Abstract
The present study examined the regulation of human skeletal muscle AMP deamination during intense exercise and quantified muscle accumulation and release of purines during and after intense exercise.
Seven healthy males performed knee extensor exercise at 64.3 W (range: 50–70 W) to exhaustion (234 s; 191–259 s). In addition, on two separate days the subjects performed exercise at the same intensity for 30 s and 80 % of exhaustion time (mean, 186 s; range, 153–207 s), respectively. Muscle biopsies were obtained from m.v. lateralis before and after each of the exercise bouts. For the exhaustive bout femoral arterio-venous concentration differences and blood flow were also determined.
During the first 30 s of exercise there was no change in muscle adenosine triphosphate (ATP), inosine monophosphate (IMP) and ammonia (NH3), although estimated free ADP and AMP increased 5- and 45-fold, respectively, during this period. After 186 s and at exhaustion muscle ATP had decreased (P < 0.05) by 15 and 19 %, respectively, muscle IMP was elevated (P < 0.05) from 0.20 to 3.65 and 5.67 mmol (kg dry weight)−1, respectively, and muscle NH3 had increased (P < 0.05) from 0.47 to 2.55 and 2.33 mmol (kg d.w.)−1, respectively. The concentration of H+ did not change during the first 30 s of exercise, but increased (P < 0.05) to 245.9 nmol l−1 (pH 6.61) after 186 s and to 374.5 nmol l−1 (pH 6.43) at exhaustion.
Muscle inosine and hypoxanthine did not change during exercise. In the first 10 min after exercise the muscle IMP concentration decreased (P < 0.05) by 2.96 mmol (kg d.w.)−1 of which inosine and hypoxanthine formation could account for 30 %. The total release of inosine and hypoxanthine during exercise and 90 min of recovery amounted to 1.07 mmol corresponding to 46 % of the net ATP decrease during exercise or 9 % of ATP at rest.
The present data suggest that AMP deamination is inhibited during the initial phase of intense exercise, probably due to accumulation of orthophosphate, and that lowered pH is an important positive modulator of AMP deaminase in contracting human skeletal muscle in vivo. Furthermore, formation and release of purines occurs mainly after intense exercise and leads to a considerable loss of nucleotides.
During intense exercise the rate of ATP utilisation in skeletal muscle is higher than the rate of ATP regeneration, which leads to an accumulation of ADP and AMP. To avoid a large accumulation of AMP within the cell, AMP is deaminated to IMP and ammonia/ammonium (in this paper we will represent both as ‘NH3’) via the enzyme AMP deaminase (Lowenstein, 1990). The maximal activity of AMP deaminase is high in skeletal muscle and the accumulation of IMP and NH3 in human muscle after intense exercise may amount to 10 mmol (kg d.w.)−1 (Sahlin et al. 1978; Graham et al. 1990; Bangsbo et al. 1992a; Tullson et al. 1995). The mechanism underlying the regulation of AMP deamination in human skeletal muscle is, however, unclear.
In contracting rat skeletal muscle AMP deaminase has been demonstrated to bind to myosin whereby the Km value of the enzyme is substantially lowered, allowing for a pronounced rate of deamination at low concentrations of AMP (Shiraki et al. 1979; Rundell et al. 1992). Attempts to demonstrate AMP deaminase binding to myosin in human skeletal muscle biopsies obtained after high intensity exercise, leading to high levels of IMP accumulation, have nevertheless failed (Tullson et al. 1995). In vitro studies have shown that the activity of AMP deaminase is enhanced by increased concentrations of ADP, AMP and H+, whereas orthophosphate, ATP and guanosine 5′-triphosphate (GTP) inhibit the enzyme (Wheeler & Lowenstein, 1979; Dudley & Terjung, 1985; Lowenstein, 1990). Orthophosphate is considered to be the primary inhibitor of AMP deaminase within muscle, with a Ki of 1–2 mmol l−1 (Lowenstein, 1990). It is not known, however, how the different modulators are altered in relation to AMP deamination and how they thereby may affect the reaction in human muscle during intense exercise.
It is furthermore unclear to what extent the AMP deaminase reaction is coupled to the major anaerobic pathways, the creatine kinase reaction and glycogenolysis/glycolysis. All the reactions are modulated by the free ADP concentration and, in addition to the AMP deaminase reaction, glycogenolysis and glycolysis are stimulated by an elevated level of free AMP. A high rate of glycolysis leads to the formation of lactate and consequently an elevated concentration of H+, which may activate the AMP deaminase reaction, at least at high H+ concentrations, as shown in rat muscle (Dudley & Terjung, 1985). The creatine kinase reaction and glycogenolysis/glycolysis have been extensively studied in humans through analysis of muscle biopsies obtained before and after muscle contractions (Bangsbo, 1996). The net use of CP and the lactate accumulation in contracting muscles have been determined after maximal exercise of various durations (Nevill et al. 1996) and during rather intense exercise at a constant intensity (Karlsson & Saltin, 1970). However, in the former experiments the metabolic responses were not monitored during the exercise and in the latter study no samples were obtained prior to 2 min of exercise. Furthermore, the release of lactate from the exercising muscle was not taken into account and no attempt was made to relate the rates of the pathways to the AMP deaminase reaction. Thus, there is still a lack of knowledge with regard to how the rates of the pathways change during intense exercise and how the reactions may interact.
A fraction of the IMP formed during exercise is degraded to inosine and hypoxanthine, which can pass through the cell membranes (Hellsten, 1994). A release of hypoxanthine from intensely exercised muscle has been observed, representing a loss of nucleotide precursors from the muscle (Bangsbo et al. 1992b; Hellsten-Westing et al. 1994). Furthermore, the ATP concentration in muscle has been found to be lowered for more than 90 min in recovery from long-term intense intermittent exercise, in part due to release of purines (Hellsten et al. 1998). It is, however, uncertain to what extent the release of purines can account for a loss in adenine nucleotides during and after a single bout of short-term intense exercise.
Thus, the aims of the present study were: (1) to examine how the rate of AMP deamination in human muscle changes in association with modulators of the reaction as well as in relation to other anaerobic reactions during high intensity dynamic exercise, and (2) to quantify purine formation and release during exercise and recovery concomitant with changes in muscle nucleotides. Subjects performed intense one-legged knee-extensor exercise to exhaustion to allow for a high power output of a well defined muscle. Changes in the accumulation of muscle metabolites and exchange of metabolites from the muscle were determined during various phases of exercise and in recovery.
METHODS
Subjects
Seven healthy male subjects ranging in age from 23 to 40 years with a mean height of 184 cm (range, 173–189 cm) and a mean weight of 78.3 kg (70.0–84.9 kg) volunteered to participate in the experiment. The subjects participated regularly in physical activity but were not involved in competitive sports. The subjects were fully informed of any risks and discomfort associated with the experimental procedures before giving their consent to participate. The study was approved by the local ethics committee.
Procedures
Main experiment
About 3 h before an experiment the subject had a light breakfast. He reported to the laboratory 2 h before the experiment. After 30 min of rest in the supine position a catheter was placed in the femoral artery of the experimental leg under local anaesthesia. The tip was positioned 1–2 cm proximal to the inguinal ligament. A catheter was also placed in the femoral vein of the experimental leg with the tip of the catheter positioned about 1–2 cm distal to the inguinal ligament. A thermistor for measurement of blood temperature was inserted through the femoral venous catheter and was advanced 8–10 cm proximal to the tip.
After the placement of the catheters the subject rested in the supine position for about 1 h. Then, the subject performed knee extensor exercise with the experimental leg at an intensity of 10 W (warm-up; Andersen et al. 1985). Following a 10 min period of rest, the subject performed knee-extensor exercise at an intensity of 64.3 W (range: 50–70 W) to exhaustion lasting 234 s (191–259 s). A biopsy was obtained from m.v. lateralis before, immediately after and 10 min after the exhaustive exercise. Blood samples of 3–4 ml volume were drawn simultaneously from the femoral artery and vein at rest, immediately before and after 10, 30, 50, 70, 100, 150 and 210 s during the exhaustive exercise as well as immediately before exhaustion. After the exercise blood samples were obtained at 45 s and at 1.5, 2.5, 3.5, 5, 7, 9.5, 11, 15, 20, 30, 45, 60, 75 and 90 min. Blood flow was measured immediately prior to blood sampling. An occlusion cuff placed just below the knee was inflated (220 mmHg) during blood sampling and blood-flow measurements.
Additional experiments
The subjects came in to the laboratory on two more occasions, separated by at least 1 week. On one occasion the subjects performed one-legged knee extensor exercise at the same intensity as that performed during the main experiment for a duration corresponding to 80 % of the exhaustion time reached during the main experiment (mean, 186 s; range, 153–207 s). On another occasion the subjects performed the exercise at the same intensity as that of the main experiment for a duration of 30 s. A muscle biopsy was obtained before and immediately after each of the two exercise bouts.
Measurements and analysis
Blood flow
Femoral venous blood flow was measured by the thermodilution technique (Andersen & Saltin, 1985). Briefly, ice cold saline was infused at a constant rate into the femoral vein for 10–15 s to achieve changes in blood temperature of 0.8–1.0°C. At rest and in late recovery, when the blood flow was low, a 30–45 s infusion period was used.
Blood analysis
Haematocrit (Hct) determinations were made in triplicates using micro centrifugation. Blood lactate was analysed using a lactate analyser (Yellow Springs Instruments). A part of each blood sample was centrifuged rapidly and the plasma was collected and stored at −20°C until analysed. Neutralised perchloric acid extracts of plasma were analysed for inosine, hypoxanthine and urate using reverse phase high performance liquid chromatography (HPLC; Tullson et al. 1990). Plasma NH3 was determined by the method of Kun & Kearney (1974).
Muscle mass
The mass of quadriceps femoris muscles was estimated based on Simpson's rule, which included measurements of thigh length, multiple circumferences of the thigh and the skin fold thickness (Jones & Pearson, 1969), and corrected based on a comparison between the anthropometric measurements and CAT-scan determinations (ratio 1:0.80). The mean knee-extensor mass of the experimental leg was 2.34 kg, with a range of 2.08–3.05 kg.
Muscle biopsies
Muscle biopsy samples were analysed for total water by weighing the samples before and after freeze drying. Changes in muscle variables were calculated on a dry weight basis and normalised to the water content of resting muscle. This enables a comparison of quadriceps muscle metabolite concentrations and the magnitude of exchange of metabolites between muscle and blood, since the muscle mass was determined at rest. Muscle specimens were extracted with perchloric acid, neutralised and analysed for nucleotides, nucleosides and nucleobases using reverse phase HPLC (Tullson et al. 1990). Muscle creatine phosphate (CP), lactate, glucose-1-phosphate (G-1-P), glucose-6-phosphate (G-6-P), and fructose-6-phosphate (F-6-P) were analysed by fluorometic assays (Lowry & Passonneau, 1972). Muscle NH3 was determined by the method of Kun & Kearney (1974). Muscle pH was measured by a small glass electrode (GK2801, Radiometer, Copenhagen, Denmark) after homogenizing the muscle sample in a non-buffering solution containing 145 mM KCl, 10 mM NaCl and 5 mM iodoacetic acid (Parkhouse et al. 1985).
Calculations
Net release of lactate, NH3 and purines by the thigh was calculated by multiplying the blood flow by the difference between femoral venous and arterial (v-adiff) concentrations of the variables. A continuous blood flow curve was constructed for each subject by linear connection of the consecutive data points to obtain ‘time-matched’ values for the blood variables and the blood flow measurements during exercise and early recovery. No difference between time-matched and measured blood flow was larger than 0.3 l min−1. Although plasma rather than blood NH3, inosine, hypoxanthine and urate concentrations were measured, it was used in combination with blood flow to estimate blood exchange of these variables because the absolute changes in plasma and whole blood concentrations for these variables are virtually identical (Harris & Dudley, 1989; Hellsten et al. 1998).
The total release of lactate, NH3 and purines during 0–30, 30–186 and 186–234 s of exercise and during 0–10, 10–30 and 30–90 min of recovery from exercise was determined as:
where x and y represent the start and end time, respectively, and f(t) the release of the compound at a given time (t) during recovery. In practice, the exchange was determined as the area under the f(t) curve, with time on the x-axis. The curves were produced on the assumption that there was a linear relationship between two measured values.
Free ADP (ADPf) and AMP (AMPf) concentrations were estimated from the creatine phosphokinase and myokinase reactions using the reactants (ATP, CP, creatine and H+) and the equilibrium constant of the near equilibrium creatine kinase reaction (Lawson & Veech, 1979). The creatine concentration was calculated based on the assumptions that the ratio CP/(CP + creatine) was 0.70 in the resting state (Spriet et al. 1987a) and that the sum of CP and creatine remained constant.
Statistics
Analysis of variance (ANOVA) with repeated measures was used to evaluate changes during exercise and recovery, and the Newman-Keuls test was used to locate differences. Differences between the values obtained during exercise or recovery and the values obtained at rest or nil were determined by Student's paired t test. Standard errors of the mean (s.e.m.) are only given in the text where these values cannot be obtained from a table or a figure.
RESULTS
Thigh blood flow
Thigh blood flow was 0.38 ± 0.05 l min−1 at rest and it increased to 2.92 ± 0.54 and 3.41 ± 0.27 l min−1 after 18 and 70 s of exercise, respectively, and at the end of exercise reaching 4.43 ± 0.47 l min−1. Thigh blood flow decreased to 3.80 ± 0.60 and 2.95 ± 0.67 l min−1 at 2.5 and 5 min after exercise, respectively, and further to 1.66 ± 0.39 and 0.65 ± 0.08 l min−1 at 9.5 and 20 min of recovery, respectively, reaching blood flow at rest after 45 min of recovery.
Muscle nucleotides and NH3
Muscle ATP levels did not change during the first 30 s of exercise, but the concentration had decreased (P < 0.05) by 15 and 19 % after 186 s and at exhaustion, respectively (Table 1). The ADP concentration was elevated (P < 0.05) after 186 s and at exhaustion, whereas the level of AMP did not change during the exercise. The estimated free ADP concentration increased from about 50 to 246 μmol (kg dry weight (d.w.))−1 after 30 s, and did not change further during the exercise (Table 1). Similarly, the estimated free AMP concentration increased from about 7 to 258 nmol (kg d.w.)−1 during the first 30 s and was unaltered during the remainder of exercise.
Table 1.
Skeletal muscle concentrations of adenine nucleotides and NH3 (mmol (kg d.w.)−1), free AMP (nmol (kg d.w.)−1), free ADP, and purine bases (mmol (kg d.w.)−1) during intense knee extensor exercise
| Exhaustive exercise (0–234 s) | 30 s exercise (0–30 s) | Exercise for 80% of time to exhaustion (0–186 s) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | 10 min post | Pre | Post | Pre | Post | |
| CP (mmol (kg d.w.)−1) | 86.5 ± 3.9 | 23.1 ± 5.5* | 87.4 ± 3.8† | 87.5 ± 3.6 | 52.7 ± 5.9* | 82.9 ± 4.5 | 28.2 ± 5.3* |
| ATP (mmol (kg d.w.)−1) | 22.52 ± 0.78 | 18.26 ± 0.76* | 20.72 ± 0.70† | 22.15 ± 0.44 | 21.89 ± 0.38 | 22.27 ± 1.00 | 18.85*± 0.81 |
| ADP (mmol (kg d.w.)−1) | 2.75 ± 0.21 | 3.13 ± 0.07* | 2.73 ± 0.11† | 2.93 ± 0.134 | 3.02 ± 0.13 | 2.83 ± 0.12 | 3.41 ± 0.11* |
| AMP (mmol (kg d.w.)−1) | 0.27 ± 0.06 | 0.19 ± 0.02 | 0.21 ± 0.04 | 0.23 ± 0.0512 | 0.24 ± 0.06 | 0.26 ± 0.03 | 0.33 ± 0.07 |
| IMP (mmol (kg d.w.)−1) | 0.20 ± 0.06 | 5.67 ± 1.54* | 2.37 ± 0.57† | 0.16 ± 0.04 | 0.18 ± 0.03‡ | 0.15 ± 0.02 | 3.65 ± 1.03* |
| TAN (mmol (kg d.w.)−1) | 25.5 ± 0.7 | 21.3 ± 1.0* | 23.3 ± 1.3† | 25.4 ± 0.7 | 24.2 ± 0.5‡ | 25.2 ± 1.3 | 19.5 ± 1.5* |
| ADPf (μmol (kg d.w.)−1) | 56.6 ± 6.5 | 264.1 ± 100.8* | 59.6 ± 13.4† | 49.4 ± 2.7 | 245.7 ± 80.0* | 50.6 ± 5.0 | 344.7 ± 146.3* |
| AMPf (nmol (kg d.w.)−1) | 7.4 ± 1.5 | 360.6 ± 251.1* | 12.9 ± 4.8† | 6.9 ± 0.8 | 257.7 ± 154.6* | 7.2 ± 1.1 | 562.1 ± 391.1* |
| NH3 (mmol (kg d.w.)−1) | 0.47 ± 0.08 | 2.55 ± 0.62* | 1.29 ± 0.23 | 0.82 ± 0.22 | 1.07 ± 0.14+ | 0.26 ± 0.06 | 2.33 ± 0.49* |
| Inosine (μmol (kg d.w.)−1) | 0.088 ± 0.024 | 0.101 ± 0.036 | 0.417 ± 0.107† | 0.055 ± 0.014 | 0.053 ± 0.008 | 0.068 ± 0.007 | 0.079 ± 0.012 |
| Hypoxanthine (μmol (kg d.w.)1) | 0.212 ± 0.021 | 0.2 27 ± 0.012 | 0.278 ± 0.021 | 0.233 ± 0.014 | 0.236 ± 0.017 | 0.205 ± 0.017 | 0.246 ± 0.022 |
| % H2O | 77.3 ± 0.7 | 78.8 ± 0.4* | 78.1 ± 0.2 | 77.0 ± 0.5 | 77.8 ± 0.4‡ | 76.5 ± 0.3 | 79.0 ± 0.9* |
Significantly different from pre-exercise (P < 0.05);
significantly different from post-exercise (P < 0.05);
significantly different from post-80% (P < 0.05).
No significant accumulation of muscle IMP was observed after 30 s of exercise but the concentration was elevated to 3.65 and 5.67 mmol (kg d.w.)−1 after 186 s and at exhaustion, respectively (Table 1). Thus, the rate of IMP accumulation was greater (P < 0.05) from 30 to 186 s and from 186 s to exhaustion than during the first 30 s of exercise (1.42 ± 0.44 and 2.13 ± 0.99 vs. 0.04 ± 0.10 mmol (kg d.w.)−1 min−1).
Muscle NH3 was unchanged during the first 30 s of exercise, but the level had increased (P < 0.05) to 2.33 and 2.55 mmol (kg d.w.)−1 after 186 s and at exhaustion, respectively (Table 1). After 50 s of exercise the NH3 concentration in femoral venous blood was elevated (P < 0.05), whereas a significant (P < 0.05) release of NH3 was observed after 30 s (Fig. 1). The total NH3 release was 0.72 mmol during the exercise (Table 2), corresponding to about 1.2 mmol (kg d.w.)−1 or 37 % of the NH3 production.
Figure 1.
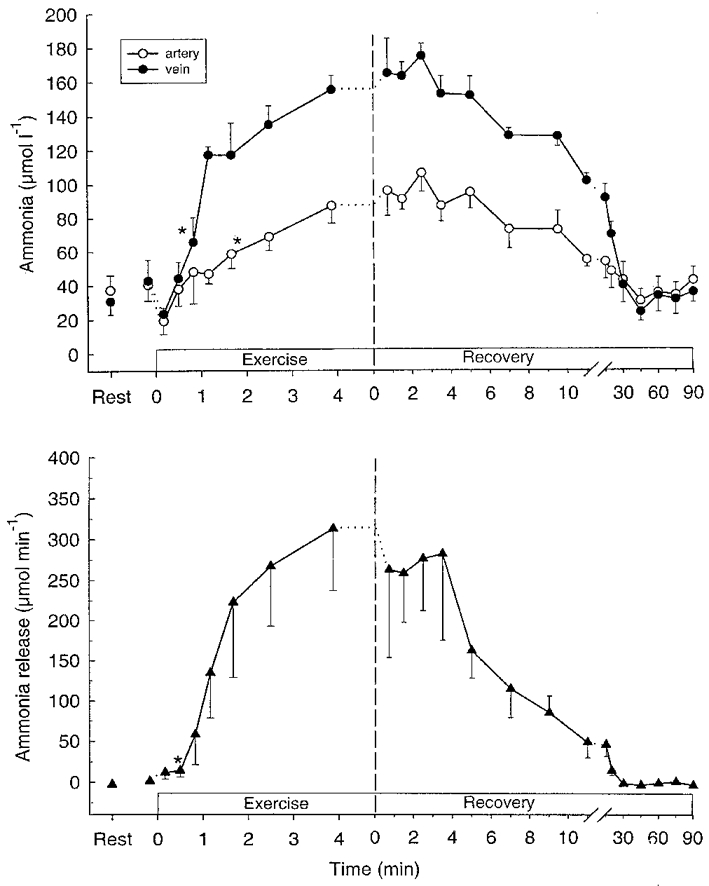
Femoral arterial (^) and venous (•) plasma ammonia (NH3) (upper panel) and net NH3 release (lower panel) during intense knee-extensor exercise. Data are means ±s.e.m.*First mean value significantly different from rest (P < 0.05).
Table 2.
Total release of purines and ammonia (mmol) from active muscle during and after intense knee-extensor exercise
| Exercise | Recovery | Exercise + Recovery | ||||
|---|---|---|---|---|---|---|
| 0–3.9 min | 0–10 min | 10–30 min | 30–90 min | 0–90 min | 0–93.9 min | |
| Inosine (μmol) | 2.8 ± 1.7 | 13.4 ± 8* | 41.5 ± 11.3* | 48.0 ± 12.2* | 102.9 ± 25.5* | 105.6 ± 26.8* |
| Hypoxanthine (μmol) | 34 ± 6 | 258 ± 43* | 430 ± 69* | 281 ± 55* | 970 ± 143* | 1004 ± 144* |
| Urate (μmol) | 216 ± 121 | −580 ± 194* | −31 ± 61 | −338 ± 264 | −949 ± 371* | −733 ± 361* |
| Ammonia (μmol) | 715 ± 196* | 1638 ± 309* | 491 ± 111* | −72 ± 77 | 2057 ± 369* | 2772 ± 528* |
Significant exchange (P < 0.05).
During the first 10 min of recovery the muscle ATP concentration increased (P < 0.05) by 13 %, but it was still 8 % lower (P < 0.05) than at rest. The muscle AMP concentration did not change, whereas muscle ADP and IMP decreased (P < 0.05) during the first 10 min after exercise (Table 1). Muscle NH3 had decreased by 47 % after 10 min of recovery, but it was still above (P < 0.05) resting level (Table 1). A significant release of NH3 was observed during the first 30 min of recovery. The total release was 2.1 mmol during 90 min of recovery (Table 2).
Muscle purines and purine release
Muscle hypoxanthine and inosine were unaltered during exercise (Table 1). After 100 s of exercise femoral venous hypoxanthine and inosine concentrations had increased (P < 0.05) (Fig 2 and Fig 3), and a significant (P < 0.05) net release of hypoxanthine and inosine was observed after 70 s (Fig. 3). Total net releases of hypoxanthine and inosine during exercise were 2.8 and 34 μmol, respectively (Table 2). No net exchange of urate was observed during exercise (Fig. 4).
Figure 2.
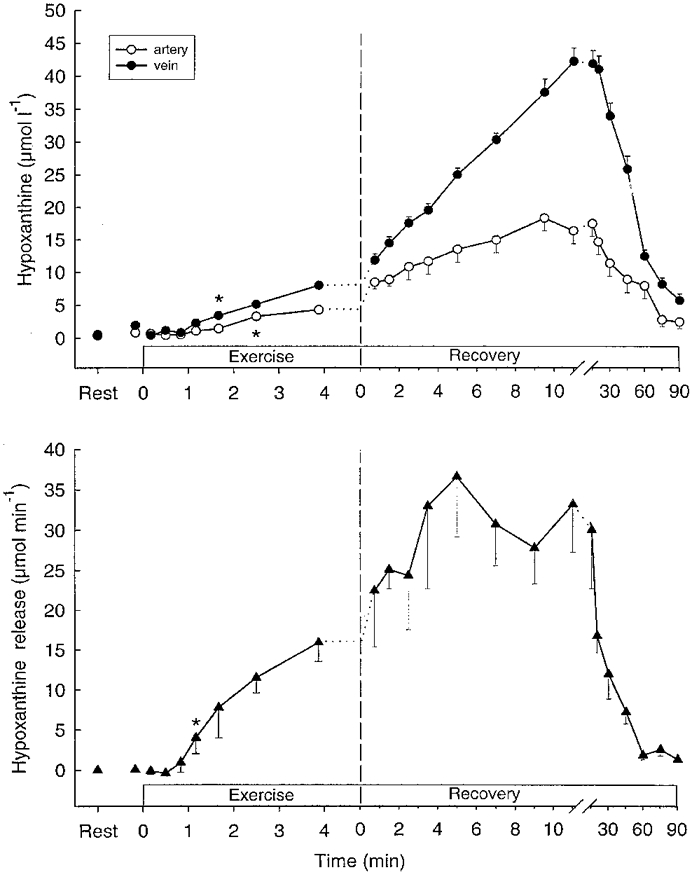
Femoral arterial (^) and venous (•) plasma hypoxanthine (upper panel) and net hypoxanthine release (lower panel) during intense knee-extensor exercise. Data are means ±s.e.m.* First mean value significantlydifferent from rest (P < 0.05).
Figure 3.
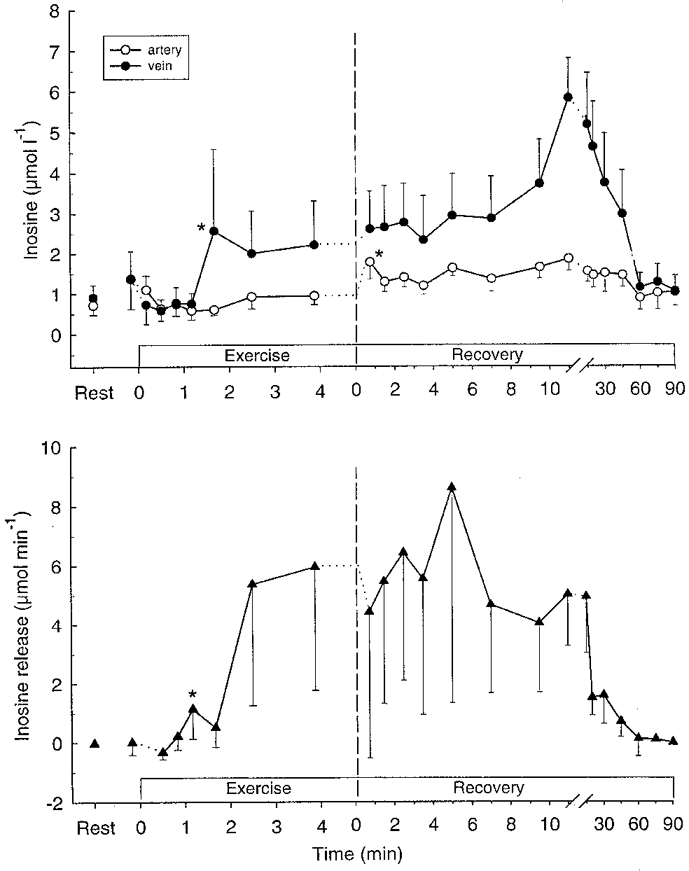
Femoral arterial (^) and venous (•) plasma inosine (upper panel) and net inosine release (lower panel) during intense knee-extensor exercise. Data are means ±s.e.m.*First mean value significantly different from rest (P < 0.05).
Figure 4.
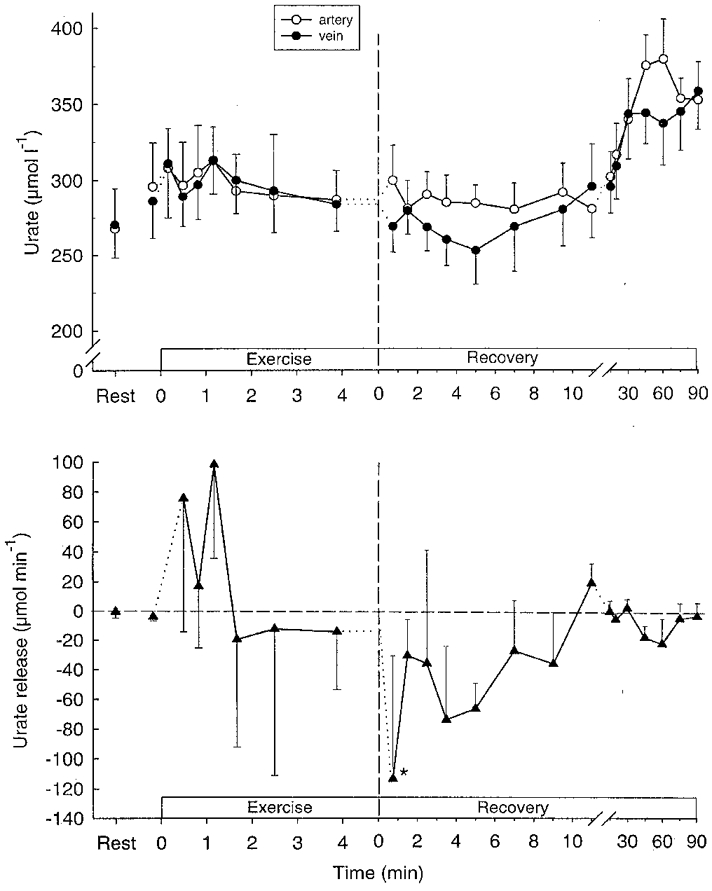
Femoral arterial (^) and venous (•) plasma urate (upper panel) and net urate release (lower panel) during intense knee-extensor exercise. Data are means ±s.e.m.*First mean value significantly different from rest (P < 0.05).
Muscle inosine increased (P < 0.05) fourfold during the first 10 min after exercise, whereas muscle hypoxanthine did not change (Table 1). In recovery, venous hypoxanthine and inosine increased (P < 0.05) further (Figs 2 and 3), and the releases of hypoxanthine and inosine peaked after 12–20 min and were still significant after 90 min of recovery (Figs 2 and 3). A significant (P < 0.05) uptake of urate was observed during the first 9.5 min of recovery as well as after 45 and 60 min of recovery (Fig. 4), with the total uptake of urate during recovery being 0.73 mmol (Table 2).
Muscle creatine phosphate, glycolytic intermediates and lactate production
Muscle creatine phosphate decreased (P < 0.05) by 40 % during the first 30 s of exercise, and had decreased by 66 % after 186 s and by 73 % at exhaustion. The rate of CP degradation was greater (P < 0.05) in the first 30 s compared to 30–186 s and 186 s to exhaustion (Fig. 5).
Figure 5.
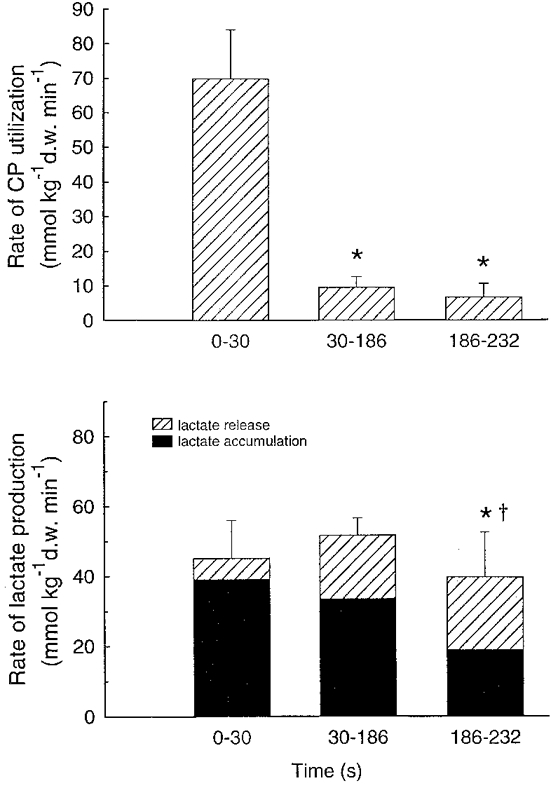
Rate of CP utilisation (upper panel) and lactate production, determined as the sum of muscle lactate accumulation and net lactate release (lower panel) during three periods (0–30, 30–186 and 186–232 s) of intense knee-extensor exercise. Data are means ±s.e.m.*Significantly different from 0–30 s (P < 0.05). †Significantly different from 30–186 s (P < 0.05).
The concentration of glucose-1-phosphate increased (P < 0.05) rapidly and reached a peak value after 30 s, whereas the concentrations of G-6-P and F-6-P rose (P < 0.05) initially and then increased (P < 0.05) further between 30 and 186 s (Table 3).
Table 3.
Skeletal muscle concentrations of glycolytic intermediates, lactate (mmol (kg d.w.)−1) and H+ (nmol l−1) during and after intense knee-extensor exercise
| Exhaustive exercise | 30 s exercise | Exercise for 80% of time to exhaustion | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | 10 min post | Pr | Post | Pre | Post | |
| G-1-P (mmol (kg d.w.−1)) | 0.67 ± 0.14 | 1.65 ± 0.46* | 1.14 ± 0.21* | 0.33 ± 0.07 | 1.97 ± 0.77* | 0.70 ± 0.18 | 1.40 ± 0.29* |
| G-6-P (mmol (kg d.w.−1)) | 0.87 ± 0.31 | 5.88 ± 1.49* | 2.14 ± 0.65*† | 0.53 ± 2.06 | 1.56 ± 1.94*‡ | 1.06 ± 0.58 | 6.60 ± 1.94* |
| F-6-P (mmol (kg d.w.−1)) | 0.12 ± 0.04 | 0.67 ± 0.21* | 0.19 ± 0.08† | 0.09 ± 0.05 | 0.28 ± 0.10‡ | 0.15 ± 0.08 | 0.54 ± 0.19* |
| Lactate (mmol (kg d.w.−1)) | 5.5 ± 1.4 | 125.6 ± 4.3* | 30.7 ± 3.3† | 4.3 ± 1.0 | 23.9 ± 5.5*‡ | 6.1 ± 2.2 | 112.0 ± 9.9* |
| H+ (nmol l−1) | 126.7 ± 18.0 | 374.5 ± 80.3* | 82.6 ± 6.2† | 122.0 ± 5.8 | 91.5 ± 16.8‡ | 114.1 ± 8.2 | 245.9 ± 69.4* |
Significantly different from pre-exercise (P < 0.05);
significantly different from post-exercise (P < 0.05);
significantly different from post-80% (P < 0.05).
The rate of muscle lactate accumulation was higher (P < 0.05) during 0–30 and 30–186 s than from 186 s to exhaustion (Fig. 5). A net lactate efflux of 2.2 mmol min−1 was observed after 30 s of exercise and the release reached 13.4 mmol min−1 at the end of exercise (Fig. 6). The release of lactate during the first 30 s was lower (P < 0.05) than during the following two periods (Fig. 5). Lactate production, determined as the sum of lactate accumulation and release, was higher (P < 0.05) from 0–30 and from 30 to 186 s than during 186 s to exhaustion, with the release accounting for 13.5, 36.6 and 53.7 % of the production, respectively (Fig. 5).
Figure 6.
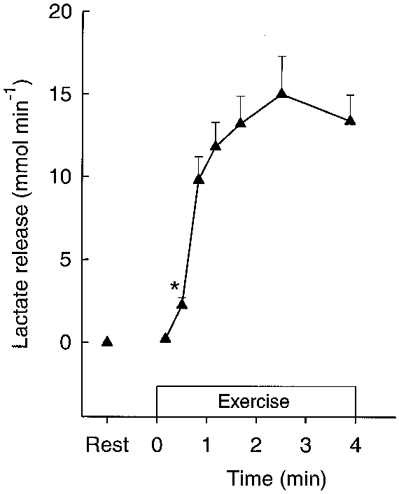
Thigh net lactate release during intense knee-extensor exercise. Data are means ±s.e.m.*First mean value significantly different from rest (P < 0.05).
Muscle H+ concentration
At rest the muscle H+ concentration was around 120 nmol l−1 (pH 6.92). The concentration did not change during the first 30 s of exercise, but had increased (P < 0.05) to 245.9 nmol l−1 (pH 6.61) after 186 s and to 374.5 nmol l−1 (pH 6.43) at exhaustion (Table 3).
DISCUSSION
The present study shows that the muscle levels of ATP, IMP and NH3 are unaltered during the initial phase of intense exercise although estimated free ADP and AMP are elevated. Furthermore, as intense exercise progresses the concentration of ATP decreases and AMP is deaminated in parallel with a lowering of pH. These findings suggest that orthophosphate, produced through the creatine kinase and ATPase reactions, is an effective inhibitor of AMP deaminase, and that an elevated H+ concentration is a major positive modulator of AMP deaminase in human skeletal muscle in vivo. The study also demonstrates that the formation of purines during intense exercise is small, whereas in recovery from exercise the purine production is pronounced and can account for a considerable loss of nucleotides from the exercised muscle.
The findings of a lack of IMP and NH3 accumulation in the muscle, as well as no NH3 release, during the first 30 s of exercise despite a significant accumulation of free ADP and AMP (which are strong activators of AMP deaminase; Wheeler & Lowenstein, 1979), suggest that AMP deaminase is inhibited in the initial phase of exercise. This inhibition may have come about by the high rate of CP utilisation leading to a stoichiometric accumulation of orthophosphate (Kushmerick & Meyer, 1985), which is known to be a major inhibitor of AMP deaminase in vitro with a Ki of 1–2 mM (Wheeler & Lowenstein, 1979; Lowenstein, 1990). It should be noted that the calculations of free ADP and AMP are based on the assumption that the creatine kinase reaction is in equilibrium during the exercise, which appears to be the case at rest (Veech et al. 1979). Moreover, the increased concentrations of free ADP and AMP were observed at 30 s of exercise, and the values do not necessarily represent the levels from 0 to 30 s of exercise. However, the greatest rate of CP utilization is observed in the early phase of exercise (Karlsson & Saltin, 1970), and thus CP probably decreased and creatine increased rapidly after onset of exercise. Even though the creatine kinase reaction may not have been in equilibrium, this most probably resulted in significant increases in free ADP and AMP early in exercise, since the concentrations of ATP and H+ were unaltered during the first 30 s. Thus, it appears reasonable to conclude that the free concentrations of ADP and AMP were elevated in the first 30 s and that AMP deaminase was inhibited.
There was a significant accumulation of IMP both between 30 and 186 s and between 186 and 234 s of exercise, although CP utilisation and, thus, probably the concentration of orthophosphate rose further. This means that the inhibitory effect of orthophosphate on the AMP-deaminase reaction was overruled. This overruling may in the first period (30–186 s) partly have been due to a significant decrease in ATP and perhaps elevated free ADP and AMP concentrations, even though the changes did not reach statistical significance probably as a result of the large variations in the concentrations between subjects (Table 1). However, the effect of the elevated free ADP and AMP concentrations did not appear to be sufficient to activate the AMP deaminase reaction, as shown for the first phase of exercise. In the last phase of exercise (186–234 s) a further IMP accumulation occurred despite a lack of change in ATP, ADP and AMP. It cannot, however, be excluded that ATP was lowered and free ADP and AMP were elevated further in compartments in the exercising muscle, which may have caused the increased IMP concentration. During both periods of exercise IMP accumulation was associated with muscle lactate accumulation leading to a lowering of muscle pH which may have played a significant role in the activation of the AMP deaminase reaction. In studies on electrically stimulated rat muscle, Dudley & Terjung (1985) observed that low muscle pH increased the AMP deaminase activity, but only when muscle pH was below 6.6. Moreover, it has been reported that the optimal pH for the enzyme is 6.5–6.1, i.e. H+ concentrations of 500–900 nmol l−1 (Setlow & Lowenstein, 1967). In the present study muscle pH was higher than 6.65 during the period from 30 to 186 s and thus, it could be questioned whether pH had an effect during this period. It should be noted that in the study by Dudley & Terjung (1985) muscle pH was estimated and that muscle pH in the present study represents an average value for a mixed muscle, probably reflecting some muscle fibres with a high and some with a low pH. Low pH levels are probably predominant in fast twitch fibres in which AMP deaminase has also been shown to be the most sensitive to pH changes (Dudley & Terjung, 1985). It is also possible that the greater AMP deamination as exercise progressed was due to a greater activation of fast twitch fibres, since these fibres have a higher AMP deaminase activity than slow twitch fibres (Jansson et al. 1987). Nevertheless, it is likely that the lowered pH was the main cause of a high rate of AMP deamination after 30 s and towards the end of exercise in part by increasing the effect of ADP on the attenuation of orthophosphate inhibition (Wheeler & Lowenstein, 1979).
In the present study the rate of net decrease in CP was the greatest in the initial phase of exercise and there was no significant change in CP between 186 s and the end of exercise. The latter finding is in accordance with the observations made by Karlsson & Saltin (1970) who found that CP had decreased by 75 % after 2 min of intense exercise and that the CP concentration was similar after 2, 6 and 16 min. The rate of CP utilisation during the first 30 s in the present study was only about half of the CP utilisation observed during 30 s of all-out cycling exercise (Bogdanis et al. 1995). Furthermore, at the end of exercise CP had not reached a minimum value and the reduction in the rate of creatine phosphate degradation occurred despite a constant elevation in the level of ADP (Tullson et al. 1995). In addition, the concentration of H+ increased progressively, which should stimulate the creatine kinase reaction. Together these findings suggest that creatine kinase became inhibited as exercise progressed or, alternatively, the rate of CP synthesis was elevated. It remains, however, unclear what could have caused these effects.
The finding of significant accumulations of G-1-P, G-6-P and F-6-P in the initial phase of exercise and of progressively increased levels of G-6-P and F-6-P suggests that the rate of glycogenolysis was higher than the rate of glycolysis throughout the exercise. The same observation has been made in studies on human muscle made to contract isometrically by electrical stimulation (Spriet et al. 1987b; Ren et al. 1988). The rate of glycolysis with lactate formation was higher from 30–186 s than in the last period of exercise, although positive modulators of phosphofructokinase (PFK), such as F-6-P, free ADP, AMP, Pi and NH3, were elevated and negative modulators like ATP and CP were lowered towards the end of exercise (Bangsbo, 1996). This finding could be explained by a lowering of pH, which has been shown in vitro to inhibit PFK (Dobson et al. 1986). It should be pointed out, however, that in studies on human muscle in vivo the effect of low pH on PFK has been found to be minor (Bangsbo et al. 1996). An alternative explanation could be found in that the activity of pyruvate dehydrogenase (PDH) and the respiratory rate are both maximal after 186 s of high intensity exercise (Bangsbo et al. 1990; Hultman, 1996). This should lead to a high rate of mitochondrial uptake of the substrates for the near-equilibrium lactate dehydrogenase (LDH) reaction, pyruvate and NADH, limiting the formation of lactate. Nevertheless, it may be that the elevated concentration of H+ inhibited glycolysis and at the same time eliminated a further increase in free ADP by stimulating the AMP deaminase reaction, when the majority of ATP was delivered from oxidative phosphorylation (Bangsbo et al. 1990).
The progessive accumulation of IMP and NH3 has been proposed to cause the development of fatigue (Sahlin, 1992), but fatigue has been shown to coincide with different levels of IMP when repeated exercise was performed at various muscle glycogen levels (Bangsbo et al. 1992a). Therefore, high IMP and NH3 concentrations are not likely to be the main cause of fatigue. In general, the finding of a relatively high muscle ATP concentration and an unaltered CP concentration in the last phase of exercise speaks against the theory of fatigue during intense exercise being caused by a lack of energy. It cannot, however, be excluded that ATP and CP are not equally distributed within the muscle cell during intense exercise, e.g. it may be that the ATP concentration falls below a critical level in the vicinity of the ATP utilisation sites (McClellan et al. 1983; Bessman & Savabi, 1988). It should be noted, however, that the data supporting ATP compartmentalisation are indirect. Low pH at the end of exercise may not be the cause of fatigue either, since it has been demonstrated that development of fatigue can occur at different levels of pH (Bangsbo et al. 1996). Therefore, at present it is unknown what reduces force production during intense exercise.
During exercise there was no accumulation of inosine and hypoxanthine in the muscle and the release of these compounds from muscle into the blood was small, suggesting that purine formation was minor during exercise. On the other hand, in recovery the muscle inosine concentration increased and a pronounced release of both inosine and hypoxanthine was found. These data indicate that dephosphorylation of IMP occurs only to a minor extent during exercise, but that the rate is markedly increased upon termination of exercise. Dephosphorylation of IMP to inosine occurs via the enzyme 5′-nucleotidase, which also dephosphorylates AMP to adenosine. Studies using the dog hindlimb model have shown that lactate increases the release of adenosine from skeletal muscle (Ballard, 1991). In vitro studies have also shown that the activity of 5′-nucleotidase may be inhibited by ATP and increased by a low adenylate charge value (Camici et al. 1985). However, in the present study IMP dephosphorylation did not appear to coincide with either the lactate production, or with the estimated free adenine nucleotide levels. Thus, it remains uncertain what factors may regulate IMP dephosphorylation in human muscle in vivo.
During the first 10 min of recovery, 73 % of the decrease in IMP and ADP could be accounted for as net resynthesis of ATP, whereas the production of purines corresponded to 25 % and accumulation of AMP within the muscle covered the remaining 1–2 % (Fig. 7). During the 90 min of recovery the combined release of hypoxanthine and inosine amounted to 1.08 mmol (kg d.w.)−1 corresponding to 37 % of the IMP accumulated during the exercise. Together with release during exercise the total purine release amounted to 44 % of the decrease in muscle ATP during exercise or a loss of about 7 % of the total adenine nucleotide pool (Table 2 and Fig. 7). This is slightly higher than observed in recovery from long term intermittent exercise and after 10 min of exhaustive cycling exercise (Hellsten et al. 1994; Hellsten et al. 1998). The lost nucleotides have to be resynthesized de novo, a process that can be estimated to take about 18 h using an average rate of 25 μmol h−1 (kg w.w.)−1 for rat mixed muscle after contraction (Tullson & Terjung, 1991). When intense exercise is performed frequently the release of purines may lead to a cumulative loss of nucleotides from the exercised muscles as was observed in two studies using high intensity training. Both studies showed an approximate 20 % reduction in the resting levels of muscle ATP after 6–8 weeks of training (Hellsten-Westing et al. 1993; Stathis et al. 1994).
Figure 7.
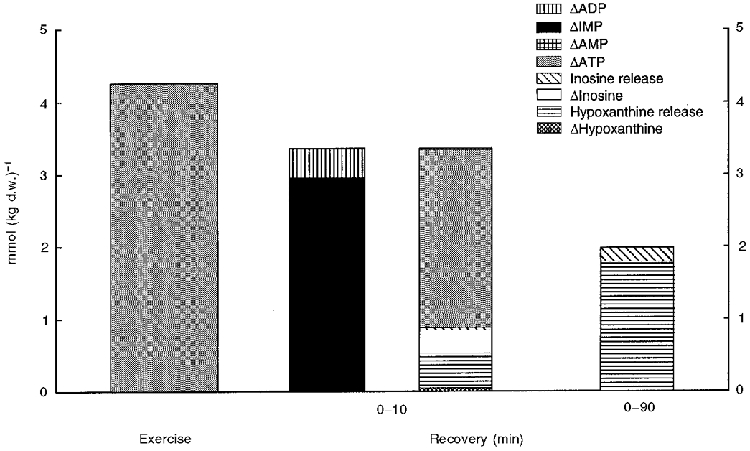
Muscle net ATP degradation during intense knee-extensor exercise (left) and changes in muscle nucleotides and purines as well as exchange of purines during recovery. Muscle net IMP decrease (▪) and ADP decrease ( ) is compared to AMP (
) is compared to AMP ( ) and ATP (
) and ATP ( ) accumulation; accumulation (□) and release (
) accumulation; accumulation (□) and release ( ) of inosine as well as accumulation (
) of inosine as well as accumulation ( ) and release (
) and release ( ) of hypoxanthine during the first 10 min of recovery (middle). In addition, total release of purines during 90 min of recovery is shown (right).
) of hypoxanthine during the first 10 min of recovery (middle). In addition, total release of purines during 90 min of recovery is shown (right).
Urate has been hypothesised to play a role in the antioxidative defence of the muscle during exercise. During intense exercise the muscle urate level is decreased and the oxidation product of urate in muscle, allantoin, is increased in parallel, indicating a radical induced oxidation of urate in the exercising human muscle (Hellsten et al. 1996). The present study shows a pronounced uptake of urate in the muscle in recovery from exercise amounting to about 0.95 mmol or 0.40 mmol (kg w.w.)−1. Although muscle urate levels were not measured in the current study, the uptake of urate corresponds well to the decrease in muscle urate levels observed in a previous study during 3–4 min of exhaustive cycling of about 0.30 mmol (kg w.w.)−1 (Tullson et al. 1995).
In summary, the present study showed that AMP deamination did not occur during the first 30 s of intense exercise, although positive modulators such as free ADP and AMP were elevated. After 30 s of intense exercise AMP deamination took place in parallel with a decrease in muscle pH, suggesting an important role of elevated H+ concentration in the regulation of AMP deamination in contracting human skeletal muscle in vivo. Formation of purines was minor during exercise, but pronounced during recovery, and the total release of purines amounted to a considerable loss of nucleotides.
Acknowledgments
We thank Merete Vannby, Ingelise Kring and Karina Olsen for excellent technical assistance. The study was supported by grants from The Danish National Research Foundation (504–14). In addition support was obtained from Team Denmark and The Sports Research Council (Idrættens Forskningsråd).
References
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for the study of an isolated exercising muscle in man. Journal of Applied Physiology. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard HJ. The influence of lactic acid on adenosine release from skeletal muscle in anaesthetized dogs. The Journal of Physiology. 1991;433:95–108. doi: 10.1113/jphysiol.1991.sp018416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J. Regulation of muscle glycogenolysis and glycolysis during intense exercise: in vivo studies using repeated intense exercise. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of Exercise IX. Champaign, IL, USA: Human Kinetics Publishers, Inc.; 1996. pp. 261–276. [Google Scholar]
- Bangsbo J, Gollnick PD, Juel C, Kiens B, Mizuno M, Saltin B. Anaerobic energy production and deficit-debt relationship during exhaustive exercise in humans. The Journal of Physiology. 1990;422:539–559. doi: 10.1113/jphysiol.1990.sp018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in humans. The Journal of Physiology. 1992a;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. The Journal of Physiology. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Sjödin B, Hellsten-Westing Y. Exhange of hypoxanthine in muscle during intense exercise in man. Acta Physiologica Scandinavica. 1992b;146:549–550. doi: 10.1111/j.1748-1716.1992.tb09465.x. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Savabi F. The role of the phosphocreatine energy shuttle in exercise and muscle hypertrophy. International Series on Sports Sciences. 1988;21:167–178. [Google Scholar]
- Bogdanis GC, Nevill ME, Boobis LH, Lakomy HKA, Nevill AM. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. The Journal of Physiology. 1995;482:467–480. doi: 10.1113/jphysiol.1995.sp020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici M, Fini C, Ipate PLL. Isolation and kinetic proterties of 5′-nucleotidase from guinea-pig skeletal muscle. Biochimica et Biophysica Acta. 1985;840:6–12. doi: 10.1016/0304-4165(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Dobson GP, Yamamoto E, Hochachka PW. Phosphofructokinase control in muscle: nature and reserval of pH-dependent ATP inhibition. American Journal of Physiology. 1986;250:R71–76. doi: 10.1152/ajpregu.1986.250.1.R71. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Terjung RL. Influence of acidosis on AMP deaminase activity in contracting fast-twitch muscle. American Journal of Physiology. 1985;248:C43–50. doi: 10.1152/ajpcell.1985.248.1.C43. [DOI] [PubMed] [Google Scholar]
- Graham TE, Bangsbo J, Gollnick PD, Juel C, Saltin B. Ammonia metabolism during intense dynamic exercise and recovery in humans. American Journal of Physiology. 1990;259:E170–176. doi: 10.1152/ajpendo.1990.259.2.E170. [DOI] [PubMed] [Google Scholar]
- Harris RT, Dudley GA. Exercise alters the distribution of ammonia and lactate in blood. Journal of Applied Physiology. 1989;66:313–317. doi: 10.1152/jappl.1989.66.1.313. [DOI] [PubMed] [Google Scholar]
- Hellsten Y. Xanthine dehydrogenase and purine metabolism in man. With special reference to exercise. Acta Physiologica Scandinavica. 1994;151(suppl. 621):21–26. [PubMed] [Google Scholar]
- Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radical Biology and Medicine. 1996;22:169–174. doi: 10.1016/s0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Sjödin B, Richter EA, Bangsbo J. Urate uptake and lowered ATP levels in human muscle after high-intensity intermittent exercise. American Journal of Physiology. 1998;274:E600–606. doi: 10.1152/ajpendo.1998.274.4.E600. [DOI] [PubMed] [Google Scholar]
- Hellsten-Westing Y, Ekblom B, Kaijser L, Sjödin B. Exchange of purines in human liver and skeletal muscle with short-term exhaustive exercise. American Journal of Physiology. 1994;266:R81–86. doi: 10.1152/ajpregu.1994.266.1.R81. [DOI] [PubMed] [Google Scholar]
- Hellsten-Westing Y, Norman B, Balsom PD, Sjödin B. Decreased resting levels of adenine nucleotides in skeletal muscle following high intensity intermittent exercise in man. Journal of Applied Physiology. 1993;74:2523–2528. doi: 10.1152/jappl.1993.74.5.2523. [DOI] [PubMed] [Google Scholar]
- Hultman E. Pyruvate dehydrogenase as a regulator of substrate utilization in skeletal muscle. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of Exercise IX. Champaign, IL, USA: Human Kinetics Publishers, Inc.; 1996. pp. 157–172. [Google Scholar]
- Jansson E, Dudley GA, Norman B, Tesch PA. ATP and IMP in single human muscle fibres after high intensity exercise. Clinical Physiology. 1987;7:337–345. doi: 10.1111/j.1475-097x.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Jones PRM, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. The Journal of Physiology. 1969;204:36. P. [PubMed] [Google Scholar]
- Karlsson J, Saltin B. Lactate, ATP and CP in working muscles during exhaustive exercise in man. Journal of Applied Physiology. 1970;29:598–602. doi: 10.1152/jappl.1970.29.5.598. [DOI] [PubMed] [Google Scholar]
- Kun E, Kearney EB. Ammonia. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1802–1805. [Google Scholar]
- Kushmerick MJ, Meyer RA. Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. American Journal of Physiology. 1985;248:C542–549. doi: 10.1152/ajpcell.1985.248.5.C542. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate tranfer reactions. Journal of Biological Chemistry. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lowenstein JM. The purine nucleotide cycle revised. International Journal of Sports Medicine. 1990;11:S37–46. doi: 10.1055/s-2007-1024853. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- McClellan G, Weisberg A, Winegrad S. Energy transport from mitochondria to myofribril by a creatine phosphate shuttle in cardiac cells. American Journal of Physiology. 1983;245:C423–427. doi: 10.1152/ajpcell.1983.245.5.C423. [DOI] [PubMed] [Google Scholar]
- Nevill ME, Bogdanis GC, Boobis LH, Lakomy HKA, Williams C. Muscle Metabolism and Performance During Sprinting. In: Maughan RJ, Shirreffs SM, editors. Biochemistry of Exercise IX. Champaign, IL, USA.: Human Kinetics Publishers, Inc.; 1996. pp. 243–260. [Google Scholar]
- Parkhouse WS, McKenzie DC, Hochachaka PW, Ovalle WK. Buffer capacity of deproteinized human vastus lateralis muscle. Journal of Applied Physiology. 1985;58:14–17. doi: 10.1152/jappl.1985.58.1.14. [DOI] [PubMed] [Google Scholar]
- Ren JM, Chasiotis D, Bergström M, Hultman E. Skeletal muscle glucolysis, glycogenolysis and glycogen phosphorylase during electrical stimulation in man. Acta Physiologica Scandinavica. 1988;133:101–107. doi: 10.1111/j.1748-1716.1988.tb08387.x. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Tullson PC, Terjung RL. Altered kinetics of AMP deaminase by myosin binding. American Journal of Physiology. 1992;263:C294–299. doi: 10.1152/ajpcell.1992.263.2.C294. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Metabolic aspects of fatigue in human skeletal muscle. In: Marconnet P, Komi PV, Saltin B, Sejersted OM, editors. Muscle Fatigue Mechanisms in Exercise and Training. Vol. 34. Basel: Karger; 1992. pp. 54–68. [Google Scholar]
- Sahlin K, Palmskog G, Hultman E. Adenine nucleotide and IMP contents of the quadriceps in man after exercise. Pflügers Archiv. 1978;374:193–198. doi: 10.1007/BF00581301. [DOI] [PubMed] [Google Scholar]
- Setlow B, Lowenstein JM. Adenylate deaminase. II. Purification and some regulation properties of the enzyme from calf brain. Journal of Biologial Chemistry. 1967;242:607–615. [PubMed] [Google Scholar]
- Shiraki H, Ogawa H, Matsuda Y, Nakagawa H. Interaction of rat muscle AMP deaminase with myosin: II. Modification of the kinetic and regulatory properties of rat muscle AMP deaminase by myosin. Biochimica et Biophysica Acta. 1979;566:345–352. doi: 10.1016/0005-2744(79)90038-x. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Söderlund K, Bergström M, Hultman E. Anaerobic energy release in skeletal muscle during electrical stimulation in men. Journal of Applied Physiology. 1987a;62:611–615. doi: 10.1152/jappl.1987.62.2.611. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Söderlund K, Bergström M, Hultman E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. Journal of Applied Physiology. 1987b;62:616–621. doi: 10.1152/jappl.1987.62.2.616. [DOI] [PubMed] [Google Scholar]
- Stathis CG, Febbraio MA, Carey MF, Snow RJ. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. Journal of Applied Physiology. 1994;76:1802–1809. doi: 10.1152/jappl.1994.76.4.1802. [DOI] [PubMed] [Google Scholar]
- Tullson PC, Bangsbo J, Hellsten Y, Richter EA. IMP metabolism in human skeletal muscle after exhaustive exercise. Journal of Applied Physiology. 1995;78:146–152. doi: 10.1152/jappl.1995.78.1.146. [DOI] [PubMed] [Google Scholar]
- Tullson PC, Terjung RL. Adenine nucleotide synthesis in exercising and endurance-trained skeletal muscle. American Journal of Physiology. 1991;261:C342–347. doi: 10.1152/ajpcell.1991.261.2.C342. [DOI] [PubMed] [Google Scholar]
- Tullson PC, Whitlock DM, Terjung RL. Adenine nucleotide degradation in slow-twitch red muscle. American Journal of Physiology. 1990;258:C258–265. doi: 10.1152/ajpcell.1990.258.2.C258. [DOI] [PubMed] [Google Scholar]
- Veech RL, Lawson JWR, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. Journal of Biological Chemistry. 1979;254:6538–6547. [PubMed] [Google Scholar]
- Wheeler TJ, Lowenstein JM. Adenylate deaminase from rat muscle. Journal of Biological Chemistry. 1979;254:8994–8999. [PubMed] [Google Scholar]


