % (w/v) agarose gel prepared with 40 mM Tris-HCl (pH 8.2) containing 20 mM acetate, 1 mM EDTA and 5 μg ml−1 ethidium bromide, until the Bromophenol Blue marker ran about two-thirds of the distance along the gel. Equal loading of DNA was confirmed under UV illumination before the gel was fixed with 7 % (w/v) trichloroacetic acid for 30 min. The gel was flattened overnight, sealed in plastic and subjected to autoradiography at -70°C using X-Omat-AR film (Kodak) and an intensifying screen. Exposure time was adjusted according to the intensity of the signal.
Presentation of data
Unless otherwise stated, data are given as means ±s.e.m.
RESULTS
Unilateral cessation of milking in lactating goats
Before single gland milking, the mean daily milk yields of the eight late-lactating goats, milked twice daily, were 0.88 ± 0.12 kg day−1 (n = 8) in the left glands and 1.06 ± 0.10 kg day−1 (n = 8) in the right glands (Fig. 1A). After milking was discontinued in the left glands, mean milk yield of the twice-daily milked right glands was maintained at 1.07 ± 0.13 (n = 6), 1.21 ± 0.13 (n = 4) and 1.06 kg day−1 (n = 2) during weeks 1, 2 and 3, respectively (Fig. 1A).
Figure 1. Milk yield and histology of goat mammary glands after unilateral cessation of milking.
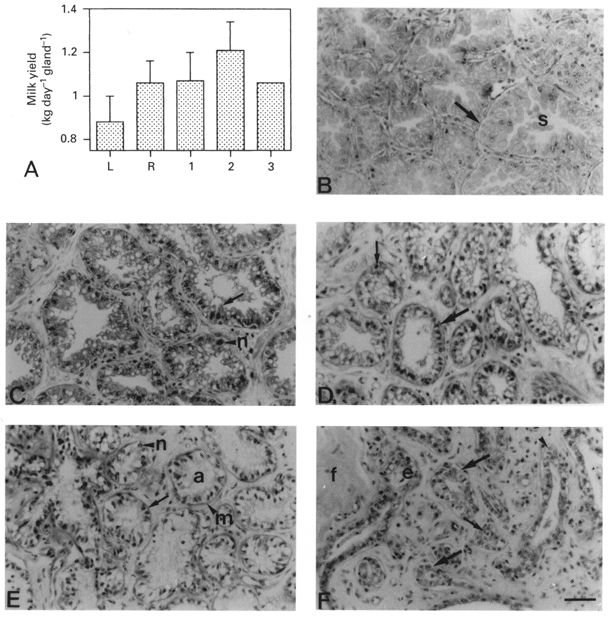
A, mean daily milk yield of left (L) and right (R) glands before and after unilateral cessation of twice-daily milking for 1, 2 or 3 weeks. Values are means ±s.e.m. when n≥ 2 (values of n are given inthe text). B-F, morphology of mammary tissue from glands milked twice daily (B) or unmilked for 3 days (C), 1 week (D), 2 weeks (E) or 3 weeks (F). B, arrow, large secretory alveolus; s, alveolar secretion. C, arrow, columnar alveolar cell; n, polymorphic neutrophil. D, large arrow, alveolar cells; arrow, alveoli; small arrow, apoptotic bodies. E, arrow, alveolar cell with pycnotic nucleus and indistinct cell membrane; a, alveolar lumen containing flocculent material; m, myoepithelial cell; n, neutrophil. F, large arrows, lobules of ductules; e cuboidal epithelial cell; f, fibrocollagenous stroma; small arrows, body defence cells; arrowhead, apoptotic bodies. Scale bar, 50 μm; applies to all panels.
Mammary tissue from glands milked twice daily was lactating in morphology, composed of closely packed secretory alveoli separated by small amounts of interstitial connective tissue (Fig. 1B). Alveolar cells were columnar in shape and possessed a large, apical secretory vesicle. This level of parenchymal organisation was maintained in glands which continued to be milked twice daily throughout the 3 weeks of unilateral milking (Fig. 1B).
After cessation of milking, the unmilked gland maintained a lactating morphology for 3 days (Fig. 1C). However, alveolar size was reduced, which resulted in less-distended myoepithelial cells and more intralobular stromal tissue separating each alveolus compared to those in twice-daily milked glands (Fig. 1B). Residual secretion was present within the lumen of unmilked alveoli, and body defence cells, in particular polymorphic neutrophils, had infiltrated the mammary parenchyma at this stage of milk stasis (Fig. 1C). One week after the cessation of milking, alveolar cells in unmilked glands had lost their columnar shape, and their pale cytoplasm contained a large apical vesicle (Fig. 1D). Their nuclei were intensely stained by haematoxylin, were shrunken in appearance and possessed an ill-defined nucleolus (Fig. 1D). Small, spherical, intensely stained structures characteristic of apoptotic bodies were observed within some alveoli, adjacent to alveolar cells (Fig. 1D). Two weeks after the cessation of milking, alveolar cells in unmilked glands were reduced to an intensely stained pyknotic nucleus and an indistinct cell membrane (Fig. 1E). The myoepithelial cells were prominent and formed a band around each alveolus (Fig. 1E). Numerous body defence cells were present amongst the structurally compromised alveolar cells. By the third week, the parenchymal structure of the unmilked mammary gland was reminiscent of ductal structures of the resting stage of mammary development, since mammary tissue consisted of distended ducts and ductules composed of a single layer of cuboidal epithelium (Fig. 1F). Apoptotic bodies were present in most ductal structures, as were localised masses of body defence cells in and around the mammary parenchyma. Both ducts and ductules were separated by substantial amounts of intra- and interlobular stroma in the form of fibrocollagenous tissue (Fig. 1F).
The staining pattern of mammary tissue from glands milked twice daily did not change during 3 weeks of unilateral milking, and was identical to that of normal lactation tissue (not shown). Despite no immediate changes in histology, immunocytochemical staining for epithelial, myoepithelial and alveolar cell markers changed in unmilked glands after the initiation of involution. Three days after the cessation of milking, less than 5 % of alveolar cells were stained by antisera raised against cytokeratin 7. After 1 week, 25-50 % of alveolar cells were moderately stained along the luminal membrane (Fig. 2A). Following a second week of milk stasis, staining for cytokeratin 7 was localised on the apical half in 50-75 % of alveolar cells. After 21 days, staining for cytokeratin 7 occurred in 25 % of epithelial cells in some ductules (Fig. 2B). In contrast, antiserum to goat β-casein failed to stain any cells 1 week after cessation of milking. Peanut lectin staining patterns in sections not treated with neuraminidase was similar to that of β-casein. The staining in both cases was present along the luminal membrane of the alveolar cells on the third day after the cessation of milking (Fig. 2C), but not thereafter (Fig. 2D). When histological sections of tissue collected 1 or 2 weeks after cessation of milking were treated with neuraminidase to remove terminal sialic acid residues, 10-70 % of alveolar cells were stained. Antisera raised against smooth muscle actin, myosin (Fig. 2E and F) and vimentin stained myo-epithelial cells in unmilked glands at all stages of induced involution (Table 1). Alveolar and epithelial cells were unstained. Myoepithelial cells possessed a distended appearance before and on day 3 of milk stasis, and thereafter became irregular in shape (Fig. 2E and F). This was mirrored by changes in the shape of the basement membrane, which became irregular and convoluted, but remained continuous around each regressing alveolus and ductule, as shown by anti-type IV collagen (Fig. 2G) and anti-laminin staining. Three weeks after cessation of milking, ductules were continuously surrounded by both the myoepithelial cells and the basement membrane (Fig. 2H). Antisera raised to cytokeratin 18 and pan-cytokeratin stained most myoepithelial cells (75-90 %) at all stages of induced involution (Table 1). The number of regressing alveolar cells stained by anti-cytokeratin 18 increased during 2 weeks of milk stasis (results not shown), whilst occasional epithelial cells (5-10 %) were strongly stained in ductules after 3 weeks (Table 1). Pan-cytokeratin antisera did not stain any regressing alveolar or epithelial cells during induced involution (Table 1).
Figure 2. Immunocytochemical and histochemical staining of tissue from unmilked glands after unilateral cessation of milking.
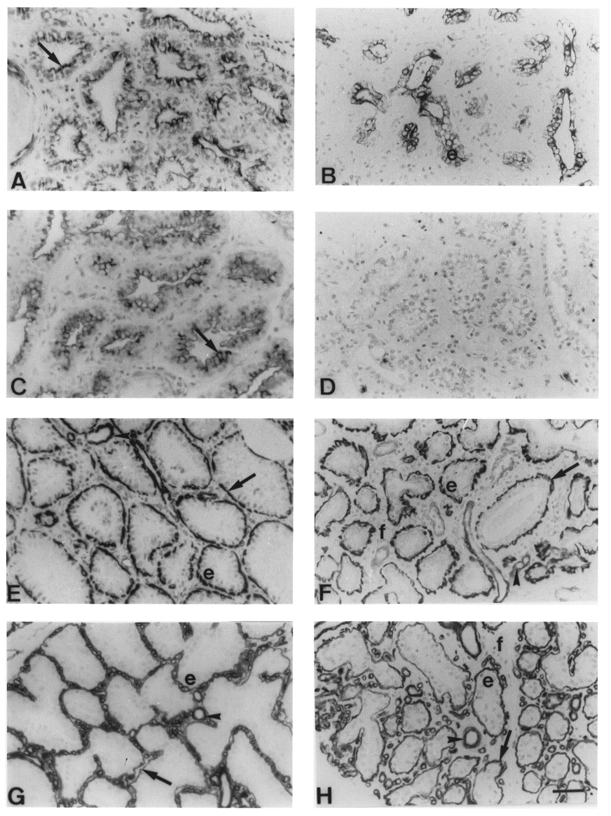
A and B, MAb OVTL 12/30 cytokeratin 7 staining 1 week (A) and 3 weeks (B) after cessation of milking. Arrow, alveolar cell luminal membrane; e, epithelial cell. C and D, horseradish peroxidase-conjugated peanut lectin staining 3 days (C) and 2 weeks (D) after cessation of milking. E and F, smooth muscle myosin Mab hSV-M staining 1 week (E) and 3 weeks (F) after cessation of milking. Arrow, myoepithelial cells; arrowheads, blood vessels; e, alveolar epithelial cells; f, stromal fibroblasts. G and H, type IV collagen MAb CIV22 staining 1 week (G) and 3 weeks (H) after cessation of milking. Arrows, basement membrane; arrowheads, blood vessels; e, alveolar epithelial cells; f, stromal fibroblasts. Scale bar, 50 μm; applies to all panels.
Table 1.
Immunocytochemical staining of lactating goat mammary tissue from glands 0 and 21 days after cessation of milking
| 0 days | 21 days, involuting | |||
|---|---|---|---|---|
| Marker/reagent | Epithelial | Myoepithelial | Epithelial | Myoepithelial |
| Epithelial related | ||||
| Cytokeratin 7 | −a | − | + | − |
| β-Casein | +++ | − | − | − |
| Peanut lectin | +++ | − | +* | − |
| Peanut lectin + N | +++ | − | + | − |
| Myoepithelial related | ||||
| Vimentin | − | + | − | ++ |
| Smooth muscle actin/myosin | − | ++ | − | +++ |
| Type IV collagen/laminin | − | ++b | − | ++b |
| Epithelial/myoepithelial related | ||||
| Cytokeratin 18 | −a | ++ | + | +++ |
| Mab LP34 | −a | ++ | − | +++ |
Abbreviations: Mab, monoclonal antibody; N, neuraminidase. Key: +++, 80–100% cells intensely stained; ++, 10–70% cells intensely stained; +, 80–100% cells diffusely stained; +* 5–10% cells intensely stained, remainder diffusely stained; -, < 5% cells stained.
Cuboidal epithelial cells in alveoli stained
basement membrane adjacent to myoepithelial cells.
The intensity of DNA laddering in lactating goat mammary tissue depended upon the frequency of milking. A higher degree of DNA laddering was present in unmilked glands compared to that in twice-daily milked glands after unilateral cessation of milking (Fig. 3A). Laddering of DNA in unmilked glands increased after the cessation of milking, and was most prominent after the second week (Fig. 3A). This result correlated with the incidence of TUNEL-positive cells in paraffin-embedded tissue sections. End-labelled, fragmented DNA was detected predominantly in alveolar cell nuclei of unmilked glands (Fig. 3B). Thus, nuclei in less than 1 % of alveolar cells were stained for end-labelled fragmented DNA in glands not milked for 3 days, whereas 1 week after cessation of milking, discrete alveolar cell nuclei (2-3 %) and isolated groups of apoptotic bodies were stained in regressing alveoli. TUNEL-positive cells accounted for ∼5 % of cells in each alveolus 2 weeks after the cessation of milking (Fig. 3B). By the third week of involution, the intensity of DNA laddering was reduced, and fewer stained apoptotic cells and bodies were detected. No myoepithelial cell nuclei were end-labelled in twice-daily milked and unmilked glands. Lactating and involuting mouse mammary glands were used as controls (Fig. 3A, C and D) (Quarrie et al. 1996).
Figure 3. DNA laddering and TUNEL-positive cells in milked and unmilked glands after cessation of milking.
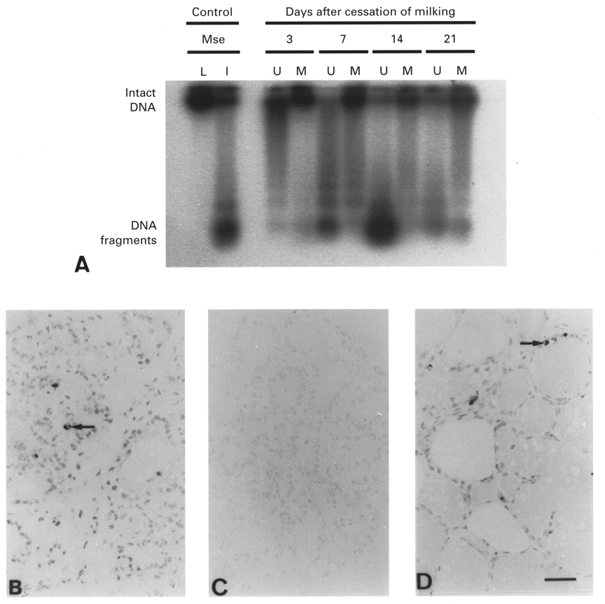
A, DNA laddering in glands milked twice daily (M) or unmilked (U) for 3, 7, 14 and 21 days. Results are representative of 2 animals at each time point. Mammary tissue from 10 day lactating (L) and 4 day involuting (I) mice (Mse) were used as controls. B and D, TUNEL detection of apoptotic cells in goat tissue 14 days after cessation of milking (B), and in mouse mammary tissue on day 10 of lactation (C) and 4 days after litter removal on day 10 (D). Arrows, TUNEL-positive cells. Scale bar, 50 μm; applies to all panels.
Effect of differential once- and thrice-daily milking in lactating goats
The mean daily milk yields of the left and right glands during twice-daily milking in the week preceding differential milking were 1.87 ± 0.11 and 1.94 ± 0.20 kg day−1 (n = 6), respectively (Fig. 4A). When once- (right glands) and thrice-daily (left glands) milking was initiated, the milk yield of the more frequently milked glands increased whilst that of the infrequently milked gland declined rapidly. Thereafter, milk yield of both once- and thrice-milked glands declined progressively (Fig. 4A), as expected at this stage of lactation, such that after 10 weeks of differential milking, the milk yield in thrice-daily and once-daily milked glands was 1.74 ± 0.12 and 1.03 ± 0.14 kg day−1, respectively (n = 6; P < 0.001; ANOVA).
Figure 4. Milk yield and mammary histology in goats milked once and thrice daily from the two glands.
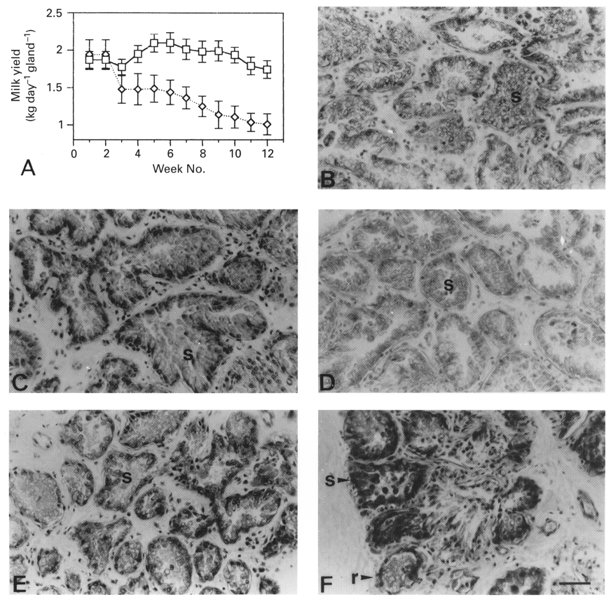
A, mean ±s.e.m. (n = 6) daily milk yield of glands milked twice daily for 2 weeks before milking of one gland thrice daily (□) and the other once daily (⋄) for 10 weeks. B-F, histology of alveoli in tissue sections from glands milked twice daily (B), then thrice daily (C and E) or once daily (D and F) for 4 weeks (C and D) or 10 weeks (E and F). s, secretory alveoli; r, regressing alveoli. Scale bar, 50 μm; applies to all panels.
Prior to differential milking, the left and right mammary glands were similar in morphology and possessed an appearance typical of lactating tissue (Fig. 4B). Mammary tissue from both glands was composed of a branched ductal system terminating in lobules of secretory alveoli which consisted of columnar alveolar cells surrounding a secretion-filled lumen (Fig. 4B). After 4 weeks of differential milking, a lactating morphology was retained by both glands, but the once-daily milked glands possessed smaller alveoli (Fig. 4D) compared to those in thrice-daily milked glands (Fig. 4C). To quantify this difference in alveolar size between glands, the numbers of alveolar cells were counted in approximately 100 separate randomly selected alveoli in once- and thrice-daily milked glands. There were more alveolar cells in alveoli from thrice-daily than in alveoli from once-daily milked glands (26 ± 7 and 19 ± 4 cells per alveolus in thrice-daily and once-daily milked glands (n = 6), respectively; P < 0.05; Student's paired t test).
When differential milking was continued for a total of 10 weeks, once-daily milked glands contained terminal structures which were heterogeneous in composition (Fig. 4F). Some structures consisted of small, regressing alveoli, which contained alveolar cells that had lost their columnar shape and appeared to be involuting, but some structures retained a lactating morphology. In addition, some terminal structures, based on their histological appearance, consisted of resting ductules and contained a single layer of cuboidal epithelial cells (Fig. 4F). The majority of terminal structures in thrice-daily milked glands after 10 weeks consisted of secretory alveoli which were also smaller than those at 4 weeks of thrice-daily milking (Fig. 4E).
Immunocytochemically, mammary tissue from glands milked twice daily was characteristic of lactating tissue (Table 2). Cytochemical staining of mammary tissue produced by once- and thrice-daily milking changed unilaterally during the 10 weeks of differential milking. Immunocytochemical staining for cytokeratin 7 was observed in 50-75 % of columnar alveolar and epithelial cells in secretory and regressing alveoli and in resting ductules of once-daily milked glands at 4 weeks (Fig. 5B) and 10 weeks (Fig. 5D) of differential milking. In contrast, only 5-25 % of alveolar cells were stained by antisera for cytokeratin 7 at 4 weeks of thrice-daily milking (Fig. 5A) and this proportion increased to 25-50 % of alveolar cells in alveoli at 10 weeks of frequent milking (Fig. 5C). HRP-conjugated peanut lectin stained columnar alveolar cells intensely in secretory alveoli from once- and thrice-daily milked glands after 4 and 10 weeks of differential milking (Fig. 5E -H). The large numbers of regressing alveoli and resting ductules which were present in glands after 10 weeks of once-daily milking did not, however, bind peanut lectin (Fig. 5H). A proportion of regressing alveolar/epithelial cells (5-25 %) in these terminal structures were stained by HRP-conjugated peanut lectin only on desialylation of the sections by neuraminidase treatment (Table 2). Antiserum to goat casein stained 90-100 % of alveolar cells in secretory alveoli in once- and thrice-daily milked glands after 4 weeks, but it did not stain alveolar/epithelial cells of involuting alveoli and regressing ductules in once-daily milked glands after 10 weeks (Table 2).
Table 2.
Immunocytochemical staining of mammary tissue from lactating goats milked twice daily at 0 weeks once and thrice daily at 10 weeks
| 0 weeks | 10 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Twice | Thrice | Once | ||||||||
| Alveolar | Alveolar | Alveolar | Alv, involuting | Ductule | ||||||
| Marker/reagent | Epithel | Myo | Epithel | Myo | Epithel | Myo | Epithel | Myo | Epithel | Myo |
| Epithelial related | ||||||||||
| Cytokeratin 7 | −a | − | ++a | − | ++ | − | ++ | − | +* | − |
| β-Casein | +++ | − | +++ | − | ++ | − | − | − | − | − |
| Peanut lectin | +++ | − | +++ | − | +++ | − | − | − | − | − |
| Peanut lectin + N | +++ | − | +++ | − | +++ | − | ++ | − | + | − |
| Myoepithelial related | ||||||||||
| Vimentin | − | + | − | + | − | + | − | ++ | − | ++ |
| Smooth muscle actin/myosin | − | ++ | − | ++ | − | ++ | − | +++ | − | +++ |
| Type IV collagen/laminin | − | +++b | − | +++b | − | +++b | − | ++b | − | ++b |
| Epithelial/myoepithelial-related | ||||||||||
| Cytokeratin 18 | −a | ++ | +a | ++ | +a | ++ | ++ | ++ | +++ | +++ |
| Mab LP34 | +*a | ++ | +*a | ++ | +*a | ++ | ++ | ++ | +++ | +++ |
| TUNELc | − | − | + | − | − | − | +* | − | − | − |
Paraffin-embedded tissue sections were obtained from both twice-daily milked mammary glands prior to differential milking (n = 6 goats). Abbreviations: Epithel, epithelial; Myo, myoepithelial; N, neuraminidase. Key: +++, 80–100% cells intensely stained; ++, 10–70% cells intensely stained; +, 80–100% cells diffusely stained; +* 5–10% cells intensely stained, remainder diffusely stained; -, < 5% cells stained.
Cuboidal epithelial cells in alveoli stained
basement membrane adjacent to myoepithelial cells
in situ end-labelled DNA strand breaks in nuclei.
Figure 5. Immunocytochemical and histochemical staining of mammary tissue from lactating goats milked once and thrice daily.
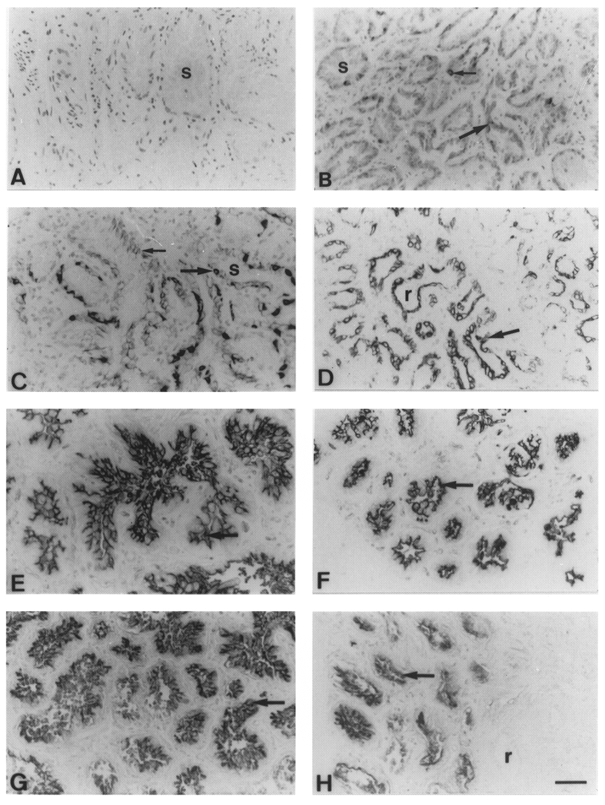
A-D, with MAb OVTL 12/30 to cytokeratin 7 and with horseradish peroxidase-conjugated peanut lectin. Cytokeratin 7 staining with MAb OVTL 12/30 in glands milked thrice daily (A and C) or once daily (B and D) for 4 weeks (A and B) or 10 weeks (C and D). s, secretory alveoli; large arrow, columnar alveolar cells; small arrow, cuboidal alveolar cells; r, resting ductules. E-H, peanut lectin staining in glands milked thrice daily (E and G) or once daily (F and H) for 4 weeks (E and F) or 10 weeks (G and H). Arrow, secretory alveoli; r, resting ductule. Scale bar, 50 μm; applies to all panels.
Antisera to smooth muscle actin, myosin and vimentin stained myoepithelial cells. These appeared smooth and distended around each alveolus in thrice-daily milked glands (Fig. 6A), whereas those in once-daily milked glands (Fig. 6B) were irregular in shape and continuous around each alveolus after 4 weeks of differential milking. After a further 6 weeks of once-daily milking, myoepithelial cells assumed a cube-like shape and their continuity round each resting ductule and secretory and regressing alveolus was maintained (Fig. 6D), whilst little or no changes in shape were observed in those of thrice-daily milked glands for the same time period (Fig. 6C). Changes in the structure of the anti-laminin-stained and anti-type IV collagen-stained basement membrane were also observed during differential milking. In thrice-daily milked glands, the basement membrane surrounding each alveolus was smooth and highly defined after 4 weeks of milking (Fig. 6E). The structures of the basement membrane after 4 weeks of once-daily milking (Fig. 6F) and after 10 weeks of thrice-daily milking (Fig. 6G) were similar, being smooth and continuous but slightly convoluted around each secretory alveolus. After 10 weeks of once-daily milking, however, the basement membrane was irregular in shape around each terminal structure (Fig. 6H).
Figure 6. Immunocytochemical staining of myosin and actin in goat mammary glands milked once and thrice daily.
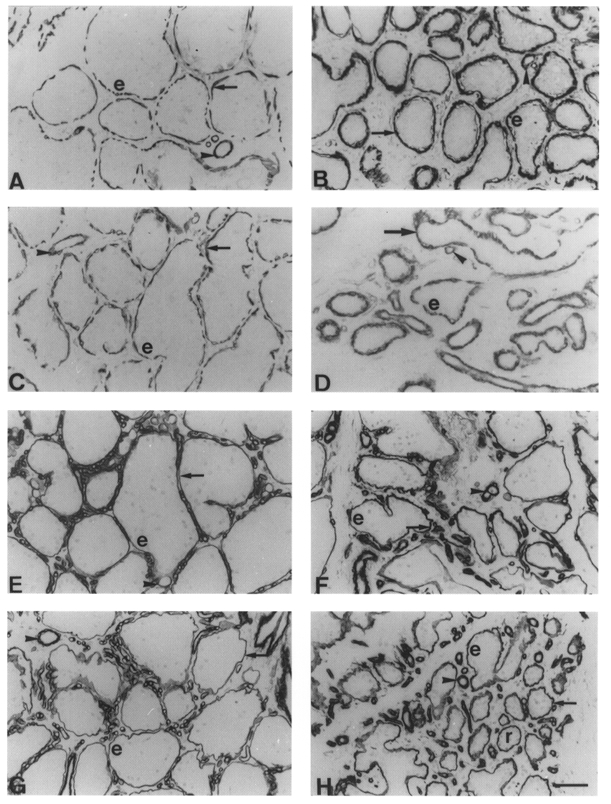
A-D, smooth muscle myosin staining with MAb hSV-Min in glands milked thrice daily (A and C) or once daily (B and D) for 4 weeks (A and B) or 10 weeks (C and D). Arrows, myoepithelial cells; arrowheads, blood vessels; e, epithelial cells. E-H, type IV collagen staining with MAb CIV22 in glands milked thrice daily (E and G) or once daily (F and H) for 4 weeks (E and F) or 10 weeks (G and H). Arrows, basement membrane; arrowheads, blood vessels; e, epithelial cells; r, resting ductules. Scale bar, 50 μm; applies to all panels.
Immunocytochemical staining of alveolar cells by antisera to cytokeratin 18 and pan-cytokeratin varied according to the morphological state of the terminal structure. Alveolar cells in secretory alveoli of once- and thrice-daily milked glands after 4 and 10 weeks were moderately stained by anti-cytokeratin 18 (Table 2). However, regressing alveolar cells in involuting alveoli and epithelial cells in ductules of once-daily milked glands were intensely stained by cytokeratin 18 antisera (Table 2). Antisera to pan-cytokeratin stained epithelial-related cells in regressing ductules and, occasionally, cuboidal epithelial cells in alveoli after 10 weeks of once-daily milking (Table 2). Both antisera to cytokeratin 18 and pan-cytokeratin strongly stained 75-90 % of myoepithelial cells in all terminal structures during once- and thrice-daily milking (Table 2).
Laddering of fragmented DNA detected by end-labelling differed between glands milked once and thrice daily. Prior to differential milking, both twice-daily milked glands contained small amounts of fragmented DNA (Fig. 7). After 4 weeks of differential milking, significant DNA laddering was present in once-daily milked glands, but not in thrice-daily milked glands (Fig. 7). This was consistent with the presence of moderately stained, end-labelled alveolar cell nuclei (∼2-5 %) and apoptotic bodies only in once-daily milked glands, and not in thrice-daily milked glands. After 10 weeks of once-daily milking, weak staining of alveolar cell nuclei and of apoptotic bodies was restricted to discrete lobules of alveoli which possessed an involuting morphology (Table 2). Isolated compacted nuclei of regressing alveolar cells were also weakly stained in alveoli from thrice-daily milked glands at this stage (Table 2). Both once- and thrice-daily milked glands had small amounts of DNA fragmentation after 10 weeks of differential milking, similar to those observed before differential milking was undertaken (Table 2). Myoepithelial cell nuclei were not end-labelled in glands milked once or thrice daily for 4 or 10 weeks (Table 2).
Figure 7. DNA laddering in goat mammary tissue before and after differential once- and thrice-daily milking.
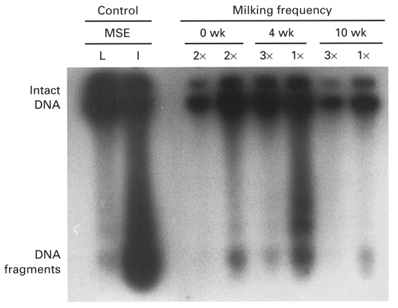
Both glands were milked twice daily before milking of one gland thrice daily and the other once daily for 10 weeks. Results are representative of tissue sampled bilaterally in 6 goats at each stage. Controls are 10 days lactating (L) and 4 days involuting (I) mouse (Mse) mammary tissue.
DISCUSSION
A heterogeneous pattern of immunocytochemical and histochemical staining by epithelial, myoepithelial and alveolar cell markers was observed in lactating goat mammary tissue influenced by unilateral and differential milking. A similar staining pattern was found in mammary tissue fixed in modified Methacarn and buffered formalin (results not shown), so this cellular heterogeneity is unlikely to be an artifact of tissue preservation. No changes in staining pattern were observed when the concentrations of antisera were increased, and different antisera raised to the same or similar molecules gave identical staining patterns. All staining was inhibited when each antiserum was blocked by the appropriate antigen/competitor. The proteolytic treatment of sections for cytoskeletal, intermediate filamental and basement membrane proteins enhanced, but did not alter the overall staining pattern of individual cells.
In late lactation, milk stasis in one gland caused rapid, ipsilateral, regression of the secretory alveoli. Alveolar regression was associated with apoptosis of the secretory cells, as demonstrated by histology, by DNA fragmentation in tissue extracts, and by the presence of TUNEL-positive alveolar cells. Apoptosis was not, however, restricted to the unmilked gland. Occasional TUNEL-positive cells and a low level of DNA laddering were observed prior to unilateral milking, and persisted in the milked gland throughout the treatment period. The presence of apoptotic cells in milked glands probably accounts for the net decrease in goat mammary cell number during declining lactation (Knight & Peaker, 1984). As falling cell number is the principal determinant of declining milk yield at this stage of lactation (Wilde & Knight, 1989), apoptosis is, therefore, likely to be an important determinant of ruminant milk yield. The late stage of lactation at which unilateral milk stasis was imposed is likely to account for the absence of any compensatory increase in milk yield in the contralateral gland, and has been ascribed to a relative insensitivity to developmental stimuli at this stage of lactation (Hamann & Reichmuth, 1990).
The apoptotic response differed from that previously reported for goat mammary tissue (Quarrie et al. 1994) in that apoptosis was induced within days, rather than after 2 weeks of milk stasis. This apparent difference is due to the sensitivity of the methods employed to detect apoptosis. Ethidium bromide staining of DNA ladders used in the previous study is considerably less sensitive than the radioisotopic labelling employed here (Gavrieli et al. 1992; Quarrie et al. 1995). Visualisation of fragmented DNA after 3 and 7 days of milk stasis indicates that in the goat, as in rodents, apoptosis is not necessarily a late response to milk stasis. In mice, teat-sealing induced apoptosis within 1-2 days, coincident with loss of differentiated gene expression (Travers et al. 1996). By comparison, milk protein gene expression in ruminants fell over 7 days when milking was stopped (Wilde et al. 1997), and DNA laddering appears to be induced by milk stasis with a similar time course.
Local control of mammary development was also evident in lactating goats milked once and thrice daily from the two glands. Whereas thrice-daily milking maintained a uniformly lactating histological appearance, once-daily milking of lactating glands resulted in regression of alveolar cells in selected alveoli, an observation consistent with gross biochemical measurements indicating that the reduction in milk yield with once-daily milking was due to partial de-differentiation of secretory alveolar cells (Wilde & Knight, 1990). The presence of a heterogeneous population of secretory and non-secretory terminal structures, i.e. involuting alveoli and resting ductules, in once-daily milked glands, was substantiated by variations in the ability of these terminal structures to bind peanut lectin, to be stained cytochemically by monoclonal antibodies against cytokeratins, by antisera to goat casein and by end-labelling of DNA in cell nuclei. However, the present study also showed that adaptation to infrequent milking also involves a decrease, within 4 weeks, in the number of alveolar cells. The temporary increase in DNA laddering evident at this time, and the presence of TUNEL-positive cells, indicates that this cell loss, during lactation, was by apoptosis. After the initial rapid divergence of the yields of thrice- and once-daily milked glands, milk yield in the two glands then declined at similar rates. This suggests that after 10 weeks of differential milking, a new dynamic balance may have been established. After an initial accelerated loss of secretory cells in the once-milked gland, the two glands may then have lost cells at similar rates, such that there was no further divergence of milk yield between glands at this point.
The heterogeneity of alveolar structures observed in goat mammary glands milked once daily has parallels in other species. Asynchronous alveolar function and development has been reported in ultrastructural studies of the mid-pregnant rodent mammary gland (Mills & Topper, 1970), and in situ hybridisation demonstrated a heterogeneous pattern of milk protein gene expression in sheep and bovine mammary tissue (Molenaar et al. 1992). Joseph & Collet (1994) observed in Tammar wallaby mammary tissue that for any secretory alveolus, all milk proteins could be produced, but not necessarily at the same levels in adjacent alveoli. This is in contrast to immuno-histochemical studies of mammary tissue from lactating women (Rudland & Hughes, 1989) which showed that mammary alveolar cells in all alveoli appeared homogeneous and were able to secrete milk proteins simultaneously. These reports may indicate interspecies differences in alveolar development and differentiation between rodents, primates and marsupials, possibly related to the hierarchy of systemic and local control mechanisms. However, because of the different experimental approaches used, the results may not be wholly comparable to those of this present study.
The results of the study show that one fate of secretory cells in lactating mammary tissue is cell death by apoptosis. Another possible fate is dedifferentiation to an uncommitted epithelial cell phenotype. This is suggested by our observation that some ductal epithelial cells bound peanut lectin cytochemically, albeit only after digestion with neuraminidase, whereas normally ductal epithelial cells do not bind to this lectin. In these cells, the carbohydrate receptor for peanut lectin, which is present on the apical surface of the alveolar cell in secretory alveoli, had been masked by sialic acid and had moved into the cytoplasm during involution. This is the converse of that observed in rodent alveolar development, where epithelial cells in alveolar buds are stained by HRP-peanut lectin only after treatment with neuraminidase but alveoli generated from alveolar buds during pregnancy do not require neuraminidase treatment for cells to be stained by the lectin. This observation is explained by the existence of a sialylated form of the peanut lectin receptor on the surface of epithelial cells in alveolar buds, and the desialylation of this receptor on the secretory cell surface in alveoli during lactation (Rudland, 1992). Goat alveolar dedifferentiation during the late stages of gradual and induced involution is also supported by the presence of ductules of epithelial cells that, like virgin and pregnant mammary epithelia (Li et al. 1999), were stained by cytokeratin antisera. Thus, staining for cytokeratin was reinstated in ductal epithelial cells which had originated by dedifferentiation of alveolar cells during involution. That a population of secretory alveolar cells revert back to an epithelial phenotype agrees with studies of mammary growth using magnetic resonance imaging, which showed that the goat's udder does not revert back to its virgin state after the first lactation (Fowler et al. 1990). The udder commenced the second lactation larger than at the start of the first cycle, a finding which suggests that some pre-alveolar epithelial cells were carried over to the succeeding lactation.
Myoepithelial cells were present in goat mammary tissue at all stages of gradual involution induced by infrequent milking and throughout induced involution precipitated by milk stasis. This is in agreement with other studies which have suggested that myoepithelial cells act as a framework to hold surviving alveolar cells together, and therefore contribute to the organisation of the glandular structure. Ultrastructural studies show that this was achieved by the myoepithelial cell processes across the gaps in alveolar structures where secretory cells had degenerated or become detached (Radnor, 1972). It is not known whether all myoepithelial cells present in lactating goat mammary gland prior to regression are also present at the end of involution, but in situ end-labelling methods did not detect any myoepithelial cell apoptosis during gradual or induced goat involution. This observation agrees with the increased cytochemical staining for myoepithelial markers, and suggests a higher proportion of myoepithelial cells to alveolar/epithelial cells are present as involution progressed. It is possible that some myoepithelial cells, particularly those that may not be completely differentiated, could revert into ductal epithelial cells during this stage of mammary development. The observation that antisera to cytokeratin 18 and pan-cytokeratin stain both myoepithelial and ductular epithelial cells during involution, but only myoepithelial cells during early lactation (Li et al. 1999), makes this interpretation plausible.
Gradual involution induced by infrequent milking, and rapid involution caused by milk stasis were each associated with changes in basement membrane structure. From a thin, well-defined appearance in lactation, the basement membrane became thicker and convoluted in shape, whilst remaining continuous around each alveolus during involution. This appears to contrast with involution in rodent mammary tissue, in which removal of the basement membrane through degradation by activated metalloproteinases (Martinez-Hernandez et al. 1976; Talhouk et al. 1992) coincides with the time of maximal cell death by apoptosis (Strange et al. 1992). It is now clear, however, that rodent mammary apoptosis following litter removal precedes basement membrane degradation (Lund et al. 1996; Quarrie et al. 1996), such that there are protease-dependent and -independent phases of mammary involution (Lund et al. 1996). Secretory cell apoptosis in bovine mammary tissue also proceeds without widespread tissue degeneration (Wilde et al. 1997). Therefore, basement membrane degradation appears not to be a prerequisite for mammary apoptosis, although it may promote it (Pullan et al. 1996). Changes in basement membrane composition not detected by immunohistochemistry in the present study may, however, influence the susceptibility of individual cells or particular alveoli to involution and apoptosis. For example, milk stasis initially causes alveolar distension and basement membrane stretching, and may thereby alter the contact between epithelial cells and the basement membrane.
The present study highlights the importance of local mechanisms in the control of mammary development, both at individual-gland and alveolar levels. It is apparent that both cellular differentiation and apoptosis are controlled at these levels by mechanisms that modulate the strategic control of mammary development by mammogenic and galactopoietic hormones. The adaptations to infrequent milking and milk stasis also substantiate circumstantial evidence that a proportion of secretory alveolar cells undergo dedifferentiation, and so contribute to the mammary cell population in the following lactation. There is at present no mechanistic explanation for the asynchronous involution observed during infrequent milking, but it may be due to localised milk stasis, and mediated by physical distension or local chemical feedback by milk constituents in poorly drained alveoli. One candidate regulator is FIL, the feedback inhibitor of lactation (Wilde et al. 1995). FIL regulates milk secretion acutely, but has also been found to influence both cellular differentiation and apoptosis in mammary cell cultures (C. J. Wilde, unpublished), an action that may be mediated by local modulation of mammogenic hormone receptors (Bennett et al. 1991). It may therefore be significant that Molenaar et al. (1992) suggested that the alveolar heterogeneity in bovine milk protein gene expression they observed in ruminant mammary tissue could be explained on the basis of interalveolar differences in prolactin binding. These putative mechanisms are the subject of further investigation.
Acknowledgments
The authors are grateful to Professor C. H. Knight, Hannah Research Institute, for performing biopsies and to Mrs Angela Platt-Higgins for help in the immunocytochemical studies. P.L. was the recipient of a BBSRC research studentship. The work was supported, in part, by the Cancer and Polio Research Fund, the NorthWest Cancer Research Fund and the Scottish Office Agriculture Environment and Fisheries Department.
References
- Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. American Journal of Pathology. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Knight CH, Wilde CJ. Regulation of prolactin binding by secreted milk proteins. Journal of Endocrinology. 1991;127:141. supplement. [Google Scholar]
- Bussolati G, Alfani V, Weber K, Osborn M. Immunocytochemical detection of actin on fixed and embedded tissues: its potential use in routine pathology. Journal of Histochemistry and Cytochemistry. 1980;28:169–173. doi: 10.1177/28.2.6986431. [DOI] [PubMed] [Google Scholar]
- Earl H, McIlhinney RAJ. Monoclonal antibodies to human casein. Molecular Immunology. 1985;22:981–991. doi: 10.1016/0161-5890(85)90086-0. [DOI] [PubMed] [Google Scholar]
- Foster CS, Edwards PAW, Dinsdale EA, Neville AM. Monoclonal antibodies to the human mammary gland: I. Distribution of determinants in non-neoplastic mammary and extra-mammary tissues. Virchows Archives of Pathology and Anatomy. 1982;394:279–293. doi: 10.1007/BF00430671. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Knight CH, Cameron GG, Foster MA. In vivo studies of mammary development in the goat using magnetic resonance imaging (MRI) Journal of Reproduction and Fertility. 1990;89:367–375. doi: 10.1530/jrf.0.0890367. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben Sasson SA. Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. Journal of Cell Biology. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson BA, Monaghan P, Mahendran R, Ellis J, O'Hare MJ. Identification of myoepithelial cells in human and rat breasts by anti-common acute lymphoblastic leukaemia antigen antibody A12. Journal of the National Cancer Institute. 1986;77:343–349. [PubMed] [Google Scholar]
- Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Research. 1982;42:4763–4770. [PubMed] [Google Scholar]
- Hamann J, Reichmuth J. Compensatory milk production within the bovine udder: effects of short-term non-milking of single quarters. Journal of Dairy Research. 1990;57:17–22. doi: 10.1017/s002202990002656x. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques. Journal of Histochemistry and Cytochemistry. 1981;29:577. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Joseph R, Collet C. Double staining in situ study of mRNAs encoding milk proteins in the mammary gland of the tammar wallaby (Macropus eugenii) Journal of Reproduction and Fertility. 1994;101:241–246. doi: 10.1530/jrf.0.1010241. [DOI] [PubMed] [Google Scholar]
- Knight CH, Peaker M. Mammary development and regression during lactation in goats in relation to milk secretion. Quarterly Journal of Experimental Physiology. 1984;69:331–338. doi: 10.1113/expphysiol.1984.sp002809. [DOI] [PubMed] [Google Scholar]
- Li P, Wilde CJ, Fernig DG, Finch LMB, Rudland PS. Identification of cell types in the developing goat mammary gland: evidence for a stem cell type during lactation. Histochemical Journal. 1999. in the Press. [DOI] [PubMed]
- Lund LR, Rømer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Danø K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Funk LM, Pierce GB. Removal of basement membrane in the involuting breast. Laboratory Investigation. 1976;34:455–462. [PubMed] [Google Scholar]
- Mills ES, Topper YJ. Mammary alveolar epithelial cells: effect of hydrocortisone on ultrastructure. Science. 1970;165:1127–1128. doi: 10.1126/science.165.3898.1127. [DOI] [PubMed] [Google Scholar]
- Molenaar AJ, Davis SR, Wilkins RJ. Expression of alpha-lactalbumin, alpha-S1-casein, and lactoferrin genes is heterogeneous in sheep and cattle mammary tissue. Journal of Histochemistry and Cytochemistry. 1992;40:611–618. doi: 10.1177/40.5.1374090. [DOI] [PubMed] [Google Scholar]
- Nagle RB, Brocker W, Davis JR, Heid HW, Kaufman M, Lucas DO, Jarasch E-D. Characterisation of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. Journal of Histochemistry and Cytochemistry. 1986;34:869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Newman RA, Klein PJ, Rudland PS. Binding of peanut lectin to breast epithelium, human carcinomas and a cultured rat mammary cell line: use of lectin as a marker of mammary differentiation. Journal of the National Cancer Institute. 1979;63:1339–1346. [PubMed] [Google Scholar]
- Ormerod EJ, Rudland PS. Cellular composition and organisation of ductal buds in developing rat mammary glands: evidence for morphological intermediates between epithelial and myoepithelial cells. American Journal of Anatomy. 1984;170:631–652. doi: 10.1002/aja.1001700408. [DOI] [PubMed] [Google Scholar]
- Peaker M, Wilde CJ, Knight CH. Local control of the mammary gland. In: Rudland PS, Fernig DG, Leinster S, Lunt GG, editors. Mammary Development and Cancer. London: Portland Press; 1998. pp. 71–79. [Google Scholar]
- Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. Journal of Cell Science. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- Quarrie LH, Addey CVP, Wilde CJ. Local regulation of mammary apoptosis in the lactating goat. Biochemical Society Transactions. 1994;22:178. doi: 10.1042/bst022178s. S. [DOI] [PubMed] [Google Scholar]
- Quarrie LH, Addey CVP, Wilde CJ. Apoptosis in lactating and involuting mouse mammary tissue detected by nick-end DNA labelling. Cell and Tissue Research. 1995;281:413–419. doi: 10.1007/BF00417859. [DOI] [PubMed] [Google Scholar]
- Quarrie LH, Addey CVP, Wilde CJ. Programmed cell death during mammary involution induced by weaning, litter removal and milk stasis. Journal of Cellular Physiology. 1996;168:559–569. doi: 10.1002/(SICI)1097-4652(199609)168:3<559::AID-JCP8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Radnor CJP. Myoepithelial cell differentiation in rat mammary glands. Journal of Anatomy. 1972;111:381–398. [PMC free article] [PubMed] [Google Scholar]
- Rudland PS. Histochemical organisation and cellular composition of ductal buds in developing human breast: evidence of cytochemical intermediates between epithelial and myoepithelial cells. Journal of Histochemistry and Cytochemistry. 1991;39:1471–1484. doi: 10.1177/39.11.1918925. [DOI] [PubMed] [Google Scholar]
- Rudland PS. Use of peanut lectin and rat mammary stem cell lines to identify a cellular differentiation pathway for the alveolar cell in the rat mammary gland. Journal of Cellular Physiology. 1992;153:157–168. doi: 10.1002/jcp.1041530120. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Hughes CM. Immunological identification of cell types in human mammary gland: variations in cellular markers are dependent on glandular topography and differentiation. Journal of Histochemistry and Cytochemistry. 1989;37:1087–1100. doi: 10.1177/37.7.2471725. [DOI] [PubMed] [Google Scholar]
- Sloane JB, Ormerod MG. Distribution of epithelial membrane antigen in normal and neoplastic tissues and its value in diagnostic tumour pathology. Cancer. 1981;47:1786–1796. doi: 10.1002/1097-0142(19810401)47:7<1786::aid-cncr2820470711>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:1383–1395. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. Journal of Cell Biology. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Lane EB. Keratin expression in the mammary gland. In: Neville MC, Daniel CW, editors. The Mammary Gland: Development and Function. New York: Plenum Press; 1987. pp. 291–323. [Google Scholar]
- Travers MT, Barber MC, Tonner E, Quarrie L, Wilde CJ, Flint DJ. The role of prolactin and growth hormone in the regulation of casein gene expression and mammary cell survival: relationships to milk synthesis and secretion. Endocrinology. 1996;137:1530–1539. doi: 10.1210/endo.137.5.8612482. [DOI] [PubMed] [Google Scholar]
- Walker NI, Harmon BV, Gobe GC, Kerr JFR. Patterns of cell death. Methods and Achievements in Experimental Pathology. 1988;13:18–54. [PubMed] [Google Scholar]
- Warburton MJ, Mitchell D, Ormerod EJ, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating and involuting rat mammary gland. Journal of Histochemistry and Cytochemistry. 1982;30:667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Addey CVP, Boddy LM, Peaker M. Autocrine regulation of milk secretion by a protein in milk. Biochemical Journal. 1995;305:51–58. doi: 10.1042/bj3050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde CJ, Addey CVP, Li P, Fernig DG. Programmed cell death in bovine mammary tissue during lactation and involution. Experimental Physiology. 1997;82:943–953. doi: 10.1113/expphysiol.1997.sp004075. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Henderson AJ, Knight CH, Blatchford DR, Faulkner A, Vernon RJ. Effects of long-term thrice-daily milking on mammary enzyme activity, cell population and milk yield. Journal of Animal Science. 1987;64:533–539. doi: 10.2527/jas1987.642533x. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Knight CH. Metabolic adaptations in mammary gland during the declining phase of lactation. Journal of Dairy Science. 1989;72:1679–1692. doi: 10.3168/jds.S0022-0302(89)79279-1. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Knight CH. Milk yield and mammary function in goats during and after once-daily milking. Journal of Dairy Research. 1990;57:441–447. doi: 10.1017/s0022029900029484. [DOI] [PubMed] [Google Scholar]


