Abstract
Hydraulic pressure in intercostal and diaphragmatic lymphatic vessels was measured through the micropuncture technique in 23 anaesthetised paralysed rabbits. Pleural lymphatic vessels with diameters ranging from 55 to 950 μm were observed under stereomicroscope view about 3–4 h after intrapleural injection of 20 % fluorescent dextrans.
Lymphatic pressure oscillated from a minimum (Pmin) to a maximum (Pmax) value, reflecting oscillations in phase with cardiac activity (cardiogenic oscillations) and lymphatic myogenic activity. With intact pleural space, Pmin in submesothelial diaphragmatic lymphatic vessels of the lateral apposition zone was −9.1 ± 4.2 mmHg, more subatmospheric than the simultaneously recorded pleural liquid pressure amounting to −3.9 ± 1.2 mmHg. In extrapleural intercostal lymphatic vessels Pmin averaged −1.3 ± 2.7 mmHg.
Cardiogenic pressure oscillations (Pmax−Pmin), were observed in all recordings; their mean amplitude was about 5 mmHg and was not dependent upon frequency of cardiac contraction, nor lymphatic vessel diameter, nor the Pmin value.
Intrinsic contractions of lymphatic vessel walls caused spontaneous pressure waves of about 7 mmHg in amplitude at a rate of 8 cycles min−1.
These results demonstrated the ability of pleural lymphatic vessels to generate pressure oscillations driving fluid from the subatmospheric pleural space into the lymphatic network.
In order to provide an efficient mechanical coupling between lung and chest wall, pleural fluid volume must be tightly controlled (Miserocchi et al. 1993; Waters et al. 1996). According to the original hypothesis put forward by Neergard (1927) and subsequently adopted by Agostoni (1972), the pleural fluid was thought to be filtered across the parietal mesothelium to be eventually drained by passive absorption into the pulmonary circulation. However, in a series of studies on pleural fluid and solute turnover, our laboratory provided ample evidence of the major role played by pleural lymphatic vessels in draining the cavity: (a) under steady-state physiological conditions most of the liquid and protein leave the pleural space via the lymphatic vessels (Negrini et al. 1985); (b) small hydrothoraces are drained at a flow rate which is independent of their protein concentration (Miserocchi & Negrini, 1986); and (c) inhibition of the intrinsic pleural lymphatic mechanism greatly reduces fluid and solute reabsorption of small hydrothoraces (Negrini et al. 1994). In addition, in the intact rabbit lung the net transcapillary pressure gradient calculated from interstitial (Miserocchi et al. 1990) and pulmonary microvascular pressure (Negrini et al. 1992b), was shown to sustain filtration into the adjacent interstitium, and not absorption into pulmonary microvasculature as expected on the basis of Neergard's hypothesis.
Pleural liquid pressure (Pliq) is subatmospheric at end expiration and it further decreases on inspiration (Miserocchi et al. 1981, 1984); hence, for lymphatic drainage to occur, intraluminar lymphatic pressure (Plymph) must be more negative than Pliq, at least during part of the respiratory cycle. Diaphragmatic peritoneal lymphatic vessels were shown to be able to drain liquid from a fluid compartment at a pressure as negative as ∼-6 mmHg (Miserocchi et al. 1989). In addition, it has been demonstrated that most of lymphatic drainage occurs in the mediastinal and diaphragmatic regions (Negrini et al. 1985), where Pliq is lower (Miserocchi et al. 1981, 1984), and lymphatic stomata and lacunae density are higher (Negrini et al. 1991, 1992a) compared with other pleural regions.
In the face of several indirect proofs of the capability of the lymphatic system to generate negative hydraulic pressure, the debate concerning the role of lymphatic vessels in controlling pleural fluid turnover is not yet completely settled. Hence, the aim of the present study was to measure, through the least invasive micropuncture technique, the intraluminar pleural lymphatic pressure. The results of this study might clarify whether lymphatic drainage of the pleural space follows hydraulic pressure gradients, thus demonstrating the capability of the lymphatic system to control fluid turnover even in interstitial spaces characterised by highly subatmospheric fluid pressure.
METHODS
General
The experiments were performed on 23 adult deeply anaesthetised rabbits of either sex (body weight 2.4 ± 0.4 kg (mean ± 1 standard deviation)). General anaesthesia was induced by injecting in an ear vein 2.5 ml (kg body weight)−1 of saline solution containing 0.25 g ml−1 of urethane plus 10 mg ml−1 of pentobarbitone sodium. Deep and stable anaesthesia was achieved by adding boluses of approximately 0.5 ml of anaesthetic over time, checking the anaesthetic level on the basis of the disappearance of the corneal reflexes. An overdose of the same anaesthetic was used to kill the animals at the end of the experiments. Animals were tracheotomised after anaesthesia and left to breathe spontaneously through an intratracheal cannula. To monitor arterial and venous systemic pressure throughout the whole experiment, a carotid artery and a jugular vein were cannulated with blunted tip saline-filled plastic catheters (100 PE) connected, via three-way stopcocks, to physiological pressure transducers (Model 4-327-I, Transamerica Delaval); pressures signals were conveyed to a thermal oscillograph (Gould 4600). The carotid line was also used throughout the experiment as a route to deliver additional anaesthetic.
Visualisation of the pleural lymphatic system
With the animal lying supine, the skin and the external intercostal muscles were removed on the right side of the thorax to expose the ribs and the internal intercostal muscles. The pleural lymphatic network was visualised by intrapleural injection of 0.2 ml of saline solution containing 20 % fluorescent dextrans (fluorescein isothiocyanate, FITC; molecular mass 70 000 Da). Injection was performed through a stainless steel cannula (external diameter 0.7 mm; internal diameter 0.4 mm) connected to plastic tubing and a three-way stopcock. To allow redistribution of the fluorescent dye into the right pleural lymphatic network, the animal was turned on its right lateral side. After 2–3 h in this position, the animal was turned back to a supine posture to proceed with the surgery, exposing the pleural lymphatic network running in the connective endothoracic fascia below the intercostal muscles.
Intercostal lymphatic vessels
The internal intercostal muscular fibres of the parasternal region of the 3rd to 7th right intercostal spaces (i.c.s.) were removed; lymphatic ducts filled with the fluorescent dye were clearly detectable though a stereomicroscope (magnification × 60–100, Zeiss SV 11, Milan) when illuminated with optical fibres (light wavelength 490 nm). The microscope image was captured by a video camera (COHU, solid-state camera, Mod. 4722–2000) equipped with a video recorder (Toshiba, Japan) and viewed on a b/w monitor, with a total magnification on the video screen of × 120–200. The diameter of the lymphatic vessels at the micropuncture site was measured with a calliper on the video screen and then converted into actual size according to the stereomicroscope total magnification. Lymphatic pressure (Plymph) at various sites along the vessels was measured by the micropuncture technique, as described below. Visualisation of the intercostal lymphatic vessels required 30–60 min; hence, micropuncture of lymph vessels began at about 200–240 min after dextran injection and 230–270 min after anaesthetic induction.
Diaphragmatic lymphatic vessels with closed pleural space
In four rabbits, after measuring Plymph in the intercostal lymphatic vessels, an ‘intact pleural window’ was prepared in the 7th to 8th i.c.s.; in these caudal lateral pleural regions, the chest wall does not face the lung surface, but is in close apposition to the lateral side of the muscular diaphragmatic dome. The pleural window was attained by resectioning a portion (∼0.5 cm2) of external and internal intercostal muscles, down to the endothoracic fascia; the latter was then carefully stripped under stereomicroscopic view until exposing the transparent underlying parietal pleura (about 30 μm thick). This approach enabled us to observe the surface of the lateral diaphragm in the apposition zone, by leaving the pleural space intact. The fluorescent lymphatic ducts of the lateral diaphragmatic surface were visualised through the stereomicroscope (Fig. 1) and micropunctured starting from about 250–300 min after fluorescent dextran pleural injection. In this animal group, pleural liquid pressure (Pliq) was recorded via a saline-filled stainless steel cannula (o.d. 0.7 mm; i.d. 0.4 mm) connected via plastic tubing to a three-way stopcock and a pressure transducer placed at the same height of the cannula. The cannula was inserted tangentially in the intercostal pleural region and its height in the pleural space was adjusted according to the height of the lymphatic vessel to be micropunctured.
Figure 1. Microphotograph of a loop of confluent lymphatic vessels on the pleural surface of the diaphragm, visualised through a stereomicroscope at about 4 h after intrapleural injection of fluorescent dextrans.

Unidirectional valves separating adjacent lymphangions are clearly detectable. The tip of the recording micropipette can be seen in the central bottom part of the microphotograph. Scale bar corresponds to 50 μm.
Diaphragmatic lymphatic vessels with open pleural space
In order to view the diaphragmatic lymph vessels with intact pleural space, resectioning of intercostal muscles and stripping of the endothoracic fascia had to be performed by illuminating the whole field with a 490 nm wavelength light, i.e. with a blue light. This made preparation of the pleural window a critical manoeuvre with a high risk of damaging the parietal pleura causing a pneumothorax. To allow an easier approach to the lymphatic vessels on the whole diaphragmatic surface, in another group of seven rabbits, after measuring Plymph in the intercostal lymphatic vessels, the right side of the chest wall was opened at the level of the 7th to 8thi.c.s. Since the right and left pleural cavities in rabbits are medially separated, a lateral pneumothorax caused collapse of the ipsilateral lung, but not of the contralateral one which remained normally expanded. The animals were turned laterally to their left side and the 7th to 9th i.c.s. were gently enlarged with bone retractors to view the right lateral diaphragmatic surface. Micropuncture of the diaphragmatic lymphatic network (Fig. 2) started at about 300 min after dextran injection.
Figure 2.
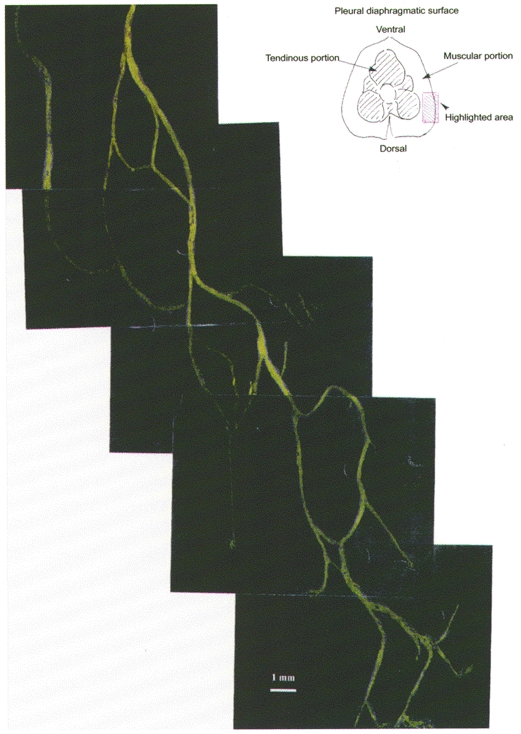
Microphotographic reconstruction of the lymphatic diaphragmatic network on the lateral right side of the pleural diaphragm (see schematic drawing at the top right).
Micropuncture of pleural lymphatic vessels
Intraluminar lymphatic pressure Plymph was measured via glass micropipettes with ∼150 μm taper bevelled down to 2–4 μm and filled with 0.5 M NaCl solution; the pipette holder was connected to a pressure transducer (Gould Instruments System, Inc.) motor driven by an electrohydraulic system (Servonull pressure measuring system, Vista Electronics Company, Ramona, CA, USA). The amplified pressure signal was displayed on the thermal oscillograph. Prior to its use, each micropipette was calibrated in a Lucite box by imposing step changes of ±5 mmHg in the box chamber; pipettes displaying a non-linear calibration in the pressure range ±30 mmHg were discarded. Once calibrated the micropipette was mounted in a three dimensional hydraulic micromanipulator (Joystick Micromanipulator MO-188 or MO-109, Narishige, Tokyo, Japan) equipped with a fourth micromanipulator movement to drive the tip of the micropipette into the tissue. Electrical zeroing of the recording system was performed prior to, and immediately after each measurement, by dipping the micropipette tip in a saline pool positioned at the same height as the pipette insertion point. Criteria for acceptance of the micropipette pressure recordings were: (a) an unchanged electrical zero of the system on withdrawal from the tissue compared with pre-insertion value; (b) a stable recording of at least 2 min; (c) repeated measurements from the same area were within 1 mmHg of each other. Plymph measurements might have been affected by the following artifacts: (1) once in the tissue, the intraluminar tip position could not be checked by dye injection through the pipette, as the injection manoeuvre would have disturbed the electrical zero of the servo-nulling pressure measuring circuit, invalidating the pressure reading; (2) Plymph might be affected by the micropipette tip position inside the vessel and by possible distortions of the tip against the vessel wall. To exclude these artifacts the pipette was driven into the vessel along its major axis to avoid distortion against the wall and at a depth of 20–30 μm, judging from the micromanipulator cursor. With these precautions, we believe that the micropipettes were recording from inside the lymphatic vessel, with minimal distortion of the wall.
During micropuncture, respiratory movements were abolished by paralysing the animal with an intravenous injection of pancuronium bromide (0.2 ml kg−1); additional boluses of the anaesthetic cocktail (0.5–1 ml of saline solution containing 0.25 g ml−1 of urethane plus 10 mg ml−1 of pentobarbitone sodium) were given every ∼30 min to maintain constant heart rate and arterial blood pressure. Blood oxygenation was guaranteed by a flow of 100 % humidified oxygen, delivered intratracheally at a pressure of ∼1 mmHg. Each micropuncture measurement lasted about 5 min and was followed by at least 5–10 min of mechanical ventilation with ambient air at a frequency of 20 breaths min−1 and a tidal volume of ∼20 ml (Mechanical ventilator model 6025, Biological Research Apparatus, Ugo Basile, Comerio, Italy). We did not measure blood gases in the present experiments. However, in a previous study in which we adopted a similar ventilatory strategy (Negrini et al. 1996) blood gases and pH remained close to control values, up to about 5 h from paralysis.
Micropuncture of intercostal and diaphragmatic lymphatic vessels was performed at time intervals ranging from 200 to 400 min after intrapleural injection of fluorescent dye and from 50 to 250 min after paralysis.
Data analysis
Pressure data were digitised and stored on a data logger for subsequent analysis. Pressure values are reported as means ± 1 s.d. and, when required, were compared using either paired or unpaired Student's t test.
RESULTS
The mean systemic arterial and venous central pressures under control conditions were 85.3 ± 15.6 mmHg (range: 50–110 mmHg) and 3.0 ± 1.1 mmHg (range: 0–7 mmHg), respectively. The mean diameter of the intercostal and diaphragmatic lymphatic vessels was 271 ± 135 μm (range: 55–950 μm; n = 94) and 285 ± 98 μm (range: 70–480 μm; n = 60), respectively. Unlike the intercostal lymphatic vessels, diaphragmatic lymph vessels are arranged in a complex network of interconnected loops (Fig. 2); in 57 % of these lymphatic vessels, clearly detectable and regularly spaced intraluminar unidirectional valves were observed (Fig. 1). The mean length and diameter of the lymphatic units between valves (lymphangions) were 1352 ± 580 μm (range 500–3150 μm; n = 34) and 318 ± 87 μm (range 190–470 μm), respectively.
Intercostal and diaphragmatic lymphatic pressure
Cardiogenic Plymph oscillations
Figure 3 presents simultaneous measurements of venous pressure, systemic arterial pressure, Pliq and diaphragmatic Plymph obtained in the intact pleural space protocol. Plymph oscillations, observed in all intercostal and diaphragmatic recordings (see also Fig. 4), were in phase with cardiac activity, as was evident when comparing Plymph with arterial and venous pressure tracings. Cardiogenic effect caused Plymph to oscillate from a minimum value (Pmin) equal to −3.7 ± 0.8 mmHg, to a maximum value (Pmax) of −1.4 ± 0.7 mmHg. The mean cardiogenic lymphatic pulse pressure, ΔP = Pmax−Pmin, in this tracing was 2.3 mmHg. The top panel of Fig. 4 shows a different Plymph tracing from a diaphragmatic lymphatic vessel with a diameter at the measuring point of 300 μm. In this tracer Pmin, Pmax and ΔP were −16.0 ± 2.2, −9.7 ± 1.9 and 6.3 mmHg, respectively. The mean Pmin, Pmax and ΔP in intercostal and diaphragmatic lymphatic vessels are presented in Table 1, distinguishing between diaphragmatic Plymph measured with intact or open pleural space. Intercostal Pliq during diaphragmatic Plymph recording in intact pleural space (see Fig. 3) averaged −3.9 ± 1.2 mmHg (n = 7). Data from Table 1 clearly indicate that: (a) there was a great variability of both intercostal and diaphragmatic Plymph values, as indicated by the wide pressure ranges; (b) notwithstanding this variability, the mean Pmin was subatmospheric in both the intercostal and the diaphragmatic pleural lymphatic networks; and (c) the lower Plymph values were found in the diaphragmatic lymphatic vessels exposed to subatmospheric pleural liquid pressure in the closed chest. In addition, in this group the mean ΔP was higher than in the intercostal or in the open-chest diaphragmatic vessels. Heart rate, in phase with cardiogenic oscillation frequency during Plymph recordings, is also reported in Table 1. The significantly lower pulse rate observed during micropuncture in diaphragmatic tracers obtained with open pleural space was probably related to the long exposure to anaesthetic and paralysing agent and to the fact that the left lung alone was expanded during these measurements.
Figure 3. Simultaneous tracings of hydraulic pressures obtained with intact pleural space.

From the top: central venous pressure, systemic arterial pressure, pleural liquid pressure (Pliq) in the costal apposition zone, pleural lymphatic pressure (Plymph) recorded in a diaphragmatic lymphatic vessel of 100 μm diameter (all values in mmHg).
Figure 4. Plymph tracers from two diaphragmatic lymphatic vessels with diameter of 300 μm (top panel) and 150 μm (bottom panel), respectively.

Top panel: the observed pressure oscillations (cardiogenic oscillations) are in phase with cardiac stroke, Plymph shifting from a minimum (Pmin) to a maximum (Pmax) value. Bottom panel: slower pressure waves due to spontaneous myogenic contraction of the lymphatic vessel walls are superimposed onto the cardiogenic oscillations.
Table 1.
Pmin, Pmax and ΔP in intercostal and diaphragmatic lymphatic vessels
| Intercostal | Diaphragmatic | ||
|---|---|---|---|
| Intact chest | Intact chest | Open chest | |
| Pmin (mmHg) | −1.3 ± 2.7 | −9.1 ± 4.2 | −2.8 ± 6 |
| Range | −10.2 to +5.9 | −16.9 to −4.1 | −16.2 to +5.2 |
| Pmax (mmHg) | 4.4 ± 4 | 0.2 ± 5.6 | 1.9 ± 6 |
| Range | −5.5 to +15.5 | −8.1 to +8.3 | −14.7 to +11.1 |
| ΔP = Pmax–Pmin (mmHg) | 5.7 ± 3.4 | 9.3 ± 4.5 | 4.7 ± 3 |
| Range | +0.7 to +13.6 | +4.1 to +16.5 | +1.5 to +11.4 |
| Heart rate (cycles min−1) | 129 ± 31 | 140 ± 28 | 66.4 ± 10 |
Intercostal (n = 94), diaphragmatic with intact chest (n = 7) and diaphragmatic with open chest (n = 56) Plymph readings were gathered in 19, 4 and 7 rabbits, respectively. Mean heart rate during Plymph measurements is also reported. Data are arithmetic means ± 1 s.d.
Plotting Pmax as a function of the corresponding Pmin (Fig. 5), the intercostal data (open circles) were described by the linear correlation: Pmax = 5.3 + 0.7Pmin (r2 = 0.3; n = 94), not shown in the figure. Diaphragmatic data with intact pleural space (filled triangles, n = 7) and open pleural space (filled squares, n = 56) fell within the correlation: Pmax = 5.0 + 0.9Pmin (r2 = 0.7; n = 63). All intercostal and diaphragmatic data fell within a single population, described by the linear correlation (continuous line): Pmax = 5.2 + 0.9Pmin (r2 = 0.6, n = 157). Analysis of variance performed on the pooled data revealed that the y-intercept was significantly different from zero (t = 10.9; degrees of freedom (d.f.) = 155; P < 0.001) and that the correlation coefficient was highly significant (t = 14.9; d.f. = 155; P < 0.001). Since the slope did not differ significantly from unity, the y-intercept of the linear Pmaxvs. Pmin relationship corresponding to ∼5 mmHg at any Pmin value, corresponded to the mean pulse pressure, Pmax−Pmin. No statistically significant correlation was found when plotting Pmin, Pmax or ΔP as a function of lymphatic vessel diameter or heart rate.
Figure 5. Effect of cardiogenic oscillations on Plymph.

Intercostal (^, n = 94), diaphragmatic with intact pleural space (▴, n = 7) and diaphragmatic with open pleural space (▪, n = 56) Pmax values are plotted as a function of the corresponding Pmin. Continuous and dashed lines correspond, respectively, to the linear correlation through the pooled data and to the 95 % confidence limit of the correlation.
Spontaneous Plymph waves
The bottom panel of Fig. 4 reports a Plymph tracing gathered from a diaphragmatic lymphatic vessel (150 μm diameter). In this recording, two distinct patterns of Plymph oscillations can be distinguished: (a) cardiogenic oscillations in phase with cardiac beat (108 cycles min−1), comparable with those observed in the bottom panel of Fig. 3 and (b) slower diaphragmatic lymphatic pressure waves with a periodicity of ∼10 s, superimposed on the cardiogenic oscillations and depending upon spontaneous myogenic contractions of the lymphatic vessel walls. Plymph was about zero in the resting phase and increased by 12 mmHg during spontaneous contractions; alternate phases of spontaneous contraction followed by relaxation to resting Plymph values were observed for time intervals lasting up to 5–6 min. In intercostal lymphatic vessels, the net spontaneous increase in baseline Plymph (ΔPmyogenic) was 6.6 ± 2.5 mmHg and occurred at a rate of 7.7 ± 1 cycles min−1 (n = 6). In diaphragmatic vessels, ΔPmyogenic and mean contraction rate were 7.3 ± 5.4 mmHg and 8.5 ± 4.8 cycles min−1 (n = 6), respectively. Spontaneous pressure oscillations seemed to be positively correlated to lymphatic contraction rate according to the linear relationship: ΔPmyogenic = −0.4 + 0.9 lymph rate (r2 = 0.6, n = 8). No relationship was found when plotting ΔPmyogenic or spontaneous contraction rate as a function of lymphatic vessel diameter.
DISCUSSION
The lymphatic system provides the most efficient mechanism for fluid drainage and the only mechanism for macromolecule removal from the pleural space under physiological conditions, acting as an efficient feedback system to offset pleural effusions (Miserocchi et al. 1993). Yet, the precise role of the lymphatic vessels in pleural liquid turnover has been questioned; indeed, at variance with other interstitial tissues where the fluid pressure is close to atmospheric, Pliq is well below atmospheric pressure (Miserocchi et al. 1981, 1984, 1988). As a result, in order to ensure pleural lymph formation, Plymph in the initial lymphatic network has to be lower than Pliq. A similar rationale applies to pulmonary interstitium, where hydraulic pressure is even lower than that of Pliq and where physiological fluid drainage is guaranteed exclusively by lymphatic drainage (Miserocchi et al. 1992; Negrini et al. 1992b).
The present study provides the first direct evidence that diaphragmatic Plymph is lower than the corresponding Pliq (Table 1 and Fig. 5), in both the intact and open pleural space; in addition, the mean diaphragmatic Plymph values were more negative and ΔP was larger in intact pleural space. Diaphragmatic Pliq is physiologically ∼2–3 mmHg lower than the hydraulic pressure measured at the same height in the subphrenic peritoneal region (Miserocchi et al. 1982), so that the pleural to peritoneal trans-diaphragmatic pressure gradient is negative. Such a gradient is reversed when the chest is opened and pleural pressure becomes atmospheric, so that the shape of the diaphragmatic dome might be different in the intact, compared with the open chest. Thus, the very negative diaphragmatic Plymph and larger ΔP observed with closed chest might suggest that diaphragmatic fibres, and thus the diaphragmatic lymph vessels, undergo a greater outward tensile stress in an intact chest. The possible mechanical effect of diaphragmatic fibres' stretch on Plymph is a complex issue that cannot be satisfactorily addressed on the basis of the present results and deserves a separate study.
Plymph was in general less negative in the intercostal than in the diaphragmatic vessels (see Fig. 5 and mean values in Table 1), even though all intercostal recordings were obtained with intact pleural space. These results might be related to the fact that Pliq is less subatmospheric in the costal than in the diaphragmatic pleural compartments (Miserocchi et al. 1981, 1982). In addition, differences between intercostal and diaphragmatic Plymph values might reflect the complex anatomical and functional arrangement of the pleural lymphatic web. Indeed, the pleural lymphatic vessels face the mechanical problem of moving fluid from the pleural cavity to the venous system, against a hydraulic pressure gradient. As schematically depicted in Fig. 6, the pleural space is directly connected, through the so called ‘stomas’ (Wang, 1974; Negrini et al. 1991), to the submesothelial lacunae (Negrini et al. 1992a), functionally comparable with the initial lymphatic vessels of other interstitial spaces. In this initial segment, including the submesothelial diaphragmatic lymphatic vessels and the deeper intercostal lymphatic vessels, Pmin is lower than Pliq (Fig. 5 and Table 1), a result that clearly indicates the ability of the initial lymphatic lacunae to sustain a hydraulic pressure gradient favouring fluid reabsorption into the lymphatic system. The fact that Pmax in the initial segment is slightly higher than Pmin in the extrapleural segment, is an interesting suggestion on the possible role of cardiogenic swings in favouring lymph propulsion. Similarly, Pmax in the extrapleural intercostal lymphatic vessels reaches a peak which is slightly higher than central venous pressure. Cardiogenic oscillations might be transmitted to lymphatic walls via local tissue deformation due to pressure transmission along the adjacent arterioles (Schmid-Schöenbein, 1990). In the diaphragm, pulse pressure might also originate from transmission of the pulse wave along the abdominal aorta.
Figure 6.

Bottom panel: schematic drawing of the functional arrangement of the pleural lymphatic vessels. Pleural fluid is drained via the stomas into the submesothelial lacunae of the initial lymphatic network. Initial lymphatic vessels empty into a net of extrapleural lymphatic vessels progressively more distant from the pleural space. Top panel: hydraulic pressures measured in the compartments presented in the bottom panel. ♦, pleural liquid pressure in the costal apposition zone. ▴, diaphragmatic Pmin and ▵, diaphragmatic Pmax with intact pleural space. •, intercostal Pmin and ^, intercostal Pmax. , central venous pressure. Bars represent ± 1 s.d.
, central venous pressure. Bars represent ± 1 s.d.
Unlike what was observed in the microvascular tree, there was no relationship between Plymph and vessel diameter, testifying to the complex fluid dynamics within the pleural lymphatic network. In addition, pleural lymphatic vessels presented spontaneous pressure oscillations with an amplitude and oscillation rate that were similar to those observed in vivo in collecting lymphatic vessels (Benoit et al. 1989; Zawieja et al. 1993; Crowe et al. 1997).
The present Plymph values were lower than those recorded in other tissues (Zweifach & Prather, 1975; Clough & Smaje, 1978; Lee, 1986; Spiegel et al. 1992). The negativity of Plymph compared with other interstitial tissues, might be partly related to the anatomical arrangement of the intercostal lymphatic vessels, lying in the cleavage plane between the endothoracic fascia and the internal intercostal muscles. At any lung volume, the ribs exert a pulling action on the intercostal tissues: hence, one might expect the wall of the intercostal lymphatic vessels to be stressed outward. Similar reasoning applies to lymphatic vessels in the diaphragm, where the fibres undergo variable tensile stresses during the respiratory cycle.
A comparison between the importance of the intrinsic and extrinsic mechanisms in the pleural lymphatic vessels under physiological conditions is at present uncertain. Indeed, on the one hand, data shown in Fig. 6 would suggest a role of cardiogenic oscillation in favouring lymph propulsion; in addition, in the present study the main component of the extrinsic mechanism, i.e. the respiratory swings, were abolished. On the other hand, although spontaneous lymphatic contraction frequency is depressed by pentobarbitone anaesthesia (McHale & Thornbury, 1988), photoactivation of FITC dextran (Zhang et al. 1997) and oxygen-derived free radicals (Zawieja et al. 1991), the intrinsic mechanism seemed to play a most important role in lymphatic drainage of pleural fluid during absorption of small hydrothoraces in spontaneously breathing rabbits (Negrini et al. 1994). This would be in keeping with the observation that the extrinsic mechanism provided by arterial pulsation was not important for lymph formation in metatarsal lymphatic of the sheep hindlimb (McGeown et al. 1988).
Acknowledgments
The authors thank Professors Giuseppe Miserocchi and Matthew Glucksberg for their suggestions and critical reviewing of the manuscript. This research was supported by grant 12/01 no. 096/00017 of the Italian Ministry of University and of Scientific and Technological Research (MURST).
References
- Agostoni E. Mechanics of the pleural space. Physiological Reviews. 1972;52:57–128. doi: 10.1152/physrev.1972.52.1.57. [DOI] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. American Journal of Physiology. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Clough G, Smaje LH. Simultaneous measurements of pressure in the interstitium and the terminal lymphatics of the cat mesentery. The Journal of Physiology. 1978;283:457–468. doi: 10.1113/jphysiol.1978.sp012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, von der Weid P-Y, Brock JA, van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. The Journal of Physiology. 1997;500:235–244. doi: 10.1113/jphysiol.1997.sp022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS. Tissue fluid pressure, lymph pressure and fluid transport in rat intestinal villi. Microvascular Research. 1986;31:170–183. doi: 10.1016/0026-2862(86)90032-4. [DOI] [PubMed] [Google Scholar]
- McGeown JG, McHale NG, Thornbury KD. Arterial pulsation and lymph formation in an isolated sheep hindlimb preparation. The Journal of Physiology. 1988;405:598–604. doi: 10.1113/jphysiol.1988.sp017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Thornbury KD. The effect of anesthetics on lymphatic contractility. Microvascular Research. 1988;37:77–104. doi: 10.1016/0026-2862(89)90073-3. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Kelly S, Negrini D. Pleural and extrapleural interstitial liquid pressure measured by cannulas and micropipettes. Journal of Applied Physiology. 1988;65:555–562. doi: 10.1152/jappl.1988.65.2.555. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Mariani E, Negrini D. Role of the diaphragm in setting liquid pressure in serous cavities. Respiration Physiology. 1982;50:381–392. doi: 10.1016/0034-5687(82)90030-5. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Nakamura T, Mariani E, Negrini D. Pleural liquid pressure over the interlobar mediastinal and diaphragmatic surfaces of the lung. Respiration Physiology. 1981;46:61–69. doi: 10.1016/0034-5687(81)90068-2. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Negrini D. Contribution of Starling and lymphatic flows to pleural liquid exchange in anesthetized rabbits. Journal of Applied Physiology. 1986;61:325–330. doi: 10.1152/jappl.1986.61.1.325. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Negrini D, Gonano C. Direct measurement of interstitial pulmonary pressure in in situ lung with intact pleural space. Journal of Applied Physiology. 1990;69:2168–2174. doi: 10.1152/jappl.1990.69.6.2168. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Negrini D, Mukenge S, Turconi P, Del Fabbro M. Liquid drainage through the peritoneal diaphragmatic surface. Journal of Applied Physiology. 1989;66:1579–1585. doi: 10.1152/jappl.1989.66.4.1579. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Pistolesi M, Miniati M, Bellina CR, Negrini D, Giuntini C. Pleural liquid pressure gradients and intrapleural distribution of an injected bolus. Journal of Applied Physiology. 1984;56:526–532. doi: 10.1152/jappl.1984.56.2.526. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Venturoli D, Negrini D, Del Fabbro M. Model of pleural fluid turnover. Journal of Applied Physiology. 1993;75:1798–1806. doi: 10.1152/jappl.1993.75.4.1798. [DOI] [PubMed] [Google Scholar]
- Neergard K. Zur Frage des Drukes in Pleuraspalt. Beiträge der Klinischen Erforschungen Tuberkulose und Lungenkranke. 1927;65:476–485. [Google Scholar]
- Negrini D, Ballard ST, Benoit JN. Contribution of lymphatic myogenic activity and of respiratory movements to pleural lymph flow. Journal of Applied Physiology. 1994;76:2267–2274. doi: 10.1152/jappl.1994.76.6.2267. [DOI] [PubMed] [Google Scholar]
- Negrini D, Del Fabbro M, Gonano C, Mukenge S, Miserocchi G. Distribution of diaphragmatic lymphatic lacunae. Journal of Applied Physiology. 1992a;72:1166–1172. doi: 10.1152/jappl.1992.72.3.1166. [DOI] [PubMed] [Google Scholar]
- Negrini D, Gonano C, Miserocchi G. Microvascular pressure profile in intact in situ lung. Journal of Applied Physiology. 1992b;72:332–339. doi: 10.1152/jappl.1992.72.1.332. [DOI] [PubMed] [Google Scholar]
- Negrini D, Mukenge S, Del Fabbro M, Gonano C, Miserocchi G. Distribution of diaphragmatic lymphatic stomata. Journal of Applied Physiology. 1991;70:1544–1549. doi: 10.1152/jappl.1991.70.4.1544. [DOI] [PubMed] [Google Scholar]
- Negrini D, Passi A, de Luca G, Miserocchi G. Pulmonary interstitial pressure and proteoglycans during development of pulmonary edema. American Journal of Physiology. 1996;270:H2000–2007. doi: 10.1152/ajpheart.1996.270.6.H2000. [DOI] [PubMed] [Google Scholar]
- Negrini D, Pistolesi M, Miniati M, Bellina CR, Giuntini C, Miserocchi G. Regional protein absorption rates from the pleural space in dogs. Journal of Applied Physiology. 1985;58:2062–2067. doi: 10.1152/jappl.1985.58.6.2062. [DOI] [PubMed] [Google Scholar]
- Schmid-Schöenbein GW. Microlymphatics and lymph flow. Physiological Reviews. 1990;70:987–1019. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Spiegel M, Vesti B, Shore A, Franzeck UK, Becker F, Bollinger A. Pressure of lymphatic capillaries in human skin. American Journal of Physiology. 1992;262:H1208–1210. doi: 10.1152/ajpheart.1992.262.4.H1208. [DOI] [PubMed] [Google Scholar]
- Wang NS. The preformed stomas connecting the pleural cavity and the lymphatics in the parietal pleura. American Review of Respiratory Disease. 1974;110:623–633. doi: 10.1164/arrd.1975.111.1.12. [DOI] [PubMed] [Google Scholar]
- Waters CM, Glucksberg MR, DePaola N, Chang JH, Grotberg JB. Shear stress alters pleural mesothelial cell permeability in culture. Journal of Applied Physiology. 1996;81:448–458. doi: 10.1152/jappl.1996.81.1.448. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Davis KI, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. American Journal of Physiology. 1993;264:H1283–1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Greiner ST, Davis KI, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contraction. American Journal of Physiology. 1991;260:H1935–1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yokoyama S, Ohhashi T. Inhibitory effects of fluorescein isothiocyanate photoactivation on lymphatic pump activity. Microvascular Research. 1997;54:99–107. doi: 10.1006/mvre.1997.2030. [DOI] [PubMed] [Google Scholar]
- Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesenthery. American Journal of Physiology. 1975;228:1326–1335. doi: 10.1152/ajplegacy.1975.228.5.1326. [DOI] [PubMed] [Google Scholar]


