Abstract
Vagal afferent input from cardiac mechanoreceptors excites neurones in the nucleus tractus solitarii (NTS), but discharge patterns evoked by physiological activation of pressure-sensitive cardiac mechanoreceptors have not been studied in vivo. The role of glutamate receptor subtypes in transmission of afferent activity to the NTS neurones has not been determined. The present study therefore has two aims: first, to characterise the discharge patterns of neurones in the NTS that receive pressure-sensitive vagal cardiac receptor input and second, to determine the roles of ionotropic glutamate receptor subtypes in the transmission of this putative cardiac mechanoreceptor-related activity to NTS neurones.
Pulse-synchronous activity of neurones in the NTS evoked by vagal afferent input was recorded extracellularly in an anaesthetised dog model using multibarrel glass electrodes, which allowed picoejection of the glutamate receptor antagonists NBQX or AP5 to block either non-NMDA or NMDA receptors, respectively, during the neuronal recording. Pressure sensitivity of the recorded neurones was examined by monitoring their response to a small increase in arterial blood pressure. Selective pressure activation of carotid sinus baroreceptors in an isolated sinus or selective denervation of aortic baroreceptors were used to test for convergent excitation of the neurones by arterial baroreceptors.
Pulse-synchronous cardiac-related neuronal activity recorded from neurones in both the right and left NTS was eliminated following section of the left (n = 17) or right (n = 1) vagus nerves. No spontaneous, non-pulsatile activity was observed in these neurones before or after vagotomy. Activity transmitted via left vagal afferents was found to be sensitive to changes in arterial blood pressure. In these neurones, activity was blocked in 13 of 17 neurones by picoejection of NBQX, with the remainder requiring both NBQX and AP5. None of the cardiac-related neurones responded to activation of carotid baroreceptors or denervation of aortic baroreceptors, indicating no convergence of activity from carotid baroreceptors or aortic baroreceptors with pressure thresholds of approximately 130 mmHg or less.
The results suggest that vagal pressure-sensitive afferent input from cardiac mechanoreceptors is transmitted primarily by left vagal afferent fibres via non-NMDA receptors to neurones in both the ipsilateral and contralateral NTS. NMDA receptors were also found to have a role in the activation of a small subpopulation of neurones.
The sites of first termination of cardiac vagal afferent fibres in the central nervous system have been located in subnuclei of the nucleus tractus solitarii (NTS), based on results from both anatomical tracing studies (Chernicky et al. 1984; Yousfi-Malki & Puizillout, 1994) and functional neuronal recording studies in which vagal afferent activity was evoked by electrical stimulation of the vagus nerve (Bennett et al. 1981; Hines et al. 1994; Zhang & Mifflin, 1995; Silva-Carvalho et al. 1998) or by chemical or mechanical activation of cardiac receptors (Hines et al. 1994; Nosaka et al. 1995; Wilson et al. 1996; Paton, 1998; Silva-Carvalho et al. 1998). Specific activation of cardiopulmonary vagal afferent fibres was found to evoke activity in neurones in the dorsomedial, medial, lateral and commissural subnuclei of the NTS (Bennett et al. 1981; Hines et al. 1994; Paton, 1998; Silva-Carvalho et al. 1998). However, the pattern of discharge of NTS neurones to natural pressure-evoked activation of cardiac receptors has not been well-characterised in an intact, in vivo preparation. In addition, the transmitter/receptors responsible for the central transmission of cardiac-related activity have not been specifically determined. Studies have identified a role for glutamate in the transmission of visceral afferent input to neurones in the NTS, including baroreceptor (Gordon & Leone, 1991; Kubo & Kihara, 1991) and chemoreceptor (Vardhan et al. 1993) activity. With regard to vagal afferent input, one study has indicated that glutamate is released in the NTS during electrical stimulation of vagal afferent fibres (Allchin et al. 1994). In addition, the broad spectrum excitatory amino acid (EAA) receptor antagonist kynurenate (KYN) was found to reduce discharge in NTS neurones evoked by vagus nerve stimulation (Zhang & Mifflin, 1995). These earlier functional studies have limitations due to the non-specific nature of the electrical stimulation of the vagal afferent fibres. The present study was undertaken to characterise the discharge patterns and responses of cardiac receptor-modulated NTS neurones in dogs evoked by physiological changes in arterial pressure, examining the contribution of ionotropic glutamate receptor subtypes to the transmission of cardiac afferent input to the central neurones.
METHODS
The discharge of cardiac receptor-modulated neurones in the NTS was studied in anaesthetised mongrel dogs weighing 12-15 kg (initial dose, 50 mg kg−1α-chloralose and 500 mg kg−1 urethane, with supplemental continuous infusion of 250 mg α-chloralose + 2.5 g urethane per hour, i.v.). All experimental procedures followed were approved by the Animal Care and Use Committees of the Medical College of Wisconsin and the Zablocki Department of Veterans Affairs Medical Centre. The left femoral artery and vein were cannulated to permit measurement of arterial blood pressure (BP) and infusion of anaesthetic, respectively. Arterial blood gases were measured using an ABL 30 Radiometer Blood Gas Analyser (Copenhagen, Denmark) and kept within normal ranges by adjustment of ventilation and infusion of bicarbonate. Arterial pressure was measured via the catheter in the left femoral artery, which was connected to a Statham pressure transducer and a Grass Model 7D polygraph (Grass Co., Quincy, MA, USA). To control baroreceptor afferent activity and permit examination of any convergent carotid baroreceptor input to cardiac-receptor modulated neurones receiving vagal input, an isolated carotid sinus preparation was prepared as previously described (Seagard et al. 1983). Briefly, the left carotid sinus was vascularly isolated to permit either a flow-through pulsatile perfusion of the sinus region at constant mean pressure (130-140 mmHg) or a slow ramp increase (1–2 mmHg s−1) in carotid sinus pressure (CSP) from 0–200 mmHg. Buffered lactated Ringer solution was used as the perfusate, oxygenated with 100 % O2 to chemically denervate any chemoreceptors not physically eliminated by the isolation technique (Seagard et al. 1990). Carotid sinus pressure was measured via a catheter in the lingual artery and recorded using a Statham pressure transducer and the Grass polygraph. Constant CSP was maintained using a servocontroller developed in this laboratory (Seagard et al. 1983). The right carotid sinus nerve was identified and sectioned to limit carotid baroreceptor afferent input to that from the isolated sinus. Completeness of right carotid sinus denervation was assured by occluding the right common carotid artery and observing no reflex change in arterial BP. The right and left vagosympathetic trunks, which include the aortic depressor nerves, were isolated from surrounding tissue and marked with loose sutures for later identification and section. In four animals, the left aortic depressor nerve was isolated from the rest of the vagosympathetic trunk and marked with a loose suture so the nerve could be sectioned independently from the vagal fibres, allowing specific elimination of aortic baroreceptor input. In four animals, the chest was opened and the left and right vagi were crushed below the apex of the heart to eliminate input from abdominal visceral receptors via the vagus nerves. In all other animals, a bilateral pneumothorax was performed to reduce movement associated with changes in thoracic pressure.
Following nerve isolation, the animal was placed in a head holder (Kopf, Tujunga, CA, USA) for stereotaxic placement of a central recording electrode. With the animal in the stereotaxic frame, an occipital craniotomy was performed, the dura opened, and the caudal portion of the fourth ventricle exposed by lifting the vermis cerebelli. This allowed the visualization of the obex, the point where the central canal opens into the fourth ventricle. Using obex as a zero reference, electrode penetrations were randomly made into the left or right NTS from 1.0 mm caudal to 2.0 mm rostral to the obex, 0.0–2.0 mm lateral to the midline, and from the surface to 2.0 mm deep. An incision was made in the pia through which the electrode was inserted at identified target co-ordinates and advanced slowly using a hydraulic microdrive. The recording sites were later verified histologically, as explained below.
Central neuronal activity was recorded using a multibarrel glass microelectrode containing a fine (7 μm) carbon filament in one barrel, connected via a high-impedance preamplifier (gain = 1000; 0.1–10 kHz passband) and filter/amplifier (4th order Butterworth; 10 Hz to 3 kHz passband). The remaining barrels were filled with artificial cerebrospinal fluid (ACSF, composition (mm): NaCl, 124; KCl, 2.0; Mg Cl2, 2.0; KH2PO4, 1.3; Ca Cl2, 2.0; NaHCO3, 24 and glucose, 11.0), or glutamate receptor antagonists or agonists diluted in ACSF for picoejection onto recorded neurones using a system designed and constructed in the laboratory. Extracellular unit activity was recorded to identify neurones that displayed pulse-synchronous discharge which correlated with arterial pressure. This focused the study on a subpopulation of NTS neurones that received a putative cardiac mechanoreceptor input. Raw central neuronal activity, along with arterial BP and CSP, were recorded on a Vetter Model 3000A PCM Recording Adapter (Vetter, Rebersburgh, PA, USA) and VCR for later analysis. Unit activity was also directed to a time/amplitude window discriminator which generated a standard pulse for each spike which fell into the preset window. The pulse output of the discriminator was then fed into a digital counter/timer whose analog output was proportional to the number of spikes per unit time. These signals were displayed on-line on the Grass polygraph in order to monitor activity during the experiment. Analysis of recorded unit neuronal activity was accomplished by (1) analog to digital sampling of the three recorded data channels at 10 kHz using a PCI-MIO-16E-1 A/D converter and LabView software (National Instruments, Austin, TX, USA) and a Power Macintosh 8500/120 (Apple Computer Inc., Cupertino, CA, USA) equipped with a Sonnet 266 MHz G3 card (Sonnet Technologies Inc., Irvine, CA, USA), and (2) creating systole-triggered post-stimulus time histograms (PSTHs) of spike activity using a Nicolet 370 digital averaging oscilloscope (Nicolet Instrument Corp., Madison, WI, USA) with PSTH capabilities in conjunction with a Hewlett Packard 310 technical workstation.
The experimental protocol was as follows. Following surgery, a constant mean pressure (140 mmHg) pulsatile perfusion was established in the isolated carotid sinus. This pressure level was chosen to ensure that baroreceptors with higher as well as lower pressure thresholds were stimulated and therefore capable of activating central neurones. Animals were given hexamethonium (20 mg kg−1, i.v.) to block reflex changes in BP and a slow infusion of phenylephrine (1.0 mg (100 ml)−1i.v.) was then used to maintain mean arterial BP at 110-130 mmHg to provide an adequate level of stimulation of cardiac mechanoreceptors at physiological levels of BP. Supplemental doses of hexamethonium were administered when reflex changes in arterial blood pressure were evident in response to changes in CSP. The protocol allowed separate activation of carotid baroreceptors via the isolated sinus, or of vagal cardiac mechanoreceptors via small increases in BP to determine the extent of convergence of input from the two receptor groups. Alteration of phenylephrine infusion rate was used to elevate BP slightly to test for pressure-sensitive modulation of recorded neurones.
With pressures controlled, the NTS was randomly explored using the multibarrel electrode until activity from a single dorsal medullary neurone that displayed a distinct cardiac rhythm was obtained. The pulse-synchronous nature of the discharge was determined by comparing neuronal discharge to the BP pulse. To test for carotid baroreceptor sensitivity of the central neurone, a step or ramp pressure change in CSP from 0 to 200 mmHg was performed. Small changes in BP were produced by changes in infusion rate of the phenylephrine in order to confirm the pressure-related sensitivity of the afferent evoked discharge of the NTS neurone. Pressures were re-established at control levels and following a control period of recording, 15 nl of ACSF was picoejected onto the neurone to test for vehicle and ejection movement effects on the discharge of the NTS neurone. For three neurones, vehicle effects were observed, possibly due to miscomposition or contamination of the ACSF. In those cases, the neuronal recording was sacrificed and a new microelectrode with a new vehicle was made and tested. The process of searching for a pulse-synchronous neurone was repeated and in the absence of vehicle effects on neuronal activity, the test protocol was followed. Either 5 mm (±)-2-amino-5-phosphonovaleric acid (AP5; RBI, Natick, MA, USA) or 100 μm 1,2, 3,4-tetrahydro-6-nitro-2,3-dioxo-benzo(f)quinoxaline-7-sulfonamide disodium (NBQX; RBI) dissolved in ACSF, pH range 7.45–7.65, was picoejected up to a volume of 15 nl to block either N-methyl-D-aspartate (NMDA) or non-NMDA receptors on the recorded neurone, respectively. This maximum volume was established by separate studies which tested for the effectiveness and selectivity of the antagonists, as explained below. Administration of the antagonist was immediately stopped if lower volumes of the agent abolished activity of the neurone. The effects of the antagonist were recorded for at least 3 min. Then, unless activity was completely eliminated, the second antagonist was picoejected as well. If discharge of the neurone was completely eliminated by the first antagonist ejected, or following picoejection of the second antagonist, the activity of the neurone was monitored until control levels of activity were re-established. Due to the long duration of action of NBQX, generally 1 h or more was needed for neuronal recovery. Following recovery, the antagonists were then administered in the opposite order, if possible. For some neurones, it was not possible to hold the neurone long enough to test the reverse order of blockade. However, the effectiveness of NBQX to block most neuronal activity, as described below, negated the absolute need for studying the effects of reverse order of blockade for most neurones. Neurones were not included in the final results unless recovery of the neurone could be confirmed.
The effectiveness of the receptor blocking protocol was tested in six neurones, utilising a slightly different protocol. For these neurones, the agonists for the glutamate receptor subtypes NMDA (RBI; 100 μm) and AMPA (non-NMDA receptor agonist; Sigma-Aldrich; 40 μm) were picoejected onto the neurone before and after administration of the receptor antagonists AP5 or NBQX. Each antagonist was tested in random order, with sufficient time given between picoejections for recovery of discharge of the neurone. These studies established 15 nl as the maximum volume needed to block receptors excited by picoejection of the agonists.
Following the final recovery of the neurone, activity of the neurone was monitored while the right and then the left vagi were sequentially sectioned. In the four animals in which the left aortic depressor nerve was isolated from the left vagal trunk, the aortic nerve was sectioned prior to the left vagus. The right aortic nerve was not separated from the right vagal trunk and was therefore eliminated in combination with sections of that trunk. At the end of the experiment, the exact location of the recording site of the cardiac receptor-modulated neurone was marked by passing a 20 μA direct positive current through the recording electrode for 30-60 s. Animals were killed at the end of the study by an overdose of anaesthetic followed by saturated KCl. The medulla was removed post mortem and frozen. Sequential 40 μm transverse sections were cut, stained with Neutral Red and examined microscopically to identify the recording site histologically. Diagrammatic representations of the locations of recording sites were compiled from all animals.
RESULTS
A total of 18 pulse-synchronous central neurones were recorded from 12 dogs. The 12 recording sites confirmed histologically were located in the medial, dorsomedial or commissural subnuclei of the NTS (Fig. 1). Discharge of 17 of the 18 NTS neurones was eliminated by left vagotomy, indicating activation by afferents with a left vagal pathway. This includes three neurones recorded from the right side of the medulla. Activity of one pulse-synchronous neurone recorded from the dorsomedial subnucleus of the left NTS at 1.2 mm rostral to obex was eliminated by right vagotomy. Regardless of the neuronal location or type of vagal afferent input, none of the neurones responded to pulsatile, step or ramp changes in CSP, indicating a lack of convergence of input from carotid baroreceptors (Fig. 2). In addition, in four animals, section of the left aortic depressor nerve alone prior to section of the left vagus did not alter the firing pattern of neurones with left vagal input, indicating a lack of aortic baroreceptor modulation of these neurones. Pulse-synchronous cardiac-related neuronal activity persisted following elimination of lower vagal input produced by crushing of the vagus nerves below the heart, indicating that the origin of the afferent input was not from abdominal structures.
Figure 1. Composite transverse sections through the dorsomedial aspect of the dog medulla illustrating the location of recording sites.
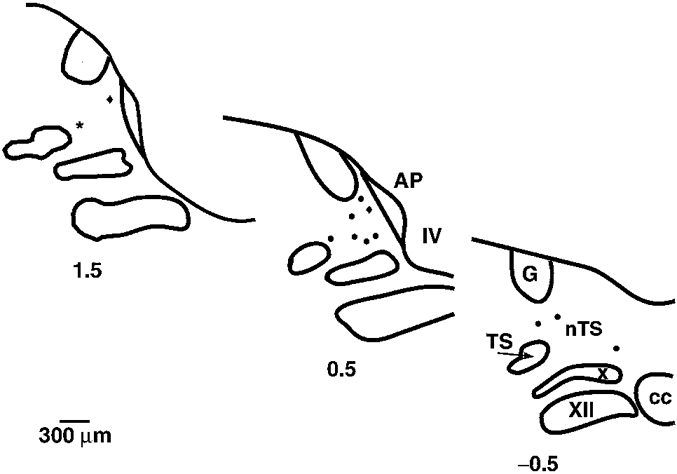
For clarity all recording sites are represented on the left side of the medulla at the closest rostro-caudal level of 0.5 mm caudal (−0.5) and 0.5 mm (0.5) and 1.5 (1.5) mm rostral to obex. •, location of neurones recorded on the left side of the medulla; ♦, location of neurones recorded on the right side of the medulla; and ⋆, location of the single neurone activated by right vagal afferent input. Abbreviations: AP, area postrema; cc, central canal; G, nucleus gracilis; nTS, nucleus tractus solitarii; TS, tractus solitarii; X, dorsal motor nucleus of the vagus; XII, hypoglossal nucleus; IV, fourth ventricle.
Figure 2. Lack of neuronal response to a step change in carotid sinus pressure.
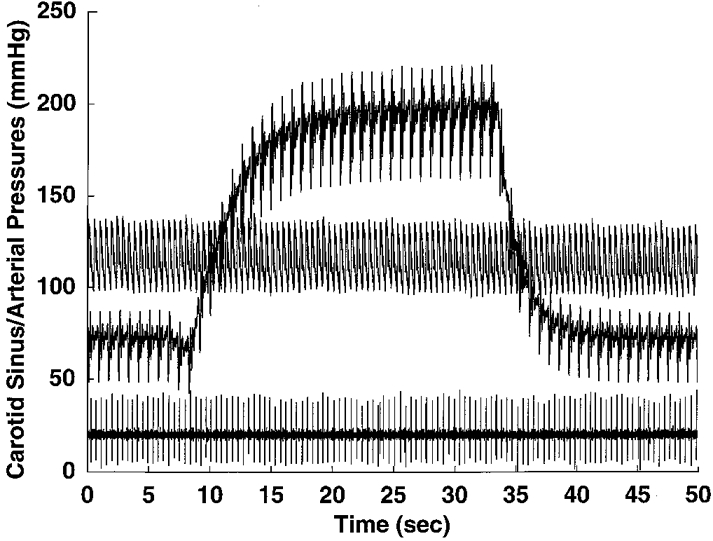
Tracings of arterial pressure (upper trace), raw neuronal activity of a pulse-synchronous NTS neurone with left vagal afferent input (lower trace) and a step change in carotid sinus pressure (pressure step; middle trace). Reflex changes in arterial pressure evoked by the carotid sinus pressure step were prevented by prior administration of hexamethonium. The lack of a neuronal response to the change in carotid sinus pressure indicated a lack of convergence of carotid baroreceptor input to the neurone. This lack of convergence was found for all neurones tested.
The frequency of discharge of neurones at mean arterial pressures of 110-130 mmHg ranged from 1 to 12 spikes (arterial pulse)−1, with a mean of 2.6 spikes pulse−1 and a median of 2.1 spikes pulse−1. Examples of discharge rates from neurones with a higher frequency discharge and lower frequency discharge are shown in Fig. 3. Little spontaneous tonic activity, indicated by spikes between pressure-induced bursts of activity, were observed in these neurones. NTS neurones with left vagal afferent drive (17 of 18) demonstrated a pressure-sensitive discharge, with increases in activity correlated to increases in BP (Fig. 4). Only small changes in arterial pressure were attempted, since movement of the brain from larger changes resulted in the loss of the neuronal recording, and therefore the saturation firing rates of the neurones could not be determined. The one neurone with right vagal input reflected heart rate, but did not demonstrate any arterial pressure sensitivity (Fig. 5).
Figure 3. Two pulse-synchronous NTS neurones.
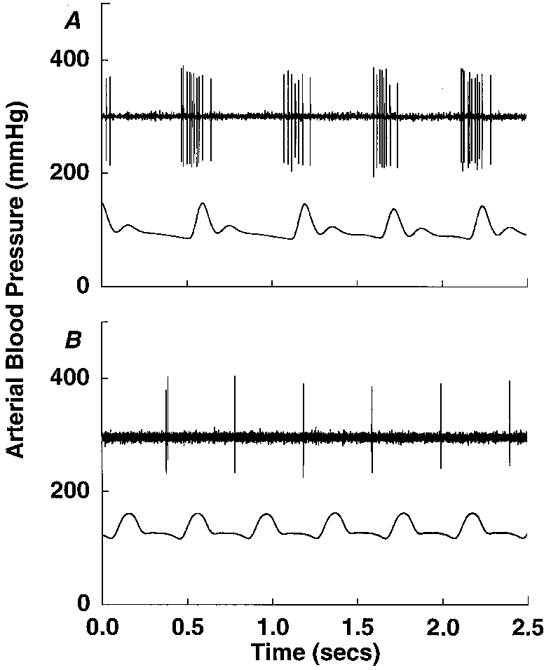
Representative tracings of discharge patterns (upper trace in each panel) of two pulse-synchronous NTS neurones with left vagal input, indicating the rates of discharge/arterial pulse (lower trace in each panel). Neuronal discharge was found to range from 1 to 12 spikes per arterial pressure pulse for all neurones studied.
Figure 4. Pressure-sensitive pulse-synchronous NTS neurone.
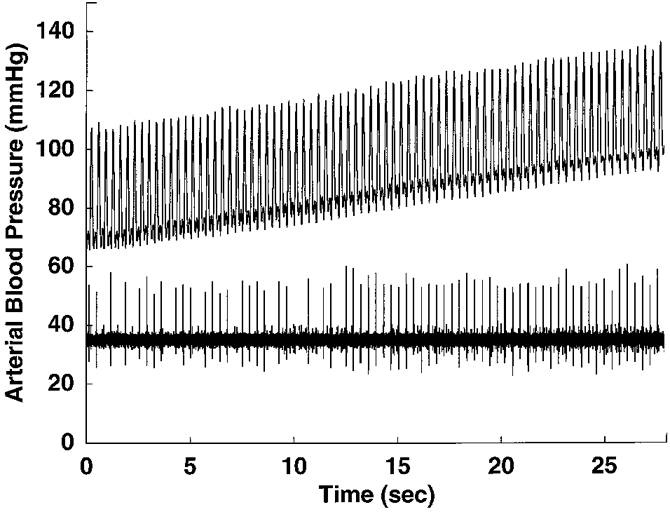
Tracings of arterial pressure (upper trace) and raw neuronal activity of a pulse-synchronous NTS neurone (lower trace) with left vagal afferent input, demonstrating the pressure sensitivity of the neuronal discharge. Firing of the neurone was found to increase with a small increase (about 30 mmHg) in mean arterial pressure.
Figure 5. Non-pressure-sensitive pulse-synchronous NTS neurone.
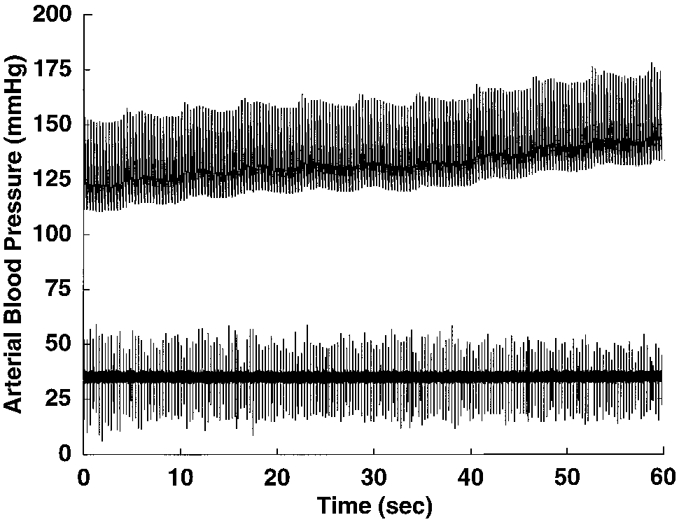
Tracings of arterial pressure (upper trace) and raw neuronal activity (lower trace) of the single neurone recorded with afferent input transmitted via the right vagus. The neurone discharged synchronously with each heart beat, but did not respond to a 25 mmHg increase in arterial pressure, unlike the pressure-sensitive neurones with left vagal afferents, an example of which is shown in Fig. 4.
The pressure-evoked nature of the discharge in pulse-synchronous neurones was verified by constructing PSTHs of neuronal firing, using systole of the arterial pressure pulse as a trigger (Fig. 6). Triggered spike activity was accumulated for 14 beats neurone−1 using 2 ms bins. Histograms for the neurones were summed and divided by the number of neurones to create an average histogram. The peak NTS neuronal discharge occurred prior to peak aortic pressure (Fig. 6), indicating that most cardiac receptors that initiated the NTS activity fired during ventricular diastole/early systole. Using the best estimates of average conduction velocity (C-fibres, 1–2 m s−1), synaptic processing and delay of the recorded aortic pressure pulse relative to ventricular pressure pulse, the peak discharge of the cardiac receptors occurred approximately 20-80 ms prior to peak ventricular systole. Close coupling between the pulse pressure and neuronal firing confirms the cardiac-related rhythm of these neurones and provides a high-fidelity signal concerning cardiac rhythm to the central nervous system.
Figure 6. Average post-stimulus time histogram of pulse-synchronous NTS neurones.

Average systole-triggered post-stimulus time histogram showing the cardiac-related nature of the pulse-synchronous NTS neuronal discharge (lower) relative to the arterial blood pressure pulse (upper). Spikes for individual neurones were accumulated for 14 triggers (bin width, 2 ms), and the resulting histograms for the neurones were averaged. The peak discharge rates of firing for individual neurones relative to the following peak aortic pressure are shown as •, with the average peak rate of discharge ± standard error shown as ▴. The average time of peak neuronal discharge preceded peak aortic pressure by 95.9 ± 6.6 ms and the average duration of discharge for the neurones was 66.6 ± 8.9 ms. There was a relatively tight cluster of individual peak discharge times around the average value.
Exposure to NBQX produced a decrease in pulse-synchronous discharge in all pressure-sensitive NTS neurones (n = 17), completely eliminating all neuronal activity in 13 (Fig. 7). Four of the neurones required exposure to both NBQX and AP5 in order to abolish discharge (Fig. 8). In the 13 neurones in which NBQX could eliminate discharge, initial exposure to AP5 alone produced some decrease in the pulse-synchronous discharge in three of these neurones. However, after recovery, NBQX alone could abolish firing in these neurones. In an additional six of these neurones, AP5 alone had no effect on the discharge rate. Although higher, there was no significant difference in the mean firing rates of the neurones that required AP5 and NBQX to eliminate activity (3.9 ± 1.3 spikes pulse−1) versus those in which discharge could be eliminated by NBQX alone (2.2 ± 1.6 spikes pulse−1).
Figure 7. Effects of NBQX on discharge of a pulse-synchronous NTS neurone.
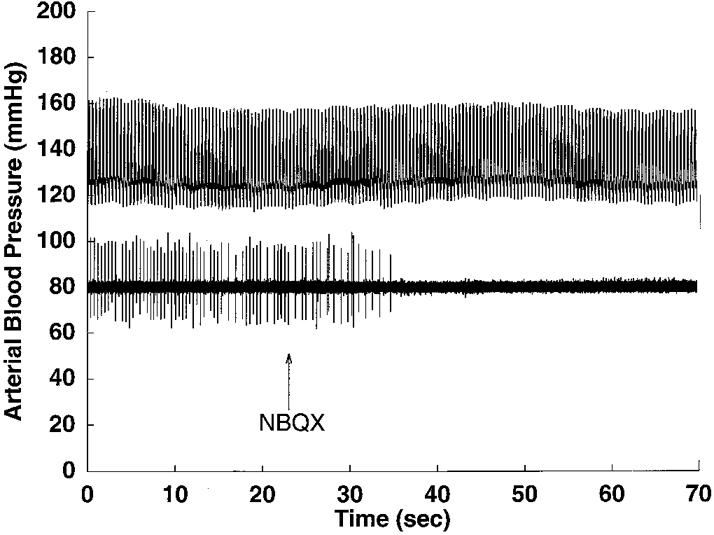
Tracings of arterial pressure (upper trace) and raw neuronal activity (lower trace) of a pulse-synchronous NTS neurone in the nucleus tractus solitarii before and after blockade of non-NMDA glutamate receptors by local exposure to NBQX. The cardiac-related discharge of the neurone was eliminated by picoejection of 7 nl of NBQX (100 μm).
Figure 8. Effects of NBQX and AP5 on discharge of a pulse-synchronous NTS neurone.
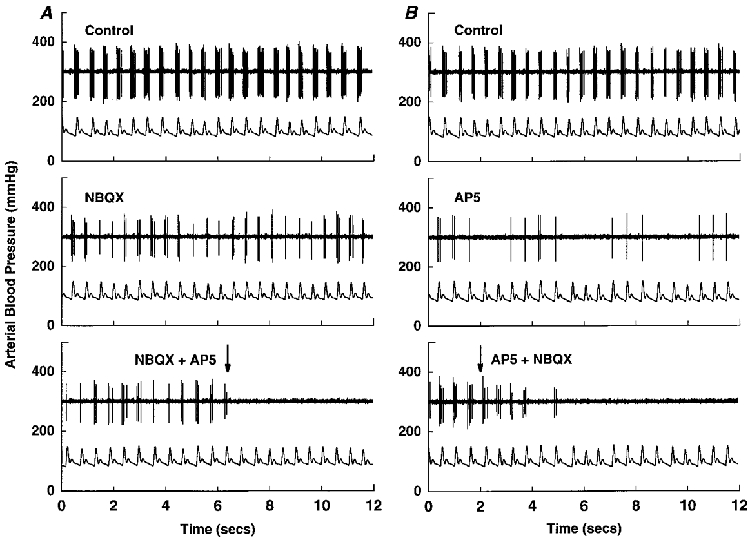
Tracings of arterial pressure (lower trace in each panel) and raw neuronal activity (upper trace in each panel) of a pulse-synchronous NTS neurone in the nucleus tractus solitarii whose discharge was decreased by both NBQX (100 μm), a non-NMDA glutamate receptor antagonist and AP5 (5 mm), an NMDA glutamate receptor antagonist. Picoejection of 15 nl of either NBQX (A) or AP5 (B) decreased discharge of the neurone (middle panels), with activity completely abolished by additional administration of the other antagonist (lower panels). Thus, cardiac-related discharge of the neurone was eliminated only after local picoejection of both ionotropic glutamate receptor blockers.
The concentrations of the antagonists employed were found to selectively block the effects of their respective agonist without altering the response to ejection of the other agonist. An example of the selectivity of the blockers is shown in Fig. 9, which uses post-stimulus triggering from systole of the arterial pressure pulse to present the firing pattern of pressure-evoked discharge of a pulse-synchronous neurone. As seen in this example, picoejection of both NMDA or AMPA increased the discharge of the neurone. Picoejection of 15 nl of NBQX eliminated discharge of the neurone and subsequent picoejection of AMPA had no effect on the unit activity, indicating blockade of the non-NMDA receptors. However, the response to NMDA was unchanged following NBQX, demonstrating that NMDA receptors were not blocked by the concentration of NBQX used in the study. A similar selectivity and effectiveness of AP5 in blocking NMDA receptors was observed when comparable trials were performed.
Figure 9. Post-stimulus time histogram showing the effects of glutamate receptor subtype agonists and antagonist on discharge of a pulse-synchronous NTS neurone.

Post-stimulus time histogram (bin width, 0.5 ms) of discharge of 1 neurone, triggered off systole of the arterial pressure pulse, which demonstrates the pulse-synchronous nature of the discharge of the neurone (A) and the selectivity of blockade of non-NMDA receptors by the concentration of NBQX utilised in the study. Picoejection of either AMPA (B) or NMDA (D) increased discharge of the neurone, indicating the presence of both non-NMDA and NMDA receptors, respectively, on the neurone. Following a return to control (not shown), picoejection of NBQX to block non-NMDA receptors completely abolished discharge of the neurone (E) and eliminated any response to an ensuing picoejection of AMPA (F), indicating an effective block of non-NMDA receptors on the neurone. However, the response to picoejection of NMDA was not changed from control (G), indicating that NMDA receptors were not affected by administration of NBQX. Comparable responses were obtained to blockade of the NMDA receptors by AP5, indicating the selectivity of both antagonists used in the present study.
DISCUSSION
The majority of neurones recorded in this study were identified in the medial and dorsomedial subnuclei of the NTS, areas which were previously reported to receive vagal afferent activity (Kalia & Mesulam, 1980; Bennett et al. 1981; Chernicky et al. 1984; Hines et al. 1994; Yousfi-Malki & Puizillout, 1994). The loss of NTS neuronal activity following left vagotomy (17 of 18 neurones) confirmed the left vagus as the primary source of input to initiate the pulse-synchronous discharge observed in these neurones. The lack of convergent input from either the carotid or aortic baroreceptors is in agreement with the minimal convergence from these receptor groups on central neurones reported by others (Silva-Carvalho et al. 1998). However, there are reports of varying degrees of convergence from vagal and baroreceptor inputs to NTS neurones in other preparations (Bonham & Hasser, 1993; Rogers et al. 1993; Nosaka et al. 1995; Zhang & Mifflin, 1995; Paton, 1998). The difference between this study and previous studies may be related to the type of stimuli used to initiate afferent input from these different receptor groups. The use of electrical stimulation of nerves in some studies may evoke afferent input from different receptor groups (e.g. chemoreceptors, pulmonary receptors, chemosensitive cardiac receptors) or different afferent patterns from the pressure-encoding receptors than more physiological changes in pressure, thus altering the ability to observe any effects of stimulation of a given receptor pathway.
All neurones recorded in this study had pulse-synchronous discharge that was eliminated following vagotomy, indicating the discharge was synaptically evoked and not due to movement of the brainstem. Small changes in arterial pressure were used to verify the pressure sensitivity of the discharge pattern of the majority of neurones (17 of 18), all of which were found to be driven by left vagal afferent input. In addition, the close relationship of peak discharge rates for all neurones relative to the arterial pressure pulse and to each other (Fig. 6) suggests that the receptors may represent a functionally similar population of neurones driven by a common type of afferent input. These results would suggest that the input driving all but one neurone originated with left cardiac mechanoreceptors, since the pressure sensitivity appeared to track changes in arterial pressure. The neurones studied were selected based on the presence of a pulse-synchronous discharge, thereby limiting the study to a subpopulation of NTS neurones. It is not known if other neurones without a pulse-synchronous discharge are also activated by cardiac mechanoreceptors.
The heartbeat-locked discharge has not been observed in NTS neurones which have been shown to receive afferent input from other pressure-sensitive cardiovascular receptors, such as the arterial baroreceptors (Lipski et al. 1975; Feldman & Moises, 1987; Mifflin et al. 1988; Rogers et al. 1993; Seagard et al. 1995). The lack of pulse-synchronous discharge in neurones that are part of the baroreflex has led to a question regarding the origin of cardiac-related discharge in efferent sympathetic and parasympathetic outflow. It is possible that at some point in the central pathways, there is convergence of information transmitted by neurones receiving baroreceptor versus left cardiac mechanoreceptor information, leading to the re-establishment of pulse-related activity in the efferent pathways. However, due to the lack of convergence found in this study between centrally directed activity from vagal cardiac mechanoreceptors and arterial baroreceptors, the site of convergence would seem to be at a higher level of the reflex arc than the NTS. One NTS neurone had a pulse-synchronous discharge pattern, but did not respond to arterial pressure changes. The pattern of discharge of the neurone plus the finding that activity was eliminated by right vagotomy suggests that this neurone was driven by input from right cardiac mechanoreceptors, possibly arising from atrial receptors. The encoding of cardiac rate by this neurone may also reflect a type of central activation that could converge at some point in the central baroreflex pathway, leading to the generation of cardiac-related efferent outflow.
The rates of discharge of the pulse-synchronous neurones evoked by left vagal input ranged from 1–12 spikes (arterial pressure pulse)−1. This discharge rate is much lower than peak rates reported for the discharge of cardiac receptors with myelinated (50-200 spikes s−1) (Coleridge et al. 1973; Thoren, 1977) or non-myelinated (14 spikes s−1) (Gupta & Thames, 1983) afferents recorded from peripheral vagal fibres. However, the discharge of regularly firing cardiac receptors has been reported to be between 1 and approximately 20 spikes (cardiac cycle)−1, occurring at various phases of the cardiac cycle, dependent on receptor location (Coleridge et al. 1964). One study found that firing occurred primarily during diastole, with increases occurring during systole with increases in arterial pressure (Gupta & Thames, 1983). Thus, while the pulse synchronicity of the cardiac receptor discharge pattern is encoded in the firing pattern of the NTS neurones, the absolute firing rates of the cardiac receptors may not be reflected at higher pressures. However, since the patterns of integration of NTS neuronal activity at higher levels of the reflex arc are not known, the relevance of this factor is not clear. In an earlier study in rats (Hines et al. 1994), it was found that NTS neuronal activity evoked through activation of cardiac mechanoreceptors by bolus saline injections demonstrated a volume threshold, with no further increases in discharge despite larger saline injections. However, the number of neurones excited by the saline injections increased with increasing volumes of injections. Investigators suggested that increased reflex responses due to mechanoreceptor activation resulted from recruitment of more NTS neurones at larger volumes, not to increases in discharge of individual neurones. A related type of integration may also be operative with the pressure-sensitive neurones recorded in the present study. While the individual neurones did demonstrate some increases in activity to increases in arterial pressure, the discharge rates never approached peak rates reported for peripheral afferent activity from the receptors, particularly those reported for receptors with myelinated afferents which also demonstrate the greatest degree of pulse-synchronous discharge (Coleridge et al. 1964; Gupta & Thames, 1983). However, the possible recruitment of more neurones at higher arterial pressure may lead to enhanced reflex responses reported for increasing activation of cardiac mechanoreceptors.
Glutamate has been proposed to be the primary neurotransmitter released by baroreceptor and vagal afferents in the NTS (Guyenet et al. 1987; Meeley et al. 1989; Kubo & Kihara, 1991; Andresen & Yang, 1993; Allchin et al. 1994; Andresen & Kunze, 1994; Lawrence & Jarrott, 1994; Lawrence, 1995; Zhang & Mifflin, 1995). However, no studies have previously examined the effectiveness of selective blocking of glutamate receptor subtypes on the discharge of NTS neurones evoked by physiological pressure stimulation. Brain slice preparations have been utilised to examine mechanisms of transmission of afferent input from putative arterial baroreceptor and vagal afferent fibres in the solitary tract to neurones in subnuclei in the NTS. Since electrical stimulation must be used as the method of evoking afferent input in these preparations, the exact origin of afferent input cannot be determined. However, results from these studies indicate roles for both NMDA and non-NMDA receptors in the initiation of activity in the NTS neurones due to primary afferent stimulation. Some results indicate that non-NMDA receptors are those primarily responsible for the transmission of afferent input to the second-order NTS neurones receiving primary afferent projections (Andresen & Yang, 1990). However, there is also evidence for a contribution from NMDA receptors in the activation of these second-order neurones, which may not be seen until the neurones become depolarised via activation of non-NMDA receptors, since the NMDA channel is blocked by Mg2+ until membrane potential reaches approximately −45 mV (Miller & Felder, 1988; Brooks & Spyer, 1993; Aylwin et al. 1997). Evidence from microinjection studies of ionotropic glutamate receptor antagonists into the NTS, which can affect neurones receiving afferent inputs from many possible peripheral receptors, also suggests that NMDA receptors, non-NMDA receptors or combinations of the subtypes contribute to the activation of NTS neurones responsible for the transmission of the depressor responses that could be attributed to either vagal cardiopulmonary or arterial baroreceptor reflexes. Depressor responses evoked by NTS microinjection of glutamate have been found to be attenuated by both NMDA and non-NMDA receptor antagonists (La Galloudec et al. 1989; Leone & Gordon, 1989; Talman, 1989; Kubo & Kihara, 1991). Similarly, reflex responses produced by pharmacologically induced changes in arterial pressure have also been shown to be blunted by NMDA or non-NMDA antagonists or combinations of the two (La Galloudec et al. 1989; Ohta & Talman, 1994). Since both of the above techniques could activate NTS neurones involved in the transmission of either arterial or cardiopulmonary baroreflexes or both, the role of each glutamate receptor subtype in the transmission of a specific reflex cannot be determined from these studies.
The contribution of glutamate receptor subtypes to the transmission of cardiac vagal afferent input has been less studied than the roles of the receptors in the transmission of baroreceptor afferent input, but the respective roles of NMDA versus non-NMDA receptors appear to be similar to those proposed for the transmission of baroreceptor input. Blockade of EAA receptors with kynurenate was found to eliminate discharge in NTS neurones evoked by either vagal or aortic nerve stimulation in rats (Zhang & Mifflin, 1995). Neurones with monosynaptic and polysynaptic inputs were similarly attenuated, but antagonists for specific ionotropic glutamate receptor subtypes were not utilised in the study. In studies that examined the transmission of vagal C-fibre chemosensitive input to the NTS, it was found that blockade of non-NMDA but not NMDA receptors significantly decreased synaptic activation of neurones primarily located in the commissural NTS (Wilson et al. 1996). However, this afferent input originated from chemosensitive C-fibre cardiac receptors, not mechanoreceptors, and the NTS neurones did not demonstrate any pulse-synchronous activity. The results of the present study indicate that non-NMDA receptors play the major role in the transmission of putative left vagal cardiac mechanoreceptor activation of NTS neurones. It was not possible to determine if the neurones recorded in the present study were second-order or higher, which could be important in evaluating the roles of the glutamate receptor subtypes. If the roles played by these receptor subtypes for transmission of vagal cardiac input are similar to those observed for transmission of activity evoked by generalised solitary tract stimulation, the non-NMDA receptors would play the largest role in the transmission of activity to second-order neurones. NMDA receptors could contribute to transmission at higher rates of afferent input, plus be more importantly involved in polysynaptic activation of neurones. Since the roles of the receptors in the transmission of vagal input at lower versus higher arterial pressures were not tested, it is not possible to determine if NMDA receptors could play a greater role at higher pressures. However, this does remain a possible area of investigation for future studies.
In conclusion, NTS neurones with pulse-synchronous discharges were recorded from subnuclei of both the right and left NTS. Activity in most neurones (17 of 18) was found to be arterial pressure sensitive and evoked by left vagal afferent input. No convergent activation was found from either carotid or aortic baroreceptors. The cardiac-related activity was either attenuated (4 of 17) or eliminated (13 of 17) by blockade of non-NMDA glutamate receptors. Remaining activity in the four neurones was eliminated with additional blockade of NMDA receptors. A single neurone, activated by right vagal input, did not demonstrate pressure sensitivity, but did reflect heart rate. These results indicate that a population of NTS neurones with a heart rate-locked pattern of discharge encodes arterial pressure-related information, primarily through activation of non-NMDA receptors.
Acknowledgments
The authors would like to acknowledge the excellent technical and histological assistance of Claudia A. Hermes, Sarah A. Botsford and Angela R. Cowan. This research was supported by VA Medical Research Funds and NIH grant HL55490.
References
- Allchin RE, Batten TFC, McWilliam PN, Vaughn PFT. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis. Experimental Physiology. 1994;79:265–268. doi: 10.1113/expphysiol.1994.sp003761. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius - Gateway to neural circulatory control. Annual Review of Physiology. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. American Journal of Physiology. 1990;259:H1307–1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Excitatory amino acid receptors and afferent synaptic transmission in the nucleus tractus solitarius. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton: CRC Press; 1993. pp. 187–192. [Google Scholar]
- Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. Journal of Neurophysiology. 1997;77:2539–2548. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Goodchild C, Kidd C, Mcwilliam PN. Neurones in the brain stem of the cat activated by myelinated and nonmyelinated fibres in the cardiac and pulmonary branches of the vagus. The Journal of Physiology. 1981;310:64P. [Google Scholar]
- Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. American Journal of Physiology. 1993;264:H1674–1685. doi: 10.1152/ajpheart.1993.264.5.H1674. [DOI] [PubMed] [Google Scholar]
- Brooks PA, Spyer KM. Evidence for NMDA receptor-mediated synaptic events in the rat nucleus tractus solitarii in vitro. The Journal of Physiology. 1993;467:21P. [Google Scholar]
- Chernicky CL, Barnes KL, Ferrario CM, Conomy JP. Afferent projections of the cervical vagus and nodose ganglion in the dog. Brain Research Bulletin. 1984;13:401–411. doi: 10.1016/0361-9230(84)90090-x. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG, Dangel A, Kidd C, Luck JC, Sleight P. Impulses in slowly conducting vagal fibres from afferent endings in the veins, atria, and arteries of dogs and cats. Circulation Research. 1973;33:87–97. doi: 10.1161/01.res.33.1.87. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG, Kidd C. Cardiac receptors in the dog, with particular reference to two types of afferent endings in the ventricular wall. The Journal of Physiology. 1964;174:323–339. doi: 10.1113/jphysiol.1964.sp007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman PD, Moises HC. Adrenergic responses of baroreceptive cells in the nucleus tractus solitarii of the rat: a microiontophoretic study. Brain Research. 1987;420:351–361. doi: 10.1016/0006-8993(87)91256-x. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Research. 1991;568:319–322. doi: 10.1016/0006-8993(91)91418-z. [DOI] [PubMed] [Google Scholar]
- Gupta BN, Thames MD. Behaviour of left ventricular mechanoreceptors with myelinated and nonmyelinated afferent vagal fibres in cats. Circulation Research. 1983;52:291–301. doi: 10.1161/01.res.52.3.291. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Research. 1987;407:272–284. doi: 10.1016/0006-8993(87)91105-x. [DOI] [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurones in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circulation Research. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. Journal of Comparative Neurology. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Unilateral blockade of excitatory amino acid receptors in the nucleus tractus solitarii produces an inhibition of baroreflexes in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1991;343:317–322. doi: 10.1007/BF00251133. [DOI] [PubMed] [Google Scholar]
- La Galloudec E, Merahi N, Laguzzi R. Cardiovascular changes induced by the local application of glutamate-related drugs in the rat nucleus tractus solitarii. Brain Research. 1989;503:322–325. doi: 10.1016/0006-8993(89)91683-1. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ. Neurotransmitter mechanisms of rat vagal afferent neurones. Clinical and Experimental Pharmacology and Physiology. 1995;22:869–873. doi: 10.1111/j.1440-1681.1995.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. L-Glutamate as a neurotransmitter at baroreceptor afferents: Evidence from in vivo microdialysis. Neuroscience. 1994;58:585–591. doi: 10.1016/0306-4522(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Leone C, Gordon FJ. Is L-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius? Journal of Pharmacology and Experimental Therapeutics. 1989;250:953–962. [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Spyer KM. The sinus nerve and baroreceptor input to the medulla of the cat. The Journal of Physiology. 1975;251:61–78. doi: 10.1113/jphysiol.1975.sp011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeley MP, Underwood MD, Talman WT, Reis DJ. Content and in vitro release of endogenous amino acids in the area of the nucleus of the solitary tract of the rat. Journal of Neurochemistry. 1989;53:1807–1817. doi: 10.1111/j.1471-4159.1989.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: Postsynaptic actions and the influence of respiration. The Journal of Physiology. 1988;399:349–367. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BD, Felder RD. Excitatory amino acid receptors intrinsic to synaptic transmission in nucleus tractus solitarii. Brain Research. 1988;456:333–343. doi: 10.1016/0006-8993(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Murase S, Murata K, Inui K. ‘Aortic baroreceptor’ neurones in the nucleus tractus solitarius in rats: Convergence of cardiovascular inputs as revealed by heartbeat-locked activity. Journal of the Autonomic Nervous System. 1995;55:69–80. doi: 10.1016/0165-1838(95)00030-2. [DOI] [PubMed] [Google Scholar]
- Ohta H, Talman WT. Both NMDA and non-NMDA receptors in the NTS participate in the baroreceptor reflex in rats. American Journal of Physiology. 1994;267:R1065–1070. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Convergence properties of solitary tract neurones driven synaptically by cardiac vagal afferents in the mouse. The Journal of Physiology. 1998;508:237–252. doi: 10.1111/j.1469-7793.1998.237br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RF, Paton JFR, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. American Journal of Physiology. 1993;265:R1355–1368. doi: 10.1152/ajpregu.1993.265.6.R1355. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Discharge patterns of baroreceptor-modulated neurones in the nucleus tractus solitarius. Neuroscience Letters. 1995;191:13–18. doi: 10.1016/0304-3940(95)11545-1. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Bosnjak ZJ, Elegbe EO, Kampine JP. Extent and mechanism of halothane sensitisation of the carotid sinus baroreceptors. Anesthesiology. 1983;58:432–437. doi: 10.1097/00000542-198305000-00007. [DOI] [PubMed] [Google Scholar]
- Seagard JL, van Brederode JFM, Dean C, Hopp FA, Gallenberg LA, Kampine JP. Firing characteristics of single-fibre carotid sinus baroreceptors. Circulation Research. 1990;66:1499–1509. doi: 10.1161/01.res.66.6.1499. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho L, Paton JFR, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurones responsive to cardiac receptor stimulation in the anaesthetised cat. Journal of Neurophysiology. 1998;79:2374–2382. doi: 10.1152/jn.1998.79.5.2374. [DOI] [PubMed] [Google Scholar]
- Talman WT. Kynurenic acid microinjected into the nucleus tractus solitarius of rat blocks the arterial baroreflex but not responses to glutamate. Neuroscience Letters. 1989;102:247–252. doi: 10.1016/0304-3940(89)90086-4. [DOI] [PubMed] [Google Scholar]
- Thoren PN. Characteristics of left ventricular receptors with nonmedullated vagal afferents in cats. Circulation Research. 1977;40:415–421. doi: 10.1161/01.res.40.4.415. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. American Journal of Physiology. 1993;264:R41–50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Zhang Z, Bonham AC. Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. The Journal of Physiology. 1996;493:773–785. doi: 10.1113/jphysiol.1996.sp021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi-Malki M, Puizillout JJ. Induction of Fos-like protein in neurones of the medulla oblongata after electrical stimulation of the vagus nerve in anaesthetised rabbit. Brain Research. 1994;635:317–322. doi: 10.1016/0006-8993(94)91454-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Excitatory amino-acid receptors contribute to carotid sinus and vagus nerve evoked excitation of neurones in the nucleus of the tractus solitarius. Journal of the Autonomic Nervous System. 1995;55:50–56. doi: 10.1016/0165-1838(95)00027-u. [DOI] [PubMed] [Google Scholar]


