Abstract
The carbonic anhydrases (CAs) participate in the maintenance of pH homeostasis in various tissues and biological fluids of the human body by catalysing the reversible reaction CO2+ H2O ⇌ HCO3−+ H+ (Davenport & Fisher, 1938; Davenport, 1939; Maren, 1967). Carbonic anhydrase isoenzyme VI (CA VI) is the only secretory isoenzyme of the mammalian CA gene family. It is exclusively expressed in the serous acinar cells of the parotid and submandibular glands, from where it is secreted into the saliva. In this review, we will discuss recent advances in research focused on the physiological role of salivary CA VI in the oral cavity and upper alimentary canal.
General aspects of salivary secretion and buffer capacity
Whole saliva is a mixture of the secretions from the parotid, submandibular, sublingual and minor salivary glands and gingival crevicular fluid. Saliva contains inorganic compounds and multiple proteins that affect conditions in the oral cavity and locally on the tooth surfaces. It brings various defence mechanisms, including leukocytes, secretory IgA, agglutinating proteins and a number of enzymes, to the actual sites of microbial growth on the tooth and mucosal surfaces. Salivation is initiated by the salivary centres in the medulla oblongata, which receive afferent signals from the sensory terminals of the oral and nasal cavities and from the higher centres in the brain. The secretion of saliva is regulated by the autonomic nervous system (Asking & Gjörstrup, 1980; Helm et al. 1982; Olsen et al. 1988; Calvert et al. 1998), and its composition follows circadian rhythms (Dawes, 1972, 1975; Parkkila et al. 1995; Kiveläet al. 1997b). Water and electrolyte secretion are mainly controlled by parasympathetic activity, whereas protein synthesis and exocytosis are mainly controlled by sympathetic activity (Jensen et al. 1991; Nederfors & Dahlöf, 1992, 1996; Nederfors et al. 1994).
Salivary buffer capacity is a factor of primary importance in maintaining oral homeostasis. The main buffer systems known to contribute to the total buffer capacity of saliva are the bicarbonate and phosphate systems and those based on proteins (Leung, 1951, 1961; Lilienthal, 1955; Izutsu & Madden, 1978; Helm et al. 1982). These systems have different pH ranges of maximal buffer capacity, the phosphate and bicarbonate systems having pK (-log of the dissociation constant) values of 6.8-7.0 and 6.1-6.3, respectively, whereas the proteins contribute to the salivary buffer capacity at very low pH values only. Most of the salivary buffer capacity operative during food intake and mastication is due to the bicarbonate system, which is based on the equilibrium CO2+ H2O ⇌ HCO3−+ H+. The concentration of bicarbonate in the saliva is greatly increased at increased flow rates (Dawes, 1969, 1974). Another essential feature of this buffer system under the conditions prevailing in the oral cavity is the phase conversion of carbon dioxide from a dissolved state into a volatile gas. When acid is added, this phase conversion considerably increases the efficacy of the neutralization reaction, as there is no accumulation of the end products but complete removal of the acid, a phenomenon referred to as ‘phase buffering’.
Phosphate makes a minor contribution to the total salivary buffer capacity relative to bicarbonate (Leung, 1951; Lilienthal, 1955). Its system is in principle analogous to that of bicarbonate but without the important phase-buffering effect. Within the pH range of the oral cavity, the phosphate buffer is based on the reversible reaction H2PO4−⇌ HPO42−+ H+. The concentration of HPO42− in saliva is relatively independent of the salivary secretion rate, and thus the capacity of the phosphate buffer system does not increase during food intake or mastication.
Evaluation of the salivary buffer effect based on proteins has produced controversial results. In general the effect has been regarded as insignificant, or at least of minor importance, although data suggesting an alternative conclusion have also been presented (Leung, 1961; Izutsu & Madden, 1978).
Carbonic anhydrase VI
The existence of CA activity in human saliva has been known for 60 years (Becks & Wainwright, 1939), but until recently only a few studies had been carried out on the physiological role of salivary CA (Rapp, 1946; Szabó, 1974; Parkkila et al. 1997; Kivelä et al. 1997a, 1999; Leinonen et al. 1999). The ovine salivary CA isoenzyme expressed in the parotid gland was described in 1979 by Fernley et al. The enzyme was purified from human saliva by Murakami & Sly (1987), and designated CA VI. In 1991, Aldred et al. cloned and characterized the cDNA encoding human carbonic anhydrase (HCA) VI. The next major step in research into salivary CA was the development of specific immunofluorometric and radioimmunoassays for HCA VI (Parkkila et al. 1993; Fernley et al. 1995), which allowed accurate quantification of CA VI in biological matrices such as saliva and serum.
CA VI is the only known secreted isoenzyme of the mammalian CA gene family, and has several properties that distinguish it from the well-characterized cytoplasmic isoenzymes. Its reported subunit molecular weight is 42 kDa (Murakami & Sly, 1987). The enzyme molecule has two N-linked oligosaccharide chains, which can be cleaved by endo-β-N-acetylglucosaminidase F but not by endo-β-N-acetylglucosaminidase H, indicating that the oligosaccharides are of a complex type (Murakami & Sly, 1987). Neuraminidase has no effect on the endo-β-N-acetylglucosaminidase F-digested protein, suggesting that HCA VI has no O-linked oligosaccharide, which contains neuraminidase-sensitive sialic acid residues. The HCA VI protein has a sequence identity of 35 % to HCA II, while residues involved at the active site of the enzyme are conserved. HCA VI has three potential N-linked glycosylation sites and two cysteine residues (Cys25 and Cys207) (Aldred et al. 1991), the latter presumably forming a disulphide bond, as in the ovine enzyme (Fernley et al. 1988). The HCA6 gene is located on chromosome 1 (Aldred et al. 1991).
In humans, immunohistochemical studies have demonstrated the location of CA VI exclusively in the secretory granules of the acinar cells of the parotid and submandibular glands (Parkkila et al. 1990), from where it is secreted into the saliva. Salivary concentrations of ovine and human CA VI have been investigated using radioimmunoassay (Fernley et al. 1991, 1995) and time-resolved immunofluorometric assay techniques (Parkkila et al. 1993, 1995; Kivelä et al. 1997a). Radioimmunoassay of ovine CA VI showed that its mean ±s.d. concentration in sheep parotid saliva is 5.61 ± 3.01 mg l−1 in the normal conscious animal, while feeding increased the concentration to 33.0 ± 19.0 mg l−1 (Fernley et al. 1991; Fernley, 1991). The mean ±s.d. concentration of CA VI in human parotid saliva was shown by the radioimmunoassay method to be 47.0 ± 39.2 mg l−1, representing about 3 % of total protein in the parotid saliva (Fernley et al. 1995). In young men, the mean ±s.e.m. concentration of CA VI in whole saliva, measured by time-resolved immunofluorometric assay, is 5.0 ± 0.2 mg l−1, and the concentration has a weak positive correlation with salivary secretion rate (Kivelä et al. 1997a).
Secretion of CA VI into the saliva has been observed to follow a circadian periodicity, its concentration being very low during sleep and rising rapidly to the daytime level after awakening and breakfast (Parkkila et al. 1995; Kiveläet al. 1997b). This circadian periodicity is very similar to that of salivary α-amylase, and a significant positive correlation is found between salivary amylase activity level and CA VI concentration (Parkkila et al. 1995; Kiveläet al. 1997b). These findings suggest that the two enzymes are secreted via similar mechanisms and may possibly be present in the same secretory granules. Since the role of the autonomic nervous system in amylase secretion is well established (Asking & Gjörstrup, 1980; Olsen et al. 1988; Nederfors & Dahlöf, 1992), it is conceivable that the autonomic pathways also control CA VI secretion. This concept was supported by Fernley et al. (1991) who demonstrated, using nerve stimulation and cholinergic drug administration, that both parasympathetic and sympathetic pathways can regulate the CA VI concentration in sheep saliva.
CA VI has been found to be transferred into the blood circulation in humans (Kiveläet al. 1997b). The mean serum concentrations of CA VI, measured by time-resolved immunofluorometric assay, are only about 1/22 of those of simultaneous saliva samples. The enzyme levels in serum show much intra-individual variation (0.15-0.33 mg l−1), although the circadian rhythm is less evident than in saliva. Western blotting of CA VI indicated that it is associated with IgG in serum, whereas it mainly occurs as a monomeric enzyme in saliva. Association with IgG may protect the enzyme from proteolytic degradation or target it to cells which do not express CA VI.
According to recent investigations, CA VI seems to be one of the key enzymes in oral physiology. It was originally predicted to regulate salivary pH or buffer capacity (Feldstein & Silverman, 1984; Kadoya et al. 1987). Interestingly, results from our laboratory indicate that these variables are not directly associated with CA VI concentration in saliva (Parkkila et al. 1993; Kivelä et al. 1997a), suggesting that the enzyme may have a different role or that it may participate in these processes together with other CAs. Another novel aspect to the function of CA VI was provided by Thatcher et al. (1998), who identified gustin, a salivary protein that has been reported to be associated with the function of taste buds, as human CA VI by protein sequencing, activity profiles and other physical data.
Role of saliva and salivary CA VI in protecting the dental hard tissues
Dental enamel is the hardest tissue in the human body, and the main challenge to it comes from acidic conditions in the oral cavity, which can cause dissolving of the mineral, i.e. dental caries or erosion. The metabolism of the microbial flora on the dental surfaces produces considerable amounts of acid, mainly in the form of lactic, acetic, formic and propionic acids (Clarke, 1924; Muntz, 1943; Stephan, 1944; Geddes, 1975, 1981). Moreover, various foods and drinks add to the acid charge on these surfaces. Saliva can be considered the oral tissue fluid of the enamel, and the maintenance of homeostasis on the dental surfaces is totally dependent on salivary factors, including inorganic compounds and multiple proteins. Saliva is involved in the clearance of food debris, detached epithelial cells and microbes. It provides inorganic ions for the neutralization of the acid and alkaline metabolic products of oral bacteria and for the remineralization of the enamel. It also brings various defence mechanisms, including leukocytes, secretory IgA, agglutinating proteins and a number of enzymes, to the actual sites of microbial adherence and growth on the tooth surfaces. The importance of saliva for dental health is demonstrated by the rampant caries seen in patients with grave salivary hypofunction (Rudney, 1995; Peeters et al. 1998).
Recent research has indicated that salivary CA VI has a remarkable role in protecting teeth from caries (Kivelä et al. 1999). It is the first salivary protein reported to be associated with the occurrence of caries in individuals as measured using the DMFT index (number of decayed, missing, or filled teeth). The negative correlation between salivary CA VI concentration and DMFT index was most distinct in the group of subjects with neglected oral hygiene. This finding, together with the observation that salivary CA VI concentration does not correlate with salivary pH or buffer capacity, has led to a search for the site of salivary CA VI function.
The enamel pellicle is a thin layer of proteins covering the enamel. Its formation is initiated by the adsorption of specific salivary proteins to the hydroxyapatite surface (Kousvelari et al. 1980; Al-Hashimi & Levine, 1989; Lamkin et al. 1996). This adsorption is assumed to be dependent on the chemical characteristics of the surface as well as the properties of the particular proteins. The enamel pellicle evidently prevents demineralization of the surface hydroxyapatite, increasing its acid resistance, although the mechanisms responsible for this effect remain elusive (Zahradnik et al. 1976, 1977, 1978; Kousvelari et al. 1980; Meurman & Frank, 1991; Featherstone et al. 1993). It is also thought to prevent calculus formation on the dental surfaces by controlling the precipitation of calcium phosphate from supersaturated saliva (Hay & Moreno, 1989).
Recent research has indicated that CA VI binds to the enamel pellicle and retains its enzyme activity on dental surfaces (Leinonen et al. 1999). In the enamel pellicle, CA VI is located at the optimal site to catalyse the conversion of salivary bicarbonate and microbe-delivered hydrogen ions to carbon dioxide and water (Fig. 1). These findings suggest that CA VI may protect teeth by catalysing the most important buffer system in the oral cavity, thus accelerating the removal of acid from the local microenvironment of the tooth surface.
Figure 1. Model illustrating the suggested function of CA VI on dental surfaces.
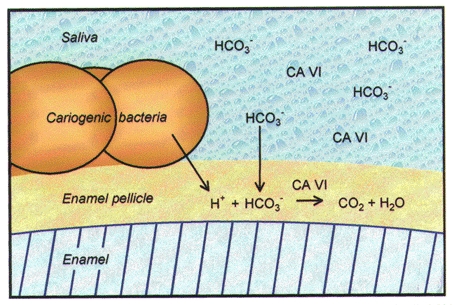
Modified from Leinonen et al. (1999).
Role of salivary CA VI on epithelial surfaces of the upper alimentary canal
The epithelia of the human oral cavity and oesophagus are exposed to widely varying conditions due to the physical and chemical properties of the ingested food, in addition to which the epithelium of the oesophagus is challenged by acid reflux from the stomach. The saliva is responsible for the luminal defence of the epithelia of the upper alimentary canal. A number of salivary proteins are known to bind to the epithelial surfaces of the oral cavity, including salivary mucins, amylase, salivary cystatins and acidic proline-rich proteins (Bradway et al. 1989, 1992). This epithelial pellicle provides a lubricatory film and an effective barrier against desiccation and environmental factors, and it is also thought to protect the epithelial cells from proteases emanating from bacteria attached to the mucosal surfaces and from degenerating polymorphonuclear leukocytes (Mandel, 1987; Vaahtoniemi et al. 1992).
Salivary bicarbonate secretion is known to be of vital importance for the maintenance of oesophageal pH homeostasis (Helm et al. 1982, 1984; Sarosiek & McCallum, 1995; Sarosiek et al. 1996), and recent observations have suggested that CA VI may also be involved in this process (Parkkila et al. 1997). CA VI has also been detected in the gastric mucus where it may contribute to the maintenance of the pH gradient on the surface epithelial cells. This view is supported by the observation that CA VI probably maintains its activity in the harsh environment of the gastric lumen and that patients with verified oesophagitis or oesophageal, gastric or duodenal ulcers have a reduced salivary CA VI concentration relative to patients with a non-acid peptic disease (Parkkila et al. 1997). The finding that CA VI is not expressed in the gastric epithelial cells (Parkkila et al. 1994) implies that gastric CA VI is of salivary origin. It has been proposed that CA VI and CA II may form a mutually complementary system for the regulation of pH homeostasis on the epithelial surfaces of the upper alimentary canal (Parkkila et al. 1990, 1994, 1997). Salivary CA VI probably catalyses the neutralization of excess acid in the mucous layer covering the oesophageal and gastric epithelial cells using endogenous and salivary bicarbonate produced by cytosolic CA II (Parkkila & Parkkila, 1996; Parkkila et al. 1997).
Conclusions
The recent advances in CA VI research suggest that salivary CA VI is one of the key enzymes maintaining homeostasis on the surfaces of the oral cavity and upper alimentary canal. Low salivary concentrations of CA VI appear to be associated with an increased prevalence of caries and acid peptic diseases. Since CA VI is present in the enamel pellicle and gastric mucus, it may function locally in the microenvironment of dental and epithelial surfaces and accelerate the neutralization of excess acid.
References
- Aldred P, Fu P, Barrett G, Penschow JD, Wright RD, Coghlan JP, Fernley RT. Human secreted carbonic anhydrase: cDNA cloning, nucleotide sequence, and hybridization histochemistry. Biochemistry. 1991;30:569–575. doi: 10.1021/bi00216a035. [DOI] [PubMed] [Google Scholar]
- Al-Hashimi I, Levine MJ. Characterization of in vivo salivary-derived enamel pellicle. Archives of Oral Biology. 1989;34:289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Asking B, Gjörstrup P. Amylase secretion in response to activation of different autonomic receptors in the rabbit parotid gland. Acta Physiologica Scandinavica. 1980;109:407–413. doi: 10.1111/j.1748-1716.1980.tb06613.x. [DOI] [PubMed] [Google Scholar]
- Becks H, Wainwright WW. Human saliva. Journal of Dental Research. 1939;18:447–456. [Google Scholar]
- Bradway SD, Bergey EJ, Jones PC, Levine MJ. Oral mucosal pellicle. Adsorption and transpeptidation of salivary components to buccal epithelial cells. Biochemical Journal. 1989;261:887–896. doi: 10.1042/bj2610887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradway SD, Bergey EJ, Scannapieco FA, Ramasubbu N, Zawacki S, Levine MJ. Formation of salivary-mucosal pellicle: The role of transglutaminase. Biochemical Journal. 1992;284:557–564. doi: 10.1042/bj2840557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PA, Heck PM, Edwards AV. Autonomic control of submandibular protein secretion in the anaesthetized calf. Experimental Physiology. 1998;83:545–556. doi: 10.1113/expphysiol.1998.sp004137. [DOI] [PubMed] [Google Scholar]
- Clarke JK. On the bacterial factor in the aetiology of dental caries. British Journal of Experimental Pathology. 1924;5:141–149. [Google Scholar]
- Davenport HW. Gastric carbonic anhydrase. The Journal of Physiology. 1939;97:32–43. doi: 10.1113/jphysiol.1939.sp003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport HW, Fisher RB. Carbonic anhydrase in the gastrointestinal mucosa. The Journal of Physiology. 1938;94:16–17P. [Google Scholar]
- Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human parotid saliva. Archives of Oral Biology. 1969;14:277–294. doi: 10.1016/0003-9969(69)90231-3. [DOI] [PubMed] [Google Scholar]
- Dawes C. Circadian rhythms in human salivary flow rate and composition. The Journal of Physiology. 1972;220:529–545. doi: 10.1113/jphysiol.1972.sp009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human submandibular saliva. Archives of Oral Biology. 1974;19:887–895. doi: 10.1016/0003-9969(74)90051-x. [DOI] [PubMed] [Google Scholar]
- Dawes C. Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. The Journal of Physiology. 1975;244:535–548. doi: 10.1113/jphysiol.1975.sp010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone JDB, Behrman JM, Bell JE. Effect of whole saliva components on enamel demineralization in vitro. Critical Reviews in Oral Biology and Medicine. 1993;4:357–362. doi: 10.1177/10454411930040031401. [DOI] [PubMed] [Google Scholar]
- Feldstein JB, Silverman DN. Purification and characterization of carbonic anhydrase from the saliva of the rat. Journal of Biological Chemistry. 1984;259:5447–5453. [PubMed] [Google Scholar]
- Fernley RT. Carbonic anhydrases secreted in the saliva. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, editors. The Carbonic Anhydrases. Cellular Physiology and Molecular Genetics. New York: Plenum Press; 1991. pp. 365–373. [Google Scholar]
- Fernley RT, Farthing J, Cooper EJ. Radioimmunoassay for salivary carbonic anhydrase in human parotid saliva. Archives of Oral Biology. 1995;40:567–569. doi: 10.1016/0003-9969(95)00001-6. [DOI] [PubMed] [Google Scholar]
- Fernley RT, Wright RD, Coghlan JP. A novel carbonic anhydrase from the ovine parotid gland. FEBS Letters. 1979;105:299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- Fernley RT, Wright RD, Coghlan JP. Complete amino acid sequence of ovine salivary carbonic anhydrase. Biochemistry. 1988;27:2815–2820. doi: 10.1021/bi00408a023. [DOI] [PubMed] [Google Scholar]
- Fernley RT, Wright RD, Coghlan JP. Radioimmunoassay of carbonic anhydrase VI in saliva and sheep tissues. Biochemical Journal. 1991;274:313–316. doi: 10.1042/bj2740313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes DA. Acids produced by human dental plaque metabolism in situ. Caries Research. 1975;9:98–109. doi: 10.1159/000260149. [DOI] [PubMed] [Google Scholar]
- Geddes DA. Studies on metabolism of dental plaque: diffusion and acid production in human dental plaque. Frontiers of Oral Physiology. 1981;3:78–87. [Google Scholar]
- Hay DI, Moreno EC. Statherin and the acidic proline-rich proteins. In: Tenovuo J, editor. Human Saliva: Clinical Chemistry and Microbiology. I. Boca Raton, FL, USA: CRC Press; 1989. pp. 131–150. [Google Scholar]
- Helm JF, Dodds WJ, Hogan WJ, Soergel KH, Egide MS, Wood CM. Acid neutralizing capacity of human saliva. Gastroenterology. 1982;83:69–74. [PubMed] [Google Scholar]
- Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. New England Journal of Medicine. 1984;310:284–288. doi: 10.1056/NEJM198402023100503. [DOI] [PubMed] [Google Scholar]
- Izutsu KT, Madden PR. Evidence for the presence of carbamino compounds in human saliva. Journal of Dental Research. 1978;57:319–325. doi: 10.1177/00220345780570022901. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Brodin P, Berg T, Aars H. Parotid secretion of fluid, amylase and kallikrein during reflex stimulation under normal conditions and after acute administration of autonomic blocking agents in man. Acta Physiologica Scandinavica. 1991;143:321–329. doi: 10.1111/j.1748-1716.1991.tb09239.x. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kuwahara H, Shimazaki M, Ogawa Y, Yagi T. Isolation of a novel carbonic anhydrase from human saliva and immunohistochemical demonstration of its related isozymes in salivary gland. Osaka City Medical Journal. 1987;33:99–109. [PubMed] [Google Scholar]
- Kivelä J, Parkkila S, Metteri J, Parkkila A-K, Toivanen A, Rajaniemi H. Salivary carbonic anhydrase VI concentration and its relation to basic characteristics of saliva in young men. Acta Physiologica Scandinavica. 1997a;161:221–225. doi: 10.1046/j.1365-201X.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- Kivelä J, Parkkila S, Parkkila A-K, Rajaniemi H. A low concentration of carbonic anhydrase isoenzyme VI in whole saliva is associated with caries prevalence. Caries Research. 1999;33:178–184. doi: 10.1159/000016514. [DOI] [PubMed] [Google Scholar]
- Kivelä J, Parkkila S, Waheed A, Parkkila A-K, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clinical Chemistry. 1997b;43:2318–2322. [PubMed] [Google Scholar]
- Kousvelari EE, Baratz RS, Burke B, Oppenheim FG. Immunochemical identification and determination of proline-rich proteins in salivary secretions, enamel pellicle, and glandular tissue specimens. Journal of Dental Research. 1980;59:1430–1438. doi: 10.1177/00220345800590081201. [DOI] [PubMed] [Google Scholar]
- Lamkin MS, Arancillo AA, Oppenheim FG. Temporal and compositional characteristics of salivary protein adsorption to hydroxyapatite. Journal of Dental Research. 1996;75:803–808. doi: 10.1177/00220345960750021101. [DOI] [PubMed] [Google Scholar]
- Leinonen J, Kivelä J, Parkkila S, Parkkila A-K, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Research. 1999;33:185–190. doi: 10.1159/000016515. [DOI] [PubMed] [Google Scholar]
- Leung SW. A demonstration of the importance of bicarbonate as a salivary buffer. Journal of Dental Research. 1951;30:403–414. doi: 10.1177/00220345510300031601. [DOI] [PubMed] [Google Scholar]
- Leung SW. The apparent first dissociation constant (pK1) of carbonic acid in saliva. Archives of Oral Biology. 1961;5:236–240. doi: 10.1016/0003-9969(61)90061-9. [DOI] [PubMed] [Google Scholar]
- Lilienthal B. An analysis of the buffer systems in saliva. Journal of Dental Research. 1955;34:516–530. doi: 10.1177/00220345550340040701. [DOI] [PubMed] [Google Scholar]
- Mandel ID. The functions of saliva. Journal of Dental Research. 1987;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiological Reviews. 1967;47:595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Meurman JH, Frank RM. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Research. 1991;25:1–6. doi: 10.1159/000261335. [DOI] [PubMed] [Google Scholar]
- Muntz JA. Production of acids from glucose by dental plaque material. Journal of Biological Chemistry. 1943;148:225–236. [Google Scholar]
- Murakami H, Sly WS. Purification and characterization of human salivary carbonic anhydrase. Journal of Biological Chemistry. 1987;262:1382–1388. [PubMed] [Google Scholar]
- Nederfors T, Dahlöf C. Effects of the β-adrenoceptor antagonists atenolol and propranolol on human whole saliva flow rate and composition. Archives of Oral Biology. 1992;37:579–584. doi: 10.1016/0003-9969(92)90141-t. [DOI] [PubMed] [Google Scholar]
- Nederfors T, Dahlöf C. Effects on salivary flow rate and composition of withdrawal of and re-exposure to the β1-selective antagonist metoprolol in a hypertensive patient population. European Journal of Oral Sciences. 1996;104:262–268. doi: 10.1111/j.1600-0722.1996.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Nederfors T, Ericsson T, Twetman S, Dahlöf C. Effects of the β-adrenoceptor antagonist atenolol and propranolol on human parotid and submandibular-sublingual salivary secretion. Journal of Dental Research. 1994;73:5–10. doi: 10.1177/00220345940730010701. [DOI] [PubMed] [Google Scholar]
- Olsen PS, Kirkegaard P, Rasmussen T, Magid E, Poulsen SS, Nexø E. Adrenergic effects on secretion of amylase from the rat salivary glands. Digestion. 1988;41:34–38. doi: 10.1159/000199729. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Kaunisto K, Rajaniemi L, Kumpulainen T, Jokinen K, Rajaniemi H. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II and I in human parotid and submandibular glands. Journal of Histochemistry and Cytochemistry. 1990;38:941–947. doi: 10.1177/38.7.2113069. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila A-K. Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scandinavian Journal of Gastroenterology. 1996;31:305–317. doi: 10.3109/00365529609006403. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila A-K, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II and VI in the human alimentary tract. Gut. 1994;35:646–650. doi: 10.1136/gut.35.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Parkkila A-K, Lehtola J, Reinilä A, Södervik H-J, Rannisto M, Rajaniemi H. Salivary carbonic anhydrase protects gastroesophageal mucosa from acid injury. Digestive Diseases and Sciences. 1997;42:1013–1019. doi: 10.1023/a:1018889120034. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila A-K, Rajaniemi H. Circadian periodicity in salivary carbonic anhydrase VI concentration. Acta Physiologica Scandinavica. 1995;154:205–211. doi: 10.1111/j.1748-1716.1995.tb09902.x. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila A-K, Vierjoki T, Ståhlberg T, Rajaniemi H. Competitive time-resolved immunofluorometric assay for quantifying carbonic anhydrase VI in saliva. Clinical Chemistry. 1993;39:2154–2157. [PubMed] [Google Scholar]
- Peeters FP, de Vries M W, Vissink A. Risks for oral health with the use of antidepressants. General Hospital Psychiatry. 1998;20:150–154. doi: 10.1016/s0163-8343(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Rapp GW. The biochemistry of oral calculus. II The presence of carbonic anhydrase in human saliva. Journal of the American Dental Association. 1946;33:191–194. doi: 10.14219/jada.archive.1946.0047. [DOI] [PubMed] [Google Scholar]
- Rudney JD. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Critical Reviews in Oral Biology and Medicine. 1995;6:343–367. doi: 10.1177/10454411950060040501. [DOI] [PubMed] [Google Scholar]
- Sarosiek J, McCallum RW. What role do salivary inorganic components play in health and disease of the esophageal mucosa? Digestion. 1995;56(suppl. 1):24–31. doi: 10.1159/000201298. [DOI] [PubMed] [Google Scholar]
- Sarosiek J, Scheurich CJ, Marcinkiewicz M, McCallum RW. Enhancement of salivary esophagoprotection: rationale for a physiological approach to gastroesophageal reflux disease. Gastroenterology. 1996;110:675–681. doi: 10.1053/gast.1996.v110.pm8608875. [DOI] [PubMed] [Google Scholar]
- Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. Journal of Dental Research. 1944;23:257–266. [Google Scholar]
- Szabó I. Carbonic anhydrase activity in the saliva of children and its relation to caries activity. Caries Research. 1974;8:187–191. doi: 10.1159/000260107. [DOI] [PubMed] [Google Scholar]
- Thatcher BJ, Doherty AE, Orvisky E, Martin BM, Henkin RI. Gustin from human parotid saliva is carbonic anhydrase VI. Biochemical and Biophysical Research Communications. 1998;250:635–641. doi: 10.1006/bbrc.1998.9356. [DOI] [PubMed] [Google Scholar]
- Vaahtoniemi LH, Räisänen S, Stenfors L-E. The age-dependence of bacterial presence on oral epithelial surfaces in vivo. Oral Microbiology and Immunology. 1992;7:263–266. doi: 10.1111/j.1399-302x.1992.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Zahradnik RT, Moreno EC, Burke EJ. Effect of salivary pellicle on enamel subsurface demineralization in vitro. Journal of Dental Research. 1976;55:664–670. doi: 10.1177/00220345760550042101. [DOI] [PubMed] [Google Scholar]
- Zahradnik RT, Propas D, Moreno EC. In vitro enamel demineralization by Streptococcus mutans in the presence of salivary pellicles. Journal of Dental Research. 1977;56:1107–1110. doi: 10.1177/00220345770560091601. [DOI] [PubMed] [Google Scholar]
- Zahradnik RT, Propas D, Moreno EC. Effect of salivary pellicle formation time on in vitro attachment and demineralization by Streptococcus mutans. Journal of Dental Research. 1978;57:1036–1042. doi: 10.1177/00220345780570110601. [DOI] [PubMed] [Google Scholar]


