Abstract
In some anaesthetized preparations, eupnoea is eliminated following a blockade or destruction of neurons in a rostral medullary pre-Bötzinger complex.
Neurons in this region might underlie the neurogenesis of eupnoea, or be the source of an input which is necessary for eupnoea to be expressed. If the latter, any apnoea following ablation of the pre-Bötzinger complex might be reversed by an augmentation in ‘tonic input.’ Contrariwise, this apnoea should be permanent if the neuronal activities of the pre- Bötzinger complex are an exclusive generator of the eupnoeic rhythm.
Decerebrate, vagotomized, paralysed and ventilated adult rats were studied. Efferent activity of the phrenic nerve was recorded as an index of ventilatory activity.
Blockade or destruction of neuronal activities of the pre-Bötzinger complex by unilateral and/or bilateral injections of muscimol or kainic acid eliminated eupnoea only transiently. Eupnoea returned following activation of the peripheral chemoreceptors and spontaneously over time.
Results do not support the concept that neuronal activities of the pre-Bötzinger complex play an exclusive role in the neurogenesis of eupnoea in vivo. Rather, these neuronal activities appear to provide a tonic input to the ponto-medullary circuit which generates eupnoea and/or appear to be one component of this circuit.
The rhythmic alterations of cranial and spinal nerves which are characteristic of eupnoeic ventilatory activity are dependent upon two interrelated processes. Neuronal mechanisms within the pontile and medullary brainstem are responsible for generating the eupnoeic rhythm. For this rhythm to be expressed in activities of cranial and spinal nerves, a level of tonic neuronal input is required (e.g. Euler, 1986; St-John, 1998a). Thus, eupnoea could be eliminated following ablation of neurons which are responsible for its neurogenesis and/or which provide for a tonic input.
The necessity of a tonic input for eupnoea to be manifested is inherent in the concept of an ‘apnoeic threshold’ for arterial partial pressures of carbon dioxide. Hence, apnoea can be induced by hyperventilation of anaesthetized or decerebrate preparations (see Cherniack et al. 1979; Euler, 1986 for discussion). The apnoea which follows a blockade of neuronal activities in extensive regions on or near the ventrolateral medullary surface is likewise considered to result from the elimination of a tonic input (see St-John, 1998a for review).
As opposed to a tonic input, neuronal activities within a rostral medullary pre-Bötzinger complex have been hypothesized to be an exclusive mechanism for generating the eupnoeic rhythm (Smith et al. 1990, 1991; Rekling & Feldman, 1998). This hypothesis was based upon the finding that ablation of neurons in this region eliminates the rhythmic activity of an in vitro brainstem-spinal cord preparation of the neonatal rat. Yet, this rhythmic activity differs markedly from eupnoea in vivo but appears identical with gasping (St-John, 1996; 1998a).
Ablation of the pre-Bötzinger complex in vivo has led to inconsistent findings. In anaesthetized or decerebrate adult cats, eupnoea continued following the placement of multiple lesions which, in sum, destroyed the entire pre-Bötzinger complex (Speck & Feldman, 1982; Speck & Beck, 1989). In decerebrate newborn rats, unilateral injections of the neurotoxin kainic acid into the pre-Bötzinger complex did not eliminate eupnoea (Huang et al. 1997).
In anaesthetized adult rats, apnoea is reported following bilateral injections of the GABA agonist muscimol into the rostral ventrolateral medulla (Koshiya et al. 1993; Koshiya & Guyenet, 1996). Recently, Ramirez et al. 1998 reported that injections of reversible blockers of synaptic activity into the pre-Bötzinger complex of anaesthetized adult cats caused transient reductions of phrenic activity. At some, but not all of these effective sites, bilateral injections of tetrodotoxin resulted in apnoea; tetrodotoxin blocks axonal conduction. In another study with anaesthetized cats, apnoea followed unilateral injections of kainic acid into a region of the rostro-ventrolateral medulla which, in some animals, appeared to include the pre-Bötzinger complex (Hsieh et al. 1998).
Authors of both these recent studies in anaesthetized cats point out that their results are compatible with neuronal activities of the pre-Bötzinger complex being responsible for the neurogenesis of eupnoea, or being the source of an input which is necessary for eupnoea to be expressed (Hsieh et al. 1998; Ramirez et al. 1998). If the latter, we hypothesize that any apnoea following ablation of the pre-Bötzinger complex might be reversed by an augmentation in ‘tonic input.’ Contrariwise, this apnoea should be permanent if neuronal activities of the pre-Bötzinger complex are an exclusive generator of the eupnoeic rhythm.
METHODS
General experimental preparation
Ninety-four adult rats of either sex were used. The surgical preparation has been described previously (Fung et al. 1994) and has been approved by the Institutional Animal Care and Use Committee of Dartmouth College and Medical School. Animals were anaesthetized with 100 mg kg−1 of ketamine and 15 mg kg−1 of xylazine, administered intraperitoneally. The vagi were sectioned bilaterally, the trachea was cannulated and cannulae were inserted into a femoral artery, a femoral vein and, in 77 rats, one or both common carotid arteries. Sodium cyanide (0.05 or 0.1 ml of 0.1 mg ml−1 in saline) was injected through this carotid cannula to activate the carotid chemoreceptors.
The animals were positioned in a stereotaxic apparatus and the brainstem was transected at a midcollicular level. All the brain rostral to the transection was removed by aspiration. In all but three animals, the caudal pole of the cerebellum was removed to expose the floor of the IVth ventricle.
Animals were paralysed with gallamine and artificially ventilated with air or oxygen; a bilateral pneumothorax was performed in most studies. End-tidal partial pressure of carbon dioxide (PET,CO2) and arterial blood pressure were continuously monitored. The minimum value of mean arterial pressure was 80 mmHg. If required, a solution of dextran and metaraminol was administered intravenously to raise arterial pressure to that level.
Recordings of neural and neuronal activities
Efferent activity was recorded from the central cut end of rootlets of the phrenic nerve. Activity was amplified, filtered (0.2- 10.0 kHz), and integrated by a RC circuit (50 ms time constant). Activities of single medullary respiratory neurons were recorded with tungsten metal electrodes, insulated with Parylene-C (A-M Systems, 2 MΩ). Amplification and filtering were similar to that for phrenic activity, except that some unit activities were filtered from 1.0 to 10 kHz. (e.g. St-John, 1998b). Activities of single respiratory-modulated neurons were extracted from those of other neurons by use of a spike sorter (St-John, 1999).
In 13 experiments, neuronal activities were recorded from one barrel of a two-barrel ‘Piggyback’ glass capillary. Injections were from the larger of the barrels, having a diameter of approximately 50 μm at the tip (see below). The smaller barrel, which was filled with 3 M NaCl, was used for recordings of neuronal activities. Amplification and filtering were as described above.
Ablations or blockade of the pre-Bötzinger complex
In order to ablate neurons, while largely sparing fibres of passage, the neurotoxin kainic acid was microinjected. It is well accepted that kainic acid produces an irreversible destruction of neurons, with histological evidence of neuronal destruction increasing progressively for many hours following injection (e.g. Denavit-Saubiéet al. 1980). Yet, as kainic acid binds to glutamate receptors, an initial augmentation of neuronal activity follows its injection. In order to preclude this neuronal excitation as underlying any changes in phrenic activity, muscimol was microinjected in a separate group of animals. Muscimol is a GABA agonist, which produces a depression of neuronal activities in vivo for a minimum of several hours (e.g. Yamada et al. 1982; Gatti et al. 1987).
Microinjections were made from a single glass capillary, having a diameter of 50 μm at the tip, in all but the 13 experiments noted, in which the double-barrel pipette was used. Pipettes were filled with 4.69 mm kainic acid or 10 mm muscimol in mock cerebrospinal fluid. This solution also contained Fast-Green FCF and fluorescent microbeads (Fung et al. 1994; Huang et al. 1997). Injections were by a WPI Nanopump. This is an oil-filled system in which fluid is ejected by movement of a piston. In initial experiments and periodically thereafter, we viewed movement of the oil-fluid interface with the reticule of a microscope and defined that the settings of 50 or 100 nanolitres per minute (nl min−1) did result in ejection of these volumes. In all experiments, the presence of microbeads in brainstem sections was taken as evidence of a successful injection.
Durations of action of kainic acid and muscimol
As noted above, there is substantial evidence from previous studies that the destruction of neurons by kainic acid is irreversible (Denavit-Saubiéet al. 1980) and the blockade of neuronal activities in vivo by muscimol is of long duration (Yamada et al. 1982). In order to confirm such findings in our preparation, studies were performed in six rats. In these studies, the micropipette containing kainic acid or muscimol was positioned in the rostral medulla. By stereotaxic co-ordinates, a tungsten metal microelectrode was advanced as close as possible to the tip of the pipette until respiratory-modulated neuronal activities could be discerned. Kainic acid or muscimol was then injected and recordings continued for a minimum of 2 h thereafter.
Activity was quantified as the mean discharge frequency per minute of the multineuronal discharges. Also, total activity was integrated over successive intervals of 1 min. Both indices of activity were normalized by expressing these as a percentage of control levels, before injections of kainic acid or muscimol.
Experimental protocol
Four different protocols were performed. In the first, involving three rats, we wished to validate that changes in phrenic activity following injections of cyanide solution into the carotid arteries reflected augmentations in eupnoea due to activation of the carotid chemoreceptors. Following injections of 0.005 or 0.010 mg of NaCN, the carotid sinus nerves were sectioned by removing all tissue surrounding the common and external carotid arteries. The injections of cyanide were then repeated. Approximately every 15 min thereafter, phrenic activity was recorded following injections of increasing doses of NaCN.
In the second series of experiments, involving 11 rats, we wished to characterize respiratory-modulated neuronal activities in the rostral medulla. Hence, activities of single medullary respiratory neurons were recorded with the tungsten electrodes. Current was passed through the electrode to mark the site of recording.
The third series, in six rats, is as described above. In these experiments, medullary neuronal activities were recorded for extended periods following injections of kainic acid or muscimol.
For the other 74 experiments, phrenic activity was recorded at normocapnoea. The solution containing NaCN was injected into the common carotid artery. Following a return to control levels of phrenic activity, the pipette was positioned in the rostral medulla, based upon co-ordinates relative to the obex, midline and depth below the surface. Kainic acid or muscimol was ejected and the pipette removed. The animals were maintained for periods up to two hours. End-tidal partial pressures of CO2 were raised to hypercapnoeic levels and/or cyanide was injected into the carotid artery. The pipette was then positioned in the same region contralaterally and the experimental sequence was repeated. At the end of the experiment animals were exposed to anoxia, by ventilation with nitrogen, or asphyxia by termination of artificial ventilation.
Histology
Sites of injection were defined by the location of microbeads, viewed with a fluorescent microscope (Huang et al. 1997). Alternate sections were stained with Cresyl Violet.
RESULTS
Neuronal activities
Respiratory-modulated activities of 175 neurons were recorded at levels of medulla reported to contain the pre-Bötzinger and Bötzinger complexes in the adult rat (Dobbins & Feldman, 1994). In these regions, 0.8–1.2 mm rostral to the obex and 1.5–1.6 mm lateral to the midline, activities were distributed from 1.0 to 3.5 mm below the surface. All but fifty activities were 2.0–2.5 mm ventral. Activities were inspiratory (n = 75), expiratory (n = 66) or phase-spanning (n = 34; Fig. 1). Phase-spanning patterns were inspiratory- expiratory (n = 8), expiratory-inspiratory (n = 12) and tonic (n = 14). While the pre-Bötzinger (0.8–0.9 mm rostral to the obex) and the Bötzinger regions (1.0–1.2 mm rostral) contained all types of activities, the proportion of expiratory activities in the Bötzinger region was higher than in the pre-Bötzinger (66 %versus 31 %). Bilateral electrolytic lesions at the sites of recording never altered the eupnoeic rhythm.
Figure 1. Types of respiratory-modulated neuronal activities.
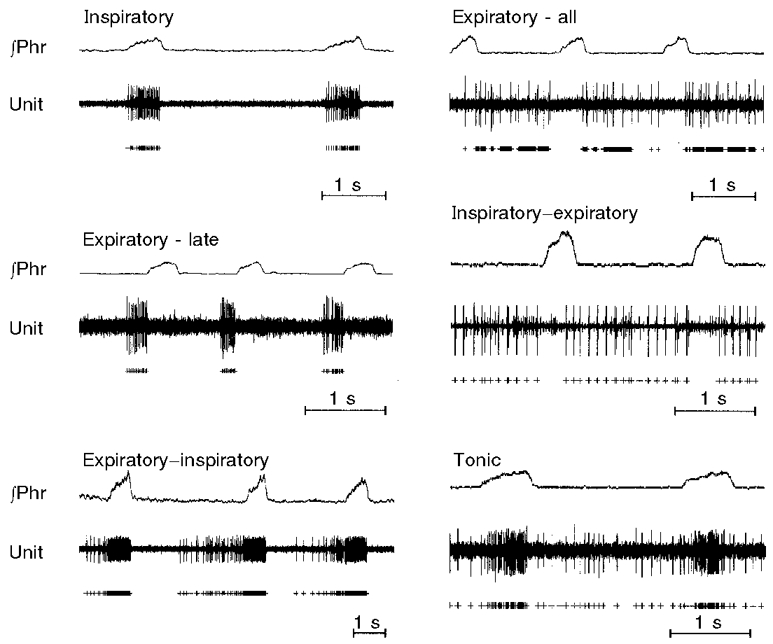
In the panels, integrated activity of the phrenic nerve (∫Phr) and neuronal activity (Unit) are shown. + below tracings are activities which were extracted from the background by a spike sorter.
Response to NaCN
As shown in Fig. 2, injections of 0.005–0.010 mg of NaCN into the carotid artery cause a transient augmentation of the peak height of phrenic bursts and the frequency of these bursts. The character of these phrenic bursts retained the ‘augmenting’ character of eupnoea (Fig. 2). Following bilateral sectioning of the carotid sinus nerves, injections of this same dose of NaCN no longer caused an alteration of phrenic activity. With further increases in the dose of NaCN, no changes in phrenic activity were recorded except for a decline in frequency. Importantly, phrenic activity retained its eupnoeic pattern.
Figure 2. Response to intracarotid injections of a solution of sodium cyanide before (CSN+) and after (CSN−) bilateral sectioning of the carotid sinus nerves.
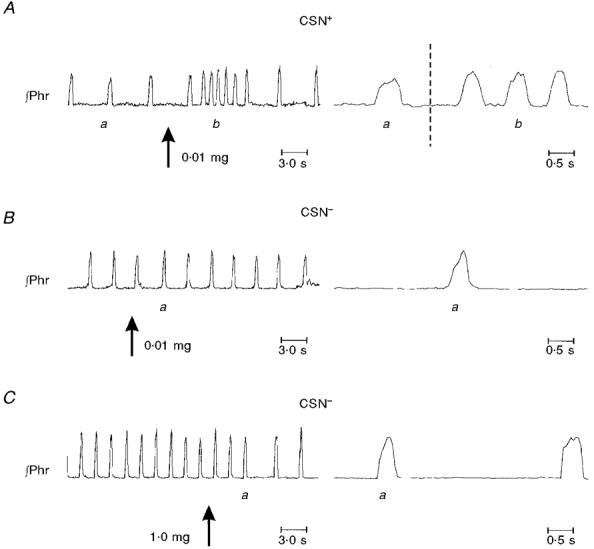
A shows augmentations in peak phrenic height and frequency of bursts following injection of 0.01 mg (kg body wt)−1 of NaCN into the left common carotid artery. Right panels show ramp-like rises of phrenic activity before (a) and after (b) the injection. B shows absence of response to a similar injection following bilateral sectioning of the carotid sinus nerves. C shows the response to injection of 1.0 mg (kg body wt)−1 of NaCN. In the right bottom panel, note that phrenic activity retained its eupnoeic pattern following injection (a).
Ablation and blockade of neurons
As only massed neuronal activities could be recorded through the double-barrel pipette, the location of microinjections was based upon the identification of fluorescent microbeads.
Kainic acid
In 13 rats, peak phrenic activity and/or the frequency of bursts gradually declined following a unilateral injection of 50 nl of kainic acid and apnoea resulted (Table 1). The time for disappearance of phasic activity varied from immediately after the injection to 26 min thereafter (mean 12.3 min). After phrenic activity had ceased, this activity did not return in hypercapnia.
Table 1.
Number of rats in which injections of kainic acid (KA) or muscimol resulted in an elimination of eupnoea and/or gasping
| Procedure | Apnoea | Gasping | No gasping | |
|---|---|---|---|---|
| KA 50 or 100 nl | Unilateral | 13 | — | — |
| n = 61 | Unilateral + bilateral | (12) | 11 | 1 |
| Bilateral only | 7 | 12 | 5 | |
| No apnoea (31) | — | 29 | 2 | |
| KA 2 ± 100 nl | Unilateral | 3 | — | — |
| n = 6 | Unilateral + bilateral | (3) | 1 | 2 |
| Bilateral only | 3 | 1 | 2 | |
| No apnoea (0) | — | — | — | |
| Muscimol | Unilateral | 2 | — | — |
| n = 7 | Unilateral + bilateral | (2) | 0 | 2 |
| Bilateral only | 2 | 2 | 0 | |
| No apnoea (0) | 3 | 3 | 0 |
Numbers in parentheses are those in which eupnoea was not eliminated or those which had a previous unilateral injection.—indicates experiment not performed.
A single injection of cyanide was effective in restoring the rhythm in seven animals. In three rats, the return of phrenic activity was transient for a number of bursts. Following cyanide, the phrenic rhythm permanently returned in the other four animals (Fig. 3). Note that this phrenic activity which recovered had the same ‘ramp-like’ rise of activity as before the injections of kainic acid. The injection of NaCN was usually made within 2 min after the onset of apnoea. However, injections made as long as 25 min after the initial apnoea were also effective in restoring phrenic activity.
Figure 3. Transient eliminations of eupnoea by injections of kainic acid into the rostral medulla.
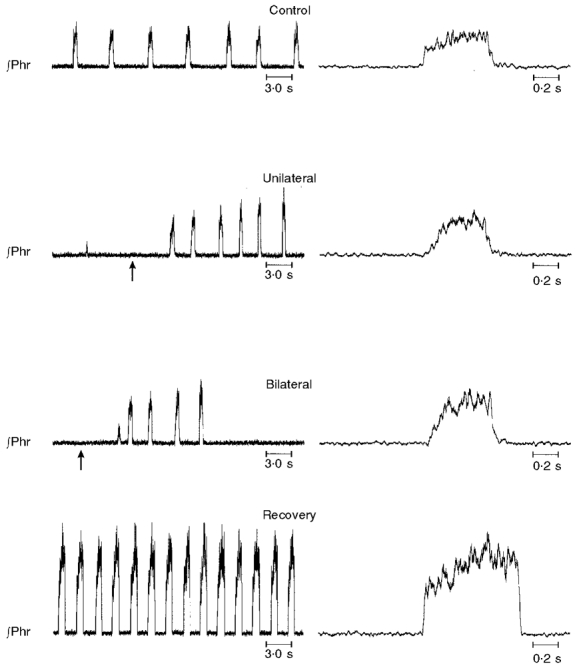
In each panel, integrated activity of the phrenic nerve (∫Phr) is shown. Right panels show phrenic activity on an expanded time scale. Control, 1 min before injection; unilateral, apnoea occurred 12 min after unilateral injection. Administration of NaCN (arrow) 2 min thereafter restored eupnoea. Bilateral, following bilateral injection. Apnoea resulted immediately but was transiently reversed by injection of NaCN (arrow) 12 min thereafter. Recovery, spontaneous recovery of eupnoea 42 min following bilateral injections.
Injections of NaCN were ineffective in restarting the eupnoeic rhythm in five rats. In these animals, such injections had produced only modest alterations in phrenic activity even before medullary lesions. A final animal received no injection of cyanide. However, in these six animals, eupnoea ultimately spontaneously returned. Likewise, eupnoea also permanently returned in all animals in which injections of cyanide had been effective in transiently restoring the phrenic rhythm. The time for recovery of eupnoea varied from 5.5 to 53 min (mean 16.9 min).
The site of effective injections of kainic acid was within that region in which respiratory-modulated neuronal activities were recorded (Fig. 4). Except for one animal, this region surrounded the nucleus ambiguus.
Figure 4. Localization of sites of injections of kainic acid and muscimol.
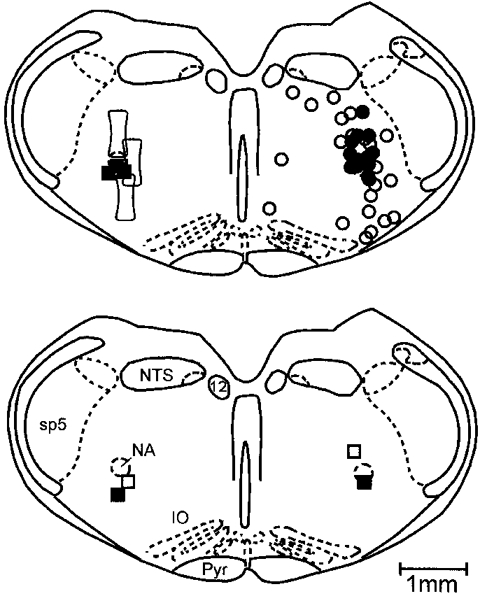
Sites between 0.7 and 1.0 mm rostral to the obex have been projected on the sections shown. In the upper panel (right), • shows sites in which a unilateral injection of kainic acid transiently eliminated eupnoea. ○ are initial sites of injection for which eupnoea was never eliminated. In the left upper panel, ▪ shows sites in which a unilateral injection of muscimol eliminated eupnoea transiently. The open outlined box shows the location, in three rats, of two injections of kainic acid which transiently eliminated eupnoea and permanently eliminated gasping. In the lower panel, □ and ▪ show sites of injection of kainic acid in two animals in which eupnoea was transiently eliminated by a unilateral and then again by a contralateral injection. IO, inferior olive; NA, nucleus ambiguus; NTS, nucleus tractus solitarius; Pyr, pyramidal tract; sp5, spinal tract of trigeminal; 12, nucleus of hypoglossal nerve.
In 12 of the animals which had exhibited apnoea, a contralateral injection of 50 nl of kainic acid again induced transient apnoea (Table 1, Fig. 3). The time for onset of this apnoea varied from immediately after the injection to 52 min thereafter (mean 12.7 min). As following unilateral injections of kainic acid, injections of cyanide were effective in restoring the eupnoeic rhythm (Fig. 3). Eupnoea ultimately returned in all rats. The time for return of eupnoea varied from 9 to 33 min. For most animals, as in Fig. 3, frequency and peak height of phrenic bursts were greater than in controls. Again, the ‘ramp-like’ rise of phrenic activity was observed. Regions of effective injections were distributed as the unilateral injection.
Apnoea was not elicited following unilateral but only after bilateral injections of 50 or 100 nl of kainic acid in another group of 17 rats. Apnoea occurred as late as 52 min following the second injection. This apnoea was transiently reversed by injections of cyanide in eight animals, and in five other rats, eupnoea permanently returned following this injection. In all 17 animals, eupnoea had returned between 9 and 90 min after phasic phrenic activity had initially disappeared. Histological evaluations revealed that at least one injection was within the region surrounding the nucleus ambiguus, as described above.
In 31 animals, apnoea was not elicited following either unilateral or bilateral injections of 50 or 100 nl of kainic acid. The location of the first of such injections is shown in Fig. 4. While some sites were clearly remote from those of effective injections, there was intermingling of injections which did or did not result in apnoea.
Upon exposure to anoxia or asphyxia, gasping was elicited in all but six of the animals in which unilateral and/or bilateral injections of kainic acid had resulted in apnoea (Table 1, Fig. 5). Note that, as opposed to eupnoea, phrenic activity in gasping had a decrementing pattern (Fig. 5). All but two of the animals in which eupnoea continued following injections likewise exhibited gasping. While there was no separation based upon region of injection of animals which did or did not exhibit gasping, seven of eight animals in which gasping was eliminated had received 100 nl injections of kainic acid.
Figure 5. Anoxia-induced gasping.
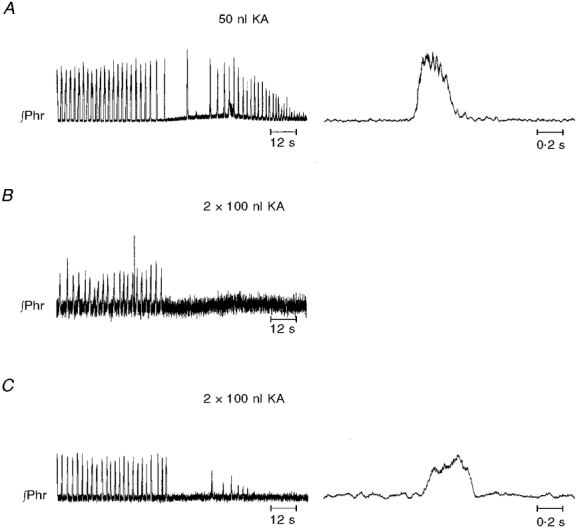
In A, eupnoea was replaced by apnoea and then gasping. This animal had received an injection of 50 nl of kainic acid bilaterally in the pre-Bötzinger complex. In the right panel, note that phrenic activity had decrementing discharge in gasp. In B and C, no gasping was elicited in anoxia. These animals had received two injections, each of 100 nl, bilaterally into the pre-Bötzinger complex. Small bursts in C had ramp-like or rounded patterns. (Phr, integrated activity of phrenic nerve.)
Six additional rats were studied in which two injections of kainic acid, each of 100 nl, were separated by 0.5 mm in the same vertical track. Alterations of eupnoea were as those following single injections in that transient and reversible apnoea followed the unilateral and/or bilateral injections. However, apnoea followed only the first and not the second injection on either side. Of these six animals, four failed to exhibit gasping in anoxia (Table 1, Fig. 5). The regions of injections are shown in Fig. 4 for three animals which failed to exhibit gasping.
Muscimol
In three of seven animals, phrenic activity declined following both unilateral and bilateral injections of 50 nl of muscimol, but apnoea was not obtained. Apnoea did occur in the other four animals (Fig. 6). Again, this apnoea was transient and reversible by injections of cyanide. The time for recovery of eupnoea following bilateral injections varied from 9–120 min. Regions of effective injections were as those of kainic acid (Fig. 4).
Figure 6. Transient eliminations of eupnoea by injections of muscimol into the rostral medulla.
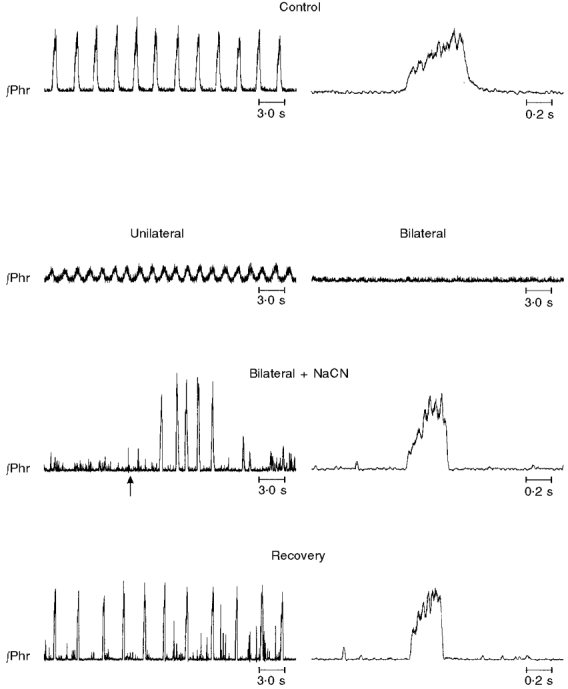
In each panel, integrated activity of the phrenic nerve (∫Phr) is shown. Control, 1 min before injection; unilateral, 10 min following unilateral injection. Peak phrenic activity was reduced. Bilateral, 10 min following bilateral injection. Apnoea was transiently reversed by injection of NaCN, which was administered 36 min following onset of apnoea (arrow). Recovery, spontaneous recovery of eupnoea. Eupnoea began to recover 120 min after onset of apnoea; record is taken 70 min thereafter. Right panels in Control, Bilateral + NaCN and Recovery show single eupnoeic phrenic bursts on an expanded scale.
Upon exposure to anoxia, gasping was elicited in two of the animals which had exhibited transient apnoea following microinjections of muscimol (Table 1).
Durations of actions of kainic acid and muscimol
Recordings of Fig. 7 show quantitative alterations in neuronal activities following injections of kainic acid. Following an injection of 50 nl, neuronal activities remained unaltered for variable periods. In one animal, a marked alteration in neuronal activities occurred within ten minutes. In the other two animals, a second injection of 50 nl was made and neuronal activities increased markedly soon thereafter. In all animals, neuronal activities then showed a decline in discharge and a cessation of activity. Concomitantly, activities of neurons which constituted a portion of the background activity also diminished (Fig. 7). Activities never returned, even after more than two hours of recording. Results were the same if data were analysed as to mean discharge frequencies of neurons (Fig. 7) or the integral of total activity. Since the latter included generalized electronic noise, it never declined to zero.
Figure 7. Sustained depressions of neuronal activities following microinjections of kainic acid (KA) or muscimol (Musc) into the rostral medulla.

A shows values of maximum discharge frequencies of multineuronal recordings following injections of kainic acid (closed symbols) or muscimol (open symbols) into the rostral medulla. Values are normalized as a percentage of control recordings, before injection. B and C show original recordings, each of 1 s duration, taken at the time indicated. Max. is maximal discharge frequencies at any time following injections of kainic acid (B) or muscimol (C). Injections of kainic acid were of 50 nl; in two animals, two injections were made. In three animals, two injections of 100 nl of muscimol were made. Time = 0 is immediately after the end of injections. Note that kainic acid caused an initial marked augmentation in neuronal activities and then a decline. Activities only declined following muscimol.
It must be noted that, if a neuronal activity was not altered within ten minutes after the second injection of kainic acid, it was assumed that the recording electrode was not close enough to the micropipette for kainic acid to reach the neuron. The pipette and recording electrode were then moved to the contralateral medulla and the experimental sequence was repeated.
Following injections of muscimol, neuronal activities only declined (Fig. 7). In one animal, some recovery was evident after sixty minutes of recording. However, even after two hours, activities had not returned to levels before injections of muscimol.
DISCUSSION
These studies establish that neuronal mechanisms within the pre-Bötzinger complex cannot represent an exclusive mechanism for the neurogenesis of eupnoea. Rather, our results are compatible with the concept that the pre-Bötzinger complex is but one of many components of a ponto-medullary circuit which can influence the expression of eupnoeic ventilatory activity.
Our results confirm those of Hsieh et al. (1998) and Ramirez et al. (1998) in that apnoea could be induced following a blockade or ablation of neuronal activities within the pre-Bötzinger complex. However, as Ramirez et al. (1998) also found, this apnoea was induced in only a limited proportion of animals following injections into seemingly identical regions. In the anaesthetized animals studied by Ramirez et al. (1998) and Hsieh et al. (1998), eupnoea never returned following injections of tetrotoxin or kainic acid, respectively, into the pre-Bötzinger complex. In the present study, eupnoea returned transiently or permanently following activation of the carotid chemoreceptors by injections of sodium cyanide. Eupnoea also spontaneously returned in all animals. Thus, our results are incompatible with neuronal activities in the pre-Bötzinger complex having an exclusive role in the neurogenesis of eupnoea.
An exclusive role for the pre-Bötzinger complex in the neurogenesis of eupnoea can be discounted only if three of our conclusions are accepted as valid. First, the injections were within the pre-Bötzinger complex, as defined by others. Second, the return of eupnoea did not reflect recovery from a transient blockade of neuronal activities within that region. Finally, intracarotid injections of sodium cyanide did elicit periods of eupnoeic ventilatory activity.
The pre-Bötzinger complex had been previously defined in adult cats in vivo based upon the neurophysiological localization of a mixed population of respiratory-modulated neuronal activities having inspiratory, expiratory and phase-spanning patterns (Connell et al. 1992; Schwarzacher et al. 1995; St-John, 1998b, 1999). As verified here in adult rats, this region lies caudal to a Bötzinger complex, containing a greater proportion of expiratory-modulated neuronal activities. The distribution of this mixed population of respiratory-modulated neuronal activities in rats included the location of those neurons which were designated as constituting the pre-Bötzinger complex from neuroanatomical studies (Dobbins & Feldman, 1994). Histological evaluations revealed that our injections encroached upon this neuroanatomical region (compare Fig. 4 here with Fig. 4 of Dobbins & Feldman, 1994). Indeed, we purposely made exceedingly large injections in order that muscimol or kainic acid would reach many or all of the neurons in this complex, as well as some in surrounding regions. Hence, we believe that evidence is substantial that our injections were within the pre- Bötzinger complex.
Also substantial is the conclusion that neuronal destruction by kainic acid is irreversible. As noted in Methods, neuroanatomical evaluations have revealed a rapid and progressive degeneration of neuronal soma following injections of kainic acid into various components of the brainstem respiratory control system (Denavit-Saubiéet al. 1980; Berger & Cooney, 1982). In the present studies, neuronal activities close to the region of injection were eliminated a few minutes after injection and no evidence of recovery of activity was evident even several hours later. However, in this same time period, an eupnoeic phrenic pattern had recovered in all animals following injections of a similar or even greater volume of kainic acid into the pre- Bötzinger complex.
The similar changes in phrenic activity which followed injections of muscimol and kainic acid demonstrate that our results cannot be equated with the transient excitation of neuronal activities by kainic acid. While the influence of muscimol on GABA receptors is ultimately reversible, its duration of action in vivo is several hours (e.g. Yamada et al. 1982; Gatti et al. 1987). A similar duration of action has been shown herein. Based upon such temporal considerations, neuronal activities within the pre-Bötzinger complex should still have been depressed by muscimol when eupnoea was re-established. Moreover, results following carotid chemoreceptor stimulation demonstrate that the return of eupnoea following injections into the pre-Bötzinger complex cannot be equated with reversible actions of either muscimol or kainic acid. Such reversibility would be expected to occur at a definitive time. Yet, the apnoea following injections of either agent was reversed at many differing times by intracarotid injections of 0.005 or 0.010 mg of sodium cyanide.
As shown in Results, intracarotid injections of 0.005 or 0.010 mg of sodium cyanide caused augmentation in peak phrenic activity and the frequency of phrenic bursts. This augmented phrenic activity still had the ramp-like rises typical of eupnoea. These responses were completely eliminated following bilateral sectioning of the carotid sinus nerves. After these nerve sections, injections of even 1.0 mg of sodium cyanide did not cause any changes from the eupnoeic pattern of phrenic activity. These results confirm many previous studies that systemic injections of cyanide cause augmentations in ventilatory activation primarily though actions upon the peripheral chemoreceptors (Heymans et al. 1931). Likewise, these results confirm that while elevated doses of cyanide may have direct actions upon the brainstem, these actions do not cause an elimination of eupnoea (Brodie & Borison, 1956).
Since the pre-Bötzinger complex cannot represent an exclusive site for the neurogenesis of eupnoea, it is possible that eupnoea can be generated in multiple regions of the brainstem. As we have discussed in a recent review (St-John, 1998a), respiratory rhythm can be generated in isolated segments of pons or medulla. However, the relationship of such rhythms to eupnoea is undefined.
Rather than generating the eupnoeic rhythm, neuronal activities of the pre-Bötzinger complex may represent one or many sources of a tonic input which is necessary for eupnoea to be expressed. A related concept would be that eupnoea is generated by a ponto-medullary neuronal circuit, of which neurons of the pre-Bötzinger complex represent one element. The latter concept has recently been advanced by Pierrefiche et al. (1998). These investigators have reported that eupnoeic ventilatory activity was severely disrupted by a blockade of inhibitory synaptic activity within the pre-Bötzinger complex in vivo in anaesthetized cats. Such a disruption is in contrast to the continuation of rhythmic discharges of the in vitro brainstem-spinal cord preparation of the neonatal rat following a similar blockade (Smith et al. 1990, 1991; Rekling & Feldman, 1998). Hence, mechanisms of rhythm generation in this preparation may have no applicability to the neurogenesis of eupnoea in vivo. Specifically, the results of Pierrefiche et al. (1998) appear to make untenable the concept that eupnoea in vivo is generated by the discharge of pacemaker neurons in the pre-Bötzinger complex (Rekling & Feldman, 1998). Rather, Pierrefiche et al. (1998) propose that any pacemaker neuronal activities, as defined in vitro, are superseded by neuronal circuits responsible for generating the eupnoeic rhythm in vivo. Pontile mechanisms are considered as a critical element of such circuits.
The possibility that neuronal activities in the pre-Bötzinger complex represent one of many sources of a tonic input which is necessary for eupnoea to be expressed is reasonable, based upon many previous results. Being the source of a tonic input would provide an explanation for the absence of change in eupnoea in some animals which Ramirez et al. (1998) and the present authors recorded following a perturbation of neuronal activities in the pre-Bötzinger complex. Concerning such a tonic input, transient apnoea similar to that reported herein follows a blockade or ablations of numerous regions on or near the ventrolateral medullary surface, including the retrotrapezoid nucleus, the retrofacial nucleus, Bötzinger complex, subretrofacial nucleus and the intermediate chemoreceptor region (see discussion in St-John, 1998a). In addition, apnoea of some hours duration has been reported following lesions of the dorsal medullary respiratory nucleus (Berger & Cooney, 1982) or the pontile pneumotaxic centre (St-John, 1979). While responses in each region have not been categorized in detail, there are some generalities. Depressions of ventilation are more severe in anaesthetized than decerebrate or unanaesthetized, intact preparations. Also, apnoea is more probable the lower the level of respiratory drive at which examinations are made. Finally, the magnitude of depression is greatest immediately after an ablation (Nattie et al. 1991, 1992; Nattie & Li, 1994; St-John, 1998a).
These generalities fit precisely with the variable changes which have been reported following interruptions of activities within the pre-Bötzinger complex. Thus, recovery following an ablation provides an explanation for the absence of change in eupnoea which Speck & Feldman (1982) and Speck & Beck (1989) reported. Since this region was gradually ablated with multiple small lesions, compensation was ongoing.
Again fitting with these generalities, laboratories which have reported seemingly irreversible apnoea have produced acute and usually bilateral interruptions of neuronal activities within the pre-Bötzinger complex of anaesthetized animals (Koshiya et al. 1993; Koshiya & Guyenet, 1996; Hsieh et al. 1998; Ramirez et al. 1998; Pierrefiche et al. 1998). A few decerebrate rats were used by Koshiya and his colleagues. Moreover, in all these studies, respiratory drive has been relatively low, as animals have been maintained at normocapnia or hypocapnia.
The present experiments differ from those reporting permanent apnoea in that unanaesthetized, decerebrate animals were studied. Moreover, we provided a powerful augmentation in respiratory drive by activation of the peripheral chemoreceptors with cyanide. Also, after initial experiments, animals were maintained for a number of hours.
Following a bilateral blockade of neuronal activities within the pre-Bötzinger complex, augmentations in respiratory drive were produced by hypoxia or asphyxia in the studies of Ramirez et al. (1998) and Pierrefiche et al. (1998). Such stimuli would concomitantly depress mechanisms for eupnoea while activating those for gasping (St-John, 1990, 1996). Ramirez et al. (1998) reported that ‘gasp-like’ bursts could be elicited in five of six animals. However, following a similar blockade of neuronal activities in the study of Pierrefiche et al. (1998), no gasp-like bursts were elicited in asphyxia, perhaps because its duration was not as long as in the study of Ramirez et al. (1998) (Richter, personal communication). Similarly, gasping could not be elicited in the studies of Hsieh et al. (1998) following injections of kainic acid into the pre-Bötzinger complex. Thus, as reported herein, a blockade or ablations of neuronal activities within the pre-Bötzinger complex can interrupt eupnoea but not gasping, but both may be eliminated together. Our present and previous results provide a possible explanation for these differing results.
In the present experiments, bilateral injections of kainic acid into the pre-Bötzinger complex, of total volumes from 50 to 200 nl on each side, were equivalent in transiently eliminating eupnoea. Gasping could not be elicited following injections of the larger volumes. Parenthetically, the absence of gasping after a return of eupnoea provides additional support for the conclusion, discussed above, that neurons were irreversibly destroyed by injections of kainic acid. Similar to the present study, unilateral injections of kainic acid into the pre-Bötzinger complex of neonatal rats only eliminated gasping if large volumes were injected (Huang et al. 1997). Eupnoea was not altered, perhaps because injections were only unilateral. Hence, it is possible that the number of neuronal activities which must be destroyed to eliminate gasping is greater than those resulting in a transient elimination of eupnoea. We have reported that gasping, but not eupnoea, is eliminated following unilateral injections of the neurotoxin kainic acid into the rostral medulla (St-John et al. 1984; Fung et al. 1994). These effective injections overlapped at the dorsomedial border with the pre-Bötzinger complex. Dendrites and/or axonal terminals of neurons in this complex are concentrated in this dorsomedial region (Schwarzacher et al. 1995; Paton, 1997). Hence, injections of kainic acid might have disrupted synaptic interactions among neurons of the pre-Bötzinger complex. Synchronization among these neuronal activities is essential for the generation of rhythmic bursts of in vitro preparations and, we hypothesize, the gasp (St-John, 1998a). In this same context, Soloman et al. (1999) have recently reported that microinjections of the potent glutamate agonist, dl-homocysteic acid, into the pre-Bötzinger complex can cause the replacement of eupnoea by gasp-like bursts. Again, this fits with the concept that neuronal activities of the pre-Bötzinger complex may generate the gasp when freed from the ponto-medullary circuit generating eupnoea or, as shown by Solomon et al. (1999), when massively activated.
Despite this uncertainty concerning the specific roles of neuronal activities in the pre-Bötzinger complex in the neurogenesis of gasping and eupnoea, results of the present study definitely establish that these neuronal activities do not play an exclusive role in the neurogenesis of eupnoea in vivo. Hence, the concept that mechanisms for the genesis of rhythmic activity in the in vitro neonatal rat brainstem preparation also underlie the neurogenesis of eupnoea in vivo is not supported.
Acknowledgments
These studies were supported by grant no. 26091 from the National Heart, Lung and Blood Institute, National Institutes of Health (USA). We thank Kaushik Dutta for his help with some initial experiments.
References
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. Journal of Applied Physiology. 1982;52:131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Brodie DA, Borison HL. Analysis of central control of respiration by the use of cyanide. Journal of Pharmacology and Experimental Therapeutics. 1956;118:220–229. [PubMed] [Google Scholar]
- Cherniack NS, von Euler C, Homma I, Kao FF. Graded changes in central chemoreceptor input by local temperature changes in the ventral surface of medulla. The Journal of Physiology. 1979;287:191–222. doi: 10.1113/jphysiol.1979.sp012654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Research. 1992;590:337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- Denavit-Saubié M, Riche D, Champagnat J, Velluti JC. Functional and morphological consequences of kainic acid microinjections into a pontine respiratory area of the cat. Neuroscience. 1980;5:1609–1620. doi: 10.1016/0306-4522(80)90025-1. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. Journal of Comparative Neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Fung M-L, Wang W, St-John WM. Medullary loci critical for expression of gasping in adult rats. The Journal of Physiology. 1994;480:597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti PJ, DaSilva AMT, Gillis RA. Cardiorespiratory effects produced by injecting drugs that affect GABA receptors into nuclei associated with the ventral surface of the medulla. Neuropharmacology. 1987;26:423–431. doi: 10.1016/0028-3908(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Heymans C, Bouckaert JJ, Dautrebande L. Sinus carotidien et réflexes respiratoires. III. Sensibilite des sinus carotidiens aux substances chemique. Action stimulante respiratoire réflexe du sulfure de sodium, du cyanure de potassium, de la licotine et de la lobeline. Archives Internationale de Pharmacodynamie et de Thérapie. 1931;40:54–91. [Google Scholar]
- Hsieh JH, Chang YC, Su CK, Hwang JC, Yen CT, Chai CY. A single minute lesion around the ventral respiratory group in medulla produces fatal apnoea in cats. Journal of the Autonomic Nervous System. 1998;73:7–18. doi: 10.1016/s0165-1838(98)00117-9. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, St-John WM. Lesions of regions for in vitro ventilatory genesis eliminates gasping but not eupnoea. Respiration Physiology. 1997;107:111–123. doi: 10.1016/s0034-5687(96)02513-3. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. The Journal of Physiology. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Research. 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Blanchford C, Li A. Retrofacial lesions: effects on CO2 sensitive phrenic and sympathetic output. Journal of Applied Physiology. 1992;73:1317–1325. doi: 10.1152/jappl.1992.73.4.1317. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respiration Physiology. 1994;97:63–77. doi: 10.1016/0034-5687(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, St-John WM. Lesions in the retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. Journal of Applied Physiology. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. The Journal of Physiology. 1997;502:623–639. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. The Journal of Physiology. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J-M, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. The Journal of Physiology. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- St-John WM. Differential alteration by hypercapnia and hypoxia of the apneustic respiratory pattern in decerebrate cats. The Journal of Physiology. 1979;287:467–491. doi: 10.1113/jphysiol.1979.sp012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM. Neurogenesis, control and functional significance of gasping. Journal of Applied Physiology. 1990;68:1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- St-John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? Journal of Applied Physiology. 1996;81:1865–1877. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Progress in Neurobiology. 1998a;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- St-John WM. Alterations in respiratory neuronal activities in ‘Pre-Bötzinger’ region in hypocapnia. Respiration Physiology. 1998b;114:119–131. doi: 10.1016/s0034-5687(98)00088-7. [DOI] [PubMed] [Google Scholar]
- St-John WM. Rostral medullary respiratory neuronal activities of decerebrate cats in eupnea, apneusis and gasping. Respiration Physiology. 1999;116:47–65. doi: 10.1016/s0034-5687(99)00030-4. [DOI] [PubMed] [Google Scholar]
- St-John WM, Bledsoe TA, Sokol HW. Identification of medullary loci critical for neurogenesis of gasping. Journal of Applied Physiology. 1984;56:1008–1019. doi: 10.1152/jappl.1984.56.4.1008. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. Journal of Neurophysiology. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Soloman IC, Edelman NH, Neubauer JH. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the Pre-Bötzinger complex in vivo. Journal of Neurophysiology. 1999;81:1150–1161. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Speck DF, Beck ER. Respiratory rhythmicity after extensive lesions of the dorsal and ventral respiratory groups in the decerebrate cat. Brain Research. 1989;482:387–392. doi: 10.1016/0006-8993(89)91206-7. [DOI] [PubMed] [Google Scholar]
- Speck DF, Feldman JL. The effects of microstimulation and microlesions in the ventral and dorsal respiratory groups in medulla of cat. Journal of Neuroscience. 1982;2:744–757. doi: 10.1523/JNEUROSCI.02-06-00744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler C. Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JF, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 1–67. part 1. [Google Scholar]
- Yamada KA, Norman WP, Hamosh P, Gillis RA. Medullary ventral surface GABA receptors affect respiratory and cardiovascular function. Brain Research. 1982;248:71–78. doi: 10.1016/0006-8993(82)91148-9. [DOI] [PubMed] [Google Scholar]


