Abstract
Isolated soleus muscle fibres from aged rats contract more slowly than those from young rats. To determine whether this effect is due to a difference between the myosin molecules, we measured the rate at which actin filaments are driven over a myosin coated surface in the presence of ATP by using a novel in vitro motility assay where myosin is extracted from single muscle fibre segments.
Motility was dependent on the myosin density on the coverslip. In regions of high myosin density, actin motility was orientated parallel and anti-parallel to the direction of flow during myosin adhesion to the coverslip. In contrast, in regions of lower myosin density, actin motility was more random. The speed was about 20% higher in the high density regions (P < 0.001). Further, the speed of filaments in the high density region, moving away or towards the fibre was less variable (P < 0.05) than that of more randomly moving filaments in the low density region.
The speed with myosin from slow soleus fibres of young adult rats (3–6 months old; v= 1.43 ± 0.23 μm s−1; mean ±s.d.) was faster (P < 0.001) than with myosin from aged rats (20–24 months old; v= 1.27 ± 0.23 μm s−1).
No difference in myosin isoforms between young adult and aged fibres could be detected using electrophoretic and immunocytochemical techniques. Fibres of both ages expressed the β/slow myosin heavy chain (MyHC) isoform and slow isoforms of essential and regulatory myosin light chains (MyLCs).
It is concluded that an age-related alteration in myosin contributes to the slowing of the maximum shortening velocity (V0) observed in soleus muscle fibres expressing the β/slow MyHC isoform.
Muscle fibre types express different isoforms of both the myosin heavy and light chains which are derived from different sets of genes (reviewed in Schiaffino & Reggiani, 1996; Pette & Staron, 1997). There is a strong relationship between the maximum speed of unloaded shortening (V0), the actin-activated ATPase activity of myosin and the myosin isoform expression of muscle fibres (e.g. Bàràny, 1967; Reiser et al. 1985; Larsson & Moss, 1993). Slow fibres express the β/slow myosin heavy chain (MyHC) with a low ATPase activity whereas fast fibres express fast MyHCs with high ATPase activities. Further, myosin has been shown to control the rate of actin translocation by in vitro motility assay in a variety of species (Homsher et al. 1992; Cuda et al. 1993; Lowey et al. 1993). There is good evidence, therefore, that different forms of myosin confer distinct contractile properties on muscle fibres.
During ageing of humans and other animals there is muscle atrophy accompanied by a marked slowing of contraction (reviewed in Larsson & Ansved, 1995). Although, in the whole organism, impaired neural function, increased passive resistance of aged muscle and delays in sarcoplasmic calcium movements may contribute to this slowing, much of the age-related change can be attributed to alteration at the level of the muscle fibre contractile proteins (Larsson & Salviati, 1989; Larsson & Ansved, 1995; Viner et al. 1996; Delbono et al. 1997). In aged rat and human muscle, slow fibres contract at approximately half the velocity of similar fibres from younger adults in tests of unloaded shortening speed (Li & Larsson, 1996; Larsson et al. 1997; Yu et al. 1998; Thompson & Brown, 1999). However, although fast to slow myosin isoform transitions have been detected at the whole muscle level in both fast- and slow-twitch muscles during ageing (see Larsson & Ansved, 1995), extensive analysis of myosin heavy chain (MyHC) and myosin light chain (MyLC) diversity has failed to reveal alterations in the isoform composition of muscle cells expressing the β/slow MyHC in aged rat soleus muscle.
In an attempt to improve our understanding of the regulatory and modulatory influence of myofibrillar protein isoforms on muscle contractility, we have used a novel in vitro motility assay which allows studies of actomyosin interactions after myosin and myosin-associated proteins have been extracted from short single muscle (1-3 mm long) fibre segments. We have recently shown that approximately 80 % of thick filament proteins (MyHC, MyLC and myosin binding protein C (MyBP-C)) are extracted (P < 0.001), while no statistically significant amounts of α-actinin or thin filament proteins (actin and TnT) are extracted from single muscle fibres by this procedure, (P. Höök & L. Larsson, unpublished observations).
The objective of the present study was to define to what extent age-related changes in the β/slow myosin contribute to the age-related slowing observed in single soleus fibres. To achieve this we have measured the speed by which unregulated actin filaments are propelled by myosin extracted from single soleus fibres from young and old rats, since motility speed is, under most conditions, a good molecular analogue of V0 (Homsher et al. 1992). The results from this study have been presented in short form elsewhere (Höök et al. 1998; Höök & Larsson, 1999; Li et al. 1999).
METHODS
Muscle fibre preparation
Male albino rats (Wistar) were fed standard laboratory food and tap water ad libitum. The animals were anaesthetised with intramuscular injection of fentanyl-fluanisone (0.2-0.3 ml kg−1) followed by pentobarbitone (30 mg kg−1) administered intraperitoneally. Soleus muscles were gently dissected from young (3-6 months, n= 3) and old (20-24 months, n= 5) animals, weighed, clamped at approximately the in situ length, frozen in Freon chilled with liquid nitrogen, and stored at -80°C pending further processing. Extensor digitorum longus (EDL) muscles were dissected from the young animals for comparison. Frozen samples were placed in glass bottles connected to a vacuum pump (20-50 mTorr) and subsequently freeze-dried (model FD-1-54D, Flexi-Dry, Stoneridge, NY, USA) for 24 h. The hearts were then dissected out. The study was approved by the local Ethical Committee, Karolinska Institute, Stockholm, Sweden.
Single muscle fibre segments (3-4 mm length) were split from the freeze-dried samples with sharp needles and forceps. After dissection, fibres were returned to the glass bottles, dried for 20 min at room temperature in the freeze drier, and stored under vacuum at -80°C until the day of the experiment (Li & Larsson, 1996).
Extraction of myosin from single muscle fibre segments
Pairs of flow cells were constructed, since this allowed us to make two measurements on each slide (only one of the flow cells is shown in Fig. 1A), by using a syringe to apply three parallel stripes of vacuum grease about 4 mm apart and 30 mm in length onto a slide. A freeze-dried muscle segment 2 mm in length was dissected, placed close to the inlet of the flow channel and parallel to it and then covered by a coverslip (18 mm2) which had been dipped in a 1 % solution of nitrocellulose in amyl acetate. Each flow cell had an internal volume of about 10 μl. The rest of the muscle fibre segment was placed in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer in a plastic microfuge tube and stored at -20°C. To extract myosin from a muscle segment, 15 μl of high salt buffer (0.5 M KCl, 25 mm Hepes, 4 mm MgCl2, 4 mm EGTA, 2 mm ATP, 1 %β-mercaptoethanol, pH 7.6) was applied to each of the flow cells and incubated for 20-30 min on ice. To wash away the unbound proteins, 3 × 20 μl of low salt buffer (25 mm KCl, 25 mm Hepes, 5 mm MgCl2, 1 mm EGTA, 1 %β-mercaptoethanol, pH 7.6) was applied to the fibre end of the coverslip and drawn through the chamber by applying filter paper to the other end. Further protein binding was blocked with 20 μl of 1 mg ml−1 BSA in low salt buffer.
Figure 1. Myosin extraction.
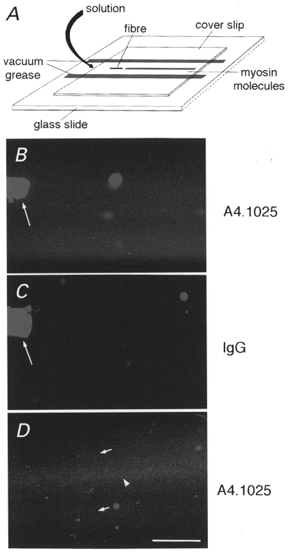
Schematic of experiment showing the location of myosin bound to the coverslip relative to the fibre segment and to solution flow (A). After incubation in extraction solution, myosin is swept out of the fibre segment by the flow of low salt buffer through the chamber (curved arrow), allowing myosin adhesion to the coverslip downstream. Myosin is unevenly distributed on the coverslip surface as revealed by indirect immunofluorescence with an anti-MyHC monoclonal antibody (A4.1025). Myosin is visible (B and D) as a dense striped track downstream of the extracted fibre segment (long arrow), whereas myosin is undetectable in regions lateral to the track (top and bottom of B and D), which are indistinguishable from a control (C) with an irrelevant first antibody (mouse IgG). In D, the myosin track 400 μm downstream from a fibre segment shows that there is a high density of myosin molecules on the central region of track (arrowhead) and a lower density on the more lateral regions (small arrows). Bar = 100 μm.
In vitro motility assay
Rabbit skeletal muscle actin was purified (Pardee & Spudich, 1982), and a portion of the actin fluorescently labelled with tetramethylrhodamine-phalloidin (Rh-Ph; Molecular Probes), before storage in the dark at 0°C. To block non-functional myosin molecules that could bind actin filaments but not detach from them in the presence of ATP, 15 μm of unlabelled F-actin filaments in low salt buffer were sonicated for 1 min and 20 μl was applied to the flow cells to bind all myosin molecules on the coverslip. To remove F-actin from functional myosin heads, 20 μl low salt buffer containing 2 mm ATP was applied to the flow cells, followed by 20 μl low salt buffer. Subsequently, 15 μl of 5 nm Rh-Ph-labelled actin filaments in low salt buffer was applied and after 1 min the cells were washed with 20 μl of low salt buffer to remove the unbound actin. Motility buffer (low salt buffer + 2 mm ATP, 0.1 mg ml−1 glucose oxidase, 23 μg ml−1 catalase, 2.5 mg ml−1 glucose, 1 mg ml−1 BSA) was then added to initiate motility. The temperature in the flow cells was thermostatically controlled at 25°C (Binomic Controller BC-100, 20/20 Technology Inc, USA), with the glass slide mounted on the stage of an inverted epifluorescence microscope (Olympus IX 70) with a ×60, 0.70 NA objective (not an immersion objective) and actin filaments were illuminated with a 200 W mercury lamp. A temperature probe (HH2Z1, Omega Engineering Inc., USA) was placed in contact with the surface of the glass slide next to the flow cell and the thermostated stage controlled temperature was confirmed at the point of observation. Actin movements were filmed with an image intensified SIT camera (SIT 66, DAGE-MIT Inc., USA), and recorded on video tape (Panasonic NV-SD40 VHS VCR).
Analysis of actin filament movement
The motion of actin filaments was analysed with an image analysis package (OPTIMAS 6.0, Optimas Corp.) to estimate the average speed for the myosin extracted from each muscle fibre. Motility speed from myosin high density regions where actin filaments moved parallel and antiparallel to the direction of flow was compared with the speed from regions of low density myosin where the filaments had a more random movement. If more than 10 % of actin filaments did not move the results from that fibre were discarded. The movement of a filament was tracked from the centre of mass, and the average speed of each filament was calculated from the x and y locations in 20 successive frames recorded at 0.2 s intervals. About 10 actin filaments were chosen from each field until a total of 20 filaments had been analysed for each muscle fibre. The standard deviation in this group of filaments was small (between 10 and 15 % of the mean) and the average velocity was taken as representative for the muscle fibre.
Detection of myosin on coverslip
After myosin extraction, unbound proteins were washed away with 3 × 20 μl low salt buffer. Twenty microlitres of 5 % horse serum in PBS solution with 0.1 % Tween (PBSTw) was applied to the flow cells to block the free surface. To detect all myosin isoforms bound to the coverslip, an anti-myosin heavy chain monoclonal antibody (15 μl of 1:10 diluted A4.1025 tissue culture supernatant, mouse IgG; Dan-Goor et al. 1990; Hughes et al. 1993) was applied into the flow cell for 1 h at room temperature. After washing several times for 20 min with low salt buffer, the primary antibody was detected with Texas Red-conjugated horse anti-mouse IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA; 1:200 diluted in 5 % horse serum in PBSTw). An epifluorescence microscope (Olympus IX70, Olympus America Inc., NY, USA) with a × 20, 0.40 NA objective was used to visualise the bound myosin.
MyHC and MyLC composition
Each muscle fibre was placed in SDS sample buffer in a plastic microfuge tube and stored at -20°C for up to 1 week or at -80°C if the gels were run later. The MyHC composition was determined by 7 % sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The total acrylamide and bis concentrations were 4 % (w/v) in the stacking gel and 7 % in the running gel, and the gel matrix included 30 % glycerol. The ammonium persulphate concentrations were 0.040 and 0.029 % in the stacking and separation gels, respectively, and the gel solutions were degassed (< 100 mTorr) for 15 min at 20°C. Polymerisation was subsequently activated by adding TEMED (Bio-Rad Laboratories, CA, USA) to the stacking (0.1 %) and separation gels (0.07 %). Sample loads were kept small to improve the resolution of the MyHC bands and electrophoresis was performed at 120 V for 22-24 h with a Tris-glycine electrode buffer (1.4 % glycine, 0.3 % Tris, pH 8.3) at 15°C (SE 600 vertical slab gel unit, Hoefer Scientific Instruments, USA).
For determinations of the MyLC composition, the acrylamide concentrations in the stacking and running gels were 3.5 and 12 % (w/v), respectively, and the gel matrix included 10 % glycerol. Sample loads were equivalent to 1.0 mm of fibre segment. The electrode buffer was twice as concentrated (2.8 % glycine, 0.6 % Tris, pH 8.3) as the buffer for the 6 % gel. A constant current (16 mA per gel) was used and the gels were run for 5 h at 15°C (see Larsson & Moss, 1993; Larsson et al. 1995). The separating gels (160 mm × 180 mm × 0.75 mm) were silver stained (see Giulian et al. 1983; Larsson & Moss, 1993) and subsequently scanned in a soft laser densitometer (Molecular Dynamics, Sunnyvale, CA, USA), with a high spatial resolution (50 μm pixel spacing) and 4096 optical density levels, to determine the relative contents of MyHCs or MyLCs. The volume integration function is used to quantify the amount of protein on 6 and 12 % gels (ImageQuant software v. 3.3, Molecular Dynamics). The silver stained SDS-PAGE used in this study has a very high sensitivity and the critical level for protein detection is 2-3 % of total MyHC content (see Larsson et al. 1995).
Statistics
The mean speed and standard deviation of the 20 filaments were calculated for each fibre and then the data were combined and the overall mean speed and standard deviation calculated. Student's two-tailed, independent t test was used for comparisons between young and old animal groups. A Mann-Whitney Rank Sum test was used for comparisons between oriented and randomly moving filaments, since equal variance test failed. Differences were considered significant at P < 0.05. Values are given as means ±s.d.
RESULTS
Single fibre in vitro motility assay
Proteins extracted from single muscle fibre segments were allowed to adhere to a nitrocellulose-coated coverslip in a flow cell. To determine the extent and distribution of myosin adhesion to the surface we employed a monoclonal antibody that recognises a conserved epitope on the head of a wide variety of MyHCs (Dan-Goor et al. 1990). Significant labelling of the surface was detected in a strip immediately downstream of the fibre segment within the flow cell, whereas labelling above background was not detected more laterally within the cell (Fig. 1A-C) as would be predicted from the direction of flow. The variation of myosin density on the surface was confirmed when we allowed labelled actin filaments to bind to, and move over, the surface. The number of actin filaments binding to the surface was much greater in the medial region than in lateral regions.
Motility speed of myosin extracted from single fibre segments of adult extensor digitorum longus (EDL) (5.83 ± 0.45 μm s−1, n= 10) was 4-fold faster than adult soleus muscle fibres (1.43 ± 0.23 μm s−1, n= 10; Fig. 2B). Five of the ten EDL fibres expressed the type IIB MyHC (5.87 ± 0.53 μm s−1) and the other five co-expressed a combination of IIX and IIB MyHCs in variable proportions (5.80 ± 0.53 μm s−1). All soleus fibres expressed only the β/slow MyHC isoform.
Figure 2. Effect of age on movement.
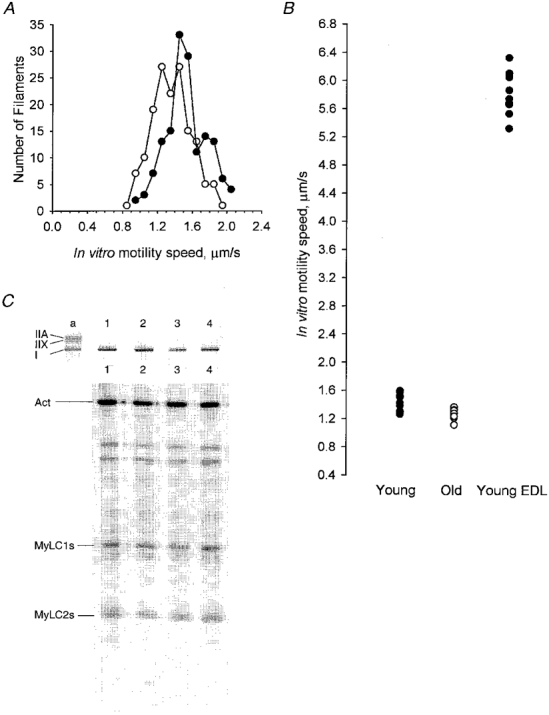
A, speed of rhodamine-phalloidin labelled actin filaments moving on myosin extract from young adult (•) and aged (^) soleus fibre segments. B, when displayed as average motility speed of all filaments on myosin from each individual fibre the whole population appears to have shifted to slower speeds with ageing (•, young; ^, old). For comparison, actin moved by myosin from fast adult extensor digitorum longus muscle fibres have been included for reference (•). C, electrophoretic protein analysis of single fibre segments from young adult (lanes 1 and 3) and old (lanes 2 and 4) soleus. Upper panel, MyHCs resolved by 7 % SDS-PAGE. Lower panel, MyLCs from the same fibres were resolved on 12 % SDS-PAGE. Only the regions of the gels corresponding to the positions of the MyHCs and MyLCs are shown. Indistinguishable slow MyHC and MyLC contents were observed in segments of the same series of individual fibres from adult and aged soleus.
The speed of actin filaments was measured at a range of distances from the fibre in order to assess the effect of myosin density (Fig. 3). Two striking differences were observed between the two regions. First, there is a high total actin filament density on the medial region downstream of the fibre segment, whereas, in lateral regions, motile filament density dropped off to a low level. Secondly, there was a polarisation of movement of actin filaments in medial high density regions.
Figure 3. Direction of filament movement.
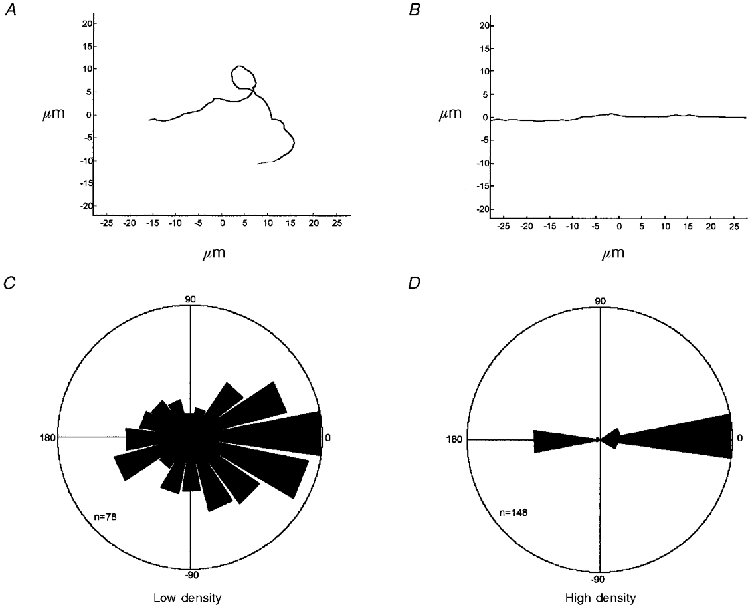
A and B show examples of trajectories of actin filaments in regions of low and high myosin density, respectively. C and D show the relative number of movements in different directions during periods of 0.2 s, the radius of each sector being proportional to the number of movements. Zero degrees corresponds to movement parallel to the direction of flow and pointing away from the fibre and 180 deg corresponds to movement towards the fibre. The outer circles correspond to 78 and 148 movements, respectively. In D the preference for moving parallel to the direction of flow is stronger than typically observed.
In medial high density regions, more than 90 % of the filaments moved with little deviation linearly either parallel or anti-parallel to the direction of flow of the myosin extraction solution. An example of this sort of trajectory is shown in Fig. 3B. In contrast, in more lateral regions movement was almost randomly orientated, the actin filaments often turning through a right angle in a few seconds. An example of such a trajectory is shown in Fig. 3A. Figures 3C and D summarise the directional data for about 10 fibres for low and high density surfaces, respectively. The radius of each sector is proportional to the number of 0.2 s movements in that direction. The degree of polarisation of movement in high density regions is dramatic (Fig. 3D). Analysis of 788 filaments from ten fibres revealed no significant difference in the direction of movement, i.e. the filaments moved either parallel or antiparallel to the flow. The effect of direction of movement on observed speed was tested by comparison of 10 filaments each in high and low density regions. An EDL fibre, expressing fast MyHC and MyLC isoforms, was chosen for this experiment because differences in motility speed related to direction are more easily detectable in faster moving filaments. Motility speed was 23 % faster (P < 0.001) in regions of high myosin density, where movement was oriented parallel to the flow (5.68 ± 0.19 μm s−1), than in low density regions, where motion was almost random (4.38 ± 0.38 μm s−1). Furthermore, the equal variance test failed (P < 0.05) when comparing motility speed from random (s.d.: 0.38 μm s−1) and oriented (s.d.: 0.19 μm s−1) movement.
The primary goal of this study was to determine whether there is an age-related difference in the speed by which actin is propelled by myosin extracted from single soleus fibres expressing the β/slow MyHC isoform. Accordingly, it was important to check the reproducibility of the measurements. Single muscle cells from soleus and EDL muscles were split in two parts and speed was measured for the two segments. The motility speed was measured in the myosin high density region and results for one fibre segment were plotted against the corresponding value of the other segment. A strong relationship was observed in motility speed of the two segments of the same fibre (r2 = 0.998; Fig. 4), demonstrating the reliability of this in vitro motility assay.
Figure 4. Motility speed in two segments of the same fibre.
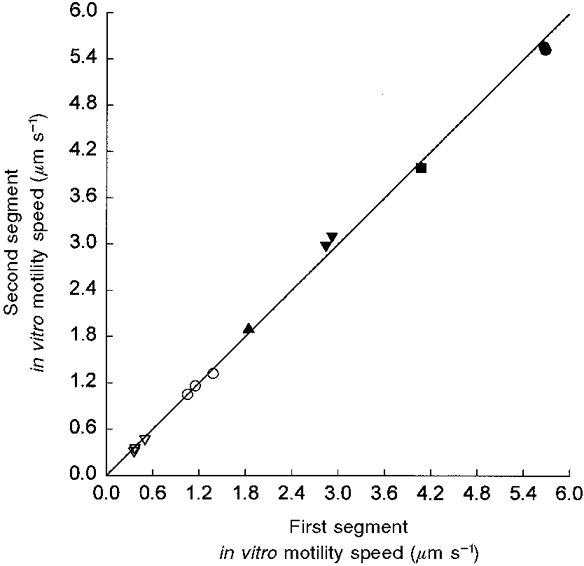
Relationship between in vitro motility speed in two segments of the same muscle fibre (r2= 0.998, P < 0.001). The muscle fibres were dissected from soleus (open symbols) and EDL (filled symbols) muscles, and the motility speed was measured at different temperatures (10 °C, triangles; 15 °C, inverted triangles; 20 °C, squares; and 25 °C, circles) in high density regions where filaments showed an oriented movement towards or away from the muscle fibre.
Effects of ageing on motility speed
The effect of ageing on motility speed of actin filaments on the myosin-coated surface was assayed from ten young and ten old rat soleus muscle fibres (Fig. 2A). The movements of about 20 actin filaments were measured for each fibre and the average speed and the standard deviation for each muscle fibre were calculated. A significant age-related difference was observed in the mean speed of sliding actin filaments; motility speed was faster (P < 0.001) in the young adult group (1.43 ± 0.23 μm s−1) than in the aged group (1.27 ± 0.23 μm s−1).
In parallel with the motility analysis, a segment from each fibre was analysed on high resolution 7 and 12 % SDS polyacrylamide gels to determine the isoforms of myosin heavy and light chains present. All slow fibres from both young and aged soleus muscle fibres contained indistinguishable slow myosin heavy and light chain isoforms (Fig. 2C). Rare fibres expressing the type IIA MyHC isoform and fast MyLC were observed to give faster speeds and were excluded from the analysis (data not shown).
DISCUSSION
The major observation of this study is the significant age-related slowing of actin filament speed moved by the β/slow MyHC isoform, by using a novel in vitro motility assay where myosin is extracted from single muscle fibre segments.
Fibre in vitro motility assay
The in vitro motility assay used in the present paper permits a wide variety of physiological and biochemical studies to be performed on single muscle fibres after long-term storage. In these and other experiments (P. Höök & L. Larsson, unpublished observations) we have documented the reliability and precision of this method. Further, the technique is not limited to rat hindlimb muscle fibres and it can be used in any single muscle cell preparation for analysis of actomyosin motility.
Motility speed has been reported to be relatively insensitive to surface myosin density (Harada et al. 1990). The present results and additional analyses (P. Höök & L. Larsson, unpublished observations) showing a lower motility speed in low than in high myosin density regions do not contradict this statement, since the changes in myosin density were parallelled by a significant difference in the directional movement of the actin filaments. We therefore suggest that the major factor underlying the difference in motility speed in the high and low myosin density regions is the random versus the oriented movement towards or away from the fibre segment. At low surface density, actin filaments show frequent changes of direction probably because actin directionality is determined by Brownian motion of the free end. Downstream of the fibre segment, where myosin bound during flow and is at high surface density, motility is polarised parallel and anti-parallel to the direction of flow. There is an equal tendency for motion to be parallel and antiparallel to the direction of flow of the myosin extracting solution. A simple interpretation would be that myosin is attached to the coverslip in a filamentous arrangement along the flow of the solution in the central part of the experimental chamber. This process is probably facilitated by the co-extraction of MyBP-C which plays an important role in the assembly of the thick filament in vitro (Koretz, 1979; Davis, 1988) and in vivo (Seiler et al. 1996).
Effects of ageing on motility speed
We have recently shown that approximately 80 % of thick filament proteins, MyHCs, MyLCs and MyBP-C, are extracted from the muscle cells, but no statistically significant extraction is observed of thin filament (actin, TnT) or cytoskeletal (α-actinin) proteins (P. Höök & L. Larsson, unpublished observations). In addition, contamination of the giant structural protein titin is unlikely, since two times higher ionic strength than used in this study is required to extract titin. Next to myosin, MyBP-C is the most abundant protein in the thick filament and 83 ± 4 % of MyBP-C is extracted from the muscle cell together with myosin (P. Höök & L. Larsson, unpublished observations). However, it has been repeatedly demonstrated by Hofmann et al. (1991a,b) that MyBP-C does not influence maximum shortening velocity, and MyBP-C contamination in the experimental chamber is accordingly an unlikely source of error in motility speed measurements. Therefore, the observation that myosin extracted directly from single aged rat soleus muscle fibres moves actin filaments at a lower speed than that from young adult animals suggests that an age-related change in the myosin molecule itself contributes to the decreased speed of unloaded shortening observed in single membrane permeabilized adult and aged muscle fibre segments (Li & Larsson, 1996; Larsson et al. 1997; Yu et al. 1998; Thompson & Brown, 1999). It is suggested that this slowing is caused by either an upregulation of a MyHC isoform in the old adult which is expressed at lower levels or is lacking in the young adult, or an age-related change in the enzymatic or mechanical properties of myosin.
The possibility of an age-related upregulation of a specific slow MyHC isoform gains credence from the identification of multiple β/slow (type I) MyHC isoforms in mammalian skeletal muscle by using S1 nuclease mapping and immunocytochemical and electrophoretic techniques (Hughes et al. 1993; Fauteck & Kandarian, 1995; Galler et al. 1997). There are several lines of evidence indicating the existence of additional MyHC isoforms in fibres previously considered homogeneous regarding their MyHC isoform composition (Schiaffino & Reggiani, 1996). However, we were unable to identify any differences in the electrophoretic mobility of MyHC and MyLC isoforms between the young adult and the old soleus fibres. High performance capillary electrophoresis and immunoblotting have also yielded negative results in our hands. The rate of cross-bridge detachment limits the rate of unloaded shortening (Huxley, 1957), which suggests that the properties of the slower myosin will be dominant and this prediction has been confirmed (Warshaw et al. 1990). In muscle cells expressing two MyHC isoforms, the slower of the two myosin isoforms will have a disproportionate influence on motility speed (Harris et al. 1994; Cuda et al. 1997) and in single muscle fibre preparations (Reiser et al. 1985; Larsson & Moss, 1993). Because the speed of the hypothetical old myosin is unknown and the effect on speed will be dependent upon what proportion of time at least one slow myosin attached, i.e. will depend upon myosin density, it is not possible to predict whether it would be detectable with gels sensitive to 2-3 % of the total myosin level. Although we cannot exclude that there is an age-related MyHC or MyLC isoform transformation in muscle cells expressing the β/slow MyHC, we presently favour an alteration in myosin function by a post-translational modification as a more probable mechanism underlying the decreased motility speed in old age.
Post-translational alterations in myosin function could be brought about by enzymatic modifications (Mooradian & Wong, 1991); or by non-enzymatic modification to individual myosin molecules, e.g. by glycation (Avigad et al. 1996), or deamination (Balagopal et al. 1997). The probability of post-translational modifications of myosin is expected to be higher in old age due to the decreased myosin synthesis rate and the slower turnover of myosin in ageing muscle. Non-enzymatic glycosylation (glycation) of proteins, by a chemical reaction of reducing sugars with primary amino groups in proteins to form a Schiff's base linkage (Watanabe et al. 1992) has been regarded as one of the biochemical bases underlying the pathophysiology of ageing (Brownlee, 1995), and glycation of myosin has been reported to increase in ageing rats (Syrovy & Hodny, 1992). In support of a post-translational modification of myosin by glycation, we have recently shown that glycation of myosin has a strong impact on motility speed, i.e. 15 and 30 min exposure of myosin to 6 mm glucose in low-salt buffer at 25°C (prior to adding the fluorescently labelled actin filaments) decreased motility speed by 13 and 100 %, respectively (Ramamurty & Larsson, 1999). To determine whether all or only some myosin molecules show altered properties on ageing it will be necessary to analyse the behaviour of individual molecules. Recent advances that permit analysis of single myosin function make this an achievable goal (Finer et al. 1994; Ishijima et al. 1998).
The viscous drag produced by the solution in the experimental chamber when fluorescently labelled actin filaments are driven by myosin is negligible compared with the force produced by the motor protein and thus in vitro motility speed would be expected, and has been shown, to be similar to unloaded shortening (V0) in single cells measured with the slack test (see Homsher et al. 1992). This has been confirmed in recent experiments in our group, i.e. a close relationship between V0 and actin motility speed propelled by the myosin extracted from the same muscle fibre segment (Höök & Larsson, 1999). The age-related changes in in vitro motility speed is qualitatively similar, but smaller in magnitude, to the changes observed in V0 (Li & Larsson, 1996). For changes of myosin to be solely responsible for the slowing of shortening speed observed in old muscle fibres a co-operative mechanism would be required. Alternatively, the age-related alteration in the myosin molecule, observed in this study, is paralleled by age-related changes in other myofibrillar proteins modulating shortening speed at the cellular level, such as regulatory thin filament proteins. This is supported by the observation of glycation of other myofibrillar proteins besides myosin, such as actin and tropomysin (Syrovy & Hodny, 1993).
Acknowledgments
We are grateful to Parinaz Pircher for performing the SDS-PAGE. This study was supported by grants from the UK Medical Research Council to J.S., the UK Medical Research Council and the EU Commission (BIOMED-2) to S.H., and the Swedish Medical Research Council (no. 08651), the EU Commission (BIOMED-2), and the GCRC, University Park (M01-RR10732-03) to L.L.
References
- Avigad G, Kniep A, Bailin G. Reaction of rabbit skeletal myosin with D glucose 6-phosphate. Biochemistry and Molecular Biology International. 1996;40:273–284. doi: 10.1080/15216549600201762. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Asey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. American Journal of Physiology. 1997;273:E790–800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Bàràny M. ATPase activity of myosin correlated with speed of muscle shortening. Journal of General Physiology. 1967;50:197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annual Review of Medicine. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Cuda G, Fananapazir L, Zhu WS, Sellers JR, Epstein NDJ. Skeletal muscle expression and abnormal function of β-myosin in hypertrophic cardiomyopathy. Journal of Clinical Investigation. 1993;9:2861–2865. doi: 10.1172/JCI116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuda G, Pate E, Cooke R, Sellers JR. In vitro actin filament sliding velocities produced by mixtures of different types of myosin. Biophysical Journal. 1997;72:1767–1779. doi: 10.1016/S0006-3495(97)78823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan-Goor M, Silberstein L, Kessel M, Muhlrad A. Localization of epitopes and functional effects of two novel monoclonal antibodies against skeletal muscle myosin. Journal of Muscle Research and Cell Motility. 1990;11:216–226. doi: 10.1007/BF01843575. [DOI] [PubMed] [Google Scholar]
- Davis JS. Interaction of C-protein with pH 8.0 synthetic thick filaments prepared from the myosin of vertebrate skeletal muscle. Journal of Muscle Research and Cell Motility. 1988;9:174–183. doi: 10.1007/BF01773739. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Excitation-Ca2+ release-contraction coupling in single aged human skeletal muscle fibre. Muscle and Nerve. 1997;(suppl. 5):S88–92. doi: 10.1002/(sici)1097-4598(1997)5+<88::aid-mus21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fauteck SP, Kandartian SC. Sensitive detection of myosin heavy chain composition in skeletal muscle under different loading conditions. American Journal of Physiology. 1995;268:C419–424. doi: 10.1152/ajpcell.1995.268.2.C419. [DOI] [PubMed] [Google Scholar]
- Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K, Gohlsch B, Pette D. Two functionally distinct myosin heavy chain isoforms in slow skeletal muscle fibres. FEBS Letters. 1997;410:150–152. doi: 10.1016/s0014-5793(97)00556-5. [DOI] [PubMed] [Google Scholar]
- Giulian GG, Moss RL, Greaser ML. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Analytical Biochemistry. 1983;129:227–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Harada Y, Sakurada K, Aoki T, Thomas DD, Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. Journal of Molecular Biology. 1990;216:49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Harris DE, Work SS, Wright RK, Alpert NR, Warshaw DM. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. Journal of Muscle Research and Cell Motility. 1994;15:11–19. doi: 10.1007/BF00123828. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Greaser ML, Moss RL. C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. The Journal of Physiology. 1991a;439:701–715. doi: 10.1113/jphysiol.1991.sp018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann PA, Hartzell HC, Moss RL. Alterations in Ca2+ sensitive tension due to partial extraction of C-protein from rat skinned cardiac myocytes and rabbit skeletal muscle fibers. Journal of General Physiology. 1991b;97:1141–1163. doi: 10.1085/jgp.97.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Wang F, Sellers JR. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. American Journal of Physiology. 1992;262:C714–723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Höök P, Larsson L. A novel in vitro motility assay to study different myosin isoforms extracted from single muscle fiber segments. Biophysical Journal. 1999;76:A51. [Google Scholar]
- Höök P, Li X, Larsson L. Effects of aging on in vitro motility speed of β/slow myosin extracted from single muscle cells. FASEB Journal. 1998;12:2430. [Google Scholar]
- Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM. Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Developmental Biology. 1993;158:183–199. doi: 10.1006/dbio.1993.1178. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics. 1957;7:255–318. [PubMed] [Google Scholar]
- Ishijima A, Kojima H, Funatsu T, Tokunaga M, Higuchi H, Tanaka H, Yanagida T. Simultaneous observation of individual ATPase and mechanical events by a single myosin molecule during interaction with actin. Cell. 1998;92:161–171. doi: 10.1016/s0092-8674(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Koretz JF. Effects of C-protein on synthetic myosin filament structure. Biophysical Journal. 1979;27:433–446. doi: 10.1016/S0006-3495(79)85227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in Neurobiology. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. American Journal of Physiology. 1997;272:C638–649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. The Journal of Physiology. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Müller U, Li X, Schiaffino S. Thyroid hormone regulation of myosin heavy chain isoform composition in young and old rats. With special reference to type IIX myosin. Acta Physiologica Scandinavica. 1995;153:109–16. doi: 10.1111/j.1748-1716.1995.tb09841.x. [DOI] [PubMed] [Google Scholar]
- Larsson L, Salviati G. Effects of age on calcium transport activity of sarcoplasmic reticulum in fast- and slow-twitch rat muscle fibres. The Journal of Physiology. 1989;419:253–264. doi: 10.1113/jphysiol.1989.sp017872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Höök P, Sleep J, Hughes SM, Larsson L. Motility speed of myosin extracted from single soleus fibres expressing slow myosin isoforms from young and old rats. Biophysical Journal. 1999;76:A36. doi: 10.1111/j.1469-7793.1999.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Larsson L. Maximum shortening velocity and myosin isoforms in single muscle fibers from young and old rats. American Journal of Physiology. 1996;270:C352–360. doi: 10.1152/ajpcell.1996.270.1.C352. [DOI] [PubMed] [Google Scholar]
- Lowey S, Waller GS, Trybus KMJ. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. Journal of Biological Chemistry. 1993;268:20414–20418. [PubMed] [Google Scholar]
- Mooradian AD, Wong NCW. Molecular biology of aging. Part II: A synopsis of current research. Journal of the American Geriatrics Society. 1991;39:717–723. doi: 10.1111/j.1532-5415.1991.tb03628.x. [DOI] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods in Enzymology. 1982;85:164–182. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. International Review of Cytology. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Ramamurty B, Larsson L. Influence of glycation on the in vitro motility of skeletal myosin. Biohysical Journal. 1999;76:A51. [Google Scholar]
- Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult-rabbit soleus muscles is correlated with myosin heavy-chain composition. Journal of Biological Chemistry. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiological Reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Seiler SH, Fischman DA, Leinwand LA. Modulation of myosin filament organization by C-protein family members. Molecular Biology of the Cell. 1996;7:113–127. doi: 10.1091/mbc.7.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovy I, Hodny Z. Non-enzymatic glycosylation of myosin: Effects of diabetes and ageing. General Physiology and Biophysics. 1992;11:301–307. [PubMed] [Google Scholar]
- Syrovy I, Hodny Z. In vitro non-enzymatic glycosylation of myofibrillar proteins. International. Journal of Biochemistry. 1993;25:941–946. doi: 10.1016/0020-711x(93)90251-9. [DOI] [PubMed] [Google Scholar]
- Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal muscle fibers from the soleus muscle. Journal of Applied Physiology. 1999;86:881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- Viner RI, Ferrington DA, Hühmer AFR, Bigelow DJ, Schöneich C. Accumulation of nitrotyrosine on the SERCA2a isoform of SR Ca-ATPase of rat skeletal muscle during aging: a peroxynitrite-mediated process? FEBS Letters. 1996;379:286–290. doi: 10.1016/0014-5793(95)01530-2. [DOI] [PubMed] [Google Scholar]
- Warshaw DM, Desrosiers JM, Work SS, Trybus KM. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding speed in vitro. Journal of Cell Biology. 1990;111:453–463. doi: 10.1083/jcb.111.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ogasawara M, Suzuki N, Nishizawa N, Ambo K. Glycation of myofibrillar protein in aged rats and mice. Bioscience, Biotechnology and Biochemistry. 1992;56:1109–1112. doi: 10.1271/bbb.56.1109. [DOI] [PubMed] [Google Scholar]
- Yu F, Degens H, Li X, Larsson L. Effects of thyroid hormone, gender and age on contractility and myosin composition in single rat soleus fibres. Pflügers Archiv. 1998;437:21–30. doi: 10.1007/s004240050741. [DOI] [PubMed] [Google Scholar]


