Abstract
Enzymatically dissociated single muscle fibres of the rat were studied under voltage clamp conditions in a double Vaseline gap experimental chamber. Intramembrane charge movement and changes in intracellular calcium concentration ([Ca2+]i) were measured and the rate of calcium release (Rrel) from the sarcoplasmic reticulum (SR) was calculated. This enabled the determination of SR permeability and thus the estimation of the transfer function between intramembrane charge movement and SR permeability.
Perchlorate (3 mm) shifted the membrane potential dependence of intramembrane charge movement to more negative voltages without any effect on the steepness or on the maximal available charge. The drug increased SR permeability at every membrane potential but did not alter the peak-to-steady level ratio. It also increased the slope of the transfer function, indicating a more efficient coupling between the voltage sensors and the ryanodine receptors.
Caffeine (1 mm), on the other hand, increased SR permeability without altering the voltage dependence of intramembrane charge movement. It neither prolonged the depolarization-induced increase in [Ca2+]i at short pulse durations nor altered the time to peak of Rrel. The augmentation of SR permeability by the drug was more pronounced during the peak caffeine response than during its steady level. This was manifested in a leftward shift of the transfer function rather than an increase in its slope.
These observations indicate that perchlorate and caffeine alter the coupling between the voltage sensors and SR calcium release channels in mammalian skeletal muscle. They do not, however, share a common mechanism for enhancing the depolarization-induced release of calcium from the SR.
Excitation-contraction coupling (ECC) in skeletal muscle involves the delicate interaction of the dihydropyridine and ryanodine receptors (DHPRs and RyRs), two proteins located in the membranes of, respectively, the transverse tubules (T-tubules) and the sarcoplasmic reticulum (SR). The primary function of the T-tubular DHPR is to relay the information arriving in the form of a potential change to the adjacent RyR calcium release channel of the SR.
Several pharmacological interventions have been used to alter the coupling between DHPRs and RyRs. Perchlorate and caffeine are probably the two most extensively studied of the molecules that somehow enhance ECC. The former was first proposed to act exclusively on the voltage sensor and shift its membrane potential dependence to more negative voltages (Lüttgau et al. 1983). This simple view was questioned following the observation that perchlorate is an effective agonist of the skeletal-type RyR (Ma et al. 1993). Ríos and co-workers (1993) interpreted the effects of perchlorate using an allosteric model, where the association of adjacent DHPRs and RyRs would be influenced by the drug.
Caffeine, on the other hand, has been shown to change the affinity of the isolated RyR for the activating calcium ions (reviewed recently by Herrmann-Frank et al. 1999). This could provide a simple explanation of the effects of the drug on amphibian skeletal muscle where a calcium-induced calcium release (CICR) mechanism has already been proposed to function during normal ECC (Ríos & Pizarro, 1988; Jacquemond et al. 1991), but a different mechanism might be required in mammals where the presence of CICR has recently been questioned (Shirokova et al. 1998).
Measurements describing the direct effects of either perchlorate or caffeine on intramembrane charge movement and on SR calcium release have been carried out mostly on amphibians (Csernoch et al. 1987; Klein et al. 1990; Györke & Palade, 1992; González & Ríos, 1993; Huang, 1998a,b). The elucidation of these effects in mammals should further clarify the role of the single RyR isoform in mammalian skeletal muscle compared to the two distinct isoforms expressed in frogs (Olivares et al. 1991) and the suggested differences in the details of ECC (Shirokova et al. 1996) for these two classes of vertebrates. It should, furthermore, give direct insight into whether CICR might play a distinct role in mammalian skeletal muscle.
Here we show that, although both perchlorate and caffeine enhance the depolarization-induced increase in SR permeability in mammalian striated muscle, the details of their actions are quite different. While perchlorate altered the voltage dependence of intramembrane charge and increased the slope of the transfer function, caffeine had no effect on these parameters. On the other hand, caffeine shifted the transfer function to the left and increased the peak-to-steady level ratio of the depolarization-induced SR permeability increase. Some aspects of these results have been presented to the American Biophysical Society (Kovács et al. 1999).
METHODS
Experimental procedures
Single skeletal muscle fibres were isolated enzymatically from the extensor digitorum communis (EDC) muscles of the rat and mounted in a double Vaseline gap chamber as described in our earlier reports (Szentesi et al. 1997; Csernoch et al. 1999). In brief, 3- to 5-month-old Wistar rats of either sex were anaesthetized with ether and killed by cervical dislocation following an approved protocol of the University Medical School of Debrecen Institutional Animal Care and Use Committee. The muscles were removed and placed in a modified Krebs solution (mm: 135 NaCl, 5 KCl, 2.5 CaCl2, 1 MgSO4, 10 Hepes, 10 glucose, 10 NaHCO3, 10 % fetal calf serum (FCS)). The fibres were separated by gently shaking the muscle for 1½ h in an incubator (IG 150, Jouan, Saint Herblain, France) at 37°C in the presence of collagenase (Sigma, Type I, 1 mg ml−1). Dissociated fibres were washed with the FCS-supplemented modified Krebs solution and stored at 4°C until further use. After transferring a fibre to the recording chamber (Kovács et al. 1983) and completing the Vaseline isolation and the saponin permeabilization, the solution in the open-end pools was changed to ‘internal solution’ (mm: 120 caesium glutamate, 5.5 MgCl2, 5 Na2-ATP, 10 sodium phosphocreatine, 10 glucose, 10 Hepes, 5 EGTA, 1.9 CaCl2) and that in the middle pool to ‘external solution’ (mm: 140 TEA-CH3SO3, 10 choline bicarbonate, 2 CaCl2, 2 MgCl2, 10 Hepes, 1 3,4-diaminopyridine, with 10−7 g ml−1 tetrodotoxin). All solutions were adjusted to pH 7.2 and 300 mosmol l−1. The internal solution also contained 1 mm antipyrylazo III (APIII) and 100 μm fura-2 for the detection of [Ca2+]i. The sarcomere length was set to 2.2–2.5 μm, while the length of the fibre segment in the middle pool was set to 500 μm. APIII was purchased from ICN Biomedicals (Aurora, OH, USA), fura-2 was from Texas Fluorescent Labs (Austin, TX, USA), and all other chemicals were from Sigma.
The experimental set-up and data acquisition have been described in detail previously (e.g. Sárközi et al. 1996). In brief, a tungsten halogen lamp was used to trans-illuminate the fibre with red light (λ > 600 nm) and a xenon arch lamp to epi-illuminate it with near-UV light (λ= 380 nm or the isosbestic wavelength). The light intensities at 510 nm and at 720 and 850 nm were measured simultaneously for the detection of fura-2 fluorescence and APIII absorbance, respectively. Fibres were voltage clamped and the holding potential was set to −100 mV. All measurements were carried out at 16-18°C.
Experimental protocol
To examine the effects of perchlorate or caffeine on the events during ECC the fibres were subjected to a series of 100 ms depolarizations to test potentials ranging from −80 to +10 mV. This protocol was then repeated after the addition of the drug to the extracellular environment and again after its removal. To obtain the value for reference conditions the data obtained before the addition of the drug and after wash were averaged.
Intramembrane charge movement
Non-linear capacitive currents representing intramembrane charge were calculated as reported earlier (Sárközi et al. 1996; Szentesi et al. 1997). In brief, depolarizing (test) and hyperpolarizing pulses (negative control) of 100 ms duration were applied and the accompanying currents were measured. The negative control was then subtracted from the test pulse after appropriate scaling. The composition of the external and internal solutions ensured that most ionic currents were blocked. However, in some fibres an inward current, presumably carried by calcium ions through L-type channels, developed at large depolarizations.
The amount of charge transferred (Q) at any given membrane potential (Vm) was calculated by integrating the ‘on’ parts of the non-linear capacitive currents. To minimize contamination from the above-mentioned calcium current, the pulse duration was decreased to 40 ms for large depolarizations. The voltage dependence of charge transfer was assessed by fitting the two-state Boltzmann function,
| (1) |
where Qmax is the maximal available charge, V′ is the voltage at equal distribution and k is the slope factor, to the calculated data. To determine an overall voltage dependence the values of Q(Vm) were normalized to Qmax determined under reference conditions on the given fibre. The normalized values were then averaged at each and every membrane potential and fitted with eqn (1) both in the absence and in the presence of the drugs. Since the values were normalized to the corresponding maximal charge in reference conditions, Qmax was set to unity for fitting the values in the absence of the drug while Qmax was fitted together with V′ and k in the presence of the drugs.
Calculation of [Ca2+]i and SR permeability
Changes in the myoplasmic free calcium concentration were calculated from APIII absorbance (Kovács et al. 1983) using the correction for intrinsic fibre absorbance (Melzer et al. 1986a) and from fura-2 fluorescence (Klein et al. 1988). To determine the concentrations of APIII and fura-2 the procedures described by Kovács et al. (1983) and by Klein et al. (1988), respectively, were used, taking the inner filter effect of APIII at 510 nm into account.
The rate of calcium release from the SR (Rrel) was calculated from the calcium transient using the procedure described by Szentesi et al. (1997). In the removal model the maximal rate of transport by the SR calcium pump (PVmax), the dissociation rate of Mg2+ from parvalbumin (koff,Mg-P) and the association and dissociation rates of Ca2+ to and from EGTA (kon,Ca-E and koff,Ca-E) were adjusted to give the best fit of the decay in [Ca2+]i.
To estimate the time course and number of open release channels in the SR membrane the calculated Rrel records were corrected for the depletion of calcium in the SR and expressed as percentage of SR content (Csernoch et al. 1993). The calcium content of the SR (C0) was estimated from the slowly declining part of the Rrel records as described by Schneider et al. (1987). The voltage (Vm) dependence of either component of SR permeability (peak or steady level) was also fitted by the two-state Boltzmann function (eqn (1)). To estimate the steady level for a given transient the values of the last 20 data points during the depolarizing pulse were averaged.
Statistical analysis
In statistical analyses all values were expressed as means ±s.e.m. The effects of a given substance were always compared to the reference value measured on the same fibre. Statistical significance was thus calculated with Student's paired t test. Differences were accepted as statistically significant if P < 0.05.
RESULTS
Perchlorate and caffeine potentiate SR calcium release
During the course of these experiments we attempted to use 3, 6 and 9 mm perchlorate and 1 and 2 mm caffeine. The fibres, however, did not tolerate the higher concentrations. In the presence of 9 mm perchlorate, calcium was rapidly (over approximately 15 min) depleted from the SR thus preventing long experiments. Similar results were obtained if 2 mm caffeine was added to the external solution. Subsequent sections thus compare results obtained with 3 mm perchlorate and 1 mm caffeine.
Figure 1 demonstrates how 3 mm perchlorate influenced the depolarization-induced changes in [Ca2+]i and the underlying SR calcium release. Although the usual protocol was followed for this fibre, the figure depicts only the results obtained by depolarizing the membrane to 0 mV. Perchlorate enhanced the calcium concentration increase, as evident both from the change in the saturation of fura-2 (Sf; Fig. 1A) and from the calcium transient (Fig. 1B). Both the rate of rise and the maximal [Ca2+]i attained were increased by the drug. The latter was on average 0.8 ± 0.2 μm in control and 1.5 ± 0.5 μm in the presence of perchlorate (n = 8). These changes were reversed after the removal of the drug (Fig. 1, wash). It should be noted that in some cases the calcium transients were smaller after wash than in control, most probably due to the decrease in SR calcium content as detailed below.
Figure 1. Perchlorate enhances SR calcium release in mammalian skeletal muscle.
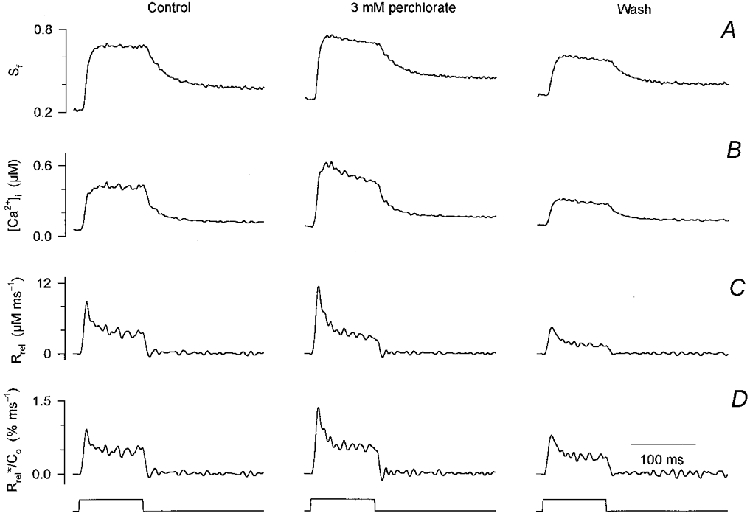
A, changes in the saturation of fura-2 during a 100 ms long depolarizing pulse to 0 mV before (control), during the presence (perchlorate) and after the removal (wash) of 3 mm perchlorate. B, calculated changes in [Ca2+]i. Note that the resting [Ca2+]i slightly increased during the experiment. C, SR calcium release fluxes calculated from the traces shown in B. The parameters of the removal model were obtained in the absence of the drug and used throughout. D, estimated SR permeability determined by correcting the records in C for the depletion of calcium in the SR and normalizing them to the calculated SR calcium content. Concentration of APIII ([APIII]) 559-652 μm, concentration of fura-2 ([fura-2]) 84-106 μm. Parameters of the removal model: koff,Mg-P = 6.0 s−1, PVmax = 1.76 mm s−1, kon,Ca-E = 1.55 μm−1 s−1, koff,Ca-E = 2.1 s−1. Estimated values for SR content (C0) before, during and after perchlorate application were 1.0, 0.9 and 0.6 mm, respectively.
To examine the effects of perchlorate on RyRs, the SR calcium release flux (Rrel; Fig. 1C) was calculated. As expected from the calcium transients, SR calcium release was greater in the presence of the drug than in control and decreased after the removal of perchlorate. This was not a consequence of altered calcium binding or calcium uptake into the SR since fitting the declining phase of the calcium transients in the absence and presence of perchlorate (not shown) did not reveal any significant change in the removal parameters.
Note that the parameters of calcium binding to EGTA obtained from fitting the decaying phase of the calcium transients led to an apparent affinity constant of EGTA for calcium that is lower than that used in the literature for equilibrium calculations (see also Figs 2 and 3). The main reason for the discrepancy is that the removal model used in the calculations did not take into account the dissociation of protons that precedes the binding of calcium. On the time scale of our measurements the effect is an apparent decrease of kon,Ca-E. If, instead, the rate constants of EGTA were not fitted but set to give an affinity of 6 × 106 M−1 the calculated release flux was increased by approximately 40 % at all voltages (data not shown). Since this selection of parameters did not influence the relative magnitude of the aumentation of Rrel by the two drugs, all calculations below were carried out by fitting kon,Ca-E and koff,Ca-E to give a better estimate of the actual amount of calcium bound to EGTA.
Figure 2. Perchlorate induced alterations in SR permeability at different membrane potentials.
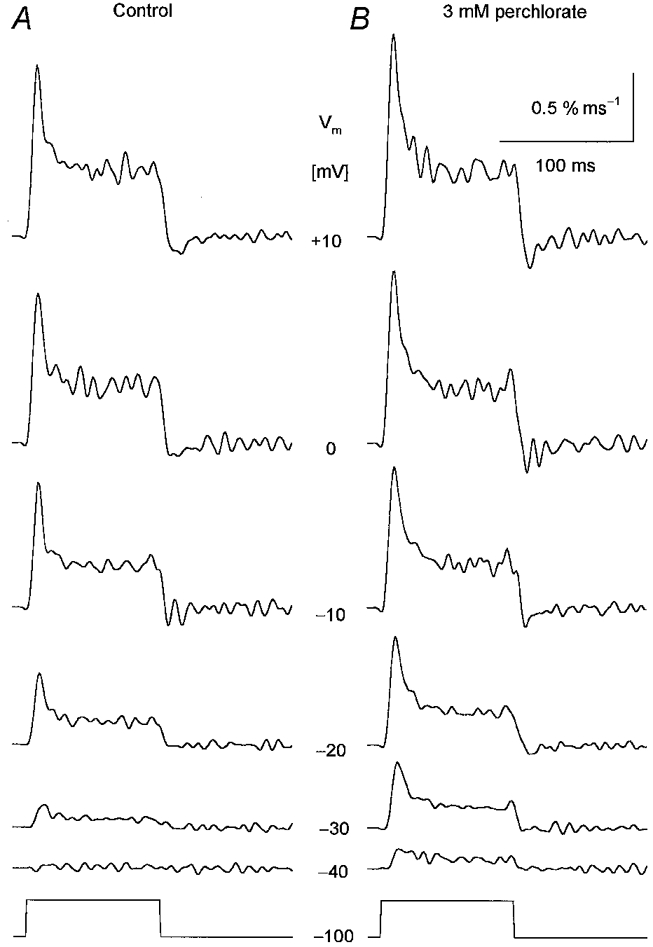
Changes in SR permeability were calculated from the calcium release records using the SR content estimated under control conditions. A, SR permeability in the absence of the drug. B, SR permeability after the addition of 3 mm perchlorate. Note that the drug enhanced the calculated permeability increase at every voltage tested. The membrane potentials during the 100 ms long depolarizations are indicated next to the traces. [APIII]= 735-881 μm, [fura-2]= 83-108 μm. Parameters of the removal model: koff,Mg-P = 8.5 s−1, PVmax = 1.86 mm s−1, kon,Ca-E = 1.1 μm−1 s−1, koff,Ca-E = 2.4 s−1. C0 = 1.5 mm.
Figure 3. Caffeine potentiates calcium transients and the depolarization-induced SR permeability increase.
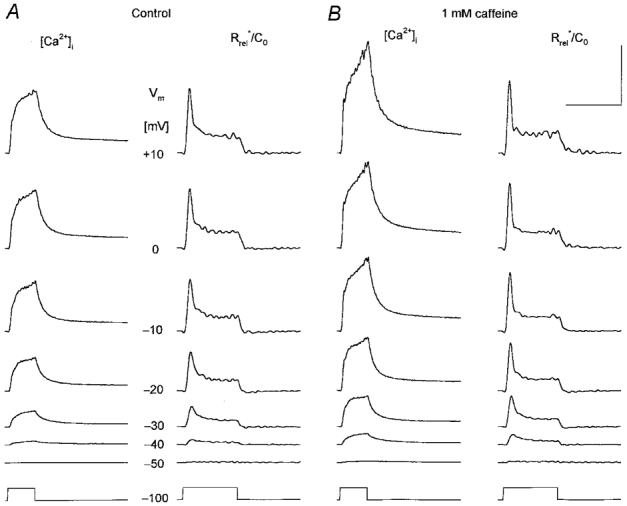
A, changes in intracellular calcium concentration ([Ca2+]i) and in SR permeability (Rrel*/C0) under control conditions at a wide range of membrane potentials as indicated for each row. The depolarization lasted for 100 ms for all traces (bottom trace). B, the effect of 1 mm caffeine on [Ca2+]i and on Rrel*/C0. Note that caffeine enhanced both the calcium concentration increase and the calculated SR permeability change at all voltages tested. Calibration bars for [Ca2+]i and Rrel*/C0 are, respectively: horizontal, 200 and 100 ms; vertical, 1 μm and 1 % ms−1. [APIII]= 829-930 μm, [fura-2]= 159-163 μm. Parameters of the removal model: koff,Mg-P = 2.2 s−1, PVmax = 3.9 mm s−1, kon,Ca-E = 0.9 μm−1 s−1 and koff,Ca-E = 5.8 s−1. C0 = 2.5 mm.
The time course of the increase in SR permeability was calculated by first correcting Rrel records for the depletion of calcium in the SR and then normalizing them to the initial SR calcium content (C0). This content was found to decrease from 1.0 to 0.6 mm during the course of the experiment presented in Fig. 1, as determined from the transients in control and after wash. Parallel with this decline in SR content, a small increase in resting [Ca2+]i was observed (from 55 to 97 nM), as is apparent from the differences in the baseline of the transients in panels A and B in Fig. 1. Although there was a small decline in SR content during some of the experiments, 1.8 ± 0.3 mm in control and 1.4 ± 0.4 mm after wash (n = 8), it was not statistically significant (P > 0.1) on average. Similarly, the average estimated SR content in the presence of perchlorate, 2.0 ± 0.4 mm, was not significantly different from that in control.
The calculated SR permeability increase displayed the usual kinetics both in the presence and absence of the drug. It rose to an early peak, 1.1 ± 0.2 % ms−1 on average for a depolarization to 0 mV in control, and declined to a steady level, 0.42 ± 0.04 % ms−1. Both the peak and the steady value were increased by perchlorate compared to control. After the removal of perchlorate, the effects were reversed. Note that the SR permeability, unlike the actual calcium flux, was similar in control and after wash, indicating that the underlying reason for the smaller calcium transient after wash was presumably the decreased SR calcium content.
Qualitatively similar results were obtained if the depolarization-induced SR permeability increases were compared at different membrane potentials in the absence and presence of perchlorate (Fig. 2). The two kinetic components were preserved by the drug and both were increased at all voltages. This increase was found to be 50 ± 11 % for the peak and 41 ± 12 % for the steady component for the depolarization to 0 mV.
Addition of 1 mm caffeine to the external solution also potentiated both the calcium transients and the underlying increase in SR permeability (Fig. 3). Calcium transients had a greater rate of rise and reached a greater maximal value in the presence of the drug. The maximum attained was found to be 1.1 ± 0.1 μm in control and 1.4 ± 0.1 μm in the presence of caffeine (n = 10). The removal of the drug (not shown) resulted in a decrease in the calcium transients to values slightly below that in control (0.9 ± 0.2 μm). As with perchlorate, neither the removal parameters nor the estimated SR content, 2.0 ± 0.2 and 1.9 ± 0.2 mm, respectively, were significantly different in the presence of caffeine compared to control. Furthermore, a slight decrease in SR content by the end of the experiment, 1.7 ± 0.1 mm after wash, was also observed.
Similar to perchlorate, the presence of caffeine did not alter the overall kinetics of the depolarization-induced change in SR permeability; it displayed an early peak followed by a fast decline to a maintained steady level. On the other hand, while it increased the early inactivating peak, caffeine left the steady level relatively unchanged. Comparing the traces obtained with depolarizations to −30 and +10 mV in Fig. 3 reveals that the effect of the drug was more pronounced at intermediate voltages.
These results clearly demonstrate that both compounds potentiate the calcium transients and the underlying SR calcium release. It should be noted that the increased flux and the increased SR permeability were due to an increase in activation rather than a decrease in calcium-dependent inactivation of the RyRs, since the rate of decline of SR permeability following the early peak was similar in the presence and absence of the drugs. For a depolarization to 0 mV the time constant of inactivation was found to be 6.2 ± 2.0 ms under reference conditions and 6.0 ± 1.9 and 5.6 ± 1.6 ms in the presence of perchlorate (P > 0.7) and caffeine (P > 0.1), respectively.
Voltage-dependent alterations in the two kinetic components of SR permeability increase
The effects of perchlorate on the peak and steady level components of the SR permeability increase as a function of the membrane potential during the test pulse are presented in Fig. 4A and B, respectively. The drug significantly increased both the peak (P < 0.03) and the steady level (P < 0.04) of SR permeability at every membrane potential, from −50 to +10 mV, where detectable calcium release was measured. Note that the potentiation of the SR permeability increase was relatively more pronounced at intermediate voltages than at large depolarizations. SR permeability in the presence of perchlorate at −20 mV was twice (2.0 ± 0.5) that in reference conditions while at +10 mV it was only 50 % greater (1.5 ± 0.1).
Figure 4. Effect of perchlorate on the two kinetic components of SR permeability increase.
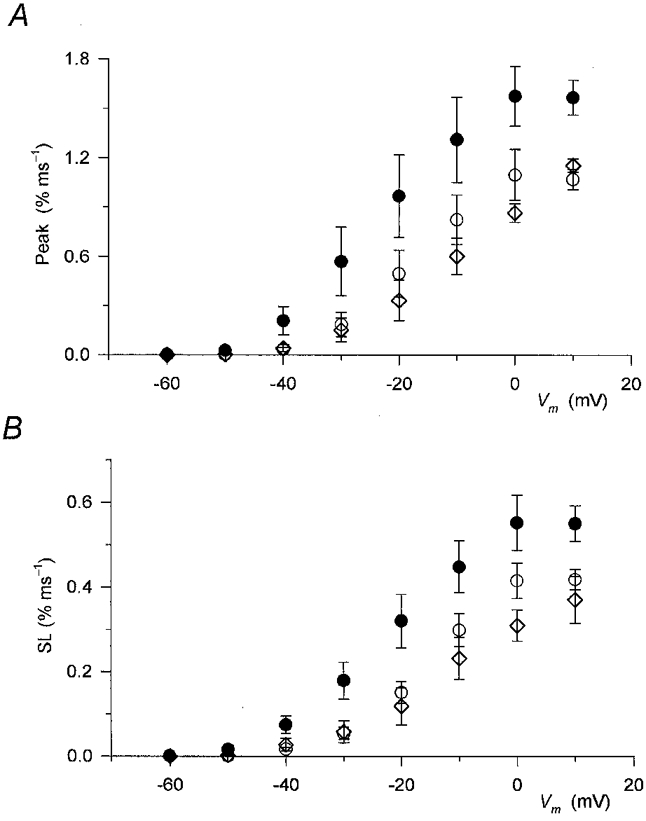
Values from 8 different fibres measured at corresponding membrane potentials were averaged before (○) and during (•) addition of perchlorate and after its removal (⋄). A, the effect of the drug on the peak, defined as the maximal value in the first 40 ms of the pulse, of SR permeability increase. B, the effect of perchlorate on the steady component (SL) of SR permeability. Note that perchlorate increased both the early peak and the maintained steady level at all voltages examined.
Table 1 presents the Boltzmann parameters, Pmax (maximum of SR permeability), V′ and k, obtained by fitting the membrane potential dependence of the two components of SR permeability with eqn (1). The drug shifted the voltage dependence to more negative membrane potentials and increased Pmax. Both the increase in permeability and the shift were found to be statistically significant (P < 0.03 and 0.02) for both components. Although the steepness of the voltage dependence was slightly decreased in both cases, this change did not prove to be significant (P > 0.2).
Table 1.
Alterations in the voltage dependence of SR permeability and intramembrane charge movement induced by 3 mm perchlorate or 1 mm caffeine
| SR permeability | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak | Steady level | Intramembrane charge | |||||||
| Pmax(% ms−1) | V′(mV) | k(mV) | Pmax(% ms−1) | V′(mV) | k(mV) | Qmax(nC μF−1) | V′(mV) | k(mV) | |
| Reference | 1.36 ± 0.11 | −4.6 ± 2.2 | 8.6 ± 0.8 | 0.50 ± 0.06 | −9.0 ± 1.3 | 7.7 ± 0.5 | 25.0 ± 0.9 | −8.1 ± 2.2 | 15.5 ± 1.1 |
| Perchlorate | 1.96 ± 0.21* | −13.3 ± 1.4* | 10.2 ± 0.9 | 0.72 ± 0.06* | −14.6 ± 1.7* | 9.7 ± 1.1 | 23.3 ± 1.1 | −14.9 ± 2.7* | 14.5 ± 1.5 |
| Reference | 1.38 ± 0.05 | −17.2 ± 1.6 | 7.5 ± 0.2 | 0.49 ± 0.03 | −17.3 ± 1.6 | 7.5 ± 0.4 | 22.8 ± 1.4 | −24.1 ± 2.5 | 13.3 ± 1.1 |
| Caffeine | 1.58 ± 0.09* | −22.5 ± 1.8* | 7.8 ± 0.2 | 0.51 ± 0.02 | −21.6 ± 1.8* | 8.7 ± 0.6 | 24.0 ± 1.7 | −25.2 ± 2.1 | 12.9 ± 0.6 |
Equation (1) was fitted to values from each individual fibre and the parameters obtained were then averaged. The number of fibres was 8 and 10 for perchlorate and caffeine, respectively.
Significantly different from reference.
The voltage-dependent effects of caffeine on the two kinetic components of SR permeability are presented in Fig. 5A and B. Caffeine increased the peak of SR permeability at all voltages, although the increase was not as pronounced as in perchlorate, being only 20 ± 6 % at 0 mV. As with perchlorate, the relative potentiation was more pronounced at intermediate voltages, averaging 73 ± 20 % at −20 mV.
Figure 5. Effect of caffeine on the two kinetic components of SR permeability increase.
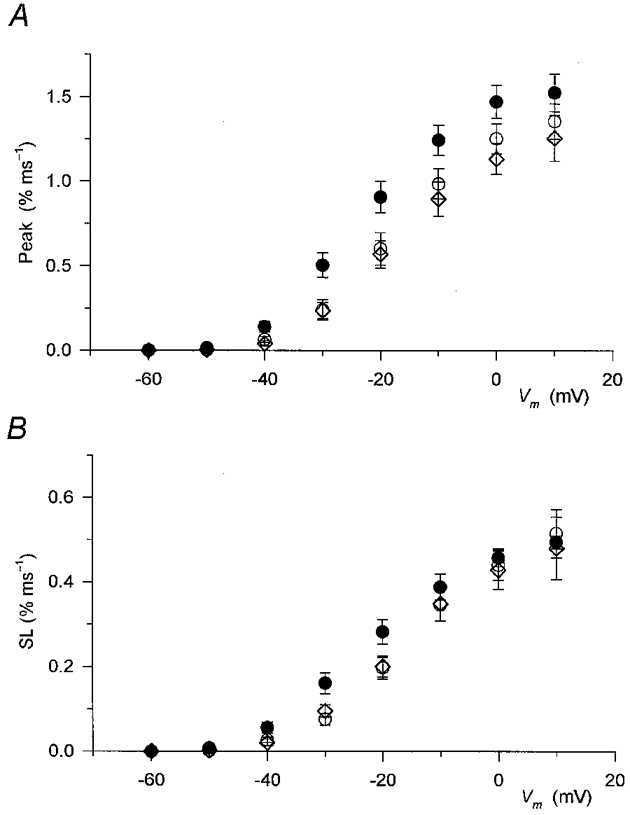
Data in the absence (○, control; ⋄, wash) and presence (•) of caffeine from 10 fibres were averaged as described in the legend to Fig. 4 for corresponding measurements. A, the effect of caffeine on the peak of SR permeability. B, the effect of the drug on the steady component (SL). Note that while the peak was increased at all voltages the effect on the steady level was not as pronounced, especially at large voltages.
The steady level was less affected by caffeine than the peak. This observation was especially evident at large depolarizations, at 0 and +10 mV, where the value after the addition of the drug was essentially identical to the values obtained either in control or after the removal of caffeine. Statistically significant increases were found only between −50 and −20 mV.
The voltage dependence of the peak and steady level of SR permeability in reference conditions and in the presence of caffeine are listed in Table 1. Caffeine increased the maximum peak SR permeability from 1.38 ± 0.05 to 1.58 ± 0.09 % ms−1 (the increase being significant, P < 0.02) but did not affect the maximum steady level (P > 0.4). The membrane potential dependence of both components was shifted to more negative voltages in the presence of the drug without any significant effect (P > 0.1) on the steepness of either function.
Thus both perchlorate and caffeine increased the peak of SR permeability, 3 mm perchlorate being more effective than 1 mm caffeine. They both shifted the voltage dependence of calcium release to smaller depolarizations while leaving its steepness unaltered. However, while perchlorate increased the steady level as much as the peak, caffeine had no effect on this component.
To demonstrate the characteristic difference between the effects of the two compounds, Fig. 6 plots the peak-to-steady level ratio of SR permeability as a function of the membrane potential. As described earlier (Shirokova et al. 1996; Szentesi et al. 1997), apart from a decrease at the smallest depolarizations, the ratio did not show any voltage dependence under reference conditions.
Figure 6. Differential alteration of the peak-to-steady level ratio of SR permeability by perchlorate and caffeine.
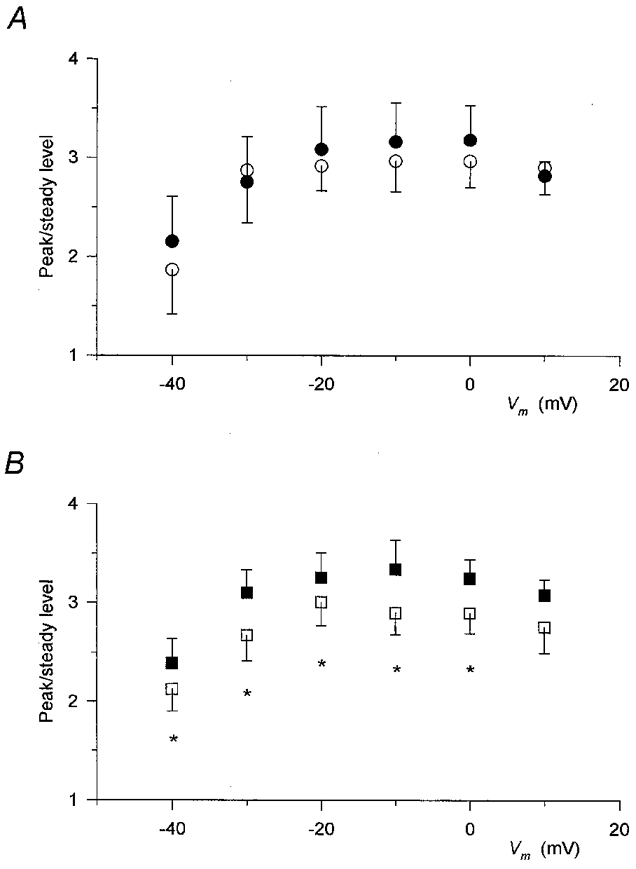
The peak-to-steady level ratio was calculated for each individual record and then the values obtained at the corresponding membrane potentials from different fibres were averaged. The average of the data obtained before the addition of the drug and after wash was used as control (open symbols). Only those fibres in which the challenge with the particular drug was carried out were included in the control and thus the control values in A and B are from different sets of experiments. A, data obtained in the presence (•) and absence (○) of perchlorate. Note that in spite of the large effect on the two components (Fig. 4) the ratio was essentially unaltered. B, values in the presence (▪) and absence (□) of caffeine. Unlike perchlorate, caffeine slightly increased the peak-to-steady level ratio. Same fibres as in Figs 4 and 5. Asterisks mark significant changes.
Figure 6A demonstrates that perchlorate not only left this voltage dependence unaltered, it actually did not change the average value at any membrane potential tested. This constant ratio was obtained in spite of the fact that the actual values of the peak and the steady level were increased by 50-100 % at different voltages. In contrast, caffeine enhanced the peak-to-steady level ratio (Fig. 6B). Although the increase was not dramatic, it was found to be statistically significant (P < 0.04) at all voltages examined except at +10 mV. It should be noted, however, that the overall voltage dependence of the ratio, which was constant at large and intermediate depolarizations but decreased slightly with small test pulses, was not altered by the drug.
Changes in intramembrane charge movement
The changes in SR calcium release demonstrated so far are consistent with an interpretation that these drugs directly influence the calcium release channels. However, the possibility that some of the changes were the consequence of altered intramembrane charge movement cannot be excluded on the basis of the data in the previous sections. We therefore compared charge movement currents in the presence and absence of both drugs.
Neither perchlorate nor caffeine had a dramatic effect on the kinetics or the amplitude of the charge movement currents in any fibre examined. Examples of non-linear capacitive currents in response to depolarizations to −20 mV, a potential where perchlorate had the largest effects (see below), are shown in the insets to Fig. 7. To assess the effects of the drugs on the voltage dependence of intramembrane charge movement, the non-linear capacitive currents were integrated and fitted with eqn (1).
Figure 7. Membrane potential dependence of intramembrane charge transfer.
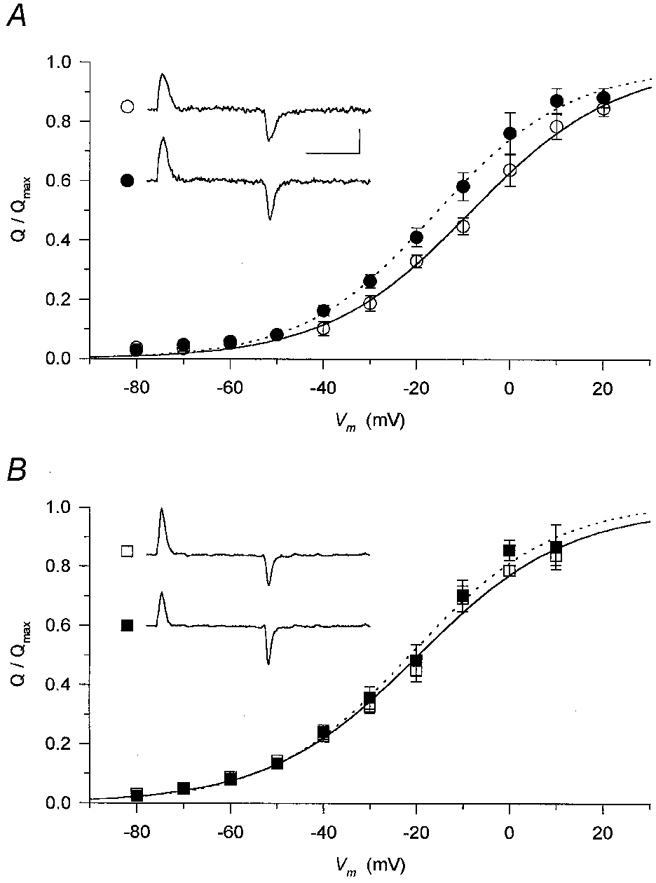
Non-linear membrane currents (shown in insets for a depolarization to −20 mV; horizontal calibration 50 ms, vertical calibration 1 A F−1) were integrated to obtain the charge moved at a given voltage. These values were then normalized to the maximal available charge in reference conditions (Q/Qmax) and averaged at each and every membrane potential. The normalized and averaged data were fitted with eqn (1) (superimposed curves) both in the absence (continuous curves) and presence (dotted curves) of the drugs. A, the effect of perchlorate (•) was to shift the membrane potential dependence to more negative voltages compared to control (○). Parameters obtained from the fits for reference conditions and in the presence of perchlorate are, respectively: V′=−8.4 and −16.0 mV, k = 15.6 and 14.3 mV. The normalized Qmax was 0.99 in the presence of perchlorate. B, caffeine (▪) did not alter the voltage dependence of intramembrane charge. Parameters obtained from the fits for reference conditions and in the presence of perchlorate are, respectively: V′=−19.6 and −20.8 mV, k = 16.1 and 15.3 mV. The normalized Qmax was 1.02 in the presence of caffeine.
Neither perchlorate (Fig. 7A) nor caffeine (Fig. 7B) altered the maximal available charge significantly, since the fitted relative maxima were 0.99 and 1.02, respectively. There was a slight shift (−7.6 mV) to more negative membrane potentials in the presence of perchlorate which was absent (−1.2 mV) when caffeine was used. The steepness of the membrane potential dependence was essentially identical to that in reference conditions, with a slight increase for both drugs (parameter k decreased by 1.3 and 0.8 mV in perchlorate and caffeine, respectively),. Similar conclusions can be drawn from the data in Table 1 representing the averages of the parameters obtained from the fits to individual fibres. The only statistically significant change was the negative shift in the voltage dependence induced by perchlorate.
These changes were thus manifested as an increase in the amount of charge moved at intermediate voltages in the presence of perchlorate, while caffeine did not alter the available charge at any membrane potential tested. These observations indicate that the alterations in SR permeability, apart from the shift in voltage dependence and all or part of the increase at intermediate voltages in the presence of perchlorate, were most probably the direct effects of these drugs on the calcium release channels.
Changes in the transfer function induced by perchlorate and caffeine
The data presented in the previous sections clearly demonstrate that both perchlorate and caffeine alter the way in which T-tubular depolarization affects the consequent increase in SR permeability. Furthermore, this alteration cannot be fully explained by changes in the properties of intramembrane charge movement. To further characterize the transmission of information from the T-tubular to the SR membrane the transfer functions of the triadic events, SR permeability versus amount of charge transferred, were calculated.
Figure 8 displays the change in transfer function induced by perchlorate (Fig. 8A) and by caffeine (Fig. 8B). To obtain the data points, mean normalized steady level SR permeability values were plotted as a function of the mean normalized charge from Fig. 7. The presence of perchlorate considerably altered the coupling between charge movement and SR permeability increase. It shifted the corresponding data points, measured at the same membrane potentials, to give both larger charge and larger permeability values. The shift along the y-axis was, however, relatively greater than along the x-axis. Due to this shift all points in the presence of perchlorate are above those in control, indicating that the same charge movement brought about a larger calcium release. Furthermore, the greater the amount of charge moved was, the greater this effect of the drug became.
Figure 8. Transfer functions in the presence of perchlorate and caffeine.
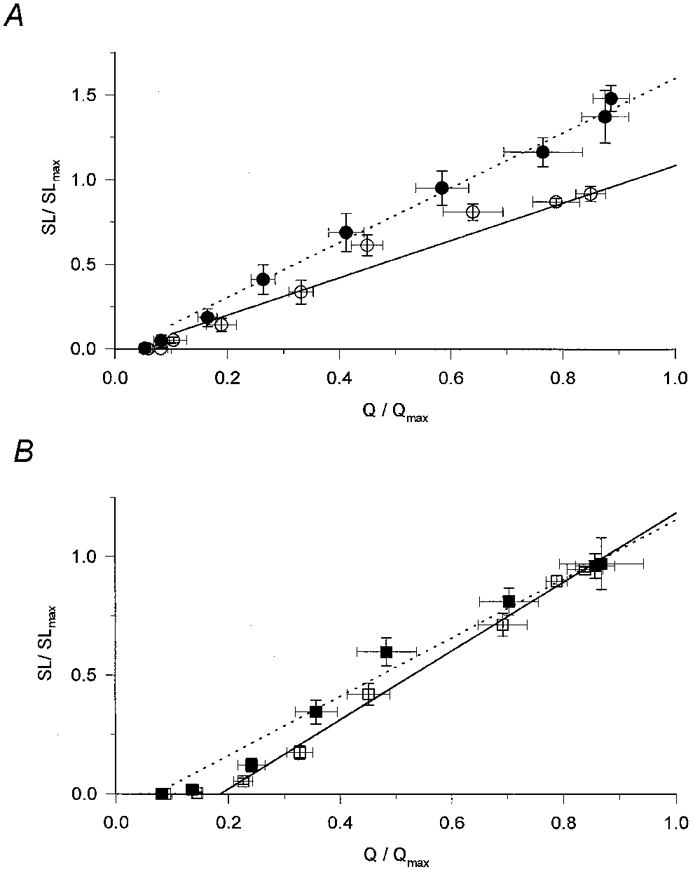
To calculate the transfer function the normalized and averaged steady SR permeability (SL/SLmax) was plotted as a function of the normalized and averaged charge (Q/Qmax). Superimposed lines represent the least squares fit of a straight line to the data points (7 points) above the threshold for the appearance of an SR permeability increase in reference conditions (continuous lines) and in the presence of the drugs (dotted lines). A, transfer function in the absence (○) and presence (•) of perchlorate. Note the increased slope (m). Parameters of the straight lines for reference conditions and in the presence of perchlorate are, respectively: m = 1.212 and 1.627, the threshold charge (Qth, the x-axis intercept) = 0.026 and 0.012, regression coefficient (r2) = 0.963 and 0.991. B, transfer functions in the absence (□) and presence (▪) of caffeine. Parameters of the straight lines for reference conditions and in the presence of perchlorate are, respectively: m = 1.454 and 1.242, Qth = 0.185 and 0.070, r2 = 0.993 and 0.982.
To quantitatively describe the transfer function and the change induced by perchlorate, a straight line was fitted to the data points above the threshold for the appearance of detectable calcium release. In this framework the transfer function is characterized by the slope (m) and the x-axis intercept (Qth) of the straight line. The former indicates the efficiency of the coupling while the latter is the charge moved before any measurable calcium release occurs. As the superimposed traces in Fig. 8A demonstrate, the most characteristic effect of perchlorate was to increase the slope of the transfer function (from 1.212 to 1.627; P < 0.02), indicating a more efficient coupling between the voltage sensors and the calcium release channels.
Caffeine had characteristically different effects on the relationship between intramembrane charge movement and SR permeability increase from those of perchlorate. It left the relationship between charge and steady level SR permeability essentially unaltered apart from a slight increase at intermediate charges (Fig. 8B). These observations were reflected in the parameters obtained by fitting the transfer functions with straight lines. Caffeine caused a small but statistically insignificant (P > 0.05) reduction in m, from 1.454 to 1.242, and a statistically significant decrease in Qth, from 0.185 to 0.070 (P < 0.01).
Control of the repolarization-induced closing of RyRs in the presence of caffeine
Caffeine has been shown to increase the calcium-induced openings of the calcium release channel in isolated SR preparations (Rousseau et al. 1988; Sitsapesan & Williams, 1990). This effect of caffeine was suspected to appear as a prolongation of SR calcium release beyond the end of the depolarizing pulse in cut amphibian skeletal muscle fibres (Klein et al. 1990). Since data reported in the previous sections showed that the effects of caffeine might be interpreted in a similar way in mammals, we examined the possibility that caffeine would alter the duration and time-to-peak value of our calcium transients and SR calcium release.
Calcium transients were elicited in the absence and presence of caffeine using depolarizing pulses of different durations ranging from 10 to 100 ms (Fig. 9). Caffeine (Fig. 9B) increased the calcium transients in a reversible manner (Fig. 9C) at all pulse durations tested. However, the time-to-peak value (Fig. 9D) remained unchanged by the drug. The slight increase, 1 ms for the fibre presented in Fig. 9, was most probably due to a change in fibre status since removing caffeine did not restore the shift in parallel with decreasing the peak. Similar results were obtained on five fibres where the times to peak of the calcium transients for a 10 ms depolarization were found to be 13.4 ± 0.4 and 13.8 ± 0.4 ms in the absence and presence of the drug, respectively.
Figure 9. Effect of caffeine on [Ca2+]i and Rrel evoked using different pulse durations.
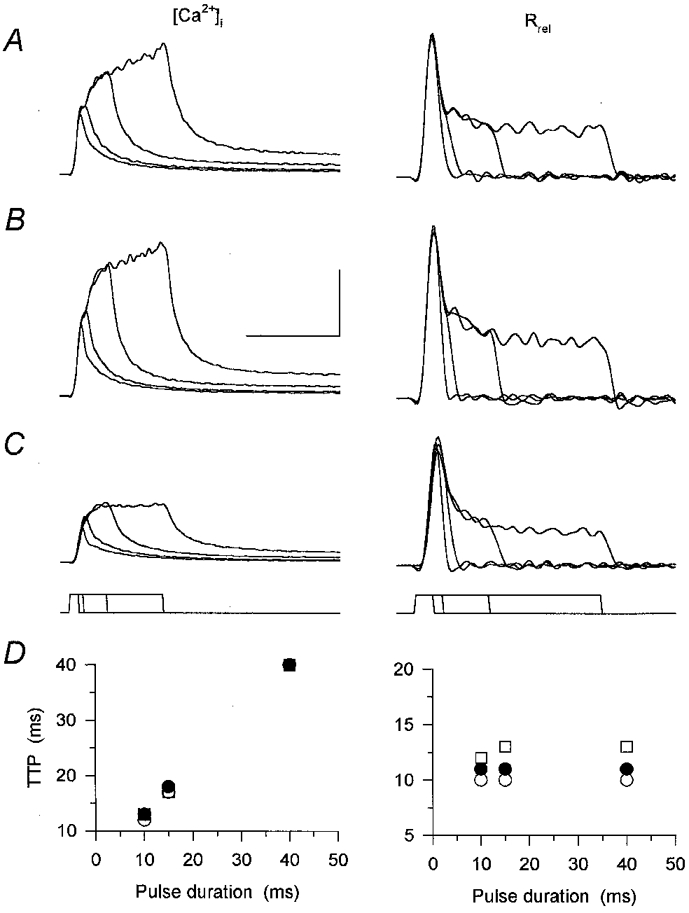
A-C, calcium transients ([Ca2+]i) and the underlying SR calcium release elicited using 10, 15, 40 and 100 ms depolarizing pulses to 0 mV. The pulse protocol is shown below the records. The measurement was carried out in control (A), in the presence of caffeine (B) and after the removal of the drug (C). Calibration bars for [Ca2+]i and for Rrel are, respectively: vertical, 0.5 μm and 15 μm ms−1; horizontal, 100 and 50 ms. D, time-to-peak (TTP) values of the calcium transients and the calculated SR calcium release for the 10, 15 and 40 ms depolarizations in control (○), after the addition of caffeine (•) and after wash (□). Values were calculated from the transients shown in A-C. Note that caffeine did not influence the TTP. [APIII]= 995-1403 μm, [fura-2]= 97.6–149.6 μm. Parameters of the removal model: koff,Mg-P = 14.5 s−1, PVmax = 1.6 mm s−1, kon,Ca-E = 2.2 μm−1 s−1 and koff,Ca-E = 1.9 s−1.
As expected from the calcium transients, the kinetics of SR calcium release was essentially unaltered by the presence of caffeine. Although the drug increased the flux, the time necessary to reach maximal calcium release rate was only shifted by 1 ms (Fig. 9D). This shift, again, is unlikely to be the consequence of caffeine; rather, it is more likely to be due to a slight change in fibre status, since removing the drug also shifted the peak to later times. These observations thus suggest that caffeine is not capable of altering the duration of SR calcium release elicited by short depolarizing pulses in mammalian skeletal muscle.
DISCUSSION
These experiments explored the effects of the calcium release modulators perchlorate and caffeine on the events of excitation-contraction coupling in mammalian muscle fibres. They focused on the alteration of the relationship between intramembrane charge movement and the increase in SR permeability and on the relationship between the two kinetic components of SR permeability increase. Both caffeine and perchlorate increased SR calcium release at all voltages tested. While caffeine achieved this without affecting intramembrane charge, perchlorate shifted the voltage dependence of charge movement to more negative membrane potentials. Differential effects were found both on the transfer function and on the peak-to-steady level ratio of SR permeability. Perchlorate increased the slope of the transfer function without any effect on the ratio. Caffeine on the other hand hardly affected the slope of the transfer function but increased the peak-to-steady level ratio. These results suggest that although there is a tight coupling in the increase of the two components of SR permeability in mammals, a clear pharmacological difference can be resolved.
Alterations in intramembrane charge movement
Both perchlorate and caffeine had qualitatively similar effects on intramembrane charge movement in the mammalian fibres examined in this study to those reported previously in cut amphibian fibres (Csernoch et al. 1987; Györke & Pallade, 1992; Huang, 1998a,b). Neither drug induced any change in the total amount of intramembrane charge nor in the steepness of its voltage dependence (Table 1).
Perchlorate shifted the membrane potential dependence to more negative voltages, similar to the shift described for amphibians (Lüttgau et al. 1983; Csernoch et al. 1987; Ma et al. 1993; Huang, 1998a), although the magnitude was less than that reported for frogs. This shift was originally (Lüttgau et al. 1983) interpreted as a direct effect on the voltage sensor but recently a more complex view has emerged. The effects of perchlorate on intramembrane charge were suggested to be secondary to its effects on the RyR calcium release channel (Ma et al. 1993). The results presented in this study show that perchlorate enhances SR calcium release at voltages (depolarization to 0 mV and above) where its effects on the intramembrane charge transfer are negligible. This confirms that the site of action of the drug has to be, at least in part, intracellular, thus strengthening the possibility that the changes in the voltage dependence of intramembrane charge movement are due to an altered interaction of the DHPR and RyR.
Both perchlorate and caffeine have been shown to enhance the late Iγ or ‘hump’ component of charge movement currents in amphibians (Csernoch et al. 1987; Szücs et al. 1991; González & Ríos, 1993; Huang, 1998a,b). In our experiments, delayed components were not observed either in control or in the presence of these drugs, similar to other measurements on mammalian fibres studied under reference conditions (e.g. Simon & Beam, 1985; Hollingworth et al. 1990). Among the several possibilities that might explain this discrepancy between the two species the most likely is that Iγ, although present, was faster under our experimental conditions and therefore merged into the early part of the charge movement currents. Iγ was indeed reported to be faster in double Vaseline gap systems than under microelectrode voltage clamp (Hui & Chandler, 1991; Pizarro et al. 1991) and, moreover, the temperature used in this study, 16-18°C, was significantly higher than those used for frogs.
Perchlorate has been shown to alter the kinetics of charge movement currents during repolarization (‘off’ tails) in amphibians (Lüttgau et al. 1983; Csernoch et al. 1987; Huang, 1987; Györke & Palade, 1992). This slowing effect of the drug was more prominent at higher (8–10 mm) concentrations (Huang, 1987; Györke & Palade, 1992), but it has been demonstrated at concentrations similar to that used in this study (Huang, 1998a). The prolongation of ‘off’ tails was not prominent in these experiments (Fig. 7), but a slight increase in the time constant of charge transfer (from 4.0 ± 0.1 to 6.2 ± 0.5 ms; P < 0.01) was indeed observed. The difference between the effects of perchlorate in rats and frogs is especially noticeable, since the effect was linked to the presence of a mechanical interaction between the key molecules of ECC (Ríos et al. 1993). Taken together with the absence of delayed components, this observation suggests that the interaction of RyRs, or the released calcium, with the voltage sensor might be different in the two classes of vertebrates.
Kinetics of SR calcium release
The SR permeability increase displays an early peak followed by a decline to a steady value in both mammalian and amphibian skeletal muscle. The drugs studied in these experiments did not alter this fundamental feature of SR calcium release (Figs 1–3).
An emerging difference between the two classes of vertebrates is that the peak-to-steady level ratio remains constant over a wide voltage range for mammals, while it has a distinct maximum at intermediate voltages in amphibians (Shirokova et al. 1996). This study confirms that this voltage-independent peak-to-steady level ratio is present under control conditions and extends the observation to include the effects on the ratio of both perchlorate and caffeine (Fig. 6). Together with results obtained using tetracaine (Csernoch et al. 1999), these data strongly suggest that the two kinetic components are tightly coupled in mammalian muscle.
Although perchlorate did not influence the peak-to-steady level ratio at the concentration used, caffeine caused a slight but significant increase in the ratio (Fig. 6). This increase, together with the observation that higher concentrations of tetracaine suppress the ratio (Csernoch et al. 1999), suggests that pharmacological interventions are capable of interfering with the tight coupling between the components of SR calcium release.
It has to be pointed out, however, that both perchlorate and caffeine increased both components of SR calcium release. This observation resembles the data described for the drugs in frogs (González & Ríos, 1993 and Klein et al. 1990, respectively), although the magnitude of response differs to some extent. The difference is more fundamental in the case of perchlorate, where the increase in steady level was much more pronounced at smaller depolarizations in frogs (González & Ríos, 1993). The underlying mechanism is not clear but apart from basic species differences the perchlorate concentration applied (8 mmvs. the 3 mm used in this study) might explain the different observations. The latter explanation is favoured since in frogs 8 mm perchlorate not only shifted the voltage dependence of intramembrane charge but increased its slope as well.
Changes in the transfer function
To describe the relationship between the amount of charge moved and SR calcium release channels opened, the transfer functions (Fig. 8) were fitted with a straight line. Only those points where the increase in SR permeability was detectable were included in the fit. This interpretation is similar to that used by Melzer and co-workers (1986b), who found a linear relationship between peak SR calcium release and suprathreshold charge movement (see also González & Ríos, 1993). Others (Simon & Hill, 1992) used a quadratic equation (RμQ4) based on the morphological data that junctional T-tubule tetrads face SR feet structures (Block et al. 1988).
To see if selecting unity as the power was valid, the least squares fits were repeated using
| (2) |
where R and Q are the normalized SR permeability and intramembrane charge, respectively, Qth is the threshold charge for initiating calcium release, m is a proportionality coefficient and n is the power representing the number of charged particles involved in opening one RyR channel.
If only those points were included in the fit where detectable SR permeability increase was measured, n was found to be between 0.8 and 1.2, justifying our choice of fitting a straight line. It should be noted that neither perchlorate nor caffeine altered n significantly. Furthermore, fitting eqn (2) to the data instead of a straight line did not influence the observation that perchlorate increased the coupling, that is the value of m (from around 1.2 to 1.7), while leaving Qth essentially unaltered (0.101 vs. 0.093). Caffeine, again in line with the interpretation in Results, decreased Qth and did not alter m.
Including all points in the fit and thus setting Qth to zero gave values of n in the range of 1.4–1.6. This is still lower than the value of 4 used by Simon & Hill (1992) based on a structure of four DHPR forming a T-tubule tetrad opposite the RyR calcium release channel.
Control of SR calcium release channel opening in mammalian muscle
One of the key observations in this study is that perchlorate augmented the SR permeability increase at all voltages. This indicates that perchlorate either increased the calcium flux through an open channel or it increased the number of open channels. The former mechanism, however, seems unlikely. Perchlorate did not influence the single channel current of reconstituted calcium release channels (Ma et al. 1993) and SR permeability was calculated by normalizing the calcium flux to the estimated SR content thus correcting for any change in the driving force of calcium through an open channel.
The result thus suggests that more channels were opened in the presence of the drug. This observation is consistent with a model in which, under control conditions, some RyRs do not open even though the facing DHPRs are in the activating conformation. Upon addition of perchlorate these, or some of these, ‘reluctant’ RyRs became activated.
Given this framework, a possible course of events in the triadic junction and the effects of perchlorate and caffeine could be summarized as follows. The conformational change of the DHPR induces the opening of the facing RyR channel via an allosteric interaction (Ríos et al. 1993). This interaction is not one-to-one, that is, some RyRs remain closed (‘reluctant’ to open) although the connecting DHPR has changed its conformation. The presence of perchlorate makes the interaction more efficient, and thus the number of ‘reluctant’ channels decreases. This more efficient coupling might also appear as a negative shift in the voltage dependence of intramembrane charge transfer (Fig. 7; Ríos et al. 1993).
The opening of the RyR channel presumably triggers the opening of additional calcium release pathways to give rise to all or part of the inactivating component of the SR permeability increase. The effects of caffeine on the transfer function and on the peak-to-steady level ratio are in line with an increase in this secondary opening. The secondary opening should be very tightly coupled to the first, as neither perchlorate (this study) nor tetracaine at low concentrations (Csernoch et al. 1999) influence the peak-to-steady level ratio even though they increase or decrease SR calcium release at every membrane potential. The tight coupling is reflected in the voltage-independent peak-to-steady level ratio of SR permeability and in the observation that caffeine did not affect the repolarization-induced closing of calcium channels (Fig. 9). The obvious candidate to mediate this process would be the released calcium, but recent confocal measurements have questioned the role of calcium-induced calcium release in mammalian skeletal muscle (Shirokova et al. 1998).
Acknowledgments
The authors wish to thank Ms R. Öri for skilful technical assistance. This work was supported by Hungarian research grants (OTKA T16957 and T030246, ETT095/1996, FKFP1289/1997).
References
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. Journal of Cell Biology. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L, Jacquemond V, Schneider MF. Microinjection of strong calcium buffers suppresses the peak of calcium release in frog skeletal muscle fibers. Journal of General Physiology. 1993;101:297–333. doi: 10.1085/jgp.101.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L, Kovács L, Szücs G. Perchlorate and the relationship between charge movement and contractile activation in frog skeletal muscle fibres. The Journal of Physiology. 1987;390:213–227. doi: 10.1113/jphysiol.1987.sp016695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L, Szentesi P, Sárközi S, Szegedi C, Jona I, Kovács L. Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibres. The Journal of Physiology. 1999;515:843–857. doi: 10.1111/j.1469-7793.1999.843ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, Ríos E. Perchlorate enhances transmission in skeletal muscle excitation-contraction coupling. Journal of General Physiology. 1993;102:373–421. doi: 10.1085/jgp.102.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Palade P. Effects of perchlorate on excitation- contraction coupling in frog and crayfish skeletal muscle. The Journal of Physiology. 1992;456:443–451. doi: 10.1113/jphysiol.1992.sp019345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A, Lüttgau HC, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. Journal of Muscle Research and Cell Motility. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Marshall MW, Robson E. The effect of tetracaine on charge movement in fast twitch rat skeletal muscle fibres. The Journal of Physiology. 1990;421:633–644. doi: 10.1113/jphysiol.1990.sp017966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL-H. ‘Off’ tails of intramembrane charge movements in frog skeletal muscle in perchlorate-containing solutions. The Journal of Physiology. 1987;384:491–509. doi: 10.1113/jphysiol.1987.sp016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL-H. The influence of perchlorate ions on complex charging transients in amphibian striated muscle. The Journal of Physiology. 1998a;506:699–714. doi: 10.1111/j.1469-7793.1998.699bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL-H. The influence of caffeine on intramembrane charge movements in intact frog striated muscle. The Journal of Physiology. 1998b;512:707–721. doi: 10.1111/j.1469-7793.1998.707bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CS, Chandler WK. Qβ and Qγ{FONT size components of intramembranous charge movement in frog cut twitch fibers. Journal of General Physiology. 1991;98:429–464. doi: 10.1085/jgp.98.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V, Csernoch L, Klein MG, Schneider MF. Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophysical Journal. 1991;60:867–873. doi: 10.1016/S0006-3495(91)82120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Schneider MF. Effects of caffeine on calcium release from the sarcoplasmic reticulum in frog skeletal muscle fibres. The Journal of Physiology. 1990;425:599–626. doi: 10.1113/jphysiol.1990.sp018120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Szücs G, Schneider MF. Simultaneous recording of calcium transients in skeletal muscle using high and low affinity calcium indicators. Biophysical Journal. 1988;53:971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L, Ríos E, Schneider MF. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. The Journal of Physiology. 1983;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L, Szentesi P, Csernoch L. Effects of perchlorate and caffeine on excitation-contraction coupling in mammalian skeletal muscle. Biophysical Journal. 1999;76:A299. doi: 10.1111/j.1469-7793.1999.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau HC, Kovács L, Gottschalk G, Fuxreiter M. How perchlorate improves excitation-contraction coupling in skeletal muscle fibers. Biophysical Journal. 1983;43:247–249. doi: 10.1016/S0006-3495(83)84346-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Anderson K, Shirokov R, Levis R, González A, Karhanek M, Hosey MM, Meissner G, Ríos E. Effects of perchlorate on the molecules of excitation-contraction coupling of skeletal and cardiac muscle. Journal of General Physiology. 1993;102:423–448. doi: 10.1085/jgp.102.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Ríos E, Schneider MF. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. The Journal of Physiology. 1986a;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Schneider MF, Simon BJ, Szûcs G. Intramembrane charge movement and calcium release in frog skeletal muscle. The Journal of Physiology. 1986b;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares EB, Tanksley SJ, Airey JA, Beck C, Ouyang Y, Deerinck TJ, Ellisman MH, Sutko JL. Nonmammalian vertebrate skeletal muscles express two triad junctional foot protein isoforms. Biophysical Journal. 1991;59:1153–1163. doi: 10.1016/S0006-3495(91)82331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G, Csernoch L, Uribe I, Rodríguez M, Ríos E. The relationship between Qγ and Ca release from the sarcoplasmic reticulum in skeletal muscle. Journal of General Physiology. 1991;97:913–947. doi: 10.1085/jgp.97.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E, Karhanek M, Ma J, González A. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. Journal of General Physiology. 1993;102:449–481. doi: 10.1085/jgp.102.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E, Pizarro G. Voltage sensors and calcium channels of excitation-contraction coupling. News in Physiological Sciences. 1988;3:223–228. [Google Scholar]
- Rousseau E, Ladine J, Liu Q-Y, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Archives of Biochemistry and Biophysics. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Sárközi S, Szentesi P, Cseri J, Kovács L, Csernoch L. Concentration-dependent effects of tetracaine on excitation-contraction coupling in frog skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1996;17:647–656. doi: 10.1007/BF00154059. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Simon BJ, Szücs G. Depletion of calcium from the sarcoplasmic reticulum during calcium release in frog skeletal muscle. The Journal of Physiology. 1987;392:167–192. doi: 10.1113/jphysiol.1987.sp016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, García J, Pizarro G, Ríos E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. Journal of General Physiology. 1996;107:1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, García J, Ríos E. Local calcium release in mammalian skeletal muscle. The Journal of Physiology. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BJ, Beam KG. Slow charge movement in mammalian skeletal muscle. Journal of General Physiology. 1985;85:1–19. doi: 10.1085/jgp.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BJ, Hill DA. Charge movement and SR calcium release in frog skeletal muscle can be related by a Hodgkin-Huxley model with four gating particles. Biophysical Journal. 1992;61:1109–1116. doi: 10.1016/S0006-3495(92)81920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Mechanism of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. The Journal of Physiology. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Jacquemond V, Kovács L, Csernoch L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. The Journal of Physiology. 1997;505:371–384. doi: 10.1111/j.1469-7793.1997.371bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szücs G, Csernoch L, Magyar J, Kovács L. Contraction threshold and the ‘hump’ component of charge movement in frog skeletal muscle. Journal of General Physiology. 1991;97:897–911. doi: 10.1085/jgp.97.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]


