Abstract
The hypothesis that potassium ions mediate activity-dependent increases of cerebral blood flow was examined in rat cerebellar cortex using ion-selective microelectrodes and laser-Doppler flowmetry. Increases of cerebellar blood flow (CeBF) and extracellular potassium concentration ([K+]o) were evoked by stimulation of parallel fibres and climbing fibres, and by microinjection of KCl into the cortex.
For parallel fibre stimulation, there was a maximal increase in [K+]o to 6.3 ± 0.5 mm and in CeBF of 122 ± 11%. Climbing fibre stimulation gave a maximal increase in [K+]o to 4.4 ± 0.2 mm and in CeBF of 157 ± 20%. This indicates different maxima for [K+]o and CeBF, dependent on the afferent system activated.
[K+]o and CeBF responses evoked by parallel or climbing fibre stimulation increased rapidly at the onset of stimulation, but exhibited different time courses during the remainder of the stimulation period and during return to baseline.
Microinjections of KCl into the cortex increased [K+]o to levels comparable to those evoked by parallel fibre stimulation. The corresponding CeBF increases were the same as, or smaller than, for parallel fibre stimulation, and much smaller than for climbing fibre stimulation. This suggests that mediators other than [K+]o are important for activity-dependent cerebral blood flow increases.
The present study showed that increased [K+]o is involved in CeBF regulation in the parallel fibre system, but is of limited importance for CeBF regulation in the climbing fibre system. The hypothesis that K+ is a major mediator of activity-dependent blood flow increases is probably not generally applicable to all brain regions and all types of neuronal stimulation.
In preceding papers we provided several categories of information about the relationship between neuronal activity and corresponding increases in cerebellar blood flow (CeBF). First we showed that activity-dependent increases of CeBF were partly dependent on postsynaptic activity and on preserved nitric oxide (NO) synthesis (Akgoren et al. 1996). Adenosine appeared to be involved as well depending on the strength of the synaptic input and the distribution of the afferent input system on the main target cell (Akgoren et al. 1997). However, neither NO nor adenosine, nor the combined action of the two vasodilators, explained the full CeBF response. In a recent paper we found that activity-dependent increases in CeBF were not necessarily linked to increased spiking activity in principal neurones, but a strong correlation could be demonstrated between the maximal amplitude of the recorded field potentials and the maximal increase in CeBF (Mathiesen et al. 1998). This suggested that transmembrane and extracellular ion fluxes associated with the generation of synaptic potentials and action potentials could be a key to an understanding of activity-dependent increases in CeBF. Accordingly we have examined whether concentration changes of extracellular K+ could account for the CeBF rise.
In order to serve as an important mediator of cerebral vasodilatation, a substance should (1) be a potent vasodilator; (2) be produced and released in response to increased neuronal activity; (3) show temporal changes in tissue concentration that closely follow the changes of blood flow; (4) reach concentrations in the vicinity of the vessels that increase blood flow to the same degree as observed with identical concentrations of exogenously administered substance.
It is well known that K+ is a potent vasodilator in the cerebral circulation (Kuschinsky et al. 1972), and several basic processes of excited nerve cells, e.g. action potentials and synaptic transmission, result in increased [K+]o (Nicholson, 1980). Increased [K+]o is immediately buffered by extracellular diffusion, ionic currents in glial cells (the spatial buffering mechanism; Gardner Medwin & Nicholson, 1983), and active uptake of K+ by glial cells due to increased activity of the Na+-K+ pump (Hertz, 1992). Increases of [K+]o induce vasodilatation in surrounding blood vessels by diffusion through the extracellular space or through astrocyte siphoning of K+ to the arteriole wall (Paulson & Newman, 1987). Elevations of [K+]o in the microenvironment of the vascular wall hyperpolarize the cell membrane of vascular smooth muscle through activation of the Na+-K+ pump, and through inwardly rectifying K+ channels (Webb & Bohr, 1978; Edwards et al. 1988; McCarron & Halpern, 1990). The delayed rectifier potassium channel also contributes to relaxation of cerebral vessels in response to modest increases in [K+]o (Faraci & Heistad, 1997). As a consequence of smooth muscle hyperpolarization, voltage-dependent Ca2+ channels close, the intracellular Ca2+ concentration decreases, and smooth muscle relaxation and vasodilatation occur (Dirnagl & Dreier, 1997). Besides its direct action on the blood vessel, elevation of [K+]o also increases metabolism by stimulating the Na+-K+-ATPase (Somjen, 1979; Walz & Hertz, 1983; Hertz, 1992), which leads to increased production of the potent vasodilators lactate, adenosine and H+ (Kuschinsky, 1988; Lassen, 1991; Magistretti, 1997). Thus, potassium fulfils the first two criteria for being the coupling agent between nerve cell activity and CeBF.
The present study examined the last two criteria by providing detailed information about the temporal and quantitative relationship between the functional increases of [K+]o and CeBF in the cerebellar cortex. We examined [K+]o with ion-selective microelectrodes and CeBF with laser-Doppler flowmetry, i.e. methods sharing the same temporal resolution (Nicholson & Rice, 1988; Nilsson, 1990). The purpose was to establish the real-time relationship between the activity-dependent increases of the two variables. We reasoned that if [K+]o regulates activity-dependent increases of CeBF, then [K+]o and CeBF should increase proportionally and roughly follow the same time course during activation. Furthermore, we hypothesized that if K+ were indeed the major vasodilator, then it should be possible to reproduce the same increases in CeBF by local microinjection of KCl into the cerebellar cortex as during stimulation of the intrinsic afferent systems, provided that similar levels of [K+]o were achieved.
The experiments were performed in rat cerebellar cortex (Fig. 1), a useful model for investigations of activity-dependent CeBF increases (Akgoren et al. 1994). In cerebellar cortex, Purkinje cells are innervated by two excitatory afferent input systems, i.e. parallel fibres and climbing fibres (Eccles et al. 1967). Parallel fibres, the axons of granule cells, connect to the distal dendrites of Purkinje cells onto which they form weak excitatory synapses, whereas climbing fibres contact the proximal dendrites of Purkinje cells onto which they form very powerful excitatory synapses (Eccles et al. 1967). Stimulation of these input systems enabled us to compare K+ and CeBF responses to activation of two input systems, which differ both in location of synaptic input onto the same target cell and in synaptic strength.
Figure 1. Schematic drawing of the experimental set-up.
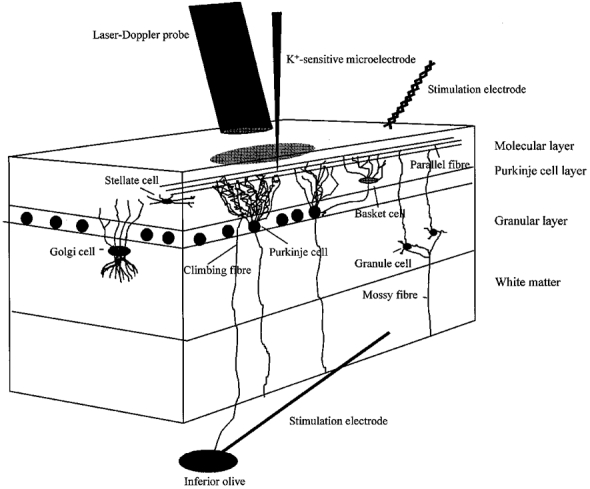
A three-dimensional sagittal view of the rat cerebellar cortex, showing the neurones of interest and the position of the laser-Doppler probe, K+-sensitive microelectrode and stimulating electrodes. The superficial parallel fibres were stimulated by a bipolar electrode positioned on the cerebellar surface, while the climbing fibres were stimulated by a monopolar electrode lowered into the inferior olive. CeBF was recorded by a laser-Doppler flowmetry (LDF) probe located 0.5 mm above the pial surface, whereas changes in [K+]o were recorded with a K+-sensitive microelectrode lowered 50-100 μm into the cortex.
METHODS
Animal preparation
Twenty-four Wistar rats (280-350 g; Panum Institute, Copenhagen) were anaesthetized with halothane (Fluotec 3 Vaporizer, CYPRANE, UK; 3.5 % induction, 1.5 % surgery and 0.7 % maintenance) in 30 % O2-70 % N2O. Additional doses of halothane were given upon pilo-erection or if the arterial blood pressure and heart rate increased by more than 10 % during the experiment. Between animals the mean arterial blood pressure varied between 90 and 120 mmHg and the heart rate between 300 and 400 beats min−1. Within animals the variation was very small during the course of the experiment. Lidocaine (5 mg ml−1s.c.) was used at the operation wounds and at the contact spots for the ear pins. The trachea was cannulated and the animals were ventilated with a volume respirator throughout the experiments to maintain the arterial blood gases at Pa,CO2= 37 mmHg, Pa,O2= 125 mmHg and pH = 7.35–7.40 (measured by ABL30, Radiometer, Denmark). Polyethylene catheters were placed in the femoral artery, for recording of blood pressure, and in the femoral vein, for slow infusion of saline during the experiment. Muscle relaxation was induced by a bolus of 7–8 mg suxamethonium chloride i.p. (Hospital Pharmacy, Denmark) followed by continuous infusion through an i.v. catheter at a rate of 2.5 mg h−1. Body temperature was monitored with a rectal probe and maintained at 37°C. Animals were placed in a head holder and the cranial bone and the dura were carefully removed over the cerebellar cortex. A pool was built around the open cranial window with 5 % agar in Ringer solution for superfusion of the surface with artificial cerebrospinal fluid (ACSF) at 37°C (composition (mm): NaCl, 120; KCl, 2.8; NaHCO3, 22.0; CaCl2, 1.45; Na2HPO4, 1.0; and MgCl2, 0.876; pH = 7.4). All surgical and anaesthetic procedures were carried out in accordance with the guidelines of the National Animal Ethics Committee. At the end of the experiment the rats were killed by i.v. injection of air.
Recording and stimulation procedures
Microelectrodes
The K+-sensitive microelectrodes were constructed from double-barrelled glass tubing (Thick Septum Theta, WPI; o.d., 1.5 mm; i.d., 1.2 mm) pulled to form microelectrodes with tip diameters of 2–4 μm (Micropipette Puller, Model P-97, Sutter Instrument Co.). The barrel serving as the ion-sensitive electrode was siliconized in dichlorodimethyl silane (D3879, Sigma) vapour for 50 s and then baked in an oven for 1 h at 110°C. The tip of the ion-selective barrel was filled with the K+ exchanger Corning 477317 (IE190, WPI) and backfilled with 150 mm KCl. The other barrel was filled with 150 mm NaCl, i.e. isotonic with the extracellular space, and acted as a reference electrode for the ion-selective barrel. Animals were grounded with a reference electrode consisting of a low-impedance Ag-AgCl wire resting in the agar pool. The electrode was calibrated in solutions with different concentrations of KCl in which NaCl was added to preserve isosmolarity.
The signals from the reference and the ion-sensitive barrels were led by Ag-AgCl wires to the inputs of a high input impedance differential amplifier (Duo 733, WPI). In addition, we used the signal from the reference barrel to record the evoked field potential which was independently amplified 1000 times with a bandwidth of 0.5 and 2 kHz (custom made, Panum Institute). The electrode was adjusted to the optimal position for recording this signal to ensure that the recording site corresponded to the site of maximal neuronal activity during parallel fibre stimulation, approximately 100 μm below the brain surface. A similar position of the recording electrode was chosen for climbing fibre stimulation to be able to compare findings obtained with the two types of stimulation.
To ensure that this recording site was optimal for both the parallel fibre and climbing fibre system we carried out laminar measurements of [K+]o during parallel fibre stimulation at 20 Hz and climbing fibre stimulation at 5 Hz. The K+ electrode was lowered to cortical depths of 0, 50, 100, 200, 300 and 400 μm (n = 3). During parallel fibre stimulation the magnitude of the [K+]o change was maximal within the upper 100 μm of the cortex and declined linearly with depth to 400 μm (Fig. 2A). During climbing fibre stimulation the increase in [K+]o was practically the same at all cortical depths measured (Fig. 2B). Thus, recordings of [K+]o taken at a cortical depth of 100 μm were taken to be representative of parallel fibre and climbing fibre stimulation and corresponded to the cortical depth at which the CeBF changes were recorded, i.e. the upper 250 μm of the cortex.
Figure 2. The laminar distribution of increases in [K+]o in response to parallel and climbing fibre activation.
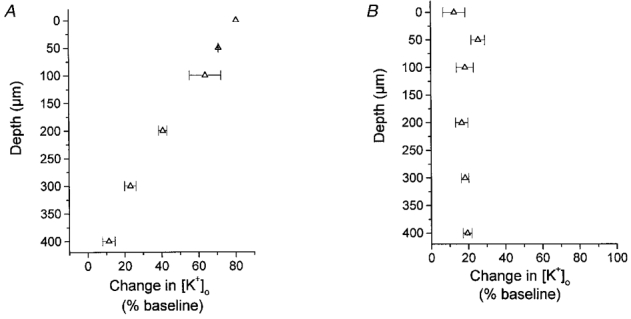
A, the laminar distribution of increases in [K+]o at cortical depths of 0, 50, 100, 200, 300 and 400 μm (n = 3) during parallel fibre stimulation at 20 Hz. B, the laminar distribution of increases in [K+]o evoked by activation of climbing fibres at 5 Hz (n = 3). Increases in [K+]o are given as percentage increases from baseline.
Laser-Doppler flowmetry
CeBF was continuously monitored by laser-Doppler flowmetry (LDF), a non-invasive technique, for on-line recordings of CeBF, as described elsewhere (Fabricius et al. 1997). In brief, laser light is detected after it is scattered by tissue, and the resultant photocurrent of the detector is processed electronically. The principle of measurement is based on the Doppler shift in frequency caused by the moving blood cells within the small volume of illuminated tissue. Although the technique records red cell flux and not blood flow, a direct relationship has been found during moderate cerebral blood flow increases as compared with the 14C-iodoantipyrine technique (Fabricius & Lauritzen, 1996). The LDF probe was used at a fixed position 0.5 mm above the pial surface (PF 415-49, Periflux 4001 Master, Perimed AB, Järfälla, Sweden; fibre separation, 140 μm; wavelength, 543 nm) and measured changes in CeBF roughly corresponding to the upper 250 μm of the cortex (Fabricius et al. 1997). All changes of CeBF were calculated as a percentage of the baseline value immediately preceding the test as described previously (Fabricius et al. 1997).
Stimulation procedures
Parallel fibres were activated by local electrical stimulation of the cerebellar cortex by a pair of Teflon-insulated, twisted platinum-iridium wires (separation, 2–5 μm; Advent Research Materials Ltd, UK). Climbing fibres were activated by stereotaxic stimulation of the inferior olive in the brain stem through a monopolar electrode (SNEX-300, RMI Inc., USA). Parallel fibres and the inferior olive were stimulated using constant current (ISO-flex, A.M.P.I., Israel) with 200 μs-long pulses at 2–3 and 150-250 μA, respectively. Microinjection of potassium (20 mm KCl in ACSF, NaCl reduced correspondingly to maintain isosmolarity) was performed with a micropipette with a tip diameter of 20-30 μm, connected to a pump (SAGE Instruments syringe pump, connected to a custom-made programmer) at an injection rate of 1.5 μl min−1. The micropipette was lowered to a depth of about 50-100 μm into the cortex.
All recordings were taken after a stable baseline was obtained. The Spike2 program with 1401plus interface (Cambridge Electronic Design Ltd) performed on-line and off-line analysis. The digital sampling rate was 10 Hz for the CeBF trace and 100 Hz for the potassium and blood pressure traces.
Experimental protocol
The study was designed to address the temporal and quantitative relationship between [K+]o and increases of CeBF.
Parallel fibres were stimulated at 5, 10, 20, 50, 75 and 100 Hz for 30 s (n = 9 rats) to examine the time course and frequency-dependent increases of [K+]o and CeBF. The K+ electrode was positioned 200 μm away from the stimulating electrode along the parallel fibres. The LDF probe was placed to cover the same area as the K+ electrode.
To assess the relative contribution of pre- or postsynaptic sources to the increased CeBF and [K+]o, we eliminated the postsynaptic responses that are mediated via glutamate (Levi & Gallo, 1986) by blocking the glutamatergic AMPA receptors with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 100 μm), a specific inhibitor of non-NMDA receptors (Blake et al. 1988). The drug was topically applied for 30-45 min prior to stimulation at 20 Hz (n = 6 rats). Control values were obtained by stimulation before application of CNQX and after washing out the drug.
The inferior olive was stimulated at 1, 2, 3, 5, 7.5, 10 and 20 Hz for 60 s (n = 5 rats) to examine the time course and frequency-dependent increases of [K+]o and CeBF during climbing fibre activation. The K+ electrode and LDF probe were placed in the same area of the vermis of lobule VIa or VIb of the cerebellar cortex.
To mimic potassium concentration elevations in response to stimulation of the intrinsic systems, we injected KCl (20 mm in ACSF) directly into the cortex, simultaneously recording elevations of [K+]o and CeBF. The injection electrode, the K+ electrode and the LDF probe were placed within 30-50 μm of one another in the vermis of lobule VIa or VIb. The electrodes were positioned at a cortical depth of 50-100 μm. The LDF probe was placed to cover the same area as the electrodes. Transient injections of 0.5, 1.0 and 1.5 μl were given (n = 4 rats).
Statistics
Results from the drug experiments with CNQX were compared by Student's paired t test. Increases in CeBF and [K+]o as a function of stimulation frequency, and the correlation between maximal increases in CeBF and [K+]o were tested by exponential and sigmoidal curve fitting (Boltzmann equation). All tests were performed using the program Origin 5.0 (Microcal, USA). Values were considered statistically significant at P < 0.05. All values are means ±s.e.m.; n indicates the number of rats. There was no overlap between rats used for the different types of stimulation.
RESULTS
[K+]o and CeBF increases evoked by stimulation of the parallel fibres
Local electrical stimulation of the cerebellar cortex to activate parallel fibres evoked frequency-dependent increases of CeBF and [K+]o. Figure 3A shows a typical recording of the simultaneous changes of CeBF and [K+]o during stimulation at 5–100 Hz.
Figure 3. Frequency-dependent increases in CeBF and [K+]o in response to parallel fibre stimulation.
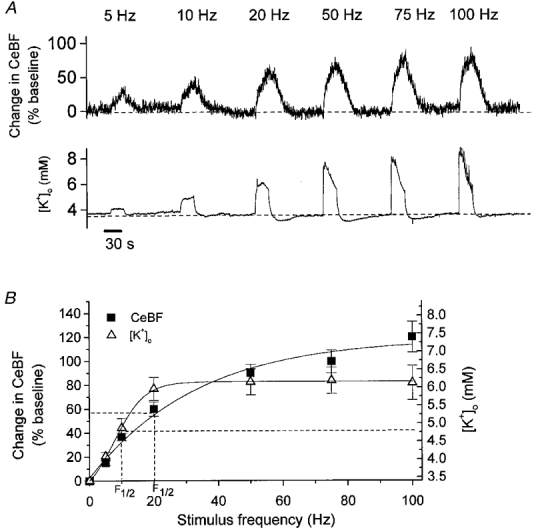
A, typical example of CeBF and [K+]o increases evoked by parallel fibre stimulation at 5, 10, 20, 50, 75 and 100 Hz for 30 s. Upper panel shows original recordings of CeBF increases as a percentage of the baseline value. Lower panel shows simultaneous [K+]o traces (mm). Bar (at bottom) indicates the duration of stimulation. B, CeBF and [K+]o increases plotted versus stimulation frequency (n = 9). Increases in CeBF are shown on the ordinate to the left as percentage increases of baseline, while maximal values to which [K+]o increased are shown on the ordinate to the right. The smooth curves represent sigmodial curve fitting. The dashed lines show the frequencies at which the half-maximum increase occurred (F½).
CeBF showed a biphasic increase, consisting of an initial fast rise within the first 2–3 s, followed by a smaller, sustained increase over the rest of the stimulation period. The [K+]o response showed a fast initial rise, attaining its peak value within milliseconds to a few seconds. At low frequencies, a plateau followed this increase, whereas at frequencies above 20 Hz, a decline of [K+]o was observed during the remainder of the stimulation period. After stimulus cessation, CeBF and [K+]o returned to baseline levels with half-times of 15-21 and 2–7 s, respectively. After reaching baseline an undershoot of [K+]o in the range of 0.3–0.4 mm was observed at frequencies above 10 Hz, lasting for up to 60 s. Thus, while both variables increased rapidly during the initial phases of stimulation, important differences were observed after the first few seconds, and the time courses of the return to baseline levels were entirely different.
CeBF and [K+]o increased as a function of stimulation frequency (Fig. 3B) (n = 9). Correlation analysis suggested a sigmoidal relationship for both the CeBF and the [K+]o increase. The largest CeBF increase, 122 ± 11 % above the baseline value, was observed at 100 Hz, while [K+]o reached its maximum value of 6.3 ± 0.5 mm at 20 Hz, with no further increase up to 100 Hz.
The frequency at which the half-maximum increase of CeBF was observed (F½) was 20 Hz whereas the half-maximum increase of [K+]o occurred at 10 Hz. This suggests that although an increase in [K+]o probably contributed to the CeBF increase in response to parallel fibre stimulation, other factors contributed as well.
[K+]o and CeBF increases evoked by electrical stimulation of the parallel fibres after inhibition of postsynaptic AMPA receptors
In order to assess the relative contribution of pre- or postsynaptic sources to increased CeBF and [K+]o, we inhibited the postsynaptic AMPA receptors with CNQX. Attenuation of the field potential evoked by electrical stimulation was used to confirm the inhibition, in that the N2 wave, corresponding to postsynaptic activity (Dow, 1949; Eccles et al. 1966), disappeared 30-45 min after topical application of 100 μm CNQX (Fig. 4A). CNQX attenuated the changes in CeBF by 47 ± 7 % and the changes in [K+]o by 44 ± 5 % (P < 0.05, n = 6) (Fig. 4B). This indicates that both pre- and postsynaptic sources contribute to the increases of CeBF and [K+]o.
Figure 4. Inhibition of postsynaptic AMPA receptors attenuates increases in CeBF and [K+]o in response to parallel fibre stimulation.
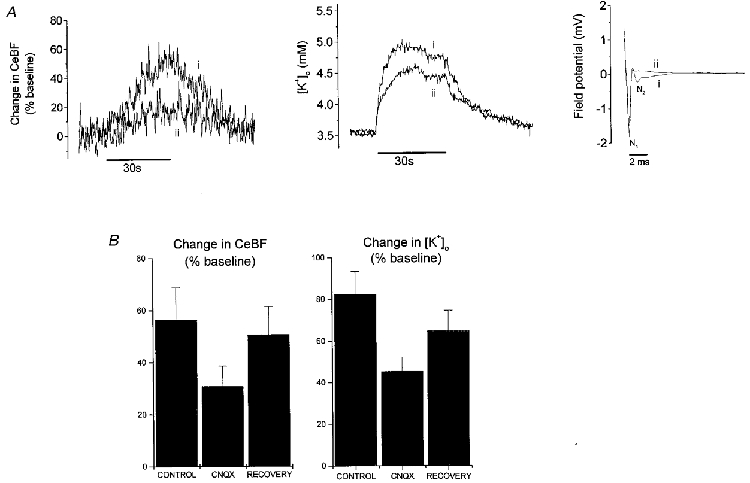
A, original records of changes in CeBF and [K+]o, and the evoked field potential during parallel fibre stimulation at 20 Hz for 30 s. i, recordings during control conditions; ii, recordings after inhibition of the postsynaptic response with the glutamate (AMPA) receptor antagonist CNQX (100 μm, topical application for 30 min). Both the CeBF response (shown as percentage increase of baseline) and the [K+]o response (mm) were attenuated during the inhibition. Bars (below traces) indicate the duration of parallel fibre stimulation. The field potential is shown on the right (mV; mean of 100 sweeps). While the control field potential (i) consists of both a presynaptic component (N1) and a postsynaptic component (N2), the field potential measured after inhibition of the postsynaptic AMPA receptors (ii) had no postsynaptic component. B, CNQX significantly attenuated the increases in CeBF and [K+]o (P < 0.05, n = 6). Increases in CeBF and [K+]o are shown as percentage increases before (control) and after application of CNQX and after washout of CNQX (recovery).
[K+]o and CeBF increases after activation of climbing fibres through electrical stimulation of the inferior olive
Activation of climbing fibres by electrical stimulation of the inferior olive evoked frequency-dependent increases of CeBF and [K+]o up to a stimulation frequency of 20 Hz. A typical recording is shown in Fig. 5A, illustrating the simultaneous changes of CeBF and [K+]o during stimulation at 1–20 Hz for 60 s.
Figure 5. Frequency-dependent increases in CeBF and [K+]o in response to climbing fibre stimulation.
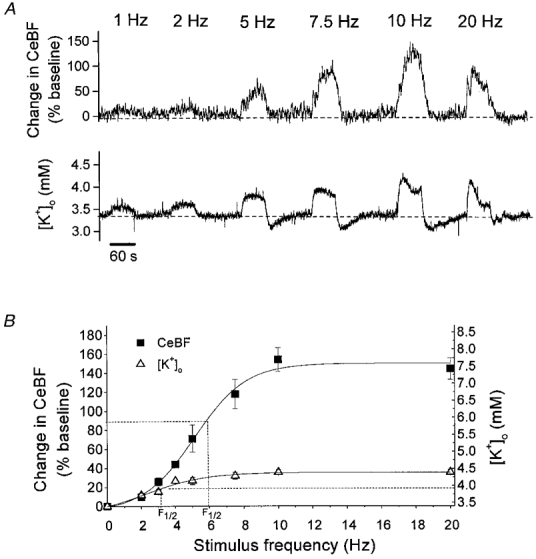
A, typical example of CeBF and [K+]o increases evoked by climbing fibre stimulation at 1, 2, 5, 7.5, 10 and 20 Hz for 60 s. Upper panel depicts recordings of CeBF increases as a percentage of the baseline value. Lower panel shows simultaneous [K+]o traces (mm). Bar (at bottom) indicates the duration of stimulation. B, CeBF and [K+]o increases plotted versus stimulation frequency (n = 5). Increases in CeBF are shown on the ordinate to the left as percentage increases of baseline and the maximal values to which [K+]o increased are shown on the ordinate to the right. The smooth curves represent sigmodial curve fitting. The dashed lines show the frequencies at which the half-maximum increase occurred (F½).
The increases of CeBF at 1 and 2 Hz were very small and monophasic throughout the period of stimulation. At 5–10 Hz the CeBF responses were biphasic with an initial steep increase lasting for 3–10 s, a decrease lasting for 5–10 s, followed by a second increase during the rest of the stimulation period. In four of the five rats the increase in CeBF at 20 Hz stimulation was followed by a decline to a lower plateau during the remainder of the stimulation period. [K+]o increased with a fast initial rise followed by a plateau during stimulation at frequencies from 1 to 7.5 Hz. At 10 and 20 Hz the rise in [K+]o was followed by a decline of [K+]o during the remainder of the stimulation period, consistent with incomplete recovery of Purkinje cell function between stimuli delivered at high frequencies (Llinas & Volkind, 1973). At the end of stimulation CeBF and [K+]o returned to baseline levels with half-times of 9–13 and 4–5 s, respectively. At frequencies higher than 5 Hz the [K+]o response was followed by an undershoot in [K+]o of 0.1–0.2 mm which lasted for 40-60 s (Fig. 5A).
Figure 5B shows the maximal amplitudes of CeBF and [K+]o plotted as a function of stimulation frequency (n = 5). Correlation analysis suggested a sigmoidal relationship for both the CeBF and the [K+]o increase. CeBF increased up to 157 ± 20 % of the baseline value, with half-maximum increase at 6 Hz. The highest [K+]o was only 4.4 ± 0.2 mm, the half-maximum value being reached at 2.5 Hz. Thus, activation of climbing fibres elicited larger CeBF increases than stimulation of parallel fibres, but smaller increases in [K+]o. This suggests that different mechanisms could be involved in producing activity-dependent CeBF increases in response to parallel fibre and climbing fibre stimulation.
[K+]o and CeBF increases after microinjection of KCl into the cortex
By microinjection of potassium into the cortex we aimed to mimic the [K+]o increases observed during activation of the two intrinsic systems. Figure 6A shows original recordings of [K+]o and CeBF in response to transient microinjection of 0.5, 1.0 and 1.5 μl of 20 mm KCl. The injection volume varied depending on the duration of injection, which was 20, 40 and 60 s, respectively.
Figure 6. Increases in CeBF and [K+]o in response to microinjection of KCl into the cortex.
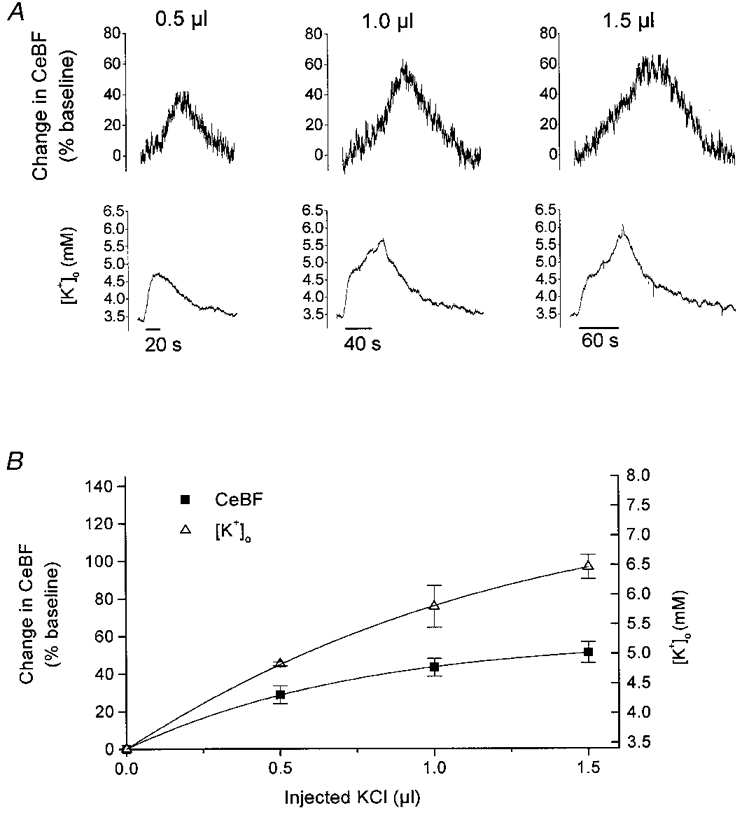
A, typical example of CeBF and [K+]o increases in response to microinjection of 0.5, 1 and 1.5 μl KCl (20 mm in ACSF) into the cortex. Upper panel shows original recordings of the CeBF increases as a percentage of the baseline value. Lower panel shows simultaneous [K+]o traces (mm). Bars (at bottom) indicate the duration of the injection. B, amplitude of CeBF and [K+]o increases (n = 4) plotted versus KCl volume. Increases in CeBF are shown on the ordinate to the left as percentage increases of baseline, while maximal values to which [K+]o increased are shown on the ordinate to the right.
[K+]o usually started to rise immediately after the onset of injection, and continued to increase throughout the period of injection, until 5–30 s after the end of injection. The decline back to baseline was very slow with a half-time of 1–2 min. CeBF usually started to increase a few seconds after the onset of injection, and continued to increase during the injection and for 40-75 s after the end of injection before declining back to baseline.
Figure 6B depicts the amplitude of CeBF and [K+]o increases versus the amount of KCl injected (n = 4). Following injections of 0.5, 1.0 and 1.5 μl KCl, [K+]o increased to 4.8 ± 0.1, 5.8 ± 0.5 and 6.5 ± 0.3 mm, respectively, with corresponding CeBF increases of 28.8 ± 6.7, 43.1 ± 6.7 and 51.1 ± 8.0 %. These values are comparable to the values of [K+]o and CeBF obtained by stimulation of parallel fibres at 1–20 Hz (see Fig. 3B). Parallel fibre stimulation at frequencies between 20 and 100 Hz gave rise to the same maximal [K+]o value of 6.3 ± 0.5 mm, but to CeBF changes of up to 122 ± 11 % (see Fig. 3B). The increases in [K+]o evoked by microinjection were larger than those evoked by climbing fibre stimulation, but the corresponding CeBF increases were smaller. Thus, injected KCl increased [K+]o and CeBF, but the vascular response was smaller than induced by stimulation of the intrinsic systems.
Overall correlation of CeBF increases to [K+]o evoked by stimulation of the intrinsic systems and through extrinsic microinjection of KCl
Figure 7 shows the CeBF increases evoked by parallel and climbing fibre activation and microinjection of KCl as a function of the simultaneously evoked [K+]o increases. Parallel fibre stimulation increased CeBF in parallel with [K+]o until [K+]o reached 6.3 ± 0.5 mm. In response to climbing fibre stimulation [K+]o did not increase above 4.4 ± 0.2 mm, but the maximum CeBF increase during climbing fibre stimulation was 40 % higher than for parallel fibre stimulation.
Figure 7. Increases in CeBF in response to climbing fibre and parallel fibre stimulation and microinjection versus corresponding increases in [K+]o.
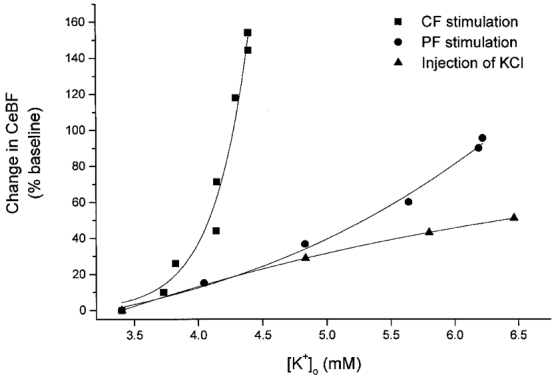
Scatter plot of the percentage increase in CeBF evoked by parallel fibre (PF) and climbing fibre (CF) activation and microinjection of KCl versus the simultaneously evoked [K+]o increase. The figure shows that the change in CeBF for a given value of [K+]o differs between the three types of stimulation.
The increase of CeBF evoked by microinjection of KCl revealed the pure effects of increased [K+]o. The difference between the CeBF increases evoked by KCl microinjection and parallel fibre stimulation was relatively small at low [K+]o but tended to increase at higher values of [K+]o. Therefore, part of the parallel fibre response could be explained by increases of [K+]o. In contrast, the difference in CeBF increases between KCl microinjections and climbing fibre stimulation was very large. This suggests that increased [K+]o probably was of limited importance for the climbing fibre-evoked CeBF response.
DISCUSSION
In this study we examined the relationship between increases of [K+]o changes and activity-dependent CeBF increases in rat cerebellum. The data showed that both [K+]o and CeBF increased in a frequency-dependent manner during stimulation of parallel and climbing fibres, but with important differences in the timing and amplitude of the two variables. First, CeBF and [K+]o both increased rapidly during onset of stimulation, independent of the afferent system stimulated, but large-scale differences in the time course were observed during maintained stimulation and return to baseline. Second, the rise of CeBF was larger in response to climbing than to parallel fibre stimulation, while the rise of [K+]o was much smaller for climbing than for parallel fibre stimulation. Third, microinjection of KCl reproduced the transient increases in [K+]o as observed during stimulation of the intrinsic systems, but the magnitude of the CeBF increase was smaller than for corresponding [K+]o increases evoked by intrinsic activation. The data suggest that the importance of [K+]o as a regulator of cerebral blood flow depends on the neural circuit investigated.
Methodological limitations
Limitations in the methodology included different spatial resolution of the laser-Doppler technique and ion-selective microelectrodes. The LDF probe covers an area of at least 140 μm across and 250 μm in depth (Jakobsson & Nilsson, 1993), whereas the area of detection of the ion-sensitive microelectrode is confined to the region of its tip, which has a diameter of 2–4 μm. Therefore, comparison of CeBF and [K+]o measurements was based on the assumption that the [K+]o increase was representative of the region in which the CeBF changes were recorded. The laminar distribution of changes in [K+]o during stimulation (see Fig. 2) was taken to support this assumption.
In experiments using microinjection of KCl from a point source, the [K+]o will decrease as a function of distance due to diffusion and uptake. Thus, the spatial distribution of [K+]o is likely to be more heterogeneous with injection than with intrinsic stimulation and the relationship between [K+]o and CeBF during microinjection may be underestimated. Brain superfusion would have produced a more homogeneous increase of [K+]o, but the increases of [K+]o and CeBF would have been sustained and would not mimic the transient and local increases evoked by parallel fibre and climbing fibre stimulation. Therefore, we chose the microinjection approach. Studies of diffusion in the cerebellar cortex have demonstrated anisotropic diffusion properties in the molecular layer with a preferred direction of diffusion along the parallel fibres while diffusion of substances perpendicular to these fibres is limited (Rice et al. 1993). This restricts diffusion of potassium away from the site of injection and thereby tends to diminish the problem of heterogeneity of [K+]o in the tissue over which CeBF is recorded. Still, caution has to be taken when correlating CeBF to [K+]o increases evoked by intrinsic and extrinsic stimulation.
Time course of increases of [K+]o and CeBF in response to parallel fibre and climbing fibre stimulation
Stimulation of both parallel and climbing fibres caused frequency-dependent and reproducible increases in CeBF and [K+]o. The increases in [K+]o were stimulus-locked, starting within a few milliseconds, while the CeBF changes had a response time in the order of seconds as reported previously (Akgoren et al. 1996; Mathiesen et al. 1998). Slow monophasic or biphasic CeBF responses with consistent delays between maxima of the potassium and vascular responses (see Figs 3A and 5A) precluded a simple relationship between the two. After the end of stimulation [K+]o returned to normal within a few seconds, while the CeBF response took a much longer time to recover, dependent on the stimulus frequency. Thus, although changes in [K+]o and CeBF did coexist in response to neuronal activation, the differences in the time course of the two indicated a complex rather than a direct relationship. The fast onset of the initial CeBF rise could be explained by a direct effect of K+ on the blood vessels during onset of nerve cell activity as suggested by others (Betz & Csornai, 1978; Leniger-Follert et al. 1978; Heuser, 1978; Leniger-Follert, 1984; Kuschinsky, 1988; Lassen, 1991). The latency differences between [K+]o and CeBF responses during the remainder of the stimulation period and return to normal could be explained if other mediators related to cell metabolism were involved in maintaining the CeBF increase during the later phases of stimulation (Leniger-Follert, 1984; Kuschinsky, 1988). Thus, important time delays between [K+]o and CeBF were observed during all but the very early phases of stimulation.
Quantitative considerations of [K+]o and CeBF in response to intrinsic stimulation and microinjection
The increases of [K+]o and CeBF during parallel fibre and climbing fibre stimulation varied as a function of stimulus frequency, but with important differences (see Figs 3B and 5B). The magnitude of the increases in [K+]o ranged between 0.2 and 1.2 mm for climbing fibre stimulation and 0.5 and 3.5 mm for parallel fibre stimulation. In contrast, the CeBF increases were larger in response to climbing fibre than to parallel fibre stimulation. To determine whether the magnitude of the [K+]o responses evoked by climbing fibre and parallel fibre stimulation was sufficient to produce the observed CeBF increases, we injected KCl into the same region of the cerebellar cortex as studied during stimulation of the two afferent systems. [K+]o and CeBF were measured at the same depth of the cortex during both intrinsic stimulation and injection over the vermis regions VIa and VIb. It is reasonable to assume that the two adjacent cerebellar regions in the different animals are supplied by the same blood vessels with similar sensitivity to changes in [K+]o. The results showed that the injected K+ increased CeBF, but that the change in CeBF per millimolar change in [K+]o was much less for injected K+ than for stimulation of the two afferent input systems, except at low frequency stimulation of the parallel fibre system. Therefore, variations in tissue concentrations of K+ could only explain a small proportion of the CeBF increases observed during climbing fibre stimulation, and only partly explained the CeBF increases observed during parallel fibre stimulation. Similar results were obtained previously, in a study examining the participation of K+ in blood flow regulation in the cerebral cortex (Iadecola & Kraig, 1991).
A possible explanation for the disparity between the changes in CeBF per millimolar change in [K+]o during the different types of stimulation (see Fig. 7) could be an interaction between K+ and other mediators (Betz & Csornai, 1978; Berne et al. 1981). Theoretically, the different CeBF responses for a given change in [K+]o could be expected to depend on the concentration of other vasoactive substances being released during activation. For example, the vasodilator effect of K+ depends on extracellular pH, being diminished at low pH and increased at high pH (Kuschinsky & Wahl, 1977). Detailed studies of extracellular pH during stimulation of the neocortex or the cerebellar cortex suggest that a brief initial alkaline shift precedes acidification with a prolonged return to normal after the end of stimulation (Kraig et al. 1983; Chesler & Kaila, 1992). Thus, the changes in pH will tend to increase the sensitivity of the blood vessels towards [K+]o in initial phases and decrease it in later phases of stimulation. Also, decreases of [Ca2+]o, which accompany neuronal activity in the cerebellar cortex, due to both pre- and postsynaptic mechanisms (Nicholson et al. 1977) will increase the sensitivity of the blood vessels to vasodilators, while raised [Ca2+]o will decrease the sensitivity (Betz & Csornai, 1978). Thus, it is difficult on the basis of measurements of the concentration changes of a single vasodilator to conclude whether or not that substance regulates the local cerebral circulation. Further studies are needed with simultaneous measurements of [K+]o, [H+]o and [Ca2+]o to elucidate the putative interactions of the aforementioned substances and their importance for the cerebellar circulation in response to increased neuronal activity.
Different importance of [K+]o as a mediator of activity-dependent CeBF increases between the parallel fibre and climbing fibre systems
The differences in magnitude of the observed [K+]o changes may be explained by the functional anatomy of the parallel fibre and the climbing fibre systems. The parallel fibres are very densely packed in the molecular layer, in which each Purkinje cell receives converging input from approximately 200 000 parallel fibres (Eccles et al. 1967). This would be expected to give rise to large [K+]o increases during activation. A large contribution of K+ from the parallel fibres was verified in the experiments in which the AMPA receptors were blocked by CNQX, which showed that approximately 50 % of the [K+]o response could be attributed to presynaptic sources. This is in accordance with other studies (Nicholson et al. 1978). Inferior olive stimulation elicits action potentials in climbing fibres and bursts of powerful excitatory synaptic potentials in Purkinje cell dendrites (Eccles et al. 1967). Climbing fibres have a slender structure and a low density (Eccles et al. 1967). Therefore, the small [K+]o change observed in response to climbing fibre stimulation is most probably due to postsynaptic activity in Purkinje cell dendrites, as noted previously (Bruggencate et al. 1976).
In a similar fashion it may be argued that the contribution of pre- and postsynaptic sources to the CeBF increase is different for the two systems. We have previously shown that inhibition of the postsynaptic glutamate receptors reduces the CeBF response by 50 % during parallel fibre stimulation (Akgoren et al. 1996), and by 85-95 % in response to climbing fibre stimulation (Mathiesen et al. 1998). This suggests that different mechanisms may be involved in producing the activity-dependent CeBF increases in response to parallel fibre and climbing fibre stimulation. The effect of a climbing fibre input on Purkinje cell spiking activity is the result of a strong monosynaptic input and the intrinsic membrane properties of the dendritic tree of the Purkinje cells. This includes several voltage-gated cation conductances that generate the so-called complex spike (Hounsgaard & Midtgaard, 1989; Mathiesen et al. 1998). The complex spike is composed of dendritic calcium spikes mediated by P-type channels, calcium-dependent plateau potentials, which produce a slow continuous increase of intracellular calcium, sodium plateau potentials, and spikes at the axon hillock (Hounsgaard & Midtgaard, 1988; Llinas & Sugimori, 1992). It is likely that the complex postsynaptic mechanisms following climbing fibre stimulation involve the activation of several Ca2+-dependent enzyme systems, e.g. phospholipases. If so, this will trigger the production of different vasoactive compounds of importance for the CeBF response, e.g. prostaglandins (Heistad & Kontos, 1983). We have previously shown that both NO and adenosine increase CeBF in response to stimulation of the climbing fibre system, while NO but not adenosine increases CeBF in response to stimulation of the parallel fibre system (Akgoren et al. 1997). This suggests that different messenger molecules mediate CeBF increases in the cerebellar cortex dependent on the afferent input system stimulated. The observations of the present study confirm this impression in that K+ probably mediates part of the CeBF response in the parallel fibre system but probably not in the climbing fibre system. Adenosine, NO and K+ are not independent as mechanisms of the CeBF increase since both K+ and adenosine may act as vasodilators via the NO-cGMP pathway (Fabricius & Lauritzen, 1994; Dreier et al. 1995).
Conclusion
In conclusion, this study compared in real time the relationship between increases of [K+]o and CeBF in rat cerebellar cortex in response to increased activity in two afferent input systems with different innervation patterns, synaptic strength and postsynaptic activity. The results indicated important differences in the time course and amplitude of changes of [K+]o and CeBF. Increased [K+]o may contribute to CeBF increases evoked by parallel fibres, but is probably not important for CeBF increases evoked by climbing fibres. Since the relationship between [K+]o and CeBF varied depending on the afferent input system stimulated, we suggest that the organization of the neuronal network should be considered when evaluating a putative mediator of activity-dependent increases of CeBF. The hypothesis that increased [K+]o explains activity-dependent increases of brain blood flow is probably not applicable to all neuronal circuits.
Acknowledgments
We thank Lillian Grøndahl for expert technical assistance. The invaluable help and assistance of the electronic and mechanical workshops are much appreciated. This work was supported by the Danish Medical Research Council, Neuroscience PharmaBiotec, The Novo-Nordisk Foundation and The Danish Medical Association Research Fund.
References
- Akgoren N, Dalgaard P, Lauritzen M. Cerebral blood increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Research. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- Akgoren N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proceedings of the National Academy of Sciences of the USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgoren N, Mathiesen C, Rubin I, Lauritzen M. Laminar analysis of activity-dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. American Journal of Physiology. 1997;42:H1166–1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- Berne RM, Winn HR, Rubio R. The local regulation of cerebral blood flow. Progress in Cardiovascular Diseases. 1981;24:243–260. doi: 10.1016/0033-0620(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Betz E, Csornai M. Action and interaction of perivascular H+, K+ and Ca2+ on pial arteries. Pflügers Archiv. 1978;374:67–72. doi: 10.1007/BF00585698. [DOI] [PubMed] [Google Scholar]
- Blake JF, Brown MW, Collingridge GL. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neuroscience Letters. 1988;89:182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Bruggencate GT, Nicholson C, Stockle H. Climbing fiber evoked potassium release in cat cerebellum. Pflügers Archiv. 1976;367:107–109. doi: 10.1007/BF00583664. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Dreier J. Regulation of cerebral blood flow by ions. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Cerebrovascular Diseases. San Diego: Academic Press; 1997. pp. 75–77. [Google Scholar]
- Dow RS. Action potentials of cerebellar cortex in response to local electrical stimulation. Journal of Neurophysiology. 1949;2:245–256. doi: 10.1152/jn.1949.12.4.245. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Gorner A, Lindauer U, Weih M, Villringer A, Dirnagl U. Nitric oxide modulates the CBF response to increased extracellular potassium. Journal of Cerebral Blood Flow and Metabolism. 1995;15:914–919. doi: 10.1038/jcbfm.1995.116. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. New York: Springer-Verlag; 1967. [Google Scholar]
- Eccles JC, Sasaki K, Strata P. The profiles of physiological events produced by a parallel fiber volley in the cerebellar cortex. Experimental Brain Research. 1966;2:18–34. doi: 10.1007/BF00234358. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. The Journal of Physiology. 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius M, Akgoren N, Dirnagl U, Lauritzen M. Laminar analysis of cerebral blood flow in cortex of rats by laser-Doppler flowmetry. A pilot sudy. Journal of Cerebral Blood Flow and Metabolism. 1997;17:1326–1336. doi: 10.1097/00004647-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Examination of the role of nitric oxide for the hypercapnic rise of cerebral blood flow in rats. American Journal of Physiology. 1994;266:H1457–1464. doi: 10.1152/ajpheart.1994.266.4.H1457. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Lauritzen M. Laser-Doppler evaluation of rat brain microcirculation: Comparison with the [C-14]-iodoantipyrine method suggests discordance during cerebral blood flow increases. Journal of Cerebral Blood Flow and Metabolism. 1996;16:156–161. doi: 10.1097/00004647-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Biology of cerebral vascular muscle. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Cerebrovascular Diseases. San Diego: Academic Press; 1997. pp. 13–17. [Google Scholar]
- Gardner Medwin AR, Nicholson C. Changes of extracellular potassium activity induced by electric current through brain tissue in the rat. The Journal of Physiology. 1983;335:375–392. doi: 10.1113/jphysiol.1983.sp014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Kontos HA. Cerebral circulation. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System. part 1. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 137–182. [Google Scholar]
- Hertz L. Autonomic control of neuronal-astrocytic interactions, regulating metabolic activities, and ion fluxes in the CNS. Brain Research Bulletin. 1992;29:303–313. doi: 10.1016/0361-9230(92)90061-2. [DOI] [PubMed] [Google Scholar]
- Heuser D. The significance of cortical extracellular H+, K+ and Ca2+ activities for regulation of local cerebral blood flow under conditions of enhanced neuronal activity. Ciba Foundation Symposium. 1978:339–353. doi: 10.1002/9780470720370.ch17. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Midtgaard J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. The Journal of Physiology. 1988;402:731–749. doi: 10.1113/jphysiol.1988.sp017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Midtgaard J. Synaptic control of excitability in turtle cerebellar Purkinje cells. The Journal of Physiology. 1989;409:157–170. doi: 10.1113/jphysiol.1989.sp017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Kraig RP. Focal elevations in neocortical interstitial K+ produced by stimulation of the fastigial nucleus in rat. Brain Research. 1991;563:273–277. doi: 10.1016/0006-8993(91)91544-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A, Nilsson GE. Prediction of sampling depth and photon pathlength in laser Doppler flowmetry. Medical and Biological Engineering and Computing. 1993;31:301–307. doi: 10.1007/BF02458050. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Ferreira Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. Journal of Neurophysiology. 1983;49:831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W. Physiology and general pathophysiology of the cerebral circulation. In: Olesen J, Edvinsson L, editors. Basic Mechanisms of Headache. Elsevier Science Publishers B.V; 1988. pp. 69–87. [Google Scholar]
- Kuschinsky W, Wahl M. Interactions between perivascular norepinephrine and potassium or osmolarity on pial arteries of cats. Microvascular Research. 1977;14:173–180. doi: 10.1016/0026-2862(77)90016-4. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W, Wahl M, Bosse O, Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. Circulation Research. 1972;31:240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Cations as mediators of functional hyperemia in the brain. In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain Work and Mental Activity. Copenhagen: Munksgaard; 1991. pp. 68–79. [Google Scholar]
- Leniger-Follert E. Mechanisms of regulation of cerebral microflow during bicuculline-induced seizures in anaesthetized cats. Journal of Cerebral Blood Flow and Metabolism. 1984;4:150–165. doi: 10.1038/jcbfm.1984.23. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E, Urbanics R, Lübbers W. Behavior of extracellular H+ and K+ activities during functional hyperemia of microcirculation in the brain cortex. Advances in. Neurology. 1978;20:97–101. [PubMed] [Google Scholar]
- Levi G, Gallo V. Release studies related to the neurotransmitter role of glutamate in the cerebellum: an overview. Neurochemical Research. 1986;11:1627–1642. doi: 10.1007/BF00967741. [DOI] [PubMed] [Google Scholar]
- Llinas R, Volkind RA. The olivo-cerebellar system: Functional properties as revealed by harmaline-induced tremor. Experimental Brain Research. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Sugimori M. The elecrophysiology of the cerebellar Purkinje cell revisited. In: Llinas RR, Sotelo C, editors. The Cerebellum Revisited. New York: Springer-Verlag; 1992. pp. 167–181. [Google Scholar]
- McCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. American Journal of Physiology. 1990;259:H902–908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Coupling of cerebral blood flow and metabolism. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Cerebrovascular Diseases. San Diego: Academic Press; 1997. pp. 70–74. [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. The Journal of Physiology. 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. Dynamics of the brain cell microenvironment. Neurosciences Research Program Bulletin. 1980;18:175–322. [PubMed] [Google Scholar]
- Nicholson C, Bruggencate GT, Steinberg R, Stöckle H. Calcium modulation in brain extracellular microenvironment demonstrated with ion-selective micropipette. Proceedings of the National Academy of Sciences of the USA. 1977;74:1287–1290. doi: 10.1073/pnas.74.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Rice ME. Use of ion-selective microelectrodes and voltammetric microsensors to study brain cell microenvironment. In: Boulton AA, Baker GB, Walz W, editors. Neuromethods. Clifton, NJ, USA: Humana Press; 1988. pp. 247–361. [Google Scholar]
- Nicholson C, ten Bruggencate G, Stöckle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. Journal of Neurophysiology. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Nilsson GE. Perimed's LDV flowmeter. In: Shepherd AP, Öberg P Å, editors. Laser-Doppler Blood Flowmetry. MA, USA: Kluwer Academic Publishers; 1990. pp. 57–73. [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Okada YC, Nicholson C. Anisotropic and heterogeneous diffusion in the turtle cerebellum - implications for volume transmission. Journal of Neurophysiology. 1993;70:2035–2044. doi: 10.1152/jn.1993.70.5.2035. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Extracellular potassium in the mammalian central nervous system. Annual Review of Physiology. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Walz W, Hertz L. Functional interactions between neurons and astrocytes. II. Potassium homeostasis at the cellular level. Progress in Neurobiology. 1983;20:133–183. doi: 10.1016/0301-0082(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Webb RC, Bohr DF. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15:198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]


