Abstract
Proteins that interact with the intracellular carboxy termini of neurotransmitter- and voltage-gated ion channels are known to control the subcellular localization of the channels, localize other proteins near those channels, and modulate channel activity. By contrast, little is known about the control of neurotransmitter transporter function by interacting proteins.
To competitively disrupt interactions of the C- and N-termini of the GLAST glutamate transporter with other proteins, we dialysed whole-cell patch-clamped retinal glia with peptides identical to the eight amino acids at the C- or N-termini of the transporter, and compared the effect on transporter-mediated currents with dialysis of scrambled versions of the same peptides.
Dialysis with the N-terminus peptide had no effect on the maximum glutamate-evoked current nor on the glutamate affinity of the transporter. Dialysis with the C-terminus peptide had no effect on the maximum current, but increased the affinity of the transporter for glutamate (compared with scrambled C-terminus peptide, and with N- and scrambled N-terminus peptides: Km decreased from 16 to 11 μm)).
These data suggest that disruption of an interaction between an intracellular protein and the last eight amino acids of the GLAST C-terminus, which have some similarity to the PDZ binding domain of ion channel C-termini, increases the glutamate affinity of GLAST. Thus, the interacting protein decreases the affinity of GLAST transporters.
Removing the GLAST C-terminus interaction increases the transporter current by 40% at low glutamate concentrations. Thus, this interaction may significantly slow the removal of low concentrations of glutamate from the extracellular space, and affect the kinetics of retinal cell light responses.
Interaction of voltage-gated K+ channels, and of neurotransmitter receptors, with other proteins may control their subcellular localization and activity, as well as locating molecules such as enzymes and IP3 receptors close to the channels or receptors (Kim et al. 1995; Kornau et al. 1995; Brenman et al. 1996; Dong et al. 1997; Horio et al. 1997; Tu et al. 1998; Garcia et al. 1998; Nishimune et al. 1998; Tezuka et al. 1999; Yamada et al. 1999). The last four to seven amino acids of the carboxy termini of many channels play a crucial role in these interactions (although more distant amino acids can also contribute: Bassand et al. 1999). The motif S/T-X-V/I, present at the C-terminus of some K+ channels and NMDA receptor subunits, allows binding of membrane proteins to the best characterised (PDZ) class of scaffolding protein, including the proteins PSD-95 (postsynaptic density-95), Dlg (Drosophila discs-large), and ZO-1 (zona occludens-1).
By contrast little is known about how neurotransmitter transporters interact with other proteins. Yeast two-hybrid experiments have suggested proteins that may interact with glutamate transporters (Lin et al. 1998; Marie et al. 1999), but functional effects of these proteins have not yet been reported. Nevertheless, such interactions may, as for ion channels, play an important role in glutamate transporter function, perhaps generating specific subcellular locations of the transporters (Chaudhry et al. 1995), or controlling their insertion into the membrane under the control of signalling systems (Davis et al. 1998). Glutamate transporters have both their N- and C-termini on the intracellular side of the cell membrane (Grunewald et al. 1998), and the neuronal EAAT5 transporter has a PDZ-binding motif at its C-terminus, suggesting that the C-terminus may be involved in protein-protein interactions (Arriza et al. 1997). The other glutamate transporters have C-termini which diverge from the strictly defined PDZ binding motif (see Discussion).
Here we use a strategy for detecting interaction of proteins with glutamate transporters which does not depend on identifying the interacting proteins. If peptides identical to the interacting part of a glutamate transporter are introduced into the cell, they should compete with the transporter for binding to the interacting protein (cf. Nishimune et al. 1998). If the interacting protein modulates transporter function, its displacement by the peptide should produce an alteration of glutamate transport. Using this approach we show for the first time that C-terminal interactions modulate a transporter's glutamate affinity.
METHODS
Glutamate transporter activity was monitored electrically, at 23-25°C, in glial (Müller) cells isolated enzymatically from the retinae of aquatic tiger salamanders (killed by stunning followed by destruction of the brain) as described by Brew & Attwell (1987).
External solution contained (mm): NaCl, 105; KCl, 2.5; CaCl2, 3; MgCl2, 0.5; glucose, 15; Hepes, 5; BaCl2 (to block inward rectifier K+ channels and improve signal to noise ratio), 6; pH adjusted to 7.4 with NaOH. The pipette solution for whole-cell clamping contained (mm): KCl, 95; NaCl, 5; Hepes, 5; MgCl2, 2; MgATP, 5; CaCl2, 1; K2EGTA, 5; pH set to 7 with KOH; salamander GLAST C- or N-terminal peptides or scrambled versions of them (see Results), 0.2; and peptidase inhibitors (to block peptide breakdown in the cell) bestatin, 0.01; pepstatin, 0.001; and leupeptin, 0.105. Pipette series resistance in whole-cell mode was around 4 MΩ, leading to series resistance voltage errors < 2 mV. Pipette junction potentials have been compensated for.
The time needed for peptides to diffuse into the cell, across an idealised barrier at the end of the pipette of width w and area A, can be calculated as follows. The rate of increase of peptide concentration C, in a cell of volume V is given by:
| (1) |
where D is the diffusion coefficient, and Cpipette is the peptide concentration in the pipette. The pipette series resistance across the barrier is:
| (2) |
where ρ is the resistivity of the pipette solution. From eqns (1) and (2),
Thus, C approaches Cpipette exponentially:
with a time constant of τ = VRs/(Dρ). For peptides with a molecular weight near 900, we estimated D= 2.6 × 10−10 m2 s−1 by interpolating (on a log-log graph of D against molecular weight) literature values for sucrose (MW = 342, D= 5.2 × 10−10 m2 s−1) and somatostatin (MW = 1638, D= 1.66 × 10−10 m2 s−1). For ρ = 0.8 Ωm, Rs= 4 MΩ, and a Müller cell volume of V= 10−14 m3, the predicted equilibration time constant is around 190 s.
Peptides were obtained from Immune Systems Limited (Paignton UK), were stored under argon (to prevent oxidation) at -20°C, and were made up freshly before experiments into pipette solution bubbled with argon. Experiments with C- and scrambled C-terminus peptides, or N- and scrambled N-terminus peptides, were carried out in an interleaved manner, with alternate cells clamped with pipette solution containing either the normal or scrambled peptide.
Data are presented as mean ±s.e.m. For glutamate dose-response data, a Michaelis-Menten curve, [Glu]oImax/([Glu]o+Km), where Imax is the maximum current and Km is the equilibrium dissociation constant, was fitted to data from each cell (Brew & Attwell, 1987) using the non-linear curve fitting routine in SigmaPlot (SPSS Inc.), and the resulting parameter values were averaged across cells, after normalising the maximum current by cell capacitance to reduce variation due to differing cell size (Barbour et al. 1991). Dose-response data in Fig. 1C and D were calculated for each cell normalised to the current obtained with 100 μm glutamate, averaged across cells (for each peptide), and then the data for each peptide were scaled to have the same maximum current at saturating glutamate concentration (since the maximum current/ capacitance was not significantly different for the different peptides).
Figure 1. Effect of including 8 amino acid long GLAST C- and N-terminal peptides in the whole-cell pipette on glutamate-evoked currents.
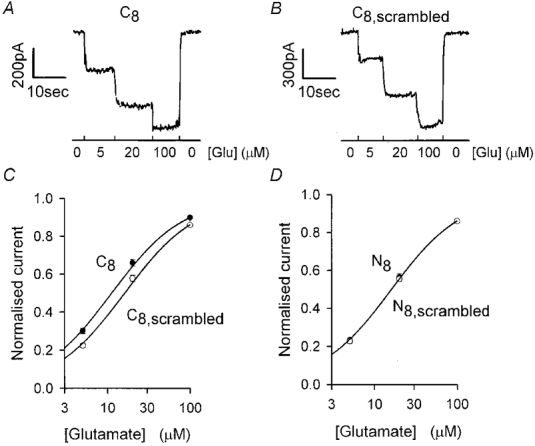
A, specimen data showing the current response of a Müller cell clamped to -63 mV with a pipette containing C-terminal peptide (last 8 amino acids: C8), during superfusion of a solution containing 5, 20 and 100 μm glutamate (record obtained 10 min after going to whole-cell mode). B, specimen data (as in A) from a cell with the pipette solution containing scrambled C-terminal peptide (C8,scrambled), normalised to the same current at 100 μm glutamate as for A for comparison. C, dose-response curves (mean current ±s.e.m., which is comparable to the symbol size) from data as in A and B (normalised and scaled as described in the Methods). Smooth curves are Michaelis-Menten curves with Km values of 16.1 μm for the scrambled C-terminus peptide and 11.2 μm for the C-terminus peptide, i.e. the mean values in Fig. 2 obtained from the 9 cells studied for each peptide; extrapolated maximum current at high [glutamate] is 1 for both curves. D, dose-response data as in C, but for pipette solutions containing N-terminus (N8) or scrambled N-terminus (N8,scrambled) peptides. Smooth curve is the same as for the scrambled C-terminus peptide in C.
RESULTS
Glutamate-evoked currents (Fig. 1A and B) in salamander retinal Müller glia are dominated by the activity of GLAST glutamate transporters, which take up extracellular glutamate released by retinal photoreceptors and bipolar cells (Brew & Attwell, 1987; Eliasof et al. 1998). These transporters generate a current due to the co-transport of 2 net positive charges with each glutamate taken up (Zerangue & Kavanaugh, 1996; Levy et al. 1998), and also a small current due to Cl− flux through an anion conductance that the transporter gates (Eliasof & Jahr, 1996; Billups et al. 1996) which is suggested to be proportional to the rate of glutamate uptake (Otis & Jahr, 1998).
The sequence of the salamander GLAST transporter has been reported by Eliasof et al. (1998). We compared the effect on the glutamate-evoked current of dialysing cells with pipette solutions containing a peptide (200 μm) with the sequence of the C- or N-terminus of the transporter or scrambled versions of them (see below). For quantitative comparison, data were measured 10 min after entering whole-cell mode to allow the peptides to enter the cell and compete with endogenous GLAST for binding to interacting proteins. In similar experiments Nishimune et al. (1998) found that 15 min dialysis of much larger neurons was sufficient for internally applied peptides to act.
Figure 1A and B shows specimen data from cells dialysed with C- and scrambled C-terminus peptides (PIDSETKM and MDKISTPE, respectively). Mean glutamate dose-response curves from such experiments are shown in Fig. 1C. Applying 5 and 20 μm glutamate to cells dialysed with C-terminus peptide evoked currents that were a larger fraction of the current evoked by 100 μm glutamate than those in cells dialysed with scrambled C-terminus peptide (P= 0.0016 for 5 μm and P= 0.034 for 20 μm, 9 cells studied for each peptide, Student's 2-tailed t test). By contrast, fractional responses to 5 and 20 μm glutamate in cells dialysed for 10 min with N- and scrambled N-terminal peptides (MTKSNGED and ENMDKGST, respectively) were not significantly different (Fig. 1D; P= 0.62 for 5 μm and P= 0.65 for 20 μm, 8-9 cells studied for each peptide).
Fitting a Michaelis-Menten curve to the dose-response data from each cell showed that the Km for glutamate activating a transporter current was not significantly different for intracellular dialysis with the N-, scrambled N- and scrambled C-terminus peptides, but was significantly reduced for the C-terminus peptide (Fig. 2A). The extrapolated maximum transporter current at high glutamate concentration (normalised by cell capacitance, a measure of cell membrane area) was not significantly different for all the peptides studied (Fig. 2B). Thus, dialysis with the C-terminus peptide specifically increases the glutamate affinity. Approximately 80 % of this affinity change had occurred 2 min after going to whole-cell mode (the earliest time at which we could measure the dose-response curve), suggesting that the values used for pipette peptide concentration and series resistance rapidly resulted in a sufficient concentration of peptide being achieved in the cell to displace endogenous proteins that bind to the GLAST C-terminus.
Figure 2. Effect of C- and N-terminal peptides on the Km and Imax of uptake.
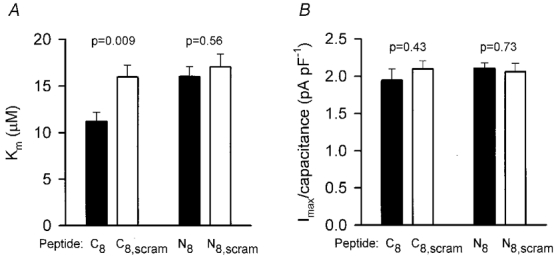
Mean values (±s.e.m.) of Km(A), and extrapolated maximum current at high [glutamate] normalised by cell capacitance (Imax/capacitance) (B), obtained from fitting Michaelis-Menten curves to dose-response data obtained as in Fig. 1, for pipettes filled with 8 amino acid peptides corresponding to the GLAST C- and N-termini (C8, N8), and scrambled versions of these peptides (C8,scram, N8,scram). P values shown are for 2-tailed t tests comparing data for normal and scrambled peptides.
The voltage dependence of the transporter current evoked by 100 μm glutamate was not significantly different when cells were dialysed with C- or scrambled C-terminus peptides (current at +20 mV/current at -80 mV was 0.129 ± 0.011 for C- and 0.134 ± 0.014 for scrambled C-terminus peptide, 7 cells each, P= 0.78), suggesting no effect on rate limiting steps of the carrier cycle involving transport of charge through the membrane field.
DISCUSSION
Dialysis of Müller cells with a peptide identical to the C-terminus of GLAST increases the affinity of GLAST for glutamate (compared with dialysis with scrambled C-terminus, N-terminus, or scrambled N-terminus peptides). Since this peptide should compete with endogenous GLAST C-terminus for binding to other proteins, the interacting proteins must normally decrease the affinity of GLAST for glutamate.
A shift of Km from 16.1 to 11.2 μm (Fig. 2A) implies a 40 % increase in transport current at low glutamate concentrations, which may significantly alter the time course of the extracellular glutamate concentration during the tail of a synaptic current. Signal transmission from bipolar cells to ganglion cells in the retina is profoundly affected by slowing the rate of glutamate uptake into Müller cells (Higgs & Lukasiewicz, 1999), with the ganglion cell response becoming greatly prolonged. Thus, the interaction we have characterised may play a role in controlling the temporal response characteristics of the retinal output. Conceivably the interaction may be removed under certain conditions, speeding retinal response kinetics.
The salamander (and human) GLAST C-terminus ends with the amino acids ETKM, which is similar to the sequence ESXV at the C-terminus of NMDA NR2 subunits. This suggests that the endogenous protein being displaced by dialysis with GLAST C-terminus peptide is likely to be in the same PDZ family as the PSD-95 protein which binds to NMDA receptors (although we cannot rule out the existence of other interacting molecules, nor the possibility that the GLAST C-terminus actually binds to part of the GLAST molecule itself as occurs for the N-termini of potassium channels: Hoshi et al. 1990). Two other members of the glutamate transporter family have related C-termini, the retinal EAAT5 ending with ETNV and the Purkinje cell EAAT4 ending with ESAM, which suggests that they may also have their affinity modulated by proteins interacting with their C-terminus. By contrast, the two other glutamate transporters, i.e. the commonest splice variant of the most abundant glial transporter GLT-1, and the neuronal EAAC1, have very different C-termini (KREK and TSQF, respectively), suggesting that they may interact with a different class of protein.
The cytoplasmic C-terminus of GLAST is unlikely to be directly involved in binding extracellular glutamate, indeed there is some evidence that the glutamate binding site is in the transporter's membrane pore loop region near residues 400-440 (Zhang & Kanner, 1999; Seal & Amara, 1998). However, chimaeric transporters in which the C-terminus of EAAT1 (the human equivalent of GLAST) is replaced by that of the lower affinity transporter EAAT2 show a decrease in glutamate affinity (Mitrovic et al. 1998), suggesting that the properties of the transporter C-terminus can modulate glutamate affinity, as is also suggested by our data. It will be interesting to see whether proteins identified as interacting with glutamate transporters (Lin et al. 1998; Marie et al. 1999) alter the glutamate affinity, as expected from our data, and whether these interactions are modulated to adjust the properties of the transporters to different situations.
Acknowledgments
This work was supported by the Wellcome Trust. Hélène Marie is in the Wellcome Trust Four Year PhD Programme in Neuroscience at UCL. We thank A. Gibb, M. Hamann, M. Hausser, P. Mobbs and R. Schoepfer for comments on the manuscript.
References
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences of the USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander retina. The Journal of Physiology. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M. Differential interaction of the tSXV motifs of the NR1 and NR2A NMDA receptor subunits with PSD-95 and SAP97. European Journal of Neuroscience. 1999;11:2031–2043. doi: 10.1046/j.1460-9568.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. Journal of Neuroscience. 1996;16:6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. Journal of Neuroscience. 1998;18:2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Arriza JL, Leighton BH, Kavanaugh MP, Amara SG. Excitatory amino acid transporters of the salamander retina: identification, localization, and function. Journal of Neuroscience. 1998;18:698–712. doi: 10.1523/JNEUROSCI.18-02-00698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, Jahr CE. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proceedings of the National Academy of Sciences of the USA. 1996;93:4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Bendahan A, Kanner BI. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron. 1998;21:623–632. doi: 10.1016/s0896-6273(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Lukasiewicz PD. Glutamate uptake limits synaptic excitation of retinal ganglion cells. Journal of Neuroscience. 1999;19:3691–3700. doi: 10.1523/JNEUROSCI.19-10-03691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. Journal of Biological Chemistry. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Levy L, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CLG, Jackson M, Jin L, Song I, Orlov W, Orlov I, Dykes-Hoberg M, Rothstein JD. Identification and characterization of Purkinje cell-specific glutamate transporter EAAT4 associated proteins (GTRAP4) Society for Neuroscience Abstracts. 1998;24:825.13. [Google Scholar]
- Marie H, Bedford F, Moss S, Attwell D. Looking for protein interactions with glutamate transporters. 1. Use of the yeast two-hybrid system to identify proteins that interact with the rat glutamate transporter GLT-1. The Journal of Physiology. 1999;518.P:19P. [Google Scholar]
- Mitrovic AD, Amara SG, Johnston GA, Vandenberg RJ. Identification of functional domains of the human glutamate transporters EAAT1 and EAAT2. Journal of Biological Chemistry. 1998;273:14698–14706. doi: 10.1074/jbc.273.24.14698. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. Journal of Neuroscience. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron. 1998;21:1487–1498. doi: 10.1016/s0896-6273(00)80666-2. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proceedings of the National Academy of Sciences of the USA. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Chochi Y, Takamiya K, Sobue K, Inui M. Modulation of the channel activity of the epsilon2/zeta1-subtype N-methyl D-aspartate receptor by PSD-95. Journal of Biological Chemistry. 1999;274:6647–6652. doi: 10.1074/jbc.274.10.6647. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kanner BI. Two serine residues of the glutamate transporter GLT-1 are crucial for coupling the fluxes of sodium and the neurotransmitter. Proceedings of the National Academy of Sciences of the USA. 1999;96:1710–1715. doi: 10.1073/pnas.96.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]


