Abstract
We have examined whether there are changes during inflammation in the membrane properties of nociceptive primary afferent neurones in the guinea-pig that might contribute to hyperalgesia. Inflammation was induced by intradermal injections of complete Freund's adjuvant (CFA) in the left leg. Intracellular voltage recordings were made from the somata of ipsilateral L6 and S1 dorsal root ganglion neurones in anaesthetised untreated guinea-pigs at 2 or 4 days after CFA treatment.
Units were classified as C, Aδ or Aα/β on the basis of their dorsal root conduction velocities (CVs). Units with receptive fields on the left leg were characterized as nociceptive, low- threshold mechanoreceptive (LTM) or unresponsive according to their responses to mechanical and thermal stimuli. The shapes of their somatic action potentials (APs) evoked by dorsal root stimulation were recorded.
Comparisons of data from nociceptive neurones recorded in CFA treated animals after 2 and 4 days with data from CFA untreated (control) animals showed the following significant changes: in C-fibre nociceptors, decreased AP duration at base, AP rise time and AP fall time, and increased maximum rates of AP rise and fall with no change in afterhyperpolarization measured to 80 % recovery (AHP80); in Aδ-fibre nociceptors, decreased AP duration at base, AP fall time and a reduction in AHP80; and in Aα7sol;β-fibre nociceptors, a decreased AHP80 but no change in AP duration. Apart from a more negative membrane potential and AHP depth below 0 mV in Aα/β nociceptors at 4 days compared with 2 days post-CFA, none of the above variables differed significantly between units recorded 2 or 4 days after CFA. Therefore the two groups were pooled and called CFA2+4d.
The reduction in AP duration in C-fibre nociceptors was apparent both in high threshold mechanoreceptor and polymodal nociceptors and also in units with either cutaneous or subcutaneous receptive fields.
No significant changes in AP duration at base or AHP80 were seen 2 or 4 days after CFA compared with control in either LTM or unresponsive neurones, although some of the latter may have become classified as nociceptors after CFA treatment.
The alterations in membrane properties of nociceptors should permit higher discharge frequencies, thus contributing to inflammatory hyperalgesia. They suggest active changes in the expression or activation of cation channels during peripheral inflammation.
Increased pain resulting from noxious or innocuous stimuli is a hallmark of inflammation. This hypersensitivity is the consequence of modifications at more than one level of the nociceptive pathway to the brain, including increased responsiveness of the peripheral terminals of nociceptive primary afferent neurones (peripheral sensitization) and changes in the central nervous system (CNS) (central sensitization) (Perl, 1985; Reeh et al. 1987; Treede et al. 1992; McMahon et al. 1993). Both these phenomena have been well studied and are thought to contribute to inflammatory hyperalgesia (see Millan, 1999). The somatic membranes of nociceptive primary afferent neurones of all conduction velocity (CV) ranges normally have both longer AP and longer AHP durations than those of LTM neurones (Koerber et al. 1988; Djouhri et al. 1998). However, the possibility that nociceptive primary afferent neurones may, during peripheral inflammation, alter their electrophysiological membrane properties in vivo in a way that may increase their ability to carry information to the CNS has not previously been studied.
We have therefore investigated whether AP configurations of physiologically identified dorsal root ganglion (DRG) neurones are altered during unilateral, localized inflammation induced by complete Freund's adjuvant (CFA) injections into one hind limb in the guinea-pig and whether any such changes are limited to nociceptive neurones. Brief reports of these studies have been presented in abstract form (Djouhri et al. 1997).
METHODS
Animals and complete Freund's adjuvant (CFA) treatment
Young female Dunkin-Hartley guinea-pigs (weight 180-300 g) were used. In the CFA treated group a unilateral hindlimb inflammation was induced by two intradermal injections, each of 70 μl of CFA (Sigma), one into the plantar surface of the left hind paw and one into the lateral region of the left knee under anaesthesia with 4 % halothane 2 days (CFA2d) or 4 days (CFA4d) prior to the electrophysiological recordings. These injections were within the cutaneous receptive fields of L6 and S1 DRGs.
Control animals
The control group had no CFA treatment (no CFA). To test whether the halothane used as an anaesthetic during CFA administration might affect the data, four no CFA animals were given halothane, two at 2 days and two at 4 days prior to electrophysiological experiments. AP and AHP80 durations were unchanged in these animals compared with no CFA animals that were not given halothane. Data from these no halothane animals were therefore included in the no CFA group.
Most animals were given the muscle relaxant gallamine during electrophysiological experiments (see below). However, in seven animals (one CFA2d, two CFA4d, and four no CFA), recordings in nociceptive units were made in the absence of gallamine in order to check whether the known K+ channel blocking activity of gallamine (Dunn et al. 1996) might affect the variables being measured. From these no-gallamine experiments, there were at least three nociceptive units in each CV group, both in CFA treated and no CFA animals. Where the number of units (n) was 4 to 7 (in no CFA C and Aδ groups, and in CFA Aδ and Aα/β groups) there was no significant difference in medians of any of the variables indicated in Fig. 1A (Mann-Whitney U test) compared with data of the same type from animals that were given gallamine during recording. Data from gallamine-free experiments were therefore pooled with those from experiments in which gallamine was used.
Figure 1. Examples of APs recorded from nociceptive neurones.
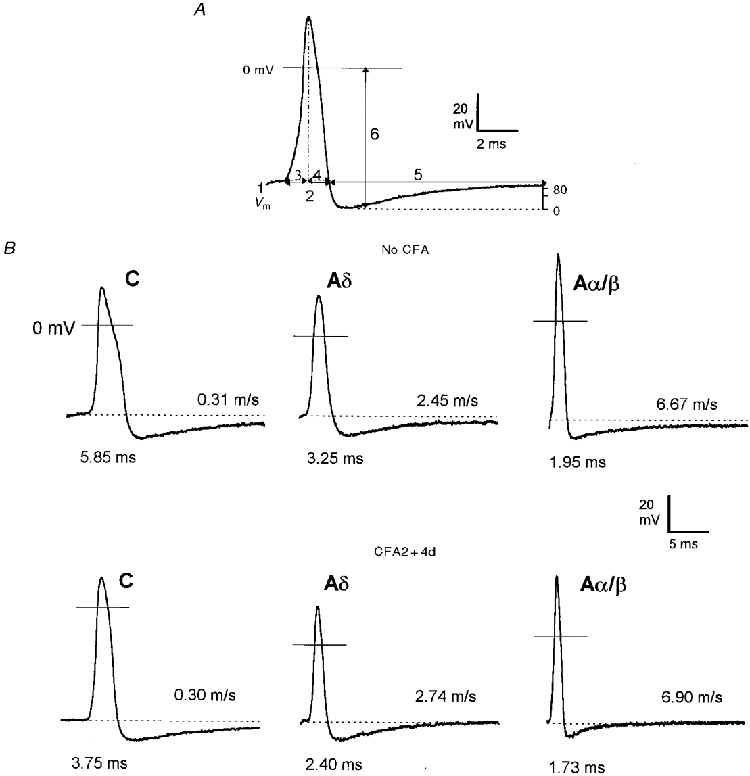
A, an intracellularly recorded somatic AP of an A-fibre neurone evoked by dorsal root stimulation showing the AP variables measured. (1) membrane potential (Vm), (2) AP duration at base, (3) AP rise time, (4) AP fall time, (5) AHP duration to 80 % recovery (AHP80) and (6) AHP depth below 0 mV. In addition, maximum rates of rise and fall of the AP, (dV/dt)max, were measured from a differential trace of the AP. B, somatic APs evoked by dorsal root stimulation and recorded intracellularly from 6 nociceptive neurones selected to represent the mean AP duration values for C (left) Aδ (middle) and Aα/β (right) groups in no CFA (top panels) and CFA2 + 4d (bottom panels) animals. The CV and AP duration at base for each unit are given to the right of, and below, each AP respectively. Note the decreased AP duration in all CV groups and decreased AHP80 duration in Aδ- and Aα/β-fibre neurones in the CFA2 + 4d group compared with the no CFA group.
Electrophysiology
Animals were anaesthetized initially with sodium pentobarbitone (50 mg kg−1, i.p). As this produced deep anaesthesia that depressed ventilation, a tracheostomy was performed immediately after induction of anaesthesia to allow artificial ventilation and continuous monitoring of end-tidal CO2. The left carotid artery was then cannulated to permit intra-arterial (i.a.) injection of drugs. Deep anaesthesia (i.e. totally lacking reflex responses (areflexic), judged by absence of limb withdrawal reflex) was maintained thereafter with supplementary doses of anaesthetic (10 mg kg−1, i.a.) each hour. Core temperature was maintained at 36 ± 0.5°C. The vertebral column was clamped at the L1 vertebra. A laminectomy allowed exposure of the left (ipsilateral) L6 and S1 DRGs. Adipose tissue over the DRG was removed with care so as not to damage any superficial blood vessels. The DRG was stabilized with a silver platform inserted beneath it. The dorsal root was sectioned near its entry to the spinal cord. When surgery had been completed and during electrophysiological recording, all animals, except for ‘no-gallamine controls’ described above, were paralysed with gallamine triethiodide (Flaxedil) (2 mg kg−1, i.a.), accompanied by 10 mg kg−1 of the anaesthetic. In order to ensure anaesthesia remained as deep as during the 3 h period of surgery, supplementary doses of pentobarbitone were given at the same rate as during the period of surgery (10 mg kg−1 each hour), each dose being accompanied by a supplementary dose of 2 mg kg−1 of gallamine triethiodide. In experiments in which blood pressure was recorded, it remained stable throughout the period of paralysis when the same anaesthetic regimen was used. Areflexia was also maintained in the no-gallamine controls that received supplementary pentobarbitone at 10 mg kg−1 each hour during the recording period. In order to test the underlying level of anaesthesia, occasionally the effects of the muscle relaxant were allowed to wear off, by delaying the dose by 30 min. For most cells from which we recorded, this resulted in loss of the cell as soon as the dorsal root was stimulated. This instability is due to muscle contraction in response to the spread of the stimulus and is especially marked with the high-intensity stimuli needed to activate C-fibre units. Nonetheless, the animal still remained completely areflexic (no response to pinch of the forelimb), indicating that the effects of the anaesthetic considerably outlasted the effects of the muscle relaxant.
Intracellular recordings from somata in DRGs were made under liquid paraffin maintained at 30°C (range ±2°C, mean 30°C) with glass micropipettes filled with 1 M KCl (80-100 MΩ), Lucifer Yellow CH (approximately 5 mg ml−1 in 0.1 M LiCl solution; 200-500 MΩ) or ethidium bromide (6 mm in 1 M KCl; 80-140 MΩ). The frequency response of the recording system including a 500 MΩ electrode was flat (±3 %) between 700 Hz and 7 kHz. The microelectrode was advanced until a membrane potential (Vm) was seen and an AP could be evoked by stimulation of the dorsal root with bipolar platinum electrodes. These electrodes delivered single 0.03 ms or 0.3 ms rectangular pulses, adjusted to twice threshold voltage for A-fibre units and suprathreshold for C-fibre units. APs were recorded on-line with a Cambridge Electronic Design (CED) 1401plus interface and SIGAV program and were subsequently analysed with the SpikeII program (CED). The conduction velocity (CV) was estimated from the latency to the rise of the dorsal root evoked AP and the conduction distance between the recording site in the ganglion and the stimulating cathode (typically 4-7 mm). Utilization time was not taken into account in calculating CVs.
AP variables and acceptance criteria
The AP variables measured are illustrated in Fig. 1A. For Table 1 and all the figures except Fig. 4, only cells with a stable Vm more negative than -40 mV and an overshooting somatic AP were included; in addition A-fibre units were accepted only if they also had a negative AHP. Furthermore, because of the very small number of C LTM units, two units (one untreated and one after CFA) with non-overshooting APs, but with AP amplitudes > 40 mV, were included. Except for unresponsive neurones, all units had a receptive field on the leg. All regions of the leg, that is, toes, foot, heel, ankle and knee regions showed clear signs of inflammation. However, units with receptive fields on the hip or trunk were excluded, as they did not appear to be inflamed. Receptive fields included in the analyses were within 1 cm of an injection site except the heel which was about 2 cm from both injection sites but which, nonetheless, showed clear signs of inflammation.
Table 1.
Effect of CFA treatment on nociceptive DRG neurones
| CV | Days post CFA | n | Vm(mV) | AP duration at base (ms) | AP rise time (ms) | AP fall time (ms) |
|---|---|---|---|---|---|---|
| P= 0.14 | P <0.0001*** | P= 0.0022** | P 0.0003*** | |||
| C | No CFA | 45 | 45.4 (41.3–49.5) | 5.5 (3.9–7.0) | 2.23 (1.7–2.8) | 3.1(2.2–3.9) |
| C | 2 + 4 | 17 | 48.0 (43.6–52.6) | 3.7 (3.1–4.0) | 1.63 (1.1–2.0) | 1.8 (1.6–2.4) |
| P= 0.58 | P= 0.009** | P= 0.14 | P= 0.0065** | |||
| Aδ | No CFA | 33 | 47.9 (43.2–52.4) | 2.75 (2.3–3.3) | 1.1 (0.96–1.4) | 1.55 (1.32–1.88) |
| Aδ | 2 + 4 | 21 | 47.4 (41.0–55.5) | 2.35 (2.0–2.5) | 1.03 (0.87–1.14) | 1.35 (1.2–1.4) |
| P= 0.34 | P= 0.08 | P= 0.21 | P= 0.057 | |||
| Aα/β | No CFA | 38 | 49.5 (44.5–56.0) | 2.03 (1.5–2.4) | 0.82 (0.6–1.04) | 1.18 (0.9–1.35) |
| Aα/β | 2 + 4 | 36 | 46.6 (42.7–54.6) | 1.79 (1.28–2.1) | 0.75 (0.53–0.98) | 1.0 (0.81–1.14) |
| CV | Days post CFA | n | Max rate of rise (V s−1) | Max rate of fall (V s−1) | AHP depth below Vm (mV) | AHP duration to 80% (ms) |
|---|---|---|---|---|---|---|
| P= 0.017* | P= 0.0005*** | P= 0.0396 | *P= 0.71 | |||
| C | No CFA | 45 (38) | 228 (178–302) | 131 (99–164) | 7.95 (4.7–12) | 17.6 (12.5–21.4) |
| C | 2 + 4 | 17 | 329 (256–373) | 169 (152–205) | 11.4 (6.5–13.2) | 16.3 (12.5–20.5) |
| P= 0.099 | P= 0 13 | P= 0.72 | P= 0.029* | |||
| Aδ | No CFA | 33 | 376 (265–430) | 211 (181–241) | 9.8 (5.0–12.2) | 13.5 (9.7–21.8) |
| Aδ | 2 + 4 | 21 | 399 (355–514) | 225 (202–267) | 11.3 (7.5–12.3) | 11.2 (9.35–12.7) |
| P= 0.067 | P= 0.0712 | P= 0.25 | P= 0.003** | |||
| Aα/β | No CFA | 38 | 405 (309–493) | 251 (225–312) | 8.1 (7.2–11.3) | 14.3 (8.3–23.0) |
| Aα/β | 2 + 4 | 36 | 460 (409–619) | 294 (241–351) | 7.2 (4.7–11.3) | 8.4 (6.2–12.2) |
A comparison of the medians of variables measured in no CFA animals and in CFA2+4d animals. As an indication of the range of the values, the 25% percentile and the 75% percentile values are given in parentheses. The overall value of P(Mann-Whitney) is shown above each section. The asterisks show the level of significance of the difference between units from no CFA and CFA2+4d animals. The number of units in each group is given under n. In parentheses the lower number (C-fibre units, no CFA) in the lower part of the Table refers to the AHP data since 7 of these C-fibre units had no negative AHPs. Note that the Table is split into two and refers to the same units in both top and bottom halves of the Table.
Figure 4. Effects of CFA on AP duration in subgroups of C-fibre nociceptive units.
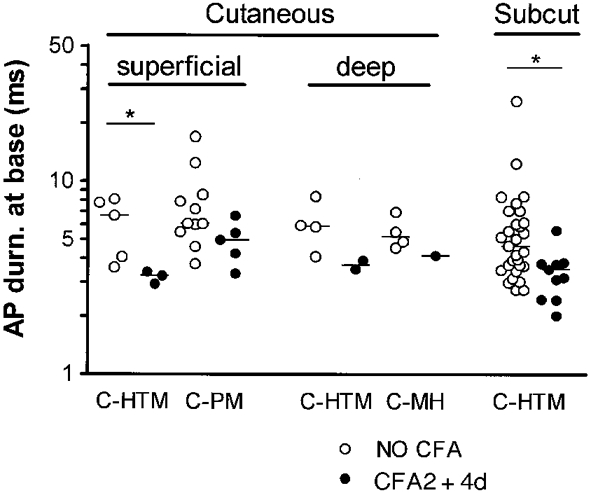
For this analysis only, units with Vm values equal to, or more negative than -35 mV were all included, and units from animals weighing 170-180 g were also included. They all had overshooting APs. Units were subdivided according to their responses to noxious mechanical and noxious heat stimuli as well as to probable depth of receptive fields in the tissues. Units were classed as having superficial cutaneous (probably epidermal or at the dermal-epidermal junction) receptive fields, deep cutaneous receptive fields (probably in dermis since they required pinch of a fold of skin to be activated), or subcutaneous (Subcut) receptive fields (requiring squeezing of deeper tissue such as fascia, muscle, bone, tendon to be activated), as previously described (Lawson et al. 1997). Of the units that responded to both noxious mechanical and noxious heat stimuli, the superficial cutaneous units were designated C-polymodal (C-PM) and deep cutaneous units were called C-mechanoheat (C-MH) units. HTM means high threshold mechanoreceptor with no response to a single application of heat, although subcutaneous units may include subcutaneous C-MH units since they were not tested with heat. The reduction in AP duration is apparent in all the subgroups shown, although there are too few units for statistical significance in the cutaneous groups.
More units were needed to examine the subgroups of C-fibre nociceptive units. AP and AHP80 durations in C-fibre neurones appear unaffected by the inclusion of units with Vm values as low as -30 mV (Djouhri et al. 1998; Fig 3 and Fig 4). Therefore, for this comparison only, all units with Vm values equal to, or more negative than, -35 mV and units from animals weighing 170-180 g were also included.
Figure 3. Effects of CFA treatment in nociceptive neurones.
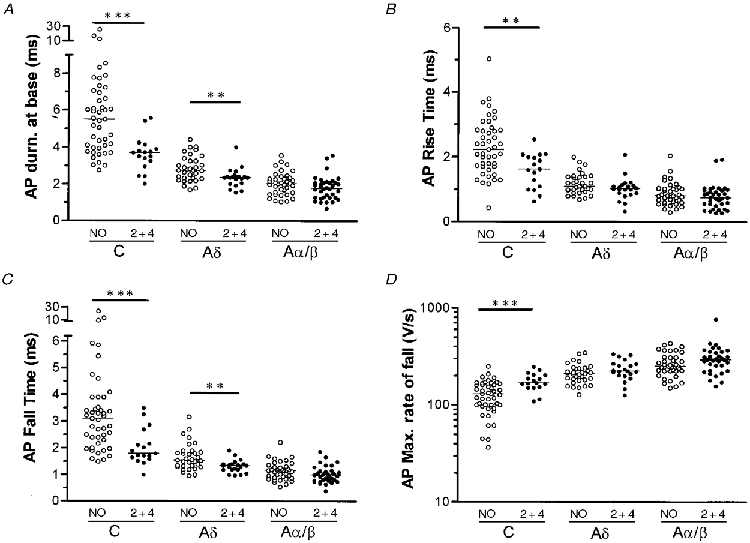
Scatterplots to show the effects of CFA treatment on the AP duration at base (A), the maximum rate of fall (D), AP rise time (B) and AP fall time (C) in C-, Aδ- and Aα/β-fibre nociceptive neurones. NO means no CFA and 2 + 4 means CFA injection 2 or 4 days prior to experiments. In each case the median (horizontal line) is superimposed. Asterisks above the graphs indicate the level of significance of any difference between the no CFA and CFA2 + 4d treated group (see Methods), no asterisks indicates no significant differences.
Sensory receptive properties
The sensory receptive properties of units were examined with hand-held stimulators and classified as previously described (Lawson et al. 1997; Djouhri et al. 1998). Three main groups of neurones were identified. (1) Low threshold mechanoreceptive (LTM) units were identified using soft brush, light pressure with a blunt object, light tap, vibration, pressure with Von Frey hairs and cooling (ice or a brief spray of ethyl chloride). (2) Nociceptive units were identified using noxious pinch with fine forceps or coarse-toothed forceps, sharp objects (needle) and heat (hot water at > 50°C or heated glass rod). Nociceptive units included: (a) high threshold mechanoreceptor (HTM) units that responded only to strong mechanical stimulation, (b) moderate pressure units that responded weakly to moderate pressure but more vigorously to strong mechanical stimulation and (c) units that responded to both strong mechanical stimuli and also promptly to a single application of noxious heat. (3) Unresponsive neurones were those not excited by any of the non-noxious or noxious stimuli listed above. The receptive properties of all subgroups of responsive neurones were easily recognizable in both no CFA and CFA treated animals, although one C-fibre unit in a CFA2 + 4d animal could not be easily classified as LTM or nociceptive, and this unit was excluded. For details of classification of subgroups of C-fibre nociceptors see legend to Fig. 4. Heat nociceptors and specific cooling receptors were not included in this study. Also, a few units with elevated mechanical thresholds that showed a response to cooling as vigorous as, or more vigorous than, their response to noxious mechanical stimuli were excluded.
Animals were killed with an overdose of anaesthetic. Experimental procedures complied throughout with Home Office Guidelines.
Statistical analyses
The non-parametric Mann-WhitneyU test was used to compare the medians of two groups of variables. Non-parametric tests were used in these cases because in some groups the data did not show a Gaussian distribution. All tests were carried out with Prism 3 software (Graphpad). The levels of significance are indicated above the graphs and in Table 1 as follows: *P < 0.05, **P < 0.01, ***P < 0.001. In Table 1, medians are shown, and variability is indicated by giving the 25 % percentile and 75 % percentile value for each data set.
RESULTS
Neurones were classified in both treated and untreated animals according to their dorsal root CVs as C (< 1.1 m s−1), Aδ (1.1-4.2 m s−1) and Aα/β (> 4.2 m s−1), established from compound APs in normal animals as previously described (Djouhri et al. 1998). These values are low because of the young age, the low temperature in the paraffin pool, the lower CVs in dorsal root than peripheral nerve fibres, and inclusion of utilization time (Waddell et al. 1989; Lawson et al. 1997; Djouhri et al. 1998). Although preliminary results of compound action potential recordings (Djouhri & Lawson, 1998) indicate that the upper limit of the Aδ wave (onset of the wave) is higher than 4.2 m s−1 after CFA treatment, the same upper limit (4.2 m s−1) was used in CFA and no CFA animals (see Discussion).
CFA treatment produced an area of localized erythema and oedema with a mean increase in girth of the ipsilateral foot compared with the contralateral foot of 19.9 ± 5.4 % at 2 days (n= 18) and 20 ± 5.8 % at 4 days (n= 11) after treatment.
Nociceptive neurones
The medians of all these variables obtained in CFA2d animals were compared with those from CFA4d animals. The n values for 2 and 4 days respectively, were 10 and 7 for C units, 16 and 5 for Aδ units, and 24 and 12 for Aα/β units. Apart from a more negative median Vm in Aα/β neurones at 2 than at 4 days (see later), no significant differences for any of the variables shown on Table 1 were found in any CV group, and the medians in 2 or 4 days tended to be close. For example the median values for AP duration at base were as follows for 2 or 4 days respectively: C-fibre units, 3.73 and 3.5 ms; Aδ-fibre units, 2.38 and 2.35 ms; and Aα/β units, 1.78 and 1.78; and the median AHP80 values were: for C-fibre units, 15.9 and 16.9 ms; for Aδ, 11.5 and 10 ms; and for Aα/β, 8.2 and 8.3 ms. Therefore the two groups were pooled for all variables in Table 1 and called CFA2 + 4d.
Selection bias
The present experiments were consciously biased towards nociceptive units by rejection of some Aα/β LTM units. The relatively high number of Aα/β nociceptive units may result from a bias towards recording from neurones with larger somata which would cause over-representation of Aα/β nociceptors and under-representation of C-fibre units. Others have, however, also found substantial numbers of Aα/β nociceptive units (Lynn & Carpenter, 1982; Ritter & Mendell, 1992).
Action potential variables
Examples of APs recorded in no CFA and in CFA2 + 4d animals are shown in Fig 1B and Fig 2. Comparisons between variables from no CFA and CFA2 + 4d animals are shown in Table 1 and Fig. 3. In C-fibre nociceptors, there were significantly lower median values in CFA2 + 4d animals compared with no CFA animals for the following variables: AP duration at base, AP rise time and AP fall time, and significantly higher values for AP maximum rate of rise and AP maximum rate of fall. In Fig. 4 the reduction in AP duration is apparent in all subgroups of C-fibre nociceptors examined (see legend). In CFA2 + 4d animals, three C-fibre nociceptive units with cutaneous receptive fields over the hip were examined. This region is outside the inflamed area, and these data were excluded from all analyses in this paper. The AP duration in these units ranged from 5.4-8.6 ms, that is, there was no evidence of the changes seen in units with receptive fields on the inflamed leg.
Figure 2. Variability in somatic AP shape of nociceptive neurones.
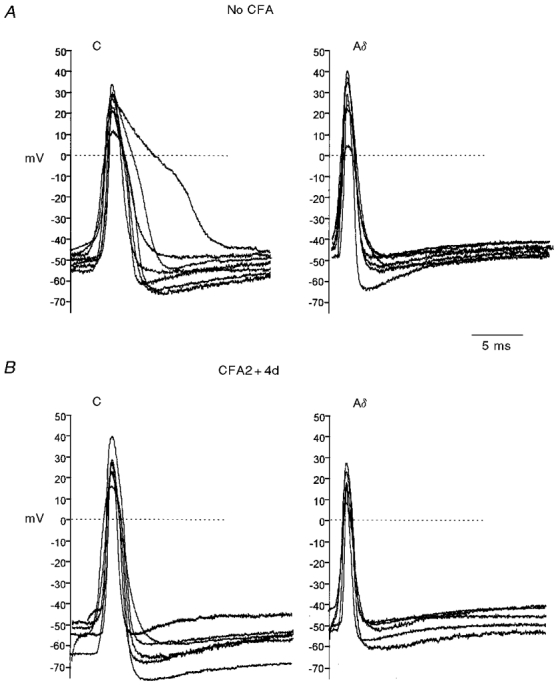
Somatic APs evoked intracellularly by dorsal root stimulation in C and Aδ nociceptive neurones from no CFA (A) and CFA2 + 4d (B) groups to show the distribution of AP shapes. APs are aligned vertically on the basis of their Vm prior to the stimulus artefact which is omitted. In each case the data were sorted according to AP duration at base, then units were selected at regular intervals throughout the data set and APs from these units were superimposed with their peaks aligned.
In Aδ-fibre nociceptors but not in Aα/β nociceptors, significantly reduced AP duration and AP fall time in CFA2 + 4d compared with no CFA animals was seen (Table 1, Fig. 3).
Afterhyperpolarization duration and depth
The duration of the afterhyperpolarization to 80 % recovery (AHP80) was significantly lower in CFA2 + 4d animals compared with no CFA animals in Aδ and highly significantly in Aα/β nociceptors (Table 1, Fig. 5A and examples in Fig. 1B).
Figure 5. Effects of CFA treatment on AHP80 duration.
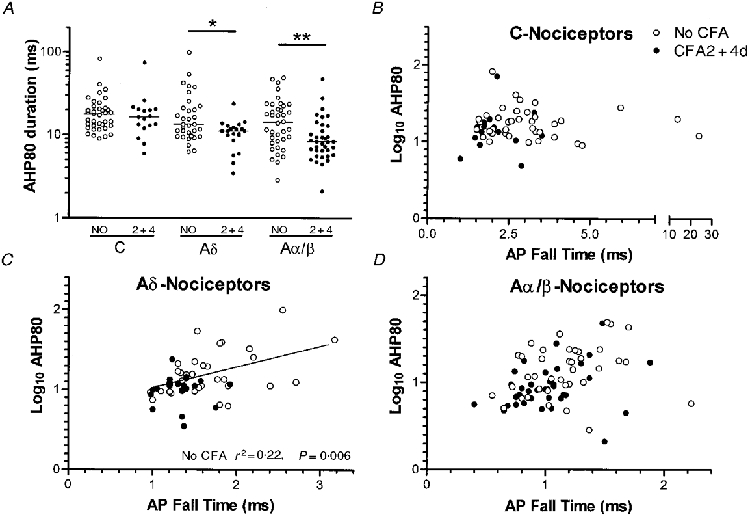
In A, scatterplots show the distribution of AHP80 values from no CFA and CFA 2 or 4 days animals. Details and statistics as in Fig. 3. In B-D, log10 AHP80 was plotted against AP fall time. Linear regression analysis was carried out on each group of nociceptive neurones, and where P < 0.05, the regression line is shown, and the P value and r2 value are given on the graph. In no CFA animals there was a significant correlation in Aδ nociceptors with an r2 of 0.22 indicating either some influence of AP fall time on AHP80 duration, or some factor that is common to both in these neurones. The shortened AP fall time may therefore have contributed to the shorter AHP80 in these units after CFA treatment.
The depth of the AHP below Vm was not significantly altered in any CV group of nociceptive neurones (not shown). The depth of the AHP below 0 mV was, however, significantly more negative in CFA2 + 4d than in no CFA animals in C-fibre nociceptors (Table 1), but in Aα/β-fibre nociceptors it was significantly (P= 0.012) more negative in CFA2d (59.9, n= 24) than in CFA4d animals (52.6, n= 12). In both cases the change in the median was matched by a change in Vm of a similar magnitude and direction (CFA2d: Vm 50.9, n= 24; CFA4d: Vm 42.6, n= 12; P= 0.015) and thus the alteration in AHP depth below Vm may be secondary to a less reduced Vm.
Since the fall time may influence the Ca2+ entry and thus the AHP80 duration, the relationship between AP fall time and log10 AHP80 duration was examined for nociceptors in each CV group in no CFA and in CFA2 + 4d animals (Fig. 5B -D). There was no correlation for C or Aα/β nociceptive cells in either group. The significant correlation found in Aδ nociceptors in no CFA animals was absent in CFA2 + 4d animals.
Possible influences other than CFA
In these experiments there was a narrow range of temperature at the DRG (29-32°C) and a greater range of animal weights (180-300 g). Values for animal weight and temperature at the DRG were available for each neurone in Table 1. The means ±s.d. of these weights and temperatures are given below and do not differ significantly (unpaired two-tailed t test) between no CFA and CFA2 + 4d nociceptors. For no CFA and CFA2 + 4d groups of nociceptive neurones the means for weight were: C-fibre units: 226 ± 26 and 229 ± 27 g, P= 0.77; Aδ units: 224 ± 24.6 and 224 ± 22.1 g, P= 0.98; and Aα/β units: 223 ± 29.4 and 230 ± 23.2 g, P= 0.25, respectively. The means for temperature were: C-fibre units: 30.2 ± 0.51 and 30.2 ± 0.45°C, P= 0.76; Aδ-fibre units: 30.2 ± 0.63 and 30.2 ± 0.46°C, P= 0.71; and Aα/β units: 30.33 ± 0.45 and 30.39 ± 0.51°C, P= 0.61. Furthermore, there was no significant correlation between AP duration at base or AHP80 and either temperature or animal weight for no CFA and CFA2 + 4d groups of nociceptive units in either C, Aδ or Aα/β CV ranges. As described in Methods the AP and AHP80 durations in nociceptive units were not influenced by the halothane anaesthesia during CFA injection, or by gallamine during recording sessions.
LTM and unresponsive neurones
There were no significant differences in the medians of Vm, AP duration at base and AHP80 (Fig. 6) between no CFA and CFA2 + 4d LTM units for either Aδ- or Aα/β-fibre LTM neurones. The only C-fibre LTM in a CFA treated animal (CFA2d) had values close to the medians for C LTMs in untreated animals (Fig. 6).
Figure 6. Effects of CFA treatment in LTM and unresponsive neurones.
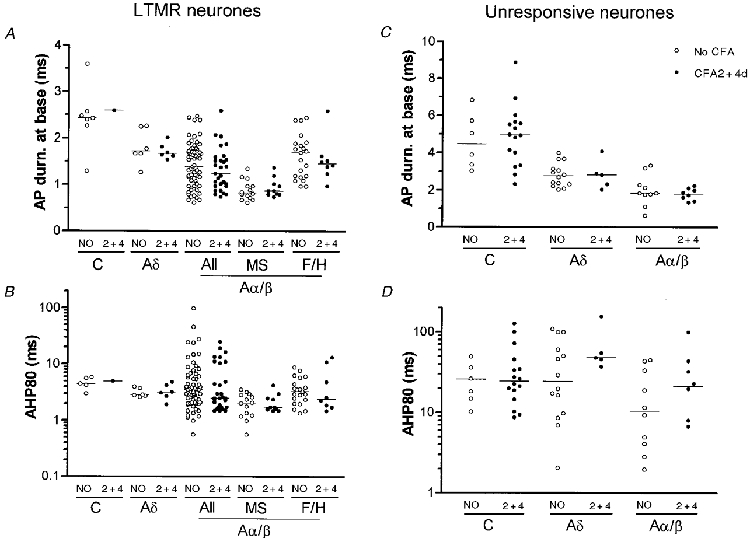
Scatterplots show the effects of CFA treatment on the AP duration at base (A and C) and the AHP80 (B and D) in LTM (A and B) and unresponsive (C and D) units. Details as in Fig. 4. All Aα/β LTM units are shown, as well as examples of subgroups of Aα/β LTM units: muscle spindle (MS) units, and field/hair (F/H) units. These were classified as previously described (Djouhri et al. 1998). No significant difference was found between medians of Vm, AP duration at base and AHP80 measured in no CFA and CFA2 + 4d units for any of the variables shown.
In unresponsive units, there were no significant differences between CFA2 + 4d and no CFA animals in the medians of the above variables (Fig. 6), although there may have been a (not significant) reduction in the number of Aδ units with the shorter AHP80 durations after CFA.
DISCUSSION
This is the first study to examine the active membrane properties of physiologically identified DRG neurones during peripheral inflammation. The results demonstrate clear changes in somatic AP configuration in nociceptive but not in LTM primary afferent neurones in guinea-pig.
Nociceptive neurones
The changes in membrane properties of nociceptive DRG neuronal somata during CFA induced peripheral inflammation include shorter duration APs in C- and Aδ-fibre nociceptive neurones with decreased rise times in C-fibre nociceptors, decreased fall times in C- and Aδ-fibre units, and shorter duration AHP80s in Aδ- and Aα/β-fibre units. These changes were not due to: variations in temperature at the DRG, animal weight, halothane anaesthesia for CFA injections, or the presence of gallamine during recording. Thus, they must have resulted from the CFA induced inflammation. Indeed, that the changes seen were due to the inflammation in CFA2 + 4d animals is supported by the finding that the AP durations in C-nociceptive units with receptive fields outside the inflamed region (over the hip) were similar to those in untreated animals. It is unlikely that all sites on the leg were equally affected by the CFA, and some of the scatter in the variables in CFA2 + 4d animals may result in part from different distances of receptive fields from the injection sites.
Although an increased incidence of nociceptive neurones with spontaneous activity was noted in CFA2 + 4d animals, the frequency of firing was low and would be unlikely to have influenced the AP variables measured. Indeed, multiple APs would be expected to cause accumulation of intracellular Ca2+ and thus greater activation of Ca2+ dependent K+ channels (KCa) (see later), which would be likely to cause increased duration of AHP80s, not the reduced duration that we observed in A-fibre nociceptors.
Of the responsive units, with receptive fields in the inflamed region, it was those that normally would have the longest AP durations (C- and Aδ-fibre nociceptive neurones) that showed the greatest proportional decrease in AP duration after CFA. Interestingly it seems that all subtypes of C-fibre neurones (including HTMs and C polymodal nociceptors, and units with either superficial or deeper receptive fields) showed evidence of a reduced AP duration. The distance of the soma from the receptive terminal (5-10 cm) makes it unlikely that the local mechanisms responsible for short term sensitization of nociceptive neurones in the periphery (see Treede et al. 1992; Millan, 1999) are responsible for the changes seen here.
Despite the increased upper limit of Aδ CVs after CFA (Djouhri & Lawson, 1998) we chose to use the same upper limit for no CFA and CFA treated animals. This was justified because: (a) it would have had no effect on AHP80 duration per se since there was no significant difference in AHP80 duration in Aδ and Aα/β neurones in untreated animals and (b) it would tend to diminish, rather than increase, the changes in AP duration seen in nociceptive neurones, because the faster Aδ units would presumably be most likely to become reclassified as Aα/β after CFA. These faster Aδ units tend to have shorter AP durations than the more slowly conducting Aδ units and longer AP durations than the Aα/β units (e.g. Waddell & Lawson, 1990). Thus the real changes in AP duration in A-fibre units may be greater than we report here.
It seems likely that the changes in membrane properties include alterations in level or activity of proteins involved in pathways regulating cation channels, e.g. channel subunits, cAMP dependent protein kinases, protein kinase C or G proteins (see Catterall, 1992). Since the AP rise time was faster in C-fibre nociceptors after CFA and since voltage gated Na+ channels are kinetically the fastest channel type contributing to the AP, an overall increase in Na+ channel density, or relative alterations in levels of Na+ channels with differing kinetics (Schild & Kunze, 1997) or modulation of pre-existing Na+ channels could contribute to the reduced AP duration. Na+ channel α subunit protein was reported to increase after CFA treatment, peaking within 1-2 days post-CFA treatment in rat DRG neurones (Gould et al. 1998). The SNS/PN3 Na+ channel α subunit (αSNS) (Akopian et al. 1996; Sangameswaran et al. 1996) carries a TTX-R Na+ current, contributing to long duration APs in small, probably nociceptive, DRG neurones (Akopian et al. 1996; Sangameswaran et al. 1996). In small DRG neurones dissociated after induction of inflammation in vivo, both an upregulation (Tanaka et al. 1998) (carageenan), and no upregulation (CFA) (Okuse et al. 1997) of αSNS mRNA have been reported. Therefore it remains unclear whether changes in SNS expression are likely to contribute to the AP duration changes reported here. Alterations of expression or activation of other types of ion channel may also contribute to the changes seen. The rate of decay (i.e. duration) of the long AHPs is thought to depend on Ca2+ influx (Sah, 1996) affecting KCa (Nowycky, 1992; Sah, 1996). AP shape (duration/rate of fall) may therefore affect AHP duration since more Ca2+ may enter during a long duration AP. Interestingly, the correlation between fall time and log10 AHP duration in Aδ nociceptors in no CFA animals was lost in CFA2 + 4d animals, indicating that the reduction of the AP fall time in these Aδ neurones may have contributed to the reduced AHP80 duration, or that some common factor may contribute to both. One possible factor is BKCa (one of the KCa channels likely to influence AHP duration) activation of which can cause a reduction in AP duration in small DRG neurones in rat (Scholz et al. 1998). The alteration in AP duration and AP fall time but not AHP duration in C-fibre nociceptors appears to indicate that these two variables alter independently, a view supported by the lack of correlation between fall time and log10 AHP80 in C- and Aα/β-fibre nociceptors in both no CFA and CFA2 + 4d units, and the loss after CFA treatment of the weak correlation seen normally in Aδ nociceptors. Several types of voltage dependent K+ channels are present in different types of DRG neurones some of which can also reduce AP duration as well as contributing to short AHPs (Nowycky, 1992; Safronov et al. 1996). For instance, the presence of I(H) (a hyperpolarization-activated current) in DRG neurones has been correlated with larger somata that have short duration APs (Scroggs et al. 1994). In addition, Ca2+ channels may affect both AP duration (since a Ca2+ inward current is associated with kinetically slow APs) and AHP duration (via the effects of intracellular Ca2+ on KCa channels). Thus there may be complex interactions between the effects of altered ion currents on AP shapes and AHP durations, and it is too early to predict exactly which types of channel are involved.
Growth factors may be important in regulating the changes described here. Nerve growth factor (NGF) is upregulated during inflammation (Donnerer et al. 1992; Woolf et al. 1994) and plays an important role in inflammatory hyperalgesia (Woolf et al. 1994). NGF has been shown in mouse to increase (acute NGF treatment; Shen & Crain, 1994), or decrease (chronic NGF treatment; Chalazonitis et al. 1987) the AP duration in cultured DRG neurones, consistent with the possibility that longer term NGF effects may contribute to the decreased AP duration in the present experiments. However, the small increase in AP duration in A-fibre nociceptors in rat (Ritter & Mendell, 1992) seen after long term (5 week) NGF treatment in vivo is hard to reconcile with the above, and while it is possible that the increase in AP duration may be related to the effects of neonatal NGF treatment on nociceptive neurones during a critical period of development, species differences cannot be ruled out.
Axotomy deprives the DRG somata of NGF and should therefore cause changes that are opposite to any changes during inflammation that are NGF dependent. Indeed peripheral nerve axotomy caused broadening of the somatic AP in DRG neurones of all CV groups in vivo (Kim et al. 1998; Stebbing et al. 1999) in contrast to the present CFA study. Since this change is in the opposite direction to that seen in the present study, it seems that NGF could play a role in regulating AP duration. Axotomy caused an increased AHP depth but no change in duration in A-fibre neurones (Kim et al. 1998; Stebbing et al. 1999). In contrast inflammation in the present study resulted in a decreased AHP depth (perhaps secondary to altered Vm) in Aα/β-fibre nociceptive neurones, but a decreased duration in A-fibre nociceptive neurones. At present it therefore seems more likely that NGF plays a role in regulating AP rather than AHP duration during inflammation in the guinea-pig.
Low threshold mechanoreceptive units
There was no detectable change in any of the variables measured in LTM units of Aδ or Aα/β neurones. There was only one C-fibre LTM in the CFA2 + 4d group, and this had values close to those of the no CFA group. Thus either there were no changes in LTM neurones, or any changes were too small to be significant. Since the AP and AHP kinetics are already relatively fast in LTM neurones, there may be no mechanisms for speeding these up significantly.
Unresponsive units
Unresponsive units (sometimes called ‘silent’ or ‘sleeping’ units) have been described in several species (Bessou & Perl, 1969; Pini et al. 1990; Handwerker et al. 1991; Meyer et al. 1991; Schmelz et al. 1994; Gee et al. 1996; Djouhri et al. 1998). Their long AP and AHP80 durations indicate that most were probably nociceptor-type units with very high thresholds (see Djouhri et al. 1998). The absence of AP configuration changes in these units after CFA was therefore surprising and may be due to units falling into one or more of the following categories: (a) units unaffected by the inflammation, (b) units with inaccessible receptive fields; (c) units with receptive properties undetected by our search stimuli and (d) units normally unresponsive but becoming sensitized by CFA (see Cohen & Perl, 1990; Davis et al. 1993) and becoming classified as nociceptive after CFA. One interpretation of the present data is that the unresponsive units with the shorter duration AHP80s might be the units that become sensitized, leaving only those units with very long AHP80s as unresponsive after the CFA treatment. Such sensitized units must have either had relatively fast AP durations normally, or undergone a reduction in AP duration similar to that in nociceptive neurones (compare Fig 3 and Fig 6). Such sensitization must have occurred in only a proportion of normally unresponsive units because unresponsive neurones with long AP and AHP80 durations remained after CFA treatment.
Possible relevance to hyperalgesia of changes in nociceptors
We predict that at least some of these changes would also occur in the fibres and possibly the terminals, since the properties of soma and fibre membranes in individual neurones are known to show certain similarities. These include adaptation properties (Harper, 1991), a correlation between maximum following frequency of soma and fibre (Lawson et al. 1996), a correlation between the AHP duration in the soma and the fibre maximum following frequency (Waddell & Lawson, 1990), and AP duration in both the fibres (Gee et al. 1999) and somata (Djouhri et al. 1998) of C cells being longer in nociceptive than in LTM units. Decreased mean AP and AHP80 durations in nociceptive neuronal somata during peripheral inflammation would allow repetitive firing at higher than normal frequencies. Indeed, preliminary unpublished data (L. Djouhri and S. N. Lawson) from guinea-pigs in this laboratory show faster maximum fibre following frequencies after CFA treatment by at least 2- to 3-fold, not only in Aδ-fibre, but also in C-fibre nociceptors in response to electrical stimulation of the nerve, indicating that there are also changes in fibre membrane properties. Decreased AP durations in jellyfish presynaptic terminals result in a larger amplitude, more transient, inward Ca2+ current accompanied by an increase in the size of the post-synaptic junctional potential despite a decreased total Ca2+ entry (Spencer et al. 1989). Thus increased post-synaptic signal per AP may well accompany a reduced AP duration, if this occurs, in the central terminals of DRG neurones. This effect could be enhanced by the increased availability of transmitters or modulators, such as substance P, during inflammation (Donnerer et al. 1992; Woolf et al. 1994).
In summary, in guinea-pig DRG neurones, decreased somatic AP and AHP durations are seen in nociceptive but not LTM neurones 2 or 4 days after CFA treatment. Unresponsive neurones, while remaining apparently unaltered, may include a subgroup that becomes sensitized and thus classified as nociceptive after CFA treatment. The changes seen in nociceptive neurons may contribute to inflammatory hyperalgesia. Understanding the mechanisms underlying these changes could be important in the development of peripherally targeted novel analgesics.
Acknowledgments
This work was supported by the Wellcome Trust UK. Thanks for technical assistance go to Carol Berry and Barbara Carruthers.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibres. Journal of Neurophysiology. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiological Reviews. 1992;72:S15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Peterson ER, Crain SM. Nerve growth factor regulates the action potential duration of mature sensory neurons. Proceedings of the National Academy of Sciences of the USA. 1987;84:289–293. doi: 10.1073/pnas.84.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RH, Perl ER. Contributions of arachidonic acid derivatives and substance P to the sensitization of cutaneous nociceptors. Journal of Neurophysiology. 1990;64:457–464. doi: 10.1152/jn.1990.64.2.457. [DOI] [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. Journal of Neurophysiology. 1993;69:1071–1081. doi: 10.1152/jn.1993.69.4.1071. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Reduced action potential (AP) duration in nociceptive dorsal root ganglion (DRG) neurones projecting to inflamed tissue in guinea-pigs. The Journal of Physiology. 1997;504.P:100P. [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurons. The Journal of Physiology. 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Increase in conduction velocity (CV) of nociceptive dorsal root ganglion (DRG) neurones during hind limb inflammation. Society for Neuroscience Abstracts. 1998;24:883–350.6. [Google Scholar]
- Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth in vivo. Neuroscience. 1992;49:693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- Dunn PM, Benton DC, Campos Rosa J, Ganellin CR, Jenkinson DH. Discrimination between subtypes of apamin-sensitive Ca2+-activated K+ channels by gallamine and a novel bis-quaternary quinolinium cyclophane, UCL 1530. British Journal of Pharmacology. 1996;117:35–42. doi: 10.1111/j.1476-5381.1996.tb15151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Basile S, Pierau FK, Cotsell B. The relationship between axonal spike shape and functional modality in cutaneous C-fibres in the pig and rat. Neuroscience. 1999;90:509–518. doi: 10.1016/s0306-4522(98)00454-0. [DOI] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Gould HJ, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund's adjuvant. Brain Research. 1998;802:69–74. doi: 10.1016/s0006-8993(98)00568-x. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. The Journal of Physiology. 1991;435:229–242. doi: 10.1113/jphysiol.1991.sp018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA. Similarities between some properties of the soma and sensory receptors of primary afferent neurones. Experimental Physiology. 1991;76:369–377. doi: 10.1113/expphysiol.1991.sp003504. [DOI] [PubMed] [Google Scholar]
- Kim YI, Na HS, Kim SH, Han HC, Yoon YW, Sung B, Nam HJ, Shin SL, Hong SK. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience. 1998;86:301–309. doi: 10.1016/s0306-4522(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. Journal of Neurophysiology. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Correlation of substance P-like immunoreactivity with sensory receptor type in guinea-pig primary afferent neurones. The Journal of Physiology. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, McCarthy PW, Prabhakar E. Electrophysiological properties of neurones with CGRP-like immunoreactivity in rat dorsal root ganglia. Journal of Comparative Neurology. 1996;365:355–366. doi: 10.1002/(SICI)1096-9861(19960212)365:3<355::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Research. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. Current Opinion in Neurobiology. 1993;3:602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Research. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Progress in Neurobiology. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Nowycky MC. Voltage-gated ion channels in dorsal root ganglion neurons. In: Scott SA, editor. Sensory Neurons Diversity, Development and Plasticity. New York, Oxford: Oxford University Press; 1992. pp. 97–115. [Google Scholar]
- Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Molecular Cell Neuroscience. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- Perl ER. Unravelling the story of pain. Advances in Pain Research Therapy. 1985;9:1–29. [Google Scholar]
- Pini A, Baranowski R, Lynn B. Long-term reduction in the number of C-fibre nociceptors following capsaicin treatment of a cutaneous nerve in adult rats. European Journal of Neuroscience. 1990;2:89–97. doi: 10.1111/j.1460-9568.1990.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Bayer J, Kocher L, Handwerker HO. Sensitization of nociceptive cutaneous nerve fibers from the rat's tail by noxious mechanical stimulation. Experimental Brain Research. 1987;65:505–512. doi: 10.1007/BF00235973. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: Effects of nerve growth factor. Journal of Neurophysiology. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. The Journal of Physiology. 1996;493:393–408. doi: 10.1113/jphysiol.1996.sp021391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends in Neurosciences. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. Journal of Biological Chemistry. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. Journal of Neurophysiology. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Ringkamp M, Handwerker HO, Torebjork HE. Sensitization of insensitive branches of C nociceptors in human skin. The Journal of Physiology. 1994;480:389–394. doi: 10.1113/jphysiol.1994.sp020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. The Journal of Physiology. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation in I(H), I(IR), and I(LEAK) between acutely isolated adult rat dorsal root ganglion neurons of different size. Journal of Neurophysiology. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Nerve growth factor rapidly prolongs the action potential of mature sensory ganglion neurons in culture, and this effect requires activation of Gs-coupled excitatory kappa-opioid receptors on these cells. Journal of Neuroscience. 1994;14:5570–5579. doi: 10.1523/JNEUROSCI.14-09-05570.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer AN, Przysiezniak J, Acosta-Urquidi J, Basarsky TA. Presynaptic spike broadening reduces junctional potential amplitude. Nature. 1989;340:636–638. doi: 10.1038/340636a0. [DOI] [PubMed] [Google Scholar]
- Stebbing MJ, Eschenfelder S, Habler H, Acosta MC, Janig W, McLachlan EM. Changes in the action potential in sensory neurones after peripheral axotomy in vivo. NeuroReport. 1999;10:201–206. doi: 10.1097/00001756-199902050-00001. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. NeuroReport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Progress in Neurobiology. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Lawson SN. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurones in vitro. Neuroscience. 1990;36:811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Lawson SN, McCarthy PW. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience. 1989;30:577–584. doi: 10.1016/0306-4522(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Safiehgarabedian B, Ma QP, Crilly P, Winters J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]


