Abstract
We have shown that the endocannabinoid anandamide and its stable analogue methanandamide relax rings of rabbit superior mesenteric artery through endothelium-dependent and -independent mechanisms that are unaffected by blockade of NO synthase and cyclooxygenase.
The endothelium-dependent component of the responses was attenuated by the gap junction inhibitor 18α-glycyrrhetinic acid (18α-GA; 50 μm), and a synthetic connexin-mimetic peptide homologous to the extracellular Gap 27 sequence of connexin 43 (43Gap 27, SRPTEKTIFII; 300 μm). By contrast, the corresponding connexin 40 peptide (40Gap 27, SRPTEKNVFIV) was inactive.
The cannabinoid CB1 receptor antagonist SR141716A (10 μm) also attenuated endothelium-dependent relaxations but this inhibition was not observed with the CB1 receptor antagonist LY320135 (10 μm). Furthermore, SR141716A mimicked the effects of 43Gap 27 peptide in blocking Lucifer Yellow dye transfer between coupled COS-7 cells (a monkey fibroblast cell line), whereas LY320135 was without effect, thus suggesting that the action of SR141716A was directly attributable to effects on gap junctions.
The endothelium-dependent component of cannabinoid-induced relaxation was also attenuated by AM404 (10 μm), an inhibitor of the high-affinity anandamide transporter, which was without effect on dye transfer.
Taken together, the findings suggest that cannabinoids derived from arachidonic acid gain access to the endothelial cytosol via a transporter mechanism and subsequently stimulate relaxation by promoting diffusion of an to adjacent smooth muscle cells via gap junctions.
Relaxations of endothelium-denuded preparations to anandamide and methanandamide were unaffected by 43Gap 27 peptide, 18α-GA, SR141716A, AM404 and indomethacin and their genesis remains to be established.
An endothelium-derived hyperpolarizing factor (EDHF) is now widely recognized to mediate endothelium-dependent vascular relaxations that are independent of nitric oxide (NO) and prostanoid synthesis (Mombouli & Vanhoutte, 1997). Although the chemical identity of EDHF remains controversial, there is accumulating evidence that this mediator normally effects relaxation following diffusion from the endothelium to smooth muscle via myoendothelial gap junctions rather than the extracellular space (Chaytor et al. 1998; Taylor et al. 1998; Dora et al. 1999; Hutcheson et al. 1999). Anatomically, this hypothesis is supported by the demonstration of myoendothelial gap junction plaques in rabbit conduit arteries (Spagnoli et al. 1982), and functional dye transfer experiments confirm direct chemical coupling between endothelium and subjacent smooth muscle (Little et al. 1995). Gap junctions are membrane structural proteins which consist of two hemichannels or connexons contributed by apposing cells, with each connexon being formed from six protein subunits or connexins arranged around an aqueous central pore that permits intercellular transfer of electrical current and molecules < 1 kDa in size (Yeager & Nicholson, 1996). Connexin 43 (Cx43) is present in both endothelial and vascular smooth muscle cells, and EDHF-mediated relaxations and hyperpolarizations of rabbit superior mesenteric artery are attenuated by a short synthetic peptide homologous to a specific region of the second extracellular loop of this connexin (43Gap 27, SRPTEKTIFII) (Chaytor et al. 1997, 1998; Dora et al. 1999; Hutcheson et al. 1999). The precise mode of action of 43Gap 27 peptide remains to be determined but is likely to involve effects on connexon docking (Warner et al. 1995; Chaytor et al. 1997). EDHF-type responses are also attenuated by the 18α and 18β isoforms of glycyrrhetinic acid, which are lipophilic aglycones that disrupt gap junction plaques (Davidson & Baumgarten, 1988; Taylor et al. 1998; Yamamoto et al. 1999). Although endothelium-dependent agonists induce endothelial hyperpolarization directly (Mehrke & Daut, 1990; Marchenko & Sage, 1993), at the present time there is little evidence that transmission of a hyperpolarizing electrical current from the endothelium contributes significantly to EDHF-mediated responses, perhaps reflecting the relatively small mass of the endothelium compared to the media (Beny & Pacicca, 1994).
EDHF-mediated responses depend on mobilization of arachidonic acid by a Ca2+-dependent phospholipase A2 (PLA2), but the pathways and mechanisms involved distal to this synthetic step remain controversial (Fulton et al. 1996; Adeagbo & Henzel, 1998; Hutcheson et al. 1999). In the rat mesenteric and coronary vasculature the arachidonic acid derivative N-arachidonylethanolamide (anandamide) displays the characteristics of an EDHF in that it stimulates hyperpolarizing calcium-activated potassium (KCa) channels and evokes NO- and prostanoid-independent relaxations that are attenuated by high extracellular [K+] (Randall et al. 1996, 1997). Relaxations to anandamide are sensitive to the cannabinoid CB1 receptor antagonist SR141716A, which also attenuates EDHF-type relaxations to muscarinic agonists (Randall et al. 1996; White & Hiley, 1997; Randall & Kendall, 1998). Since endothelial cells synthesize anandamide (Deutsch et al. 1997), these findings have led to the proposal that this endocannabinoid is an EDHF (Randall & Kendall, 1997). It has become apparent, however, that EDHF- and anandamide-induced relaxations exhibit differential susceptibility to K+ channel blockers (White & Hiley, 1997; Plane et al. 1997; Petersson et al. 1997; Zygmunt et al. 1997a; Randall & Kendall, 1998), and in certain artery types anandamide stimulates endothelium-dependent relaxations that involve NO and/or prostanoids (Ellis et al. 1995; Deutsch et al. 1997; Bilfinger et al. 1998).
In view of the uncertainties regarding anandamide as a putative EDHF, in the present study we have examined the role of CB1 receptors, the high-affinity anandamide transport mechanism and gap junctional communication in cannabinoid-induced relaxations of the rabbit superior mesenteric artery. As anandamide may be converted into other vasoactive arachidonate derivatives within the endothelial cell (Pratt et al. 1998), experiments were also conducted with its stable chiral analogue methanandamide to investigate the possible role of intracellular metabolism (Abadji et al. 1994). The effects of two different CB1 receptor antagonists (SR141716A and LY320135), an inhibitor of the high-affinity anandamide transporter (AM404), 43Gap 27 peptide and 18α-glycyrrhetinic acid were evaluated to define the mechanisms contributing to mechanical relaxations. In parallel experiments the ability of these agents to modulate intercellular transfer of Lucifer Yellow dye was investigated in confluent COS-7 cells. This African green monkey kidney fibroblast cell line expresses Cx43 as its only functional gap junction protein (George et al. 1998), and Western blot analysis has shown that the only connexin protein expressed in smooth muscle from the rabbit superior mesenteric artery is Cx43 (Chaytor et al. 1997). Since mRNA for Cx40 is detectable in the rabbit superior mesenteric artery (Chaytor et al. 1997), and in other species Cx40 forms functional channels in vascular cells (Li & Simard, 1999), the effects of a Gap 27 construct possessing homology with Cx40 (40Gap 27, SRPTEKNVFIV) were compared to those of 43Gap 27 peptide.
METHODS
Isolated ring preparations
Male New Zealand White rabbits (2-2.5 kg) were killed by administration of sodium pentobarbitone (120 mg kg−1 intravenously). The superior mesenteric artery was removed, stripped of adherent connective and adipose tissue and placed in Holman's buffer (composition, mm: 120 NaCl, 5 KCl, 2.5 CaCl2, 1.3 NaH2PO4, 25 NaHCO3, 11 glucose and 10 sucrose). Rings 2-3 mm wide were dissected and suspended in oxygenated (95 % O2-5 % CO2) 3 ml tissue baths at 37°C and attached using hooks and thread to FT102 force transducers with isometric force being recorded on a MacLab/4e (ADInstruments, Hastings, UK). In experiments investigating the direct smooth muscle effects of anandamide or methanandamide the endothelium was removed by gentle abrasion of the intimal surface with a roughened probe. Each ring was placed under 0.6 g tension and allowed to equilibrate for ∼1 h with the baseline being reset at intervals following stress relaxation.
Experimental protocol
Endothelium-intact rings were submaximally contracted by phenylephrine (10 μm) and subsequently allowed to stabilise for 20-30 min before cumulative concentration-response curves to anandamide or methanandamide were constructed in the presence or absence of NG-nitro-L-arginine methyl ester (L-NAME, 300 μm) and/or indomethacin (10 μm). Following these initial responses and washout of all drugs, a second series of responses was obtained subsequent to incubation with 43Gap 27 peptide (300 μm) for 20 min. The effects of 18α-glycyrrhetinic acid (18α-GA, 50 μm for 60 min) against anandamide-induced relaxations were similarly determined. Some rings were not challenged with the peptide or 18α-GA in order to obtain time-matched controls. Further experiments were conducted in the presence of the CB1 receptor antagonists N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride (SR141716A) or (6-methoxy-2-(4-methoxyphenyl) benzo[b]thien-3-yl)(4-cyanophenyl) methanone (LY320135), or the high-affinity anandamide transporter inhibitor N-(4-hydroxyphenyl) arachidonylamide (AM404), each agent being administered at a concentration of 10 μm, 20 min before the response curves were constructed. Responses to cannabinoids were also investigated in endothelium-denuded preparations in the presence or absence of 43Gap 27 peptide (300 μm), 18α-GA (50 μm), SR141716A (10 μm), LY320135 (10 μm), AM404 (10 μm) or indomethacin (10 μm). At the end of each experiment involving denuded rings ACh (1 μm) was added to confirm the absence of endothelium.
In a further series of experiments, concentration-relaxation curves were constructed for ACh in endothelium-intact rings submaximally precontracted with phenylephrine (10 μm). Responses to ACh were investigated following preincubation with 43Gap 27 peptide (300 μm) or 40Gap 27 peptide (300 μm and 500 μm) for 20 min in the presence of L-NAME (300 μm) and indomethacin (10 μm). Relaxations to ACh were also studied following preincubation for 20 min with SR141716A (10 μm) in the absence or presence of L-NAME (300 μm) and indomethacin (10 μm).
Dye transfer
Lucifer Yellow CH (charge -2, MW 457; 5 % w/v in 0.3 M LiCl) was used to evaluate cell-cell coupling in COS-7 cells (ECACC, Wiltshire, UK) following intracytoplasmic injection. Confluent monolayers were formed after 3 days’ incubation at 37°C in 5 % CO2 following seeding at a density of 1 × 106 cells per 60 mm2 tissue culture dish in complete Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum, 100 mg l−1 penicillin- streptomycin-glutamine and 250 mg l−1 amphotericin B (Gibco BRL). The monolayers were washed twice with phosphate-buffered saline (120 mm NaCl; 2.7 mm KCl; 10 mm Na2HPO4.2H2O, pH 7.4) and incubated in Leibowitz L-15 medium (supplemented as above) at 37°C. The cells were then exposed to 43Gap 27 peptide (300 and 500 μm), 40Gap 27 peptide (300 μm and 500 μm), anandamide (100 μm), LY320135 (10 μm), SR141716A (10 μm) and AM404 (10 μm) prior to Lucifer Yellow injection. 18α-GA (50 μm) was used to provide a positive control (Munari-Silem et al. 1995). In all cases monolayers were exposed to these agents for 60 min at 37°C.
Single cells were selected and Lucifer Yellow injected into the cytoplasmic compartment using an Eppendorff micromanipulator 5170 and microinjector 5242 at a pressure of 200 hPa and an injection time of 0.3 s. Following dye injection, the cells were returned to the incubator for 15 min and then fixed in 4 % paraformaldehyde. Transfer of Lucifer Yellow was determined by fluorescence microscopy using filter set 05 (395-440 nm/ 460-470 nm; Zeiss, UK) on an Axiostat microscope (Zeiss, UK). Inhibition of dye transfer was quantified by determining the percentage of injected cells that communicated Lucifer Yellow to < 5, 5-10 and > 10 neighbouring cells (Nadarajah et al. 1998).
Materials
Acetylcholine, 18α-glycyrrhetinic acid, indomethacin, L-NAME, phenylephrine and Lucifer Yellow were supplied by Sigma, UK. 43Gap 27 peptide (SRPTEKTIFII) and 40Gap 27 peptide (SRPTEKNVFIV) were synthesised by Genosys Biotechnologies (Pampisford, UK). Anandamide was supplied by Tocris, UK and LY320135 from Eli Lilly, UK. We thank Dr A. Makriyannis, School of Pharmacy, University of Connecticut, Connecticut, USA and Dr D. Piomelli, The Neurosciences Institute, San Diego, USA for the gift of AM404. Methanandamide and SR141716A were supplied by Research Biochemicals International, UK. All compounds were dissolved in buffered Holman's solution except AM404, anandamide, methanandamide and SR141716A (all ethanol) and indomethacin (5 % bicarbonate solution).
Statistics
Concentration-response curves and Lucifer Yellow dye transfer were analysed using one-way analysis of variance (ANOVA) with the Bonferroni multiple comparisons test as a further method of analysis. Maximal relaxations were compared using Student's paired t test. P < 0.05 was considered significant. Data are expressed as the mean ±s.e.m.
RESULTS
Effects of 43Gap 27 peptide, L-NAME and indomethacin on endothelium-dependent relaxations to anandamide and methanandamide
Phenylephrine (10 μm) induced a constriction of 2.25 ± 0.14 g in rings of endothelium-intact superior mesenteric artery (n= 35; data pooled from all experiments). Anandamide evoked a concentration-dependent relaxation in endothelium-intact rings that was maximal at 10-30 μm, with the maximal response being 41.5 ± 5.0 % of phenylephrine-induced contraction (Fig 1A and Fig 2A). Preincubation with 43Gap 27 peptide (300 μm) significantly attenuated this relaxation and caused a rightward shift in the concentration-response curve, such that maximal relaxation was reduced by 47.0 ± 3.6 % and the EC50 value increased from 3.3 ± 1.1 to 9.8 ± 1.8 μm (P < 0.05, n= 5; Fig 1B and Fig2 A). Similarly, methanandamide-induced relaxation of endothelium-intact rings was maximally 55.2 ± 6.3 % of phenylephrine-induced tone at a concentration of 30 μm, and this was reduced by 38.2 ± 4.8 % following preincubation with 43Gap 27 peptide with a rightward shift in the concentration-response curve such that the EC50 value increased from 4.4 ± 0.8 to 9.7 ± 1.1 μm (P < 0.05, n= 5; Fig. 2C). Maximal relaxations and EC50 values did not differ significantly for the anandamide- and methanandamide-induced responses. Indomethacin (10 μm) had no significant effect on the concentration-response curves to anandamide or methanandamide (n= 4; Fig. 2A and C).
Figure 1. Representative traces showing anandamide-induced relaxation in endothelium-intact rings.
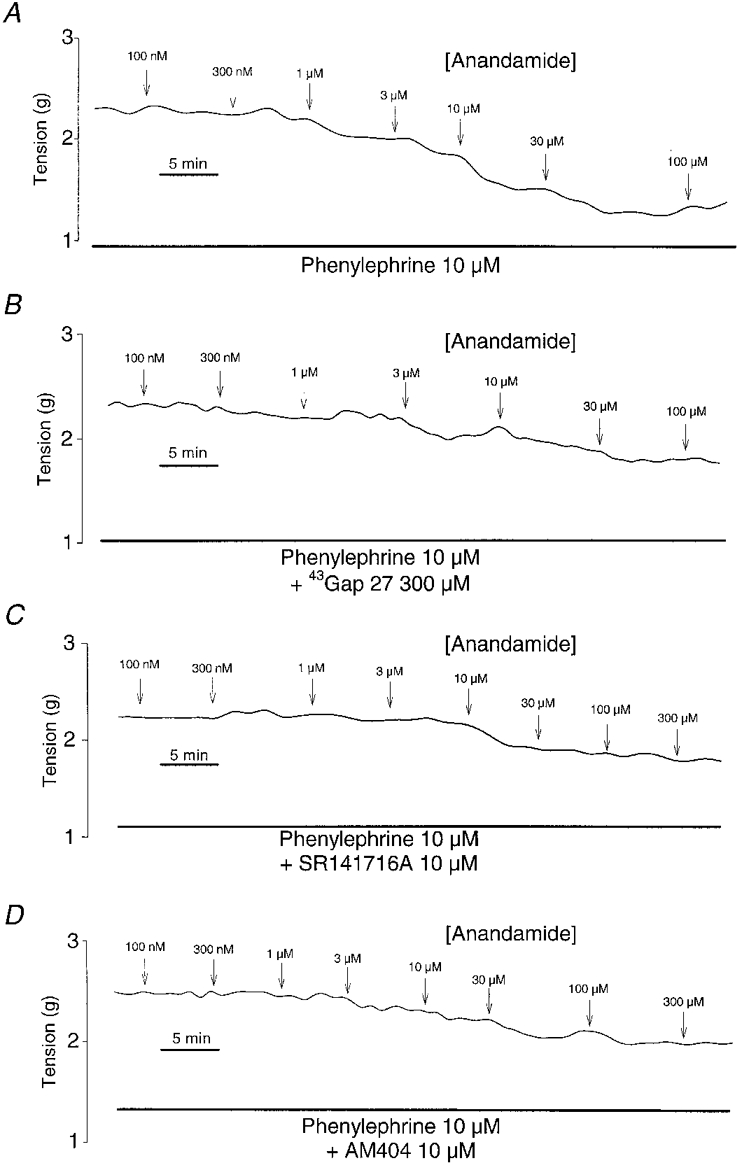
Anandamide-induced relaxation in endothelium-intact rings (A) and the effects of preincubation with 300 μm43Gap 27 peptide (B), 10 μm SR141716A (C) and 10 μm AM404 (D).
Figure 2. Concentration-response curves showing the effects of 43Gap 27 peptide (300 μm), indomethacin (10 μm) and L-NAME (300 μm) on anandamide- and methanandamide-induced relaxations.
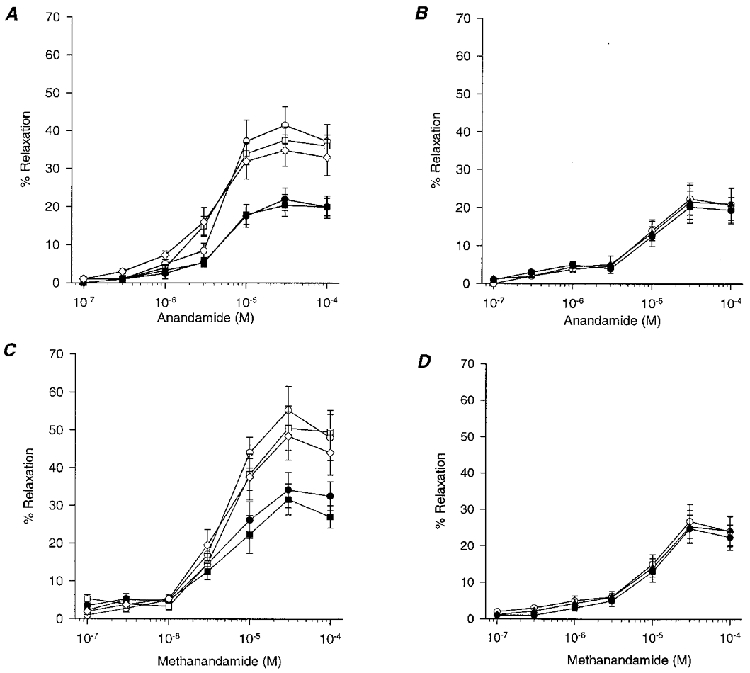
A, in endothelium-intact rings 43Gap 27 peptide (300 μm; •) caused a significant attenuation of responses to anandamide, whereas preincubation with L-NAME (300 μm; n= 5; □) or indomethacin (10 μm; n= 4; ⋄) was without effect when compared to control (n= 5; ^). When 43Gap 27 peptide (300 μm) was used in combination with L-NAME (300 μm; ▪), responses did not differ significantly from those obtained with the peptide alone (P < 0.05; n= 5). B, in endothelium-denuded rings anandamide-induced relaxation was not attenuated by 43Gap 27 peptide (300 μm; n= 5; •) or SR141716A (10 μm; n= 5; ▴) when compared to control (^). C, methanandamide-induced relaxations exhibited similar characteristics to those obtained with anandamide, preincubation with 43Gap 27 peptide (300 μm; n= 5; •) attenuating relaxation in endothelium-intact rings. Relaxations were not affected by L-NAME (300 μm; n= 5; □) or indomethacin (10 μm; n= 4; ⋄). Other symbols as in A. D, in endothelium-denuded rings responses to methanandamide were unaffected by 43Gap 27 peptide (300 μm; n= 5; •) or SR141716A (10 μm; n= 5; ▴). ^, control (n= 5).
L-NAME (300 μm) had no significant effect on anandamide- or methanandamide-induced relaxations, either alone, or when administered in the presence of 43Gap 27 peptide (300 μm). In the presence of L-NAME, maximal relaxations were 37.5 ± 3.6 and 50.5 ± 5.9 % of phenylephrine-induced contraction with EC50 values of 3.8 ± 0.8 and 4.7 ± 0.9 μm for the two cannabinoids, respectively (n= 5; Fig. 2A and C). When 43Gap 27 peptide and L-NAME were used in combination the inhibitory effect of the peptide was not enhanced (n= 5; Fig. 2A and C).
Endothelium-independent relaxations to anandamide and methanandamide
Phenylephrine (10 μm) induced a constriction of 2.50 ± 0.20 g in rings of endothelium-denuded superior mesenteric artery (n= 18; data pooled from all experiments). Anandamide and methanandamide also evoked concentration-dependent relaxations in endothelium-denuded rings of superior mesenteric artery, maximal responses being obtained at concentrations of 10-30 μm and representing 22.4 ± 4.2 and 26.8 ± 4.7 % of phenylephrine-induced contraction, respectively (Fig. 2B and D). Preincubation with 43Gap 27 peptide (300 μm) did not significantly affect concentration-response curves to either agent, maximal relaxations then being 20.3 ± 4.2 and 24.8 ± 3.8 %, respectively. EC50 values were 9.2 ± 1.2 and 9.6 ± 1.1 μm for control responses and, following preincubation with 43Gap 27 peptide, 9.6 ± 0.8 and 10.1 ± 1.2 μm for anandamide- or methanandamide-induced relaxations, respectively (n= 5; Fig 2B and D). No significant inhibitory effect against anandamide- and methanandamide-induced relaxation was found following preincubation with SR141716A (10 μm). In this series of experiments maximal relaxations to the two cannabinoids were 21.6 ± 4.5 and 25.3 ± 4.5 % with EC50 values of 10.1 ± 0.9 and 10.3 ± 1.3 μm, respectively, and these values were not significantly different from each other (n= 5; Fig 2B and D). Indomethacin (10 μm), LY320135 (10 μm) and AM404 (10 μm) similarly did not affect relaxations to anandamide or methanandamide in endothelium-denuded rings, maximal relaxations being 24.4 ± 3.8, 21.8 ± 5.2 and 26.2 ± 5.4 % with EC50 values of 10.2 ± 2.6, 9.8 ± 1.8 and 10.1 ± 2.4 μm, respectively (n= 4 in each case; data not plotted in Fig. 2 for simplicity).
Effects of 18α-glycyrrhetinic acid on endothelium-dependent and -independent relaxations to anandamide
The effects of 18α-glycyrrhetinic acid (18α-GA; 50 μm) were also investigated in endothelium-intact and -denuded rings. Preincubation with this gap junction inhibitor significantly attenuated maximal anandamide-induced relaxations by 54.3 ± 4.3 %, with EC50 values increasing to 14.8 ± 1.2 μm (P < 0.05, n= 5; Fig. 3). Anandamide-induced relaxations of endothelium-denuded rings were not significantly affected in magnitude or EC50 value following preincubation with 18α-GA (50 μm; n= 5; Fig. 3).
Figure 3. Concentration-response curves showing effects of preincubation with 18α-GA.
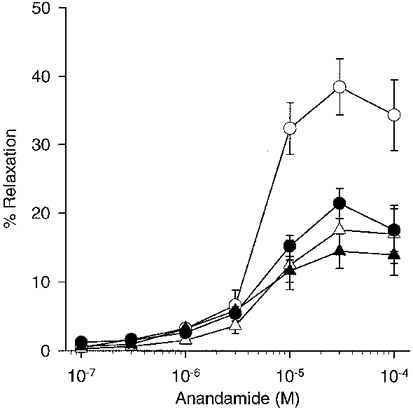
18α-GA (50 μm; n= 5; ▵) caused attenuation of anandamide-induced relaxation in endothelium-intact rings of superior mesenteric artery when compared to control rings (n= 5; ^). In endothelium-denuded preparations 18α-GA (n= 5; ▴) did not significantly affect anandamide-evoked responses when compared to denuded controls (n= 5; •).
Effects of SR141716A, LY320135 and AM404 on endothelium-dependent relaxation to anandamide and methanandamide
The effects of the CB1 receptor antagonists SR141716A (10 μm) and LY320135 (10 μm) against anandamide- and methanandamide-induced relaxation, were investigated in endothelium-intact rings (Fig. 4). SR141716A caused a rightward shift in the concentration-response curves, such that maximal relaxations were reduced by 52.9 ± 5.8 and 57.9 ± 6.2 %, with the EC50 values increasing to 30.4 ± 4.1 and 32.0 ± 2.8 μm, respectively (P < 0.05, n= 4; Fig 1C, and Fig 4A and B). By contrast, LY320135 did not significantly alter maximal relaxation or EC50 values for the anandamide- or methanandamide-induced responses (n= 4; Fig. 4C and D).
Figure 4. Concentration-response curves showing the effects of SR141716A, LY320135 and AM404 on anandamide- and methanandamide-induced relaxations in endothelium-intact rings.
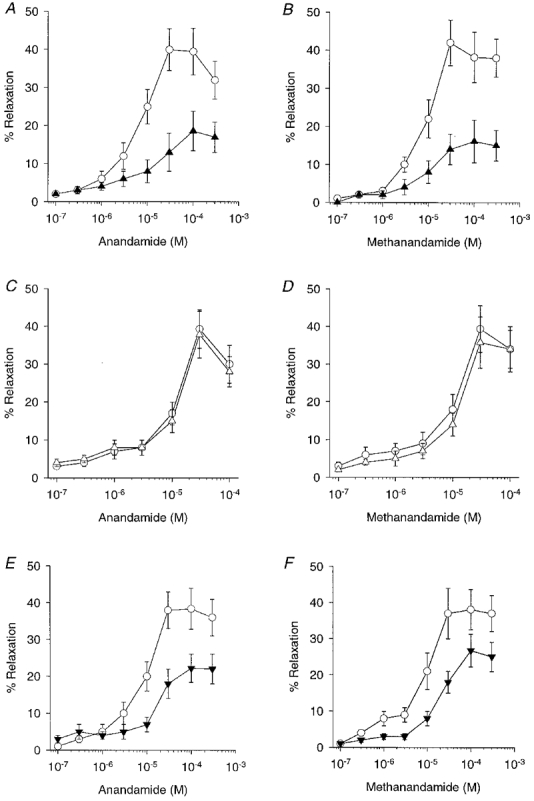
A and B, control responses to both agents (^) were significantly attenuated by preincubation with the CB1 receptor antagonist SR141716A (10 μm; n= 5; ▴). C and D, LY320135 (10 μm; n= 5; ▵), also a CB1 receptor antagonist, did not significantly affect relaxations when compared to control. E and F, preincubation with the transporter inhibitor AM404 (10 μm; n= 5; ▾) caused significant attenuation of both anandamide- and methanandamide-induced relaxations.
The cannabinoid transporter inhibitor AM404 caused significant inhibition of both anandamide and methanandamide-induced responses. Maximal relaxations to the two agents were reduced by 42.2 ± 5.2 and 29.9 ± 4.1 % and EC50 values increased to 34.8 ± 1.8 and 56.2 ± 4.1 μm, respectively (P < 0.05, n= 4; Fig 1D and Fig 4E and F).
Effects of SR141716A on acetylcholine-induced relaxations
Acetylcholine evoked concentration-dependent relaxations that were maximal at 3 μm, being then equivalent to 86.9 ± 5.4 % of the phenylephrine-induced contraction. Preincubation with SR141716A (10 μm) for 20 min caused a significant reduction in maximal relaxation by 48.9 ± 4.2 % and a rightward shift in the concentration-response curve with the EC50 value increasing from 357.4 ± 28 to 942.0 ± 56 nm (P < 0.05, n= 4; Fig. 5). The combination of SR141716A (10 μm), L-NAME (300 μm) and indomethacin (10 μm) caused a further significant decrease in maximal relaxation when compared to the effect of SR141716A alone, with the maximal response being inhibited by 66.9 ± 7.2 % (P < 0.05, n= 4; Fig. 5). Although there was a corresponding rightward shift in the concentration-response curve, with the EC50 value increasing to 994 ± 62 nm, this did not achieve statistical significance compared to SR141716A alone.
Figure 5. Concentration-response curves to acetylcholine showing the effects of preincubation with SR141716A.
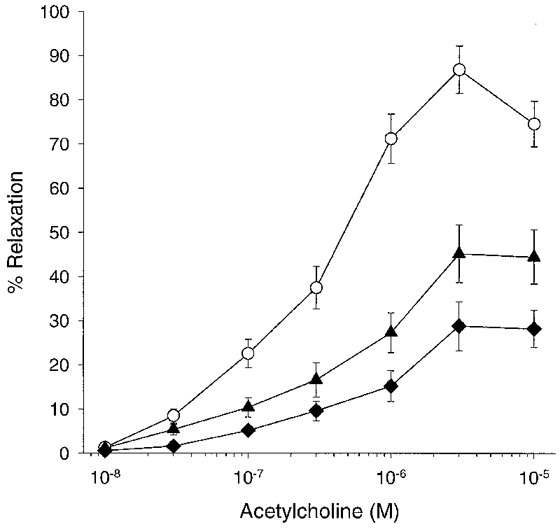
SR141716A (10 μm; n= 4; ▴) caused attenuation of acetylcholine-induced relaxation when compared to control (^). The combined presence of SR141716A (10 μm), L-NAME (300 μm) and indomethacin (10 μm) caused further inhibition of relaxation (n= 4; ♦).
Effects of anandamide, AM404, LY320135, SR141716A, 43Gap 27 peptide and 18α-GA on Lucifer Yellow dye transfer
Confluent monolayers of COS-7 cells injected with Lucifer Yellow exhibited rapid spread of the dye to neighbouring cells (Fig. 6A and 6A′), with efficient gap junctional coupling permitting 72.4 ± 5.7 % of injected cells to transfer the dye to > 10 neighbouring cells with 12.8 ± 10.3 and 9.0 ± 2.2 % transferring to < 5 and 5-10 cells, respectively, in the control situation (n= 61 injected cells; Fig. 7). In most instances the dye appeared in 20-30 neighbouring cells within 15 min (Fig. 6A′). Anandamide, the cannabinoid transporter inhibitor AM404 and the CB1 receptor antagonist LY320135 (100, 10 and 10 μm, respectively) did not significantly affect the patterns of dye transfer observed in control cells (n= 21, 20 and 56 injected cells; Fig 6B-D and Fig 7). By contrast, COS-7 cells incubated with SR141716A (10 μm) demonstrated almost complete loss of intercellular communication with dye transfer being restricted to < 5 cells in 97.9 ± 1.1 % of cases (n= 103 injected cells, P < 0.001; Fig 6E and Fig 7). The known gap junction inhibitor 18α-GA (50 μm) caused a significant reduction in cell-cell coupling such that only 38.0 ± 3.5 % of cells permitted dye transfer to > 10 neighbouring cells with an increase to 53.8 ± 5.5 % of cells transferring Lucifer Yellow to < 5 cells compared to control (n= 25 injected cells, P < 0.005 and P < 0.005, respectively; Fig 6I and Fig 7). In a similar fashion, 43Gap 27 peptide (300 and 500 μm) attenuated dye transfer with 55.0 ± 5.0 and 26.8 ± 3.3 % transferring dye to > 10 neighbouring cells (n= 34 and 17 injected cells, P < 0.05 and P < 0.01, respectively; Fig 6H and Fig 7) with a corresponding increase in the number of injections following which dye transferred to < 5 cells of 35.4 ± 0.4 and 49.7 ± 2.3 %, respectively (P < 0.05 and P < 0.005, respectively; Fig 6H and Fig 7).
Figure 6. Representative photographs demonstrating the effects of preincubation with anandamide, AM404, LY320135, SR141716A, 40Gap 27 peptide, 43Gap 27 peptide and 18α-GA on Lucifer Yellow dye transfer in COS-7 cells.
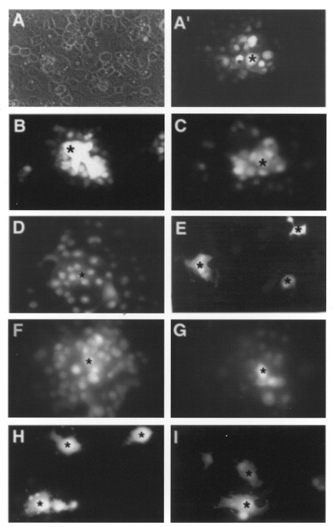
A, typical phase view of COS-7 cells showing confluence. A′, Lucifer Yellow dye transfer in the control situation demonstrating efficient gap junctional communication between > 20 cells. Anandamide (100 μm; B), the cannabinoid transporter inhibitor AM404 (10 μm; C) and the CB1 receptor antagonist LY320135 (10 μm; D) did not affect dye transfer. E, preincubation with SR141716A (10 μm) markedly inhibited dye transfer. F and G, 40Gap 27 peptide (300 and 500 μm, respectively) did not attenuate Lucifer Yellow dye transfer. H and I, following preincubation with 43Gap 27 peptide (500 μm) or 18α-GA (50 μm), dye transfer was markedly reduced. * denotes individual cells injected with Lucifer Yellow. Magnification × 40.
Figure 7. Histogram showing effects on Lucifer Yellow dye transfer in COS-7 cells.
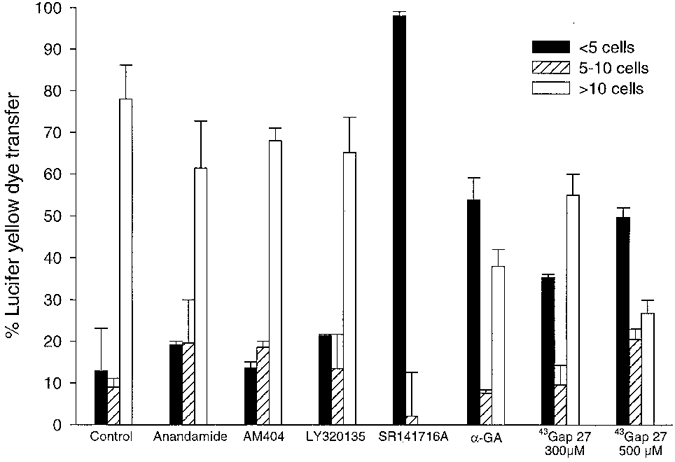
Preincubation with SR141716A (10 μm) caused almost complete abolition of dye transfer to more than 5 cells, and 18α-GA (50 μm) and 43Gap 27 peptide (300 μm and 500 μm, respectively) caused a significant shift in the pattern of dye transfer seen following intracytoplasmic injection when compared to control. Anandamide (100 μm), the transporter inhibitor AM404 (10 μm) and the CB1 receptor antagonist LY321035 (10 μm) did not affect dye transfer.
Comparison of the effects of 40Gap 27 peptide and 43Gap 27 peptide on endothelium-dependent relaxations to acetylcholine in the presence of L-NAME
In the presence of L-NAME (300 μm) and indomethacin (10 μm), ACh evoked concentration-dependent relaxations in endothelium-intact rings of superior mesenteric artery that were maximal at 1-3 μm, with the maximal response being 44.4 ± 3.6 % of phenylephrine-induced contraction (n= 5). Preincubation with 43Gap 27 peptide (300 μm) markedly inhibited this response with maximal relaxations being reduced by 85.4 ± 4.2 % and the EC50 value increasing to 455 ± 24 nm (n= 4; P < 0.05; Fig. 8A). In marked contrast, preincubation with 40Gap 27 peptide (300 μm and 500 μm) was completely without effect on the relaxations induced by ACh (n= 4; Fig. 8A).
Figure 8. Concentration-response curves and histogram showing the effects of 40Gap 27 peptide on ACh-induced relaxations in the superior mesenteric artery and Lucifer Yellow dye transfer in COS-7 cells.
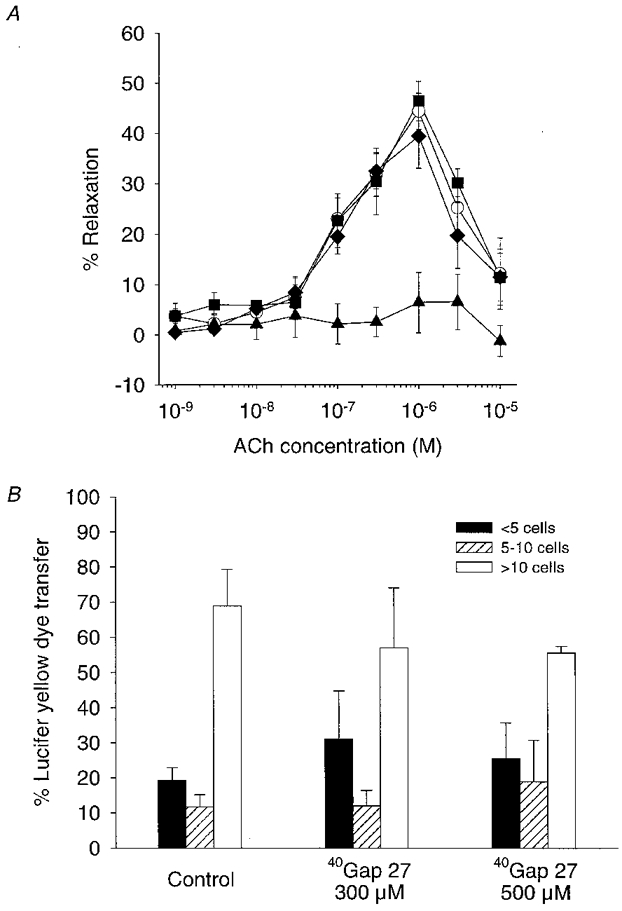
A, 40Gap 27 peptide (300 μm; n= 4; ▪, and 500 μm; n= 4; ♦) did not significantly affect relaxation responses evoked by ACh in the presence of L-NAME (300 μm) and indomethacin (10 μm) when compared to control (^; n= 5). Preincubation with 43Gap 27 peptide (300 μm; n= 4; ▴) caused marked attenuation of ACh-induced relaxation. B, 40Gap 27 peptide at concentrations of 300 and 500 μm did not significantly alter the pattern of dye transfer following intracytoplasmic injection of Lucifer Yellow.
Effects of 40Gap 27 peptide on Lucifer Yellow dye transfer
In a further set of experiments with COS-7 monolayers, 63.7 ± 10.4 % of cells injected with Lucifer Yellow transferred dye to > 10 neighbouring cells in the control situation, with 19.3 ± 3.5 and 11.7 ± 3.5 % of cells transferring to < 5 and 5-10 neighbours, respectively (n= 30 injected cells; Fig. 8B). Preincubation with 40Gap 27 peptide (300 and 500 μm) did not affect this pattern of dye transfer (n= 30 injected cells; Fig. 8B).
DISCUSSION
The major finding of the present study is that direct heterocellular communication, rather than NO or prostanoids, underpins the endothelium-dependent component of the relaxations evoked by anandamide and methanandamide in the rabbit superior mesenteric artery. Relaxations of endothelium-intact rings in the presence of 43Gap 27 peptide and 18α-glycyrrhetinic acid, which both interrupt gap junction communication, were thus equivalent in magnitude to those observed in denuded vessels. The study also suggests that the action of cannabinoids can involve facilitated entry into the endothelial cell via a high-affinity transport mechanism, rather than activation of endothelial CB1 receptors.
Inhibition of EDHF-type vascular relaxations by SR141716A has previously been attributed to selective blockade of smooth muscle CB1 receptors (Randall et al. 1996; White & Hiley, 1997). An unexpected finding, therefore, was that preincubation with SR141716A at concentrations which inhibited the endothelium-dependent component of responses to anandamide, methanandamide and ACh, markedly attenuated intercellular transfer of Lucifer Yellow in a COS-7 cell system that allows quantification of intercellular communication via gap junctions constructed from Cx43. The inability of the CB1 receptor antagonist LY320135 (Felder et al. 1998) to attenuate cannabinoid-induced relaxations in either endothelium-intact or -denuded vessels confirms that their action is not dependent on activation of endothelial or smooth muscle receptors in rabbit mesenteric arteries. The findings reveal a previously unsuspected action for SR141716A and question its specificity as a CB1 receptor antagonist in the vascular system. Observations that the antagonism of relaxations to anandamide by SR141716A was non-competitive support this conclusion.
Analogous findings have recently been reported in the perfused rat mesentery in which anandamide induced both endothelium-dependent and -independent relaxations, with only the former component being sensitive to SR141716A (Wagner et al. 1999). Furthermore, two potent synthetic CB1 agonists were devoid of vasodilator activity (Wagner et al. 1999). In the literature, however, there are apparently conflicting reports concerning the effects of SR141716A, including: (i) inhibition of EDHF-type responses to acetylcholine and A23187 in the presence of L-NAME and indomethacin (Randall et al. 1996), (ii) no effect on anandamide-induced relaxations or EDHF-type responses evoked by acetylcholine and A23187 (Plane et al. 1997), (iii) attenuation of anandamide-induced relaxation with no effect on acetylcholine-induced EDHF-type responses (Zygmunt et al. 1997b), and (iv) no effect on anandamide-induced relaxation but inhibition of the Ca2+ ionophore A23187-induced release of radiolabelled arachidonic acid from endothelial cells, which also suggests that SR141716A is not purely a CB1 receptor antagonist (Pratt et al. 1998). Variability in the effects of SR141716A in different vascular beds may be attributable to differences in the contributions of gap junctional communication, populations of CB1 receptors, or poorly characterized effects on arachidonate metabolism. Indeed, in some vessel types anandamide stimulates NO release through a mechanism that is blocked by SR141716A, consistent with the involvement of endothelial CB1 receptors (Deutsch et al. 1997; Bilfinger et al. 1998).
As anandamide does not appear to activate CB1 receptors in the rabbit superior mesenteric artery, we investigated whether its entry into the endothelial cell was necessary for vasorelaxant activity using a selective inhibitor of the high-affinity anandamide transporter, AM404, that does not activate CB1 receptors (Beltramo et al. 1997). In the guinea-pig, AM404 enhances CB receptor-mediated vascular responses by preventing the uptake and subsequent metabolism of anandamide, thus enhancing its in vivo depressor effects (Calignano et al. 1997). By contrast, in the present study AM404 selectively abolished endothelium-dependent relaxations to anandamide and methanandamide, suggesting that both are transported into the endothelium before activating more distal mechanisms of relaxation. The observation that AM404 inhibited, rather than enhanced, the endothelium-dependent component of the relaxations induced by the cannabinoids confirms that their action does not involve stimulation of endothelial CB1 receptors in rabbit mesenteric arteries, given that the COS-7 cell system failed to demonstrate direct inhibition of dye transfer by AM404. By contrast, the direct smooth muscle effects of anandamide and methanandamide did not require facilitated transport, as AM404 did not antagonize relaxation in endothelium-denuded preparations. The mechanisms that underlie this direct action on smooth muscle remain unknown, but can be distinguished from endothelium-mediated responses by complete resistance to 43Gap 27 peptide, SR141716A and AM404.
The connexin subtypes that contribute to direct endothelial-smooth muscle communication are presently unknown. Since endothelium expresses Cx37, 40 and 43 proteins, and vascular smooth muscle expresses either Cx43 or Cx40 and 43, a variety of homotypic, heterotypic and heteromeric channels could in theory be formed (Elfgang et al. 1995; Carter et al. 1996; Chaytor et al. 1997; Li & Simard, 1999). In mammals (including rat, human and mouse) the 43Gap 27 sequence used in the present study is identical to the corresponding Gap 27 sequence in Cx37, so that this peptide cannot distinguish between heterocellular communication via homotypic Cx43/Cx43 channels or heterotypic channels constructed from Cx37/Cx43. However, the lack of effect of 40Gap 27 peptide against endothelium-dependent relaxations and dye transfer in the present experiments would appear to exclude the participation of Cx40 and is consistent with observations that neither smooth muscle from rabbit mesenteric arteries nor COS-7 cells express Cx40 protein (Chaytor et al. 1997; George et al. 1998). These findings also suggest that small differences in the Gap 27 peptide sequence leading into the fourth transmembrane domain can markedly affect the connexon selectivity of such peptide constructs.
Differences between freshly isolated arteries and cultured COS-7 cells were nevertheless apparent. Firstly, the attenuation of Lucifer Yellow dye transfer by 43Gap 27 peptide was concentration dependent but still incomplete at 500 μm, whereas at 300 μm the peptide effectively abolished the EDHF-mediated component of the mechanical relaxations induced by anandamide. Secondly, 10 μm SR141716A was more effective in attenuating dye transfer than 300 μm43Gap 27 peptide or 50 μm 18α-glycyrrhetinic acid in COS-7 cells, whereas all three agents effectively abolished EDHF-type relaxations at these concentrations. The explanation for these findings must await a more complete mechanistic understanding of the effects of these agents on gap junction permeability and delineation of the characteristics of the connexon subtypes that couple endothelial and smooth muscle cells. Indeed, in neurones and astrocytes, anandamide and its precursor arachidonic acid have been reported to reduce gap junctional communication through a mechanism that involves altered voltage gating in the case of arachidonic acid (Venance et al. 1995; Schmilinsky-Fluri et al. 1997; Weingart & Bukauskas, 1998). In the present study, however, dye transfer was unaffected by anandamide in the COS-7 model cell system and anandamide-induced relaxations were not susceptible to inhibition by 43Gap 27 peptide. These findings indicate that closure of myoendothelial gap junctions is not an important action of anandamide in the arterial wall.
Other endothelial metabolites of arachidonic acid such as cytochrome P450-derived epoxyeicosatrienoic acids (EETs) also possess the characteristics of EDHFs and may activate large-conductance KCa channels (Hecker et al. 1994; Randall et al. 1996; Campbell et al. 1996; Mombouli et al. 1996; Randall & Kendall, 1998). Indeed, it is possible that the actions of anandamide and EETs are not completely distinct, as anandamide may be converted into EETs following hydrolysis by the fatty acid amide hydrolase (Beltramo et al. 1997; Pratt et al. 1998). Bovine endothelial cells incubated with radiolabelled anandamide thus demonstrate ∼15 % conversion of anandamide to arachidonic acid, EETs and prostanoids after 30 min (Pratt et al. 1998). In the present study, however, maximal relaxations to anandamide, and their potency, were similar to methanandamide, which is not susceptible to breakdown by the fatty acid amide hydrolase (Abadji et al. 1994). Furthermore, certain epoxyeicosatrienoic acid regioisomers may stimulate a strictly endothelium-dependent relaxation in rabbit mesenteric arteries that involves synthesis of NO in addition to mechanisms that depend on gap junctional communication, thus exhibiting characteristics that differ from anandamide (Hutcheson et al. 1999). The present findings also suggest that there is no significant intracellular metabolism of anandamide to vasoactive prostanoids as both endothelium-dependent and -independent relaxations were unaffected by indomethacin. Taken together, these findings indicate that anandamide hydrolysis is unlikely to contribute to a pool of biologically active EETs or prostanoids that are available for relaxation in rabbit mesenteric arteries.
In conclusion, anandamide stimulates endothelium-dependent relaxations of the rabbit superior mesenteric artery that are exclusively dependent on heterocellular gap junctional communication and likely to involve transport into the endothelial cell rather than activation of CB1 receptors. Analogous observations were made with the stable analogue methanandamide, thus suggesting that intracellular metabolism is not essential for vasorelaxation. Both cannabinoids also mediate effects on smooth muscle, whose genesis remains to be determined. The role of gap junctional communication in the vascular actions of cannabinoids in other species remains to be evaluated.
Acknowledgments
The study was supported by the Medical Research Council.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. Journal of Medicinal Chemistry. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Adeagbo AS, Henzel MK. Calcium-dependent phospholipase A2 mediates the production of endothelium-derived hyperpolarizing factor in perfused rat mesenteric prearteriolar bed. Journal of Vascular Research. 1998;35:27–35. doi: 10.1159/000025562. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Bény J-L, Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. American Journal of Physiology. 1994;266:H1465–1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Bilfinger TV, Salzet M, Fimiani C, Deutsch DG, Tramu G, Stefano GB. Pharmacological evidence for anandamide amidase in human cardiac and vascular tissues. International Journal of Cardiology. 1998;64:S15–22. doi: 10.1016/s0167-5273(98)00031-x. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor, AM404. European Journal of Pharmacology. 1997;337:R1–2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation Research. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Carter TD, Chen XY, Carlile G, Kalapothakis E, Ogden D, Evans WH. Porcine aortic endothelial gap junctions: identification and permeation by caged InsP3. Journal of Cell Science. 1996;109:1765–1773. doi: 10.1242/jcs.109.7.1765. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. The Journal of Physiology. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. The Journal of Physiology. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap junctional intercellular communication. Structure-activity relationships. Journal of Pharmacology and Experimental Therapeutics. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. Journal of Clinical Investigation. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Martin PEM, Chaytor AT, Evans WH, Garland CJ, Griffith TM. Role of heterocellular gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochemical and Biophysical Research Communications. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. Journal of Cell Biology. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, Moore SF, Willoughby KA. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. American Journal of Physiology. 1995;269:H1859–1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, Cullinan GJ, Hunden DC, Johnson DW, Chaney MO, Koppel GA, Brownstein M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. Journal of Pharmacology and Experimental Therapeutics. 1998;284:291–297. [PubMed] [Google Scholar]
- Fulton D, McGiff JC, Quilley J. Role of phospholipase C and phospholipase A2 in the nitric oxide-independent vasodilator effect of bradykinin in the rat perfused heart. Journal of Pharmacology and Experimental Therapeutics. 1996;278:518–526. [PubMed] [Google Scholar]
- George CH, Kendall JM, Campbell AK, Evans WH. Connexin-aequorin chimerae report cytoplasmic calcium environments along trafficking pathways leading to gap junction biogenesis in living COS-7 cells. Journal of Biological Chemistry. 1998;273:29822–29829. doi: 10.1074/jbc.273.45.29822. [DOI] [PubMed] [Google Scholar]
- Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. The Journal of Physiology. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson IR, Chaytor AT, Evans WH, Griffith TM. NO-independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication: role of gap junctions and phospholipase A2. Circulation Research. 1999;84:53–63. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- Li X, Simard JM. Multiple connexins form gap junction channels in rat basilar artery smooth muscle cells. Circulation Research. 1999;84:1277–1284. doi: 10.1161/01.res.84.11.1277. [DOI] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circulation Research. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. The Journal of Physiology. 1993;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G, Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. The Journal of Physiology. 1990;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Bissiriou I, Agboton VD, Vanhoutte PM. Bioassay of endothelium-derived hyperpolarizing factor. Biochemical and Biophysical Research Communications. 1996;221:484–488. doi: 10.1006/bbrc.1996.0621. [DOI] [PubMed] [Google Scholar]
- Mombouli J-V, Vanhoutte PM. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends in Pharmacological Sciences. 1997;18:252–256. [PubMed] [Google Scholar]
- Munari-Silem Y, Lebrethon MC, Morand I, Rousset B, Saez JM. Gap junction-mediated cell-to-cell communication in bovine and human adrenal cells. A process whereby cells increase their responsiveness to physiological corticotropin concentrations. Journal of Clinical Investigation. 1995;95:1429–1439. doi: 10.1172/JCI117813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Makarenkova H, Becker DL, Evans WH, Parnavelas JG. Basic FGF increases communication between cells of the developing neocortex. Journal of Neuroscience. 1998;18:7881–7890. doi: 10.1523/JNEUROSCI.18-19-07881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson J, Zygmunt PM, Hogestatt ED. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. British Journal of Pharmacology. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Holland M, Waldron GJ, Garland CJ, Boyle JP. Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. British Journal of Pharmacology. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-Arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. American Journal of Physiology. 1998;274:H375–381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochemical and Biophysical Research Communications. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. European Journal of Pharmacology. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Endocannabinoids: a new class of vasoactive substances. Trends in Pharmacological Sciences. 1998;19:55–58. doi: 10.1016/s0165-6147(97)01161-9. [DOI] [PubMed] [Google Scholar]
- Randall MD, McCulloch AI, Kendall DA. Comparative pharmacology of endothelium-derived hyperpolarizing factor and anandamide in rat isolated mesentery. European Journal of Pharmacology. 1997;333:191–197. doi: 10.1016/s0014-2999(97)01137-0. [DOI] [PubMed] [Google Scholar]
- Schmilinsky-Fluri G, Valiunas V, Willi M, Weingart R. Modulation of cardiac gap junctions: the mode of action of arachidonic acid. Journal of Molecular and Cellular Cardiology. 1997;29:1703–1713. doi: 10.1006/jmcc.1997.0409. [DOI] [PubMed] [Google Scholar]
- Spagnoli LG, Villaschi S, Neri L, Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982;38:124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Taylor HJ, Chaytor AT, Evans WH, Griffith TM. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18-α-glycyrrhetinic acid. British Journal of Pharmacology. 1998;125:1–4. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Warner A, Clements DK, Parikh S, Evans WH, Dehaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. The Journal of Physiology. 1995;488:721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R, Bukauskas FF. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism. Pflügers Archiv. 1998;435:301–309. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. British Journal of Pharmacology. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Imaeda K, Suzuki H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. The Journal of Physiology. 1999;514:505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Nicholson BJ. Structure of gap junction intercellular channels. Current Opinion in Structural Biology. 1996;6:183–192. doi: 10.1016/s0959-440x(96)80073-x. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Edwards G, Weston AH, Larsson B, Högestätt ED. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. British Journal of Pharmacology. 1997a;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Högestätt ED, Waldeck K, Edwards G, Kirkup AJ, Weston AH. Studies on the effects of anandamide in rat hepatic artery. British Journal of Pharmacology. 1997b;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]


