Abstract
To investigate the functional significance of different troponin T (TnT) isoforms in the Ca2+ activation of muscle contraction, transgenic mice have been constructed with a chicken fast skeletal muscle TnT transgene driven by a cardiac α-myosin heavy chain gene promoter.
Cardiac muscle-specific expression of the fast skeletal muscle TnT has been obtained with significant myofibril incorporation. Expression of the endogenous cardiac muscle thin filament regulatory proteins, such as troponin I and tropomyosin, was not altered in the transgenic mouse heart, providing an authentic system for the functional characterization of TnT isoforms.
Cardiac muscle contractility was analysed for the force vs. Ca2+ relationship in skinned ventricular trabeculae of transgenic mice in comparison with wild-type litter-mates. The results showed unchanged pCa50 values (5.1 ± 0.04 and 5.1 ± 0.1, respectively) but significantly steeper slopes (the Hill coefficient was 2.0 ± 0.2 vs. 1.0 ± 0.2, P < 0.05).
The results demonstrate that the structural and functional variation of different TnT isoforms may contribute to the difference in responsiveness and overall cooperativity of the thin filament-based Ca2+ regulation between cardiac and skeletal muscles.
The activation of striated (cardiac and skeletal) muscle contraction by intracellular Ca2+ is a highly cooperative process involving a series of myofilamental protein conformational changes. An μ2-fold higher cooperativity is apparent during the activation of skeletal muscle compared with that of cardiac muscle (Brandt et al. 1984; Babu et al. 1987; Sweitzer & Moss, 1990). The thin filament-based Ca2+ activation of muscle contraction requires conformational transitions of the troponin-tropomyosin-F-actin assembly (Leavis & Gergely, 1984; Tobacman, 1996). Troponin T (TnT) is the tropomyosin-binding subunit of the troponin complex and interacts with tropomyosin, troponin C (TnC), troponin I (TnI) and F-actin (Zot & Potter, 1987; Perry, 1998). Therefore, TnT may be considered as a molecular organizer of the thin filament regulatory system (Potter et al. 1995; Tobacman, 1996). TnT is at a central position in this allosteric Ca2+ signalling system, and thus its structure-function characteristics may contribute to the characteristics of muscle contraction.
Cardiac and skeletal muscle TnTs are conserved in their primary structure, especially in the central and COOH-terminal regions (Cooper & Ordahl, 1985; Breitbart & Nadal-Ginard, 1986; Gahlmann et al. 1987; Smillie et al. 1988; Jin et al. 1992,1996; Briggs & Schachat, 1993; Wang & Jin, 1997). The main structural difference between cardiac and skeletal muscle TnTs exists in the NH2-terminal domain, which is also highly variable among the alternative mRNA splicing-generated isoforms of each of the muscle type-specific TnTs. Alternative splicing of an NH2-terminal exon produces a large to small, acidic to basic cardiac TnT isoform switch during heart development (Jin & Lin, 1988). Complex alternative splicing of multiple NH2-terminal exons also results in an acidic to basic switch of the fast skeletal muscle TnT isoforms during development (Wang & Jin, 1997).
The functional significance of the different TnT isoforms is not fully understood. Differences have been observed in the activation of the actomyosin ATPase by reconstituted thin filaments containing TnT isoforms with different NH2-terminal primary structures (Tobacman, 1988). We have demonstrated that the physical properties and 3-dimensional structure of the NH2-terminal variable region can modulate the global conformation of the TnT molecule and its binding affinities to TnI and tropomyosin (Ogut & Jin, 1996; Wang & Jin, 1998). The expression of cardiac TnT isoforms with NH2-terminal structural differences is associated with myocardial hypertrophy and failure (Akella et al. 1995; Saba et al. 1996). The NH2-terminal charge of TnT isoforms also contributes to the tolerance of the thin filament regulatory system to acidosis (Ogut & Jin, 1998). Point mutations generating single amino acid substitutions in the cardiac TnT polypeptide chain are related to the molecular etiopathology of human familial hypertrophic cardiomyopathies with dominant phenotypic effects (Watkins et al. 1995; Lin et al. 1996; Sweeney et al. 1998). These data support the concept that TnT isoforms with minor structural differences may play a role in modulating the contractile properties of different muscle types both during development and under pathological conditions. Although it has been previously demonstrated that regulated expression of the thin filament proteins is not responsible for the developmental change of maximal shortening velocity of muscle (Reiser et al. 1989), others have suggested that TnT and/or tropomyosin isoform expression may contribute to the cooperativity of fast skeletal muscle contraction (Schachat et al. 1987).
Extensive biochemical and biophysical studies have laid a foundation for further investigation of the structure-function relationships of TnT isoforms within native muscle fibres. In the present study, we have established specific expression of a fast skeletal muscle TnT in the cardiac muscle of transgenic mice with significant myofibril incorporation. To characterize the effects of TnT structure and function on the regulation of muscle contraction, force vs. Ca2+ relationships were studied in skinned cardiac trabeculae. The results show that the transgenic mouse ventricular trabeculae conferred a significantly increased Hill coefficient compared with that obtained from wild-type mouse cardiac muscle. The data provide strong evidence that the structure-function relationship of TnT isoforms may be a factor in determining the overall cooperativity of the Ca2+ activation of skeletal and cardiac muscle contraction.
METHODS
The core molecular cloning and recombinant DNA techniques used in this study have been described previously (Jin et al. 1998; Huang et al. 1999).
Cloning of full length cDNA encoding an adult chicken breast muscle TnT
To investigate the functional difference between TnT isoforms in an integrated muscle system, our strategy was to first compare two distinct TnT isoforms, the cardiac and the fast skeletal muscle TnTs, in a transgenic mouse muscle consisting of a uniform fibre type. In contrast to most skeletal muscles containing mixed fibre types, the myocardium contains purely cardiac muscle and thus is an ideal tissue environment for this investigation. Chicken breast muscle has been studied extensively as a representative fast white skeletal muscle and, therefore, we have chosen to compare adult chicken breast muscle fast TnT with the cardiac TnT. It was then essential in this study to specifically identify the exogenous skeletal muscle TnT and the endogenous cardiac TnT expressed in the heart of transgenic mice. To distinguish the cardiac and skeletal muscle TnTs of largely conserved structures, we have developed a highly specific anti-chicken breast muscle TnT monoclonal antibody (mAb), 6B8, which has no cross-reaction with cardiac TnT (Wang & Jin, 1998), as well as an anti-cardiac TnT mAb, CT3, which has no cross-reaction with fast skeletal muscle TnT (Jin et al. 1998).
cDNA encoding the chicken fast skeletal muscle TnT was cloned by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from the breast muscle (pectoralis major) of an adult White Leghorn chicken, which was killed by decapitation using a guillotine, with the TRIzol reagent (Gibco/BRL) according to the manufacturer's protocol. The upstream (forward) (GCCCATATGTCTGATACCGAR) and downstream (reverse) (TTACTTCCAGCGCCCGCCAAC) oligonucleotide primers were synthesized according to the published chicken fast skeletal muscle TnT cDNA sequence (Smillie et al. 1988) corresponding to the regions around the translation initiation and termination codons, respectively. The first strand cDNA was synthesized from the total RNA templates using Moloney murine leukemia virus reverse transcriptase (Pharmacia Biotech, Inc.). Double-stranded cDNA including the entire coding region of the chicken breast muscle TnT was then amplified by PCR and cloned into the pAED4 plasmid as previously described (Jin, 1995; Ogut & Jin, 1996). Full length chicken fast skeletal muscle TnT cDNA including the 3′-untranslated regions and the poly(A) tail was then reconstituted by pasting a restriction enzyme fragment isolated from the chicken TnT2 cDNA (Smillie et al. 1988, kindly provided by Dr Larry Smillie, University of Alberta). The native 5′-untranslated region containing the consensus eukaryotic translation initiation sequence was restored using oligonucleotide-mediated site-specific mutagenesis to reverse the sequence changes generated during the RT-PCR cloning (the introduction of an Nde I restriction site) for bacterial expression using the pAED4 vector. Briefly, a synthetic primer corresponding to the authentic sequence flanking the translation initiation codon of chicken fast TnT cDNA (Smillie et al. 1988) was used together with a vector sequence-derived 3′-flanking primer in a standard PCR reaction to amplify the cDNA insert for reconstruction of the original cDNA. The resulting cDNA template was mapped by restriction endonuclease digestion and sequenced to verify the fidelity of cloning.
Production of transgenic mice and genotypic characterization
To investigate the functional effects of the fast skeletal muscle TnT on the contraction of cardiac muscle, we have constructed a transgene using the cloned promoter of the mouse cardiac α-myosin heavy chain (α-MHC) gene (Subramanian et al. 1993; generously provided by Dr Jeffrey Robbins, University of Cincinnati) to direct a heart-specific, post-natal expression of the chicken fast skeletal muscle TnT cDNA in transgenic mice. The construction of the transgene (Fig. 2A) was carried out by standard recombinant DNA techniques. The transgene DNA segment was cleaved from the recombinant plasmid by restriction enzyme digestion at flanking sites and isolated by agarose gel electrophoresis. After being recovered from the gel slice by electrophoresis elution and purified by the QIAfilter mini-column (Qiagen, Chatsworth, CA, USA), the linear transgene DNA fragment was used in the production of transgenic mice. Using fertilized eggs collected from the oviducts of C57BL/6 mice after gonadotropin-induced superovulation, the pronucleus injection and embryo reimplantation were performed at the Transgenic Core Facility at the Cleveland Clinic Foundation (Cleveland, OH, USA). For the screening of the transgenic genotypes, genomic DNA was purified from mouse tail snips by proteinase K digestion (200 μg ml−1 in 100 mm Tris-HCl, pH 8.0, containing 0.2 M NaCl, 5 mm EDTA and 0.2 % sodium dodecyl sulfate (SDS)) at 50°C overnight, followed by phenol-CHCl3 extraction and ethanol precipitation. The transgenic founders were initially identified by PCR using a pair of transgene specific oligonucleotide primers (Fig. 2A) and then verified by Southern blotting using the chicken fast skeletal muscle TnT cDNA probe (Fig. 2A). The copy number of the transgene integrated into the genome of each founder transgenic mouse was estimated from the Southern blots in comparison with the quantitative controls. The transgenic founders were bred with wild-type C57BL/6 mice and the progenies were screened by PCR as described above. The positive transgenic progenies from each founder line were maintained and used for phenotypic characterization.
Figure 2. Production of transgenic mouse with a heart-specific transgene encoding a fast skeletal muscle TnT isoform.
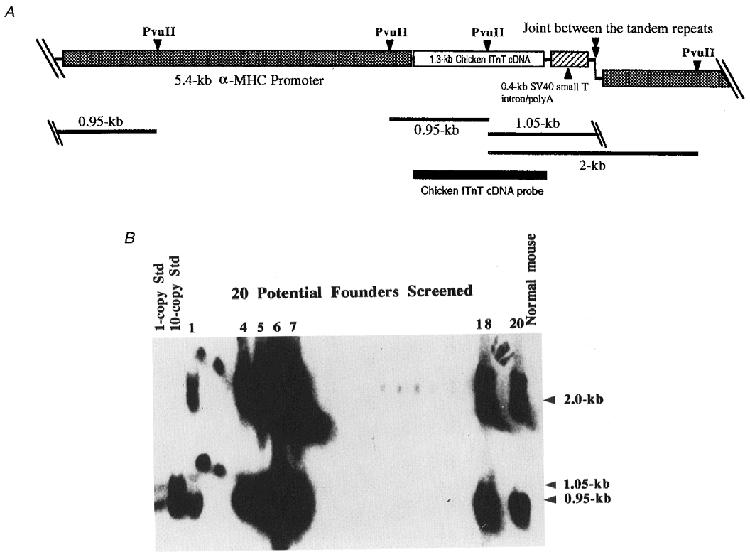
A, transgene construct for the expression of adult chicken breast muscle TnT under the control of the mouse α-MHC gene promoter. Position of the chicken TnT cDNA probe used in Southern blot genotype analysis is outlined on the map. For the Southern blot genotyping, the mouse genomic DNA and the transgene DNA fragment prepared for the pronucleus injection were digested by the restriction enzyme Pvu II. In the transgenic mouse genome which contains tandem repeats of the transgene, two positive Pvu II fragments of 0.95 and 2.0 kb are expected to hybridize with the cDNA probe. The 1.05 kb transgene construct-specific band will not be seen after integration into the chromosome. B, Southern blot genotyping of the transgenic mouse lines. Only the samples from seven positive founders are labelled with their identification numbers to show the various levels of transgene copies integrated into the mouse genome as compared with the 1- and 10-copy standards digested together with normal (wild-type) mouse genomic DNA.
SDS-polyacrylamide gel electrophoresis and Western blotting
Mice were killed by cervical dislocation as previously described (Ogut & Jin, 1996). Total protein extracts from whole muscle tissues or extensively washed myofibrils were prepared in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 1 % SDS to ensure the solubilization of myofibril proteins. The samples were resolved by Laemmli SDS-PAGE using a Bio-Rad Lab minigel apparatus. The protein bands resolved on the SDS gel were transferred to a nitrocellulose membrane using a Bio-Rad Lab semidry electrotransfer apparatus at a current of 5 mA cm−2 for 30 min. The nitrocellulose membranes were blocked with 1 % bovine serum albumin (BSA) in Tris-buffered saline (TBS: 150 mm NaCl, 50 mm Tris-HCl, pH 7.5). To examine the expression of TnT isoforms and other sarcomere thin filament proteins, the membranes were incubated with the following primary antibodies diluted in TBS containing 0.1 % BSA: (a) an mAb, 6B8, specifically against the chicken breast muscle TnT (Wang & Jin, 1998), (b) an anti-cardiac TnT mAb, CT3 (Jin et al. 1998), (c) an mAb against TnI (TnI-1, Huang et al. 1999), (d) an mAb against tropomyosin (CH1; Lin et al. 1985; a gift from Dr Jim Lin, University of Iowa), and (e) a rabbit polyclonal antiserum RATnT raised against chicken breast muscle TnT with cross-reactions to both skeletal muscle and cardiac TnTs (Wang & Jin, 1998). Subsequent washing, incubation with alkaline phosphatase-labelled anti-mouse IgG or anti-rabbit IgG second antibodies (Sigma), and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)-nitro blue tetrazolium (NBT) substrate reaction were performed as described previously (Ogut & Jin, 1998; Wang & Jin, 1998).
To examine the effect of exogenous TnT expression on the distribution of other thin filament proteins, transgenic and control mouse ventricular muscle was cut into fine pieces approximately the size of isolated trabeculae (see below) with a sharp razor blade and washed in skinning solution at 4°C with rotation for 30 min. After centrifugation at 14 000 g at 4°C for 20 min, the supernatant and pellet were analysed for the free and myofibril-integrated proteins by SDS-PAGE and Western blotting using the anti-TnT, TnI and tropomyosin antibodies as described above.
Immunofluorescence microscopy
The preparation of mouse cardiac and chicken breast muscle myofibrils and indirect immunofluorescence microscopy were carried out as previously described (Jin, 1995). The myofibrils were settled in relaxing buffer, fixed with 3.7 % formaldehyde, and blocked with 1 % BSA and 0.05 % Tween-20 in phosphate-buffered saline (PBS: 137 mm NaCl, 2.7 mm KH2PO4, 8 mm Na2HPO4, pH 7.4) before incubation with the anti-chicken fast skeletal muscle TnT mAb 6B8 at room temperature for 2 h. After washing with PBS containing 0.05 % Tween-20, TRITC-conjugated anti-mouse IgG second antibody (Sigma) was applied to the slides to detect the 6B8 mAb staining. After incubation and washing as above, the slides were mounted with a coverslip and viewed under a Zeiss Axiovert 100H phase-contrast-epifluorescence microscope. A Plan-Neo phase fluorescence × 100 objective lens (oil, NA 1.30) was used for the photography of both phase-contrast and fluorescence images.
Measurement of Ca2+ activation of cardiac muscle contraction
The Ca2+ sensitivity of force for myocardial trabeculae of five mature male transgenic mice from four founder lines (1, 4, 5 and 18, Fig. 2B) and five of their wild-type male litter mates were determined in a blind experimental setting.
Triton-skinned trabeculae were prepared as described by Smith & Barsotti (1993). Briefly, mice were killed by CO2 asphyxiation and their hearts were rapidly removed and washed in ice-cold Ca2+-free physiological saline solution containing (mm): 140 NaCl, 4.7 KCl, 0.3 Na2HPO4, 1.2 MgCl2, 5.6 glucose, 2.0 Mops (3-[N-morpholino]propane-sulfonic acid) and 0.5 EGTA, to remove blood from the cardiac chambers. The heart was then transferred to a Sylgard-coated Petri dish containing Ca2+-free physiological saline solution. Under a dissecting microscope, the trabeculae, approximately 50 μm in diameter, were located and removed. The trabeculae were pinned at their in vivo length and covered with cold (10°C) skinning solution containing (mm): 5 MgATP, 5 EGTA, 25 methane sulfonic acid (MS), 6.9 MgCl2, 25 creatine phosphate, 2 glutathione; and 0.5 % Triton X-100; pH 7. After incubation at 4°C for 2 h, the skinning solution was replaced with storing solution containing 50 % glycerol and (mm): 5 MgATP, 5 EGTA, 25 MS, 6.9 MgCl2, 25 creatine phosphate, 2 glutathione; and 10 μg ml−1 leupeptin. The pH of all solutions was adjusted with KOH to 7.0 at room temperature. The skinned trabeculae in storing solution were placed at −20°C for no more than 2 days before use. Two to three trabeculae were obtained from the left ventricle of each transgenic or control mouse heart.
To measure Ca2+ sensitivity of force development, trabeculae were mounted at their in vivo length between a force transducer (Akers, AME801, Horten, Norway) and a piezoelectric length driver (Physic Instrumente, Walbronn, Germany) using small aluminum foil T-clips (Goldman et al. 1984). The resting force of the preparation was 250 ± 10 μN or 3.5 kN m−2. Force was then recorded as a function of pCa (-log[Ca2+]). The trabeculae was first bathed in relaxing solution (pCa 9) containing (mm): 5 MgATP, 5 EGTA, 25 MS, 0.01 CaCl2, 6.9 MgCl2 and 25 creatine phosphate; then sequentially transferred to activating solutions, each containing higher [Ca2+] until a new steady-state force was reached. The [Ca2+] was increased until maximal Ca2+ activation occurred in an activating solution (pCa 4) containing (mm): 5 MgATP, 5 EGTA, 25 MS, 5.3 CaCl2, 6.9 MgCl2 and 25 creatine phosphate. Methods and procedures for mixing individual solutions have been previously described (Brozovich & Yamakawa, 1993). A total of 13 trabeculae from the transgenic mouse heart were analysed together with 12 wild-type controls.
Data of force was recorded on a Nicolet 320 digital oscilloscope (12-bit resolution) with a sampling rate of 10 Hz and stored on floppy disks. For each pCa, the steady-state force was measured and then normalized to the maximum level at pCa 4. Data of relative force vs. pCa were averaged for the transgenic and control trabeculae, and the average relative force vs. pCa was fitted to a 4-parameter Hill equation:
where F0 is a constant, Fmax is the maximum predicted relative force, pCa50 is the pCa at the half-maximum force level of the Hill fit, and n is the Hill coefficient.
To verify the TnT isoform contents, total protein extracts from the ventricular muscle of each of the transgenic and control mouse hearts were analysed by Western blotting as above using the anti-chicken fast skeletal muscle TnT mAb 6B8 and the polyclonal antibody RATnT which recognizes both skeletal and cardiac muscle TnTs.
To compare pCa-tension relationships for the transgenic mouse cardiac muscle to a muscle naturally expressing the fast skeletal muscle TnT, single fibres of chicken pectoralis were prepared as described by Reiser et al. (1992). Briefly, a White Leghorn chicken was killed by CO2 asphyxiation and the pectoralis was removed and placed in cold relaxing solution. Then, 1 mm bundles of fibres were dissected from the muscle, tied onto capillary tubes, and placed in storing solution at −20°C. Prior to each experiment, a single fibre was dissected from the bundle in relaxing solution and the ends were placed in aluminum foil T-clips and mounted in the experimental chamber. The mechanical measurements were performed as described for the trabeculae.
RESULTS
Primary structure comparison between chicken breast muscle TnT and mouse cardiac TnT
By a combination of molecular biology methods, we have cloned and reconstituted the full length cDNA coding template for the major TnT isoform in adult chicken breast muscle (GenBank/EMBL Data Bank accession number AF044922) and verified its authenticity by DNA sequencing. An amino acid sequence alignment of the chicken fast skeletal muscle TnT and the adult mouse cardiac TnT (Jin et al. 1996) is shown in Fig. 1 to compare their primary structures. The sequence alignment demonstrates that the most significant difference between the two TnT isoforms is in the alternatively spliced NH2-terminal hypervariable region. Compared with the most conserved central region, the COOH-terminal portion of the cardiac and fast TnTs also shows some structural variation, especially the segment encoded by the mutually exclusive exons 16 and 17 in fast skeletal muscle TnT (Breitbart & Nadal-Ginard, 1986; Smillie et al. 1988; Wang & Jin, 1997). The predicted molecular weight and isoelectric point (pI) of the chicken breast muscle fast TnT (287 amino acid residues) and the major isoform of adult mouse cardiac TnT (288 residues) are 33 798 and 6.63, and 34 547 and 5.03, respectively (calculated using the PCGENE computer program, Intelligenetics, Mountain View, CA, USA). As shown by the sequence alignment, the significant difference in the pIs of the two TnT isoforms is due to their NH2-terminal charge difference.
Figure 1. Primary structural comparison between cardiac and skeletal muscle TnTs.

The amino acid sequences of the adult chicken breast muscle fast TnT (CfTnT) and adult mouse cardiac TnT (McTnT) were deduced from cDNA sequences. In the primary structure alignment, identical residues are represented by dashes and the gaps inserted to maximize the alignment are represented by asterisks. The exon boundaries are outlined according to rat cardiac (Jin et al. 1992) and fast skeletal muscle (Breitbart & Nadal-Ginard, 1986) TnT genes.
Establishment of transgenic mouse lines bearing α-MHC promoter-directed transgene encoding the chicken fast skeletal muscle TnT
To establish an integrated physiological system to investigate the role of different TnT isoforms in modulating muscle contraction, we have constructed seven transgenic mouse lines bearing the α-MHC promoter-directed chicken breast muscle fast TnT transgene (Fig. 2). The results of Southern blot genotyping showed that these transgenic founders bear various dosages of the chicken fast skeletal muscle TnT transgene in their genome (Fig. 2B). Two-dimensional densitometry analysis of the Southern blot autoradiographs by a SciScan 5000 densitometer (US Biochemical, Cleveland, OH, USA) determined that the transgene is integrated into the genome of the seven transgenic mouse lines with the following copy numbers (in heterozygotes): no. 1, 4; no. 4, 21; no. 5, 27; no. 6, 49; no. 7, 23; no. 18, 17; and no. 20, 11, respectively. The Southern blot pattern indicates the integration of the transgene in the mouse genome as tandem repeats. Transgene-positive progenies were obtained from all seven transgenic mouse founders, indicating a successful transmission of the transgene allele. Genotyping of large numbers of progeny from the founders demonstrated a μ50 % segregation ratio of the transgene, reflecting a single allele. Due to the greater availability of F1 offspring obtained from male founders than that from female founders during the initial breeding, heterozygous transgenic progeny (7–9 weeks of age) from the four male founders were used for phenotypic characterization in the present study.
Heart-specific expression of fast skeletal muscle TnT in transgenic mice with significant myofibril incorporation
Expression of the chicken fast skeletal muscle TnT was detected in the heart of the transgene-positive progenies of all four of the transgenic mouse lines characterized in this study. No expression of the chicken skeletal muscle TnT was found in their skeletal muscle (Fig. 3A) or the various other tissues tested (data not shown). This result proves that the intron-less fast skeletal muscle TnT cDNA can be expressed in transgenic mouse cardiac muscle. The results also add evidence for the advantage of using the promoter of α-MHC, a myocardial protein gene, to direct the transcription of the transgene. This promoter has produced heart-specific, post-natal expression in the transgenic animal without developmental or systemic side-effects. In contrast to the coordinated regulation of α- and β-tropomyosin genes reported for the transgenic mouse heart over-expressing β-tropomyosin controlled by the α-MHC promoter (Muthuchamy et al. 1995), the steady level of endogenous cardiac TnT gene expression in the transgenic mouse heart over-expressing fast skeletal muscle TnT transgene driven also by the α-MHC promoter precludes any feedback regulation by the exogenous TnT protein or mRNA. The Western blots in Fig. 3A further show that the expression of two other major cardiac muscle thin filament regulatory proteins, tropomyosin and TnI, was not altered in the transgenic mouse heart expressing the exogenous fast skeletal muscle TnT, confirming a native myofibril environment for the functional comparison of the different TnT isoforms.
Figure 3. Heart-specific expression of fast skeletal muscle TnT in transgenic mice and incorporation into the cardiac myofibril.
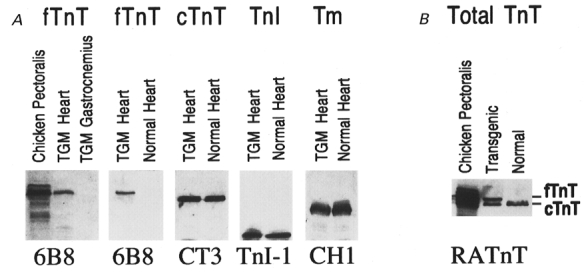
A, expression of fast skeletal muscle TnT in the transgenic mouse heart. Total protein extracts from transgenic mouse heart bearing the α-MHC promoter-directed chicken breast muscle TnT transgene (TGM) were analysed together with normal mouse heart and chicken breast muscle (pectoralis) controls by 14 % SDS-PAGE with an acrylamide/bisacrylamide ratio of 180:1. To monitor specific protein expression, Western blots were done using four specific mAbs against chicken breast muscle TnT (6B8), cardiac TnT (CT3), TnI (TnI-1), or tropomyosin (CH1), respectively. The blots revealed a high level expression of the chicken fast skeletal muscle TnT in the transgenic mouse heart but not skeletal muscle (gastrocnemius). The expression of TnI, tropomyosin and the endogenous cardiac TnT was not affected as compared with the normal mouse heart control. B, significant incorporation of the exogenous fast skeletal muscle TnT into the cardiac myofibrils of transgenic mice. The extensively washed transgenic mouse ventricular myofibrils were dissolved in SDS-PAGE sample buffer and analysed by Western blotting using the RATnT polyclonal antiserum. The result shows a significant myofibril incorporation of the fast skeletal muscle TnT in the transgenic mouse cardiac muscle. fTnT, chicken fast skeletal muscle TnT; cTnT, mouse cardiac TnT.
While the transgenic mouse hearts express a normal level of endogenous cardiac TnT, over-expression of exogenous fast skeletal muscle TnT significantly changed the TnT isoform contents in the transgenic cardiac muscle. This can be seen by superimposition of the Western blots using the anti-cardiac TnT mAb CT3 and anti-chicken breast muscle TnT mAb 6B8 (Fig. 3A). To examine the incorporation of the exogenous fast skeletal muscle TnT in the transgenic mouse cardiac myofibrils, Western blots using the RATnT polyclonal antibody recognizing both cardiac and skeletal muscle TnT demonstrated that the chicken fast skeletal muscle TnT was present in significant amounts in the extensively washed myofibrils in additon to the endogenous cardiac TnT (Fig. 3B). This result demonstrates that exogenous fast skeletal muscle TnT is incorporated into the contractile assembly when expressed in cardiac muscle.
The incorporation of chicken fast skeletal muscle TnT into the transgenic mouse cardiac sarcomere was further demonstrated by immunofluorescence microscopy using the specific anti-chicken fast TnT mAb 6B8 (Fig. 4). The sarcomeres of the transgenic mouse cardiac myofibril were normal in appearance, indicating that there was no detectable alteration of the sarcomeric structure in the transgenic cardiac muscle. The 6B8 mAb fluorescence staining demonstrated an I-band localization of the fast skeletal muscle TnT in the transgenic, but not wild-type, mouse cardiac sarcomeres. The 6B8 mAb staining pattern on the transgenic mouse cardiac myofibril was similar to that of the chicken breast muscle myofibril, demonstrating a native incorporation of the fast skeletal muscle TnT into the transgenic mouse cardiac muscle thin filaments. Altogether, the data indicate that the transgenic mouse myocardium provides a novel model system for the functional characterization of TnT isoforms.
Figure 4. Incorporation of chicken fast skeletal muscle TnT in the transgenic mouse cardiac muscle thin filament.

Phase-contrast and immunofluorescence microscopy was carried out on cardiac myofibrils from the transgenic and wild-type mice. The chicken fast skeletal muscle TnT-specific 6B8 mAb fluorescence staining demonstrated an I-band localization of the fast skeletal muscle TnT in the transgenic, but not wild-type, mouse cardiac sarcomeres similar to that in the chicken breast muscle myofibril, indicating a native thin filament association.
Increased cooperativity in Ca2+-activated contraction of transgenic mouse cardiac muscle containing fast skeletal muscle TnT
To investigate the functional significance of different TnT isoforms in the Ca2+ activation of muscle contraction, we carried out skinned fibre experiments using ventricular trabeculae from the hearts of transgenic mice. Multiple lines of the transgenic mice were analysed to avoid the effect of line to line variation. Representative data records are displayed in Fig. 5A and the average force vs. Ca2+ relationship is shown in Fig. 5B. The pCa50 value of the force-Ca2+ curves of the transgenic trabeculae was identical to that of the wild-type mouse trabeculae (5.1 ± 0.04 and 5.1 ± 0.1, mean±s.d., respectively). However, the slope of the force-pCa curves of the transgenic mouse cardiac muscle containing fast skeletal muscle TnT was much steeper than that of the normal cardiac muscle containing only cardiac TnT (Hill coefficient = 2.0 ± 0.2 for the transgenic mice vs. 1.0 ± 0.2 for the control hearts, P < 0.05), reflecting a significant increase in the overall cooperativity of Ca2+ activation of contraction. Under the same experimental conditions, we also determined the Ca2+ sensitivity of skinned chicken pectoralis fibres and found a pCa50 of 5.36 ± 0.07 and a Hill coefficient of 3.2 ± 0.2 (n = 6), verifying the higher cooperativity of skeletal muscle in comparison with cardiac muscle.
Figure 5. Ca2+-activated contraction of permeabilized cardiac muscle containing exogenous fast skeletal muscle TnT.
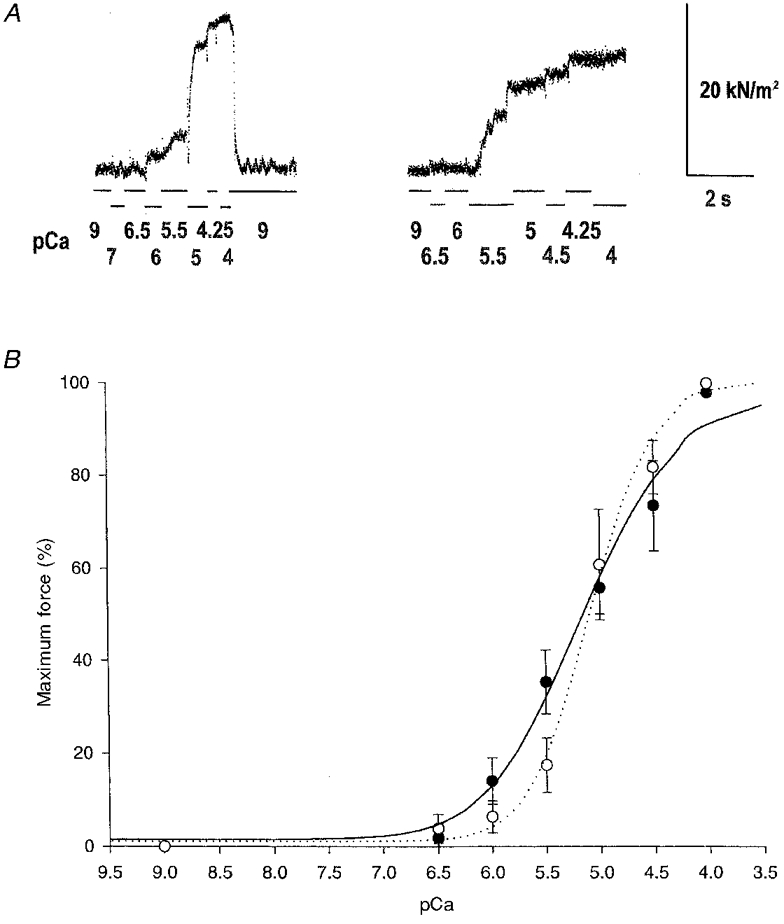
A, representative data trace of force during Ca2+ activation of skinned trabeculae from control (left) and transgenic (right) hearts. Solution was changed at the breakpoints of the lines below the data traces and the pCa of each solution is displayed. B, the force-pCa curves were normalized to the maximal Ca2+- (pCa 4) activated force as 1 (the two superimposed pCa 4 data points are slightly offset for display). The results demonstrate that the transgenic mouse cardiac muscle (○, dotted line) containing the exogenous fast skeletal muscle TnT is activated by Ca2+ with identical pCa50 values as compared with the normal mouse cardiac muscles (•, continuous line). However, the slope of the force-pCa curve was significantly steeper for the transgenic vs. normal cardiac muscle (P < 0.05), indicating an increased cooperativity (Hill coefficient = 2.0 ± 0.2 vs. 1.0 ± 0.2).
The Ca2+ sensitivity of force for cardiac muscle has been shown to be influenced by sarcomere length; sarcomere length changes of as little as 5 % significantly change the pCa50 without changing the Hill coefficient (Kentish et al. 1986). As noted in the Methods, we mounted all the trabeculae at their in vivo length. While the Hill coefficient of the force-Ca2+ relationship was doubled in the transgenics vs. the controls, no difference existed in the pCa50. In addition, the maximal Ca2+-activated force was not different for the trabeculae of the transgenic and control mice (30 ± 8 vs. 21 ± 6 kN m−2, respectively). These data suggest that the sarcomere length of both the control and transgenic mice were not significantly different.
Western blots of the ventricular muscle protein extracts from each mouse heart used in the contractility assays confirmed the heart-specific expression of exogenous fast skeletal muscle TnT in all of the transgenic animals and the wild-type nature of the control mice (data not shown). Under the skinning conditions, the free TnI and tropomyosin present in the transgenic mouse cardiac muscle cells were at similar levels to that in the wild-type cardiac muscle, despite the presence of additional free TnT due to the transgenic over-expression (Fig. 6). The presence of TnT, TnI and tropomyosin in the soluble fraction of wild-type cardiac muscle suggests that the thin filament-associated proteins are normally in excess in muscle cells, and pools of free TnT, TnI and tropomyosin exist in the cardiac muscle to maintain this equilibrium. This assumption is supported by the report that there is a cytoplasmic pool of unassembled TnI in the rat cardiac muscle where TnT and TnI have similar turnover rates (Martin, 1981). This observation indicates that these proteins are probably assembled into the thin filament in a saturable manner. Therefore, the over-expression of TnT would not significantly alter the stoichiometry or distribution of the thin filament regulatory proteins in the sarcomere and a native assembly of the thin filament can be maintained in the transgenic cardiac muscle.
Figure 6. Distribution of thin filament proteins in the cardiac muscle cells of transgenic and control mice.
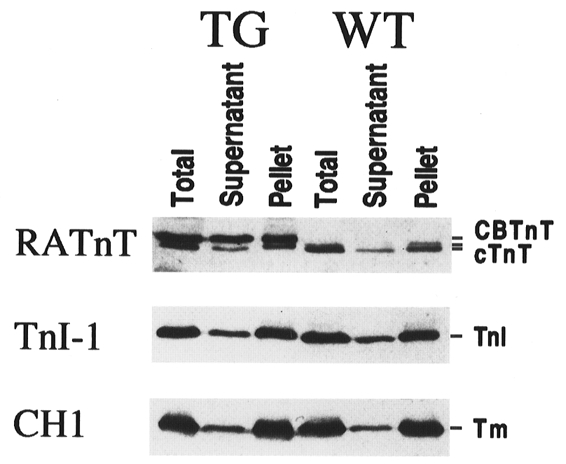
The dissected transgenic (TG) and wild-type (WT) mouse ventricular muscle tissue strips were extracted with the skinning solution used for the contractility experiments. The distribution of exogenous and endogenous TnT, TnI and tropomyosin was examined by Western blotting using specific antibodies as described in Fig. 3, except that the RATnT antiserum was used at a lower concentration and, therefore, reacted more weakly with the cardiac TnT. The blots demonstrate that there were significant amounts of TnT, TnI and tropomyosin in the soluble fraction. The decrease of cardiac TnT incorporation in the transgenic mouse cardiac myofibril with an increase in the soluble fraction can be visualized. Despite the presence of over-expressed free fast skeletal muscle TnT in the transgenic cardiac myocytes, no significant difference was seen for the distribution of TnI and tropomyosin as compared with the wild-type cardiac muscle.
DISCUSSION
Primary structure difference between the cardiac and fast skeletal muscle TnT isoforms
The primary structure of vertebrate TnT is better conserved in each of the muscle type-specific (cardiac, fast or slow skeletal muscle) TnTs across species than among the three TnTs in one species (Jin et al. 1998). Therefore, the comparison between cardiac and fast skeletal muscle TnTs mainly illustrates their structure-function relationship for the contractility of different muscle fibre types. The hypervariable, developmentally regulated NH2-terminal region is the predominant difference between the two TnT isoforms; however, its functional significance is unclear. We have demonstrated that the structure of the NH2-terminal variable region may modulate both the global conformation and function of TnT (Ogut & Jin, 1996; Wang & Jin, 1998). While the size difference between the NH2-terminal variable domains of the chicken fast skeletal muscle TnT and the adult mouse cardiac TnT is minimal, their difference in NH2-terminal amino acid composition results in a significant difference in charge. This may be the underlying mechanism for the physiological role of TnT isoforms in determining the sensitivity of muscle to Ca2+ activation (Ogut et al. 1999) and the tolerance of the thin filament regulatory system to pH (Ogut & Jin, 1998). Nevertheless, there are other structural differences between cardiac and fast TnT isoforms, such as the fast skeletal muscle TnT-specific exon 16-encoded segment in the COOH-terminal domain (Wang & Jin, 1997). Because single amino acid substitutions in cardiac TnT can produce significant phenotypes in human familial hypertrophic cardiomyopathy (Watkins et al. 1995), the functional effects of the minor structural variations should not be overlooked. Therefore, this transgenic mouse model may also be useful in the investigation of the pathological effects of subtle structural changes in TnT.
Expression of fast skeletal muscle TnT in the cardiac muscle of transgenic mice and its incorporation into the myofibrils
By applying the α-MHC gene promoter that has been used for the transgenic expression of several sarcomeric proteins in the heart, such as skeletal muscle TnC (Metzger et al. 1993), β-tropomyosin (Muthuchamy et al. 1995) and COOH-terminal truncated cardiac TnT (Tardiff et al. 1998), we have obtained a high level cardiac muscle-specific expression of the chicken fast skeletal muscle TnT in multiple transgenic mouse lines. Although the level of exogenous TnT expression is generally dependent on the copy number of the transgene, it does not simply correlate to the transgene dosage as verified by Western blots on transgenic mouse hearts from different transgenic lines (data not shown). This result suggests that the chromosomal integration site of the transgene may have effects on its expression. As demonstrated by Western blots on proteins extracted from isolated cardiac myofibrils of the transgenic mice (Fig. 3B), significant amounts of the exogenous fast skeletal muscle TnT isoform was incorporated into the cardiac myofibril. The I-band location of the chicken fast skeletal muscle TnT shown by immunofluorescence microscopy indicates that the fast skeletal muscle TnT transgenically expressed in the mouse cardiac muscle is able to integrate into the troponin complexes along the thin filament. Since the antibodies applied in this study have different affinities to the exogenous skeletal muscle TnT and endogenous cardiac TnT in the Western blots, it is difficult to accurately quantify the exact percentage of skeletal muscle TnT incorporation in the cardiac myofibril. However, it is clear that a fraction of the contractile units in the transgenic mouse cardiac muscle has been altered to show the functional significance of different TnT isoforms.
Structure and function of TnT isoforms in determining the cooperativity of Ca2+-activated contraction of striated muscle
A previous study has shown that transgenic mouse cardiac muscle with exchange of β- for α-tropomyosin had increased sensitivity during Ca2+ activation (Palmiter et al. 1996). Another study demonstrated in transgenic mouse cardiac muscle expressing skeletal muscle TnC that at pH 7.0 there is no difference in pCa50 or Hill coefficient, whereas at pH 6.2 the incorporation of skeletal muscle TnC conferred a higher tolerance to the acidosis environment (Metzger et al. 1993). While we did not see a change in pCa50 in our study on the transgenic mouse cardiac muscle expressing fast skeletal muscle TnT, the incorporation of fast skeletal muscle TnT into the cardiac muscle resulted in significantly steeper slopes of the force-Ca2+ activation relationship as compared with normal cardiac muscles (Fig. 5). Due to the sample size examined in this initial study, a quantitative relationship between the incorporation of exogenous fast TnT and the contractile phenotype of the transgenic cardiac muscle could not be determined. However, it is worth noting that point mutation-generated cardiac TnT structure changes have highly dominant effects on the phenotypes of muscle (Watkins et al. 1995; Tardiff et al. 1998). Therefore, the transgenic expression of fast skeletal muscle TnT in cardiac muscle at a wide range of levels may result in similar phenotypic effects.
It is known that skeletal muscle fibres have significantly higher cooperativity than cardiac muscle during Ca2+ activation of contraction. Our data show significantly increased cooperativity of Ca2+ activation of force in the transgenic mouse cardiac muscle which differs from the wild-type mouse heart only in the incorporation of fast skeletal muscle TnT. Although the presence of endogenous cardiac TnT precludes an accurate quantification of the effect of the fast skeletal muscle TnT, the result provides clear evidence showing roles of the different TnT isoforms in modulating the allosteric process of striated muscle contraction, e.g. via interactions with the tropomyosin head-tail overlap region (Heeley et al. 1987; Schaertl et al. 1995). The role of TnT isoforms in determining the cooperativity of thin filament regulation in striated muscle may further support the hypothesis that the alternatively spliced NH2-terminal domain of TnT functions as a conformational tuning site for the fibre type-specific and developmentally regulated cardiac and skeletal muscle TnT isoforms (Jin & Lin, 1988; Wang & Jin, 1997).
Our value for the Hill coefficient was low compared with those reported in the literature for the ventricular trabeculae (Kentish et al. 1986). The Ca2+ sensitivity of chicken pectoralis fibres in our experimental system also had a lower Hill coefficient (3.2 ± 0.2) compared with our data obtained using a different buffer system and experimental apparatus (4.5 ± 0.6, Ogut et al. 1999). These results show that the absolute pCa50 and Hill coefficient values calculated for the force-Ca2+ relationship are difficult to compare between studies as it has been demonstrated that both the pCa50 and the Hill coefficient vary with the ionic composition of the solutions (Godt, 1974; Andrews et al. 1991). Nevertheless, in our experiments using the same solutions and apparatuses, cooperativity (the Hill coefficient) is higher in the transgenic cardiac muscle than normal controls by a factor of 2, clearly reflecting the importance of the structure-function difference between cardiac and fast skeletal muscle TnT isoforms in determining the cooperativity during the Ca2+ activation of striated muscle contraction.
Pathological significance
Investigating the physiological and pathological role of TnT isoforms using the transgenic mouse models will further contribute to our understanding of the TnT isoform switches during cardiac development (Jin & Lin, 1988) and found in failing hearts (Anderson et al. 1995; Saba et al. 1996) and the molecular etiopathology of myocardial failure. The incorporation of fast skeletal muscle TnT in the cardiac muscle may increase the rate of rise of force due to the increase in cooperativity or, alternatively, decrease in the absolute force during the initial stage of activation (Fig. 5B). The co-presence of two classes of TnT (the endogenous cardiac TnT and the transgene-encoded fast skeletal muscle TnT) in the myocardium may result in two populations of contractile units which are regulated non-homogenously during contraction of the transgenic mouse cardiac muscle. This heterogeneity of myocardium may have dominant pathological effects on the function of cardiac muscle.
Recently, it has been demonstrated in transgenic mouse cardiac muscle that a very low amount of expression and incorporation of a COOH-terminal truncated cardiac TnT, which is known to cause human familial hypertrophic cardiomyopathy, into the myofibril caused neonatal lethality (Tardiff et al. 1998). Less than 5 % incorporation of the COOH-terminal truncated TnT can produce a significant change in muscle diastolic mechanics. This highly dominant effect supports our hypothesis that it may be the heterogeneity of the thin filament regulatory system, rather than the quantity of contractile units containing abnormal TnT, that results in the change of the mechanical properties of cardiac muscle.
Studies of the effect of fast skeletal muscle TnT on cardiac muscle contractility may contribute to our understanding of the pathogenesis of familial hypertrophic cardiomyopathy. Using the experimental design which introduces a normal fast skeletal muscle TnT isoform (instead of a mutant cardiac TnT) into the cardiac muscle to elucidate the structure-function relationship of TnT, we are able to examine the effects of conformational changes of TnT caused by scattered structural alterations on the structure and function of the transgenic mouse heart. Similar to that seen in the mutant cardiac TnT-caused human familiar cardiomyopathy, we found no significant myocardial hypertrophy in these transgenic mice. Without the addition of stress, the young mice are apparently ‘normal’ in their daily life activities. However, myocardial degeneration and changes in diastolic properties of the ventricle have been observed in older transgenic mice (data not shown). To understand the chronic effects of the altered myocardial contractility on cardiac function, the long term pathological consequence of the TnT heterogeneity in the transgenic cardiac muscle is currently being studied.
Acknowledgments
We thank Aihua Chen for assistance in the Western blot shown in Fig. 3, Laura Hobzek for technical assistance in the ventricular trabeculae contractility assays, Jennifer Wang and Ozgur Ogut for participation in the reconstruction of the full length adult chicken breast muscle TnT cDNA, Dr Jeffrey Robbins for providing the mouse cardiac α-MHC gene promoter, Dr Larry Smillie for providing the chicken TnT2 cDNA, Dr Jim Lin for providing the CH1 mAb, and Dr Clemencia Colmenares for advisement during the transgenic mouse production. This study was supported in part by Grants-in-Aid from the Heart and Stroke Foundation of Canada and American Heart Association to J.-P.J and a grant from the National Institutes of Health (HL44181) to F.V.B.
References
- Akella AB, Ding X-L, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T band shifts in the diabetic rat. Circulation Research. 1995;76:600–606. doi: 10.1161/01.res.76.4.600. [DOI] [PubMed] [Google Scholar]
- Anderson PAW, Greig A, Mark TA, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circulation Research. 1995;76:681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- Andrews MAW, Maughan DW, Nosek TM, Godt RE. Ion-specific and general ionic effects on contraction of skinned fast twitch skeletal muscle from the rabbit. Journal of General Physiology. 1991;98:1105–1128. doi: 10.1085/jgp.98.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A, Scordilis SP, Sonnenblick EH, Gulati J. The control of myocardial contraction with skeletal fast muscle troponin C. Journal of Biological Chemistry. 1987;262:5815–5822. [PubMed] [Google Scholar]
- Brandt PW, Diamond MS, Schachat FH. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. Journal of Molecular Biology. 1984;180:379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Breitbart RE, Nadal-Ginard B. Complete nucleotide sequence of the fast skeletal troponin T gene: Alternative spliced exons exhibit unusual interspecies divergence. Journal of Molecular Biology. 1986;188:313–324. doi: 10.1016/0022-2836(86)90157-9. [DOI] [PubMed] [Google Scholar]
- Briggs MM, Schachat F. Origin of fetal troponin T: developmentally regulated splicing of a new exon in the fast troponin T gene. Developmental Biology. 1993;158:503–509. doi: 10.1006/dbio.1993.1208. [DOI] [PubMed] [Google Scholar]
- Brozovich FV, Yamakawa M. Agonist activating modulates cross-bridge states in single vascular smooth muscle cells. American Journal of Physiology. 1993;264:C103–108. doi: 10.1152/ajpcell.1993.264.1.C103. [DOI] [PubMed] [Google Scholar]
- Cooper TA, Ordahl CP. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternative splicing. Journal of Biological Chemistry. 1985;260:11140–11148. [PubMed] [Google Scholar]
- Gahlmann R, Troutt AB, Wade RP, Gunning P, Kedes L. Alternative splicing generates variants in important functional domains of human slow skeletal troponin T. Journal of Biological Chemistry. 1987;262:16122–16126. [PubMed] [Google Scholar]
- Godt RE. Calcium activated tension of skinned fibres of the frog: dependence on magnesium-adenosine triphosphate concentration. Journal of General Physiology. 1974;63:722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, Trentham DR. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5′-triphosphate. The Journal of Physiology. 1984;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley DH, Golosinska K, Smillie LB. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. Journal of Biological Chemistry. 1987;262:9971–9978. [PubMed] [Google Scholar]
- Huang Q-Q, Chen A, Jin J-P. Complete sequence and genomic organization of mouse slow skeletal muscle troponin T gene. Gene. 1999;229:1–10. doi: 10.1016/s0378-1119(99)00051-7. [DOI] [PubMed] [Google Scholar]
- Jin J-P. Cloned rat cardiac titin class I and class II motifs: expression, purification, characterization and interaction with F-actin. Journal of Biological Chemistry. 1995;270:6908–6916. [PubMed] [Google Scholar]
- Jin J-P, Chen A, Huang Q-Q. Three alternatively spliced mouse slow skeletal muscle troponin T isoforms: conserved primary structure and regulated expression during postnatal development. Gene. 1998;214:121–129. doi: 10.1016/s0378-1119(98)00214-5. [DOI] [PubMed] [Google Scholar]
- Jin J-P, Huang Q-Q, Yeh H-I, Lin JJ-C. Complete nucleotide sequence and structural organization of rat cardiac troponin T gene. A single gene generates embryonic and adult isoforms via developmentally regulated alternative splicing. Journal of Molecular Biology. 1992;227:1269–1276. doi: 10.1016/0022-2836(92)90540-z. [DOI] [PubMed] [Google Scholar]
- Jin J-P, Lin JJ-C. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat heart during development. Journal of Biological Chemistry. 1988;263:7309–7315. [PubMed] [Google Scholar]
- Jin J-P, Wang J, Zhang J. Expression of four alternatively spliced exons of the mouse cardiac troponin T gene: characterization of a large number of full length cDNA clones. Gene. 1996;168:217–221. doi: 10.1016/0378-1119(95)00803-9. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HEDJ, Ricciardi L, Bocx JJJ, Noble MIM. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Circulation Research. 1986;58:755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Leavis PC, Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Critical Reviews in Biochemistry. 1984;16:235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Lin D, Babkova A, Homsher E, Tobacman LS. Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. Journal of Clinical Investigation. 1996;97:2842–2848. doi: 10.1172/JCI118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ-C, Chou CS, Lin JL-C. Monoclonal antibodies against chicken tropomyosin isoforms: production, characterization, and application. Hybridoma. 1985;3:223–242. doi: 10.1089/hyb.1985.4.223. [DOI] [PubMed] [Google Scholar]
- Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin I. Journal of Biological Chemistry. 1981;256:964–968. [PubMed] [Google Scholar]
- Metzger JM, Parmacek MS, Barr E, Pasyk K, Lin W-I, Cochrane KL, Field LJ, Leiden JM. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proceedings of the National Academy of Sciences of the USA. 1993;90:9036–9040. doi: 10.1073/pnas.90.19.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, O'Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular physiological effects of overexpressing striated muscle β-tropomyosin in the adult murine heart. Journal of Biological Chemistry. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Ogut O, Granzier H, Jin J-P. Acidic and basic troponin T isoforms in mature fast skeletal muscle and their effect on contractility. American Journal of Physiology. 1999;276:C1162–1170. doi: 10.1152/ajpcell.1999.276.5.C1162. [DOI] [PubMed] [Google Scholar]
- Ogut O, Jin J-P. Expression, zinc-affinity purification and characterization of a novel metal-binding cluster in troponin T: metal-stabilized α-helical structure and effects of the NH2-terminal variable region on the conformation of intact troponin T and its association with tropomyosin. Biochemistry. 1996;35:16581–16590. doi: 10.1021/bi961712y. [DOI] [PubMed] [Google Scholar]
- Ogut O, Jin J-P. Developmentally regulated, alternative RNA splicing-generated pectoral muscle-specific troponin T isoforms and role of the NH2-terminal hypervariable region in the tolerance to acidosis. Journal of Biological Chemistry. 1998;273:27858–27866. doi: 10.1074/jbc.273.43.27858. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of β- for α-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross bridge binding, and protein phosphorylation. Journal of Biological Chemistry. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Perry SV. Troponin T: genetics, properties and function. Journal of Muscle Research and Cell Motility. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- Potter JD, Sheng Z, Pan B-S, Zhao J. A direct regulatory role for troponin T and dual role for troponin C in the Ca2+ regulation of muscle contraction. Journal of Biological Chemistry. 1995;270:2557–2562. doi: 10.1074/jbc.270.6.2557. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Giulian GG, Greaser ML, Moss RL. Determination of the functional significance of transitions in contractile protein isoforms during development. In: Kedes LH, Stockdale FE, editors. Cellular and Molecular Biology of Muscle Development. New York, NY: Alan R. Liss, Inc.; 1989. pp. 881–891. [Google Scholar]
- Reiser PJ, Greaser ML, Moss RL. Developmental changes in troponin T isoform expression and tension production in chicken single skeletal muscle fibres. The Journal of Physiology. 1992;449:573–588. doi: 10.1113/jphysiol.1992.sp019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba Z, Nassar R, Ungerleider RM, Oakeley AE, Anderson PAW. Cardiac troponin T isoform expression correlates with pathophysiological descriptors in patients who underwent corrective surgery for congenital heart disease. Circulation. 1996;94:472–476. doi: 10.1161/01.cir.94.3.472. [DOI] [PubMed] [Google Scholar]
- Schachat FH, Diamond MS, Brandt PW. Effect of different troponin T-tropomyosin combinations on thin filament activation. Journal of Molecular Biology. 1987;198:551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Schaertl S, Lehrer SS, Geeves MA. Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry. 1995;34:15890–15894. doi: 10.1021/bi00049a003. [DOI] [PubMed] [Google Scholar]
- Smillie LB, Golosinska K, Reinach F. Sequences of complete cDNA's encoding four variants of chicken skeletal muscle troponin T. Journal of Biological Chemistry. 1988;263:18816–18820. [PubMed] [Google Scholar]
- Smith JP, Barsotti RJ. A computer-based servo system for controlling isotonic contractions of muscle. American Journal of Physiology. 1993;265:C1424–1432. doi: 10.1152/ajpcell.1993.265.5.C1424. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Gulick J, Neumann J, Knotts S, Robbins J. Transgenic analysis of the thyroid-responsive elements in the α-cardiac myosin heavy chain promoter. Journal of Biological Chemistry. 1993;268:4331–4336. [PubMed] [Google Scholar]
- Sweeney HL, Feng HSS, Yang ZH, Watkins H. Functional analysis of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proceedings of the National Academy of Sciences of the USA. 1998;95:14406–14410. doi: 10.1073/pnas.95.24.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer NK, Moss RL. The effect of altered temperature on Ca2+-sensitive force in permeabilized myocardium and skeletal muscle. Journal of General Physiology. 1990;96:1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familiar hypertrophic cardiomyopathy. Journal of Clinical Investigation. 1998;101:2800–2811. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman LS. Structure-function studies of the amino-terminal region of troponin T. Journal of Biological Chemistry. 1988;263:2668–2672. [PubMed] [Google Scholar]
- Tobacman LS. Thin filament mediated regulation of cardiac contraction. Annual Review of Physiology. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- Wang J, Jin J-P. Primary structure and developmental acidic to basic transition of 13 alternatively spliced mouse fast skeletal muscle troponin T isoforms. Gene. 1997;193:105–114. doi: 10.1016/s0378-1119(97)00100-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Jin J-P. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry. 1998;37:14519–14528. doi: 10.1021/bi9812322. [DOI] [PubMed] [Google Scholar]
- Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. New England Journal of Medicine. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- Zot AS, Potter JD. Structure aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annual Review of Biophysics and Biophysical Chemistry. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]


