Abstract
Receptor-mediated endocytosis is an important mechanism for transport of macromolecules and regulation of cell-surface receptor expression. In renal proximal tubules, receptor-mediated endocytosis mediates the reabsorption of filtered albumin. Acidification of the endocytic compartments is essential because it interferes with ligand-receptor dissociation, vesicle trafficking, fusion events and coat formation.
Here we show that the activity of Na+−H+ exchanger isoform 3 (NHE3) is important for proper receptor-mediated endocytosis of albumin and endosomal pH homeostasis in a renal proximal tubular cell line (opossum kidney cells) which expresses NHE3 only.
Depending on their inhibitory potency with respect to NHE3 and their lipophilicity, the NHE inhibitors EIPA, amiloride and HOE694 differentially reduced albumin endocytosis. The hydrophilic inhibitor HOE642 had no effect.
Inhibition of NHE3 led to an alkalinization of early endosomes and to an acidification of the cytoplasm, indicating that Na+−H+ exchange contributes to the acidification of the early endosomal compartment due to the existence of a sufficient Na+ gradient across the endosomal membrane.
Exclusive acidification of the cytoplasm with propionic acid or by removal of Na+ induced a significantly smaller reduction in endocytosis than that induced by inhibition of Na+−H+ exchange.
Analysis of the inhibitory profiles indicates that in early endosomes and endocytic vesicles NHE3 is of major importance, whereas plasma membrane NHE3 plays a minor role.
Thus, NHE3-mediated acidification along the first part of the endocytic pathway plays an important role in receptor-mediated endocytosis. Furthermore, the involvement of NHE3 offers new ways to explain the regulation of receptor-mediated endocytosis.
Receptor-mediated endocytosis is an essential mechanism for the transport of a variety of macromolecules into cells as well as across epithelia (Mukherjee et al. 1997). Besides transport of macromolecules, endocytosis is also involved in antigen presentation, maintenance of cell polarity and regulation of cell-surface receptor expression. The endocytic mechanisms underlying receptor-mediated endocytosis can be roughly subdivided into two types: (i) endocytosis via clathrin-coated pits and (ii) non-clathrin-mediated endocytosis, consisting mainly of caveolae-mediated endocytosis (Mukherjee et al. 1997; Schmid, 1997). Clathrin-mediated endocytosis is the best characterised endocytic mechanism and is the predominant pathway for macromolecule uptake along epithelia (Mukherjee et al. 1997; Schmid, 1997; Marshansky et al. 1997; Christensen et al. 1998). One example of clathrin-mediated endocytosis is the uptake of filtered serum albumin across the apical membrane of renal proximal tubular cells (Gekle et al. 1997; Gekle, 1998; Christensen et al. 1998). In the present study we used this model to study receptor-mediated endocytosis. Receptors undergoing clathrin-mediated endocytosis are concentrated in coated pits and subsequently delivered to the early endosomal compartment by endocytic vesicles (Mukherjee et al. 1997; Schmid, 1997). In sorting endosomes, internalised receptors and ligands are directed either to recycling endosomes or to the late endosomal compartment and further on to the lysosomes, where they undergo degradation. Serum albumin, for example, is directed mainly to lysosomes (Cui et al. 1996; Czekay et al. 1997; Christensen et al. 1998).
An important process along the endocytic pathway is the acidification of endosomal compartments (Mellman et al. 1986; Gruenberg & Maxfield, 1995; Mukherjee et al. 1997). Adequate acidification is a crucial process because endosomal pH interferes, for example, with ligand-receptor dissociation, vesicle trafficking, endosomal fusion events, recycling to the plasma membrane and COP-coat formation (Mellman et al. 1986; Gekle et al. 1995a, 1996a; Papkonstanti et al. 1996; Storrie & Desjardins, 1996; Mukherjee et al. 1997). Acidification is accomplished, at least in part, by the vacuole-type H+-ATPase which works in parallel with a counterion conductance, in order to limit the formation of an endosomal-positive membrane potential (Rybak et al. 1997). In most cases the counterion conductance consists of Cl− channels (Mellman et al. 1986; Gekle et al. 1995a; Steinmeyer et al. 1995; Marshansky & Vinay, 1996). In proximal tubular cells CLC-5-type Cl− channels play an important role in counterion conductance (Steinmeyer et al. 1995; Devuyst et al. 1998). Recently, evidence was presented for the involvement of Na+−H+ exchange (NHE), especially via isoform 3 (NHE3), in endosomal acidification (Kapus et al. 1994; Marshansky & Vinay, 1996; D'Souza et al. 1998). Na+−H+ exchangers are ubiquitous plasma membrane transport proteins involved in cellular pH homeostasis and volume regulation. NHE3 seems to cycle between the plasma membrane and the early endosomal compartment, contributing on its way to endosomal acidification (Janecki et al. 1998; Kurashima et al. 1998).
In the present study we used a cell line derived from opossum renal proximal tubule (OK cells) which shows a well-characterised apical endocytic uptake activity for albumin as well as apical expression of NHE3, but no basolateral expression of NHE (Noel et al. 1996; Gekle et al. 1997; Brunskill et al. 1998). Renal proximal tubular albumin reabsorption is of major importance because it prevents the loss of valuable amino acids, but at the same time it can induce tubulointerstitial inflammation and fibrosis (Burton & Harris, 1996; Jerums et al. 1997; Gekle, 1998). In this study we determined the contribution of NHE to the regulation of endosomal pH and to reabsorptive albumin endocytosis in renal proximal tubular cells. In addition we performed experiments in order to estimate the Na+ gradient across the endosomal membrane which is of importance for NHE activity. Our data show that NHE3 is an important determinant of receptor-mediated endocytosis in renal proximal tubular cells due to its contribution to endosomal acidification and its role in cytosolic pH homeostasis.
METHODS
Materials
Minimal essential medium (MEM) and fetal calf serum were obtained from Biochrom, 12213 Berlin, Germany. HOE694, HOE642 and 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) were generous gifts from Dr H. J. Lang (Hoechst AG, Frankfurt, Germany). All other applied chemicals were obtained from Sigma. Hepes Ringer solution contained (mmol l−1): NaCl 122.5, KCl 5.4, CaCl2 1.2, MgCl2 0.8, Na2HPO4 0.8, NaH2PO4 0.2, glucose 5.5 and Hepes 10.
Cell culture
Opossum kidney (OK) cells were kindly provided by Dr J. Biber (Department of Physiology, Zürich, Switzerland) and LLCPK1 cells by Dr G. G. Straunthaler (Department of Physiology, Innsbruck, Austria). Cells were grown in plastic culture flasks (Falcon, 69042 Heidelberg, Germany) as described previously (Gekle et al. 1995a,b). Cells were used 9 days after plating (confluent monolayers).
Measurements of intracellular pH
The intracellular pH of single cells was determined using the pH-sensitive dye BCECF (Molecular Probes) as described elsewhere (Thomas et al. 1979; Weiner & Hamm, 1989; Gekle et al. 1995a, 1996b). Briefly, cells were incubated with BCECF at a final concentration of 3 μmol l−1 for 5 min, rinsed 4 times with superfusion solution to remove the BCECF-containing solution and transferred to the stage of an Axiovert 100 TV microscope (Zeiss). Excitation wavelengths were 460 and 488 nm, and the emitted light was filtered through a bandpass filter (515–565 nm). Images were digitised on-line using video-imaging software (Attofluor, Zeiss, Oberkochen, Germany). Calibration was performed after each experiment using the nigericin technique (Thomas et al. 1979; Weiner & Hamm, 1989; Gekle et al. 1995a). Because BCECF may also label acidic compartments within the cell, leading to the possible underestimation of cytosolic pH, we restricted the areas of measurement to regions with a homogeneous, non-punctate dye distribution. The value obtained for cytosolic pH under control conditions (∼7.45) indicates that the observed signal was indeed derived from the cytosol.
Measurement of endosomal pH
Measurement of the fluorescence intensity ratio in endosomes (Fuchs et al. 1989; Gekle et al. 1995a,b, 1998b) of single OK cells was performed after 5 min incubation in Hepes Ringer solution (pH 7.4, 37°C) containing 0.1 mg ml−1 fluorescein isothiocyanate (FITC)-labelled bovine serum albumin (FITC-albumin), as described previously (Zen et al. 1992; Gekle et al. 1995a). Endosomal pH was calculated using the formula:
where k is the affinity constant of FITC-albumin for H+ (k = 4.1× 10−7), Rmax and Rmin represent the maximum and minimum calibration ratios, respectively, and R is the actual ratio. The affinity constant was determined by us in a previous study (Gekle et al. 1995a) in intact OK cells clamped to different pH values. Single cell measurements were performed using the above-described system under continuous superfusion with Hepes Ringer solution (pH 7.4, 37°C). Excitation wavelengths were 436 and 488 nm, and the emitted light was filtered through a bandpass filter (515–565 nm). Images were digitised on-line using video-imaging software (Attofluor). Because the spectral properties of FITC and BCECF are similar, endosomal and cytosolic pH could not be determined simultaneously on the same coverslip. The area of fluorescence measurement was restricted to groups of endosomes in single cells (∼10–20 μm2). Thus, the pH of a group of endosomes containing FITC-albumin (albumin-containing endosomal compartment) was determined. Since the cells were pulse-labelled for 5 min the majority of the albumin-containing endosomal compartments should belong to the early endosomal compartment (Mukherjee et al. 1997). This assumption is furthermore supported by the albumin-containing endosomal compartment pH (∼6.3) lying in the expected range for early endosomes but not for late endosomes. Of course we do not exclude the possibility that a minor part of the albumin had already reached late endosomes.
Measurement of cytosolic and endosomal sodium
Sodium concentrations were determined using the sodium-sensitive dye SBFI (Molecular Probes) as described previously (Gekle et al. 1998a), using the above-mentioned set-up. Briefly, cells were incubated with MEM containing 5 × 10−6 mol l−1 SBFI-AM (cytosolic measurements) or 4 × 10−5 mol l−1 SBFI-tetrazolium salt (endosomal measurements) for 60 or 10 min, respectively. Because the SBFI-tetrazolium salt is membrane impermeable, it is taken up by fluid-phase endocytosis only. Thus, the fluorescent signal obtained using the SBFI-tetrazolium salt is a function of endosomal sodium. As the KD of SBFI for sodium is ∼11 mmol l−1, measurements of high sodium concentrations are less precise than measurements made in the cytosolic sodium concentration range. Furthermore, SBFI is pH sensitive, leading to the possible underestimation of sodium as pH decreases. Therefore, we estimated the endosomal sodium concentration only roughly, in order to gain some information about the endosome-to-cytosol sodium gradient. The excitation wavelengths were 334 and 380 nm, and the emitted light was filtered through a bandpass filter (515–565 nm). Images were digitised on-line using video-imaging software (Attofluor). Sodium calibration was performed after each experiment with nystatin as described previously (Schlatter et al. 1996; Gekle et al. 1998a).
Uptake studies
Uptake experiments were performed as described earlier (Gekle et al. 1995a, 1997). After three acidic washes (pH 6.0), the monolayers grown on plastic Petri dishes (9 days) were incubated with Hepes Ringer solution containing 10 mg l−1 FITC-labelled bovine serum albumin at 37 or 4°C for the time periods indicated the the text and figures. In a previous study we have already shown that at 4°C the substrates bind to the plasma membrane but are not internalised (Gekle et al. 1995a). At 37°C albumin is taken up by receptor-mediated endocytosis (Gekle et al. 1995a,b, 1997). Less than 10 % of FITC-albumin uptake is non-specific (Gekle et al. 1997). The amount of internalised substrate was determined by subtracting the portion of bound albumin from total cell-associated albumin. Unbound FITC-albumin was removed by rinsing 8 times with ice-cold Hepes Ringer solution (Gekle et al. 1995a). Cells were disaggregrated by detergent (Triton X-100, 0.1 % v/v in Mops solution, which guaranteed that all fluorescence measurement were performed at pH 7.4) and the cell-associated fluorescence was measured using a spectrofluorometer according to the method described by Gekle et al. (Gekle et al. 1995a, 1997). Protein was determined as described elsewhere (Lowry et al. 1951). The rate of fluid-phase endocytosis was determined by the uptake of FITC-dextran using the same protocol as for FITC-albumin uptake (Gekle et al. 1995a).
Calculations and statistics
The inhibition constant (IC50) was calculated according to DeLean et al. (1978). Curve fitting was performed using the least-squares-method using the Sigma Plot for Windows software (Jandel Scientific, Corte Madera, CA, USA). Data are presented as mean values ±s.e.m. and n represents the number of Petri dishes (for uptake) or cells studied (for pH and Na+). Cells of at least three passages were used for each experimental series. Significance of difference was tested by Student's t test or ANOVA as appropriate. Differences were considered significant if P < 0.05.
RESULTS
Time course of inhibition of endocytosis by the Na+−H+ exchange inhibitor EIPA
We determined the time course of the effect of 100 μmol l−1 EIPA (5-(N-ethyl-N-isopropyl)-amiloride) on endocytosis of albumin. Albumin uptake was determined after a 10 min preincubation period with EIPA, in order to determine endocytosis when the new steady-state of pH was reached (see below and in Fig. 2). As shown in Fig. 1A, EIPA exerted a strong and rapid effect on endocytic uptake of albumin in OK cells. Under control conditions uptake was almost linear during the first 15 min (Fig. 1A). In the presence of EIPA, uptake of albumin showed a time-dependent saturation after 5–10 min (Fig. 1A). Comparison of the time course under control conditions and in the presence of EIPA (Fig. 1B) revealed that EIPA inhibited early steps along the endocytic pathway. Even the very initial uptake rates were reduced dramatically in the presence of EIPA (the half-maximum effect was achieved after 2 min; Fig. 1B), consistent with an effect between the plasma membrane and the early endosomal compartment. When EIPA was added together with albumin at time 0 min, there was a delay in the action of EIPA of 2 min corresponding to the time necessary for EIPA to alter pH (Fig. 2). After this delay period, uptake was inhibited with virtually the same time course as that of the preincubation experiment described above (Fig. 1B, inset). To exclude the possibility that the albumin label (FITC) influenced the effect of EIPA, we repeated the experiments with 14C-bovine serum albumin and obtained virtually the same results (100 μmol l−1 EIPA reduced 14C-bovine serum albumin uptake to 23 ± 5 % of control, n = 4). Fluid-phase endocytosis, which accounts for 1–2 % of total albumin uptake (Gekle et al. 1995a), was not affected by 100 μmol l−1 EIPA (FITC-dextran uptake was 96 ± 7 % of control in the presence of EIPA, n = 6).
Figure 2. Representative tracings of EIPA-induced pH changes.

A, cytosolic pH (pHc) in the presence of increasing concentrations of EIPA. B, endosomal pH (pHe) in the presence of increasing concentrations of EIPA.
Figure 1. Inhibitors of Na+−H+ exchange block receptor-mediated endocytosis of FITC-albumin.

The albumin concentration was 10 mg l−1. A, time course of albumin uptake under control conditions and in the presence of 100 μmol l−1 EIPA. EIPA was present 10 min prior to as well as during uptake (n = 10). B, uptake in the presence of 100 μmol l−1 EIPA as a percentage of control uptake. The half-maximum effect was achieved after 2 min. The inset shows the time course when EIPA was added only at the beginning of the uptake period (n = 6). C, dose-response curves for the bath concentrations of three different inhibitors of Na+−H+ exchange (n = 6–12). D, dose-response curve for the endosomal concentrations of two lipophilic inhibitors of Na+−H+ exchange. The endosomal concentrations were calculated using the measured pH values (n = 6–12) and pK values from literature (Kleyman & Cragoe, 1988).
Action profile of different Na+−H+ exchange inhibitors with respect to albumin endocytosis
Next, we compared the action of three inhibitors of NHE with different inhibitory constants and different lipophilicity (Kleyman & Cragoe, 1988; Noel & Pouysségur, 1995; Wakabayashi et al. 1997) on albumin endocytosis. Figure 1C shows the dose-response curves of the three inhibitors EIPA, HOE694 and amiloride with respect to albumin endocytosis. The three inhibitors differed with respect to the IC50 value as well as with respect to the maximum extent of inhibition. EIPA reduced endocytosis to ∼20 % of control with a half-maximum effect at ∼21 μmol l−1 extracellular EIPA (IC50ec). Amiloride and HOE694 reduced endocytosis only partially and with higher IC50ec values. Because EIPA and HOE694 are weak organic bases (pK≡ 8.7) with relative high lipophilicity (Kleyman & Cragoe, 1988; manufacturer's information), they will accumulate in endosomes due to ionic trapping and the local concentrations at the endosomal NHE will be higher. Thus, we calculated the concentration of EIPA and HOE694 in the early endosomal compartment using the measured pH values ((Ce/Cec = (1 + 10(pK− pHe))/(1 + 10(pK− pHec)), where Ce is endosomal concentration and Cec the extracellular concentration) and determined the endosomal IC50 values (IC50e, Fig. 1D). The IC50e values for EIPA and HOE694 were ∼85 and ∼250 μmol l−1, respectively. For amiloride ionic trapping is negligible over the short period under investigation in this study, because its lipophilicity is ∼20 times lower than that of EIPA (Kleyman & Cragoe, 1988).
In order to test whether NHE activity also influences albumin uptake in another proximal tubule-derived cell line we tested the effect of EIPA on porcine LLCPK1 cells (Ling et al. 1996). EIPA reduced albumin uptake to ∼20 % of control with an IC50ec of ∼20 μM. Thus, the observed effect are not cell line specific.
Effect of Na+−H+ exchange inhibitors on endosomal and cytosolic pH
Figure 2 shows two original tracings of the changes in cytosolic pH (pHc; Fig. 2A) and endosomal pH (pHe; Fig. 2B) after application of EIPA. The mean values for cytosolic and endosomal pH under control conditions were ∼7.45 and ∼6.30, respectively. EIPA induced concentration-dependent acidification of the cytosol and an alkalinization of endosomes. Figure 3A-C shows the dose-response curves of the three NHE inhibitors with respect to endosomal and cytosolic pH. EIPA induced a concentration-dependent alkalinization of the albumin-containing endosomal compartment with an IC50ec of 13 μmol l−1 and an IC50e of ∼65 μmol l−1. These values are similar to the values for inhibition of endocytosis (Fig. 1). Since pHc and pHe change in opposite directions, the effect of EIPA on pHe is not a secondary one, but due to a direct interaction with endosomal Na+−H+ exchange. Yet, part of the cytosolic acidification is secondary to endosomal alkalinization: since the endosomal membrane is leaky for H+, any reduction in the activity of H+ import into endosomes will lead to an increased net efflux of H+ into the cytosol (Mellman et al. 1986). Because endosomes have a substantial buffer capacity which can contribute up to 20 % of total cellular buffer capacity in OK cells (Gekle & Silbernagl, 1995; Rybak et al. 1997), an increased H+ efflux may indeed alter cytoplasmic pH. Thus, in order to determine the contribution of the inhibition of plasma membrane NHE to cytosolic acidification we estimated and subtracted the endosomal-derived H+ load after exposure to NHE inhibitors (Gekle & Silbernagl, 1995). Consequently, EIPA-induced inhibition of plasma membrane NHE was responsible for a cytosolic acidification by ∼0.4 pH units. EIPA-induced inhibition of endosomal NHE was responsible for an endosomal alkalinization of ∼0.6 pH units (Fig. 3A), which accounts for a drop in cytosolic pH by ∼0.1 pH units. HOE694 induced an endosomal alkalinization similar to EIPA (Fig. 3B). The IC50 values for HOE694 were similar to those for the inhibition of albumin endocytosis (Fig. 1). However, the effect of HOE694 on cytosolic pH (∼-0.18 pH units) was much smaller than that of EIPA. Thus, inhibition of plasma membrane NHE contributed to cytosolic acidification by ∼0.08 pH units only, in the case of HOE694. The difference between EIPA and HOE694 results, at least in part, from the lower affinity of HOE694 than EIPA for NHE3 (Noel & Pouysségur, 1995; Noel et al. 1996). Thus, with HOE694 we can inhibit mainly endosomal NHE3 under our experimental conditions. By contrast, superfusion of labelled cells with amiloride led to an acidification of the cytosol but virtually no change in endosomal pH (Fig. 3C). Due to its low lipophilicity (see below), the early endosomal amiloride concentrations are most probably too low to affect NHE3 (Kleyman & Cragoe, 1988; Noel & Pouysségur, 1995). Of course, the contribution of amiloride taken up via fluid-phase endocytosis to the inhibition of albumin uptake has to be taken into consideration, too, as will be described below. However, this mechanism of uptake does not lead to a rapid pH change in early endosomes.
Figure 3. pH changes induced by Na+−H+ exchange inhibition.

Summary of the pH changes induced by EIPA (A), HOE694 (B) and amiloride (C). IC50ec, inhibitory constant derived from extracellular concentrations. IC50e, inhibitory constant derived from endosomal concentrations (n = 6–18). D, representative tracing of the determination of endosomal [Na+] using the dye SBFI. After a short control period endosomal [Na+] was clamped to different values in order to estimate the range of endosomal [Na+].
Changes in late endosomal/lysosomal pH induced by Na+−H+ exchange inhibitors as an indirect measure for their lipophilicity
In order to investigate the differences in lipophilicity of EIPA, HOE694 and amiloride in intact cells, we investigated their effects on late endosomal/lysosomal pH. For this purpose the late endosomal/lysosomal compartment was labelled with FITC-dextran (1 g l−1) for 12 h, followed by a period of 12 h incubation without FITC-dextran. This protocol leads to a preferential labelling of the late endosomal/lysosomal compartment (Ohkuma & Poole, 1978; Gekle & Silbernagl, 1995; Ling et al. 1996; Gekle et al. 1998b). The rationale for these experiments is as follows. The late endosomal/lysosomal compartment most probably does not contain NHE (Janecki et al. 1998; D'Souza et al. 1998). Yet if NHE was expressed in this compartment it would not contribute to acidification but rather to alkalinization due to the H+ gradient (Hinside+/Houtside+≥ 100), which cannot be exceeded by the Na+ gradient (Nass & Rao, 1998). Thus, inhibitors of NHE should exert either no specific effect on pH or induce acidification. By contrast, non-ionic trapping of NHE inhibitors as lipophilic weak organic bases (pK = 8.7) could induce alkalinization of the late endosomal/lysosomal compartment. The pH in the late endosomal/lysosomal compartment is 5.5 or smaller (Mukherjee et al. 1997). Therefore, the weak organic bases should accumulate at least ∼80-fold. Thus, in the presence of 100 μmol l−1 extracellular weak organic base the concentration in the late endosomal/lysosomal compartment should reach ≥ 8 mmol l−1. Because the buffer capacity in the late endosomal/lysosomal compartment is in the range of 20 mmol/(l ×ΔpH), ionic trapping of lipophilic bases could induce an alkalinization ≥ 0.4 pH units (Gekle & Silbernagl, 1995). As shown in Fig. 4, EIPA and HOE694 induced a substantial alkalinization of the late endosomal/lysosomal compartment, whereas amiloride exerted only a very small effect. These data support the view that EIPA and HOE694 access the endosomal compartment rapidly via diffusion, whereas amiloride seems to be virtually excluded from this process.
Figure 4. Alkalinization of late endosomes/lysosomes.

Because Na+−H+ exchange cannot contribute to the acidification of endosomes/lysosomes, the changes in pH in the late endosomes/lysosomes (pHLE/LY) induced by the inhibitors are due to the weak base effect. The lipophilic substances EIPA (A) and HOE694 (B) induce a substantial and reversible alkalinization, whereas the hydrophilic substance amiloride (C) exerts only a minor effect. D, summary of the pH changes induced by 100 μmol l−1 of EIPA, HOE694, amiloride or the hydrophilic analogue of HOE694, HOE642 (n = 40).
Estimation of the endosomal/cytosolic Na+ gradient
The cytosolic Na+ concentration was 7 ± 1 mmol l−1 (n = 40) under our experimental conditions. As shown in Fig. 3D, the endosomal Na+ concentration was indeed higher than the cytosolic concentration and ranged between 70 and 100 mmol l−1. Thus, the Na+ gradient is sufficient to establish a gradient of 1–1.2 pH units across the membrane of early endosomes. Additional work is needed to determine the mechanisms underlying the establishment of this gradient. An involvement of Na+-K+-ATPase as well as the delivery of sodium via endocytic vesicles is conceivable (Mukherjee et al. 1997; D'Souza et al. 1998).
Analysis of the Na+−H+ exchange inhibitor profiles
Figure 5A and B summarise the IC50 values and the maximum effects of the three inhibitors. Comparison of the determined IC50 values (Fig. 5A) with known values from the literature (Tse et al. 1993; Noel & Pouysségur, 1995; Noel et al. 1996; Wakabayashi et al. 1997; Robertson et al. 1997; Amemiya et al. 1998) confirms that it is the isoform 3 of NHE which is responsible for the observed effects (Fig. 5C; the IC50 values for NHE4 are much higher than those for NHE3; Noel & Pouysségur, 1995). These data are consistent with the expression of NHE3 in OK cells (Amemiya et al. 1998). The different maximum effects of the three inhibitors (Fig. 5B) indicate that both endosomal and plasma membrane NHE may influence albumin uptake, because endosomal alkalinization as well as cytosolic acidification could interfere with receptor-mediated endocytosis (Schmid, 1997). In order to address the relative contribution of endosomal and plasma membrane NHE further, we performed experiments with propionic acid and after removal of Na+.
Figure 5. Summary of inhibitor actions.
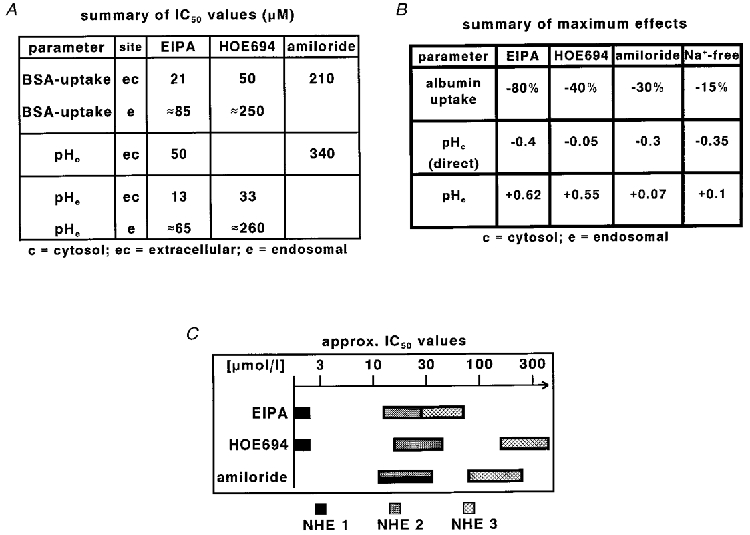
Summary of the IC50 values (A) and the maximum effects (B) of the different Na+−H+ exchange inhibitors. pHc (direct) represents the change in cytosolic pH attributable to the inhibition of plasma membrane Na+−H+ exchange. C, summary of published IC50 values for different Na+−H+ exchange isoforms in Na+-containing solutions (taken from Tse et al. 1993; Noel & Pouysségur, 1995; Noel et al. 1996; Robertson et al. 1997; Wakabayashi et al. 1997; Amemiya et al. 1998).
Effects of propionic acid and removal of Na+ on pH and endocytosis
Propionic acid is a weak organic acid (pK≡ 4.9) able to cross the cell membrane in the non-dissociated form. Inside the cell, propionic acid dissociates into H+ and propionate−, thereby acidifying the cytosol (Fig. 6A). By contrast, endosomal pH remains virtually constant in the presence of propionic acid (Fig. 6B). Thus, application of propionic acid is a suitable manoeuvre for exclusive cytosolic acidification without altering endosomal pH. Figure 6C shows the time course of albumin uptake in the presence of propionic acid compared to control conditions. Cytosolic acidification with propionic acid led to only a slight decrease in albumin uptake. Thus, the effect of propionic acid was different from the effect of amiloride (Fig. 1C). Amiloride in the presence of propionic acid reduced the uptake rate (Fig. 6C), but had only a minor effect on pHc under these conditions (Fig. 6D). Although, amiloride prevented the slow recovery of pHc, there was a substantial cytosolic acidification during the entire uptake period in the absence as well as in the presence of amiloride.
Figure 6. Effect of propionic acid.

The weak organic acid induced a sustained cytosolic acidification (A, representative tracing of 50 cells) but virtually no change in early endosomal pH (B, representative tracing of 60 cells). At the same time 40 mmol l−1 propionic acid led to a slight but significant inhibition of albumin uptake (C, n = 12). Amiloride (1 mmol l−1) reduced albumin uptake in the presence of 40 mmol l−1 propionic acid significantly (C), but affected propionic acid-induced pH changes only to a minor extent (D, representative tracing of 25 cells), indicating that amiloride did not act via changes in cytosolic pH alone.
Amiloride can be taken up by endocytosis and thereby reach endocytic vesicles and the endosomal compartment (Fig. 7C) where it may impair acidification. We determined pHe in the endosomal compartment labelled with FITC-albumin in the presence of 1 mmol l−1 amiloride, i.e. amiloride was present only during the 5 min incubation period prior to the determination of pHe. Indeed, we found that pHe after labelling in the presence of amiloride was 6.36 ± 0.03 (n = 80), a value slightly but significantly higher than that of the respective controls (6.22 ± 0.03; n = 91).
Figure 7. Site of action of Na+−H+ exchange inhibitors.

A, HOE694 and amiloride exert an additive effect on albumin endocytosis (n = 6). The experimental additive value is close to the calculated one. B, the hydrophilic HOE694 analogue, HOE642, did not affect albumin uptake significantly at concentrations up to 300 μmol l−1. This is explained by the difference in lipophilicity, leading to a lower accumulation of HOE642 in acidic compartments (n = 9). C, model for the sites of action of the NHE inhibitors. In the concentration range used in this study EIPA acted at the plasma membrane as well as in the endosomal compartment to which it had access by non-ionic diffusion and endocytic uptake. Amiloride, which acted also at the plasma membrane, had access to the endosomal compartment mainly via endocytic uptake. HOE694 acted mainly in the endosomal compartment to which it had access by non-ionic diffusion and endocytic uptake. EV, endocytic vesicle; EE, early endosome; RE, recycling endosome; ECV, endocytic carrier vesicle; LE, late endosome.
The complete removal of extracellular Na+ (Na+ was replaced by N-methyl-D-glucamine) reduced albumin uptake to 85 ± 6 % of control (n = 6; P < 0.05versus control). Cytosolic pH dropped by 0.35 ± 0.06 pH units (n = 24; P < 0.05versus control) and endosomal pH showed a small increase of 0.10 ± 0.03 pH units (n = 32; P < 0.05versus control).
Comparison of HOE694 with its hydrophilic analogue HOE642
According to our present hypothesis, application of the hydrophilic HOE694 analogue HOE642 (Fig. 4D) − which has a similar inhibitory profile to HOE694 (Rußet al. 1996) − should not affect albumin uptake because of its weak endosomal accumulation. Figure 7B compares the effects of HOE694 and HOE642 on albumin uptake. In accordance with our hypothesis, HOE642 did not affect albumin uptake significantly at concentrations up to 300 μmol l−1.
Because HOE694 seems to act only after ionic trapping in acidic compartments, which is not the case for amiloride, there should be an additive action of both inhibitors. Figure 7C shows that simultaneous addition of amiloride and HOE694, at concentrations that exerted maximum effects if applied alone, led to a significantly stronger inhibition of albumin endocytosis compared to the individual effect of each of the inhibitors. The experimental additive value was close to the calculated additive value.
EIPA reduces the number of apical binding sites
In order to test whether inhibition of NHE3 leads to a decrease in binding sites, as shown for other manoeuvres that induce endosomal alkalinization (Gekle et al. 1995a), we determined albumin binding after 15 min preincubation at 37°C in the presence of 100 μmol l−1 EIPA and compared it with albumin binding after 15 min preincubation in the absence of EIPA. Preincubation with EIPA reduced albumin binding to 77 ± 4 % of control (n = 5; P < 0.05versus control), possibly via disturbed ligand receptor dissociation (Gekle et al. 1995a; Mukherjee et al. 1997).
Exclusion of non-specific effects of the inhibitors
Two possible other effects of the inhibitors have to be excluded. Because the inhibitors are weak bases (Kleyman & Cragoe, 1988), they can accumulate in acidic compartments and thereby buffer H+, leading to alkalinization (weak base effect). As an example, we calculated the contribution of the weak base effect on endosomal alkalinization for 100 μmol l−1 extracellular EIPA. At an endosomal pH of ∼6.2, there is an 15-fold accumulation of EIPA, leading to an endosomal concentration of 1.5 mmol l−1 of EIPA. Because endosomal buffer capacity is ∼40 mmol/(l ×ΔpH) (Gekle & Silbernagl, 1995; Rybak et al. 1997), the accumulation of EIPA induces an alkalinization of only 0.038 pH units. Thus, the weak base effect is negligible. This conclusion is furthermore supported by the observation that another weak base (100 μmol l−1 NH4Cl) exerted only a very small effect on albumin uptake (93 ± 3 % of control, n = 4). At higher concentrations NHE inhibitors may also inhibit Na+-Ca2+ exchange, although the IC50 values for Na+-Ca2+ exchange are at least 10-fold higher than those for NHE (Kleyman & Cragoe, 1988). Inhibition of Na+-Ca2+ exchange would result in an increase in cytosolic Ca2+. However, in a previous study we showed that an increase in cytosolic Ca2+ does not inhibit albumin endocytosis in OK cells (Gekle et al. 1995b). Therefore, we can exclude the possibility that the NHE inhibitors acted via inhibition of Na+-Ca2+ exchange.
Of course, we have to be aware of the possibility that other, yet unknown, side effects may have contributed to a certain extent to the observed effects. However, the analysis of four different compounds makes this possibility unlikely.
DISCUSSION
In the present study we show that NHE3 contributes substantially to the maintenance of endosomal pH and to receptor-mediated endocytosis in proximal tubule-derived opossum kidney (OK) cells (Schwegler et al. 1991; Gekle et al. 1997, 1998b; Brunskill et al. 1998). Our data indicate that NHE3 interacts with receptor-mediated endocytosis mainly via its role in the maintenance of endosomal pH homeostasis.
An important prerequisite with respect to the contribution of NHE in endosomal acidification is the existence of an appropriate Na+ gradient. Our estimates of the endosomal/ cytosolic Na+ gradient (≥ 10/1) indicate that there indeed exists a sufficient Na+ gradient to drive acidification in the early endosomal compartment. The inhibitory action of the specific inhibitors of NHE on endosomal acidification, with IC50 values corresponding to the NHE3, show that the existing Na+ gradient is indeed used by NHE3 in order to acidify the early endosomal compartment. Thus, in OK cells there is an endosomal/cytosolic Na+ gradient which provides the driving force for H+ import into early endosomes via Na+−H+ exchange. The mechanisms establishing this Na+ gradient have not been identified so far. Delivery of Na+ via Na+-K+-ATPase in the endosomal membrane, as well as delivery of Na+ via endocytic vesicles carrying Na+-rich extracellular fluid, is conceivable (Mukherjee et al. 1997; D'Souza et al. 1998).
The activity of NHE3 is furthermore relevant for physiological endocytic uptake rates. This is clearly shown by the inhibitory effects of the three substances on albumin uptake. Again, the IC50 values are in good agreement with an inhibition of NHE3 (Tse et al. 1993; Noel & Pouysségur, 1995; Noel et al. 1996; Wakabayashi et al. 1997; Robertson et al. 1997; Amemiya et al. 1998) and correspond to the IC50 values for the observed pH changes, indicating the importance of an appropriate pH homeostasis for endocytosis. The rapid time course of the inhibitory action – the decrease in the relative uptake rate in the presence of EIPA occurs during the first 5 min (Fig. 1) – supports the hypothesis that NHE plays a role in transport from the plasma membrane to the early endosomal compartment.
The differences in the effects of the NHE inhibitors, taken together with the differences in lipophilicity and IC50 values, enables us to draw some further conclusions regarding the importance of NHE in the endosomal membrane for endocytosis. EIPA, an inhibitor with high lipophilicity (Kleyman & Cragoe, 1988) and low IC50 values, exerted the strongest effect on albumin uptake. Furthermore, EIPA induced a strong endosomal alkalinization and cytosolic acidification. Thus, EIPA may inhibit NHE at the endosomal membrane, at the plasma membrane and in endocytic vesicles. Thus, the effects of EIPA alone do not allow us to estimate the contribution of NHE at the different sites. However, when we examined the inhibitory profiles of HOE694 and amiloride as well as the effect of exclusive cytosolic acidification using propionic acid, we found that the activities of NHE at the endosomal membrane and at the plasma membrane may influence endocytosis. Yet, in quantitative terms, the activity of NHE at the endosomal membrane seems to be the most important component. (a) Exclusive acidification of the cytosol with propionic acid led to a small (∼-15 %), but significant, reduction of endocytosis. (b) Amiloride, a relatively hydrophilic inhibitor, exerted a smaller effect than EIPA (Kleyman & Cragoe, 1988). The greater effect of amiloride than propionic acid is most probably due to uptake of amiloride by endocytosis, whereby amiloride reaches endocytic vesicles where it may inhibit NHE (Fig. 7C). (c) HOE694, a lipophilic inhibitor with low affinity for NHE3 which acts mainly at the endosomal membrane due to ionic trapping, exerted a stronger inhibition than either propionic acid and amiloride.
The possibility that the inhibitors exerted their effects not via an interaction with NHE3, but via the weak base effect have been ruled out. Our calculations predicted that there is insufficient accumulation of the weak bases in early endosomes to explain the observed pH changes. Furthermore, the calculations predicted that 100 μM EIPA or 100 μM HOE694 should lead to a substantial alkalinization of the late endosomal/lysosomal compartment (where NHE cannot contribute to alkalinization because the organelle/cytosol proton gradient is ≥ 100) via the weak base effect. As shown in Fig. 4, this was indeed the case, supporting the validity of the calculations. Furthermore, these data confirm the difference in lipophilicity between EIPA and HOE694 on the one side (high lipophilicity) and amiloride on the other (low lipophilicity) in intact cells (Kleyman & Cragoe, 1988).
Our data also indicate that there are two ways for the inhibitors to reach the endosomal NHE (Fig. 7C). Lipophilic inhibitors gain access to the early endosomal compartment via non-ionic diffusion, leading to an accumulation of the substances and therefore to a higher local concentration compared to the extracellular space. Thus, the inhibitors applied simultaneously with the substrate may change endosomal pH before the substrate for endocytosis is delivered. The second possibility for reaching the endocytic pathway, albeit without accumulation, is uptake via endocytosis, as seems to be the case for amiloride and, of course for EIPA and HOE694, too. If they enter by this route the inhibitors act simultaneously with delivery of the endocytic substrate. The existence of these two pathways also contributes to the explanation of why EIPA exerted a much stronger inhibition than amiloride or HOE694, besides the different actions of EIPA and HOE694 at the plasma membrane. EIPA reaches the endocytic compartments via both pathways at concentrations that inhibit NHE3. Therefore, EIPA disturbs pH homeostasis in the incoming endocytic vesicles as well as in the accepting endosomal compartment. By contrast, HOE694 acts mainly in the accepting endosomal compartment, whereas amiloride may act in the incoming endocytic vesicles. These data support the hypothesis that NHE3 activity in early endosomes as well as in endocytic vesicles is important for albumin uptake.
Taken together, our data show that endosomal NHE3 plays an important role in receptor-mediated endocytosis of albumin due to its contribution to endosomal pH homeostasis. Inhibition of NHE3 disturbs endosomal acidification and thereby retards endocytosis. Impaired receptor recycling seems to be one mechanism responsible for the observed effects. However, it explains reduced albumin uptake only in part. Other possible mechanisms for transduction of endosomal alkalinization to reduced albumin uptake include fusion events, vesicle trafficking or the formation of carrier vesicles (Mukherjee et al. 1997). The current data do not allow us to discriminate between these mechanisms in more detail and will be investigated in further studies.
Because NHE3 is regulated by a variety of stimuli (Wakabayashi et al. 1997), its involvement in receptor-mediated endocytosis offers a new mechanism to explain the regulation of endocytosis and possibly its malfunction under pathophysiological conditions (Gekle et al. 1997; Wakabayashi et al. 1997). For example, activation of protein kinase A by cAMP, a manoeuvre known to inhibit NHE3 (Wakabayashi et al. 1997), has been shown to reduce albumin endocytosis in OK cells (Gekle et al. 1997). In addition, we have shown that cAMP leads to an alkalinization of the endosomal compartment in these cells (Gekle et al. 1995b). These data indicate that cAMP may regulate endocytosis, at least in part, via its action on NHE3 activity. More work is required in order to determine the precise role of NHE3 in the regulation of receptor-mediated endocytosis and to investigate the mechanisms underlying the establishment of the Na+ gradient across the endosomal membrane.
Acknowledgments
This study was supported by the Deutsche-Forschungsgemeinschaft (SFB 176/A6).
References
- Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. American Journal of Physiology. 1998;269:C126–133. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]
- Brunskill NJ, Stuart J, Tobin AB, Walls J, Nahorski S. Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. Journal of Clinical Investigation. 1998;101:2140–2150. doi: 10.1172/JCI1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C, Harris KPG. The role of proteinuria in the progression of chronic renal failure. American Journal of Kidney Diseases. 1996;27:765–775. doi: 10.1016/s0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H, Verroust P, Moestrup SK. Membrane receptors for endocytosis in the renal proximal tubule. International Review of Cytology. 1998;180:237–284. doi: 10.1016/s0074-7696(08)61772-6. [DOI] [PubMed] [Google Scholar]
- Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. American Journal of Physiology. 1996;271:F900–907. doi: 10.1152/ajprenal.1996.271.4.F900. [DOI] [PubMed] [Google Scholar]
- Czekay R-P, Orlando RA, Woodward L, Lundstrom M, Farquhar MG. Endocytic trafficking of megalin/RAP complexes: dissociation of the complexes in late endosomes. Molecular Biology of the Cell. 1997;8:517–532. doi: 10.1091/mbc.8.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. Journal of Biological Chemistry. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- Delean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassey, radioligand assey, and physiological dose-response curves. American Journal of Physiology. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV. Expression studies of CLC-5 in human kidney and opossum kidney (OK) cells yield a pathophysiological basis for Dent's disease. Journal of the American Society of Nephrology. 1998;9:33A. [Google Scholar]
- Fuchs R, Schmid S, Mellman I. A possible role for Na+, K+-ATPase in regulating ATP-dependent endosome acidification. Proceedings of the National Academy of Sciences of the USA. 1989;86:539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M. Renal proximal tubular albumin reabsorption: The daily prevention of albuminuria. News in Physiological Sciences. 1998;13:5–11. doi: 10.1152/physiologyonline.1998.13.1.5. [DOI] [PubMed] [Google Scholar]
- Gekle M, Golenhofen N, Oberleithner H, Silbernagl S. Rapid activation of Na+/H+-exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proceedings of the National Academy of Sciences of the USA. 1996b;93:10500–10504. doi: 10.1073/pnas.93.19.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Schwerdt G, Silbernagl S. Albumin endocytosis in OK cells: Dependence on actin and microtubules, regulation by protein kinases. American Journal of Physiology. 1997;272:F668–677. doi: 10.1152/ajprenal.1997.272.5.F668. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. American Journal of Physiology. 1995a;268:F899–906. doi: 10.1152/ajprenal.1995.268.5.F899. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Kinetics of receptor-mediated endocytosis of albumin in cells derived from the proximal tubule of the kidney (Opossum kidney cells): Influence of Ca2+ and cAMP. Pflügers Archiv. 1995b;430:374–380. doi: 10.1007/BF00373912. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Functional characterization of albumin binding to the apical membrane of OK cells. American Journal of Physiology. 1996a;271:F286–291. doi: 10.1152/ajprenal.1996.271.2.F286. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Long-term protein exposure reduces albumin binding and uptake in proximal tubule-derived opossum kidney cells. Journal of the American Society of Nephrology. 1998b;9:960–968. doi: 10.1681/ASN.V96960. [DOI] [PubMed] [Google Scholar]
- Gekle M, Silbernagl S. Comparison of the buffer capacity of endocytotic vesicles, lysosomes and cytoplasm in cells derived from the proximal tubule of the kidney (opossum kidney cells) Pflügers Archiv. 1995;429:452–454. doi: 10.1007/BF00374165. [DOI] [PubMed] [Google Scholar]
- Gekle M, Silbernagl S, Wünsch S. Non-genomic action of the mineralocorticoid aldosterone on cytosolic sodium in cultured kidney cells. The Journal of Physiology. 1998a;511:255–263. doi: 10.1111/j.1469-7793.1998.255bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield Membrane transport in the endocytic pathway. Current Opinion in Cell Biology. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Journal of Biological Chemistry. 1998;273:8790–8798. doi: 10.1074/jbc.273.15.8790. [DOI] [PubMed] [Google Scholar]
- Jerums G, Panagiotopoulos S, Tsalamandris C, Allen TJ, Gilbert RE, Comper WD. Why is proteinuria such an important risk factor for progression in clinical trials. Kidney International. 1997;52:S-87–S-92. [PubMed] [Google Scholar]
- Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. Journal of Biological Chemistry. 1994;269:23544–23552. [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. Journal of Membrane Biology. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Szabó EZ, Lukacs G, Orlowski J. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. Journal of Biological Chemistry. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- Ling H, Vamvakas S, Gekle M, Schaefer L, Teschner M, Schaefer RM, Heidland A. Role of lysosomal cathepsin activities in cell hypertrophy induced by NH4Cl in cultured renal proximal tubule cells. Journal of the American Society of Nephrology. 1996;7:73–80. doi: 10.1681/ASN.V7173. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marshansky V, Bourgoin S, Londono I, Bendayan M, Maranda B, Vinay P. Receptor-mediated endocytosis in kidney proximal tubules: Recent advances and hypothesis. Electrophoresis. 1997;18:2661–2676. doi: 10.1002/elps.1150181423. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Vinay P. Proton gradient formation in early endosomes from proximal tubules. Biochimica et Biophysica Acta. 1996;1284:171–180. doi: 10.1016/s0005-2736(96)00123-x. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annual Review of Biochemistry. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nass R, Rao R. Novel localization of a Na+/H+exchanger in a late endosomal compartment of yeast. Journal of Biological Chemistry. 1998;273:21054–21060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- Noel J, Pouysségur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. American Journal of Physiology. 1995;268:C283–296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Noel J, Roux D, Pouysségur J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. Journal of Cell. Science. 1996;109:929–939. doi: 10.1242/jcs.109.5.929. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proceedings of the National Academy of Sciences of the USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkonstanti EA, Emmanouel DS, Gravanis A, Stournaras C. Na+/Pi co-transport alters rapidly cytoskeletal protein polymerization dynamics in opossum kidney cells. Biochemical Journal. 1996;315:241–247. doi: 10.1042/bj3150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MA, Woodside M, Foskett JK, Orlowski J, Grinstein S. Muscarinic agonists induce phosphorylation-independent activation of the NHE-1 isoform of the Na+/H+ antiporter in salivary acinar cells. Journal of Biological Chemistry. 1997;272:287–294. [PubMed] [Google Scholar]
- Ruß U, Balser C, Scholz W, Albus U, Lang HJ, Weichert A, Schölkens BA, Gögelein H. Effects of the Na+/H+-exchange inhibitor Hoe 642 on intracellular pH, calcium and sodium in isolated rat ventricular myocytes. Pflügers Archiv. 1996;433:26–34. doi: 10.1007/s004240050244. [DOI] [PubMed] [Google Scholar]
- Rybak SL, Lanni F, Murphy RF. Theoretical considerations on the role of membrane potential in the regulation of endosomal pH. Biophysical Journal. 1997;73:674–687. doi: 10.1016/S0006-3495(97)78102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter E, Haxelmans S, Ankorina I. Correlation between intracellular activities of Ca2+ and Na+ in rat cortical collecting duct – a possible coupling mechanism between Na+-K+-ATPase and basolateral K+ conductance. Kidney and Blood Pressure Research. 1996;19:24–31. doi: 10.1159/000174042. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annual Review of Biochemistry. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schwegler JS, Heppelmann B, Mildenberger S, Silbernagl S. Receptor-mediated endocytosis of albumin in cultured opossum kidney cells: a model for proximal tubular protein reabsorption. Pflügers Archiv. 1991;418:383–392. doi: 10.1007/BF00550876. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. Journal of Biological Chemistry. 1995;270:31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? BioEssays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tse CM, Levine SA, Yun CHC, Montrose MH, Little PJ, Pouysségur J, Donowitz M. Cloning and expression of a rabbit cDNA encoding a serum-activated ethylisopropylamiloride-resistant epithelial Na+/H+ exchanger isoform (NHE-2) Journal of Biological Chemistry. 1993;268:11917–11924. [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouysségur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77:51–67. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. American Journal of Physiology. 1989;256:F957–964. doi: 10.1152/ajprenal.1989.256.5.F957. [DOI] [PubMed] [Google Scholar]
- Zen K, Biwersi J, Periasamy N, Verkman AS. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. Journal of Cell Biology. 1992;119:99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]


