The maintenance of a constant cell volume is of fundamental importance for survival and normal functions in animal cells. Cell volume regulation mechanisms participate not only in a variety of cell functions but also in the pathophysiology of diverse diseases and cell death. Activation of K+ and Cl− channels is responsible for the regulatory volume decrease, volume regulation after osmotic swelling, in most cell types. The phenotypic properties of the major volume-regulatory Cl− channel have been well characterized (Okada, 1997). However, the molecular identity or nature of the volume-sensitive Cl− channel (VSCC) has not as yet been precisely established (Okada et al. 1998). Also, our understanding of the molecular mechanisms of the gating of the VSCC is still rudimentary.
Okada (1997) put forward a hypothesis that the activation mechanism of the VSCC is associated with unfolding of plasma membrane invaginations (such as caveolae), on the basis of following facts. (1) Osmotic cell swelling can be attained at the expense of loss of multiple invaginations or infoldings of the plasma membrane. (2) The single-channel events could be recorded only when the patch pipette was attached to cells which were already swollen, whereas it could not be observed in the patch sealed prior to cell swelling even though whole-cell VSCC currents could be simultaneously recorded from the same cell.
The report by Trouet et al. (1999) in this issue of The The Journal of Physiology provides clear evidence of an involvement of caveolins, the principal protein of caveolae, in the modulation of the VSCC activity. No ultrastructural study was performed, however, to establish whether the caveolin transfection caused de novo formation of caveolae. Transient expression of caveolin-1β gave rise to marked upregulation of whole-cell VSCC currents in caveolin-1-deficient cell lines. Based on their qualitative observation that the caveolin expression little affected the extent of osmotic cell swelling, the authors explain their results in the following two alternative ways: either caveolins increased the availability of VSCCs in the plasma membrane or they played a crucial role in the signal transduction cascade activating VSCCs.
Caveolins form stabilized sphingolipid- cholesterol microdomains, termed ‘rafts’, and function as scaffolding proteins to organize, bind and concentrate specific lipids (cholesterol and glycosphingolipids), specific proteins (annexin II, adenylate cyclase, nitric oxide synthase and other signal transduction molecules), and a variety of lipid-modified signalling molecules (Harder & Simons, 1997). Caveolins are known to be involved in recruitment and sorting of a specific set of membrane proteins and exclude others. At the raft or caveolae (namely, large and stable raft domains), caveolins are also known to function as a negative regulator of three classes of signal transduction molecules: G protein-mediated signalling components (such as G proteins and G protein-coupled receptors), Ca2+-mediated signalling components (such as Ins(1,4,5)P3 receptors, Ca2+-ATPase and calmodulin), and tyrosine kinase-mitogen-activated protein kinase pathway components (Shaul & Anderson, 1998). Therefore, it is possible that the caveolin isoform facilitates delivery of the VSCC proteins and/or their regulatory molecules to the plasma membrane and then provides scaffolding on which these molecules can assemble to form the pre-activated state or complexes (Fig. 1).
Figure 1. Schematic model for possible mechanisms of caveolin-associated regulation of volume-sensitive Cl− channel.
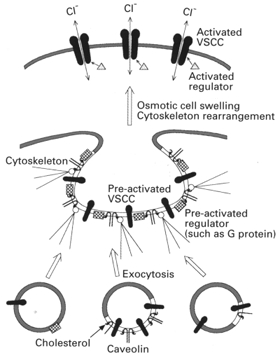
The plasma membrane is envisaged as a mosaic of caveolin-associated raft domains (open regions) and non-raft domains (shaded grey regions). Caveolins (hairpin structures) directly interact with cholesterol (tadpole structures) and regulatory signals such as G proteins (squares). Linker proteins for cytoskeletons such as annexin II (open circles) may interact with cholesterol and/or caveolins on the cytoplasmic face of plasma membrane (see text for details).
Osmotic cell swelling is associated with rearrangement of submembrane F-actin-based cytoskeleton (see citations in Okada, 1997). Even during shrinkage or no discernible swelling, the VSCC is activated by certain intracellular manoeuvres, such as lowered ionic strength (Cannon et al. 1998; also see citation in Trouet et al. 1999), GTP-γ-S application (see citations in Okada, 1997) and tyrosine kinase introduction (Lepple-Wienhues et al. 1998), all of which may induce cytoskeletal rearrangement. Thus, it is likely that submembrane cytoskeletal rearrangement may release or redistribute the VSCC protein itself and its regulators from caveolin-associated microdomains (rafts or caveolae). Thus, at least two possible mechanisms, though not mutually exclusive, could be envisaged for VSCC activation after osmotic swelling or cytoskeletal rearrangement (Fig. 1): stimulation of the signal transduction cascade of VSCCs and oligomerization or assembly of the VSCC proteins with the counterpart subunits.
Further investigation is required to determine the precise activation mechanism to decisively send other hypotheses to the scaffold. However, this study by Trouet et al. (1999) has set up a robust framework for future studies.
References
- Cannon CL, Basavappa S, Strange K. American Journal of Physiology. 1998;275:C416–422. doi: 10.1152/ajpcell.1998.275.2.C416. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Current Opinion in Cell Biology. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F. Journal of Cell Biology. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y, Oiki S, Hazama A, Morishima S. Journal of General Physiology. 1998;112:365–367. doi: 10.1085/jgp.112.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW, Anderson RGW. American Journal of Physiology. 1998;275:L843–851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- Trouet D, Nilius B, Jacobs A, Remacle C, Droogmans G, Eggermont J. The Journal of Physiology. 1999;520:113–119. doi: 10.1111/j.1469-7793.1999.t01-1-00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


