Abstract
Human myometrial smooth muscle cells (HMSMCs) in culture were exposed to recombinant human interleukin-1β (IL-1β, 10 ng ml−1) for 1 to 24 h. Cyclooxygenase-2 (COX-2) mRNA and protein were rapidly induced, with expression sustained at 24 h.
Cycloheximide (10 μg ml−1, 6 h) blocked IL-1β-induced COX-2 protein expression and super-induced COX-2 mRNA expression. Induction of COX-2 mRNA and protein was blocked by dexamethasone (1 μm, 6 h).
IL-1β-induced COX-2 expression was accompanied by a 3-fold increase of prostaglandin E2 release into the culture medium.
IL-1β induced a transient (5–30 min) activation of p42/44 and p38 mitogen-activated protein kinase (MAPK) enzymes in HMSMCs. Activity of p38 MAPK was monitored by in-gel activity of its substrate MAP kinase-activated protein kinase-2 (MAPKAP kinase-2). Induction of MAPKAP kinase-2 activity was prevented by the p38 MAPK inhibitor SB 203580 (10 μm, 5–30 min).
COX-2 protein expression detected after 6 h IL-1β stimulation was blocked by SB 203580 (10 μm). Exposure of HMSMCs to 10 ng ml−1 IL-1β for only 30 min induced a level of COX-2 protein expression at 6 h culture similar to that detected in cells exposed to the cytokine for 6 h.
Exposure of cells to SB 203580 (10 μm) during only the first 30 min of IL-1β stimulation was effective in blocking COX-2 protein expression assayed after 6 h in culture.
This study has established that a transient activation of the p38 MAPK cascade is involved in IL-1β-stimulated COX-2 expression in human myometrial smooth muscle cells. Induction of COX-2 by IL-1β in HMSMCs provides support for the hypothesis that autocrine prostaglandin signalling in the myometrium, initiated by elevated intrauterine cytokine concentrations, plays a role in regulating myometrial contractility during labour.
The precise mechanisms underlying the initiation of labour, at term or earlier, are not known. However, intrauterine infection is one of the principal causes of pre-term labour (Brockelhurst, 1999), and there is convincing evidence implicating inflammatory cytokines in the normal biochemical mechanisms of parturition (Steinborn et al. 1996; Tanaka et al. 1998). A major target of these signals in a variety of cell types is increased production and release of prostaglandins (Higgs et al. 1984). The rate-limiting step in the synthesis of prostaglandins is the conversion of arachidonic acid (AA) to the precursor prostaglandin H2 (PGH2), catalysed by cyclooxygenase (COX) enzymes (also known as prostaglandin endoperoxide H synthases). COX is a homodimeric, bifunctional enzyme, and two isoforms have been identified (see Smith & DeWitt, 1996). COX-1 is present in nearly all tissues, and its expression is usually not regulated by external stimuli, whereas COX-2 is an inducible enzyme that is normally undetectable, but whose expression is rapidly induced in response to growth factors, tumour promoters, cytokines and bacterial cell wall products (Kujubu et al. 1991; Seibert & Masferrer, 1994; Smith & DeWitt, 1996). Activity of induced COX-2 is implicated in the overproduction of prostaglandins observed in inflammatory situations (Crofford et al. 1994; Onoe et al. 1996; Hendel & Neilsen 1997; Baker et al. 1999).
Prostaglandins act through specific G-protein-coupled membrane receptors, and acutely regulate smooth muscle tone principally by modulating levels of IP3 and cAMP, which in turn lead to alterations in intracellular calcium (Negishi et al. 1995). Prostaglandins E2 (PGE2) and F2α (PGF2α) have long been identified as crucial mediators in the maintenance and progression of labour contractions (Challis & Lye 1994). COX-1 and COX-2 isoforms have been detected during human pregnancy in fetal membranes, placenta, decidua and myometrium, with expression of COX-2 (rather than COX-1) increasing in the myometrium, amnion, chorion and placenta prior to labour (Zuo et al. 1994; Slater et al. 1998, 1999). Elevated levels of prostaglandins in uterine tissues, produced by COX-2 induced in response to inflammatory signals, may contribute to increased contractile frequency and strength during labour.
Thus, inflammatory cytokines provide a potential mechanism for increased COX-2 expression and prostaglandin release by intrauterine tissues. Elevated levels of cytokines such as IL-1β are found in decidua, chorion and amniotic fluid from women with normal and pre-term labour (Cox et al. 1997), and elevated fetal-serum levels of IL-6 and IL-8 resulting from infection can predict pre-term delivery (Romero et al. 1998). Cervico-vaginal concentrations of IL-1β and IL-6 in excess of 10 ng ml−1 have been reported in cases of pre-term rupture of membranes and labour contractions in the absence of infection (Steinborn et al. 1996). Moreover, IL-1β, IL-6, and IL-8 levels in lower uterine segment biopsies increase with gestational age, the degree of cervical dilation, and the onset of labour (Tanaka et al. 1998).
Investigations in a number of cell types have demonstrated that IL-1β-induced COX-2 expression involves the activation of members of the nuclear factor kappa B (NF-κB) family of cellular transcription factors, and the c-jun NH2-terminal (JNK), p42/44 and p38 mitogen-activated protein kinase (MAPK) cascades (Newton et al. 1997a; Guan et al. 1998; LaPointe & Isenovic 1999). The degree of regulation by p38 MAPK appears to depend on cell type, with inhibition leading to complete or partial inhibition of COX-2 expression in rat mesangial cells and the macrophage cell line RAW 264.7, respectively (Guan et al. 1997, 1998; Hwang et al. 1997).
In human myometrial cells, IL-1β, tumour necrosis factor α (TNFα), and epidermal growth factor (EGF) elicit increased AA metabolism and release of PGE2, PGF2α and prostacyclin (Pollard & Mitchell 1996; Todd et al. 1996). We describe here the induction of COX-2 and its regulation by p38 MAPK in human myometrial smooth muscle cells in culture. Similar findings have been presented recently in abstract form by Belt et al. (1998), who noted that IL-1β activated all three MAP kinase cascades in an immortalised human myometrial cell line, and described the blockade of COX-2 expression with the p38 MAP kinase inhibitor SB 203580. In this study we present findings which establish that activation of p38 MAP kinase is essential for IL-1β-induced COX-2 expression in human myometrial smooth muscle cells established form primary culture. A preliminary account of part of this work has been presented in abstract form (Bartlett et al. 1998a,b).
METHODS
Isolation and primary culture of human myometrial smooth muscle cells
Myometrial tissue was obtained from biopsies at caesarean section from ten non-labouring women at term (gestation 38.4 ± 0.7 weeks). The indications for elective section were breech delivery or previous caesarean section. There was no evidence of uterine contractions, cervical change or uterine dysfunction. The study was approved by the local Ethics Committee of St Thomas’ Hospital, London, where all tissues were collected, and all patients gave written, informed consent. Biopsies were obtained from the mid-line of the upper edge of the lower uterine segment incision and collected in sterile culture medium.
In order to obtain rapid outgrowth, cells were initially cultured in MCDB 131 culture medium (Gibco Life Technologies). Explants (2 mm3, visibly free of blood vessels) were dissected and placed onto the surface of 25 cm2 culture flasks for 2 h (37°C humidified 5 % CO2-95 % air atmosphere), before being submerged in MCDB 131 medium (with 10 % fetal calf serum (FCS), 10 mm L-glutamine, 100 u ml−1 penicillin, 100 μg ml−1 streptomycin) supplemented with 2.5 μg ml−1 amphotericin B, and 50 μg ml−1 gentamicin, 10 ng ml−1 human recombinant epidermal growth factor (EGF), and 1 μg ml−1 hydrocortisone. After 4 days the medium was replaced with MCDB 131 without added amphotericin B and gentamicin. Primary human myometrial smooth muscle cell (HMSMC) cultures were established within 10 days. After passage 1, cells were cultured in MCDB 131 without EGF or hydrocortisone. Cells exhibited typical smooth muscle morphology by phase contrast microscopy, and cultures stained positively with a monoclonal anti-smooth muscle α-actin antibody (data not shown). Experiments were performed with confluent HMSMCs in passage 2 and 3, within 3 weeks of delivery.
For experiments, cells were seeded into 6 cm or 9 cm diameter culture dishes for protein and RNA analysis, respectively. In initial experiments, cells cultured in MCDB 131 medium showed a weak response to IL-1β (data not shown). Therefore, before addition of recombinant human IL-1β (R&D Systems), confluent HMSMCs were serum deprived for 16-24 h in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies), supplemented with 0.5 % FCS, 4 mm L-glutamine, 100 u ml−1 penicillin, and 100 μg ml−1 streptomycin. Cells were stimulated by the addition of IL-1β (0.1-100 ng ml−1) for the specified times. For inhibitor studies, 10 μm SB 203580 (Alexis Biochemicals, Nottingham, UK), 100 μm indomethacin, 10 μg ml−1 cycloheximide, or 1 μm dexamethasone were added (as a 1000 × stock in dimethyl sulphoxide (DMSO)) to the overlying culture medium 30 min prior to addition of IL-1β. Blots are representative of a minimum of two different HMSMC cultures.
Northern blotting
Cells for harvesting were washed twice with ice cold phosphate buffered saline (PBS) and total RNA was extracted with a Qiagen ‘RNAeasy’ spin column kit in accordance with the manufacturer's instructions (Qiagen, Crawley, UK). RNA was concentrated by ethanol precipitation and redissolved in 10 μl ribonuclease-free water. Twenty micrograms of each RNA sample were run on denaturing 1.2 % (w/v) agarose gels (20 mm MOPS, pH 7.0, 8 mm sodium acetate, 1 mm EDTA, pH 8.0, 2.2 M formaldehyde). RNA was blotted from the gel onto Hybond N + nylon membranes (Amersham Pharmacia Biotech). Random primed 32P-labelled probes were generated using a ‘Megaprime’ labelling kit (Amersham Pharmacia Biotech), with a 2 kb fragment containing the open reading frame of human COX-2 cDNA or a human glyceraldehyde 3-phosphate dehydrogenase (G3PDH) cCNA sequence (Clontech) as template. Blots were washed at 50°C with 0.1 % SDS, 0.1 × SSC (saline sodium citrate; 150 mm NaCl, 17 mm sodium citrate, pH 7.0) and exposed to X-ray film overnight at -70°C.
Immunoblotting
At the time of harvest, cells were washed twice with ice cold PBS containing 0.4 mm NaVO4, and lysed on ice with 200 μl lysis buffer (20 mm Tris-HCl, pH 7.5, 0.15 M NaCl, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm MgCl2, 1 % Triton X-100, 2.5 mm sodium pyrophosphate, 1 mmβ-glycerophosphate, 100 μm NaVO4, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin, 5 μg ml−1 chymostatin, 1 μg ml−1 aprotinin, 1 mm phenylmethylsulphonyl fluoride). Lysates were scraped with a cell scraper, combined with 70 μl 4 × Laemmli sample buffer (62.5 mm Tris-HCl, pH 6.8, 10 % v/v glycerol, 5 % v/v β-mercaptoethanol, 2.3 % w/v SDS) and boiled for 5 min. Equal amounts of protein were run on 10 % SDS-polyacrylamide gel electrophoresis. Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore), and blocked overnight at 4°C in blocking solution (3 % w/v BSA in PBS, 0.1 % v/v Tween 20 (PBS-T)). For analysis of COX protein expression, blots were incubated with agitation at room temperature for 1 h with polyclonal anti-COX-1 or COX-2 antibodies (Santa Cruz) diluted 1:1000 in blocking solution. After washing in PBS-T solution, blots were incubated further for 1 h at room temperature with a horseradish peroxidase (HRP) conjugated anti-goat secondary antibody diluted 1:2000 (Santa Cruz). Blots were then washed four times in PBS-T, and antibody-bound protein was visualised with an Enhanced Chemiluminescence (ECL) kit (Amersham Pharmacia Biotech). Expression of smooth muscle α-actin was assessed with a monoclonal antibody used at 1:1000 dilution, with an HRP-conjugated goat anti-mouse antibody at 1:10000 as the secondary antibody (Pierce).
Immunoprecipitation
Cell lysates were subjected to three cycles of freezing and thawing on dry ice, and centrifuged at 13 000 r.p.m. for 5 min at 4°C. Supernatants were transferred to fresh tubes, and 200 μg protein incubated at 4°C on a rotor overnight with 3 μl polyclonal anti-pan p38 MAP kinase antibody (New England Biolabs). Immunoglobulins were precipitated with 20 μl Protein A/G-agarose (Santa Cruz) for 3 h at 4°C, and complexes washed three times with lysis buffer. Immunoprecipitates were then combined with an equal volume of 2 × Laemmli sample buffer and boiled for 5 min. Samples were blotted, and blots probed with a polyclonal anti-phosphorylated p38 (pp38) MAP kinase antibody at 1:1000 dilution (New England Biolabs). The secondary antibody was an HRP conjugated goat anti-rabbit antibody at 1:10 000 dilution (Pierce).
In-gel kinase assay
Protein kinase assays were performed as described by Cano et al. (1996). Briefly, harvested cells in 1 × Laemmli sample buffer were run on 14 % acrylamide gels polymerised in the presence of 500 μg ml−1 myelin basic protein (MBP) or 250 μg ml−1 of a polymer of glutamate and tyrosine (4:1 poly-Glu-Tyr). After electrophoresis, SDS was removed by incubation in 20 % isopropanol in 50 mm Tris-HCl, pH 8.0, for 1 h at room temperature. The gel was then washed for 1 h with 1 mm dithiothreitol (DTT), 50 mm Tris-HCl, pH 8.0. To denature the proteins, gels were incubated in 6 M guanidine-HCl, 20 mm DTT, 2 mm EDTA, 50 mm Tris-HCl, pH 8.0, for 1 h. Proteins were then renatured by overnight incubation at 4°C in 1 mm DTT, 2 mm EDTA, 0.04 % Tween 20, 50 mm Tris-HCl, pH 8.0, without agitation. For the protein kinase assays, gels were equilibrated at room temperature for 1 h in kinase buffer containing 1 mm DTT, 0.1 mm EGTA, 20 mm MgCl2, 100 μm NaVO4, 40 mm Hepes-NaOH, pH 8.0. The kinase reaction was carried out for 1 h in kinase buffer with 30 μm ATP and 10 μCi ml−1[γ-32P]ATP (Amersham Pharmacia Biotech). Finally, the gels were washed extensively with 5 % (w/v) trichloroacetic acid, 1 % (w/v) sodium pyrophosphate until washes were free of radioactivity. Autoradiography of dried, stained gels was performed at -70°C.
PGE2 determination
PGE2 in overlying culture media was measured with an enzyme immunoassay kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Chemicals
All chemicals were from Sigma unless otherwise stated above.
RESULTS
Induction of COX-2 expression in HMSMCs by IL-1β
HMSMC cultures exhibited either low or undetectable basal COX-2 expression. Stimulation for 6 h with IL-1β induced a dose-dependent (1-100 ng ml−1) expression of COX-2 protein detected by immunoblot (data not shown). Maximal band intensity was achieved with a concentration of 10 ng ml−1 IL-1β. This concentration, similar to levels reported in cervico-vaginal secretions during active labour (Steinborn et al. 1996), was used for all subsequent experiments. Analysis of the time course of COX-2 expression revealed a rapid induction, with mRNA and protein detectable within 1-2 and 2-4 h, respectively. Representative Northern and immunoblots are shown in Fig. 1A and B. Steady-state mRNA levels declined after 4 h stimulation but COX-2 mRNA expression was sustained at 24 h. Protein expression reached a maximum after 6 h and was sustained at 24 h, with an accompanying elevation of PGE2 accumulation in the culture medium. PGE2 increased from a basal level of 171 ± 17 to 452 ± 7 ng (mg protein)−1 (24 h)−1 in IL-1β-stimulated cells (mean ±s.e.m., n= 6 cell cultures). COX-1 protein was expressed basally but was unaffected by IL-1β treatment.
Figure 1. Induction of COX-2 in human myometrial smooth muscle cells (HMSMCs) by IL-1β.
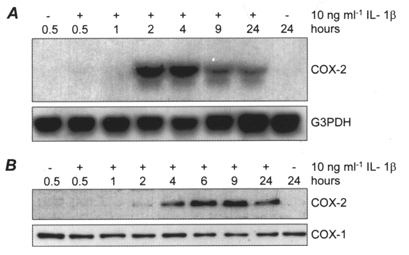
Representative time course of IL-1β-induced COX-2 mRNA (A) and protein (B) expression in HMSMCs compared to the expression of G3PDH and COX-1, respectively.
The protein synthesis inhibitor cycloheximide and the corticosteroid dexamethasone were tested for their effects on COX-2 expression after 6 h exposure to 10 ng ml−1 IL-1β (Fig. 2A and B). Cycloheximide (10 μg ml−1) prevented synthesis of COX-2 protein, and increased the level of COX-2 mRNA, indicative of super-induction characteristic of immediate/early genes such as COX-2 (Edwards & Mahadevan 1992; Newton et al. 1997b). Dexamethasone (1 μm) blocked IL-1β-induced COX-2 mRNA and protein expression, consistent with the effect of corticosteroids on COX-2 expression in other systems (Smith & DeWitt, 1996; Newton et al. 1998).
Figure 2. The effects of cycloheximide and dexamethasone on IL-1β-induced COX-2 expression in HMSMCs.
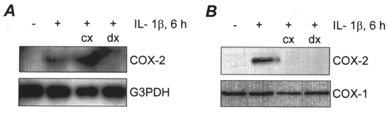
Representative Northern blot (A) and immunoblot (B) showing the effect of 10 μg ml−1 cycloheximide (cx) and 1 μm dexamethasone (dx) on COX-2 expression in HMSMCs exposed to 10 ng ml−1 IL-1β for 6 h.
Activation of MAP kinase cascades by IL-1β in HMSMCs
Quiescent HMSMCs were stimulated with IL-1β for between 1 and 60 min. Activity of p42 and p44 MAP kinase isoforms was directly assessed by in-gel phosphorylation of myelin basic protein (MBP). IL-1β caused a marked increase in activity between 10 and 30 min exposure (Fig. 3A). p38 MAPK is not effective at phosphorylation of MBP in in-gel kinase assays, but kinase activity was also evident at 50 kDa. This is the size of human MAP kinase-activated kinase-2 (MAPKAP kinase-2), which is a substrate of p38 MAPK, and thus may indicate p38 MAPK activation in IL-1β-stimulated cells (Freshney et al. 1994; Rouse et al. 1994). Immunoprecipitation and immunoblotting of p38 MAPK isoforms confirmed a transient phosphorylation of the enzyme first detectable after 5 min, with maximal intensity at 15-20 min (Fig. 3B). The kinase inhibitor SB 203580 blocks the activity but not the phosphorylation of p38 MAPK (Cohen 1997). The effectiveness of 10 μm SB 203580 as an inhibitor of p38 MAPK activity in HMSMCs was therefore assessed by autophosphorylation of MAPKAP kinase-2 in the presence of poly-Glu-Tyr (see Methods). Stimulation with IL-1β elicited a strong signal at 50 kDa (Fig. 3C), which has been identified previously as MAPKAP kinase-2 (Cano et al. 1996). Addition of 10 μm SB 203580 to the culture medium blocked this activity, demonstrating the effectiveness of this concentration in inhibiting p38 MAPK. A similar result was obtained with a species at approximately 60 kDa. The identity of this species is unknown, but it is similar in size to MAP kinase interacting kinase-1 (Mnk-1), which is activated by p38 MAPK and p42/44 MAPKs (Cohen 1997).
Figure 3. MAP kinase signalling in IL-1β-stimulated HMSMCs.
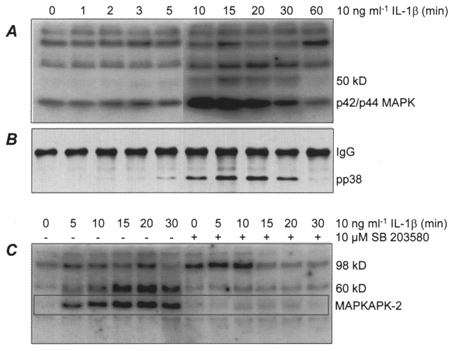
A, in-gel phosphorylation of myelin basic protein (MBP) by HMSMC extracts. The 50 kDa species is putatively identified as MAPKAP kinase-2 (see text). B, immunoprecipitation/immunoblot showing phosphorylated (active) p38 MAPK (pp38). IgG indicates immunoglobulins. C, in-gel kinase activity in the presence of poly-Glu-Tyr (4:1). Cells were stimulated in the absence or presence of SB 203580 as indicated. The boxed area marks the position of auto-phosphorylated MAPKAP kinase-2.
The effect of inhibition of p38 MAP kinase on IL-1β-induced COX-2 expression in HMSMCs
HMSMCs, pretreated (30 min) with 10 μm SB 203580, 100 μm indomethacin or DMSO vehicle, were then stimulated with 10 ng ml−1 IL-1β for 6 h. In the presence of the p38 MAPK inhibitor induced COX-2 protein expression was blocked, with the signal reduced to control levels (Fig. 4A). Indomethacin had no effect on induced COX-2 protein expression. The steady state expression of COX-1 and smooth muscle α-actin were unaffected by these treatments. In further experiments, the medium was changed to medium without cytokine or inhibitor 30 min after the initial application of IL-1β, and the cells cultured for a further 6 h (Fig. 4B). Short exposure to 10 ng ml−1 IL-1β was effective in inducing COX-2 protein expression detected after a further 6 h in culture, with a similar band intensity observed to that produced by constant exposure to IL-1β. Short exposure of stimulated HMSMCs to 10 μm SB 203580 was as effective as prolonged exposure in reducing induced COX-2 protein expression measured 6 h later.
Figure 4. Inhibition of IL-1β-induced COX-2 protein expression by the p38 MAP kinase inhibitor SB 203580.
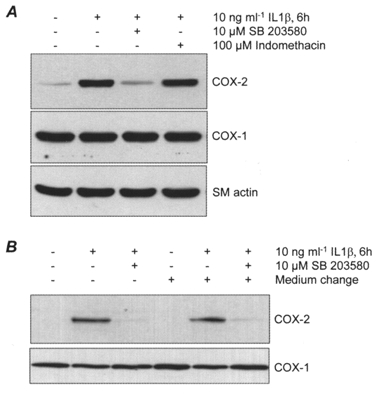
HMSMCs were stimulated with IL-1β following 30 min pre-incubation with SB 203580 or the COX inhibitor indomethacin. A, immunoblot of COX-2 expression, with COX-1 and smooth muscle (SM) actin expression for comparison. B, immunoblot of COX-2 expression in stimulated cells with and without replacement of medium 30 min after addition of IL-1β (with COX-1 expression for comparison).
DISCUSSION
The present study has characterised signalling pathways involved in cytokine-induced COX-2 expression and activity in human myometrial smooth muscle cells (HMSMCs) in vitro. Induction of COX-2 by IL-1β in human myometrial smooth muscle cells provides support for the hypothesis that elevated intrauterine cytokine concentrations play a major role in the onset and maintenance of uterine contractions via increased synthesis of contractile prostaglandins by uterine tissues, including the myometrium. Moreover, we provide convincing evidence that a transient induction of p38 MAPK activity is essential for IL-1β-induced COX-2 expression in HMSMCs.
The amnion and placental trophoblasts are rich sources of prostaglandins and COX-2 in late pregnancy (Dudley et al. 1996; Slater et al. 1998), and there is evidence that increased fetal membrane synthesis of PGE2 precedes clinical labour (Brown et al. 1998). Production of PGE2 and PGF2α by decidual stromal cells may provide a further important source in labour. However, it is uncertain how much of these prostanoids reach the myometrium. Our results support the hypothesis that autocrine prostaglandin signalling in the myometrium, initiated by cytokine action, plays a role in regulating myometrial contractility. It is possible that phenotypic changes resulting from the transition to cell culture may lead to synthetic changes within the cells, and thus our findings in cultured myometrial cells may need to be interpreted with some caution. However, our results are consistent with the recent identification of increased COX-2 expression in the myometrium in vivo associated with labour (Slater et al. 1999).
The rapid induction of COX-2 expression and activity we describe is consistent with the work of Belt et al. (1998) and with reports on other cell types, where a role for signalling via p38 MAPK has also been identified. MAPKs have been implicated as a mechanism by which signals are transduced from the cell surface to the nucleus in response to a variety of stimuli, and participate in intracellular processes by further inducing the phosphorylation of substrates such as other protein kinases and transcription factors (Cohen, 1997). Several reports implicate MAPK signalling in the regulation of prostaglandin biosynthesis. In the mouse macrophage cell line RAW 264.7, inhibition of p38 MAP kinase results in only a partial blockade of COX-2 induction, suggesting that in this cell type p38 MAPK plays a non essential role and that other factors such as NF-κB are more important (Hwang et al. 1997). Our results in HMSMCs are similar to findings in rat mesangial cells, where blockade of p38α MAPK, as well as c-jun N-terminal kinase (JNK), prevents COX-2 expression (Guan et al. 1997, 1998). The finding that induction of COX-2 via this signalling cascade is differently regulated in different cell types has implications for the tissue specific regulation of stress responses and for potential therapeutic interventions in uterine prostaglandin synthesis.
Our findings implicating p38 MAPK in IL-1β-induced COX-2 expression are based on pharmacological inhibition with the pyridinyl imidazole SB 203580. Kinase assays of p38 MAPK commonly measure activity of cell lysates or the immunoprecipitated enzyme. The effective inhibitor concentrations may be altered in these assays, or the inhibitor removed during washes. The kinase assay we used in the present study measures the in-gel autophoshorylation of MAPKAP kinase-2, itself phosphorylated and activated by p38 MAPK during the stimulation of cells prior to lysis (Cano et al. 1996). Thus the measured activity is an index of p38 MAP kinase activity in intact cells. In our experiments, 10 μm SB 203580 was an effective inhibitor of p38 MAPK activity and COX-2 expression. SB 203580 has recently been shown also to act as an inhibitor of purified COX proteins in vitro (Borsch-Haubold et al. 1998), implying that some of its effects may be mediated through blocking of COX activity. Inhibition of COX activity with 100 μm indomethacin did not affect IL-1β-induced COX-2 protein expression detected by immunoblot 6 h later. It is therefore likely that the effects of SB 203580 were mediated via inhibition of p38 MAPK rather than by altered prostaglandin synthesis. The p38 MAPK isoforms p38α and p38β, but not p38γ or p38δ, are sensitive to SB 203580 (Cohen, 1997). Thus, the effectiveness of this kinase inhibitor in our experiments suggests that IL-1β is signalling through p38α and p38β. Several substrates of p38 MAPK have been identified (Cohen, 1997), and the principal among these is MAPKAP kinase-2, employed in our experiments as a marker of p38 MAPK activity. We have not yet identified the 60 kDa kinase activity observed in our in-gel kinase assay, but it may correspond to the related substrate MAPK-interacting kinase-1 (Fukunaga & Hunter, 1997). The COX-2 promoter region has been characterised partially, and includes sites for NF-κB, AP-2 and SP1, and an activating transcription factor (ATF)/cAMP response element (ATF/CRE) (Appleby et al. 1994). The mechanisms involved in transcriptional activation of COX-2 downstream from p38 MAPK are still unclear, but have been proposed to include phosphorylation of the transcription factor ATF-2 (Guan et al. 1998). p38 MAPK also induces transcriptional activity via phosphorylation of ATF-1 and CRE binding protein (CREB) (Iordanov et al. 1997). Recent evidence suggests that, in addition to activation of transcription, p38 MAPK plays a role in the stabilisation of COX-2 mRNA (Ridley et al. 1998; Dean et al. 1999).
Current models of parturition propose that, prior to the onset of labour contractions, the myometrium undergoes activation whereby alterations in the hormonal/cytokine environment progressively modify the expression of groups or ‘cassettes’ of genes encoding contraction associated proteins (CAPs) (Challis & Lye, 1994). These include the gap junctional protein connexin 43, ion channels, COX-2, and prostaglandin receptors (PGRs). On the basis of this, it might be predicted that a given signal acting on the myometrium could act as a switch, moving the tissue onto the next stage in the activation process. Consistent with this hypothesis, we observed that exposure of HMSMCs to 10 ng ml−1 IL-1β for 30 min was as effective in inducing COX-2 protein expression after 6 h culture as exposure to this cytokine during the entire culture period. This brief exposure corresponded to the peak activation of p38 MAPK, and 10 μm SB 203580 was effective in blocking COX-2 induction in these experiments. The concentrations of SB 203580 and IL-1β after medium change are unknown, but it is reasonable to assume that they were greatly reduced. Thus we conclude that a transient activation of p38 MAPK in myometrial cells exposed to 10 ng ml−1 IL-1β induces sustained expression of COX-2.
In human granulosa-luteal cells, COX-2 and the PGF2α receptor FP are co-ordinately upregulated by IL-1β (Narko et al. 1997), and cytokines may similarly regulate these and other CAP genes in the myometrium. Myometrial expression of FP declines with gestational age, before increasing during labour at term and pre-term, whereas the relaxatory PGE2 receptor EP2 is highly expressed earlier in pregnancy and then declines at term (Brodt-Eppley & Myatt, 1999). Thus, cytokine mediated induction of COX-2, coupled with the co-ordinated alteration in the expression of other CAPs such as prostaglandin receptor subtypes, could play an important role in normal labour at term, and may account for the initiation of premature labour associated with intrauterine infection.
Acknowledgments
We gratefully acknowledge the Tommy's Campaign (Grant no. 29) for support of this work and thank Professor Lucilla Poston for her helpful discussions. This research group is a member of the London Myometrial Group.
References
- Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochemical Journal. 1994;302:723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CSR, Hall RJC, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arteriosclerosis Thrombosis and Vascular Biology. 1999;19:646–655. doi: 10.1161/01.atv.19.3.646. [DOI] [PubMed] [Google Scholar]
- Bartlett SR, Sawdy R, Dennes W, Bennett P, Mann GE. Cytokine-induced regulation of nitric oxide synthase and cyclooxygenase 2 in cultured human myometrial cells. Journal of the Society for Gynecologic Investigation. 1998a;5(suppl.):183A. [Google Scholar]
- Bartlett SR, Sawdy R, Mann GE. Cytokine-induced regulation of cyclooxygenase-2 expression in cultured human myometrial smooth muscle cells. Prenatal and Neonatal Medicine. 1998b;3(suppl.):25. [Google Scholar]
- Belt AR, Baldessare JJ, Romero R, Hertelendy F. IL-1 activates the MAP kinase family of enzymes in human myometrial cells: Evidence for the involvement of p38 kinase in IL-1-cyclooxygenase-2 (Cox-2) expression. Journal of the Society for Gynecologic Investigation. 1998;5(suppl.):64A. [Google Scholar]
- Borsch-Haubold AG, Pasquet S, Watson SP. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. Journal of Biological Chemistry. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- Brockelhurst P. Infection and premature delivery. British Medical Journal. 1999;318:548–549. [Google Scholar]
- Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstetrics and Gynecology. 1999;93:89–93. doi: 10.1016/s0029-7844(98)00378-0. [DOI] [PubMed] [Google Scholar]
- Brown NL, Alvi SA, Elder MG, Bennett PR, Sullivan MHF. A spontaneous induction of fetal membrane prostaglandin production precedes clinical labour. Journal of Endocrinology. 1998;157:R1–6. doi: 10.1677/joe.0.157r001. [DOI] [PubMed] [Google Scholar]
- Cano E, Doza YN, Benlevy R, Cohen P, Mahadevan LC. Identification of anisomycin-activated kinases p45 and p55 in murine cells as MAPKAP kinase-2. Oncogene. 1996;12:805–812. [PubMed] [Google Scholar]
- Challis JRG, Lye SJ. Parturition. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 985–1031. [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends in Cell Biology. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1 beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Human Reproduction Update. 1997;3:517–527. doi: 10.1093/humupd/3.5.517. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Wilder RL, Ristimaki AP, Sano H, Remmers EF, Epps HR, Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1β, phorbol ester, and corticosteroids. Journal Clinical Investigation. 1994;93:1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JLE, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. Journal of Biological Chemistry. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Edwin SS, Mitchell MD. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labour. Journal of the Society for Gynecological Investigation. 1996;3:328–335. [PubMed] [Google Scholar]
- Edwards DR, Mahadevan LC. Protein-synthesis inhibitors differentially superinduce c-fos and c-jun by 3 distinct mechanisms - lack of evidence for labile repressors. EMBO Journal. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein-kinase cascade that results in the phosphorylation of hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Hunter T. Mnk1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO Journal. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZH, Baier LD, Morrison AR. p38 Mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1β. Journal of Biological Chemistry. 1997;272:8083–8089. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- Guan ZH, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1 beta-induced cyclooxygenase-2 expression requires activation of both c-jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. Journal of Biological Chemistry. 1998;273:28670–28676. doi: 10.1074/jbc.273.44.28670. [DOI] [PubMed] [Google Scholar]
- Hendel J, Nielsen OH. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. American Journal of Gastroenterology. 1997;92:1170–1173. [PubMed] [Google Scholar]
- Higgs GA, Moncada S, Vane JR. Eicosanoids and inflammation. Annals of Clinical Research. 1984;16:287–299. [PubMed] [Google Scholar]
- Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide - mediation through both mitogen-activated protein kinase and NF-κB signaling pathways in macrophages. Biochemical Pharmacology. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf HJ, Herrlich P. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO Journal. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. Tis10, a phorbol ester tumor promoter-inducible messenger-RNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase cyclooxygenase homolog. Journal of Biological Chemistry. 1991;266:12866–12872. [PubMed] [Google Scholar]
- LaPointe MC, Isenovic E. Interleukin-1β regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signalling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- Narko K, Ritvos O, Ristimaki A. Induction of cyclooxygenase-2 and prostaglandin F-2α receptor expression by interleukin-1β in cultured human granulosa-luteal cells. Endocrinology. 1997;138:3638–3644. doi: 10.1210/endo.138.9.5388. [DOI] [PubMed] [Google Scholar]
- Negishi M, Yukihoko S, Ichikawa A. Molecular mechanisms and diverse actions of prostanoid receptors. Biochimica et Biophysica Acta. 1995;1259:109–120. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- Newton R, Kuitert LME, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochemical and Biophysical Research Communications. 1997a;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- Newton R, Seybold J, Kuitert LME, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. Journal of Biological Chemistry. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- Newton R, Stevens DA, Hart LA, Lindsay M, Adcock IM, Barnes PJ. Superinduction of cox-2 mRNA by cycloheximide and interleukin-1β involves increased transcription and correlates with increased NF-κB and JNK activation. FEBS Letters. 1997b;418:135–138. doi: 10.1016/s0014-5793(97)01362-8. [DOI] [PubMed] [Google Scholar]
- Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, Seo H, Ohta H, Nozawa S, Kudo I, Suda T. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. Journal of Immunology. 1996;156:758–764. [PubMed] [Google Scholar]
- Pollard JK, Mitchell MD. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. American Journal of Obstetrics and Gynecology. 1996;174:682–686. doi: 10.1016/s0002-9378(96)70450-7. [DOI] [PubMed] [Google Scholar]
- Ridley SH, Dean JLE, Sarsfield SJ, Brook M, Clark AR, Saklatvala J. A p38 map kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Letters. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- Romero R, Gomez R, Ghezzi F, Yoon BY, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. American Journal of Obstetrics and Gynecology. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonsollamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat-shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat-shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Seibert K, Masferrer J. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;94:17–23. [PubMed] [Google Scholar]
- Slater D, Allport V, Bennett P. Changes in the expression of the type-2 but not the type-1 cyclooxygenase enzyme in chorion-decidua with the onset of labour. British Journal of Obstetrics and Gynaecology. 1998;105:745–748. doi: 10.1111/j.1471-0528.1998.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Slater DM, Dennes WJB, Campa JS, Poston L, Bennett PR. Expression of cyclo-oxygenase types 1 and 2 in human myometrium throughout pregnancy. Molecular Human Reproduction. 1999;5:880–884. doi: 10.1093/molehr/5.9.880. [DOI] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL. Prostaglandin endoperoxide h synthases-1 and -2. Advances in Immunology. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- Steinborn A, Kuhnert M, Halberstadt E. Immunmodulating cytokines induce term and preterm parturition. Journal of Perinatal Medicine. 1996;24:381–390. doi: 10.1515/jpme.1996.24.4.381. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Narahara H, Takai N, Yoshimatsu J, Anai T, Miyakawa I. Interleukin-1β and interleukin-8 in cervicovaginal fluid during pregnancy. American Journal of Obstetrics and Gynecology. 1998;179:644–649. doi: 10.1016/s0002-9378(98)70058-4. [DOI] [PubMed] [Google Scholar]
- Todd HM, Dundoo VL, Gerber WR, Cwiak CA, Baldassare JJ, Hertelendy F. Effect of cytokines on prostaglandin E2 and prostacylin production in primary cultures of human myometrial cells. Journal of Maternal and Fetal Medicine. 1996;5:161–167. doi: 10.1002/(SICI)1520-6661(199607/08)5:4<161::AID-MFM1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zuo J, Lei ZM, Rao CV, Pietrantoni M, Cook VD. Differential cyclooxygenase-1 and cyclooxygenase-2 gene-expression in human myometria from preterm and term deliveries. Journal of Clinical Endocrinology and Metabolism. 1994;79:894–899. doi: 10.1210/jcem.79.3.8077379. [DOI] [PubMed] [Google Scholar]


