Abstract
We examined the effects of P2X purinoceptor agonists and P2 purinoceptor antagonists on mesenteric afferent nerves supplying the jejunum in the pentobarbitone sodium-anaesthetised rat.
ATP (0.01–10 mg kg−1, i.a.) and α,β-methylene-ATP (1–30 μg kg−1, i.a.) each induced dose-dependent increases in afferent nerve discharge and intrajejunal pressure. The effect on afferent nerves comprised an early (< 2 s after administration) intense burst of activity followed by a later increase (> 2 s after administration), less pronounced in comparison, which coincided with elevated intrajejunal pressure.
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (20 mg kg−1, i.v.) and suramin (80 mg kg−1, i.v.) each antagonised both the early and later increases in afferent nerve discharge elicited by α,β-methylene-ATP (30 μg kg−1, i.a.).
Co-administration of ω-conotoxin MVIIA and ω-conotoxin SVIB (each at 25 μg kg−1, i.v.), or treatment with the selective 5-HT3 receptor antagonist alosetron (30 μg kg−1, i.v.), did not affect the rapid burst of afferent nerve activity elicited by α,β-methylene-ATP (30 μg kg−1, i.a.). However, toxin treatment did attenuate the elevations in intrajejunal pressure and the corresponding later phases of evoked afferent discharge, while alosetron inhibited basal afferent nerve activity.
In summary, ATP and α,β-methylene-ATP each evoke excitation of mesenteric afferent nerves in the anaesthetised rat. We propose that the early increase in mesenteric afferent nerve activity represents a direct effect on the nerve ending, mediated by P2X receptors, whereas the later increase reflects activation of mechanosensitive fibres secondary to elevated intrajejunal pressure.
A multiplicity of ionotropic P2X and metabotropic P2Y purinoceptors mediate the now well-established extracellular role of ATP as a neurotransmitter (for reviews see Humphrey et al. 1998; Harden et al. 1998). Amongst its many important physiological actions, ATP may subserve as an extracellular transducer of sensory stimuli (see Burnstock & Wood, 1996, for review; Nakamura & Strittmatter, 1996; Cook et al. 1997). For example, ATP and analogues activate inward currents in cells dispersed from amphibian and mammalian sensory ganglia (Krishtal et al. 1988; Bean, 1990; Evans et al. 1992; Khakh et al. 1995; Robertson et al. 1996). The biophysical characteristics of these currents suggested that they were mediated by ionotropic P2X receptors and, indeed, mRNA for several P2X receptors is present in these neural tissues (Chen et al. 1995; Lewis et al. 1995; Kidd et al. 1995; Collo et al. 1996). Moreover, ATP stimulates mammalian dorsal horn neurones (Jahr & Jessell, 1983), depolarises isolated vagus nerve trunks (Trezise et al. 1994) and evokes excitation of cutaneous (Bleehen, 1978), visceral (Pelleg & Hurt, 1996) and knee joint afferent nerves (Dowd et al. 1998a) and carotid chemoreceptors (McQueen et al. 1998). These latter four effects would also appear to be mediated through the activation of ionotropic P2X receptors (Trezise et al. 1994; Pelleg & Hurt, 1996; Dowd et al. 1998a; McQueen et al. 1998). Furthermore, both the ability of exogenous ATP and analogues to stimulate pain (Bleehen & Keele, 1977; Bland-Ward & Humphrey, 1997; Hamilton et al. 1999) and the analgesic effect of P2 receptor antagonists in human and animal pain models (Ho et al. 1992; Driessen et al. 1994) are consistent with a role for ATP as a physiological mediator of a form of nociception.
Within the gastrointestinal tract, there is abundant evidence that ATP acts as a neurotransmitter, being released from either extrinsic sympathetic efferent nerves (Burnstock, 1990; Vanner & Surprenant, 1996) or from intrinsic or enteric neurones (Galligan & Bertrand, 1994). Patch-clamp experiments performed on cells obtained from nodose and dorsal root ganglion cells (the locations of the cell bodies of afferent nerves which project to the intestines along vagal and spinal pathways, respectively) suggest that ATP, via the stimulation of P2X receptors, may be involved in the perception of intraluminal and, perhaps, even nociceptive stimuli arising from the gut (Lewis et al. 1995; Khakh et al. 1995; Robertson et al. 1996). However, there is no direct evidence for the presence of functional P2X receptors on the terminals of either intrinsic or extrinsic intestinal afferent nerves. Since ATP is released from cells at sites of tissue injury (see Kennedy & Leff, 1995; Burnstock & Wood, 1996), an effect of this substance at P2X receptors present on intestinal afferent nerve endings may contribute to the abdominal discomfort and pain associated with functional and organic disorders of the bowel. Thus it was pertinent to determine whether ATP affected intestinal afferent nerve discharge and to establish the mechanism(s) involved. Therefore, in the present study we examined the effects of ATP and the selective P2X receptor agonist α,β-methylene-ATP and the P2 receptor antagonists pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and suramin on the activity of extrinsic intestinal afferent nerves supplying the jejunum in the anaesthetised rat.
METHODS
Animals
Experiments were performed using 46 Sheffield-strain male Wistar rats (300-450 g), which were allowed free access to food and water. General anaesthesia was produced with an intraperitoneal injection of pentobarbitone sodium (60 mg kg−1) and maintained by intravenous (i.v.) infusion (0.5-1 mg kg−1 min−1). The depth of general anaesthesia was assessed (every 30-45 min) by observing whether the animal's blood pressure was maintained at a constant level and/or whether it was perturbed after pinching a forepaw. The infusion rate of anaesthetic was adjusted accordingly. After completion of an experiment, animals were killed by an anaesthetic overdose followed by exsanguination.
Surgical procedures
The surgical techniques and other experimental methods are documented extensively elsewhere (Kirkup et al. 1998). Briefly, a mid-line incision was made in the neck and the trachea was intubated to maintain a patent airway. The right external jugular vein was bi-cannulated with two saline-filled cannulae, one to enable anaesthetic infusion and the other for the systemic administration of drugs. The left common carotid artery was cannulated with a heparinised catheter (200 units ml−1 heparin in 0.9 % w/v NaCl solution) to facilitate arterial pressure recording (Elcomatic EM760). The heart rate was electronically derived from this pressure record. A fine, heparinised cannula was passed up the left femoral artery to about 5 mm rostral to the junction of the abdominal aorta with the coeliac artery (the position of the tip of the cannula was confirmed post mortem). This was in order to permit the close intra-arterial (i.a.) administration of drugs. Body temperature was monitored and maintained at around 37°C by a rectal thermistor-controlled heating blanket.
A mid-line laparotomy was performed and the caecum was excised in order to create a greater operating area within the peritoneal cavity. A 10-15 cm length of proximal jejunum was located (typically 1-5 cm from the ligament of Trietz). This was isolated with ligatures and incisions were made on the anterior mesenteric border at each end. The loop was intubated with Portex tubing (PP100) passed through the right abdominal wall via two small incisions. The saline-filled aboral cannula contained two smaller bore saline-filled Portex tubes (PP30), one for the introduction of saline to produce distension (applications were drained via the oral cannula) and the other was connected to a transducer (Elcomatic EM760) to record intrajejunal pressure. The jejunal cannulae were closed to provide isovolumic recording conditions and the isolated loop was filled with saline to a pressure of around 5 cmH2O. This pressure was maintained by saline perfusion (0.4-1.0 ml h−1) through the aboral cannula. The small abdominal wall incisions were sutured and the muscle and skin of the perimeter of the large abdominal incision were sewn to a steel ring and the resulting well was subsequently filled with pre-warmed (37°C) liquid paraffin.
Nerve preparation and afferent recording
A mesenteric arcade supplying the isolated loop of jejunum was placed on a black Perspex platform and a single paravascular nerve bundle was dissected out, severed distally from the wall of the jejunum (approximately 1-1.5 cm) in order to eliminate the recording of efferent nerve activity, and cleaned. It was then attached to one of a pair of platinum recording electrodes, with a length of connective tissue wrapped around the other to act as a reference. The electrodes were connected to a Neurolog head stage (NL100; Digitimer, Welwyn Garden City, UK) and the signal was differentially amplified (NL103) and filtered with a bandwidth of 100-1000 Hz (NL125). This filtered afferent nerve recording was digitised (PCM-2 A/D VCR adaptor; Medical Systems Corp., Greenvale, NY, USA) and recorded on videotape (Saisho, VR3300X) for post-experimental analysis. The afferent nerve recording was also relayed to a spike processor (Digitimer D130) and to enable on-line viewing and data capture, the outputs from this, together with the signals from the arterial and intrajejunal pressure transducers, were relayed, via a 1401 plus interface board (Cambridge Electronic Design (CED), Cambridge, UK), to a PC running Spike 2 software (CED).
Experimental protocols
After a 45-60 min stabilisation period, the viability of the preparation was assessed with an i.v. bolus dose of 2-methyl-5-hydroxytryptamine (2-methyl-5-HT; 10 μg) and, after a further 5-10 min, the intestinal loop was distended for 15 s with the rapid introduction (< 1 s) of 0.5 and/or 1.0 ml of isothermic saline, which was subsequently drained away. Afferent nerve preparations which failed to respond to the test stimuli were discarded. For dose- response studies, sequential doses of the P2X receptor agonist in use were administered with the interval between incremental doses being sufficient to permit the recovery of variables to their basal values. In the studies with the P2 receptor antagonists, preparations were challenged every 15 min with the selective P2X receptor agonist α,β-methylene-ATP, in order to determine the reproducibility of the responses evoked by the nucleotide. The animal was then treated i.v. with either the vehicle or the antagonist (infused slowly over a period of 1 min in a 1.6 ml injection volume), 5 min prior to a subsequent challenge with the agonist. α,β-Methylene-ATP was then administered every 15 min on at least two more occasions in order to assess the duration of action of the anatgonist in use. In the investigations of the effects of the selective 5-HT3 receptor antagonist alosetron, or the ω-conotoxins MVIIA and SVIB, preparations were initially challenged with α,β-methylene-ATP. Alosetron or the toxins were then administered (in the same injection volume and manner as the P2 receptor antagonists) 5 min before the next challenge with the nucleotide. In some experiments, after completion of the experimental protocol, the preparations were then challenged with the initial test stimuli. Agonists were administered either i.a. or i.v. in a 0.05 or 0.10 ml dose volume, respectively, followed by a flush of 0.5 ml saline.
Analysis of data
Effects of agents on the parameters of blood pressure, heart rate, intrajejunal pressure and mesenteric afferent nerve activity, expressed in units of millimetres of mercury, beats per minute, centimetres of water and spikes per second, respectively, were calculated as described previously (Kirkup et al. 1998). Data are presented as the arithmetic mean of either the absolute or percentage of control ±s.e.m. from four or more animals per vehicle-, antagonist- or toxin-treated or -untreated group. Where n values are given they refer to the number of animals. Significant differences between data points were determined by appropriate use of either parametric or non-parametric ANOVA followed by Student's paired t test (with Bonferroni corrections where applicable) or the Mann-Whitney U test, respectively. A probability of P < 0.05 was considered statistically significant.
Drugs
ATP (sodium salt), α,β-methylene-ATP (lithium salt), bethanechol (chloride salt) and L-phenylephrine (hydrochloride salt) were obtained from Sigma. ω-Conotoxin MVIIA was obtained from Latoxan, Rosans, France. ω-Conotoxin SVIB was purchased from Alomone Laboratories, Jerusalem, Israel. Alosetron (hydrochloride salt) was obtained from GlaxoWellcome Research & Development Ltd, Stevenage, UK. 2-Methyl-5-HT (maleate salt) and suramin (hexasodium salt) were purchased from Research Biochemicals Inc., Poole, Dorset, UK. PPADS (tetrasodium salt) was obtained from both Research Biochemicals Inc. and Tocris Cookson Ltd, Bristol, UK.
ATP, α,β-methylene-ATP, alosetron, bethanechol, ω-conotoxin MVIIA, ω-conotoxin SVIB, 2-methyl-5-HT, L-phenylephrine, PPADS and suramin were each dissolved in saline. Intravenous administration of corresponding volumes of saline vehicle produced transient (lasting for < 5 s) disturbances in blood pressure and heart rate. In addition, in some preparations, i.a. injection of saline vehicle induced a small (< 0.5 cmH2O), short-lived (< 10 s) increase in intrajejunal pressure.
RESULTS
Effects of ATP and α,β-methylene-ATP
In this initial series of experiments, all of the mesenteric afferent nerve bundles tested (15 out of 15) responded to either ATP (0.01-10 mg kg−1, i.a.; n= 7) or the selective P2X receptor agonist α,β-methylene-ATP (1-30 μg kg−1, i.a.; n= 8), in a dose-dependent manner (Fig. 1). For both agonists, the peak effects did not achieve maxima over the dose ranges tested but α,β-methylene-ATP was apparently 30-100-fold more potent than ATP (Fig. 1). The pattern of the mesenteric afferent nerve response to the nucleotides comprised an early (primary) burst of action potentials which, at doses of 3 mg kg−1 ATP and 30 μg kg−1α,β-methylene-ATP, occurred 1.4 ± 0.3 s (range, 0.8-2.4 s) and 1.5 ± 0.2 s (range, 0.5-2.3 s), respectively, after administration (Fig. 2). The overall duration of this primary response was 3.3 ± 0.7 s (range, 1.2-7.0 s) and 3.7 ± 0.9 s (range, 0.9-7.7 s) for ATP and α,β-methylene-ATP, respectively (Fig. 2). The early burst of afferent nerve discharge induced by ATP and α,β-methylene-ATP was followed in seven out of seven and five out of eight preparations, respectively, by a delayed and more sustained later (secondary) phase of afferent nerve firing which was less intense in comparison to the primary burst (Fig. 2). This later phase of afferent nerve discharge evoked by ATP and α,β-methylene-ATP peaked 13.3 ± 1.7 s (range, 5.9-16.1 s; n= 7) and 36.3 ± 12.4 s (range, 8.8-64.0 s; n= 5) after administration, respectively.
Figure 1. Dose-response curves of the effects of close arterial administration of ATP and α,β-methylene-ATP.
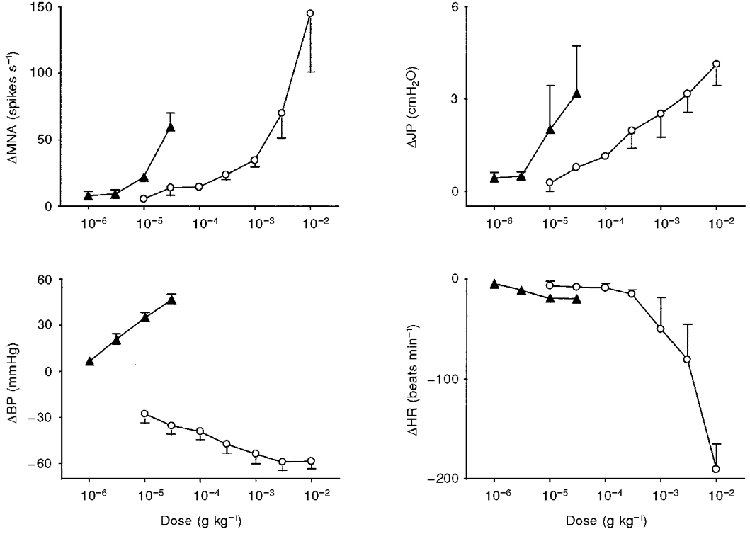
Values are means ±s.e.m. for 7-8 experiments of the peak change in these variables induced by either ATP (^) or α,β-methylene-ATP (▴). Abbreviations: MNA, mesenteric afferent nerve activity; JP, intrajejunal pressure; BP, blood pressure; HR, heart rate.
Figure 2. Representative traces of simultaneous recordings of the effects of ATP and α,β-methylene-ATP on intrajejunal pressure and whole mesenteric afferent nerve activity in the anaesthetised rat.
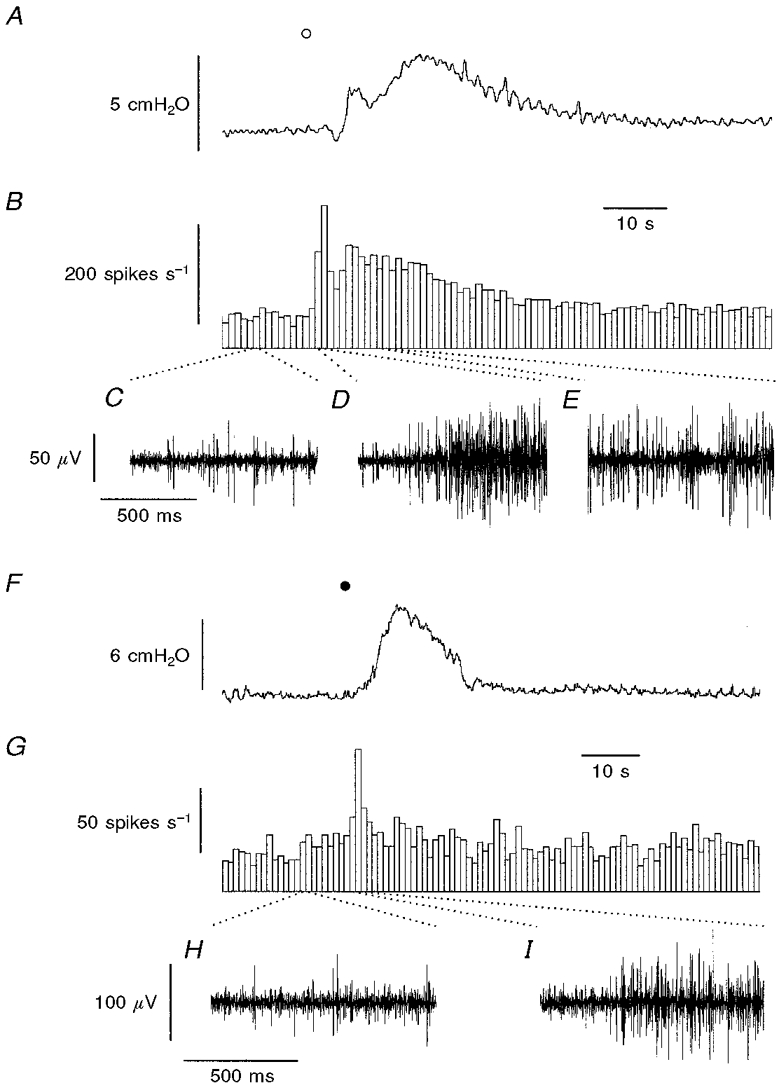
A shows the effects of ATP (^; 3 mg kg−1, i.a.) on intrajejunal pressure. B, sequential frequency histogram depicting whole mesenteric nerve activity, below which are 1 s recordings of the afferent nerve activity before (C) and during the early (D) and late phases (E) of discharge evoked by the agonist. F and G illustrate the effects of α,β-methylene-ATP (•; 30 μg kg−1, i.a.) on intrajejunal pressure (F) and whole nerve activity (G), below which are 1 s samples of basal afferent discharge (H) and the early burst (I) of activity induced by the nucleotide. The responses were obtained from different preparations.
In addition to these effects on mesenteric afferent nerve activity, ATP and α,β-methylene-ATP each induced dose-dependent increases in intrajejunal pressure which, again, failed to achieve maxima over the dose ranges tested (Fig. 1). There were, however, temporal distinctions between the initiation of the elevations in mesenteric afferent nerve activity and intrajejunal pressure produced by the agonists (Fig. 2). The latencies from administration for the rises in intrajejunal pressure evoked by ATP (3 mg kg−1) and α,β-methylene-ATP (30 μg kg−1) were significantly greater than those for the onset of the primary burst of afferent nerve discharge (ATP, 4.6 ± 0.6 s; range, 2.4-6.4 s; P < 0.001, n= 7; α,β-methylene-ATP, 8.0 ± 1.8 s; range, 3.5-16.8 s; P < 0.05, n= 8; Fig. 2). The elevations in intrajejunal pressure induced by ATP comprised two phases (6 out of 7 animals; Fig. 2A), whilst α,β-methylene-ATP typically induced a monophasic response (6 out of 8 preparations; Fig. 2F). Nevertheless, the increases in intrajejunal pressure evoked by ATP and α,β-methylene-ATP peaked 9.0 ± 2.4 s (range, 6.0-22.4 s; n= 7) and 28.4 ± 5.2 s (range, 9.1-53.0 s; n= 8), respectively, after administration, similar times to the peak of the late burst of afferent nerve discharge induced by the nucleotides in these preparations.
ATP and α,β-methylene-ATP also produced marked cardiovascular effects (Fig. 1). Each nucleotide evoked a dose-dependent bradycardia although over the dose ranges tested this effect was most profound for ATP (Fig. 1). Furthermore, each agent had contrasting effects on blood pressure. The dominant effect of ATP on this variable was a dose-dependent hypotension (Fig. 1), although at higher doses (> 100 μg kg−1) this effect was preceded by a transient elevation in blood pressure (not shown). α,β-Methylene-ATP induced only a dose-dependent hypertension in comparison (Fig. 1). To determine the effects of elevations in blood pressure on the discharge of mesenteric afferent nerves, the effects of the selective α1-adrenoceptor agonist L-phenylephrine (10 μg kg−1, i.a.), and α,β-methylene-ATP (30 μg kg−1, i.a.), were assessed in four other preparations. Each agonist induced a hypertension of a similar magnitude (L-phenylephrine, 50 ± 5 mmHg; range, 40-57 mmHg; α,β-methylene-ATP, 48 ± 6 mmHg; range, 37-59 mmHg). However, L-phenylephrine did not evoke a discharge of jejunal afferent nerve action potentials on administration and did not affect ongoing afferent nerve activity (mean basal discharge rate, 38 ± 12 spikes s−1; range, 21-63 spikes s−1; mean discharge rate over 30 s period after administration of L-phenylephrine, 38 ± 12 spikes s−1; range, 21-60 spikes s−1). In contrast, α,β-methylene-ATP rapidly evoked (< 2 s) a volley of afferent nerve firing after injection which attained a mean peak discharge frequency of 70 ± 14 spikes s−1 (range, 34-86 spikes s−1).
Effects of PPADS and suramin
To obtain evidence that the effects of the nucleotides were mediated through an interaction with P2 receptors, we sought to investigate their sensitivity to the P2 receptor antagonists PPADS and suramin. The actions of the antagonists were only investigated on the responses produced by α,β-methylene-ATP since this analogue is less susceptible than ATP to breakdown by nucleotidases which themselves are also inhibited by the P2 receptor antagonists. A phenomenon often encountered when using α,β-methylene-ATP in vitro is desensitisation. However, pilot experiments showed that the effects of α,β-methylene-ATP (30 μg kg−1, i.a.) displayed no desensitisation when the nucleotide was administered every 15 min (Figs 3A and B and 4). Furthermore, treatment with saline vehicle (5 min prior to the next challenge with the agonist) in these seven preparations did not significantly affect the peak amplitude of the responses evoked by α,β-methylene-ATP (Fig. 4). Moreover, treatment with saline did not modulate the late peak afferent nerve discharge evoked by of α,β-methylene-ATP which was observed in five of these seven preparations (the magnitude of this response after saline vehicle was 126 ± 11 % (range, 94-150 %) of the response prior to treatment). The effects of PPADS (20 mg kg−1, i.v.) were assessed in five experiments. Administration of PPADS 5 min before the next dose of α,β-methylene-ATP significantly attenuated the peak effects of the nucleotide on the early peak afferent nerve discharge and on all of the other variables measured (Figs 3C and 4). In addition, treatment with PPADS attenuated the late peak burst of afferent nerve activity which occurred in four of these preparations to 40 ± 18 % of the response before treatment (range, 2-80 %; P < 0.05 compared with saline-treated group). The antagonism produced by PPADS was relatively short-lived and the peak responses to α,β-methylene-ATP exhibited some recovery 20 min after treatment with the antagonist (Fig. 4). In one experiment, the peak effects of α,β-methylene-ATP had almost regained their initial magnitude 65 min after injection of PPADS (Fig. 3D). The antagonistic effects of suramin (80 mg kg−1, i.v.) were examined in another six experiments. Treatment with suramin 5 min before the next administration of α,β-methylene-ATP inhibited the peak effects of the agonist on the early peak mesenteric afferent nerve activity and intrajejunal pressure (Fig. 4). Furthermore, in the five preparations which produced a late discharge of action potentials in response to α,β-methylene-ATP, suramin treatment abrogated the peak level to 57 ± 8 % (range, 44-85 %; P < 0.01, compared with saline-treated group) of the response which occurred prior to treatment. Similar to PPADS, the inhibitory effect of suramin was transient, but more so (Fig. 4). Treatment with vehicle or suramin did not affect the baseline values of the variables (Table 1). PPADS, however, produced small effects on the basal values of the blood pressure and heart rate (Table 1). Nevertheless, administration of the vehicle or either of the P2 receptor antagonists did not significantly affect any of the peak responses induced either by injection of 2-methyl-5-HT (10 μg, i.v.) or by distension of the isolated segment of jejunum with 0.5 ml of saline (Table 2).
Figure 3. Representative traces of simultaneous recordings of the effect of PPADS on responses produced by α,β-methylene-ATP.
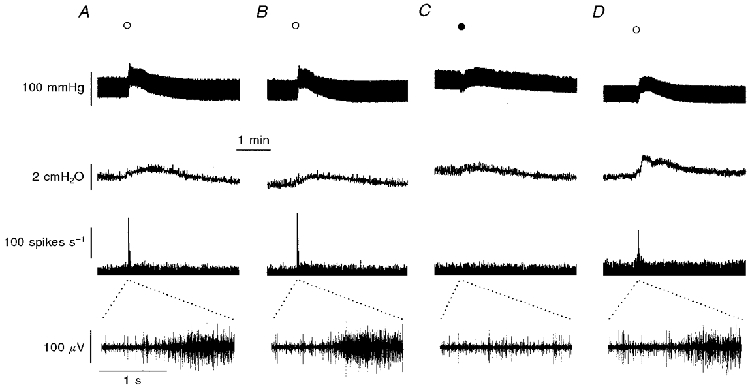
A and B, prior to treatment with PPADS (20 mg kg−1, i.v.), administration of α,β-methylene-ATP (^; 30 μg kg−1, i.a.) at 15 min intervals evoked reproducible effects on (from uppermost to lowermost trace) blood pressure, intrajejunal pressure and whole mesenteric afferent nerve activity. The lowermost trace is a 2 s extracellular recording of afferent nerve activity from the time point on the whole nerve activity trace as denoted by the dotted lines. C, PPADS administered 5 min before the next challenge with α,β-methylene-ATP (•; 30 μg kg−1, i.a.) attenuated the effects of the nucleotide on blood and intrajejunal pressure and abolished the mesenteric afferent nerve response. D, the antagonism produced by PPADS was not long-lasting, and 65 min after its injection, the responses evoked by α,β-methylene-ATP (^; 30 μg kg−1, i.a.) had almost recovered.
Figure 4. Effects of treatment with vehicle, PPADS or suramin on responses evoked by α,β-methylene-ATP.
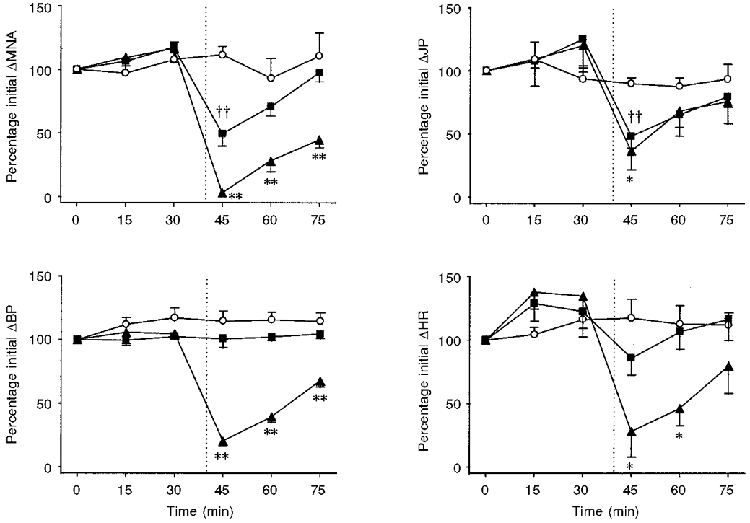
Values are means ±s.e.m. for 5-7 experiments of the peak change induced by α,β-methylene-ATP (30 μg kg−1, i.a.) as a percentage of the initial response evoked by the nucleotide in animals treated with either the vehicle (^; 0.9 % saline, i.v.), PPADS (▴; 20 mg kg−1, i.v.) or suramin (▪; 80 mg kg−1, i.v.). The vertical dotted lines denote the time point of administration of vehicle or antagonist. **P < 0.01, *P < 0.05, significant difference between vehicle- and PPADS-treated group. ††P < 0.01, significant difference between vehicle- and suramin-treated group. For abbreviations, see Fig. 1.
Table 1.
Effects of i.v. administration of saline vehicle, PPADS (20 mg kg−1) or suramin (80 mg kg−1) on baseline values of the variables of mesenteric afferent nerve activity (MNA), intrajejunal pressure (JP), blood pressure (BP) and heart rate (HR)
| MNA(spikes s−1) | JP(cmH2O) | BP(mmHg) | HR(beats min−1) | |
|---|---|---|---|---|
| Saline vehicle (n= 7) | ||||
| Pre | 22 ± 3 (11–33) | 5.3 ± 0.7 (3.0–8.7) | 105 ± 4 (85–115) | 359 ± 16 (315–419) |
| Post | 22 ± 3 (10–32) | 5.5 ± 0.7 (3.1–8.8) | 108 ± 5 (89–119) | 358 ± 15 (305–417) |
| PPADS (n= 5) | ||||
| Pre | 33 ± 8 (21–62) | 4.4 ± 0.7 (3.0–6.7) | 108 ± 13 (84–150) | 350 ± 21 (313–409) |
| Post | 36 ± 8 (28–64) | 4.3 ± 0.2 (3.8–4.6) | 124 ± 15 (88–168)* | 333 ± 17 (304–378)* |
| Suramin (n= 6) | ||||
| Pre | 26 ± 6 (13–43) | 4.7 ± 0.6 (2.9–6.6) | 104 ± 4 (91–111) | 383 ± 11 (358–417) |
| Post | 32 ± 9 (13–56) | 5.6 ± 0.9 (3.1–9.0) | 114 ± 7 (86–133) | 372 ± 14 (333–428) |
Pre, baseline value 1 min before treatment with vehicle or an antagonist; Post, baseline value 5 min after treatment with vehicle or an antagonist. Values are means ±s.e.m. (n, number of animals). The ranges are given in parentheses.
P < 0.05, significantly different from values determined before an antagonist treatment.
Table 2.
Effects of i.v. administration of saline vehicle, PPADS (20 mg kg−1) or suramin (80 mg kg−1) on peak changes induced by 2-methyl-5-HT (10 μg, i.v.) or by distension of the jejunum by 0.5 ml saline on the variables of mesenteric afferent nerve activity (μmNA), intrajejunal pressure (ΔJP), blood pressure (ΔBP) and heart rate (ΔHR)
| μmNA(spikes s−1) | ΔJP(cmH2O) | ΔBP(mmHg) | ΔHR(beats min−1) | |
|---|---|---|---|---|
| Saline vehicle (n= 7) | ||||
| 2-methyl-5HT | ||||
| Pre | 110 ± 23 (76–112) | 2.2 ± 0.2 (1.6–2.9) | −21 ± 6 (−33 to -5) | −117 ± 43 (−245 to -17) |
| Post | 137 ± 24 (81–208) | 2.1 ± 0.3 (1.4–2.7) | −13 ± 4 (−24 to -5) | −106 ± 36 (−202 to -20) |
| Distension | ||||
| Pre | 82 ± 12 (44–127) | 33.3 ± 8.3 (9.6–56.1) | 1 ± 1 (0–4) | 0 ± 1 (−2 to 2) |
| emsp;Post | 76 ± 17 (47–136) | 32.3 ± 8.9 (6.9–55.7) | −1 ± 1 (−3 to 1) | 0 ± 1 (−5 to 3) |
| PPADS (n= 5) | ||||
| emsp;2-methyl-5-HT | ||||
| Pre | 113 ± 23 (79–194) | 4.0 ± 1.9 (1.7–10.9) | −20 ± 7 (−33 to -5) | −69 ± 44 (−226 to -17) |
| Post | 142 ± 20 (93–194) | 4.2 ± 2.2 (1.8–12.0) | −13 ± 5 (−26 to -5) | −86 ± 38 (−202 to -20) |
| Distension | ||||
| Pre | 101 ± 23 (44–164) | 34.7 ± 7.2 (16.6–56.1) | 1 ± 1 (−2 to 4) | 1 ± 1 (−2 to 4) |
| Post | 91 ± 23 (54–146) | 27.2 ± 9.5 (6.9–49.2) | 0.4 ± 0.4 (−1 to 1) | −1 ± 2 (−8 to 2) |
| Suramin (n= 6) | ||||
| 2-methyl-5-HT | ||||
| Pre | 164 ± 37 (87–310) | 4.4 ± 1.2 (1.4–9.1) | −17 ± 3 (−24 to -4) | −88 ± 28 (−189 to -18) |
| Post | 168 ± 36 (84–280) | 3.3 ± 1.0 (1.4–7.2) | −17 ± 3 (−26 to -5) | −108 ± 37 (−236 to -24) |
| Distension | ||||
| Pre | 90 ± 17 (53–156) | 27.2 ± 7.2 (10.8–55.7) | −1 ± 1 (−3 to 3) | 1 ± 1 (0–3) |
| Post | 107 ± 27 (49–215) | 35.5 ± 7.3 (12.4–55.4) | 0 ± 1 (−3 to 1) | 3 ± 1 (−1 to 6) |
Pre, peak response evoked by 2-methyl-5-HT or distension 10 min before treatment with vehicle or an antagonist; Post, peak response evoked by 2-methyl-5-HT or distension 5 min after treatment with vehicle or an antagonist. Values are means ±s.e.m. (n, number of animals). The ranges are given in parentheses. No significant differences were found between the peak responses evoked before and after treatment with either the vehicle or antagonist.
Effects of ω-conotoxin MVIIA and ω-conotoxin SVIB
The responses evoked by α,β-methylene-ATP (30 μg kg−1, i.a.) in the jejunum may have been secondary to the excitation of enteric neuronal circuits. In order to establish whether this was the case, we investigated the effects of co-administration of the Ca2+ channel toxins, ω-conotoxin MVIIA (25 μg kg−1, i.v.; N-type selective) and ω-conotoxin SVIB (25 μg kg−1, i.v.; P- and Q-type selective) on the events elicited by the nucleotide. In the four preparations used, injection of α,β-methylene-ATP (30 μg kg−1, i.a.) prior to treatment with the toxins evoked an early burst of action potentials and an elevation in intrajejunal pressure with a corresponding late phase of mesenteric afferent nerve discharge (Fig. 5A), in addition to haemodynamic effects (not shown). Administration of the combination of toxins, 5 min before the next challenge with the nucleotide, produced significant effects on the cardiovascular variables (Table 3). Nevertheless, the peak early bursts of mesenteric afferent nerve discharge initiated by α,β-methylene-ATP were unaffected by the toxin treatment (Fig. 5B and C) although the peak elevations in intrajejunal pressure produced by the agonist were attenuated and the associated bursts of afferent activity (which occurred in 3 of these 4 preparations) were also reduced to 52 ± 7 % (range, 44-64 %; P < 0.05, compared with saline-treated group) of the response prior to toxin administration (Fig. 5B and C). Furthermore, the toxin treatment attenuated the peak bradycardia evoked by α,β-methylene-ATP (pre-toxins, -45 ± 7 beats min−1; range, -58 to -31 beats min−1; post-toxins, -15 ± 3 beats min−1; range, -17 to -8 beats min−1; P < 0.01). The peak hypertension induced by the nucleotide was, however, augmented by the ω-conotoxins (pre-toxins, 43 ± 2 mmHg; range, 38-45 mmHg; post-toxins, 52 ± 3 mmHg; range, 46-59 mmHg; P < 0.05). Treatment with the toxins also modulated some of the peak responses elicited by 2-methyl-5-HT (10 μg, i.v.). The peak primary burst of afferent discharge induced by the amine was unchanged by the toxin treatment (Fig. 5D). However, administration of ω-conotoxins MVIIA and SVIB inhibited the 2-methyl-5-HT-evoked increases in intrajejunal pressure (Fig. 5D), bradycardia (pre-toxins, -46 ± 12 beats min−1; range, -68 to -27 beats min−1; post-toxins, -8 ± 1 beats min−1; range, -10 to -6 beats min−1; P < 0.05) and hypotension (pre-toxins, -18 ± 2 mmHg; range, -21 to -12 mmHg; post-toxins, 9 ± 2 mmHg; range, 7-11 mmHg; P < 0.05). In one experiment, toxin treatment had no observable effect on the peak increases in mesenteric afferent nerve discharge and intrajejunal pressure and bradycardia evoked by the muscarinic receptor agonist bethanechol (100 μg kg−1, i.v.; not shown).
Figure 5. Effects of treatment with both ω-conotoxin MVIIA and ω-conotoxin SVIB on the responses elicited by α,β-methylene-ATP and 2-methyl-5-HT.
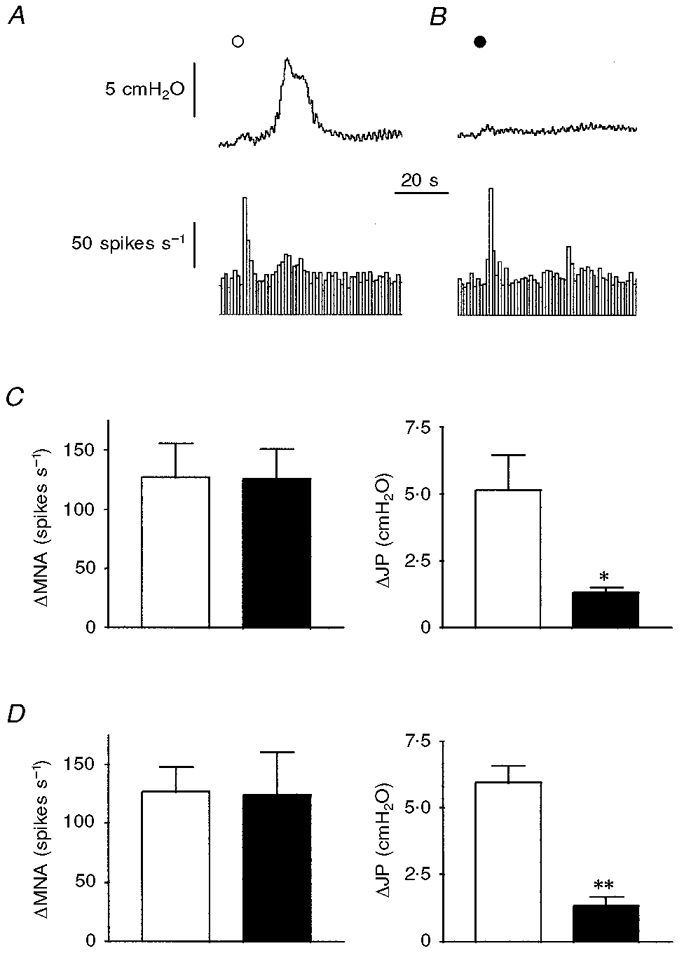
A, administration of α,β-methylene-ATP (^; 30 μg kg−1, i.a.) evoked an increase in intrajejunal pressure (upper trace) and a rapid burst of afferent discharge (lower trace) followed by a later burst which corresponded temporally with the pressure elevation. B, after treatment with ω-conotoxin MVIIA and ω-conotoxin SVIB (each at 25 μg kg−1, i.v.) for 5 min, the early burst of afferent activity elicited by α,β-methylene-ATP (•; 30 μg kg−1, i.a.) was unaffected whereas the elevation in intrajejunal pressure and concomitant afferent activity were abolished. C and D, summarised data of the peak effects of α,β-methylene-ATP (30 μg kg−1, i.a.) and 2-methyl-5-HT (10 μg, i.v.), respectively, on afferent discharge and intrajejunal pressure before (□) and after (▪) treatment with the conotoxins. Note that only the peak increase in intrajejunal pressure evoked by either α,β-methylene-ATP (C) or 2-methyl-5-HT (D) was affected after treatment with toxin. The bars in C and D represent means ±s.e.m. for 4 experiments. **P < 0.01, *P < 0.05, significant difference between magnitude of response before and after toxin treatment. For abbreviations, see Fig. 1.
Table 3.
Effects of I.V. administration of ω-conotoxins MVIIA and SVIB (each at 25 μg kg−1) or alosetron (30 μg kg−1) on baseline values of the variables of mesenteric afferent nerve activity (MNA), intrajejunal pressure (JP), blood pressure (BP) and heart rate (HR)
| MNA(spikes s−1) | JP(cmH2O) | BP(mmHg) | HR(beats min−1) | |
|---|---|---|---|---|
| ω-Conotoxins MVIIA and SVIB (n= 4) | ||||
| Pre | 50 ± 8 (32–65) | 9.2 ± 1.1 (7.4–10.9) | 99 ± 15 (63–124) | 387 ± 28 (352–457) |
| Post | 59 ± 12 (31–72) | 9.3 ± 0.8 (7.6–10.7) | 67 ± 9 (49–85)* | 319 ± 17 (290–359)** |
| Alosetron (n= 5) | ||||
| Pre | 46 ± 9 (24–68) | 7.0 ± 1.0 (4.2–9.8) | 105 ± 15 (54–134) | 430 ± 35 (374–550) |
| Post | 36 ± 6 (23–52)* | 6.9 ± 1.1 (4.0–8.9) | 107 ± 16 (53–127) | 433 ± 35 (365–552) |
Pre, value 1 min before treatment with toxin or antagonist; Post, value 5 min after treatment with toxin or antagonist. Values are means ±s.e.m. (n, number of animals). The ranges are given in parentheses.
P < 0.01
P < 0.05 significantly different from values determined before treatment with toxin or antagonist.
Effects of alosetron
We have shown previously that exogenous 5-HT evokes a brief but intense stimulation of mesenteric afferent nerves through the activation of 5-HT3 receptors (Hillsley et al. 1998; Hillsley & Grundy, 1998). Thus, the possibility existed that the excitation of mesenteric afferent nerves elicited by the nucleotides may have occurred secondary to the release of 5-HT stored within enterochromaffin (EC) cells present within the intestinal mucosa. We therefore investigated, in five experiments, the responses elicited by α,β-methylene-ATP (30 μg kg−1, i.a.) before and 5 min after treatment with the potent and selective 5-HT3 receptor antagonist alosetron (30 μg kg−1, i.v.). Administration of the antagonist produced a significant depression of basal mesenteric afferent nerve activity but did not affect the baseline values of the other variables (Table 3). Nevertheless, treatment with alosetron did not significantly affect the peak early bursts of mesenteric afferent nerve activity or the peak elevations in intrajejunal pressure evoked by the nucleotide (Fig. 6A-C). In addition, the late peak burst of afferent nerve discharge associated with the pressure increases was unaffected after alosetron (129 ± 29 %; range, 88-204 %; P > 0.05, compared with saline-treated group) in the four out of five preparations that generated this response. Furthermore, alosetron did not modulate the α,β-methylene-ATP-evoked pressor response (pre-alosetron, 45 ± 5 mmHg; range, 37-61 mmHg; post-alosetron, 46 ± 7 mmHg; range, 33-62 mmHg) or bradycardia (pre-alosetron, -51 ± 13 beats min−1; range, -82 to -21 beats min−1; post-alosetron, -52 ± 13 beats min−1; range, -95 to -31 beats min−1). However, alosetron inhibited the peak effects of exogenous 2-methyl-5-HT (10 μg, i.v.) on mesenteric afferent nerve activity and intrajejunal pressure (Fig. 6D) and on blood pressure (pre-alosetron, -39 ± 12 mmHg; range, -81 to -26 mmHg; post-alosetron, 7 ± 3 mmHg; range, 2-18 mmHg) and heart rate (pre-alosetron, -108 ± 50 beats min−1; range, -285 to -39 beats min−1; post-alosetron, 4 ± 3 beats min−1; range, -2 to 12 beats min−1).
Figure 6. Effects of administration of alosetron on responses evoked by α,β-methylene-ATP and 2-methyl-5-HT.
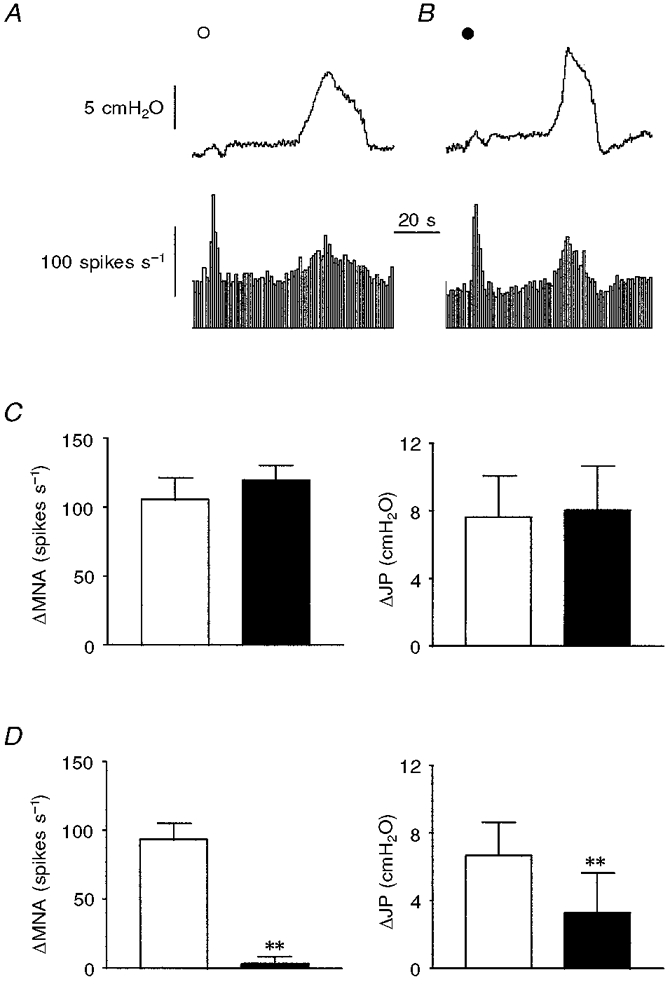
A and B, α,β-methylene-ATP (^; 30 μg kg−1, i.a.)-elicited increases in intrajejunal pressure (upper trace) and afferent activity (lower trace) (A) were not attenuated after a 5 min treatment with alosetron (•; 30 μg kg−1, i.v.) (B). C and D, pooled data of the peak effects of α,β-methylene-ATP (30 μg kg−1, i.a.) and 2-methyl-5-HT (10 μg, i.v.), respectively, on afferent discharge and intrajejunal pressure before (□) and after (▪) treatment with alosetron. Note that only the peak increases in afferent discharge and intrajejunal pressure produced by 2-methyl-5-HT (D) were affected after administration of the selective 5-HT3 receptor antagonist. The bars in C and D represent means ±s.e.m. for 5 experiments. **P < 0.01, significant difference between magnitude of response before and after alosetron. For abbreviations, see Fig. 1.
DISCUSSION
The major finding from our present study is that intra-arterial administration of ATP and the selective P2X receptor agonist α,β-methylene-ATP elicited excitation of afferent nerves supplying the jejunum in the anaesthetised rat, in conjunction with elevations in intrajejunal pressure and alterations in cardiovascular variables. With specific reference to these latter effects, ATP and α,β-methylene-ATP both produced a bradycardia which probably involves vago-vagal reflex mechanisms and, in the case of the former, a contribution from its enzymatic breakdown product, adenosine (see McQueen et al. 1998). Our present observations with the α,β-methylene-ATP-induced bradycardia are consistent with those of McQueen et al. (1998), since this effect of the nucleotide was attenuated after administration of the ω-conotoxins MVIIA and SVIB. The remaining, respective, vasopressor/depressor and vasopressor effects of ATP and α,β-methylene-ATP mirror those previously observed for these agents in pithed rats (Schlicker et al. 1989) and they thus presumably reflect the activation of receptors present on vascular smooth muscle. Interestingly, Schlicker and co-workers (Schlicker et al. 1989) demonstrated antagonism by suramin of the vasopressor effects of α,β-methylene-ATP. However, this group employed a dose of suramin (100 μmol kg−1, i.v.) approximately twice that utilised in the present study (80 mg kg−1, i.v.; which is about 53 μmol kg−1, i.v.).
The effects of ATP and α,β-methylene-ATP on intestinal afferent nerves were qualitatively similar to responses produced by 5-HT in this preparation (Hillsley et al. 1998). Namely, an initial (primary) intense burst of action potentials with a rapid onset followed by a later (secondary) burst which was less pronounced and more prolonged in comparison. In the case of 5-HT, further experiments suggested that the initial response to this agonist represented a direct action on the terminals of afferents in the bowel wall since the response was of short latency, mediated entirely by 5-HT3 receptors, which are ligand-gated cation channels and thus mediate fast responses and insensitive to pre-treatment with L- and N-type Ca2+ channel inhibitors (Hillsley et al. 1998). From the present investigation, several lines of evidence indicate a possible mechanism for the initial or primary response produced by ATP and α,β-methylene-ATP. Firstly, the initial response to the nucleotides developed rapidly, occurring within 2 s of intra-arterial administration and, crucially, before the evoked elevations in intrajejunal pressure commenced. This observation supports the view that the initial burst of firing elicited by these agonists was not secondary to motor events in the intestines. Secondly, it is unlikely that the initial response was secondary to effects on the vasculature and the heart since falls in blood pressure and heart rate per se do not evoke rapid bursts of intestinal afferent nerve activity (Kirkup et al. 1997, 1998). Furthermore, in the present study, L-phenylephrine did not have any effect on the discharge of the afferent nerves despite producing a similar pressor response to α,β-methylene-ATP. In addition, suramin produced a significant antagonism of the initial burst of action potentials induced by α,β-methylene-ATP without affecting the haemodynamic effects of the nucleotide. Thirdly, the initial response evoked by α,β-methylene-ATP was unaffected by pre-treatment with a combination of toxins which collectively inhibit N-, P- and Q-type Ca2+ channels. This suggests that the initial burst of afferent discharge evoked by α,β-methylene-ATP was not secondary to activation of enteric neuronal circuits. Finally, local release of 5-HT is unlikely to mediate the initial response since it was unaffected by administration of the selective 5-HT3 receptor antagonist alosetron at a dose which attenuated the afferent nerve discharge induced by the selective 5-HT3 receptor agonist 2-methyl-5-HT. Interestingly, alosetron inhibited basal afferent nerve activity. This confirms our previous observations with another selective 5-HT3 receptor antagonist granisetron (Hillsley et al. 1998), and supports our postulate that there is a spontaneous release of endogenous 5-HT, possibly from EC cells, which continually affects the activity of these mesenteric afferent nerves. Nevertheless, all these observations strongly suggest that the initial burst of discharge induced by ATP and α,β-methylene-ATP is a direct effect and occurs through the stimulation of receptors present on the endings of primary afferent fibres supplying the intestines.
The population(s) of intestinal afferent nerves and the type(s) of afferent fibre, whether myelinated (Aδ) or unmyelinated (C), at which the nucleotides are acting remains to be resolved. Three populations of afferent fibres are known to be present at the level of the mesenteric nerve bundle. There are two populations of first order neurones which project to the brain stem and spinal cord along vagal and splanchnic pathways, respectively, and an additional population of predominantly second order neurones, termed intestinofugal fibres, which project to autonomic ganglia and are synaptically driven by intrinsic sensory neurones (Anthony & Kreulen, 1990; Miller & Szurszewski, 1997). Since the initial burst of discharge produced by α,β-methylene-ATP was unaffected by treatment with ω-conotoxins MVIIA and SVIB, this would suggest that activation of intestinofugal fibres may occur in the absence of input from enteric nerves. Thus, all of these populations of afferent neurones remain as potential sites of action and experiments are in progress to delineate their respective contribution to the initial burst of afferent discharge evoked by ATP and α,β-methylene-ATP. In view of this, the physiological significance of the initial response remains uncertain at present and it could represent either (or both) the transduction of nutrient/luminal or noxious signals following vagal and spinal pathways, respectively, and/or the stimulation of peripheral reflex pathways.
Adenosine, administered either intravenously (Kirkup et al. 1998) or intra-arterially (A. J. Kirkup & D. Grundy, unpublished observations), does not produce a similar initial intense burst of intestinal afferent nerve discharge as that observed in response to administration of either ATP or α,β-methylene-ATP. This would therefore seem to preclude the possibility that adenosine receptors, following enzymatic degradation of the nucleotides, mediate this response. Indeed, since this initial burst of discharge evoked by α,β-methylene-ATP is sensitive to PPADS and suramin, it is more plausible that a P2 receptor is responsible. Our present observations with α,β-methylene-ATP would favour the involvement of a P2X rather than a P2Y receptor since this agonist exhibits considerable selectivity for the former class of purinoceptor (Humphrey et al. 1998). In support of this postulate, immunoreactivity and mRNA for P2X1 and P2X3 receptors are present in the nodose and dorsal root ganglia, and these are the only homomeric recombinant receptors which exhibit significant sensitivity to α,β-methylene-ATP, PPADS and suramin (Collo et al. 1996; Xiang et al. 1998). However, the discriminative ability of the ATP analogue β,γ-methylene-L-ATP would appear to rule out the functional expression of P2X1 receptors in nodose and dorsal root ganglia (Trezise et al. 1995; Rae et al. 1998). Indeed, the ATP- and α,β-methylene-ATP-induced currents produced by the native receptor in capsaicin-sensitive acutely dispersed (Ueno et al. 1999), and short-term cultured, dorsal root ganglion cells (Robertson et al. 1996; Rae et al. 1998) closely resemble the currents evoked by the nucleotides in cells heterologously expressing P2X3 receptors (α,β-methylene-ATP sensitive, rapidly desensitising; Lewis et al. 1995). However, the native P2X receptors in nodose ganglion cells (Khakh et al. 1995; Lewis et al. 1995) and in capsaicin-insensitive acutely dissociated dorsal root ganglion cells (Ueno et al. 1999) mediate a current which exhibits slow desensitisation in the continued presence of either ATP or α,β-methylene-ATP. Moreover, the P2X2/P2X3 heteropolymer is associated with a slowly desensitising current in response to α,β-methylene-ATP (Lewis et al. 1995). In the present study, the initial stimulation of afferent nerve discharge produced by ATP and α,β-methylene-ATP persisted for approximately 2-5 s and, in the case of α,β-methylene-ATP, exhibited no desensitisation when the nucleotide was administered every 15 min. Although caution must be exercised when comparing in vitro findings with experiments performed in whole animals, such properties would not be consistent with the involvement of homomeric P2X3 receptors, which desensitise within milliseconds. Thus, it is feasible that heteromeric P2X2/3 receptors underlie the initial burst of mesenteric afferent nerve activity induced by ATP and α,β-methylene-ATP. In this respect, our postulate concurs with that of Dowd and colleagues (Dowd et al. 1998a) for the effects of these nucleotides on knee joint afferent nerves whose cell bodies reside in the dorsal root ganglia. However, in view of the lack of more selective pharmacological agents we are unable to characterise definitively the subtype of P2X receptor which might mediate the initial response induced by either ATP or α,β-methylene-ATP. As an added complication, recent studies have demonstrated the presence of multiple types of native P2X receptors in nodose (Thomas et al. 1998) and dorsal root ganglia cells (Grubb & Evans, 1999; Ueno et al. 1999). Moreover, it remains to be determined what functional characteristics are displayed by other heteropolymeric combinations (Torres et al. 1999) and whether these concur with other phenotypes of P2X-mediated responses found in nature.
The later or secondary responses evoked by ATP and α,β-methylene-ATP in the present investigation typically occurred after the initial response had decayed. This response was further characterised by a persistent volley of afferent discharge that corresponded temporally with the elevations in intrajejunal pressure (in contrast to the initial response). In a previous study characterising the mesenteric afferent nerve responses to 5-HT, this delayed afferent nerve response constituted the firing of mechanosensitive afferents consequent to 5-HT-evoked contractile activity in the jejunum (Hillsley et al. 1998). A similar mechanism may underlie the secondary burst of afferent discharge elicited by ATP and α,β-methylene-ATP. This view is supported by our finding that treatment with either the P2 receptor antagonists or the ω-conotoxins not only inhibited the α,β-methylene-ATP-elicited rises in intrajejunal pressure but also the associated late burst of afferent nerve discharge. This latter finding, in addition, indicates that the intrajejunal pressure increases induced by α,β-methylene-ATP (and possibly ATP), like those produced by 5-HT in vivo (Hillsley et al. 1998), possibly comprise a reflex-mediated component in addition to a direct effect on the intestinal smooth muscle. This reflex neural pathway would appear not to involve 5-HT3 receptor-containing synapses since the elevations in intrajejunal pressure were insensitive to alosetron. It therefore seems possible that the effects of ATP and α,β-methylene-ATP on intrajejunal pressure involve a direct effect of the nucleotides on enteric motor neurones, since ATP depolarises neurones in the myenteric plexus (Katayama & Morita, 1989; Galligan & Bertrand, 1994). The secondary burst of afferent nerve discharge evoked by ATP, but not by α,β-methylene-ATP, may possibly entail a contribution from another mechanism. In an earlier study, we observed adenosine-induced elevations in mesenteric afferent nerve discharge (Kirkup et al. 1998) in conjunction with rises in intrajejunal pressure. Thus the breakdown of ATP into adenosine, which would be expected to occur readily in vivo, and the ensuing activation of adenosine receptors, may contribute to both the ATP-evoked late bursts of afferent nerve activity and the concomitant rises in pressure within the jejunum. Indeed, the observations of Dowd and co-workers (Dowd et al. 1998a, b) would appear to support this notion. They obtained delayed increases in knee joint afferent nerve discharge in response to intra-arterial administration of ATP (Dowd et al. 1998a). Further experiments demonstrated that this slow response was consistent with the activation of adenosine receptors present on the afferent nerve endings, subsequent to hydrolysis of ATP, presumably by ectonucleotidases, to adenosine (Dowd et al. 1998b).
In conclusion, in the anaesthetised rat, ATP and α,β-methylene-ATP each elicit early and late bursts of excitation of intestinal afferent nerves and elevate intrajeunal pressure. We propose that the initial burst of intestinal afferent nerve discharge represents a direct effect on mesenteric afferent nerve endings and is evoked by stimulation of ionotropic P2X receptors. In contrast, we postulate that the later burst reflects the discharge of mechanosensitive afferent nerves following increased pressure within the jejunum and, furthermore, may involve a contribution from adenosine receptor-mediated mechanisms. These phenomena may contribute to the sensations of abdominal discomfort and pain which are predominant in functional and organic disorders of the intestines.
Acknowledgments
A.J.K. is a GlaxoWellcome Research Fellow. C.E.B. is a research student supported by GlaxoWellcome. A.J.K. wishes to thank Drs D. Trezise, A. Brunsden and W. Jiang for helpful discussion.
References
- Anthony TL, Kreulen DL. Volume-sensitive synaptic input to neurons in guinea pig inferior mesenteric ganglion. American Journal of Physiology. 1990;259:G490–497. doi: 10.1152/ajpgi.1990.259.3.G490. [DOI] [PubMed] [Google Scholar]
- Bean BP. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. Journal of Neuroscience. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PPA. Acute nociception mediated by hindpaw P2X receptor activation in the rat. British Journal of Pharmacology. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T. The effects of adenine nucleotides on cutaneous afferent nerve activity. British Journal of Pharmacology. 1978;62:573–577. doi: 10.1111/j.1476-5381.1978.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Annals of the New York Academy of. Sciences. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Current Opinion in Neurobiology. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PPA. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. British Journal of Pharmacology. 1998a;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PPA. Adenosine A1 receptor-mediated excitation of nociceptive afferents innervating the normal and arthritic rat knee joint. British Journal of Pharmacology. 1998b;125:1267–1271. doi: 10.1038/sj.bjp.0702185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen B, Reimann W, Selve N, Friderichs E, Bultmann R. Antinociceptive effect of intrathecally administered P2-purinoceptor antagonists in rats. Brain Research. 1994;666:182–188. doi: 10.1016/0006-8993(94)90770-6. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. European Journal of Neuroscience. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. British Journal of Pharmacology. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Barnard EA, Boeynaems H-M, Burnstock G, Dubyak G, Hourani SMO, Insel PA. P2Y receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. Cambridge, UK: Burlington Press; 1998. pp. 209–217. [Google Scholar]
- Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. The Journal of Physiology. 1998;509:717–727. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. The Journal of Physiology. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BT, Huo YY, Lu JG, Newman RA, Levin VA. Analgesic activity of anticancer agent suramin. Anticancer Drugs. 1992;3:91–94. doi: 10.1097/00001813-199204000-00003. [DOI] [PubMed] [Google Scholar]
- Humphrey PPA, Khakh BS, Kennedy C, King BF, Burnstock G. P2X receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. Cambridge, UK: Burlington Press; 1998. pp. 195–208. [Google Scholar]
- Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Morita K. Adenosine 5′-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. The Journal of Physiology. 1989;408:373–390. doi: 10.1113/jphysiol.1989.sp017464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Leff P. Painful connection for ATP. Nature. 1995;377:385–386. doi: 10.1038/377385a0. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Humphrey PPA, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. The Journal of Physiology. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PPA. Localization of P2X purinoceptor transcripts in the rat nervous system. Molecular Pharmacology. 1995;48:569–573. [PubMed] [Google Scholar]
- Kirkup AJ, Eastwood C, Grundy D, Chessell IP, Humphrey PPA. Activation of adenosine receptors increases rat mesenteric afferent nerve discharge. British Journal of Pharmacology. 1997;120:18P. doi: 10.1038/sj.bjp.0702202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Eastwood C, Grundy D, Chessell IP, Humphrey PPA. Characterization of adenosine receptors evoking excitation of mesenteric afferents in the rat. British Journal of Pharmacology. 1998;125:1352–1360. doi: 10.1038/sj.bjp.0702202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Obukhov AG. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988;27:995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Bond SM, Moores C, Chessell I, Humphrey PPA, Dowd E. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. The Journal of Physiology. 1998;507:843–855. doi: 10.1111/j.1469-7793.1998.843bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Szurszewski JH. Colonic mechanosensory afferent input to neurons in the mouse superior mesenteric ganglion. American Journal of Physiology. 1997;272:G357–366. doi: 10.1152/ajpgi.1997.272.2.G357. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proceedings of the National Academy of Sciences of the USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. The Journal of Physiology. 1996;490:265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae MG, Rowan EG, Kennedy C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. British Journal of Pharmacology. 1998;124:176–180. doi: 10.1038/sj.bjp.0701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Rae MG, Rowan EG, Kennedy C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. British Journal of Pharmacology. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Urbanek E, Gothert M. ATP, α,β-methylene ATP and suramin as tools for characterization of vascular P2X receptors in the pithed rat. Journal of Autonomic Pharmacology. 1989;9:357–366. doi: 10.1111/j.1474-8673.1989.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Virginio C, North RA, Surprenant A. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. The Journal of Physiology. 1998;509:411–417. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits: specificities exist with regard to possible partners. Journal of Biological Chemistry. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Trezise DJ, Kennedy I, Humphrey PPA. The use of antagonists to characterize the receptors mediating depolarization of the rat isolated vagus nerve by α,β-methylene adenosine 5′-triphosphate. British Journal of Pharmacology. 1994;112:282–288. doi: 10.1111/j.1476-5381.1994.tb13065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise DJ, Michel AD, Grahames CB, Khakh BS, Surprenant A, Humphrey PPA. The selective P2X purinoceptor agonist, β,γ-methylene-L-adenosine 5′-triphosphate, discriminates between smooth muscle and neuronal P2X purinoceptors. Naunyn-Schmiedeberg's Archives of Pharmacology. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:603–609. doi: 10.1007/BF00170159. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. British Journal of Pharmacology. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. American Journal of Physiology. 1996;271:G223–230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neuroscience Letters. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]


